Abstract
Glycation and glycoxidation of proteins and peptides have been intensively studied and are considered as reliable diagnostic biomarkers of hyperglycemia and early stages of type II diabetes. However, glucose can also react with primary amino groups present in other cellular components, such as aminophospholipids (aminoPLs). Although it is proposed that glycated aminoPLs can induce many cellular responses and contribute to the development and progression of diabetes, the routes of their formation and their biological roles are only partially revealed. The same is true for the influence of glucose-derived modifications on the biophysical properties of PLs. Here we studied structural, signaling, and biophysical properties of glycated and glycoxidized phosphatidylethanolamines (PEs). By combining high resolution mass spectrometry and nuclear magnetic resonance spectroscopy it was possible to deduce the structures of several intermediates indicating an oxidative cleavage of the Amadori product yielding glycoxidized PEs including advanced glycation end products, such as carboxyethyl- and carboxymethyl-ethanolamines. The pro-oxidative role of glycated PEs was demonstrated and further associated with several cellular responses including activation of NFκB signaling pathways. Label free proteomics indicated significant alterations in proteins regulating cellular metabolisms. Finally, the biophysical properties of PL membranes changed significantly upon PE glycation, such as melting temperature (Tm), membrane surface charge, and ion transport across the phospholipid bilayer.
Keywords: advanced glycation end products (AGE), aminophospholipids glycation, cellular metabolism, mass spectrometry, membrane surface charge, NMR spectroscopy
Introduction
Glycation refers to a non-enzymatic reaction between aldoses (or ketoses) and amino groups of biomolecules yielding initially Schiff bases and after an Amadori rearrangement ketamine derivatives. Glycation of amino groups at the N-termini or the side chains of lysine residues in proteins has been intensively studied and was linked to the development and progression of diabetes mellitus [1, 2]. The level of N-terminally glycated hemoglobin (HbA1c), for example, is a well established clinical marker indicative for long-term plasma glucose concentrations. Additionally, aminophospholipids (aminoPLs) like phosphatidylethanolamines (PEs) and - serines (PSs) are also glycated in vivo (gPEs and gPSs, respectively) [3–5]. Most studies have focused on in vitro gPSs, although glycoxidized PSs were identified and quantified in lipid extracts from human erythrocytes [6].
PE, a main component of mammalian membranes, are glycated in animal models and human plasma under hyperglycemic conditions [7]. Furthermore, several reports indicate a pro-oxidative role of gPEs, which contributes to the pathogenesis of diabetic complications, such as neuropathy, retinopathy, and nephropathy [8–11]. In the presence of reactive oxygen species (ROS) gPEs can be oxidatively degraded to advanced glycation end products (AGEs) of which carboxymethylamine (CM-PE) and carboxyethylamine (CE-PE) are the most studied. Similar to AGE modifications of proteins, several pathways yielding PE-AGEs have been proposed. Common is a reaction between the amino group of PE and dicarbonyls, such as methylglyoxal and glyoxal, produced by oxidation (Wolff’s pathway) of sugars [12] or Schiff bases (Namiki pathway) [13]. Additionally, ROS can oxidize Amadori compounds yielding CM-PE and CE-PE (oxidative cleavage; Hodge pathway) [14, 15]. Although several groups have studied the relative contributions of each pathway forming AGE-modified proteins, the corresponding mechanisms yielding AGEs of PEs have not been investigated yet. It remains even open, if Schiff base or Amadori products contribute mostly to glycated aminoPLs.
Glycation and glycoxidation of aminoPLs alter the physiology of cells and tissues. Glycated and AGE-modified PE induce the production of pro-inflammatory cytokines in monocytes and myeloid dendritic cells [16]. Furthermore, AGE-PEs appear to play a role in apoptotic cell signaling [17–19]. Besides altered cell signaling pathways, modifications of the lipid head group in combination with peroxidation of the unsaturated fatty acyl esters can considerably change the biophysical properties of PL membrane bilayers, such as plasticity and permeability, ultimately disintegrating cell membranes. Changes of membrane fluidity and membrane curvature were reported for PEs modified by saturated aldehydes or γ-keto-aldehydes [20]. Recently, we could show that alkyl modified PEs induce a negative membrane curvature in lipid vesicles [21].
In recent years these modifications have been intensively studied by several groups, due to the high biological relevance of glycated and glycoxidized aminoPLs in hyperglycemia related disorders [22]. Liquid chromatography (LC) and mass spectrometry (MS) allowed the characterization of molecular species formed by incubating aminoPLs with glucose in the presence of ROS [23]. In vitro studies provided the basis for different targeted strategies to quantify gPEs and glycoxidized PE (goxPEs) in human plasma and red blood cells [24]. However, glycation, glycoxidation, and oxidation of unsaturated fatty acyl chains in aminoPLs results in a large variety of modified species with largely unknown biological effects. Therefore, structural, biological, and biophysical aspects of gPE should be simultaneously investigated to understand the role of modified lipids in hyperglycemia related pathologies.
Here we combined cell biology with analytical, biochemical, and biophysical techniques to address diverse glycated and glycoxidized PE lipids. Nuclear magnetic resonance spectroscopy (NMR) showed that glycation of PEs yields dominantly the Amadori product in vitro. Mass spectrometry (MS) of glycoxidized lipids confirmed CM-PE and CE-PE as main AGEs formed by Amadori degradation and identified a variety of diverse fatty acyl oxidation products associated with AGE formation. The pro-oxidative effects of gPEs were judged by peroxide formation and further evaluated in activation of cellular stress response pathways. Systemic effects of modified lipids on cellular proteome were studied using label free LC-MS/MS. Finally, differential scanning calorimetry, measurement of the Zeta-potential, and lipid bilayer membrane conductance were used to address the effect of modified PE on biological membranes.
Material and Methods
Chemicals
1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphoethanolamine (PLPE), 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphoethanolamine (PAPE), and diphytanoylphosphatidylcholine (DPhPC) were purchased from Avanti Polar Lipids. Inc. (Alabaster, Alabama). Iron (II) sulfate, d-1,2-13C2-glucose, m-aminophenylboronic acid–agarose, hydrogen peroxide, trichloroethanol, hexane, hexadecane, 2-(N-morpholino)ethanesulfonic acid (MES), tris(hydroxymethyl)aminomethane (TRIS), ethylene glycol tetraacetic acid (EGTA), cardiolipin (CA), valinomycin, 7-(diethylamino)-coumarin-3-carbohydrazide (CHH), sodium deoxycholate, tris (2-carboxyethyl) phosphine (TCEP), urea, thiourea, all sodium, potassium, and ammonium salts were obtained from Sigma Aldrich GmbH (Germany). Sodium dodecyl sulfate, glycerol, dithiothreitol, ethanol, and d(+)-glucose were from Carl Roth GmbH & Co (Karlsruhe, Germany) and iodoacetamide from AppliChem GmbH (Darmstadt, Germany). Trypsin was obtained from Promega GmbH (Mannheim, Germany). d-13C6-glucose was purchased from Cambridge Isotope laboratory, Inc (Saarbrücken, Germany). Acetonitrile (ULC-MS grade), formic acid, and methanol were obtained from Biosolve GmbH (Valkenswaard, Netherlands), chloroform from Merck KGaA (Darmstadt, Germany). Bromophenol blue, glycine, tetramethylethylenediamine (TEMED), acrylamide/bis solution, and ammonium persulfate were obtained from Serva Electrophoresis, GmbH (Heidelberg, Germany). Dulbecco’s modified Eagle’s medium (DMEM/Ham’s F12), fetal bovine serum (FBS), phosphate buffer saline (PBS), trypan Blue (0.1%) and antibiotic (penicillin/streptomycin) solutions were obtained from Life Technologies, GmbH (Darmstadt, Germany). Low fluorescent PVDF membranes, immunoblot blocking solution (AdvanBlock) and immunoblot washing solution (AdvanWash) were from Advansta (Biozym Scientific GmbH, Germany). Water was purified in-house (18.2 MΩ-cm; PURELAB Ultra, Celle, Germany).
PE glycation and oxidation
DPPE, POPE, PLPE, and PAPE were glycated as described previously [23]. Briefly, PE (1 mmol/L in methanol) was incubated with d-glucose or d-glucose-1,2-13C2 (5 mmol/L in methanol; gPE) or in the absence (control, cPE) under nitrogen atmosphere in a boiling water bath for 30 min. Samples were dried under a stream of nitrogen. Glycated PE vesicles (0.5 mmol/L in 5 mmol/L aqueous ammonium bicarbonate) were oxidizedwith FeSO4 (80 µmmol/L) and H2O2 (50 mmol/L) at 37°C for 2 hours. Lipids were extracted with a mixture of chloroform and methanol (1:1, v/v). The lower organic phase was collected, five-fold diluted (v/v) in a mixture of methanol and chloroform (2:1, v/v) containing 5 mmol/L ammonium formate (ESI solution) and directly analyzed by ESI-LTQ-Orbitrap-MS.
Boronate affinity chromatography
GlycatedPE (100 µg) was loaded in sample buffer (1 mL, mixture of methanol and ammonium hydroxide, 95:5, v/v) on a m-aminophenylboronic acid–agarose column (1 mL propylene column; Qiagen GmbH) [25] and washed with sample buffer (2 mL). Glycated lipids were eluted with methanol (5 mL) and dried under a stream of nitrogen.
Mass spectrometry
Glycated and glycoxidized PE were analyzed by direct injection using robotic nanoflow ion source TriVersa NanoMate (AdvionBio Sciences, Ithaca NY) equipped with nano-electrospray chips (1.4 kV ionization voltage, 0.4 psi back pressure) coupled to a LTQ Orbitrap XL ETD mass spectrometer (Thermo Fischer Scientific GmbH, Bremen, Germany). The temperature of the transfer capillary was set to 200°C and the tube lens voltage was 115 V. Mass spectra were acquired with a target mass resolution of 100,000 at m/z 400. CID fragmentation experiments were performed in a linear ion trap (isolation width 1-1.5 u, normalized collision energy 30%, activation time 30 ms, activation Q 0.25). Gas-phase fractionation (GPF) was applied using five different m/z ranges (280–450, 450–600, 600–750, 750–900, and 900–1100) for 3 min each, i.e. 15 min total acquisition time. The five most intense signals of a survey scan were selected in data-dependent acquisition (DDA) mode for consecutive fragmentation in the LTQ (top 5) [26]. A m/z selected for DDA was excluded afterwards for 45 s using the maximal size of the dynamic exclusion list (500 m/z values). Acquired data were analyzed by using Xcalibur software (version 2.0.7).
NMR
Glycated samples (dried under nitrogen atmosphereand stored at -20°C) were dissolved in a mixture of CDCl3 and CD3OD (4:1, v/v; 600 µL), transferred into 5 mL NMR tubes (P507, Wilmad Inc. Vineland, NJ 08360, USA), and NMR-measurements started within 1 h. All NMR measurements were performed on a Bruker DRX 600 NMR-spectrometer (14.0954T field strength, 600.13 MHz 1H NMR frequency, Bruker GmbH, Rheinstetten, Germany) at a temperature of 310K. A x-channel optimized 5 mm broad band observe (BBO) probe was used with measurement parameters set to: center frequency for 13C sfo1=150.92 MHz, 13C 90° excitation pulse length p1=11 µs, sweep width sw=238.35 ppm i.e. 35.971 kHz, number of complex acquisition points td=65536, repetition time TR≥10s, and number of scans in the pulse acquire experiments (zg) ns=6000. NMR data were processed and plotted using GNU octave version 3.8.2 [27]. NMR-simulations were performed using the “spinach” library version 1.2.1437 [28] locally adopted to run under GNU octave instead of MATLAB.
Quantification of H2O2
Water peroxide concentrations were determined in gPE (1 mmol/L) and cPE (1 mmol/L) standards using the Red hydrogen peroxide assay kit (Enzo Life Science; Lörrach, Germany) following the manufacturer´s protocol. Briefly, gPE and cPE were dissolved in the assay buffer and transferred into a 96-well plate. A solution composed of red peroxidase substrate and 0.8 U/mL peroxidase was added and fluorescence (λexc=540 nm, λem=590 nm) was recorded using the Paradigm™ Detection Platform (Molecular devices, Salzburg, Austria).
Cell culture
Primary rat cardiomyocytes (Innoprot, Spain) were cultured until 80% confluence in DMEM/F12 medium supplemented with FBS (20%), horse serum (5%), L-glutamine (2 mmol/L), non-essential amino acids (0.1 mmol/L), sodium pyruvate (3 mmol/L), and antibiotics (1%) at 37 °C (95% air and 5% CO2, humidified atmosphere). The medium was replaced by serum-free medium 24 h prior to addition of DPPE, cDPPE, gDPPE, goxDPPE (1 mmol/L in DMEM/F12) or glucose (5 mmol/L in DMEM/F12) followed by an incubation period of 30 min or 16 h.
Total protein extract
After treatment the medium was discarded, cells were harvested and centrifuged (1000 x g, 10 min, 4°C). Cell pellets were washed (ice cold PBS, 3 times), disrupted in lysis buffer (7 mol/L urea, 2 mol/L thiourea, 1% (w/v) sodium deoxycholate, in 50 mmol/L Tris-HCl, pH7.5), sonicated (30 sec, 30% amplitude; 20 kHz ultrasonic processor; Vibra-Cell [Sonics & Materials, Inc. Connecticut, United States]), and centrifuged (10 000 × g, 10 min, 4°C).
Nuclear protein extraction
After treatment the medium was discarded, cells were harvested and centrifuged (1000 x g, 10 min, 4°C). Cell pellets were washed (ice cold PBS, 3 times),disrupted in Tris-HCl buffer (1.5 mmol/L, pH 8.8), and centrifuged (1000 × g, 10 min). The supernatants containing cytoplasmic proteins were discarded, the pellets were suspended in lysis buffer (62.5 mmol/L Tris-HCl, pH 6.8, 50 mmol/L DTT, 2% w/v SDS, 20% w/v glycerol), and disrupted by sonication (30 sec, 30% amplitude; 20 kHz ultrasonic processor; Vibra-Cell [Sonics& Materials, Inc. Connecticut, United States]).
Western blot
Nuclear extract proteins were dissolved in Laemmli sample buffer (62.5 mmol/L Tris-HCl, pH 6.8, 50 mmol/L DTT, 2% w/v SDS, 20% w/v glycerol, 0.2% w/v bromophenolblue) and separated by SDS-PAGE (10 or 18% T; BioRad mini protean III cell; BioRad Laboratories GmbH, Munich, Germany). Based on label free quantification [29–31], the protein amounts were adjusted among the samples, separated by SDS-PAGE, and blotted onto low fluorescent polyvinylidenedifluoride (PVDF) membranes (Mini Trans-Blot® Cell, BioRad Laboratories). Membranes were blocked overnight (4°C, Immunoblot Blocking solution, AdvanBlock, Advansta), incubated with primary antibodies (1:10,000; in blocking buffer, 1 h, RT; rabbit polyclonal anti-Nrf2, rabbit polyclonal anti-NFκB p65 (pSer 536) [Santa Cruz Biotechnology, Heidelberg, Germany] or mouse polyclonal anti-H2AX (pSer 139) [Thermo Fisher Scientific, München, Germany]) and washed (Immunoblot Washing solution, AdvanWash, Advansta). Peroxidase-conjugated donkey anti-rabbit (1:2,500; in blocking buffer) or anti-mouse Ab (1:10,000; in blocking buffer) was added (1H, RT). Membranes were washed (Immunoblot Washing solution, AdvanWash, Advansta), WesternBright Sirius HRP substrate (Advansta) added, and the blot imaged on a Fusion FX7 Imaging system (PeqlabBiotechnologie GmbH, Erlangen, Germany).
SDS-PAGE of CHH-labeled carbonylated proteins [32]
Total protein extracts (5 µL in lysis buffer) were mixed with Laemmli sample buffer (10 µL) before CHH (1.6 µL of 1 mmol/L stock in methanol) was added to label carbonylated proteins. Samples were incubated (2 h, 37°C) and separated by SDS PAGE (12% T), as described above. CHH-labeled proteins were visualized on a ChemiDoc™ MP (Bio-Rad Laboratories GmbH, Munich, Germany), using the Image Lab™ software and DyLight 488 channel filter for Blue Epi illumination. Gels were additionally stained with Coomassie Brilliant Blue G250(2 h, RT), washed with deionized water (2 h, RT) and an image taken on the ChemiDoc™ MP. fluorescence and Coomassie stained gel images were used for normalization by ImageJ software [33].
Tryptic digest and label free protein quantification
The total protein extracts (10 µg in lysis buffer; three experimental replicates) were reduced with TCEP (5 mmol/L, 37°C, 1 h), alkylated with iodoacetamide (10 mmol/L, 37°C, 30 min, dark), and the excess of iodoacetamide quenched with dithiothreitol (10 mmol/L, 37°C, 30 min). Samples were diluted with eight volumes of ammonium bicarbonate (50 mmol/L) and trypsin added (enzyme to protein ratio of 1:25, 50 mol/L ammonium bicarbonate; 37°C, 16 h). The digest was stopped by adding formic acid (final concentration of 0.5% v/v). Precipitated sodium deoxycholate was removed by centrifugation [34].
A nano-Acquity UPLC (Waters GmbH, Eschborn, Germany) was coupled on-line to an LTQ Orbitrap XL ETD mass spectrometer equipped with a nano-ESI source (Thermo Fischer Scientific, Bremen, Germany). Eluent A was aqueous formic acid (0.1% v/v) and eluent B was formic acid (0.1% v/v) in acetonitrile. Tryptic peptides (100 ng) were loaded onto a trap column (nanoAcquity Symmetry C18, internal diameter 180 µm, length 20 mm, particle diameter 5 µm) at a flow rate of 10 µL/min. Peptides were separated on a BEH 130 column (C18-phase, internal diameter 75 µm, length 100 mm, particle diameter 1.7 µm) with a flow rate of 0.4 µL/min using the following gradient: 3% B for 1 min, 3% to 35% B in 90 min and to 85% B in 5 min (hold for 5 min). Together with an equilibration time of 15 min, samples were injected every 2 h. The transfer capillary temperature was set to 200°C and tube lens voltage to 110 V. An ion spray voltage of 1.5 kV was applied to a PicoTip™ on-line nano-ESI emitter (New Objective, Berlin, Germany). The precursor ion survey scans were acquired in the orbitrap (resolution of 60,000) for an m/z-range from 400 to 2,000. The CID-tandem mass spectra (isolation width 2, activation Q 0.25, normalized collision energy 35%, activation time 30 ms) were recorded by data dependent acquisition (DDA) for the six most abundant ions of each survey scan with dynamic exclusion for 60 sec using Xcalibur software (Version 2.0.7).
For relative label free protein quantification “.raw” files were uploaded into Progenesis QI for proteomics (Waters GmbH) for feature detection, alignment, and quantification. Proteins were identified by Sequest (Proteome Discoverer 1.4, Thermo Fisher Scientific) using “.mgf” files generated by Progenesis QI and the following parameter settings: maximum of two missed cleavage sites, peptide mass tolerance of 10 ppm, peptide fragment tolerance of 0.8 Da, variable modifications for oxidation of Met and carbamidomethylation of Cys. Protein identification results (at least three rank 1 peptides per protein) were imported back into Progenesis QI and used for protein quantification. Only proteins down- or upregulated at least twofold (ANOVA p < 0.01) based on at least three non-conflicting peptides were considered. Differentially regulated proteins were classified based on their biological functions using protein descriptions provided in UniProtKB (www.uniprot.org).
Differential scanning calorimetry (DSC)
DPPE (5 g/L) was dissolved with or without modified PE (gDPPE, goxDPPE or cDPPE; 50 mg/L) in chloroform, dried under nitrogen, and suspended in HEPES (10 mmol/L,pH 7.1) by vortexing and sonication to form multilamellar vesicles. The measurements were performed on a Nano DSC (TA Instruments, Eschborn, Germany) using a 340-µL cell, constant pressure (3 bar), a scan rate of 1°C/min from 10°C to 100°C. Three heating and cooling scans were acquired per sample with a delay of 15 min in between in order to allow thermal equilibration. Calorimetry curves were analyzed with NanoAnalyze Data Analysis version 2.1. Melting point, delta entropy (∆S), and delta enthalpy (∆H) were calculated for each reaction by base peak area integration of three independent measurements.
Planar bilayer formation and total membrane conductance measurements
Planar lipid bilayers (diameter 200-250 μm) were formed from liposomes in the presence or absence of antibiotic valinomycin (90 pmol/L) on the tip of plastic pipettes (Eppendorf) [35]. The final lipid concentration was 1.12 g/L (in 0.2 mol/L KCl, 10 mmol/L TRIS, 10 mmol/L MES, 0.6 mmol/L EGTA, pH 7.35). Membrane formation and bilayer quality was monitored by capacitance measurements (0.73±0.3 µF/cm2). Current-voltage (I-V) characteristics were measured by a patch-clamp amplifier (EPC 10, HEKA Elektronik Dr.Schulze GmbH, Germany) at 32° C. Total membrane conductance was calculated from a linear fit of experimental data (I) for voltages ranging from -50 to 50 mV [36].
Measurement of the Zeta-Potential
Lipids were dissolved in chloroform (DPhPC) or a mixture of chloroform and methanol (1:1, v/v; DPPE and modified DPPE) and the solvents were evaporated. Buffer (50 mmol/L Na2SO4, 10 mmol/L MES, 10 mmol/L TRIS, 0.6 mmol/L EGTA, pH 7.32) was added to reach a lipid concentration of 0.2 g/L and vortexed (5 min). Large unilamellar vesicles (LUVs) were obtained using a small-volume extruder (Avanti Polar lipids Inc) with a filter size of 200 nm. The electrophoretic mobility of LUVs was measured in an electrical field using a Malvern Zetasizer Nano ZS device (Malvern, UK) at 25°C.
Results
Analysis of glycated PE
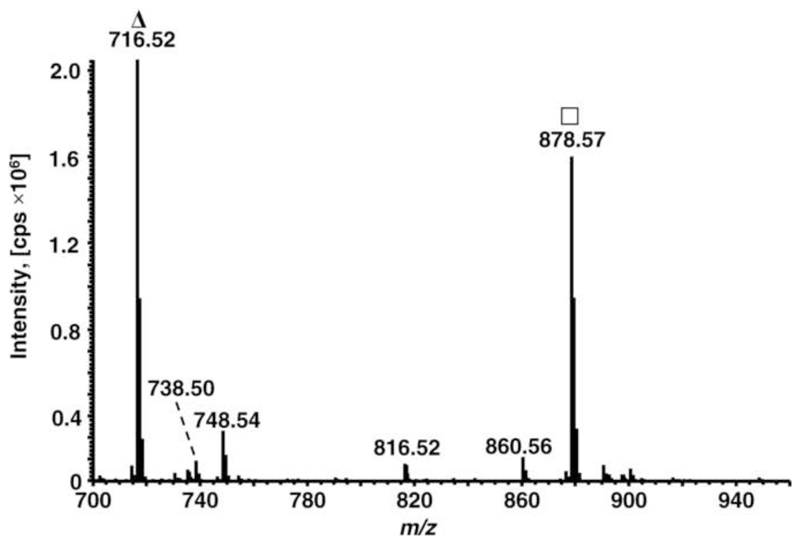
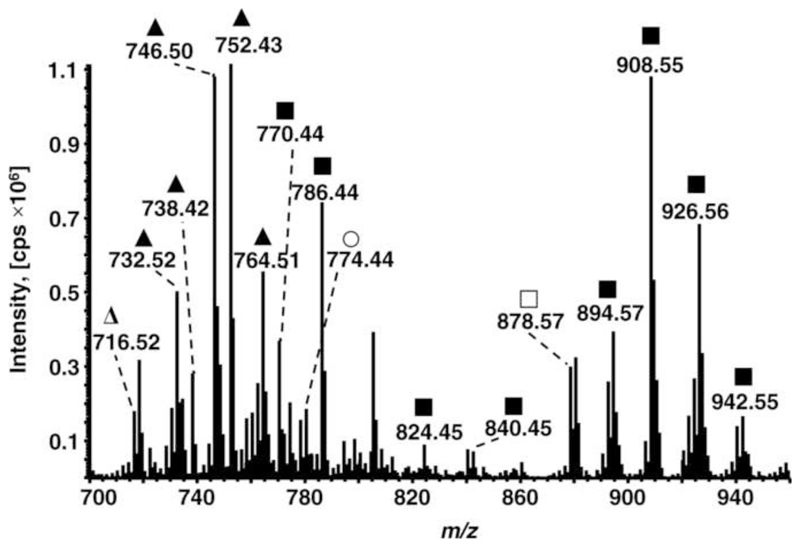
Previous studies demonstrated that the yields of in vitro glycation depend on several parameters, such as solvent composition, temperature, and reaction time[37, 38]. When PE and glucose were incubated in methanol, the glycation yields were higher than in phosphate buffer, which is most commonly applied in protein studies. However, in both cases glycation was not quantitative [37, 38]. In favor of short reaction times, PE lipids (DPPE, POPE, PLPE, and PAPE) were co-incubated with glucose in methanol at 100°C for 30 min. The formed gPE were identified by ESI-LTQ-Orbitrap-MS (positive ion mode) searching for signal pairs with characteristic mass shifts of 162 Da (+C6H10O5) and 144 Da (+C6H10O5-H2O). The mass spectrum of gPLPE (Fig. 1) displayed besides the unmodified PLPE (m/z 716.52, base peak) two major signals at m/z 878.57 (78%) corresponding to gPLPE and m/z 860.56 (4.5%) indicating the expected loss of water. Two minor signals at m/z 748.54 (14%) and 816.52 (3.6%) were assigned to oxidation of PLPE (+32 u) and oxidative cleavage of gPLPE at the C10-position of linoleoyl, respectively. Similarly, the mass spectra of the glycated DPPE, POPE, and PAPE samples displayed two dominant signals corresponding to glycated and unmodified PE at signal intensity ratios I(gPE)/I(PE) of 3.1, 1.6, and 0.78, respectively.
Figure 1. Mass spectrum of glycated PLPE acquired on an ESI-Orbitrap-MS in positive ion mode; Δ unmodified PLPE, □ glycated PLPE.
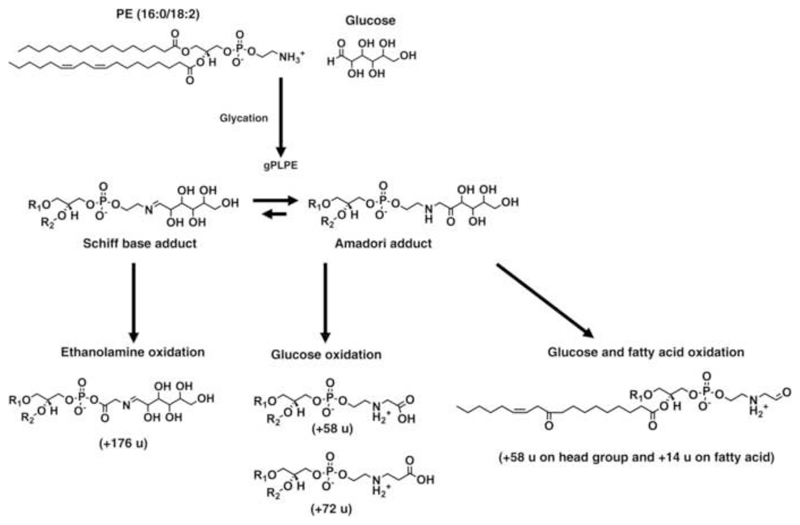
Glycation reactions can yield two structurally different isomeric products - the Schiff base and Amadori adducts (Scheme 1). Schiff bases can be distinguished from the isomeric Amadori adducts in tandem mass spectra by the specific neutral loss of 120 u corresponding to the erythrose-moiety (Fig. S1) [39]. However, quantitative investigations to determine the product ratios requires separation of both compounds by chromatography or ion mobility spectrometry, which is difficult to achieve. Instead, we applied13C NMR spectroscopy (without 1H decoupling) to analyze thegPLPE glycated using d-1,2-13C2-glucose. To the best of our knowledge, 13C-labelled compounds will produce the same products as educts with the natural isotopes, although the kineticsis typically reduced due to the isotope effect.
Scheme 1. Possible reaction pathways yielding the modified lipids produced by PE glycation and glycoxidation.
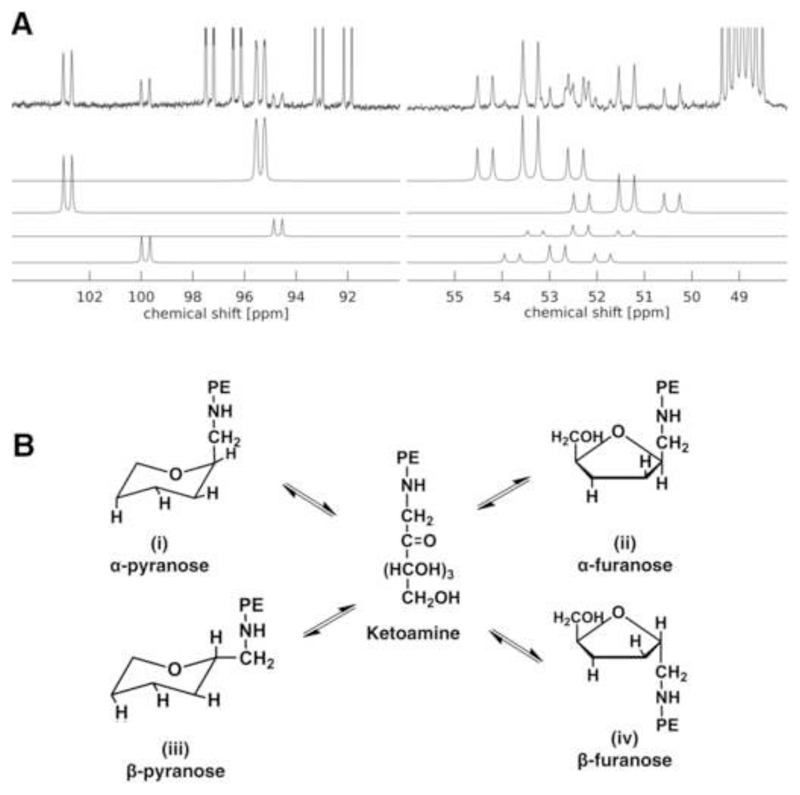
The spectrum displayed the resonances corresponding to the Amadori keto- and methylenegroups in the chemical shift ranges from 103 to 94 ppm and 55 to 50 ppm, respectively (Fig. 2A). Signals at 92.46 and 96.72 ppm were assigned as 2-13C of α- and β-1,2-13C2-glucose as doublet of doublets due to the carbon-carbon 1J(1-13C2,-2-13C2) coupling of 46.5 Hz and a carbon-proton 1J(13C,1H) coupling of 169.8 and 161.2 Hz. Four different glucose cyclic adducts were present in the reaction mixture: (i) α-1-(N-PLPE)-d-glucopyranose (95.29 and 53.32 ppm), (ii) α-1-(N-PLPE)-d-glucofuranose (102.75 and 51.29 ppm), (iii) β-1-(N-PLPE)-d-glucopyranose (94.61 and 52.26 ppm), and (iv) β-1-(N-PLPE)-d-glucofuranose (99.93 and 52.75 ppm) (Fig. 2A and B). Although the Schiff base was present in the samples, as confirmed by its characteristic neutral loss of 120 u in MS/MS (Fig. S1), its concentration was too low for recording reasonable 13C NMR spectra.
Figure 2. 13C NMR spectrum (A) and identified glycated PLPE (B).
A - 13C pulse experiment (i.e. without 1H decoupling) of the Amadori products built upon reaction of 1,2-13C2-Glucose with PLPE (top). Resonances of four Amadori products were identified and simulations were fitted to the experimental spectrum (bottom). The 13C heptett of the CD3OD solvent was referenced to 48.5 ppm but truncated at the top. B - Possible conformations of Amadori products identified in the NMR spectrum: (i) α-1-(N-PLPE)-d-glucopyranose, (ii) α-1-(N-PLPE)-d-glucofuranose, (iii) β-1-(N-PLPE)-d-glucopyranose, and (iv) β-1-(N-PLPE)-d-glucofuranose. Only two rotamers are shown for the α- and β-glucopyranose forms.
The manual fit of the four Amadori product simulations allowed to calculate the relative contents of each isoform and the unreacted 1,2-13C2-glucose (Tables S1 and S2). Pyranose and furanose resonances and coupling constants in the spectra match closely with published data [40]. The integral intensities indicated that the main cyclic adducts formed on the aminophospholipid are α-pyranose and α-furanose, whereas the β-isoforms were of minor intensities.
Pro-oxidative effect of glycated PE
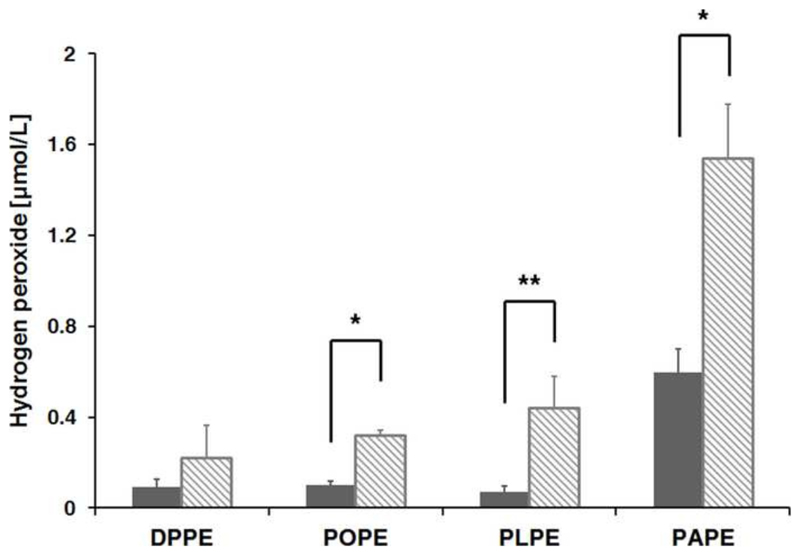
Glycated proteins and lipids were reported as a source of ROS that may enhance oxidative stress (OS) in vivo [41, 42]. Here, peroxides release by glycated lipids containing fatty acyl chains with different number of double bounds was studied. When DPPE, POPE, PLPE, and PAPE were incubated with d-glucose in methanol for 30 min at 100°C they produced hydrogen peroxide at concentrations of 216, 310, 430, and 1530 nmol/L, respectively (Fig. 3), whereas the control samples treated without d-glucose contained only 90, 100, 70, and 590 nmol/L of H2O2. Since the peroxide assay applied here detects also lipid-bound hydroperoxides [43], the peroxide levels of the control samples were attributed to the initial amounts of lipid peroxidation products. Thus, it can be assumed that 1 mmol of gDPPE, gPOPE, gPLPE, and gPAPE produces 126, 210, 360, and 940 nmol peroxides. As all four lipids were added at similar concentrations, the higher peroxide quantities produced by gPOPE, gPLPE, and gPAPE containing unsaturated fatty acids compared to gDPPE (saturated fatty acids) were attributed to the formation of 84, 234, and 810 nmol lipid-bound peroxides, respectively. Thus, the glucose moiety in the lipid head group produced H2O2 and additionally lipid-bound peroxides nearly proportional to the number of double bounds, i.e. one, two, and four unsaturated bonds in POPE, PLPE, and PAPE, respectively.
Figure 3. Water peroxide content in aqueous solution of glycated PE.
H2O2 was quantified in aqueous solutions of glycated DPPE, POPE, PLPE, PAPE (1 mmol/L; dashed bars) and the corresponding non-glycated controls (grey bars; samples incubated at 100°C without sugar). Experiments were performed in triplicate. Significant differences were assessed using t-test (* <0.001; **< 0.0005).
Glycoxidized PE
Formationof advanced glycation end products (AGE) was studied by oxidizing gPE lipids using the Fenton reaction. The high mass accuracy of the orbitrap allowed to assign elemental compositions to all products. In combination with tandem mass spectrometry it was possible to deduce the structures of new products (Tables 1 and S3), as exemplified for goxPLPE (Fig. 4). This mass spectrum still contained weak signals of unmodified and glycated PE, similar to all other goxPE. Additionally, goxPE containing unsaturated fatty acyl residues, i.e. goxPOPE, goxPLPE, and goxPAPE, displayed signals indicating 3, 14, and 24 oxidative cleavage products (OCP), respectively, and 7, 9, and 12 oxygen addition products (OAP). Furthermore, 15 and 22 glycated OCPs of PLPE and PAPE, respectively, and 6, 8, and 6 glycated OAPs of POPE, PLPE, and PAPE, respectively, were identified. Interestingly, oxidation of gDPPE (m/z 854.58) yielded besides the unmodified DPPE (m/z 692.52) four products at m/z 750.52, 764.54, 866.57, and 868.55. The mass increment of 58m/z units corresponded most likely to CM-PE. The second mass shift of 72 m/z units was assigned by MS/MS to two isomers, i.e. CE-PE and a second compound modified at both the head group (+58 m/z units attributed to CM-PE) and diacylglycerol (DAG; + 14 m/z units). The elemental composition as well as MS2 and MS3confirmed the modification at DAG (Fig. S2), but it was impossible to assign a valid structure. The tandem mass spectra recorded for the third and fourth oxidation products with mass increments of 174 and 176 m/z units suggested again different isomers. An increment mass of 162 m/z units was assigned to the head group in combination with mass shifts of 12 and 14 m/z units at the ethanolamine, which was reported before as oxidation of the Schiff base adduct [23]. Additionally, increment masses of 174 (C6H7O6) and 176 m/z units (C6H9O6) at the head group corresponding to multiple oxidations of the glucose moiety were identified in agreement with Melo et al [44].
Table 1. Overview of all in vitro lycated and glycoxidized PE species identified using high resolution mass spectrometry.
OCP - oxidative cleavage products; OAP - oxygen addition products; HG - head group.
| Modifications | DP | PO | PL | PA |
|---|---|---|---|---|
| PE | PE | PE | PE | |
| OCP | 3 | 14 | 21 | |
| OAP | 7 | 9 | 12 | |
| Glycated | ||||
| Glycated (+ 162 u) | 1 | 1 | 1 | 1 |
| Glycated OCP | 15 | 12 | ||
| Glycated OAP | 6 | 8 | 6 | |
| Glycoxidized | ||||
| + 58 u | 1 | 1 | 1 | |
| +72 u | 1 | 1 | ||
| +174 u | 1 | |||
| +176 u | 1 | |||
| Glycoxidized and OCP | ||||
| + 58 u | 1 | 3 | 3 | |
| +72 u | 3 | 3 | ||
| +132 u | 1 | |||
| Glycoxidized and OAP | ||||
| + 58 u | 1 | 4 | ||
| + 72 u | 2 | |||
| Total | 8 | 26 | 54 | 59 |
Figure 4. Mass spectrum of glycoxidized PLPE acquired on an ESI-Orbitrap-MS in positive ion mode.
Δ unmodified PLPE, ▲ oxidized PLPE, ■ glycated and oxidized PLPE, ○ glycoxidated PLPE and ● glycoxidated and oxidized PLPE.
Oxidation of gPE containing unsaturated fatty acyl residues yielded several glycoxidation products with CM-PE being the most prominent one including POPE and PLPE with intact PUFA, whereas PAPE did not yield any CM-PE species with an intact arachidonoyl residue. Additionally, CM-POPE contained one OCP and four OAP of oleic acid, CM-PLPE three OCP, and CM-PAPE three OCP. CE-PE was the second most abundant glycoxidation product: CE-POPE with intact oleoyl chain and two OAP as well as three OCP of CE-PLPE and CE-PAPE were detected. Additionally, a product with a mass increment of 132 m/z units relative to PAPE was detected that may correspond to an oxidative cleavage of the glucose moiety at C5.
OCP of gPOPE were truncated at C8, C9, or C11 of the oleoyl chain, i.e. at vinyl and allyl positions. Similarly, the fatty acyl chains of gPLPE and gPAPE were cleaved at the double bonds or in allyl position. It is important to note, that oxidation of gPE resulted in species containing both glycated/glycoxidized head groups and oxidized (OCP or OAP) fatty acyl chains, whereas the signals of goxPE with intact PUFA chains were much weaker. For instance, goxPAPE with unmodified arachidonoyl chain was not detected.
Mechanism of AGE formation
Formation of AGEs has been intensively studied in proteins and peptides, but little is known about the pathways generating AGEs from glycated aminoPLs. In general, three pathways leading to AGEs are described in the literature. The Wolf pathways describes autoxidation of glucose in the presence of transition metals producing ketoaldehydes (e.g. glyoxal and methylglyoxal), which can irreversibly modify primary amino groups to form carboxymethyl and carboxyethyl derivatives, respectively [12]. The Namiki pathways propose that the same dicarbonyls can be released from glucose-derived Schiff base via retro-aldol condensation [13]. Finally, Amadori degradation assumes AGE formation via oxidative cleavage of the Amadori product, which can be initiated by free radicals and transition metals (e.g. Fe2+ or Cu+; Hodge pathway)[45].
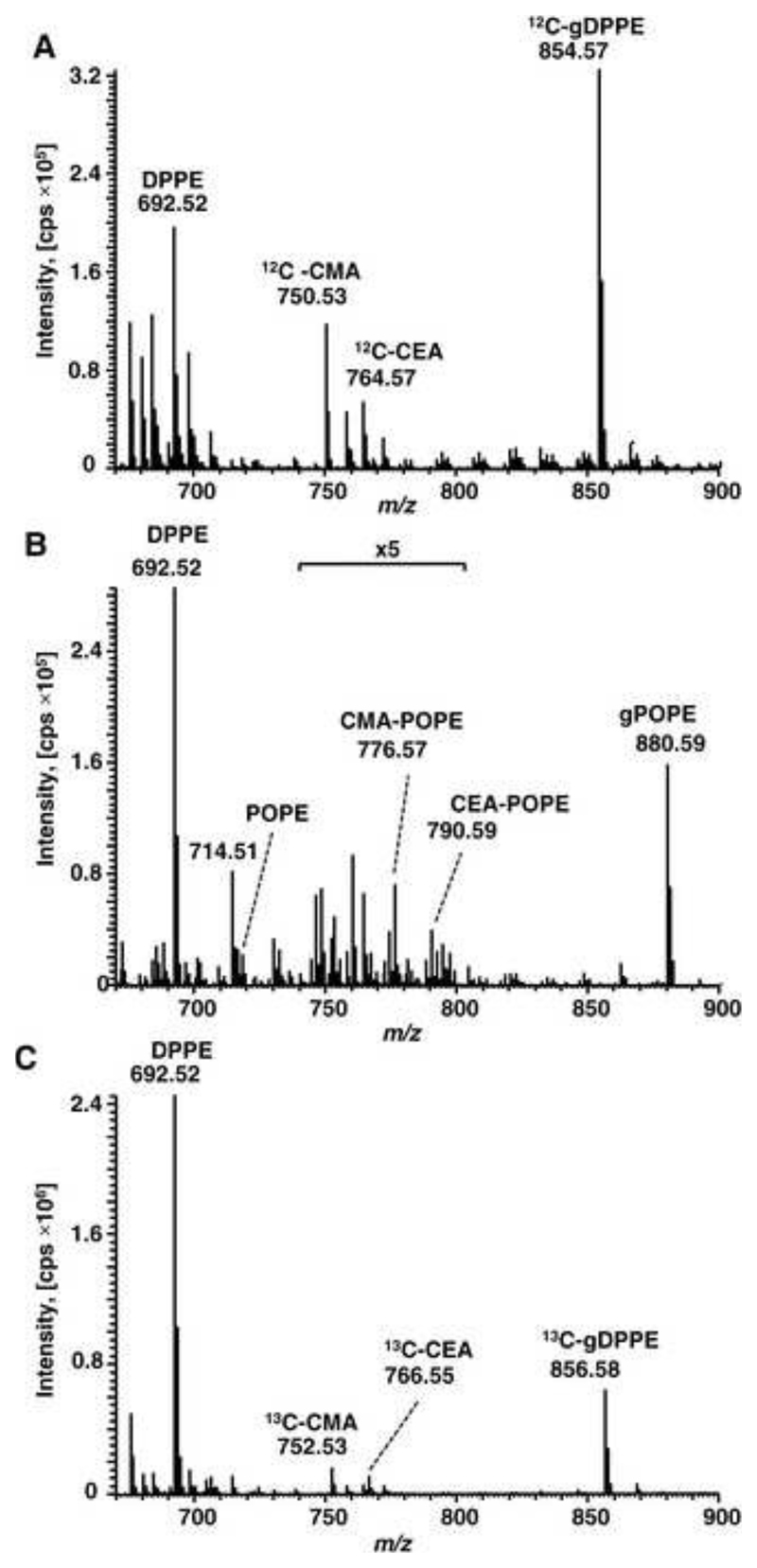
Here we evaluated the contribution of each pathway to the formation of CM- and CE-PE. Thus, DPPE glycated with unlabeled d-glucose was incubated with 13C6 d-glucose using Fenton reaction conditions (Fig. 5A). Besides DPPE (m/z 692.52) and gDPPE (m/z 854.57), CM-PE and CE-PE were detected at m/z 750.53 (+58 u) and m/z 764.57 (+72 u), respectively, excluding the Wolf pathway as both AGE were degradation products of unlabeled glucose and not derived from 13C6-labeled glucose. The Namiki pathway was evaluated for gPOPE purified by boronate affinity chromatography and incubated with DPPE in the presence of Fenton reagents (Fig. 5B). The mass spectrum displayed signals indicating DPPE (m/z 692.52), gPOPE (m/z 880.59), and small amounts of POPE (m/z 718.51), most likely formed by retro-aldol condensation reaction. The expected CM-PE and CE-PE adducts of POPE were observed at m/z 776.57 and 790.59, respectively, whereas signals of AGE-modified DPPE were missing. Taken together, glucose was slightly released from gPOPE via retro-aldol condensation (confirmed by unmodified POPE), but the possibly formed reactive dicarbonyls did not yield detectable quantities of CM- or CE-DPPE.
Figure 5. MS evaluation of carboxymethyl- and carboxyethylamine formation during PE glycoxidation.
Orbitrap mass spectra acquired for different glycoxidations of PE. A –DPPE was glycated with d-glucose and oxidized in the presence of 13C6-d-glucose. B - Purified gPOPE was incubated with DPPE using oxidative conditions of the Fenton reaction. C - DPPE glycated with 13C2-d-glucose and oxidized using the Fenton reaction.
Finally, when purifiedd-1,2-13C2-glucose derived DPPE was oxidized (Fenton reaction) (Fig. 5C), the mass spectrum displayed gDPPE at m/z 856.58 (+164 u) and the corresponding CM-PE and CE-PE adducts at m/z 752.53 (+60 u) and 766.55 (+74 u) indicating both AGE contained 13C. This confirmed their formation via β-scission of the Amadori compound between C2-C3 and C3-C4.
gPE and goxPE trigger cellular responses in vitro
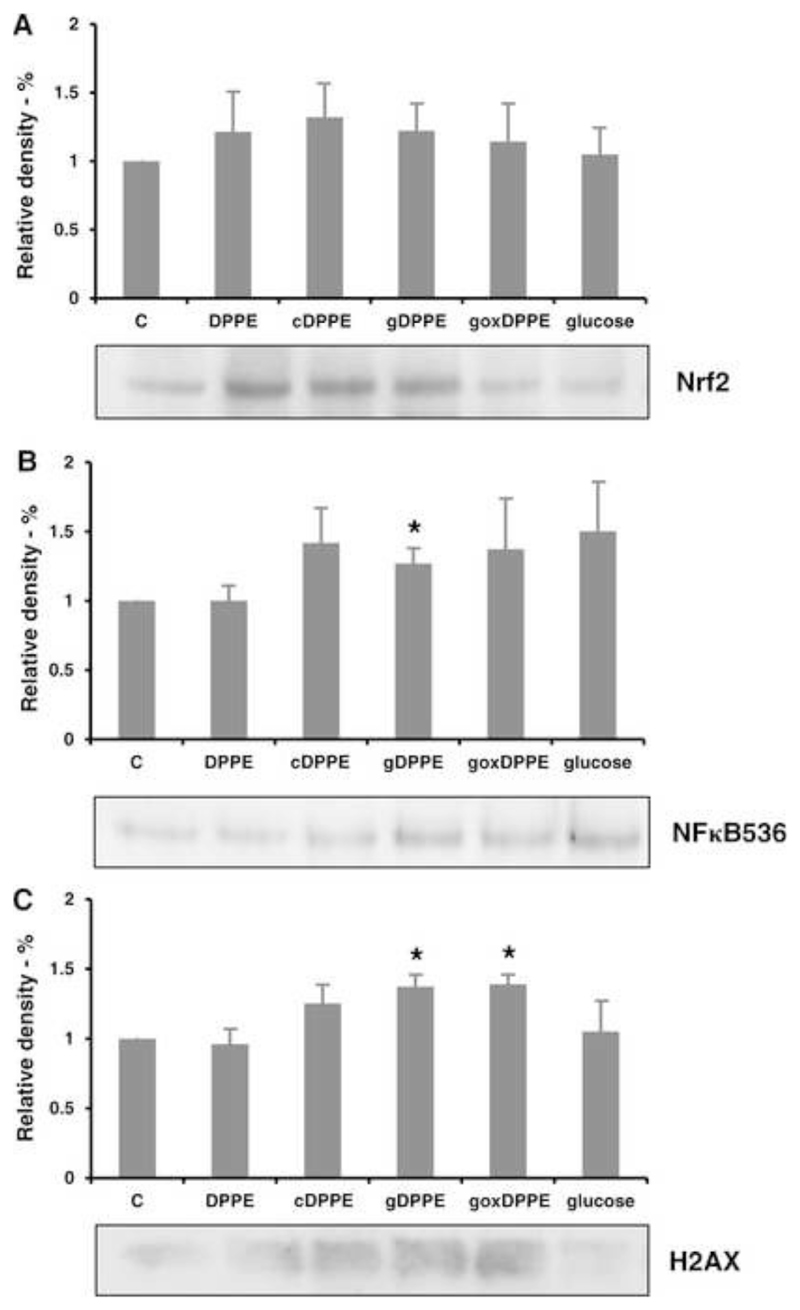
Possible cellular effects triggered by modified lipids were studied by treating rat cardiomyocytes with DPPE, cDPPE, gDPPE, and goxDPPE (1 mmol/L each), or d-glucose (5 mmol/L) for 30 min. Moderate peroxide concentrations, produced by glycated PE (Fig. 3), might trigger activation and nuclear translocation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) transcriptional factor, which is responsible for activating the transcription of antioxidant defense genes. Incubation of primary cardiomyocytes with DPPE, cDPPE, and goxDPPE slightly increased Nrf2 nucleus translocation (Fig. 6A). However, no significant difference was observed between treated and untreated cells.
Figure 6. Effect of glycated and glycoxidized DPPE on signaling pathways in rat primary cardiomyocytes.
Cell nuclear extracts were separated by SDS-PAGE, blotted, and probed with antibodies directed against Nrf2 (A), pSer536 NFκB (B), and pSer139 H2AX (C). Quantitative data represent means of four independent experiments. *p < 0.05 between groups (untreated vs treated) was assessed using t-test.
Activation of the NFκB pathway, which is responsible for a wide range of stress responses including induction of pro-inflammatory genes, was monitored with an antibody recognizing p65 protein phosphorylated at Ser536 (Fig. 6B). Cells treated with gDPPE showed significantly increased band intensities, whereas cDPPE, goxDPPE, and glucose had only a weak effect. Additionally, potential stress effects indicated by the phosphorylation status of histone H2AX were studied for modified PE lipids (Fig. 6C). Originally, H2AX phosphorylation was attributed to DNA double strand cleavage, but it relates also to many other stress derived events. Primary cardiomyocytes incubated with gDPPE and goxDPPE initiated H2AX phosphorylation compared to untreated cells, whereas glucose and DPPE had no effects and cDPPE only a minor influence.
Longer lasting effects of modified lipids on the OS-status were studied also on rat cardiomyocytes by incubating these cells with the above mentioned substances and concentrations for 16 h. Protein carbonylation, a well-known marker of OS, was quantified using a carbonyl-specific fluorescent tag (Fig.S3). No differences in comparison to control levels of carbonylated proteins were observed for DPPE, cDPPE, and gDPPE, whereas goxDPPE significantly increased the carbonylation degree by 50%.
gPE and goxPE mediated alteration of cellular proteome
Possible effects of gPE and goxPE on cellular homeostasis were studied proteome-wide by relative label free quantification. Considering all treatment conditions, 169 proteins were at least two fold up- or downregulated in comparison to the control (Tables S4). The lowest number of regulated proteins was detected in DPPE treated cells (22 proteins of which 4 were up- and 18were down-regulated), followed by glucose (32 proteins, 13 up and 19 down) and cDPPE (40 protein, 9 up and 31 down). Incubation of cardiomyoctes with gDPPE resulted up- (13proteins) and down-regulation (40 proteins) of 53 proteins. The highest number of differentially regulated proteins (128) was identified in cells treated with goxDPPE with 70 and 58 proteins up- and down-regulated, respectively.
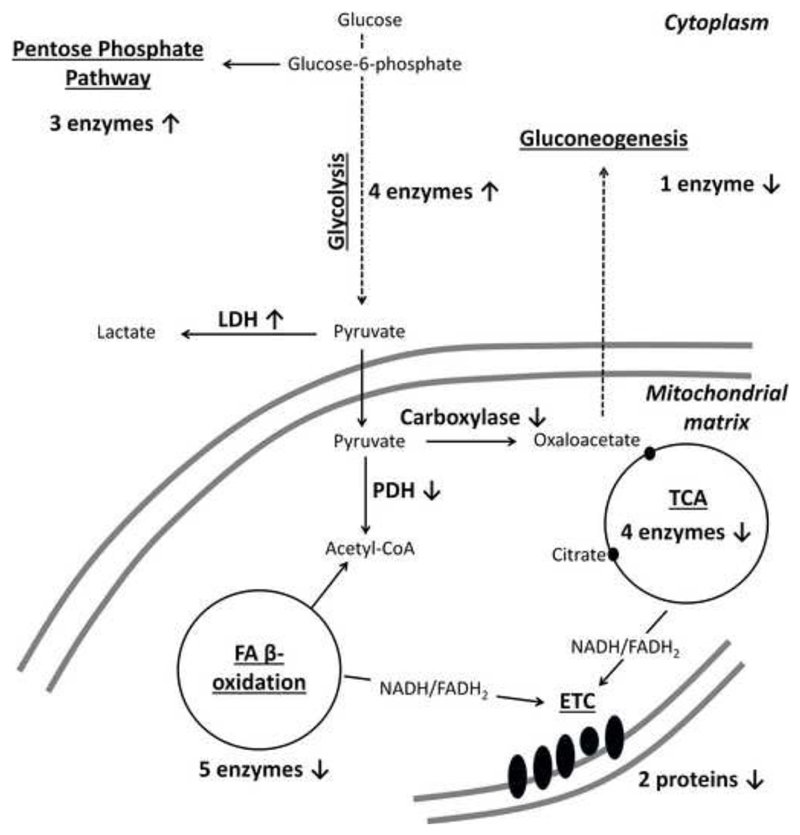
Based on the biological functions provided by UniProtKB, the 169 proteins were attributed to 11 groups: regulation of metabolism and energy production, stress response, protein turnover, cytoskeleton/muscle contraction, protein secretion and extracellular matrix (ECM) proteins, channels/receptors, cell signaling, DNA replication/chromatin, lipid transport/metabolism, and mitochondria protein translocation (Tables 2 and S4). This clearly demonstrates significant metabolic reconfigurations initiated by gDPPE and especially goxDPPE in cardiomyocytes. Thus, up-regulation of glycolysis can be proposed for goxPE treated cells based on the increased quantities of four pathway specific enzymes. Furthermore, two subunits of pyruvate dehydrogenase were downregulated, whereas lactate dehydrogenase was upregulated indicating a shift from aerobic to anaerobic metabolism, which was further supported by significant downregulation of fatty acid β-oxidation, tricarboxylic acid cycle (TCA) and mitochondrial electron transport chain (ETC) proteins. A remarkable change was detected for ETC proteins upon gDPPE treatment with six proteins of Complexes I, III, and V being downregulated.
Table 2. Functional characterization of proteins differentially regulated in rat cardiomyocytes treated with glucose, DPPE, cDPPE, glycated, and glycoxidized DPPE for 16 h.
Regulation levels are shown relative to a control sample. ARE - antioxidant response element; ECM - extracellular matrix; HSP - heat shock proteins; PTM - post-translational modifications.
| Glucose | DPPE | cDPPE | gDPPE | goxDPPE |
|---|---|---|---|---|
| Regulation of metabolism and energy production | ||||
| Glycolysis (5proteins) | ||||
| - | - | - | - | 5 ↑ |
| Pentose Phosphate Pathway (3 proteins) | ||||
| - | - | 1 ↑ | 1 ↑ | 3 ↑ |
| Gluconeogenesis (1 protein) | ||||
| - | - | - | - | 1 ↓ |
| TricarboxylicAcid Cycle (6proteins) | ||||
| 1 ↓ | 1 ↓ | 1 ↓ | 2 ↓ | 6 ↓ |
| Mitochondrial Electron Transport Chain (6 proteins) | ||||
| 2 ↓ | - | 3 ↓ | 6 ↓ | 2↓ |
| Fatty Acid Beta-Oxidation (5 proteins) | ||||
| - | 1 ↓ | 1 ↓ | 1 ↓ | 5 ↓ |
| AminoAcidMetabolism (7 proteins) | ||||
| 1 ↓ | 1 ↓ | 1 ↓ | 1 ↓ | 1 ↑/6 ↓ |
| Purine/pyrimidinemetabolism (3 proteins) | ||||
| - | - | - | - | 3 ↑ |
| Ketone bodiesmetabolism (2proteins) | ||||
| - | - | - | - | 2 ↓ |
| Polysaccharides metabolism (1 protein) | ||||
| 1 ↓ | 1 ↓ | 1 ↓ | 1 ↓ | 1 ↑ |
| Stress response | ||||
| Antioxidants (6 proteins) | ||||
| - | - | - | 1 ↓ | 2 ↑/4 ↓ |
| Metabolism of xenobiotics and drugs (6 proteins) | ||||
| 1 ↓ | - | - | - | 4 ↑/1 ↓ |
| Proteins under control of ARE (2 proteins) | ||||
| - | - | - | - | 2 ↑ |
| Control of inflammatory response (2 proteins) | ||||
| 1 ↑ | 1 ↑ | 1 ↑ | 1 ↑ | 2 ↑ |
| Protein Folding/HSPs (11proteins) | ||||
| - | 1 ↓ | 1 ↑/1 ↓ | 1↑/1 ↓ | 5 ↑/5 ↓ |
| Protein turnover | ||||
| Protein degradation/Proteasome (3proteins) | ||||
| 1 ↓ | 1 ↓ | - | 1 ↓ | 1 ↑/1 ↓ |
| Protein synthesis (11proteins) | ||||
| 2 ↑ | 1 ↑ | 1 ↑/1 ↓ | 1 ↑ | 9 ↑ |
| Cytoskeleton/Musclecontraction | ||||
| Muscle contraction related proteins (2 proteins) | ||||
| - | 1 ↓ | 1 ↓ | 1 ↑/1 ↓ | - |
| Reorganization of actin cytoskeleton (7 proteins) | ||||
| - | - | 1 ↑/1 ↓ | 2 ↑/1 ↓ | 5 ↑ |
| Microtubulesreorganization (5 proteins) | ||||
| - | - | - | - | 5 ↑ |
| Protein secretionand ECM | ||||
| Protein secretion/endocytosis (14 proteins) | ||||
| 3 ↓ | 5 ↓ | 6 ↓ | 7 ↓ | 3 ↑/6 ↓ |
| ECM/celladhesion (10 proteins) | ||||
| - | 1 ↓ | 4 ↓ | 5 ↓ | 3 ↑/6 ↓ |
| Channels/Receptors | ||||
| Membrane Channels (2 proteins) | ||||
| - | - | - | - | 1 ↑/1 ↓ |
| Receptors (3proteins) | ||||
| - | - | 1 ↓ | 1 ↓ | 1 ↑/1 ↓ |
| Cellsignaling | ||||
| Protein kinases (5 proteins) | ||||
| 1↑/3 ↓ | 2 ↓ | 2 ↓ | 1 ↑/1 ↓ | 3 ↑ |
| Regulation of Ca-signaling (3 proteins) | ||||
| - | - | 1 ↓ | 1 ↓ | 1 ↑/2 ↓ |
| Regulation of cell signaling (6 proteins) | ||||
| - | - | 2 ↑ | 2 ↑ | 6 ↑ |
| DNA replication/chromatin | ||||
| DNA replication/DNA repair (3 proteins) | ||||
| 2 ↑/1 ↓ | - | 1 ↓ | 1 ↓ | - |
| mRNASplicing (4 proteins) | ||||
| 2 ↑ | 1 ↑ | 2 ↑ | 2 ↑ | 2 ↓ |
| Histones/histones PTMs (4 proteins) | ||||
| 1 ↑/1 ↓ | - | 2 ↓ | 2 ↓ | - |
| Lipid transport/metabolism (4 proteins) | ||||
| 1 ↓ | 1 ↓ | - | 1 ↓ | 1 ↑/1 ↓ |
| Mitochondria protein translocation (3 proteins) | ||||
| 1 ↓ | 1 ↓ | 1 ↓ | 2 ↓ | 2 ↓ |
| Miscellaneous (14 proteins) | ||||
| 4 ↑/2 ↓ | 1 ↑/1 ↓ | 2 ↓ | 1 ↑/3 ↓ | 3 ↑/4 ↓ |
Antioxidant and detoxification systems were upregulated after goxDPPE treatment, as indicated by three enzyme of the pentose phosphate pathway (PPP) including the rate limiting enzyme glucose-6-phosphate dehydrogenase (3.1 fold). Furthermore, cytoplasmic antioxidant proteins, such as glutathione S-transferase P (2.1 fold) and peroxiredoxin 1 (2.8 fold), were upregulated in response to goxPE. The same was true for heme oxygenase I (2.8 fold), a well known marker of ARE controlled gene transcription. Interestingly, upregulation of inducible nitric oxide synthase (iNOS) was detected for all conditions with the highest values after DPPE (6-fold) and gDPPE (8.6-fold) treatments. Many proteins involved in metabolism of xenobiotics, such as NADP+-dependent alcohol dehydrogenase (3.3 fold) and aldose reductase (2.5 fold), were upregulated. Remarkably, mitochondrial antioxidant proteins (e.g. peroxiredoxin 5) and detoxification enzymes (e.g. mitochondrial aldehyde dehydrogenase) were significantly downregulated. Similar changes were obtainedf or cytoplasmic vs endoplasmic reticulum chaperons and heat shock proteins in gPE and goxPE treated cells, i.e. seven cytoplasmic proteins including HSP β1, 70 1A/1B, 90α and 90β were upregulated and all ER resident chaperons (e.g. calreticulin and three isoforms of protein disulfide-isomerase) were downregulated.
Another hallmark of goxPE treated cells was the significant upregulation of proteins involved in protein synthesis including four ribosomal proteins, two tRNA ligases, two elongation factors, and translation initiation factor 3D. Furthermore, five proteins involved in organization of actin cytoskeleton and five proteins belonging to microtubules organization were upregulated in goxPE treated cells. Many proteins involved in protein secretion, endocytosis, cell adhesion and ECM were differentially regulated in cells incubated with cPE, gPE, and goxPE, most of which showed significant downregulation. Finally, significant changes were obtained for proteins involved in signaling pathways for all treatment conditions with goxPE influencing the most proteins. Several protein kinases including calcium/calmodulin-dependent protein kinase IIβ, integrin-linked protein kinase, Rho associated protein kinase 2, phosphatidylinositol 4-kinase α, and receptor tyrosin-protein kinase erbB2 were differentially regulated. The less enriched functional groups included proteins involved in lipid transport/metabolism, mitochondria protein translocation system, DNA replication and repair, mRNA splicing, histones and regulation of histone modifications, membrane channels and receptors.
gPE and goxPEs affect the phase transition of multi-lamellar DPPE vesicles
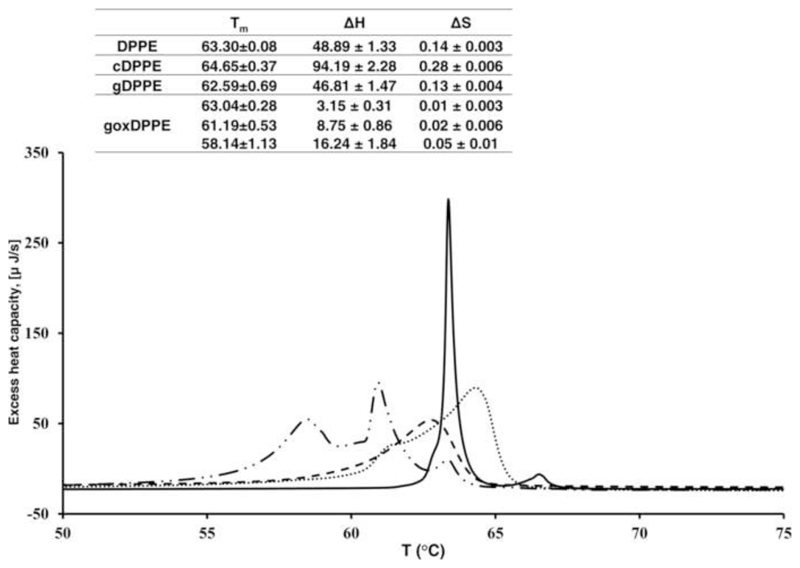
Head group modifications of PE will affect signaling events and also the biophysical properties of cell membranes. To study macroscopic changes induced by Amadori and AGE modified PE in phospholipid bilayers we applied differential scanning calorimetry (DSC). DSC heating curves of DPPE vesicles revealed a clear phase transition with a melting temperature (Tm) of 63.3 °C corresponding to transition enthalpy and entropy of 48.8 J and 0.14 J K-1, respectively (Fig. 7). Addition of gDPPE reduced Tm to 62.59 °C with a significant peak broadening, which corresponded to a transition ∆H of 46.81 J and a transition ∆S of 0.13 J K-1. The DSC heating curve of DPPE vesicles containing goxDPPE showed three main signals with maxima at 63.04, 61.19, and 58.14°C. Based on MS, the goxDPPE sample represented a mixture of compounds including unmodified, glycated, and glycoxidized species. Thus, the peak at 63.04°C may indicate unmodified DPPE vesicles (Tm = 63.3 °C), whereas the other two signals were even below the Tm of gDPPE specifying even more disordered phases. Interestingly, cDPPE (treated in the same manner as gDPPE but without glucose) displayed a completely different thermogram showing a broad signal with a maximum at 64.64°C. The effect might be attributed to diacylglycerols formed during thermal degradation of PE [46]. Furthermore, the loss of the ethanolamine moiety may increase the PL bilayer rigidity.
Figure 7. Differential scanning calorimetry curves of DPPE incubated with glycated or glycoxidized DPPE.
– Heating DSC plots of DPPE vesicle (full line) and DPPE vesicles containing cDPPE (dotted line), glycated DPPE (dashed line), and glycoxidated DPPE (dashed-dotted line). Table insert: ΔH and ΔS were calculated based on three independent measurements.
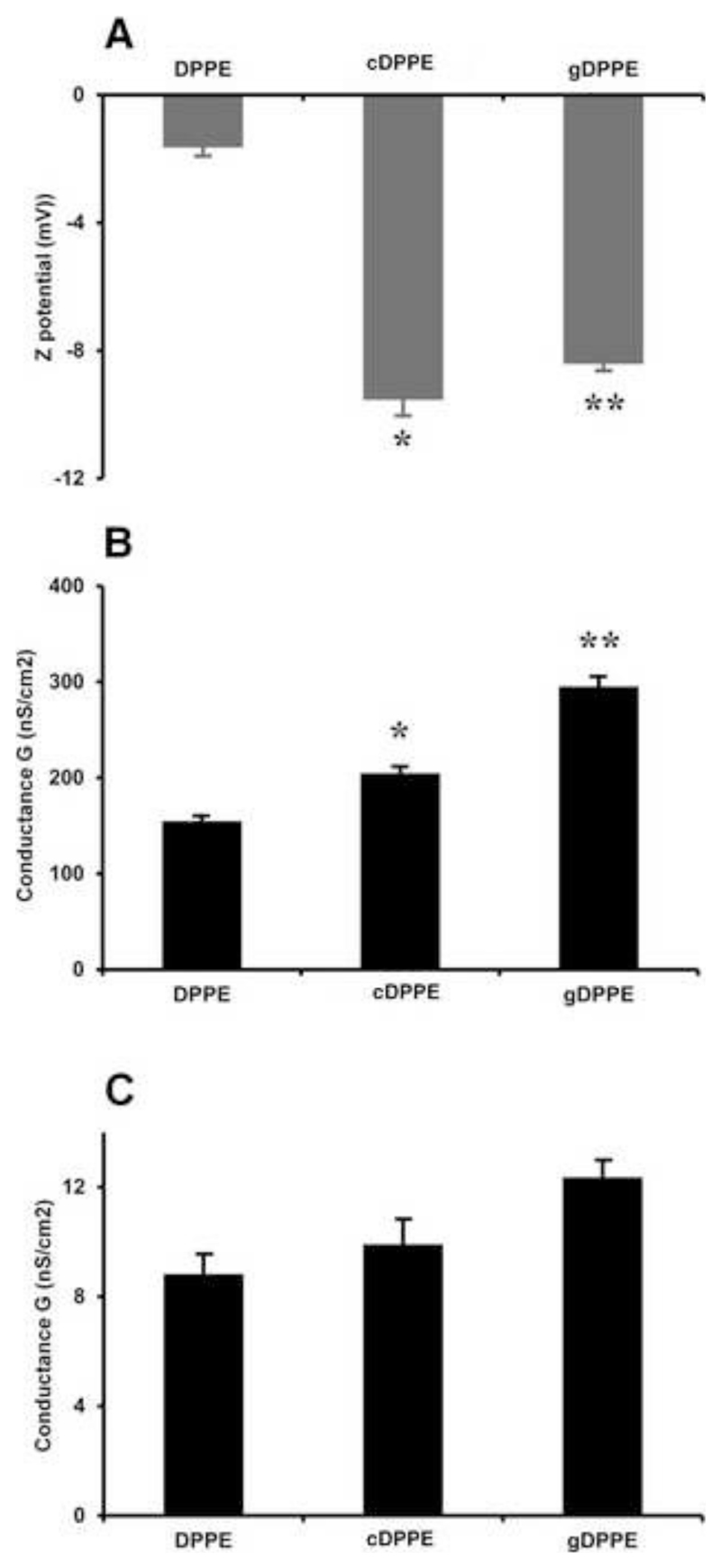
Effect of glycated aminoPL on Zeta-potential and total membrane conductance
The electrical parameters of biological membranes influence numerous cellular processes, such as regulation of membrane enzyme activity or translocation of metabolites and ions. To the best of our knowledge, the impact of glycated PE on the electrical properties of biological membranes was studied here for the first time. Liposomes composed of diphytanoylglycerophosphocholine (DPhPC) and equimolar amounts of either cDPPE or gDPPE significantly increased the negative Zeta-potential of the bilayer membrane compared to DPhPC/DPPE membranes (Fig.8A). Unfortunately, it was not possible to obtain stable unilamellar vesicles with goxDPPE. In case of gDPPE, the increase might be attributed to the hydroxyl groups of glucose, whereas for cDPPE it might be related to the products formed by thermal degradation. Further on, the conductance of membranes composed of DPhPCand cardiolipin (CL) with cDPPE or gDPPE (molar ratios of 9:2:9) in the presence of valinomycin, an ionophore transporting potassium ions across the membrane, increased by 32% and 90%, respectively, relative to DPPE as control (Fig. 8B). Measurements of the membrane conductance without valinomycin were comparable for all three membrane lipid compositions (Fig. 8C), with slightly higher values for gDPPE containing membranes.
Figure 8. Effect of glycated and glycoxidized DPPE on Zeta-potential and membrane conductance.
A - Zera-potentials in the presence of gDPPE and cDPPE. Liposomes were composed of DPhPC and equimolar amounts of DPPE, cDPPE or gDPPE. The influence of gDPPE on the total membrane conductance was measured in the presence (B) or absence (C) of valinomycin (90 pmol/L). The membranes consisted of DPhPC, CL, and DPPE, cDPPE or gDPPE (molar ratios of 9:2:9). Diagrams represent means and standard deviations from 3–5 independent experiments. Statistical significance was determined using Student’s t-test, * < 0.05 between groups (DPPE vs cDPPE) and **<0.05 between groups (cDPPE and gDPPE).
Discussion
Structural characterization of gPE and goxPE
Glycation and glycoxidation, collectively termed the Maillard reaction, can affect all classes of biomolecules. It was estimated that up to 1% of lysine and arginine residues in proteins, 0.1% of aminoPLs and one of 107 residues in DNA are modified by glycation or glycoxidation. The Maillard reaction attracted much attention due to its role in both food chemistry and the pathophysiology of ageing and hyperglycemia related human disorders, mainly diabetes mellitus and its complications [47–49]. The first reaction step is the reversible condensation of an aldose or ketose with a primary amine (Schiff base product), followed by the Amadori rearrangement yielding a ketamine (Amadori product), which can finally produce after further oxidative fragmentation steps a variety of advanced glycation end products (AGEs). Alternatively, free sugars can be oxidized by ROS to reactive dicarbonyls, such as glyoxal (GO) and methylglyoxal (MGO), which can react with amines leading to de novo AGEs. AGEs facilitate numerous biological activities and are often associated with pro-inflammatory and immune responses. Hyperglycemic conditions correlate with elevated levels of protein and lipid glycation/glycoxidation linking sugar-mediated AGEs formation to the severity of a disease. In order decrease the production of AGEs and prevent their presumed disease-related effects, precursors of AGEs (free sugars vs Schiff base vs Amadori product) need to be defined. Studies at the amino acid and protein levels indicate that ketamines (Amodori product) predominate during the initial Maillard reactions and are present mostly in the cyclic pyranose and furanose forms [40, 50]. However, to the best of our knowledge, no data on glycated lipid structures have been reported.
In gPLPE the ketamine exists in four different isomeric cyclic forms, i.e. α/β-pyranose and α/β-furanose, with the α-isoforms dominating. Similar observations were reported for glycated proteins and amino acids [40]. Neglia et al. confirmed the correct assignments of the mutarotational isomers additionally for cyclic glucose adducts on lysine and free fructose [50]. However, in these cases the β anomers dominated both pyranose and furanose isoforms. Although the anomer ratio might depend slightly on the temperature and solvent, the opposite rations are most likely related to the different substances.
Although Schiff bases were detected in all samples by their characteristic neutral losses of 120 u in MS/MS (Fig. S1), the concentration were too low for recording reasonable 13C NMR spectra. The open chain forms of Amadori products and Schiff bases are most likely intermediates of the initial glycation reaction. Besides the much lower sensitivity of NMR compared to MS it should also be noted that NMR spectra are recorded in solution while mass spectra relies on protonated species in vacuum that may influence the ratio of Amadori and Schiff base products. The Schiff base might be detected after reduction or by using advanced 2D NMR techniques, such as 1H-1H COSY and 13C-1H COSY that were successfully applied to distinguish the open and closed chain structures of glycated Ac-Lys-OH [51], but this was not pursued here.
Our observation of the dominating Amadori product as early glycation derivative of PLPE was further confirmed by investigating the pathways yielding AGEs, i.e. formation of CM-PE and CE-PE via β-scission of Amadori products between C2-C3 and C3-C4, respectively. The applied conditions favored oxidative cleavage as main pathway of CM-PE and CE-PE formation in aminoPLs, similar to glycated amino acids and proteins [45]. It is important to note, that AGEs can be formed in vivo also by other reactions, for example by reactive dicarbonyls formed via lipid peroxidation or several metabolic pathways, such as triose phosphate fragmentation or acetone oxidation [52]. MGO and GO react 50,000 faster with free amino groups than glucose and thus might represent the main physiological contributors of AGE formation [53]. Thus, it is difficult to anticipate an in vivo route of AGEs formation that would strongly depend on the affected tissues and pathological condition in question.
Pro-oxidative effect of gPE and structural variety of glycoxidized PE
Glycated proteins and lipids represent a rich source of ROS that may enhance oxidative stress (OS) in vivo [41, 42]. The mechanism of ROS formation by glycated proteins and lipids is closely linked to autoxidation of monosaccharides, which consumes O2 and produces eneaminol and O2●- leading to hydrogen peroxide [12]. This is in agreement with previous observations that reaction mixtures containing superoxide dismutase and catalase decrease ROS production [54]. Furthermore, incubating the low density lipoprotein with glucose increased both glycation and lipid peroxidation levels [41].
Here even 1 mmol of glycated PEs produced approximately 120 nmol of H2O2. Furthermore, the number of double bounds in PE fatty acyl chains significantly influenced the formation of peroxides indicating that PUFA in glycated PEs are more prone to peroxidation. Hicks and coworkers demonstrated that lipid peroxides react with Amadori enediols subsequently forming a glycoxidized product and alkoxyl radicals, which are responsible for propagation of the peroxidative chain reaction and elevated levels of peroxidation products [55]. This clearly indicates synergetic effect between glycation and oxidation of aminoPLs supporting the positive correlation between lipid peroxides and gPE observed in human plasma and erythrocytes of diabetic patients [5].
We identified more than hundred substances derived from oxidation (Fenton reaction) of four different gPE species, which can be subdivided into five groups: (i) PE with oxidized fatty acyl chains and unmodified head group, (ii) glycated PE, (iii) glycated PE with oxidized fatty acyl chains, (iv) glycoxidized PE, and (v) glycoxidized PE with oxidized fatty acyl chains (Tables 1 and S3). As expected, the lowest number of products occurred for saturated DPPE and the highest for PAPE containing a quadruply unsaturated fatty acid. Importantly, glycoxidized PAPE with intact arachidonyl residue was not detected. Instead, all identified AGE-PAPE species contained oxidized fatty acyl chains. The gPE and goxPE identified here are in a good agreement with previous reports, such as oxygen adducts produced upon UV irradiation and Fenton oxidation of glycated PE [23, 44].
These results support significant synergies between glycation and oxidation of aminoPLs, which is usually not considered in analyses of biological samples. Mixed oxidized/glycoxidized PE species characterized here and in a previous study [23], should be considered when targeting modified PE in biological samples. The m/z values of precursor and product ion pairs specific for PUFA-oxidized gPE and goxPE reported here (Table S3) can be used for targeted quantification, such as multiple reaction monitoring (MRM).
Cellular effects of g/goxPE
Glycated aminoPLs and proteins participate in cell signaling. Glycated LDL increases ROS production, affects some antioxidant pathways, and impairs the mitochondrial electron transport chain in vascular endothelial cells [56]. Amadori compounds can induce apoptosis in human peritoneal mesothelial cells and activate c-Jun N-terminal kinases (JNK) and p38 mitogen-activated protein kinases [57]. Recently, Simoes et al. showed that glycated lipids can induce cytokine production in monocytes and dendritic cells [16]. Furthermore, Amadori PEs participate in angiogenic differentiation in human umbilical vein endothelial cells (HUVEC) and increase the expression of vascular endothelial growth factor (VEGF) and matrix metalloproteinases-2 and -9 (MMP2/9) [58].
Here, we would differentiate short and long lasting effects of modified lipids on cellular homeostasis in rat cardiomyocytes (CM). After 30 min glycated and glycoxidized lipids did not significantly activate antioxidant responses mediated via the Nrf2 transcriptional factor despite the production of H2O2 by gPEs. However, both lipids triggered phosphorylation of histone H2AX, which was previously used to monitor DNA damage but nowadays is more generally associated with chromatin remodeling triggering different stress response events [59]. Furthermore, gDPPE activated the NFκB pathway. It is tempting to speculate that gPE and goxPE are recognized by RAGE or other scavenger receptors and may thus induce cellular stress responses via JNK activation followed by H2AX phosphorylation and/or induction of the NFκB pathway [60, 61]. Longer incubation times (16 h) with goxDPPE, increased protein carbonylation by 50% in contrast to all other lipids indicating disturbed redox balances. However, the mechanism responsible for protein oxidation (direct ROS production or/and receptor mediated signaling) upon goxDPPE treatment remain unclear.
Quantitative proteomics provided a more systematic view on changes in cellular homeostasis induced by gDPPE and goxDPPE indicating significant regulatory changes for proteins involved in main metabolic and energy production pathways in treated cells. Thus, tricaboxylic acid cycle (TCA), fatty acid β-oxidation, mitochondrial electron transport chain (ETC) and amino acid catabolism were downregulated in goxPE treated cells (Fig. 8). This reconfiguration was accompanied by significant upregulation of glycolitic enzymes indicating a shift from aerobic to anaerobic metabolism. Cardiomyocytes are post-mitotic cells characterized by high levels of oxygen consumption, and thus strongly rely on oxidative metabolisms at steady state conditions. CM utilize lipids via fatty acid β-oxidation as a major fuel for ATP production, whereas the mitochondria supply almost 90% of ATP through fatty acid β-oxidation. However, hypoxia or mitochondrial dysfunction can induce a shift from aerobic to anaerobic metabolism [62]. As the present study applied standard oxygen concentrations, the observed effects most likely indicate impaired mitochondrial functions. Negative effects of hyperglycemia in general and glycated or glycoxidized biomolecules in particular on mitochondrial functions were reported for endothelial cells incubated with glycated LDL: reduced oxygen consumption by mitochondria complexes, decreased activity of Complex I-IV, attenuated mitochondrial membrane potential, and increased abundance of mitochondria associated ROS [56]. Treatment of β-islet cells with carboxymethyllysine (CML) and CML-BSA also induced ROS formation, triggered mitochondrial dysfunction and selective mitophagy [63].
Specifically cardiac tissue show drastic metabolic reconfigurations accompanied by mitochondrial dysfunction for several cardiopathologies, such as ischemia-reperfusion, heart hypertrophy, myocardial infarction, and heart failure [62, 64]. Diabetic cardiomyopathy also correlates with increased OS, impaired glucose metabolism, altered mitochondrial function, and calcium signaling regulation [65]. Low ATP levels initiate opening of ATP-sensitive potassium channels leading to a significant loss of calcium in the cytoplasm and inhibition of muscle contraction. Thus, upregulation of the glycolytic pathway at low oxygen concentrations or in case of mitochondrial dysfunction was proposed to promote cell survival [66]. However, the underlying mechanisms still remains unclear; it might be a direct glycation effect of biomolecules or a down-stream consequence of receptor mediated signaling cascades. Few recent studies provide a link between RAGE and the attenuation of mitochondrial activity and contractile function in CM. For example, mouse CM incubated with glyceraldehyde-derived AGE-BSA directly affected cellular calcium homeostasis [67]. MGO-derived AGEs directly up-regulated cardiac RAGE mRNA levels and were linked to impairment of contractile function in CM of mice with streptozotocin induced diabetes. Importantly, this dysfunction was associated to mitochondrial membrane potential depolarization, which was interrupted by RAGE gene knockdown [65]. Treatment with CML impaired mitochondrial respiration, which was attributed to AGE-RAGE interactions triggering downstream signaling via the RAGE-ceramide axis [68]. Despite many reports on protein and amino acid AGEs, the ability of RAGE receptors to recognize gPE or goxPE has not reported so far to the best of our knowledge. However, recently it was demonstrated that isolevuglandin-modified PEs, i.e. lipids modified by highly reactive gamma-ketoaldehydes produced by lipid peroxidation, can activate the NF-κB pathway in macrophages via RAGE [69]. Considering our quantitative proteomic data and the literature, it is reasonable to speculate that incubation of CM with goxPE resulted in impairment of mitochondrial function, up-regulation of glycolysis to compensate cellular energy demands, and possibly increased production of ROS followed by protein carbonylation. Further experiments targeting mitochondrial membrane potential and ROS production are required for a detailed functional evaluation of the omics results.
Increased ROS levels upon goxDPPE treatment are further supported by upregulation of antioxidant and detoxification systems. Enzymes of pentose phosphate pathway (PPP), one of the main NADPH source required to sustain high levels of reduced glutathione, were up-regulated including the rate limiting enzyme glucose-6-phosphate dehydrogenase (G6PDH). Up-regulation of G6PDH activity by OS was previously demonstrated in adult CM and correlated with improved contractile function [70]. Several cytoplamic but not mitochondrial or ER resident anti-oxidant enzymes, chaperons and proteins involved in metabolism of xenobiotics were up-regulated as well. Finally, up-regulation of heme oxygenase I and aryl hydrocarbon receptor nuclear translocator-like protein 1confirmed activation of anti-oxidant response element genes.
Another interesting observation here was upregulation of proteins involved in protein synthesis upon goxDPPE treatment. Interestingly, HUVEC cells incubated with Amadori-PE increase proliferation [71], whereas the elevated protein expression in CM as post-mitotic cells contribute more likely to cell hypertrophy. Cardiac hypertrophy is a well-know pathological outcome initiated by a wide range of stress conditions including hypertension and ischemia-induced cardiac remodeling [64]. Adaptation to cardiac hypertrophy is mediated through induction of a fetal genetic program enhancing protein synthesis and increasing the size of CM. When not successful at the compensating stages, cardiac hyperthrophy will progress to heart failure. The transition from cardiac hypertrophy to heart failure is characterized by increased hypertrophic signals and declined bioenergetics, mitochondrial content, ETC complex amount and activity, and significant reduction in oxidative phosphorylation [64], which closely resembles cellular conditions revealed here by quantitative proteomics.
A large number of proteins involved in reorganization of actin and microtubule cytoskeleton, protein secretion and regulation of extracellular matrix, DNA replication and chromatin remodeling were differentially regulated especially in goxDPPE treated cells. Interestingly, over ten proteins involve in cell signaling were up- and downregulated, again mostly by gDPPE and goxDPPE. Among them were several interesting proteins participating in calcium signaling regulation and protein kinases opening a new avenues for evaluating effects of glycated and glycoxidzied lipids on cell physiology.
Effect of gPE and gox PE on model lipid membranes
Head group modifications of PE can affect signaling events and additionally the biophysical properties of cell membranes. Using model lipid vesicles we confirmed earlier reports that head group modified alkanal- and isolevuglandin-PE, for example, change the membrane curvature and plasticity [72]. Specifically, pyridine ring containing PE head group adducts of hexanal induce a negative curvature on phospholipid membranes [21].
Here glycation reduced the energy necessary to induce phase transitions in DPPE vesicles from a solid gel to a disordered liquid phase, very similar to changes induced by addition of detergents or ethanol [73]. This indicates that Amadori-PE might contribute to the formation of non-homogeneous lipid bilayers promoting a liquid disordered phase. Interestingly, goxPE reduced the Tm of lipid vesicles further indicating even more disordered phases. Electrical parameters of biological membranes, which influence numerous cellular processes including regulation of membrane enzyme activity and translocation of metabolites and ions, were studied here for glycated PE for the first time, to the best of our knowledge. Glycation of PE lipids altered the Zeta-potential and the translocation of potassium ions in the presence of valinomycin across lipid bilayer membranes. The latter indicates that modified PEs decrease the membrane energy barrier in the lipid bilayer for cations, which coincides with changes induced by the membrane dipole modifier phloretin [74]. Furthermore, a decreased boundary potential due to oxidized and glycated DPPE can be partially explained by alterations of the surface potential, as previously described for the calcium channel blocker verapamil [75]. Unfortunately, it was not possible to obtain stable unilamellar vesicles with goxDPPE.
However, these results should be treated with caution, since data obtained on model lipid vesicles might not fully reflect the properties of real biological membranes. In vitro vesicles are simple systems that can be easily manipulated under controlled condition [76], but their permeability, substrate transport, lipid components, and lipid dynamics might differ from biological membranes. Additionally, the protein-free synthetic bilayers lack specific recognition systems and the lipid asymmetry is not fully represented. These limitations can be partially overcome using lipid bilayers reconstructed with membrane proteins of interest. Recently, it was shown that PE head groups modified by reactive α,β-unsaturated aldehydes affect the activity of membrane proteins. Lipid bilayer membranes containing reconstituted uncoupling protein 1 (UPC1) significantly alter their conductivity when the PE head groups are modified, but not for nucleophilic amino acid residues modified in UPC1, altering the transport activity of UPC1 [77]. Glycation of lipids in lipid micelles containing plasma-membrane Ca2+-ATPase decreased the affinity of lipids to the trans-membrane surface of the protein and the thermal stability of the enzyme, which was not observed upon protein glycation [78].
Conclusion
This study revealed a high complexity of glycated and oxidized products in glycoxidated PE samples, which all may have different biological and biophysical properties than unmodified PE. The Amadori compounds were the main products of PE glycation, whereas CM-PE and CE-PE were the major products of PE glycoxidation, formed mostly by Amadori cleavage. Glycated gPE showed pro-oxidative effects generating hydrogen peroxide and lipid-bound peroxides, in addition to multiple substances resulting from oxidative cleavage or oxygen addition at the fatty acyl chains and modifications of the head group. Glycated and glycoxidated PE stimulated directly or via mediators like H2O2 or receptor mediated signaling different cell pathways. Furthermore, the expression levels of several proteins involved in regulation of metabolic pathways, energy productions, and protein synthesis changed. The altered electrical properties of membranes induced by gPE may significantly affect the ion transport across cell membranes.
Supplementary Material
Highlights.
PE glycoxidation produces highly complex mixtures of glycated and oxidized products
NMR spectroscopy identified Amadori compounds as major glycation products of PE
Pro-oxidative effects of glycated PE were associated with increased lipid peroxide levels
AGEs are formed prevalently via the Amadori cleavage pathway
Glycated and glycoxidized PEs trigger reconfiguration of cellular proteome
Glycated and glycoxidized PEs alter electrical properties of biological membranes
Figure 9. Influence of goxPE on the regulation levels of proteins involved in metabolic pathways and energy production in rat cardiomyocytes after 16 h.
ETC - electron transport chain; FA - fatty acids; LDH - lactate dehydrogenase; PDH - pyruvate dehydrogenase; TCA - tricarboxylic acid cycle.
Acknowledgement
Financial support from the European Regional Development Fund (ERDF, European Union and Free State Saxony, EFRE; 100146238 and 100121468 to MF; 100105139 and 100127675 to RH), the “Bundesministerium für Bildung and Forschung” (BMBF), German Research Council (DFG; FE-1236/3-1 to MF; Schi 476/16-1 to JS), Austrian Research Fund (FWF, P25123 to EP), and the Leipzig Interdisciplinary Research Cluster of Genetic Factors, Clinical Phenotypes and Environment (LIFE Center, Universität Leipzig) is gratefully acknowledged. This project was funded by means of the European Social Fund and the Free State of Saxony.
References
- [1].Nass N, Bartling B, Navarrete Santos A, Scheubel RJ, Borgermann J, Silber RE, Simm A. Advanced glycation end products, diabetes and ageing. Z Gerontol Geriatr. 2007;40:349–356. doi: 10.1007/s00391-007-0484-9. [DOI] [PubMed] [Google Scholar]
- [2].Jenkins AJ, Best JD, Klein RL, Lyons TJ. 'Lipoproteins, glycoxidation and diabetic angiopathy'. Diabetes Metab Res Rev. 2004;20:349–368. doi: 10.1002/dmrr.491. [DOI] [PubMed] [Google Scholar]
- [3].Nakagawa K, Oak JH, Higuchi O, Tsuzuki T, Oikawa S, Otani H, Mune M, Cai H, Miyazawa T. Ion-trap tandem mass spectrometric analysis of Amadori-glycated phosphatidylethanolamine in human plasma with or without diabetes. J Lipid Res. 2005;46:2514–2524. doi: 10.1194/jlr.D500025-JLR200. [DOI] [PubMed] [Google Scholar]
- [4].Breitling-Utzmann CM, Unger A, Friedl DA, Lederer MO. Identification and quantification of phosphatidylethanolamine-derived glucosylamines and aminoketoses from human erythrocytes-influence of glycation products on lipid peroxidation. Arch Biochem Biophys. 2001;391:245–254. doi: 10.1006/abbi.2001.2406. [DOI] [PubMed] [Google Scholar]
- [5].Suzuki K, Nakagawa K, Miyazawa T. Augmentation of blood lipid glycation and lipid oxidation in diabetic patients. Clin Chem Lab Med. 2014;52:47–52. doi: 10.1515/cclm-2012-0886. [DOI] [PubMed] [Google Scholar]
- [6].Fountain WC, Requena JR, Jenkins AJ, Lyons TJ, Smyth B, Baynes JW, Thorpe SR. Quantification of N-(glucitol)ethanolamine and N-(carboxymethyl)serine: two products of nonenzymatic modification of aminophospholipids formed in vivo. Anal Biochem. 1999;272:48–55. doi: 10.1006/abio.1999.4147. [DOI] [PubMed] [Google Scholar]
- [7].Sookwong P, Nakagawa K, Fujita I, Shoji N, Miyazawa T. Amadori-glycated phosphatidylethanolamine, a potential marker for hyperglycemia, in streptozotocin-induced diabetic rats. Lipids. 2011;46:943–952. doi: 10.1007/s11745-011-3588-3. [DOI] [PubMed] [Google Scholar]
- [8].Hiebert LM, Han J, Mandal AK. Glycosaminoglycans, Hyperglycemia, and Disease. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5695. [DOI] [PubMed] [Google Scholar]
- [9].Lapolla A, Fedele D, Traldi P. Glyco-oxidation in diabetes and related diseases. Clin Chim Acta. 2005;357:236–250. doi: 10.1016/j.cccn.2005.03.032. [DOI] [PubMed] [Google Scholar]
- [10].Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci. 2011;1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. Biochem J. 1987;245:243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Namiki M. Advances in the Maillard reaction and glycation researches--mainly on the Namiki pathway. Seikagaku. 2003;75:37–42. [PubMed] [Google Scholar]
- [14].Gobert J, Glomb MA. Degradation of glucose: reinvestigation of reactive alpha-Dicarbonyl compounds. J Agric Food Chem. 2009;57:8591–8597. doi: 10.1021/jf9019085. [DOI] [PubMed] [Google Scholar]
- [15].Nagai R, Ikeda K, Higashi T, Sano H, Jinnouchi Y, Araki T, Horiuchi S. Hydroxyl radical mediates N epsilon-(carboxymethyl)lysine formation from Amadori product. Biochem Biophys Res Commun. 1997;234:167–172. doi: 10.1006/bbrc.1997.6608. [DOI] [PubMed] [Google Scholar]
- [16].Simoes C, Silva AC, Domingues P, Laranjeira P, Paiva A, Domingues MR. Modified phosphatidylethanolamines induce different levels of cytokine expression in monocytes and dendritic cells. Chem Phys Lipids. 2013;175-176:57–64. doi: 10.1016/j.chemphyslip.2013.07.008. [DOI] [PubMed] [Google Scholar]
- [17].Pan Y, Liang H, Liu H, Li D, Chen X, Li L, Zhang CY, Zen K. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J Immunol. 2014;192:437–446. doi: 10.4049/jimmunol.1301790. [DOI] [PubMed] [Google Scholar]
- [18].Zhou LL, Cao W, Xie C, Tian J, Zhou Z, Zhou Q, Zhu P, Li A, Liu Y, Miyata T, Hou FF, et al. The receptor of advanced glycation end products plays a central role in advanced oxidation protein products-induced podocyte apoptosis. Kidney Int. 2012;82:759–770. doi: 10.1038/ki.2012.184. [DOI] [PubMed] [Google Scholar]
- [19].Alikhani M, Maclellan CM, Raptis M, Vora S, Trackman PC, Graves DT. Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor. Am J Physiol Cell Physiol. 2007;292:C850–856. doi: 10.1152/ajpcell.00356.2006. [DOI] [PubMed] [Google Scholar]
- [20].Guo L, Davies SS. Bioactive aldehyde-modified phosphatidylethanolamines. Biochimie. 2013;95:74–78. doi: 10.1016/j.biochi.2012.07.010. [DOI] [PubMed] [Google Scholar]
- [21].Annibal A, Schubert K, Wagner U, Hoffmann R, Schiller J, Fedorova M. New covalent modifications of phosphatidylethanolamine by alkanals: mass spectrometry based structural characterization and biological effects. J Mass Spectrom. 2014;49:557–569. doi: 10.1002/jms.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res. 2013;47(Suppl 1):3–27. doi: 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- [23].Simoes C, Simoes V, Reis A, Domingues P, Domingues MR. Oxidation of glycated phosphatidylethanolamines: evidence of oxidation in glycated polar head identified by LC-MS/MS. Anal Bioanal Chem. 2010;397:2417–2427. doi: 10.1007/s00216-010-3825-2. [DOI] [PubMed] [Google Scholar]
- [24].Henning C, Smuda M, Girndt M, Ulrich C, Glomb MA. Molecular basis of maillard amide-advanced glycation end product (AGE) formation in vivo. J Biol Chem. 2011;286:44350–44356. doi: 10.1074/jbc.M111.282442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Oak J, Nakagawa K, Miyazawa T. Synthetically prepared Aamadori-glycated phosphatidylethanolaminecan trigger lipid peroxidation via free radical reactions. FEBS Lett. 2000;481:26–30. doi: 10.1016/s0014-5793(00)01966-9. [DOI] [PubMed] [Google Scholar]
- [26].Milic I, Hoffmann R, Fedorova M. Simultaneous Detection of Low and High Molecular Weight Carbonylated Compounds Derived from Lipid Peroxidation by Electrospray Ionization-Tandem Mass Spectrometry. Anal Chem. 2012;85:156–162. doi: 10.1021/ac302356z. [DOI] [PubMed] [Google Scholar]
- [27].Eaton JW. GNU Octave and reproducible research. J Process Cont. 2012;22:1433–1438. [Google Scholar]
- [28].Hogben HJ, Krzystyniak M, Charnock GTP, Hore PJ, Kuprov I. Spinach – A software library for simulation of spin dynamics in large spin systems. J Magn Reson. 2011;208:179–194. doi: 10.1016/j.jmr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- [29].Colella AD, Chegenii N, Tea MN, Gibbins IL, Williams KA, Chataway TK. Comparison of Stain-Free gels with traditional immunoblot loading control methodology. Anal Biochem. 2012;430:108–110. doi: 10.1016/j.ab.2012.08.015. [DOI] [PubMed] [Google Scholar]
- [30].Susnea I, Bernevic B, Wicke M, Ma L, Liu S, Schellander K, Przybylski M. Application of MALDI-TOF-mass spectrometry to proteome analysis using stain-free gel electrophoresis. Top Curr Chem. 2013;331:37–54. doi: 10.1007/128_2012_321. [DOI] [PubMed] [Google Scholar]
- [31].Kreisig T, Prasse AA, Zscharnack K, Volke D, Zuchner T. His-tag protein monitoring by a fast mix-and-measure immunoassay. Sci Rep. 2014;4:5613. doi: 10.1038/srep05613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vemula V, Ni Z, Fedorova M. Fluorescence labeling of carbonylated lipids and proteins in cells using coumarin-hydrazide. Redox biology. 2015;5:195–204. doi: 10.1016/j.redox.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bollineni RC, Fedorova M, Bluher M, Hoffmann R. Carbonylated plasma proteins as potential biomarkers of obesity induced type 2 diabetes mellitus. J Proteome Res. 2014;13:5081–5093. doi: 10.1021/pr500324y. [DOI] [PubMed] [Google Scholar]
- [35].Beck V, Jaburek M, Breen EP, Porter RK, Jezek P, Pohl EE. A new automated technique for the reconstitution of hydrophobic proteins into planar bilayer membranes. Studies of human recombinant uncoupling protein 1. Biochim Biophys Acta. 2006;1757:474–479. doi: 10.1016/j.bbabio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- [36].Rupprecht A, Sokolenko EA, Beck V, Ninnemann O, Jaburek M, Trimbuch T, Klishin SS, Jezek P, Skulachev VP, Pohl EE. Role of the transmembrane potential in the membrane proton leak. Biophys J. 2010;98:1503–1511. doi: 10.1016/j.bpj.2009.12.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344(Pt 1):109–116. [PMC free article] [PubMed] [Google Scholar]
- [38].Martins SIFS, Marcelis ATM, van Boekel MAJS. Kinetic modelling of Amadori N-(1-deoxy-d-fructos-1-yl)-glycine degradation pathways. Part I—Reaction mechanism. Carbohydr Res. 2003;338:1651–1663. doi: 10.1016/s0008-6215(03)00173-3. [DOI] [PubMed] [Google Scholar]
- [39].Utzmann CM, Lederer MO. Identification and quantification of aminophospholipid-linked Maillard compounds in model systems and egg yolk products. J Agric Food Chem. 2000;48:1000–1008. doi: 10.1021/jf9911489. [DOI] [PubMed] [Google Scholar]
- [40].Neglia CI, Cohen HJ, Garber AR, Ellis PD, Thorpe SR, Baynes JW. 13C NMR investigation of nonenzymatic glucosylation of protein. Model studies using RNase A. J Biol Chem. 1983;258:14279–14283. [PubMed] [Google Scholar]
- [41].Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci U S A. 1993;90:6434–6438. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bucala R, Vlassara H. Advanced glycosylation end products in diabetic renal and vascular disease. Am J Kidney Dis. 1995;26:875–888. doi: 10.1016/0272-6386(95)90051-9. [DOI] [PubMed] [Google Scholar]
- [43].Shirasaka N, Ohnishi H, Sato K, Miyamoto R, Terashita T, Yoshizumi H. Horseradish peroxidase degrades lipid hydroperoxides and suppresses lipid peroxidation of polyunsaturated fatty acids in the presence of phenolic antioxidants. J Biosci Bioeng. 2005;100:653–656. doi: 10.1263/jbb.100.653. [DOI] [PubMed] [Google Scholar]
- [44].Melo T, Silva EM, Simoes C, Domingues P, Domingues MR. Photooxidation of glycated and non-glycated phosphatidylethanolamines monitored by mass spectrometry. J Mass Spectrom. 2013;48:68–78. doi: 10.1002/jms.3129. [DOI] [PubMed] [Google Scholar]
- [45].Glomb MA, Monnier VM. Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem. 1995;270:10017–10026. doi: 10.1074/jbc.270.17.10017. [DOI] [PubMed] [Google Scholar]
- [46].Roper H, Roper S, Meyer B. Amadori- and N-nitroso-Amadori compounds and their pyrolysis products. Chemical, analytical and biological aspects. IARC Sci Publ. 1984:101–111. [PubMed] [Google Scholar]
- [47].Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox biology. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nowotny K, Jung T, Hohn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5:194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stirban A, Gawlowski T, Roden M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol Metab. 2014;3:94–108. doi: 10.1016/j.molmet.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Neglia CI, Cohen HJ, Garber AR, Thorpe SR, Baynes JW. Characterization of glycated proteins by 13C NMR spectroscopy. Identification of specific sites of protein modification by glucose. J Biol Chem. 1985;260:5406–5410. [PubMed] [Google Scholar]
- [51].Li C, Wang H, Juarez M, Ruan ED. Structural Characterization of Amadori Rearrangement Product of Glucosylated Nalpha-Acetyl-Lysine by Nuclear Magnetic Resonance Spectroscopy. Int J Spectrosc. 2014;2014:6. [Google Scholar]
- [52].Phillips SA, Thornalley PJ. The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur J Biochem / FEBS. 1993;212:101–105. doi: 10.1111/j.1432-1033.1993.tb17638.x. [DOI] [PubMed] [Google Scholar]
- [53].Rabbani N, Thornalley PJ. Dicarbonyls linked to damage in the powerhouse: glycation of mitochondrial proteins and oxidative stress. Biochem Soc Trans. 2008;36:1045–1050. doi: 10.1042/BST0361045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jones AF, Winkles JW, Thornalley PJ, Lunec J, Jennings PE, Barnett AH. Inhibitory effect of superoxide dismutase on fructosamine assay. Clin Chem. 1987;33:147–149. [PubMed] [Google Scholar]
- [55].Hicks M, Delbridge L, Yue DK, Reeve TS. Catalysis of lipid peroxidation by glucose and glycosylated collagen. Biochem Biophys Res Commun. 1988;151:649–655. doi: 10.1016/s0006-291x(88)80330-9. [DOI] [PubMed] [Google Scholar]
- [56].Sangle GV, Chowdhury SK, Xie X, Stelmack GL, Halayko AJ, Shen GX. Impairment of mitochondrial respiratory chain activity in aortic endothelial cells induced by glycated low-density lipoprotein. Free Radic Biol Med. 2010;48:781–790. doi: 10.1016/j.freeradbiomed.2009.12.017. [DOI] [PubMed] [Google Scholar]
- [57].Sanchez-Rodriguez C, Peiro C, Vallejo S, Matesanz N, El-Assar M, Azcutia V, Romacho T, Sanchez-Ferrer CF, Rodriguez-Manas L, Nevado J. Pathways responsible for apoptosis resulting from amadori-induced oxidative and nitrosative stress in human mesothelial cells. Am J Nephrol. 2011;34:104–114. doi: 10.1159/000329107. [DOI] [PubMed] [Google Scholar]
- [58].Oak J-H, Nakagawa K, Oikawa S, Miyazawa T. Amadori-glycated phosphatidylethanolamine induces angiogenic differentiations in cultured human umbilical vein endothelial cells. FEBS Letters. 2003;555:419–423. doi: 10.1016/s0014-5793(03)01237-7. [DOI] [PubMed] [Google Scholar]
- [59].Turinetto V, Giachino C. Multiple facets of histone variant H2AX: a DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 2015;43:2489–2498. doi: 10.1093/nar/gkv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- [61].Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285–293. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]
- [62].Opie LH, Sack MN. Metabolic plasticity and the promotion of cardiac protection in ischemia and ischemic preconditioning. J Mol Cell Cardiol. 2002;34:1077–1089. doi: 10.1006/jmcc.2002.2066. [DOI] [PubMed] [Google Scholar]
- [63].Lo MC, Lu CI, Chen MH, Chen CD, Lee HM, Kao SH. Glycoxidative stress-induced mitophagy modulates mitochondrial fates. Ann N Y Acad Sci. 2010;1201:1–7. doi: 10.1111/j.1749-6632.2010.05630.x. [DOI] [PubMed] [Google Scholar]
- [64].Rosca MG, Tandler B, Hoppel CL. Mitochondria in cardiac hypertrophy and heart failure. J Mol Cell Cardiol. 2013;55:31–41. doi: 10.1016/j.yjmcc.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ma H, Li SY, Xu P, Babcock SA, Dolence EK, Brownlee M, Li J, Ren J. Advanced glycation endproduct (AGE) accumulation and AGE receptor (RAGE) up-regulation contribute to the onset of diabetic cardiomyopathy. Cell Mol Med. 2009;13:1751–1764. doi: 10.1111/j.1582-4934.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [66].Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM. Redox signaling in cardiac myocytes. Free Radic Biol Med. 2011;50:777–793. doi: 10.1016/j.freeradbiomed.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Petrova R, Yamamoto Y, Muraki K, Yonekura H, Sakurai S, Watanabe T, Li H, Takeuchi M, Makita Z, Kato I, Takasawa S, et al. Advanced glycation endproduct-induced calcium handling impairment in mouse cardiac myocytes. J Mol Cell Cardiol. 2002;34:1425–1431. doi: 10.1006/jmcc.2002.2084. [DOI] [PubMed] [Google Scholar]
- [68].Nelson MB, Swensen AC, Winden DR, Bodine JS, Bikman BT, Reynolds PR. Cardiomyocyte mitochondrial respiration is reduced by receptor for advanced glycation end-product signaling in a ceramide-dependent manner. Am J Physiol Heart Circ Physio. 2015;309:H63–69. doi: 10.1152/ajpheart.00043.2015. [DOI] [PubMed] [Google Scholar]
- [69].Guo L, Chen Z, Amarnath V, Yancey PG, Van Lenten BJ, Savage JR, Fazio S, Linton MF, Davies SS. Isolevuglandin-type lipid aldehydes induce the inflammatory response of macrophages by modifying phosphatidylethanolamines and activating the receptor for advanced glycation endproducts. Antioxid Redox Signal. 2015;22:1633–1645. doi: 10.1089/ars.2014.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jain M, Brenner DA, Cui L, Lim CC, Wang B, Pimentel DR, Koh S, Sawyer DB, Leopold JA, Handy DE, Loscalzo J, et al. Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ Res. 2003;93:e9–16. doi: 10.1161/01.RES.0000083489.83704.76. [DOI] [PubMed] [Google Scholar]
- [71].Nakagawa K, Oak JH, Miyazawa T. Angiogenic potency of Amadori-glycated phosphatidylethanolamine. Ann N Y Acad Sci. 2005;1043:413–416. doi: 10.1196/annals.1333.048. [DOI] [PubMed] [Google Scholar]
- [72].Guo L, Chen Z, Cox BE, Amarnath V, Epand RF, Epand RM, Davies SS. Phosphatidylethanolamines Modified by γ-Ketoaldehyde (γKA) Induce Endoplasmic Reticulum Stress and Endothelial Activation. J Biol Chem. 2011;286:18170–18180. doi: 10.1074/jbc.M110.213470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Huang C, McIntosh TJ. Probing the ethanol-induced chain interdigitations in gel-state bilayers of mixed-chain phosphatidylcholines. Biophys J. 1997;72:2702–2709. doi: 10.1016/S0006-3495(97)78913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pohl P, Rokitskaya TI, Pohl EE, Saparov SM. Permeation of phloretin across bilayer lipid membranes monitored by dipole potential and microelectrode measurements. Biochim Biophys Acta. 1997;1323:163–172. doi: 10.1016/s0005-2736(96)00185-x. [DOI] [PubMed] [Google Scholar]
- [75].Pohl EE, Krylov AV, Block M, Pohl P. Changes of the membrane potential profile induced by verapamil and propranolol. Biochim Biophys Acta. 1998;1373:170–178. doi: 10.1016/s0005-2736(98)00098-4. [DOI] [PubMed] [Google Scholar]
- [76].Chan YH, Boxer SG. Model membrane systems and their applications. Curr Opin Chem Biol. 2007;11:581–587. doi: 10.1016/j.cbpa.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Jovanovic O, Pashkovskaya AA, Annibal A, Vazdar M, Burchardt N, Sansone A, Gille L, Fedorova M, Ferreri C, Pohl EE. The molecular mechanism behind reactive aldehyde action on transmembrane translocations of proton and potassium ions. Free Radic Biol Med. 2015;89:1067–1076. doi: 10.1016/j.freeradbiomed.2015.10.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Levi V, Villamil Giraldo AM, Castello PR, Rossi JP, Gonzalez Flecha FL. Effects of phosphatidylethanolamine glycation on lipid-protein interactions and membrane protein thermal stability. Biochem J. 2008;416:145–152. doi: 10.1042/BJ20080618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.