Abstract
Research over the past decade on the cell–biomaterial interface has shifted to the third dimension. Besides mimicking the native extracellular environment by 3D cell culture, hydrogels offer the possibility to generate well-defined 3D biofabricated tissue analogs. In this context, gelatin-methacryloyl (gelMA) hydrogels have recently gained increased attention. This interest is sparked by the combination of the inherent bioactivity of gelatin and the physicochemical tailorability of photo-crosslinkable hydrogels. GelMA is a versatile matrix that can be used to engineer tissue analogs ranging from vasculature to cartilage and bone. Convergence of biological and biofabrication approaches is necessary to progress from merely proving cell functionality or construct shape fidelity towards regenerating tissues. GelMA has a critical pioneering role in this process and could be used to accelerate the development of clinically relevant applications.
Hydrogels and the Paradigm Shift to the Third Dimension
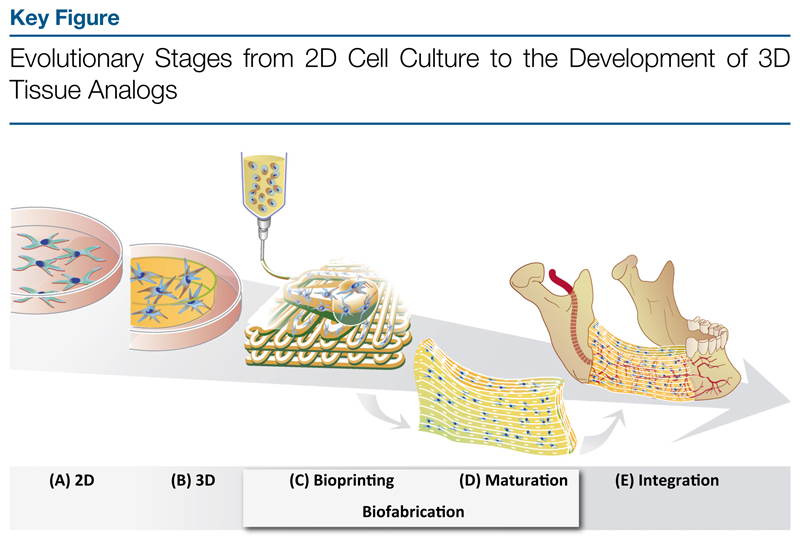
Over the past decade, cell culture research has witnessed a paradigm shift into the third dimension. 3D cultured cells behave differently compared with those cultured in monolayers (2D) and their responses better resemble those in the native tissue [1]. In this shift from the second to the third dimension, hydrogel-based approaches are driving current biomaterial research in tissue engineering. In tissue engineering, hydrogels are used that ideally resemble the natural extracellular matrix (ECM) to stimulate cells to form functional tissue with mechanical integrity to ensure survival of the graft upon implantation. While current synthetic hydrogels are often still too reductionist compared with biopolymers and, therefore, lack important biological cues [2,3], biological materials generally lack the necessary strength and precise mechanical tunability. In present-day biomaterial research, there is a strong need for a merger of both biologically active and physicochemically tailorable hydrogels [3]. Gelatin modified by methacryloyl (methacrylamide and methacrylate) side groups (gelMA) has recently gained increasing attention, because it satisfies the requirements of biofunctionality and mechanical tunability to a reasonable extent, particularly compared with other available hydrogel-forming biomaterials [4–9]. By using this 3D cell culture platform, not only is the natural extracellular environment represented, but it also provides the possibility to generate well-defined 3D tissue constructs [10–12]. In this respect, conventional 3D casting techniques for cell-laden hydrogels are replaced by advanced fabrication techniques. The emerging field of biofabrication (see Glossary) has as its aim the automated generation of biologically functional, hierarchical 3D constructs using living cells, bioactive molecules, biomaterials, cell aggregates, or hybrid cell-material and their subsequent maturation [13]. This advanced technology, which encompasses both bioassembly and bioprinting, allows for the generation of architecturally complex tissue analogs, which comprise a spatially organized assembly of various cell types potentially mimicking the native situation. This development of 3D tissue analogs reflects the evolutionary stages from cell culture in monolayers to 3D culture in disc-shaped hydrogels, to biofabricating 3D constructs undergoing biological maturation to ultimately repair a tissue defect in vivo (Figure 1, Key Figure).
Glossary.
Biofabrication: ‘The automated generation of biologically functional products with structural organization from living cells, bioactive molecules, biomaterials, cell aggregates such as micro-tissues, or hybrid cell-material constructs, through Bioprinting or Bioassembly and subsequent tissue maturation processes’ [13].
Bioink: fluid or gel containing living cells to be used for printing of tissue constructs.
Endothelial colony-forming cells (ECFC): endothelial progenitors that are able to differentiate into functional endothelial cells. Although they are present in adult blood, they can be obtained with higher yield from umbilical cord blood for engineering endothelial networks or for coating the luminal side of vascular structures.
Habeeb method: method to determine the number of free amino groups in proteins.
Irgacure 2959: water-soluble, cytocompatible radical photoinitiator for the UV curing of unsaturated monomers and prepolymers.
Microfluidics: passive or active fluid handling or manipulation within micrometer-sized channels.
Micromolding: production of objects with micrometer-sized features within a mold.
Micropatterning: patterning of (bio) materials to control the fate and geometry of adhering cells.
Microtissue: hydrogels in the millimeter range with encapsulated cells that are used for 3D cultivation.
Mesenchymal stem cells or multipotent stromal cells (MSC): adult stem cells that can differentiate towards at least the osteogenic, adipogenic, and chondrogenic lineages. MSCs from human bone marrow aspirates are the gold standard human cell source used in tissue engineering and regenerative medicine of bone, fat and cartilage tissue.
Photo-crosslinking: covalent binding of molecules using light as an initiating system.
Poloxamer: a triblock copolymer with typical trade names ‘Lutrol’ or ‘Pluronics’. It is used for defined printing processes for creating sacrificial layers or fibers in biofabrication approaches.
Polycaprolactone (PCL): a biodegradable and biocompatible polyester that is often used as a scaffolding material in tissue engineering.
Soft lithography: combines various fabrication techniques that use elastomeric materials to fabricate constructs typically on the micrometer or nanometer scale. Photopatterning is an example of soft lithography that uses molds and/or photomasks.
Stereolithography: an additive manufacturing technique to fabricate scaffolding materials by spatially controlled photo-crosslinking of polymers in a bath of resin.
Figure 1.
(A) 2D cell culture on plastic; (B) 3D cell culture inside hydrogel constructs; (C) bioprinting of 3D constructs; (D) biological maturation of the 3D bioprinted construct forming a tissue analog; and (E) implantation and integration of the tissue analog into the defect site.
In this review, we provide an overview of the uses of gelMA as a cell-encapsulating hydrogel, serving as a base material for a multitude of tissue-engineering strategies. We provide a picture of the diverse modifications of gelatin and its crosslinking systems, detailing the trends in gelatin-based biomaterial research, and the place of gelMA therein. In particular, we describe the use of gelMA in state-of-the-art biofabrication approaches to obtain complex tissue analogs, and we highlight the functional aspects of these developments. By doing so, we put into perspective the usefulness of gelMA-based engineered constructs in terms of the translational aspect of regenerative medicine.
Gelatin-Based Hydrogels for Cell Encapsulation
Gelatin is widely used in applications ranging from the food industry [14] to medicine and pharmaceutical processing [15]. In tissue engineering and regenerative medicine, gelatin is an attractive base material for engineering ‘smart’ hydrogels for drug delivery (e.g., [16,17]). Increasing interest in the use of gelatin in these fields stems from its various desirable features, including biocompatibility, biodegradability, low cost, and ease of manipulation [18]. Additionally, gelatin is a material that is generally recognized as safe (GRAS) by the US Food and Drug Administration (FDA) for food processing. Furthermore, it is routinely used in the clinic as a plasma expander and as a stabilizer in several protein formulations, including vaccines [16]. Gelatin is a proteinaceous substance comprising denatured and partially hydrolyzed native collagen, mainly type I [17] (Box 1). In contrast to collagen, gelatin exhibits limited antigenicity due to heat denaturation [19]. Importantly, the bioactive sequences of collagen [e.g., the arginine-glycine-aspartic acid (RGD) peptide] for cell attachment and matrix metalloproteinase (MMP)-sensitive degradation sites are retained in the gelatin backbone [20]. As such, essential cellular functions, such as migration, proliferation, and differentiation, can be facilitated via integrin-mediated cell adhesion and cell-mediated enzymatic degradation [21,22].
Box 1. Gelatin: A Versatile Biomaterial.
Typically, for the extraction of gelatin, collagen is obtained from bovine or porcine skin or bone as a by-product of the meat-processing industry. Extracts from collagen compositions are commonly obtained under either acidic or basic conditions, which are referred to as type A or type B gelatin, respectively. These collected protein fragments form a gelatinous mixture. The Bloom strength, which typically ranges from 90 g to 300 g for porcine skin, is a measure of the strength of the physical gel that is formed upon cooling. This depends on species and molecular weight, among other factors. For example, fish gelatin is characterized by lower Bloom strength compared with porcine or bovine gelatin [68] and, therefore, is less suitable for biofabrication purposes, because these often make use of these gelation properties.
To use gelatin as a biomaterial, its instability at body temperature is overcome by covalent crosslinking methods [23]. Gelatin can be crosslinked either without prior modification or after functionalization of its side groups. Unmodified gelatin can be crosslinked in various ways to form a covalent network, such as by chemical or enzymatic crosslinking (Box 2). The application of gelatin-based hydrogels based on prior modification, such as gelMA, are the main focus of this review.
Box 2. Covalent Crosslinking without Chemical Modification.
Unmodified gelatin is crosslinkable without any prior modification, which is the strategy traditionally used to fabricate gelatin hydrogels. For instance, aldehydes are well-known crosslinking agents for proteins [69,70], but are typically not suitable for simultaneous cell encapsulation due to the cytotoxicity, immunogenicity, and inflammatory effects of their degradation products [71]. In addition, genipin, a natural crosslinking agent, which is considered less cytotoxic compared with aldehydes, must be used at a low dose when the hydrogel is employed to encapsulate cells [72]. Overall, most crosslinking agents that enable generation of gels with high mechanical stability exhibit considerable cytotoxicity [70]. By contrast, enzymatic crosslinking of gelatin under physiological conditions by means of transglutaminases or tyrosinases provides a more cell-friendly approach [73–75]. However, this crosslinking system exhibits limited tailorability in the design of the hydrogels. Major disadvantages of direct crosslinking methods (without prior modification of gelatin) include poor control over the crosslinking density and the resulting stiffness of the hydrogel. For these reasons, using functionalized gelatin has become a favored approach over the direct crosslinking of gelatin.
Covalent Crosslinking after Chemical Modification
The addition of functional groups to the gelatin backbone is a crosslinking strategy with a high degree of control over hydrogel design and properties, compared with direct crosslinking techniques. Crosslinking of functional groups can be initiated using various systems. However, only a few are suitable for simultaneous crosslinking and cell encapsulation [24] (Table 1). Both (photo)radical-initiating systems and enzymatic crosslinking of functionalized gelatin are frequently used. In contrast to indirect enzymatic crosslinking, photoinitiation provides good temporal and spatial control over the crosslinking process [25], which is essential for creating an architecturally complex tissue analog. For this, both ultraviolet light (UV) and visible light (VIS) are used for photoinitiation [10,26].
Table 1. Modifications of Gelatin and Crosslinking Systems Used for Cell Encapsulationa.
| Functional Group | Initiating System | Biological Response after Cell Encapsulation | Refs |
|---|---|---|---|
| Acrylamide | Irgacure 2959 (UV-A, 365 nm) | >90% viability after 1 day (HepG2) | [76] |
| Ferulic acid | Laccase + O2 | >91% viability (fibroblasts, ECFCs), angiogenesis | [77] |
| Furfurylamine | Rose Bengal (VIS) | 87% viability after 1 day (MSCs), used for osteochondral tissue formation | [26] |
| Methacryloyl | APS/TEMED | >80% viability after 1 day (chondrocytes) | [51] |
| Irgacure 2959 (UV-A, 365 nm) | 70% to >90% viability depending on, e.g., crosslinking conditions, cell type, macromer concentration; various differentiations investigated | E.g., [3,4,6–8, 10,11,21,28, 33,35,37,38, 40,42–45,48, 50,52,55,58, 62,76,78] | |
| VA-086 (UV-A, 365 nm) | MSC/HUVEC coculture, vascularization; >97% viability after extrusion printing (HepG2) | [5,12] | |
| LAP (VIS 430–490 nm) | >96% viability after 1 day (MSCs), chondrogenic differentiation; cell proliferation increase of 23% over 2 weeks (MSC); adipocyte culture | [9,79,80] | |
| G2CK or P2CK (near-infrared femtosecond laser 800 nm) | 26% viability (MG63 cells) after two-photon polymerization | [81] | |
| Methacryloyl and acetylation | Irgacure 2959 (UV) | Chondrocyte encapsulation, used for inkjet printing | [28] |
| Methacryloyl galactosylation | Irgacure 2959 | 90% viability (HepG2), functional testing of hepatocytes | [27] |
| Norborene | DTT or LAP (UV 365 nm) | >91% viability (MSCs) | [82] |
| Phenolation | HRP + H2O2 | >94% viability or not quantified; various differentiations investigated (e.g., neurogenesis, osteogenesis, and vascularization) | E.g., [83–89] |
| Styrenation | Camphor-quinone (VIS 400–520 nm) | 26% viability (chondrocytes) | [90] |
Abbreviations: APS/TEMED, ammonium persulfate/tetramethylethylenediamine; DTT, dithiothreitol; ECFC, endothelial colony-forming cell; G2CK and P2CK, benzylidene cycloketone-based photoinitiators; HepG2, human hepatocellular carcinoma cell line; HRP, horseradish peroxidase; HUVEC, human umbilical vein endothelial cells; LAP, lithium acylpho-sphinate; MG63, human osteosarcoma cell line; MSC, mesenchymal stromal cells; VA-086, 2,2’-azobis[2-methyl-N-(2-hydroxyethyl)propionamide].
A variety of functional groups have been used for crosslinking in (photo)radical-initiating or enzymatic-catalyzing systems (Table 1). Moreover, some double modifications have been used to improve cell behavior [27] or enhance processability [28]. Yet, most of the reported literature uses gelMA with the photoinitiator 1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one, which is better known under its trade name Irgacure® 2959 from BASF (formerly Ciba Specialty Chemicals). This water-soluble initiator, which dissociates into a benzoyl and ketyl-free radical upon UV light irradiation through an ∝-cleavage reaction [29], has a relatively low cytotoxicity compared with other photoinitiators [30]. Interestingly, deviations from the gold standard of using gelMA with Irgacure 2959, except for the use of styrenated gelatin with camphorquinone (which showed low cell viability), are all recent and one-off demonstrations. The introduction of acrylamide, furfurylamine, and norborene-substituted gelatin may have specific advantages compared with gelMA, although these await further research.
The use of VIS (with suitable initiators) has a strong rationale, since UV is known to have detrimental effects on biological components. Although cell viability is generally assessed 1 day after crosslinking, more subtle damage may be incurred by UV that could affect cell functionality and tissue formation in the longer term [31]. Moreover, the long-term effects of Irgacure 2959, albeit relatively cell friendly compared with other UV photoinitiators [32], have not yet been studied fully.
Gelatin-Methacryloyl Hydrogels for Cell Encapsulation
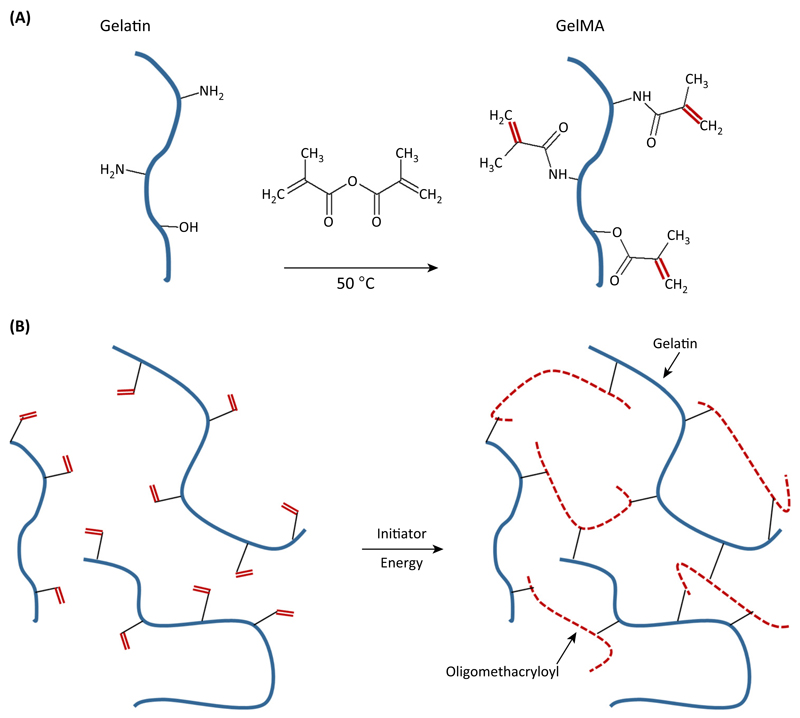
GelMA was first introduced in 2000 by Van den Bulcke and coworkers [33]. Subsequently, it gained considerable interest in the tissue-engineering community due to its inherent bioactivity and physicochemical tailorability [34]. The first step in the hydrogel design of gelMA is the selection of an appropriate degree of functionalization (DoF) of gelatin (Box 3). This is tailored by the amount of methacrylic anhydride that is used for the synthesis of gelMA macromers (Box 3). By using these macromers, hydrogels can be fabricated in the presence of a (light) initiator and an energy source. Via radical polymerization, gelatin chains are connected thorough short polymethacryloyl chains (Figure 2).
Box 3. GelMA Synthesis.
GelMA is generally prepared by reacting gelatin with methacrylic anhydride in phosphate buffered saline (PBS) at pH 7.5. Methacrylic anhydride is added slowly to the gelatin solution under vigorous stirring at 50 °C. During the reaction, methacrylic acid is formed. After 1 h, the reaction is diluted with water. To remove unreacted methacrylic anhydride from the reaction mixture, it is dialyzed against distilled water. The obtained reaction product, gelMA, is freeze-dried to a white porous foam [33]. The DoF of the synthesized gelMA batch can be tailored by varying the methacrylic anhydride:gelatin ratio [21,33]. The DoF was characterized by van den Bulcke et al. as the percentage of functionalized primary amine groups over total primary amine groups [33] and is generally determined by the Habeeb method [91].
Figure 2. Gelatin-Methacryloyl (GelMA) Synthesis and Hydrogel Formation.
(A) Reaction of methacrylic anhydride with amine and hydroxyl groups on gelatin gives rise to gelMA macromers. (B) Upon generation of a free radical (e.g., by light exposure in the presence of a photoinitiator), the methacrylamide and methacrylate side groups on the gelMA chains polymerize via radical addition-type polymerization to yield a network of gelatin chains connected through short polymethacryloyl chains.
The biomaterial can be further tailored to form specific tissues by designing the desired physicochemical properties. As an example, spreading of cartilage cells needs to be prevented within the gel. This can be achieved by using increased polymer concentrations (conventionally 10%) that may sterically prevent cells from spreading. In addition, highly functionalized gels with a DoF approaching 80% can hamper cell spreading, possibly by extensive crosslinks throughout the hydrogel. Typically, gelMA macromers with DoF of 20–80% are used to generate stable hydrogels [10,21,35], with increasing percentages of methacryloyl substitution leading to hydrogels that are stiffer and more durable, with smaller pore sizes [35]. Typically, by varying the macromer concentration from 5% to 20%, hydrogels are generated with compressive moduli in the range of 5–180 kPa [10]. Next to the DoF of a synthesized gelMA batch and its macromer concentration, the parameters of photo-crosslinking critically influence the properties of the resulting hydrogel. These parameters include the light exposure time, light intensity, and initiator concentration. Over time, these parameters can be affected by degradation. The gelMA network is susceptible to local degradation by enzymes, most notably by MMPs, which are secreted by the (embedded) cells [3,36].
All design parameters need to be carefully balanced for each specific application. These include the stiffness, degradation profile, and intended cellular behavior in the resulting hydrogel. For a detailed summary on the design parameters, the reader is referred to a recent review by Khademhosseini and colleagues [34]. Due to this tunability of gelMA properties, it is used in a variety of strategies in tissue engineering. Indeed, gelMA has been applied in approaches aiming to regenerate neural tissue, vascularization, cartilage, bone, skin, skeletal muscle, cardio, liver, and kidney [34].
Biofabrication Techniques
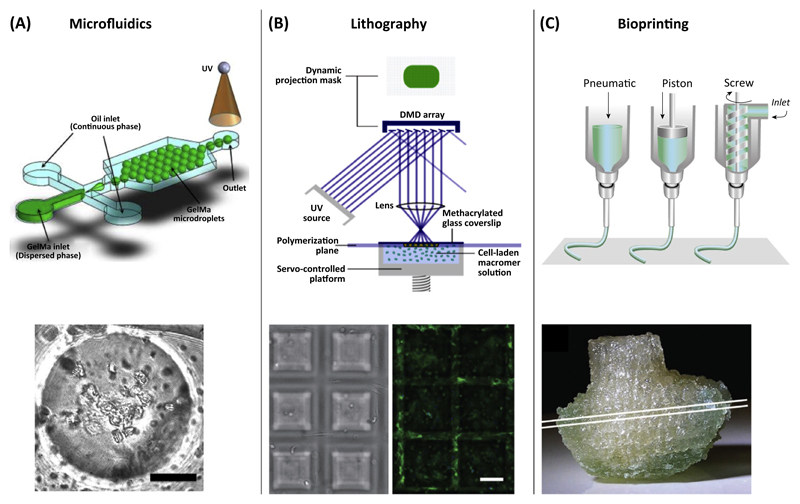
Conventionally, research on cell-encapsulating gelMA hydrogels is often based on casted or molded disk-shaped microtissues that serve as models to study cell–material interactions. To obtain a tissue-like construct with a defined 3D structure, more advanced technologies have now emerged. The excellent spatial and temporal control over gelMA crosslinking, and its rheological behavior enable deposition by various biofabrication-related techniques (Figure 3).
Figure 3. Categories of Gelatin-Methacryloyl (GelMA)-Based Biofabrication-Related Techniques and their Generated Constructs.
(A) Preparation of cell-laden microspheres by microfluidics (scale bar = 30 μm). (B) Stereolithographic fabrication of pyramid-shaped scaffolds by spatially controlled light-initiated crosslinking of a gelMA macromer-cell mixture in a computer-controlled platform. By using this approach, cells are encapsulated while building the construct. Encapsulated cells stained for actin expression (scale bar = 100 μm). (C) Computer-controlled robotic dispensing of cell-laden gelMA to build a 3D construct, for instance, a bioprinted analog of the distal femur from a human knee. Reproduced, with permission, from [92] (A), [42] (B), and (upper picture) [93] and (lower picture) [45] (C). Abbreviation: UV, ultraviolet.
Fabricating Cell-Laden Modules by Microfluidic Strategies
Microfluidic strategies were developed to encapsulate cells in gelMA droplets (Figure 3A) for a bottom-up tissue-engineering approach, or as micromodule for advanced assembling strategies to build more complex tissues [37]. Furthermore, a microfluidic spinning technique was introduced to fabricate photo-crosslinkable gelMA fibers with encapsulated cells [38]. It was shown that engravement of gelMA fibers induced cell alignment on the surface of the fibers [38]. To enhance the potential of these cell-laden fibers, encapsulation within a bulk hydrogel may be beneficial. Such 3D patterns in the fibers can be used as templates for creating tissues that exhibit preferential cell orientations, such as blood vessels or muscle fibers. Recently, an alternative set-up was introduced to create highly viable cell-laden microfibers in a straightforward and high-throughput manner. Upon stretching of the loaded fibers, cell alignment within the constructs was achieved [39].
Using Soft- and Stereolithography for Cell Encapsulation
GelMA is also used in various soft lithography techniques to fabricate micropatterned tissues that involve cell encapsulation. Construct features on the micrometer scale, down to 100 μm in resolution, were successfully fabricated, resulting in robust cell-laden gelMA microtissues [21]. Such a micropatterning procedure was also used in a ‘layer-by-layer’ bottom-up approach by means of masks to build an osteon-like hydrogel with microchannel networks based on gelMA [40,41]. These approaches demonstrate the localized deposition of cells to form the vasculogenic and osteogenic parts of bone tissue. However, when moving towards creating larger constructs for tissue repair strategies, such a mask-based approach in micromolding is limited due to high costs, its time-consuming nature, and lack of automation.
In contrast to micromolding, stereolithography circumvents these challenges since it can be performed as a maskless photopatterning technique able to directly build up 3D structures. The design is processed by software and sliced into several layers. By a dynamic stereolithographic technique, 100–250 μm-thin slices with various shapes could be fabricated (Figure 2B) with high cell survival of approximately 80% after 8 h of cell encapsulation [42]. Overall, stereolithography is a valuable means to create complex 3D architectures to guide cell alignment and behavior within a generated construct. However, stereolithography is limited to one resin-composition containing one biomaterial (mixture) and homogeneously distributed cells.
Bioprinting of Tissues with Cell-Containing GelMA-Based Inks
In addition to lithographic approaches, tissue analogs can be also generated in a layer-by-layer fashion with bioprinting. Tissue construction by 3D printing of cells by means of a hydrogel-based ink has recently become an attractive approach in tissue engineering [10,43]. By a direct-write bioprinting strategy, researchers showed that it was possible to build gelMA-based constructs with varying architectures [44]. To embrace the complexity of a tissue in a printed analog, a bioprinting approach was proposed that comprises heterogeneous subunits [43]. In this approach, a poloxamer gel was used as a sacrificial material to create the vascular luminal space for seeding of endothelial cells. Around the vascular bed, fibers were coprinted containing heterogeneous cell types with high cell viability and the bulk material was molded using gelMA [43]. Furthermore, for engineering bone, a microcarrier technology was combined with printing technology. Mesenchymal stromal cells (MSCs) were seeded on polylactide microspheres for extensive cell expansion and these multicellular aggregates were printed within a gelMA-gellan gum ink [8].
Several strategies were introduced to allow for the well-defined deposition of cell-laden gelMA. To improve the rheological characteristics of gelMA for printing, viscosity-enhancing components were mixed into the bioink. For instance, adding gellan gum [11] or hyaluronic acid [10] to the gelMA-precursor solution optimized the ink rheological properties for dispensing. Another method for improving biofabrication of gelMA is codeposition with reinforcing biomaterials. Thermoplastics, such as polycaprolactone (PCL), can serve a dual role here. First, the deposition of gelMA is more defined because the PCL can delineate the boundaries of the gelMA compartment and, second, constructs can be generated with enhanced mechanical properties [10,45]. A third approach to improving the printability of cell-laden gelMA hydrogels relies on the inherent temperature-dependent sol–gel transition of gelatin and not on viscosity-enhancing materials or codeposition techniques [12]. Cooling of the printed fibers on the collecting plate to 5 °C, immediately after deposition, enhanced physical crosslinking of gelMA and provided sufficient mechanical integrity to build up layers. However, the rapid change in temperature may affect the behavior of more fragile cell types. This approach was suitable for high gelMA concentrations between 10% and 20% and allowed encapsulation of a liver cell line (HepG2) with high viability.
To generate complex anatomically shaped constructs, sacrificial components, such as poly (vinylalcohol) (PVA) and alginate, have been codeposited with gelMA and PCL. These sacrificial materials, which were removed in aqueous solution after the fabrication process, were used as temporary structures for the support of overhanging geometries. By using this approach, porous constructs were obtained of clinically relevant sizes without affecting cell viability during the fabrication process [45].
GelMA Composite Structures for Enhanced Tissue-Specific Functionality
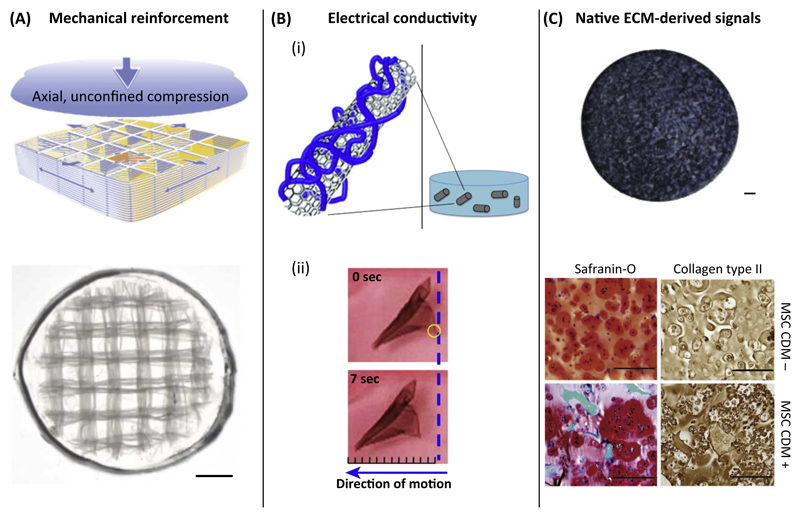
Analogous to the use of gelatin [46], gelMA is increasingly being used in combination with other materials. GelMA will not always be suitable on its own and may need further biologic stimuli for improved cell behavior or enhanced mechanical properties (Figure 4). In composites, a synergistic effect of the materials can be achieved that enhances the (bio)functionality of gelMA-based hydrogels. For example, GelMA composites were developed with calcium phosphates [40], polysaccharides [47], hyaluronan [10,48], silk [49], and ECM particles [50]. Furthermore, synthetic polymers, such as PCL [45,51] and poly(ethylene glycol) (PEG) [52–54], and nanoparticles have also been combined with gelMA [55].
Figure 4. Examples of Combining Gelatin-Methacryloyl (GelMA) with Different Materials to Obtain Tissue-Specific Functionalities.
(A) Mechanical reinforcement of hydrogels by combination with electrospun box structures that form a macroscopic network structure (scale bar = 1 mm). (B) (i) Providing gelMA with electrical conductivity by the addition of carbon nanotubes to the bulk hydrogel. (ii) The cardiomyocyte-seeded composite showed improved contraction behavior, resulting in movement of the construct of about 2.5 mm. (C) Optimizing gelMA by addition of cartilage-derived matrix particles [1.5% (w/v)] to the hydrogel (scale bar = 500 μm). Mesenchymal stromal cells (MSCs) produced pronounced cartilage-specific matrix, of GAGs and collagen type II. compared with non-laden gelMA (scale bar = 200 μm). Reproduced, with permission, from [51] (A), [55] (B, i), [58] (B, ii), and [50] (C). Abbreviation: ECM, extracellular matrix.
Mechanical Reinforcement
The mechanical properties of gelMA can be tailorable, resulting in considerable strength and stiffness. However, this will not be sufficient for some applications, particularly when low stiffness is chosen for the sake of biofunctionality. In these cases, reinforcement strategies are available, including codeposition with reinforcing biomaterials (hybrid printing) [56], infusion of gelMA into 3D printed scaffolds followed by covalent binding between gel and scaffold [6], and reinforcement of cell-laden gels with 3D printed microfibers, leading to an increase in stiffness of up to 54 times compared with hydrogel or fiber mesh alone [51] (Figure 4A).
Engineering Vascular Networks in gelMA
A major hurdle in tissue engineering is the limited supply of oxygen and nutrients in generated tissue constructs. This limitation is addressed by introducing a minute vascular network in tissue-engineered constructs to prevent a necrotic core. The feasibility to engineer vascular-like networks in gelMA constructs has been investigated mainly by two approaches. First, by using a scaffold-based strategy, relatively large-diameter vascular beds are engineered that are seeded with endothelial cells after fabrication of the construct [57]. Second, smaller, capillary-like structures are generated by encapsulation of endothelial cells within the bulk material [57]. The latter approach is based on the intrinsic capability of endothelial cells to self-assemble de novo into capillary-like structures.
Capillary-like structures have been formed by self-organization of MSCs and endothelial colony-forming cells (ECFCs) [7,35] or human umbilical vein endothelial cells (HUVECs) [5] that were combined within a gelMA bulk hydrogel. The next step in engineering vasculature-like structures within a tissue-engineered construct could be taken by offering appropriate (blood) flow conditions for improved cell maturation. Accordingly, a coculture of ECFCs and MSCs, embedded in a pure gelMA carrier, was implanted subcutaneously into immunodeficient mice [7,35]. After 7 days, an evenly distributed endothelial network was formed throughout the construct. This provided proof of concept of functional anastomoses of bioengineered vascular-like structures in gelMA [7].
Tissue-Specific Differentiation in gelMA
Next to general approaches for vascularizing cell-laden constructs, gelMA has been used in a broad spectrum of tissue-specific applications. For the engineering of cardiac patches, gelMA was combined with carbon nanotubes and graphene oxide microspheres for introducing electrical conductivity [55,58,59]. Functional assessment of neonatal rat cardiomyocytes on a 2D composite patch highlighted higher and more synchronous beating rates and a lower excitation threshold compared with a culture on pure gelMA [58]. These 2D patches are thought to be rolled up or folded to form 3D tissues [58]. For a direct 3D approach that encapsulates cells within the composite, a cell line (NIH-3T3 fibroblasts) was used that demonstrated good cellular function [59].
In liver tissue engineering, hepatocyte microaggregates were generated in a high-throughput manner and encapsulated in gelMA [60]. Analysis confirmed that the encapsulation did not interfere with cell viability, and primary hepatocytes could be maintained with a stable phenotype for 21 days. Furthermore, gelMA containing the cell aggregates could serve as a bioink for 3D liver printing [60].
In bone tissue engineering, the combination of cells, minerals, and proteins, such as occurs in the native tissue, is increasingly used [46]. GelMA has been combined with calcium phosphates and human osteoblast-like cells (MG63) [40]. Although the addition of ceramics resulted in higher mechanical strength, no significant effect on osteogenicity has been shown so far.
In addition to the direct intramembranous route, bone can also be formed via an indirect endochondral route, with cartilage tissue as an intermediate stage. Endochondral ossification was shown in gelMA constructs in a subcutaneous rat model [50]. First, gelMA-encapsulated MSCs were cultured in vitro for 2 weeks to provide a cartilage template that was subsequently remodeled in vivo into mineralized bone tissue harboring bone marrow cavities. The gelMA hydrogel was almost completely degraded during this process, while the newly formed matrix assured construct integrity [50].
Cartilage is another load-bearing tissue that requires prolonged mechanical performance of tissue-engineered constructs, for which gelMA has been demonstrated useful. Although dedifferentiation of chondrocytes can occur within gelMA gel of low stiffness (1.5 kPa) [61], in stiffer gelMA gels (approximately 30 kPa) chondrogenic redifferentiation occurs, both in vitro [10] and in vivo [6]. Yet, hyaluronic acid has been shown to be a valuable additive to gelMA-based constructs for cartilage tissue engineering, because it directly influences chondrocyte differentiation in a concentration-dependent manner [10,48,62]. Moreover, for cartilage engineering, a sophisticated construct was designed with high mechanical strength. In this approach, methacrylated PCL was 3D printed and covalently crosslinked with chondrocyte-laden gelMA [6]. It is vital that reinforcing strategies do not impede tissue formation. This broad application of gelMA for numerous tissue-engineering strategies underscores its versatility. However, it still remains to be determined which tissue analogs gelMA is most suitable for (see Outstanding Questions). This may largely depend on the potency of gelMA within composite structures that can give tissue-specific functionality to gelatin.
Outstanding Questions.
Is gelMA suitable for engineering analogs of all human tissues?
Will gelMA serve as a stepping-stone and be eventually replaced by new synthetic gels with a sufficient level of biofunctionality required for a tissue analog?
How can multiple cell types be combined, each requiring their specific cues, within one construct to generate multitissue-type, vascularized, clinical-sized, and functional tissue analogs?
How much predefined architectural complexity does a 3D biofabricated tissue analog require and to what extent can this architecture be created and modulated by encapsulated cells?
Tissue Architecture
The architecture of a tissue analog is mainly dictated by (bio)functional and mechanical aspects. Currently, the main challenge lies in up-scaling microtissues to clinically relevant-sized constructs. This cannot be achieved by simply applying the same methods and creating a larger tissue. The complexity of the construct is increased, together with the number of challenges. For example, up scaling of a construct comprises nutrient transfer throughout the construct and provision of the required mechanical stability. Whereas gelMA has proven its potency in creating microtissues, in future research yet more focus is expected on up-scaling.
Furthermore, an essential aspect of tissue architecture is to embrace the complexity of a tissue in its engineered analog. For example, Kolesky and coworkers divided different tissue components (vasculature, ECM, and specific cells) over multiple bioinks [43]. However, this biofabrication approach, similar to most others, focused on short-term cell behavior rather than on long-term features, such as matrix remodeling and tissue maturation. Such long-term outcomes of biofabricated tissues will be of great value to determine the level of architectural complexity that will need to be imposed to obtain functional human tissue analogs.
GelMA from A (Pre-)Clinical Perspective
Promising results were obtained in a preclinical study, demonstrating the potential of gelMA for clinical application. The aforementioned endochondral bone regeneration [50] is an example of impressive balance between degradation of a biomaterial and replacement by neotissue, which is one of the key and most challenging goals in tissue engineering. GelMA degradability can be tailored to the remodeling rate of the target tissue within limits. Increasing the DoF will improve mechanical properties and extend the required degradation period [36,63].
For clinical translation, gelMA as a base material has to meet several requirements. First, the in vitro and in vivo biocompatibility of gelMA and its degradation products, particularly oligomethacrylates, has to be considered. An extensive in vitro study showed good biocompatibility for gelatin type B-based gelMA, while type A-based gelMA elicited inflammatory reactions [64], possibly caused by high levels of endotoxins in the latter material. So far, only one immunocompetent animal (mouse) model has been used to test gelMA biocompatibility. The absence of proinflammatory activity provided a first proof of immunocompatibility for type B-based gelMA [64]. While endotoxin-free gelatin was used (type B-based gelMA) in this study, most research is currently conducted with gelMA that is based on gelatin with high endotoxin levels. These endotoxins can cloud the observations by influencing cell behaviour (e.g., stimulation of osteogenesis [65]), or may elicit other undesired effects. This aspect is generally underestimated in the field.
Other challenges in clinical translation are in batch-to-batch variations and possible disease transfer associated with animal-derived materials. Nonetheless, clinical grade gelatin is now routinely used in the clinic, which indicates that the benefits are believed to outweigh the risks.
Future Directions
The current major bottleneck in the translation of tissue-engineered constructs to the clinic is to convert a successful regenerative approach to procedures adhering to good manufacturing practice (GMP) while retaining the intended regenerative capacity. The conversion extends from the gelMA synthesis and bioprinting to the cell-culturing protocols. For example, the gold standard for gelMA crosslinking is now by Irgacure 2959 and UV light. However, to circumvent the associated drawbacks of UV light, alternative crosslinking systems, such as by VIS, may receive more attention for crosslinking gelMA. The incorporation of cells further complicates translation because tissue-engineering products need to conform to the legislation for advanced therapy medicinal products (ATMPs), which is still an underexplored field [66]. Thus, given its general potency, gelMA might not only be a pioneer for translating semisynthetic biomaterials to ATMPs, but could also act as a ‘transitional technology’ [67]. In this way, we could understand further how to accelerate the translation of the technology from bench to bedside. During this process, gelMA could serve as a stepping-stone for the design of next-generation tissue analogs.
Concluding Remarks
GelMA has become an attractive biomaterial in recent years for engineering various tissues since the gelatin backbone provides cells with biological cues and its functionalization enables one to tailor specific physicochemical properties. At present, research is either mainly focused on the generation of viable well-defined 3D constructs or on long-term cell performance in nonbio-fabricated constructs.
The greatest challenge is to scale up construct dimensions to clinically relevant sizes. Therefore, future research with gelMA should focus on converging biofabrication and tissue-engineering technologies to create large, well-defined, and functional tissue equivalents upon maturation. The design of smart geometries, combinations of various materials and tissue types, and maintenance of the complex tissues under ATMP guidelines will be next. In conclusion, gelMA has a valuable pioneering role and is likely to accelerate the clinical translation of biofabrication-based tissue repair.
Trends.
In gelMA hydrogels, the inherent bioactivity of gelatin is combined with the tailorability of photo-crosslinking.
3D-generated tissue analogs need to be geometrically natural mimics that are biofunctionally and mechanically stable.
GelMA will accelerate the development of cell-laden biofabricated constructs and will have a pioneering role in their translation to clinically relevant applications.
Acknowledgments
The authors’ research work is supported by the Dutch Arthritis Foundation; the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement 309962 (HydroZONES); and the European Research Council under grant agreement 647426 (3D-JOINT).
References
- 1.Pampaloni F, et al. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 2.Ehrbar M, et al. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials. 2007;28:3856–3866. doi: 10.1016/j.biomaterials.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Benton JA, et al. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng Part A. 2009;15:3221–3230. doi: 10.1089/ten.tea.2008.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertassoni LE, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–2211. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Occhetta P, et al. VA-086 methacrylate gelatine photopolymerizable hydrogels: a parametric study for highly biocompatible 3D cell embedding. J Biomed Mater Res Part A. 2014;103:2109–2117. doi: 10.1002/jbm.a.35346. [DOI] [PubMed] [Google Scholar]
- 6.Boere KWM, et al. Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater. 2014;10:2602–2611. doi: 10.1016/j.actbio.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 7.Lin RZ, et al. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials. 2013;34:6785–6796. doi: 10.1016/j.biomaterials.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levato R, et al. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication. 2014;6:035020. doi: 10.1088/1758-5082/6/3/035020. [DOI] [PubMed] [Google Scholar]
- 9.Lin H, et al. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng Part A. 2014;20:2402–2411. doi: 10.1089/ten.tea.2013.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuurman W, et al. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol Biosci. 2013;13:551–561. doi: 10.1002/mabi.201200471. [DOI] [PubMed] [Google Scholar]
- 11.Melchels FPW, et al. Development and characterisation of a new bioink for additive tissue manufacturing. J Mater Chem B. 2014;2:2282–2289. doi: 10.1039/c3tb21280g. [DOI] [PubMed] [Google Scholar]
- 12.Billiet T, et al. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35:49–62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 13.Groll J, et al. Biofabrication: reappraising the definition in an evolving field. Biofabrication. 2016;8:013001. doi: 10.1088/1758-5090/8/1/013001. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Guillen MC, et al. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocolloids. 2011;25:1813–1827. [Google Scholar]
- 15.Djagny KB, et al. Gelatin: a valuable protein for food and pharmaceutical industries: review. Crit Rev Food Sci. 2001;41:481–492. doi: 10.1080/20014091091904. [DOI] [PubMed] [Google Scholar]
- 16.Elzoghby AO. Gelatin-based nanoparticles as drug and gene delivery systems: reviewing three decades of research. J Controlled Release. 2013;172:1075–1091. doi: 10.1016/j.jconrel.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Young S, et al. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Controlled Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Lai J-Y, Li Y-T. Functional assessment of cross-linked porous gelatin hydrogels for bioengineered cell sheet carriers. Biomacromolecules. 2010;11:1387–1397. doi: 10.1021/bm100213f. [DOI] [PubMed] [Google Scholar]
- 19.Gorgieva S, Kokol V. Collagen- vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. INTECH Open Access Publisher; 2011. [Google Scholar]
- 20.Van den Steen PE, et al. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 21.Nichol JW, et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heino J, et al. Evolution of collagen-based adhesion systems. Int J Biochem Cell Biol. 2009;41:341–348. doi: 10.1016/j.biocel.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Reddy N, et al. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015;33:362–369. doi: 10.1016/j.tibtech.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Mironi-Harpaz I, et al. Photopolymerization of cell-encapsulating hydrogels: crosslinking efficiency versus cytotoxicity. Acta Biomater. 2012;8:1838–1848. doi: 10.1016/j.actbio.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 26.Mazaki T, et al. A novel, visible light-induced, rapidly crosslinkable gelatin scaffold for osteochondral tissue engineering. Sci Rep. 2014;4:4457. doi: 10.1038/srep04457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gevaert E, et al. Galactose-functionalized gelatin hydrogels improve the functionality of encapsulated Hepg2 cells. Macromol Biosci. 2014;14:419–427. doi: 10.1002/mabi.201300320. [DOI] [PubMed] [Google Scholar]
- 28.Hoch E, et al. Chemical tailoring of gelatin to adjust its chemical and physical properties for functional bioprinting. J Mater Chem B. 2013;1:5675–5685. doi: 10.1039/c3tb20745e. [DOI] [PubMed] [Google Scholar]
- 29.Liu M, et al. Time-resolved spectroscopic and density functional theory study of the photochemistry of irgacure-2959 in an aqueous solution. J Phys Chem A. 2014;118:8701–8707. doi: 10.1021/jp506099n. [DOI] [PubMed] [Google Scholar]
- 30.Williams CG, et al. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26:1211–1218. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Fedorovich NE, et al. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30:344–353. doi: 10.1016/j.biomaterials.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 32.Bryant SJ, et al. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomaterials Sci Polym Ed. 2000;11:439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 33.Van Den Bulcke AI, et al. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 34.Yue K, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YC, et al. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater. 2012;22:2027–2039. doi: 10.1002/adfm.201101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koshy ST, et al. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials. 2014;35:2477–2487. doi: 10.1016/j.biomaterials.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung J, Oh J. Swelling characterization of photo-cross-linked gelatin methacrylate spherical microgels for bioencapsulation. e-Polymers. 2014;14:161–168. [Google Scholar]
- 38.Shi X, et al. Microfluidic spinning of cell-responsive grooved microfibers. Adv Funct Mater. 2015;25:2250–2259. [Google Scholar]
- 39.Li Y, et al. Chinese-noodle-inspired muscle myofiber fabrication. Adv Funct Mater. 2015;25:5999–6008. [Google Scholar]
- 40.Zuo Y, et al. Photocrosslinkable methacrylated gelatin and hydroxyapatite hybrid hydrogel for modularly engineering biomimetic osteon. ACS Appl Mater Interfaces. 2015;7:10386–10394. doi: 10.1021/acsami.5b01433. [DOI] [PubMed] [Google Scholar]
- 41.Zuo Y, et al. Bottom-up approach to build osteon-like structure by cell-laden photocrosslinkable hydrogel. Chem Commun (Camb) 2012;48:3170–3172. doi: 10.1039/c2cc16744a. [DOI] [PubMed] [Google Scholar]
- 42.Soman P, et al. Digital microfabrication of user-defined 3D microstructures in cell-laden hydrogels. Biotechnol Bioeng. 2013;110:3038–3047. doi: 10.1002/bit.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolesky DB, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 44.Bertassoni LE, et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication. 2014;6:024105. doi: 10.1088/1758-5082/6/2/024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Visser J, et al. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication. 2013;5:035007. doi: 10.1088/1758-5082/5/3/035007. [DOI] [PubMed] [Google Scholar]
- 46.Santoro M, et al. Gelatin carriers for drug and cell delivery in tissue engineering. J Control Release. 2014;190:210–218. doi: 10.1016/j.jconrel.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, et al. Cell-laden photocrosslinked GelMA–DexMA copolymer hydrogels with tunable mechanical properties for tissue engineering. J Mater Sci Mater Med. 2014;25:2173–2183. doi: 10.1007/s10856-014-5261-x. [DOI] [PubMed] [Google Scholar]
- 48.Levett PA, et al. Hyaluronic acid enhances the mechanical properties of tissue-engineered cartilage constructs. PLoS ONE. 2014;9:e113216. doi: 10.1371/journal.pone.0113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao W, et al. Synthesis and characterization of photo-crosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011;7:2384–2393. doi: 10.1016/j.actbio.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visser J, et al. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials. 2015;37:174–182. doi: 10.1016/j.biomaterials.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 51.Visser J, et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat Commun. 2015;6:6933. doi: 10.1038/ncomms7933. [DOI] [PubMed] [Google Scholar]
- 52.Cha CY, et al. Structural reinforcement of cell-laden hydrogels with microfabricated three dimensional scaffolds. Biomater Sci. 2014;2:703–709. doi: 10.1039/C3BM60210A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutson CB, et al. Synthesis and characterization of tunable poly (ethylene glycol): gelatin methacrylate composite hydrogels. Tissue Eng Part A. 2011;17:1713–1723. doi: 10.1089/ten.tea.2010.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daniele MA, et al. Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds. Biomaterials. 2014;35:1845–1856. doi: 10.1016/j.biomaterials.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Shin SR, et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano. 2011;6:362–372. doi: 10.1021/nn203711s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuurman W, et al. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication. 2011;3:021001. doi: 10.1088/1758-5082/3/2/021001. [DOI] [PubMed] [Google Scholar]
- 57.Novosel EC, et al. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Shin SR, et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7:2369–2380. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin SR, et al. Cell-laden microengineered and mechanically tunable hybrid hydrogels of gelatin and graphene oxide. Adv Mater. 2013;25:6385–6391. doi: 10.1002/adma.201301082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gevaert E, et al. High throughput micro-well generation of hepatocyte micro-aggregates for tissue engineering. PLoS ONE. 2014;9:e105171. doi: 10.1371/journal.pone.0105171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levett PA, et al. Chondrocyte redifferentiation and construct mechanical property development in single-component photocrosslinkable hydrogels. J Biomed Mater Res Part A. 2014;102:2544–2553. doi: 10.1002/jbm.a.34924. [DOI] [PubMed] [Google Scholar]
- 62.Levett PA, et al. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014;10:214–223. doi: 10.1016/j.actbio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen AH, et al. Gelatin methacrylate microspheres for controlled growth factor release. Acta Biomater. 2015;13:101–110. doi: 10.1016/j.actbio.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sirova M, et al. Immunocompatibility evaluation of hydrogel-coated polyimide implants for applications in regenerative medicine. J Biomed Mater Res Part A. 2014;102:1982–1990. doi: 10.1002/jbm.a.34873. [DOI] [PubMed] [Google Scholar]
- 65.Croes M, et al. Proinflammatory mediators enhance the osteogenesis of human mesenchymal stem cells after lineage commitment. PLoS ONE. 2015;10:e0132781. doi: 10.1371/journal.pone.0132781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leijten J, et al. Cell based advanced therapeutic medicinal products for bone repair: keep it simple? Adv Drug Deliv Rev. 2015;84:30–44. doi: 10.1016/j.addr.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 67.Evans CH. Barriers to the clinical translation of orthopedic tissue engineering. Tissue Eng Part B: Rev. 2011;17:437–441. doi: 10.1089/ten.teb.2011.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farris S, et al. Development of polyion-complex hydrogels as an alternative approach for the production of bio-based polymers for food packaging applications: a review. Trends Food Sci Technol. 2009;20:316–332. [Google Scholar]
- 69.Bigi A, et al. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials. 2001;22:763–768. doi: 10.1016/s0142-9612(00)00236-2. [DOI] [PubMed] [Google Scholar]
- 70.Sisson K, et al. Evaluation of cross-linking methods for electrospun gelatin on cell growth and viability. Biomacromolecules. 2009;10:1675–1680. doi: 10.1021/bm900036s. [DOI] [PubMed] [Google Scholar]
- 71.Speer DP, et al. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J Biomed Mater Res. 1980;14:753–764. doi: 10.1002/jbm.820140607. [DOI] [PubMed] [Google Scholar]
- 72.Wang C, et al. Cytocompatibility study of a natural biomaterial crosslinker: Genipin with therapeutic model cells. J Biomed Mater Res Part B: Appl Biomater. 2011;97B:58–65. doi: 10.1002/jbm.b.31786. [DOI] [PubMed] [Google Scholar]
- 73.Babin H, Dickinson E. Influence of transglutaminase treatment on the thermoreversible gelation of gelatin. Food Hydrocolloids. 2001;15:271–276. [Google Scholar]
- 74.Chen P-Y, et al. Fabrication of large perfusable macroporous cell-laden hydrogel scaffolds using microbial transglutaminase. Acta Biomater. 2014;10:912–920. doi: 10.1016/j.actbio.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 75.Das S, et al. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015;11:233–246. doi: 10.1016/j.actbio.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 76.Billiet T, et al. Quantitative contrasts in the photopolymerization of acrylamide and methacrylamide-functionalized gelatin hydrogel building blocks. Macromol Biosci. 2013;13:1531–1545. doi: 10.1002/mabi.201300143. [DOI] [PubMed] [Google Scholar]
- 77.Park KM, Gerecht S. Hypoxia-inducible hydrogels. Nat Commun. 2014;5:4075. doi: 10.1038/ncomms5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou L, et al. Biomimetic mineralization of anionic gelatin hydrogels: effect of degree of methacrylation. RSC Adv. 2014;4:21997–22008. [Google Scholar]
- 79.Henrikson KJ, et al. Nucleous pulposus tissue engineering using a novel photopolymerizable hydrogel and minimally invasive delivery. Spine J. 2014;14:S173. [Google Scholar]
- 80.Huber B, et al. Methacrylated gelatin and mature adipocytes are promising components for adipose tissue engineering. J Biomater Appl. 2015;30:699–710. doi: 10.1177/0885328215587450. [DOI] [PubMed] [Google Scholar]
- 81.Ovsianikov A, et al. Laser photofabrication of cell-containing hydrogel constructs. Langmuir. 2014;30:3787–3794. doi: 10.1021/la402346z. [DOI] [PubMed] [Google Scholar]
- 82.Mũnoz Z, et al. Gelatin hydrogels formed by orthogonal thiol-norbornene photochemistry for cell encapsulation. Biomater Sci. 2014;2:1063–1072. doi: 10.1039/c4bm00070f. [DOI] [PubMed] [Google Scholar]
- 83.Lee Y, et al. Enzyme-catalyzed in situ forming gelatin hydrogels as bioactive wound dressings: effects of fibroblast delivery on wound healing efficacy. J Mater Chem B. 2014;2:7712–7718. doi: 10.1039/c4tb01111b. [DOI] [PubMed] [Google Scholar]
- 84.Amini AA, Nair LS. Enzymatically cross-linked injectable gelatin gel as osteoblast delivery vehicle. J Bioact Compat Pol. 2012;27:342–355. [Google Scholar]
- 85.Lee Y, et al. In situ forming gelatin-based tissue adhesives and their phenolic content-driven properties. J Mater Chem B. 2013;1:2407–2414. doi: 10.1039/c3tb00578j. [DOI] [PubMed] [Google Scholar]
- 86.Wang LS, et al. Enzymatically cross-linked gelatin-phenol hydrogels with a broader stiffness range for osteogenic differentiation of human mesenchymal stem cells. Acta Biomater. 2012;8:1826–1837. doi: 10.1016/j.actbio.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 87.Lee SH, et al. In situ crosslinkable gelatin hydrogels for vasculogenic induction and delivery of mesenchymal stem cells. Adv Funct Mater. 2014;24:6771–6781. doi: 10.1002/adfm.201401110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Z, et al. Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis. Acta Biomater. 2015;13:88–100. doi: 10.1016/j.actbio.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chuang C-H, et al. Enzymatic regulation of functional vascular networks using gelatin hydrogels. Acta Biomater. 2015;19:85–99. doi: 10.1016/j.actbio.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoshikawa A, et al. Encapsulation of chondrocytes in photopolymerizable styrenated gelatin for cartilage tissue engineering. Tissue Eng. 2006;12:2333–2341. doi: 10.1089/ten.2006.12.2333. [DOI] [PubMed] [Google Scholar]
- 91.Habeeb ASA. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966;14:328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 92.Jung J, Oh J. Cell-induced flow-focusing instability in gelatin methacrylate microdroplet generation. Biomicrofluidics. 2014;8:036503. doi: 10.1063/1.4880375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malda J, et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater. 2013;25:5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]