Abstract
Objective
Voiding cystourethrography (VCUG) has been considered as the gold standard technique for the diagnosis of vesicoureteral reflux (VUR). But, it requires fluoroscopic guidance which expose children to radiation. Voiding urosonography (VUS) is technically analogous to VCUG and has the major advantage of zero radiation exposure. This study aims to determine the efficacy of contrast enhanced-VUS (ce-VUS) with respect to VCUG in diagnosing VUR.
Material and methods
This study involves 30 children over a period of 3 years. All patients underwent a VCUG followed by the ce-VUS on the same day. All VUS studies were done by the same sonologist in the sonography department. The images were recorded and reviewed by the same sonologist before reporting.
Results
The median age of the patients was 51.53 months. There were 21 males and 9 females. On VCUG, 16 patients had no reflux, and 14 patients had reflux. On ce-VUS, 14 patients had no VUR, and 16 patients had VUR. Of the total 58 kidney-ureter units (KUUs), VUR was detected in 17 KUUs on VCUG and in 21 KUUs on ce-VUS. Thus, ce-VUS detected 4 refluxing units that were not seen on VCUG. In right KUUs, ce-VUS detected VUR in 3 units where no reflux was found in VCUG. In the 28 left KUUs, 25 units on ce-VUS showed concordance with the grade of VUR as detected by VCUG; 3 were discordant. Two units on ce-VUS showed a VUR one grade higher than the corresponding grade on VCUG and in one unit it was one grade lower. Thus, in total, ce-VUS picked up 4 cases which were missed by VCUG.
Conclusion
ce-VUS is a good imaging modality when compared to voiding cystourethrography to assess pediatric vesicoureteral reflux, in view of its superior diagnostic performance, feasibility and radiation safety for children.
Keywords: Vesicoureteral reflux, voiding cystourethrography, voiding urosonography
Introduction
Vesicoureteral reflux (VUR) is the retrograde flow of urine from the bladder into the ureter and towards the kidney. It is an important cause for urinary tract infections (UTIs). Its prevalence varies from 1.3% in healthy children to 8–50% among children evaluated after an UTI.[1–3] A cross-sectional study by Fong et al.[4,5] has demonstrated that vesicoureteral reflux is present in 30% of boys and 43% of girls presenting with symptomatic UTI in infancy. VUR is caused both by a developmental anomaly related to inadequate length of intravesical submucosal ureter and a dysfunctional problem where many patients have associated dysfunction of bladder and bowel emptying.[4,6] It is associated with reflux nephropathy and renal scarring.[4,7] VUR needs to be excluded in patients with hydronephrosis, renal scarring and other findings that suggest high-grade vesicoureteral reflux or obstructive uropathy on renal ultrasound.[4,8] Early detection and effective treatment of UTI is important to prevent sequelae. The conventional reflux imaging modalities for diagnosing VUR are voiding cystourethrography (VCUG), radionuclide cystography (RNC) and voiding urosonography (VUS).[4]
Voiding cystourethrography has been considered as the gold standard technique for the diagnosis of VUR.[4] However, it requires urinary catheterization and fluoroscopic imaging which expose children to radiation. VUR is graded by the International Reflux Grading System (IRGS), which classifies VUR into five grades- Grade I: Reflux into ureter, Grade II: Reflux into non-dilated renal pelvis, Grade III: Reflux into mildly dilated renal pelvis, Grade IV: Reflux into moderately dilated renal pelvis, Grade V: Reflux into severely dilated renal pelvis with tortuous ureter.[4,9] The major drawback of VCUG is the patient’s exposure to the radiation, which may be repeated more than once during the course of VUR management.[4] During a single VCUG procedure, the child is exposed to a radiation dose of approximately 0.64–0.807 mSv, which is equal to 20–35 chest X-rays.[4,10]
Radionuclide cystography involves bladder catheterization and intravesical administration of radiopharmaceuticals and has the advantages of continuous examination of kidneys and bladder during filling phase, with lower gonadal radiation dose.[4,11,12] The diagnostic performance is comparable to VCUG and the cost is also lower.[4,11,13] But, owing to its lower spatial resolution and impaired anatomical delineation of the urethra, RNC is generally used for follow-up of patients with known vesicoureteral reflux.[4,12]
To avoid radiation exposure during diagnostic imaging for VUR, the first attempts began in the mid-1970s which were presented by Darge.[14] Previously known as reflux sonography, echocystography, cystosonography, and echo-enhanced cystography, the most widely applied term Voiding Urosonography with the abbreviation “VUS” was proposed for the first time in 2000.[4,15–18] VUS is technically analogous to VCUG- an ultrasound contrast agent is administered intravesically via the urinary catheter, followed by continuous, alternate examination of the kidneys, urinary bladder, and retrovesical region during filling and voiding phases, as well as the urethra via transperineal or interscrotal approach during voiding phase.[4] The diagnosis of vesicoureteral reflux is determined by the presence of moving echogenic microbubbles arising from contrast material used during ultrasonographic examination of the upper urinary tract.[4] The grading of VUR is done by the five-tier grading system by Darge and Troeger[4,19]:
Grade I: Microbubbles only in the ureter,
Grade II: Microbubbles in the renal pelvis; no significant renal pelvic dilation,
Grade III: Microbubbles in the renal pelvis + significant renal pelvic dilation + moderate calyceal dilatation,
Grade IV: Microbubbles in the renal pelvis + significant renal pelvic dilation + significant calyceal dilatation,
Grade V: Microbubbles in the renal pelvis + significant renal pelvic dilation and calyceal dilatation + loss of renal pelvis contour + dilated tortuous ureters.
In VUS, the contrast agents used are normal saline, albumin and first-generation contrast agent like Levovist® (Schering Health Care Co, Germany).[4] There are some limitations of Levovist including low shelf life, early disintegration time and high quantity of dye required.[4,18–20] This has led to widespread use of second- generation contrast agent containing sulphur hexafluoride molecules (SonoVue®, Bracco, Milan, Italy) in VUS.[4,19,20] The major advantage of VUS is the zero radiation exposure even during multiple procedures for the management of VUR in children.
This study aims to determine the efficacy of contrast enhanced-VUS (ce-VUS) using second generation contrast agent in comparison with VCUG in diagnosing VUR.
Material and methods
This is a cross sectional analytical study involving 30 children over a period of 3 years. Children with first attack of febrile UTI below one year of age, recurrent urinary tract infections with suspect underlying VUR, antenatally diagnosed hydronephrosis (which required evaluation for the presence of a VUR), known cases of posterior urethral valves or neurogenic bladder necessitating follow-up evaluation for the presence of a VUR and medically and surgically treated children that needed follow-up re-evaluation were included in the study. Children with active UTI were excluded from this study.
The study was started after receiving approval from the hospital ethics committee. Written informed valid consent of the patient was taken for the participation in the study including both procedures.
A detailed history was obtained concerning primary, current, and past medical problems. For each new patient, laboratory and imaging studies were performed as indicated including routine urinalysis, culture, renal function tests, CBC and ultrasonography of the kidneys and bladder. For previously diagnosed patients, the previous medical records, laboratory data and imaging studies were reviewed.
Rutine urinalyses were performed anew in all cases to ensure absence of active UTI prior to the study. Antibiotic prophylaxis was given to all patients before the procedure which consisted of amoxicillin-clavulinic acid, given at the dose of 40 mg/kg/day. The first dose was administered one hour prior to the procedure followed by additional 5 doses at 12 hourly intervals.
All patients underwent a VCUG followed by ce-VUS on the same day. Ce-VUS was performed using the Phillips IU-22 (Eindhoven, The Netherlands) ultrasound machine equipped with a C5–2MHz convex transducer. Specific contrast detection software with a mechanical index of 0.7 was used. All VUS studies were done by the same sonologist in the sonography department.
Using a 50 cc syringe and depending on the age of the child, an appropriate volume of normal saline was drawn into the syringe, and mixed with the 5–10 drops of second- generation contrast agent which was instilled into the bladder slowly. The examination was done by using both the tissue harmonic and contrast harmonic modes. The bladder, kidney and ureters were monitored. The presence of microbubbles in the kidney-ureter unit (KUU), which appeared as strong hyperechoic signals both in tissue harmonic and contrast harmonic imaging studies was considered diagnostic for VUR. Finally, a post-voiding check of the bladder and kidneys was performed. The images were recorded and reviewed by the same sinologist before reporting. The results were graded according to the classification system by Darge et al.[4,19].
The estimated sample size for detection of VUR in children with urinary tract infections requires a minimum of 30 patients with H0: incidence of VUR =25% H1:50% with power of 80% and alpha=0.05. The formula used is Z test for Binomial Proportion. Sample size calculation was performed using SAS 9.2 software.
Results
Thirty children who required an imaging study to detect VUR were included in the study. The mean age of the patients was 51.53 months (range, 1–146 months). The patients (male, n=21, and female, n=9) were <1 (n=5), 1–5 (n=14), and >5 (n=11) years old.
VCUG was indicated for the patients with recurrent UTI (n=16), and for the follow-up evaluation of surgically (n=8) or medically treated (n=2) VUR patients, and for the evaluation of antenatally detected hydronephrosis in 2 patients. One patient was being followed up after bladder stone removal and the other one had a history of abdominal pain and pyuria (Table 1).
Table 1.
Indications of radiological evaluation in children with VUR
| Indications | Frequency | % |
|---|---|---|
| Febrile UTI | 16 | 53.33 |
| Follow-up of post-op VUR | 8 | 26.67 |
| Follow-up of medically treated VUR | 2 | 6.67 |
| Follow-up of antenatally detected HN | 2 | 6.67 |
| Follow-up of bladder stone removal | 1 | 3.33 |
| Abdominal pain with pyuria | 1 | 3.33 |
| Total | 30 | 100 |
UTI: urinary tract infection; VUR: vesicoureteral reflux; HN: hydronephrosis
Spina bifida (n=2), post- traumatic urethral stricture (n=1), and hydrocephalus (n=1) were associated comorbidities in indicated number of patients. The remaining patients had no comorbidities. Eighteen patients had recurrent episodes of UTI, whereas 7 had one previous episode of UTI. Fifteen patients had no prior VCUG study. Of the 15 patients who had a previous VCUG study, there were a total of 28 kidney-ureter units (KUUs) as 2 patients had only a single kidney each. Of these 28 KUUs, 23 had a reflux involving the right (n=14), and left (n=9) KUUs.
Of these 15 patients, 4 had been treated medically (26.67%) and 11 had received both surgical and medical treatment. Nine patients had no associated anomalies. Two patients had solitary kidney due to a previous nephro-ureterectomy (n=1) and congenital solitary ectopic kidney (n=1). Two patients had bladder diverticula. One patient had an associated ureterocele and the other one had neurogenic bladder.
In this study, all 30 patients underwent VCUG and ce-VUS sequentially on the same day. On VCUG, 16 patients had no reflux, while 14 patients had either primary (n=11) or secondary (n=3) VUR. On ce-VUS, 14 patients had no reflux, while 16 patients had either primary (n=12) or secondary (n=4) VUR. ce-VUS detected reflux in 2 patients that were not seen on VCUG (Table 2).
Table 2.
Types of VUR detected on both VCUG and ce-VUS in 30 patients
| VUR type | VCUG | ce-VUS |
|---|---|---|
| No reflux | 16 | 14 |
| Primary | 11 | 12 |
| Secondary | 3 | 4 |
| Total | 30 | 30 |
VUR: vesicoureteral reflux; VCUG: voiding cystourethrography; VUS: voiding urosonography
A total of 58 KUUs were evaluated in 30 patients including 30 right KUUs, and 28 left KUUs ( 2 patients had solitary right kidneys). VUR was detected in 17 KUUs on VCUG and in 21 KUUs on ce-VUS. Thus, ce-VUS detected 4 refluxing units that were not seen on VCUG (Figure 1).
Figure 1.
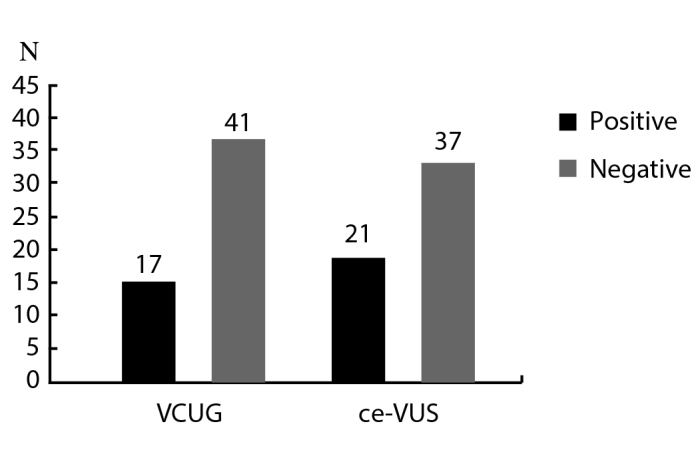
Comparison of KUUs detected on VCUG and ce-VUS
KUU: kidney-ureter units; VCUG: voiding cystourethrography; VUS: voiding urosonography
Of the total 30 right KUUs, VCUG detected VUR in 11 KUUs [grade III (n=5), I (n=1), IV (n=2, and V (n=1) refluxes] and ce-VUS detected VUR in 14 KUUs [grade III (n=5), I (n=5), IV (n=3), and V (n=1)] (Table 3). Of the total 28 left KUUs, VCUG detected VUR in 6 KUUs [ grade III (n=3), and n=3 for grades I, IV and V each) and ce-VUS detected VUR in 7 KUUs [n=2 for grades III and grade V each and grade II, n=1)] (Table 4).
Table 3.
Comparison of right KUUs regarding grades of VUR detected on VCUG and ce-VUS
| Grade of VUR | VCUG | ce-VUS | Discordant |
|---|---|---|---|
| 0 | 19 | 16 | 2 Grade I 1 Grade 4 |
| I | 3 | 3 | |
| II | 0 | 0 | |
| III | 5 | 5 | |
| IV | 2 | 2 | |
| V | 1 | 1 | |
| Total | 30 | 27 | 3 |
KUU: kidney- ureter units; VUR: vesicoureteral reflux; VCUG: voiding cystourethrography; VUS: voiding urosonography
Table 4.
Comparison of left KUUs regarding grades of VUR detected on VCUG and ce-VUS
| Grade of VUR | VCUG | ce-VUS | Discordant |
|---|---|---|---|
| 0 | 22 | 21 | 1 Grade I |
| I | 1 | 1 | |
| II | 0 | ||
| III | 3 | 2 | 1 Grade II |
| IV | 1 | 1 Grade V | |
| V | 1 | 1 | |
| Total | 28 | 25 | 3 |
KUU: kidney-ureter units; VUR: vesicoureteral reflux; VCUG: voiding cystourethrography; VUS: voiding urosonography
Thus in right KUUs, the severity of VUR assessed by ce-VUS was identical to that assessed by VCUG in 27 out of 30 units. However, ce-VUS detected VUR in 3 units where no reflux was found in VCUG. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of ce-VUS in right KUUs were 100%, 84.2%, 78.5%, and 100%, respectvely. Cohen’s kappa cost-effectiveness was 0.8, and the level of statistical significance was set at p<0.001. Hence there was a strong agreement between ce-VUS and VCUG regarding right KUUs.
In the 28 left KUUs, 25 units on ce-VUS showed concordance with the grade of VUR as detected by VCUG, while discordant results were detected in 3 cases. Two units on VUS showed a VUR one grade higher than the corresponding grade on VCUG and in one unit it was one grade lower. The sensitivity, specificity, PPV and NPV of ce-VUS in left KUUs were 100%, 90.9%, 75%, and 100%, respectvely. Cohen’s kappa coefficient was 0.90, and level of statistical significance was set at p<0.001 which indicated a strong agreement between ce-VUS and VCUG regarding left KUUs.
Thus, in total, among 58 KUUs, VCUG detected 17. while ce-VUS 21 cases of VUR. Thus ce-VUS picked up 4 cases which were missed by VCUG. ce-VUS had 100% sensitivity, 87.8% specificity, 77.27% PPV and 100% NPV. Cohen’s kappa coefficient was 0.84, and level of statistical significance was set at p<0.001. There was a strong agreement between VCUG and ce-VUS regarding both right and left KUUs.
Discussion
Voiding cystourethrography is considered as the gold standard for the diagnosis or exclusion of VUR, despite the relatively high dose of ionizing radiation delivered during the procedure.[4] Pulsed fluoroscopy is the standard mode in many modern pediatric radiology departments which has resulted in significant reduction in radiation doses. However, most urological patients who have a VUR undergo several repeat VCUG procedures to evaluate the outcome of the VUR in terms of resolution or worsening of VUR. This exposes the child to a cumulative high dose of ionizing radiation even when adequate precautions are taken to deliver as low a radiation dose as possible.[4,10]
In view of the increased life-time risk of malignancy associated with cumulative exposure to ionizing radiation, there is a growing interest in the use of radiation-free imaging techniques. Contrast-enhanced VUS is an attractive option to demonstrate the presence, grade, and severity of VUR.[4] It can be repeated frequently if needed, as there is no radiation exposure. A further advantage is the availability of second-generation contrast agents for ce-VUS which allows the use of substantially lower volumes compared to the first-generation contrast agents, leading to a significant reduction in cost of the procedure.[4,18]
With the recent development of contrast-specific ultrasound techniques and the availability of stable US contrast agents; ce-VUS needs to be evaluated in comparison with VCUG to determine its efficacy in detecting and grading VUR and to assess its suitability to replace VCUG as an imaging modality of choice for VUR.[4,21,22] Very few studies have so far looked at the comparison of these two techniques.[4,21–26]
In this study, the sensitivity of ce-VUS in detecting VUR was 100% when compared to VCUG. ce-VUS did not miss any VUR which was detected by VCUG. This is in concordance with studies done earlier by Ascenti et al.[26], Ključevšek et al.[25] and Tse et al.[23] who also found that ce-VUS had 100% sensitivity. Tse et al.[23] compared the two procedures and reported that ce-VUS did not miss any VUR that was detected on VCUG.[4] However, few studies have shown a lesser sensitivity ranging from 80 to 86%.[22,28,29] The number of refluxing units missed by ce-VUS in these studies was small, ranging from 1 to 14 accounting for <1% to 3.8% of all refluxing units.[22,27,28]
In this study, the specificity of ce-VUS was 90.24%. Studies by Tse et al.[23] and Kis et al.[28] showed a slightly lesser specificities of 85 and 86%, respectively. Studies by Darge et al.[27] and Ascenti et al.[26] showed a relatively higher specificity of 92 to 97%. The sensitivity and specificity were similar for both the right and left KUUs.
In this study, ce-VUS detected a total of 22 refluxing units while VCUG detected 17 units. This finding is similar to other studies in literature.[4,23–28] Among the five cases detected by only ce-VUS in this study, four were grade I in severity which may not have major clinical implications. However, in a single case, ce-VUS detected a grade IV reflux which was not seen on VCUG that changed the plan of management.
In this study, there was an excellent correlation between ce-VUS and VCUG for identical grades of reflux in 52 of the 58 (93%). Discrepancy was seen in 6 units. In all these six cases, ce-VUS documented a higher grade of reflux when compared to VCUG. In 5 out of 6 cases the grade differed by one. In only one case the difference was of a magnitude of four. This slightly higher grade noted in five cases might have been due to the fact that contrast-specific US harmonic techniques are more sensitive at depicting even a few refluxing microbubbles of contrast agent compared to VCUG that demands a larger volume of refluxing contrast agent diluted in urine, especially in cases with dilated systems.[4]
There was one case with VUR grade IV detected on VUS that was entirely missed on VCUG. The review of the VCUG suggested this could have been a technical fault in the performance of the VCUG as the bladder did not appear full in the initial phase.
This study is, however, not in agreement with the findings of the study done by Darge et al.[24] and Tse et al.[23] which stated that the refluxes missed by VCUG were predominantly of higher grade. The fact that VCUG might underestimate or miss reflux may be partly explained by the intermittent nature of VUR, the potential for marked dilution of contrast agent in a dilated system, and the shorter fluoroscopic guidance during VCUG.[4] Conversely, prolonged observation is an advantage of ce-VUS that allows a higher number of patients with VUR and possibly higher grades of VUR to be diagnosed compared to VCUG. Due to the increased sensitivity of harmonic imaging, even sporadic microbubbles can be reliably visualized.[4] Hence, ce-VUS is a modality in which VUR detection can be done better than relative to the standard VCUG technique.[4]
The major attraction in using ce-VUS is its lack of ionizing radiation. In a recent study on radiation dose of pediatric VCUG by Sulieman et al.[29], the mean entrance surface dose for VCUG with positive reflux was 1.45 mGy, and negative reflux was 1.05 mGy. As gonads were inside the radiation field during the examination, there was a higher organ equivalent dose to ovaries (0.44 mSv) and testes (0.33 mSv) than to thyroid (0.006 mSv).[29] The estimated risks of malignancy of ovaries and testes were 4.4 × 10−7 and 3.3 × 10−7, respectively.[29] Considering that many of these children are likely to undergo more than one VCUG, in addition to other imaging modalities using ionizing radiation, it becomes even more imperative to consider imaging modalities with no radiation exposure such as ce-VUS.
ce-VUS is a new procedure and many sonologists may currently lack the experience of doing and interpreting the test results. According to a recent review by Prasad and Cheng, the techniques of ce-VUS are operator-dependent and require highly skilled sonographers.[4,30] Cost of ce-VUS is a second limiting factor. Because after reconstitution, microbubbles are stable for approximately 6 hours, a 5 mL vial could be potentially used for the examination of reflux in four to five children which implies that children needing a VUR study can be examined in the same session in order to reduce the cost of the procedure. No complications related to the use of the contrast agents have been reported in the literature.[4,23,31] Recently, Babu et al.[32], Sidhu et al.[33], Sellars et al.[34], Darge et al.[35], Yusuf et al.[36] and Colleran et al.[37] have published articles describing the safety, cost-effectiveness and need of implementing ce-VUS in assessing VUR in pediatric age group.
In conclusion, in an era of heightened radiation awareness and protection, radiation doses to infants and children should be kept as low as reasonably achievable. This study indicates 100% sensitivity of the ce-VUS, and it is superior over VCUG in that it can detect extra 4 refluxes. In most of the cases, ce-VUS detects either the same or higher grade of reflux when compared to VCUG with equal efficacy of detecting other anomalies.
With improved operator learning curve for ce-VUS, it has the potential to replace VCUG. The longer dynamic imaging acquisition of ce-VUS also suits the intermittent nature of VUR. ce-VUS using second-generation contrast agent may be introduced as a valid alternative diagnostic modality for detecting vesicoureteral reflux, based on its radiation free, highly efficacious, reliable, and safe characteristics.[4,22,23]
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of TNMC and BYL Nair Hospital.
Informed Consent: Written informed consent was obtained from patients’ parents who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - N.M., A.S., H.P.; Design - N.M., A.S., C.G., A.P., M.A., H.P.; Supervision - N.M., A.S., C.G., A.P., M.A., H.P.; Resources - N.M., A.S., C.G., A.P., M.A., H.P.; Materials - N.M., A.S., C.G., A.P., M.A., H.P.; Data Collection and/or Processing - N.M., A.S.; Analysis and/or Interpretation - N.M., A.S., C.G., A.P., M.A., H.P.; Literature Search - N.M., A.S., C.G., A.P., M.A., H.P.; Writing Manuscript - N.M., A.S., C.G., A.P., M.A., H.P.; Critical Review - N.M., A.S., C.G., A.P., M.A., H.P.; Other - N.M., A.S., C.G., A.P., M.A., H.P.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ransley PG. Vesicoureteral reflux: continuing surgical dilemma. Urology. 1978;12:246–55. doi: 10.1016/0090-4295(78)90387-4. [DOI] [PubMed] [Google Scholar]
- 2.Wennerström M, Hansson S, Jodal U, Stokland E. Disappearance of vesicoureteral reflux in children. Arch Pediatr Adolesc Med. 1998;152:879–83. doi: 10.1001/archpedi.152.9.879. [DOI] [PubMed] [Google Scholar]
- 3.Dick PT, Feldman W. Routine diagnostic imaging for childhood urinary tract infections: a systematic overview. J Pediatr. 1996;128:15–22. doi: 10.1016/S0022-3476(96)70422-5. [DOI] [PubMed] [Google Scholar]
- 4.Tse KS, Wong LS, Lau HY, Fok WS, Chan YH, Tang KW, et al. Pediatric vesicoureteral reflux imaging: where are we? Novel ultrasound-based voiding urosonography. Hong Kong Med J. 2014;20:437–43. doi: 10.12809/hkmj144215. [DOI] [PubMed] [Google Scholar]
- 5.Fong KW, Wong SN. Symptomatic urinary tract infection in children: experience in a regional hospital in Hong Kong. Hong Kong J Paediatr. 2004;9:30–6. [Google Scholar]
- 6.Koff SA, Wagner TT, Jayanthi VR. The relationship among dysfunctional elimination syndromes, primary vesicoureteral reflux and urinary tract infections in children. J Urol. 1998;160:1019–22. doi: 10.1016/S0022-5347(01)62686-7. [DOI] [PubMed] [Google Scholar]
- 7.Bailey RR. The relationship of vesicoureteralreflux to urinary tract infection and chronic pyelonephritis-reflux nephropathy. Clin Nephrol. 1973;1:132–41. [PubMed] [Google Scholar]
- 8.Ditchfield MR, De Campo JF, Cook DJ, Nolan TM, Powell HR, Sloane R, et al. Vesicoureteral reflux: an accurate predictor of acute pyelonephritis in childhood urinary tract infection? Radiology. 1994;190:413–5. doi: 10.1148/radiology.190.2.8284391. [DOI] [PubMed] [Google Scholar]
- 9.Lebowitz RL, Olbing H, Parkkulainen KV, Smellie JM, Tamminen-Mobius TE. International system of radiographic grading of vesicoureteral reflux. International Reflux Study in Children. Pediatr Radiol. 1985;15:105–9. doi: 10.1007/BF02388714. [DOI] [PubMed] [Google Scholar]
- 10.Perisinakis K, Raissaki M, Damilakis J, Stratakis J, Neratzoulakis J, Gourtsoyiannis N. Fluoroscopy-controlled voiding cystourethrography in infants and children: are the radiation risks trivial? Eur Radiol. 2006;16:846–51. doi: 10.1007/s00330-005-0072-6. [DOI] [PubMed] [Google Scholar]
- 11.Unver T, Alpay H, Biyikli NK, Ones T. Comparison of direct radionuclide cystography and voiding cystourethrography in detecting vesicoureteral reflux. Pediatr Int. 2006;48:287–91. doi: 10.1111/j.1442-200X.2006.02206.x. [DOI] [PubMed] [Google Scholar]
- 12.Fettich J, Colarinha P, Fischer S, Frökier J, Gordon I, Hahn K, et al. Guidelines for direct radionuclide cystography in children. Eur J Nucl Med Mol Imaging. 2003;30:39–44. doi: 10.1007/s00259-003-1137-x. [DOI] [PubMed] [Google Scholar]
- 13.Medina LS, Aquirre E, Altman NR. Vesicoureteral reflux imaging in children: comparative cost analysis. Acad Radiol. 2003;10:139–44. doi: 10.1016/S1076-6332(03)80037-5. [DOI] [PubMed] [Google Scholar]
- 14.Darge K. Diagnosis of vesicoureteral reflux with ultrasound. Pediatr Nephrol. 2002;17:52–60. doi: 10.1007/s004670200010. [DOI] [PubMed] [Google Scholar]
- 15.Darge K, Troeger J. Sonographic examination of vesicoureterorenal reflux: one method many names!. Proceedings of the Second European Meeting on Sonographic Diagnosis of Vesicoureteral Reflux; Heidelberg, Germany. 2000. [Google Scholar]
- 16.Radmayr C, Klauser A, Pallwein L, Zurnedden D, Bartsch G, Frauscher F. Contrast enhanced reflux sonography in children: a comparison to standard radiological imaging. J Urol. 2002;167:1428–30. doi: 10.1016/S0022-5347(05)65335-9. [DOI] [PubMed] [Google Scholar]
- 17.Escape I, Martinez J, Bastart F, Solduga C, Sala P. Usefulness of echocystography in the study of vesicoureteral reflux. J Ultrasound Med. 2001;20:145–9. doi: 10.7863/jum.2001.20.2.145. [DOI] [PubMed] [Google Scholar]
- 18.Bosio M. Cystosonography with echocontrast: a new imaging modality to detect vesicoureteral reflux in children. Pediatr Radiol. 1998;28:250–5. doi: 10.1007/s002470050343. [DOI] [PubMed] [Google Scholar]
- 19.Darge K, Troeger J. Vesicoureteral reflux grading in contrast-enhanced voiding urosonography. Eur J Radiol. 2002;43:122–8. doi: 10.1016/S0720-048X(02)00114-6. [DOI] [PubMed] [Google Scholar]
- 20.Fritzsch T, Schlief R. Levovist. Drugs Fut. 1995;20:1224–7. doi: 10.1358/dof.1995.020.12.329398. [DOI] [Google Scholar]
- 21.Schneider M. SonoVue, a new ultrasound contrast agent. Eur Radiol. 1999;9(Suppl 3):S347–8. doi: 10.1007/PL00014071. [DOI] [PubMed] [Google Scholar]
- 22.Papadopoulou F, Anthopoulou A, Siomou E, Efremidis S, Tsamboulas C, Darge K. Harmonic voiding urosonography with a second-generation contrast agent for the diagnosis of vesicoureteral reflux. Pediatr Radiol. 2009;39:239–44. doi: 10.1007/s00247-008-1080-x. [DOI] [PubMed] [Google Scholar]
- 23.Tse KS, Wong LS, Lau HY, Fok WS, Chan YH, Tang KW, et al. Paediatric vesicoureteric reflux imaging: where are we? Novel ultrasound-based voiding urosonography. Hong Kong Med J. 2014;20:437–43. doi: 10.12809/hkmj144215. [DOI] [PubMed] [Google Scholar]
- 24.Darge K. Voiding urosonography with US contrast agents for the diagnosis of vesicoureteral reflux in children. II. Comparison with radiological examinations. Pediatr Radiol. 2008;38:54–63. doi: 10.1007/s00247-007-0529-7. quiz 126–7. [DOI] [PubMed] [Google Scholar]
- 25.Ključevšek D, Battelino N, Tomažič M, Kersnik Levart T. A comparison of echo-enhanced voiding urosonography with X-ray voiding cystourethrography in the first year of life. Acta Paediatr. 2012;101:e235–9. doi: 10.1111/j.1651-2227.2011.02588.x. [DOI] [PubMed] [Google Scholar]
- 26.Ascenti G, Zimbaro G, Mazziotti S, Chimenz R, Fede C, Visalli C, et al. Harmonic US imaging of vesicoureteral reflux in children: usefulness of a second generation US contrast agent. Pediatr Radiol. 2004;34:481–7. doi: 10.1007/s00247-004-1190-z. [DOI] [PubMed] [Google Scholar]
- 27.Darge K, Beer M, Gordjani N, Riedmiller H. Contrastenhanced voiding urosonography with the use of a 2nd generation US contrast medium: preliminary results. Pediatr Radiol. 2004;34:97. [Google Scholar]
- 28.Kis E, Nyitrai A, Varkonyi I, Mattyus I, Cseprekal O, Reusz G, et al. Voiding urosonography with second-generation contrast agent versus voiding cystourethrography. Pediatr Nephrol. 2010;25:2289–93. doi: 10.1007/s00467-010-1618-7. [DOI] [PubMed] [Google Scholar]
- 29.Sulieman A, Theodorou K, Vlychou M, Topaltzikis T, Kanavou D, Fezoulidis I, et al. Radiation dose measurement and risk estimation for pediatric patients undergoing micturating cystourethrography. Br J Radiol. 2007;80:731–7. doi: 10.1259/bjr/16010686. [DOI] [PubMed] [Google Scholar]
- 30.Prasad MM, Cheng EY. Radiographic evaluation of children with febrile urinary tract infection: bottom-up, top-down, or none of the above? Adv Urol. 2012;2012:716739. doi: 10.1155/2012/716739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riccabona M. Application of a second-generation US contrast agent in infants and children-a European questionnaire-based survey. Pediatr Radiol. 2012;42:1471–80. doi: 10.1007/s00247-012-2472-5. [DOI] [PubMed] [Google Scholar]
- 32.Babu R, Gopinath V, Sai V. Voiding urosonography: Contrast-enhanced ultrasound cystography to diagnose vesicoureteralreflux: A pilot study. J Indian Assoc Pediatr Surg. 2015;20:40–1. doi: 10.4103/0971-9261.145548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sidhu PS, Cantisani V, Deganello A, Dietrich CF, Duran C, Franke D, et al. Role of contrast-enhanced ultrasound (CEUS) in pediatric practice: an EFSUMB position statement. Ultraschall Med. 2017;38:33–43. doi: 10.1055/s-0042-110394. [DOI] [PubMed] [Google Scholar]
- 34.Sellars ME, Deganello A, Sidhu PS. Pediatric contrast-enhanced ultrasound (CEUS): a technique that requires co-operation for rapid implementation into clinical practice. Ultraschall Med. 2016;35:203–6. doi: 10.1055/s-0034-1366567. [DOI] [PubMed] [Google Scholar]
- 35.Darge K, Papadopoulou F, Ntoulia A, Bulas DI, Coley BD, Fordham LA, et al. Safety of contrast-enhanced ultrasound in children for non-cardiac applications: a review by the Society for Pediatric Radiology (SPR) and the International Contrast Ultrasound Society (ICUS) Pediatr Radiol. 2013;43:1063–73. doi: 10.1007/s00247-013-2746-6. [DOI] [PubMed] [Google Scholar]
- 36.Yusuf GT, Sellars ME, Deganello A, Cosgrove DO, Sidhu PS. Retrospective analysis of the safety and cost implications of pediatric contrast-enhanced ultrasound at a single center. AJR Am J Roentgenol. 2016;208:446–52. doi: 10.2214/AJR.16.16700. [DOI] [PubMed] [Google Scholar]
- 37.Colleran GC, Barnewolt CE, Chow JS, Paltiel HJ. Intrarenal reflux: diagnosis at contrast-enhanced voiding urosonography. J Ultrasound Med. 2016;35:1811–9. doi: 10.7863/ultra.15.09056. [DOI] [PubMed] [Google Scholar]


