Abstract
Introduction
Tobacco and alcohol often are used simultaneously by young adults, and their co-use is associated with greater health consequences than from single use. Social media platforms offer low cost and highly accessible channels to reach and engage young people in substance use interventions. The current trial seeks to compare the Facebook Tobacco Status Project (TSP) smoking cessation intervention to an intervention targeting both tobacco use and heavy episodic drinking (TSP+ALC) among young adults who use both substances.
Methods
This randomized clinical trial will evaluate the feasibility and initial efficacy of TSP+ALC compared to TSP with 225 US young adult smokers reporting heavy drinking. Participants will be recruited online and randomized to one of two conditions (TSP or TSP+ALC), both with assignment to a Facebook group tailored to readiness to quit smoking. Groups will receive a 90-day intervention including daily Facebook postings and weekly live counseling sessions. The TSP+ALC group will include content related to alcohol use. All participants will be offered a 2-week introductory supply of nicotine patch. Participants will complete baseline, 3-, 6-, and 12-month online assessments of substance use and other health risk behaviors. The primary efficacy outcome is biochemically-verified 7-day point prevalence abstinence. Secondary outcomes include alcohol and tobacco use, combined use, and thoughts about each substance.
Discussion
This trial examines an innovative and scalable approach to engaging young adults online in tobacco and alcohol use treatment. Study findings will inform digital health interventions and best practices for treating multiple substance use in young adults.
Keywords: Tobacco, Smoking, Heavy episodic drinking, Alcohol, Clinical trial, Young adults, Social media, Facebook, Behavioral intervention
1. Introduction
There are strong links between tobacco use and heavy episodic drinking (HED; 4+ drinks for women, 5+ drinks for men in a single episode) [1]. Treating tobacco use and HED simultaneously can lead to better tobacco cessation outcomes than targeting each substance separately. For example, addressing alcohol use during tobacco quit line counseling resulted in significantly greater tobacco abstinence at 7-month follow-up (13.5%) compared to tobacco counseling alone (10.3%) [2]. In young adults, an integrated tobacco/alcohol treatment was acceptable and resulted in more participants biochemically confirmed abstinent from tobacco at 3 months compared to standard tobacco treatment (but no differences in drinking outcomes) [3]. In a follow-up trial, young adults who received integrated intervention were more likely to have biochemically confirmed abstinence from tobacco, consumed fewer drinks and had fewer HED episodes per month at 6 months than those who received standard treatment [4].
Extending an intervention that addresses both smoking and HED to a digital environment could maximize reach and utility. A trial testing the effectiveness of a 14-day mobile feedback intervention jointly targeting smoking and HED resulted in a decrease in the number of cigarettes smoked compared to a minimal assessment control condition, but did not reduce HED or concurrent smoking and drinking at 1 month follow-up [5]. Given the ongoing, significant public health impact of alcohol and tobacco use, more research is needed to determine how best to harness digital tools to target smoking and HED in young adults.
Facebook, the most popular social media platform, is used by 88% of US adults aged 18 to 29, 79% of whom access it daily [6]. Reports of smoking cessation support groups on Facebook have shown the platform to be useful for sharing experiences and providing encouragement and information [7] and with engaging young adults around tobacco prevention [8]. Quit rates, reported as 7-day abstinence, with Facebook interventions were 25% at two weeks in a pilot feasibility study of adults motivated to quit smoking [N=15; [9]] and 47% at 3 months in a trial including web and Facebook components for young adults ready to quit smoking [N=102; [10]]. While these findings are encouraging, there is a need to further investigate social media to promote tobacco use and HED reduction, especially among those not yet ready to quit smoking.
Our research group has developed the Tobacco Status Project (TSP), an intervention tailored to readiness to quit smoking with young adult smokers showing strong participant engagement and promising initial smoking quit rates [11]. In a randomized controlled trial (N=225), we will test TSP against a modified version targeting both tobacco and alcohol (TSP+ALC). The primary outcome will be biochemically-verified 7-day point prevalence abstinence, and secondary outcomes will include days of HED, sum of days using either tobacco or alcohol in the past 30 days, dependence symptoms, quit attempts, motivation, and thoughts about abstinence. Given the high rates of tobacco use and HED in young adults and the extent of social media use in the population, results are expected to provide an evidence-base from which to disseminate broadly effective low-cost interventions.
2. Methods
2.1. Study design
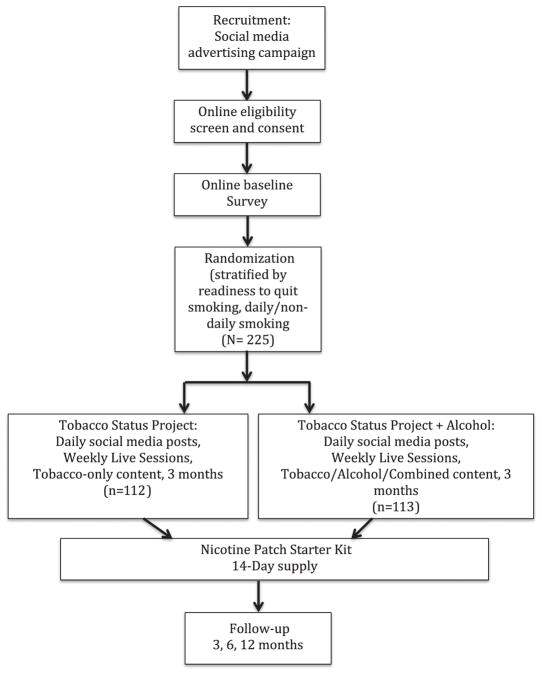
This study is a randomized controlled trial (RCT) with 225 adults aged 18 to 25 recruited through social media (Fig. 1). Participants will be randomized to one of two conditions: (1) the Tobacco Status Project motivationally-tailored smoking cessation intervention delivered through Facebook (TSP); or (2) the Tobacco Status Project+Alcohol (TSP+ALC) intervention tailored to address tobacco use and HED. Participants in both groups will be eligible to receive a 14-day start pack of nicotine patch and a monetary incentive for engagement in their assigned intervention. We will compare TSP+ALC to TSP participants on biochemically-verified 7-day point prevalence abstinence from tobacco (primary outcome), reported abstinence from tobacco, days of HED, sum of days using both tobacco and alcohol in the past 30 days, and dependence symptoms, quit attempts, motivation, and thoughts about abstinence for both substances (secondary outcomes) assessed at 3, 6, and 12 months follow-up. Additionally, through analysis of Facebook data, the project aims to determine patterns of engagement, correlates of active participation, types of posts that attract the most user activity (e.g., likes and comments), and the relationship between user activity and tobacco use and heavy drinking in each group.
Fig. 1.
Participant flow through a trial testing the efficacy of tobacco-only Facebook intervention (Tobacco Status Project; TSP) vs. intervention addressing tobacco and heavy episodic drinking (TSP+ALC; N=225).
2.2. Participants
Participants will be 225 adult men and women who: 1) read English; 2) are between 18 and 25 years of age; 3) indicate they use Facebook at least 3 days per week; 4) have smoked ≥100 cigarettes in their lifetime and currently smoke at least 1 cigarette per day on 4 or more days of the week; and 5) have had at least one HED episode (5+ for men, 4+ for women in a single drinking episode) in the past month. Exclusion criteria are non-English language proficiency or cognitive impairment.
2.3. Sample size and power analysis
Given limited prior research comparing two Facebook interventions, the sample size of N=225 in this pilot trial was not driven by estimated effect sizes or hypothesis testing, but rather was set to compare groups on all outcomes and estimate expected quit rates useful for powering a larger trial. We expect to have representation (although not equal) among men and women, and across ethnic groups consistent with our prior and current trials [12]. We also expect to have representation across levels of smoking and drinking, and readiness to quit using each substance. We anticipate an attrition rate of 30% by 12 months, yielding a final sample size of 158 (79 in each group). Assuming a small effect size (odds ratio 1.5), five predictors in a logistic model testing our primary outcome (verified 7-day abstinence; see Analysis section), we will have 78% power to detect an effect. We expect to generate effect size estimates to inform a larger, fully-powered clinical trial.
2.4. Procedures
Participants will be recruited online primarily through social media advertisements using a design and targeting strategy employed successfully by our group with young adult smokers [13]. All advertisements will provide a link to the study’s website, with eligibility questions. Eligible respondents will be invited to give informed consent with signature online. The informed consent process will include indication that participants understand risks of using the nicotine patch. Those consenting to participate in the study will be asked to verify age by sending proof (e.g., photo of their driver’s license) through email or social media. Verified participants will be emailed a baseline assessment. Those completing the baseline assessment will be randomized to one of two intervention groups, stratified based on readiness to quit smoking (ready to quit in the next 30 days or not) and smoking pattern (daily vs. non-daily), variables known to be related to outcomes and addressed by the interventions [14]. All participants within each condition will then be assigned to a private Facebook group (either TSP or TSP+ALC) tailored to their readiness to quit tobacco. Groups will begin on a rolling basis, minimizing wait times (approximately 2 weeks maximum), and maximizing groups size (aiming for at least 12 participants enrolled per group based on our prior work; [15,16]) Groups will receive one social media post daily for 90 days and weekly “The Dr. Is In” interactive counseling sessions with a postdoctoral-level trainee managed through the study’s private group pages. Study staff will check Facebook daily for messages from participants, address issues as they arise, and can facilitate an interaction with a counselor outside of weekly live sessions as necessary. To date, no participant has requested to interact with a counselor privately outside of weekly live sessions in our previous work. Three-, 6, and 12-month follow-up assessments will be emailed to participants upon completion of treatment.
Informed consent, baseline and 3-, 6-, and 12-month assessments will be delivered using the online survey program Qualtrics [17]. Verification of non-smoking status will be conducted for all participants reporting no smoking in the past 7 days at the post-test assessment using mailed saliva cotinine test kits. Participants will receive gift cards in the amount of $20 for each 20–30-minute online survey, and a bonus $20 for completing all assessments, for a total of $100 possible compensation for participation. Recruitment is anticipated to begin on December 1, 2017, and results are anticipated to be reported in early 2019. All study procedures have been approved by the University of California, San Francisco (UCSF) Institutional Review Board.
2.5. Treatment conditions
2.5.1. Tobacco Status Project (TSP)
The TSP is a smoking cessation intervention implemented entirely through private Facebook groups. The intervention has two main features. First, Facebook posts were designed to be delivered each day for 90 days to intervention groups on Facebook. Posts are based on the US Clinical Practice Guidelines for smoking cessation [18], and the Transtheoretical Model (TTM) of behavior change [19], both of which indicate that treatment should be tailored to participants’ readiness to quit (ready in the next 30 days, not ready in the next 30 days). For those not ready to quit in the next month, posts are based on the core TTM concepts of decisional balance and self-efficacy. Counseling strategies include the 5R’s (relevance, risks, rewards, roadblocks, and repetition), shown to increase likelihood of tobacco quit attempts [20,21], and Motivational Interviewing (MI), a directive patient-centered counseling intervention recommended by the Clinical Practice Guidelines and complementary to TTM [18,22]. Posts elicit participants’ motivation and importance of changing tobacco use, problems associated with use, and use open-ended questions to elicit “change talk” (e.g., desire/commitment to change). For those ready to quit in the next month, posts incorporate cognitive and behavioral coping skills found effective for long-term smoking [23], as well as the TTM processes of self-liberation (e.g., making a commitment to quit), stimulus control (e.g., removing smoking paraphernalia from the home), and counter conditioning (e.g., engaging in alternative behaviors). Posts also encourage setting a quit date and making a detailed quit plan. In both groups, additional posts focus on assessing use (e.g., dependence symptoms, previous quit attempts), learning about strategies to support quitting (e.g., medication), and awareness of the tobacco industry’s efforts to target young adults through marketing. Posts include a combination of images, videos, and text designed to reflect the experience of young adults and all pose a question designed to elicit a response from participants (e.g., “What are your triggers to smoke?”).
The intervention also incorporates weekly “The Dr. Is In” live sessions with a study counselor (using Facebook commenting features), during which the counselor provides some limited content for discussion and participants can ask questions and get supplemental support. The content for the “Dr. Is In” sessions is tailored to readiness to quit tobacco and is based on motivational interviewing and cognitive behavioral coping skills for smoking cessation. This strategy has been successful in yielding participation in interactive discussion, and setting of goals related to quitting tobacco [11]. As needed, referrals to more intensive treatment in participants’ local area will be provided.
2.5.2. Tobacco Status Project+Alcohol (TSP+ALC)
The TSP+ALC intervention design was informed by the results from focus groups with the target population [24] and findings from evaluation of the existing TSP intervention. Content targeting HED is based on the NIAAA Guides Rethinking Drinking [25] and the Guide to Alcohol Screening and Brief Intervention for Youth [26], which recommends use of the 5A’s to address alcohol use. Content is similar, although not identical, in both the ready to quit smoking and not ready to quit smoking groups, based on feedback from focus groups that most young adults who engage in HED were not ready to change their alcohol use. Early posts include questions to assess alcohol use patterns and provide normative feedback about risks based on use. Recognizing that ambivalence is part of many young adults’ experience with changing substance use behavior, early posts also incorporate MI strategies [22]. Later posts focus on combining MI with cognitive behavioral therapy (CBT) coping skills to empower participants to prepare for reduction or cessation of alcohol use, set a specific goal, and make a plan for achieving that goal. Core CBT skills include coping with cravings and urges to drink heavily, managing thoughts about alcohol use, problem-solving, drink refusal skills, and elective skills topics including planning for emergencies, recognizing seemingly irrelevant decisions, managing negative moods and demonstrating assertiveness. Some posts integrate smoking and drinking content (e.g., “If you tried to quit smoking in the past, did drinking get in the way? How?”)
TSP+ALC also includes weekly “The Dr. Is In” sessions. Strategies used in the sessions, as in TSP, are based primarily on MI and CBT skills with the goal here of changing both tobacco use and HED. Stimulus material for these sessions addresses use of both substances. Content is tailored to readiness to quit tobacco (Ready/Not Ready), while the alcohol content is largely focused on motivating people who are not ready to quit/change drinking.
2.6. Monetary incentive for intervention engagement
Analyses of our TSP feasibility trial showed that a monetary incentive increased engagement in the Facebook intervention among those who had some basic level of engagement (at least one Facebook comment) [15]. Thus, in this trial, comments to posts will be tallied weekly, tallies will be posted in groups for motivation, and participants who comment every day of the intervention (all 90 days) will be provided a $20 gift card at the end of the intervention period (3 months).
2.7. Nicotine replacement therapy (NRT)
We will provide 14-day starter packs of nicotine patch to participants regardless of intervention condition. Smokers receiving a 14-day starter pack showed more satisfaction with a telephone quitline and had higher 7-day quit rates compared to those not receiving patch [27]. All participants who smoke an average of 5 or more cigarettes per day in the past 30 days and who join the Facebook secret group before the study begins will be offered the nicotine patch. At the start of the trial, all participants for whom it is not contraindicated (e.g., pregnant, nursing, recent cardiovascular trauma, uncontrolled hypertension) will be offered, through the mail, a two-week supply of nicotine patch based on smoking rate at intake. Participants in both conditions will be encouraged to discuss nicotine replacement therapy use and to ask questions during weekly counseling sessions. Those not willing to receive the patch at the start of the intervention period can be sent the patch at any time during the three-month intervention if they become interested.
2.8. Measures
As this trial is meant to determine the ideal measures for a larger trial we have included multiple measures of substance use and other similar constructs (e.g., thoughts about tobacco and alcohol abstinence) and plan to examine psychometric properties at the end of the trial.
2.8.1. Primary outcome (tobacco)
The primary outcome is biochemically-verified 7-day point prevalence abstinence from all tobacco products at 3, 6, and 12 months. Those reporting no smoking in the past 7 days will be mailed a saliva cotinine test kit and will be asked to provide two pictures: one of the participant giving a saliva sample, and another of the test result. Participants with a salivary cotinine level < 11 ng/ml, indicating nonsmoking [28], will be considered confirmed nonsmokers. Biochemical verification is recommended in randomized trials with sample sizes under 500 [29]. Saliva cotinine test kits have been successfully mailed to participants in previous studies [30], and the research team has used this method previously [11,12]. If participants indicate use of an electronic nicotine delivery-system to aid in smoking cessation, saliva cotinine and reported use of that product will be recorded and reported separately from biochemically verified abstinence.
2.8.2. Secondary outcomes (tobacco)
Days of smoking and cigarettes smoked per day will be assessed with the Timeline Followback (TLFB), which has good reliability and validity when administered online [31,32]. For validation, single items will assess number of cigarettes smoked in the past 24 h and 7 days, the average cigarettes per day in four categories (“10 or less” to “31 or more”), and average cigarettes smoked per day in the past month. These measures will be used to calculate 7-day reported abstinence from smoking (y/n) and reduction of cigarette consumption by 50% or more between baseline and each follow-up (y/n). The number of 24 h quit attempts since the last assessment will be used to calculate presence of at least one quit attempt in the assessment time period. The Pro-Change Health Risk Assessment [HRI; [33]] will assess readiness to quit smoking by categorizing participants into Transtheoretical Model stage of change categories at each time point (precontemplation, contemplation, preparation, action, and maintenance; [19]), predictive of quit attempts and cessation [34]. Desire to quit smoking, abstinence self-efficacy, perceived difficulty of quitting, and abstinence goal will be assessed with the 4-item Thoughts About Abstinence Form [35], measuring each construct on a scale from 1 (“least”) to 10 (“most”), and categorizing goals as no goal, intermediary goal (e.g., reduced smoking), or total abstinence. At 12 months, the 6-item Fagerström Test of Cigarette Dependence (FTCD) [36,37] will assess nicotine dependence (Table 1).
Table 1.
Outcome measures for a trial comparing Tobacco Status Project to Tobacco Status Project+Alcohol (N=225).
| Outcome | Outcome | Measure | Follow-up timepoint (months) |
|---|---|---|---|
| Primary | |||
| Tobacco | Biochemically-verified 7-day abstinence from tobacco (y/n) | Saliva cotinine-verified abstinence | 3, 6, 12 |
| Secondary | |||
| Tobacco | 7-day reported abstinence from tobacco (y/n) | Timeline Followback (Tobacco) | 3, 6, 12 |
| Cigarettes smoked in the past 7 days | |||
| Reduction of cigarette consumption by 50% or more (y/n) | Cigarettes smoked in the past 7 days | 3, 6, 12 | |
| Timeline Followback (Tobacco) | |||
| Presence of at least one quit attempt in the assessment period | Number of 24 h quit attempts since the last assessment | 3, 6, 12 | |
| Readiness to quit smoking | Health Risk Assessment (Tobacco item) | 3, 6, 12 | |
| Desire to quit smoking, | Thoughts About Abstinence Form (Tobacco) | 3, 6, 12 | |
| Abstinence self-efficacy | |||
| Perceived difficulty of quitting | |||
| Long-term smoking goal | |||
| Tobacco Dependence Symptoms | Fagerstrom Test for Cigarette Dependence | 12 | |
| Alcohol | Days of heavy episodic drinking, | Timeline Followback (Alcohol) | 3, 6, 12 |
| Number of drinks per week | |||
| Presence at least one attempt to cut down or quit drinking in the assessment period | Number and longest length of purposeful attempts to reduce/quit drinking since the last assessment | 3, 6, 12 | |
| Readiness to change alcohol use | Readiness Ruler | 3, 6, 12 | |
| Health Risk Assessment (Alcohol item) | |||
| Desire to reduce drinking | Importance and confidence rulers | 3, 6, 12 | |
| Importance of quitting drinking | Thoughts About Abstinence Form (Alcohol) | ||
| Self-efficacy to quit drinking | |||
| Long-term drinking goal | |||
| Misuse of alcohol in the past year | AUDIT-C | 12 | |
| Tobacco and Alcohol | Proportion of days using both tobacco and alcohol tobacco or binge drinking in the past month | Timeline Followback (Tobacco and Alcohol) | 3, 6, 12 |
| Combined smoking and drinking during last drinking episode | Single item to assess co-use in last drinking episode during follow-up time period (y/n) | 3, 6, 12 | |
| Feasibility | |||
| Cost, length of time to recruit full sample | Facebook Ads Manager statistics | 3 | |
| Retention | Proportion of participants completing each follow-up assessment | 3, 6, 12 | |
| Engagement | Comment volume by participant and by post/counseling session during the intervention (from Facebook) | 3 | |
| Biochemical verification of abstinence procedure | Proportion of reported abstinence completing verification procedure | 3, 6, 12 | |
| Disocrdance between report and verification | |||
| Usability | |||
| Ease, comprehension, helpfulness, and likability of each intervention | Usability measure | 3 months | |
2.8.3. Secondary outcomes (alcohol)
Past 30 day HED episodes, defined as 4+ drinks in an occasion for women and 5+ drinks for men, [38] will be assessed with the (TLFB), and validated with a single item assessing number of days of HED. Two items will assess the number of active attempts to cut down or quit drinking and longest period of remaining alcohol free since the last assessment. Current readiness to change alcohol use will be assessed with a single-item Readiness Ruler, scored on a Likert scale from 1 (“I never think about drinking less”) to 4 (“I am already trying to cut down on my drinking”) developed and validated for use with young adult heavy drinkers participating in brief interventions [39]. The alcohol item from the Staging Health Risk Assessment [33] will assess current drinking in accordance with NIAAA low-risk guidelines (≤14 drinks per week and no>4 drinks in an occasion for men; (≤7 drinks per week and no>3 drinks in an occasion for women), and, will categorize participants within stage of change to reduce drinking to low-risk guidelines to be consistent with the tobacco measure. Thoughts about cutting down or stopping alcohol use will include desire to quit drinking, importance and confidence rulers measuring each construct on a scale from 1 (“least”) to 10 (“most”) [39,40], and long-term goals for drinking in six categories from “I have no goals” to “Abstain”. At the 12 month assessment, the Alcohol Use Disorders Identification Test-C (AUDIT-C; 3-items; range: 0–12) will assess misuse of alcohol in the past year [41]. Used widely in the literature, cut-off scores of 3 in women and 4 in men have demonstrated good sensitivity and specificity compared to longer clinical interviews [42].
2.8.4. Secondary outcomes (tobacco and alcohol)
TLFB data will be used to calculate the sum and proportion of any using days reporting both tobacco and any alcohol, as well as HED in the past month. A single item will assess whether the last drinking episode also included cigarette smoking (y/n).
2.8.5. Feasibility measures
Advertising statistics available online through Facebook will be used to track costs and recruitment over time at each study stage (e.g., study interest, meeting criteria, signing consent, randomized). Retention will be evaluated by the proportion of participants that completes the 3-, 6-, and 12-month assessments. Engagement will be measured by the total number of Facebook “likes” and comments, summed by participant and by post/counseling session. Implementation of biochemical verification of tobacco abstinence: will be measured by the proportion of participants in each condition reporting 7-day abstinence at 3-month follow-up that completes a saliva cotinine test and the rate of discordance between saliva cotinine results and self-report.
2.8.6. Usability
A usability measure, adapted from our previous research [11], will assess ease of use, comprehension, helpfulness, and likability of the TSP and TSP+ALC interventions at 3 months, combining both quantitative responses (8 items assessing usability, likability, perceived helpfulness, with responses on Likert scale from “Strongly agree” to “Strongly disagree”), and qualitative responses (e.g., open-ended questions assessing most and least liked features of the intervention).
2.9. Additional measures
2.9.1. Demographics
Age, gender, ethnicity, sexual identity, parental and personal education/income, work/school status, and military involvement, will be assessed at baseline with a Demographic Questionnaire used in our prior research with young adults [43].
2.9.2. Smoking history
At baseline we will assess age of initiation and years of smoking; prior quit attempts and cessation strategies; other nicotine (e.g., electronic nicotine delivery systems) and tobacco use (e.g., cigars) [44]; nicotine dependence using the FTCD, and social smoking with 3-items used with young adults [45,46].
2.9.3. Alcohol use history
At baseline we will assess age of first drink and first heavy drinking episode, prior attempts to change HED, and any change/cessation strategies used.
2.9.4. Combined alcohol and tobacco use
Two items assessing subjective effects of smoking while drinking (estimated proportion of smoking episodes occurring under the influence of alcohol and pleasure from smoking under the influence of alcohol), based on [47], and used in our prior work [48] will be given at baseline and all follow-up timepoints.
2.9.5. Drug use severity
At baseline and 12 months, the Drug Abuse Screening Test-10 (DAST; 10 items) [49], will assess problems associated with illicit drug use and misuse of prescription drugs in the past year. Used widely in the literature, a cut-off score of 2 has demonstrated good sensitivity and specificity in identifying substance use disorders compared to longer clinical interviews [50].
2.9.6. Other health risk behaviors
The 34-item Staging Health Risk Assessment [33] will screen and assesses readiness to change for 9 health risk behaviors in addition to tobacco and alcohol (mentioned above), including illicit drug use, poor sleep quality, sedentary behavior, poor diet (high fat, low fruit and vegetable intake), poor stress management, depression, risky driving, and high-risk sexual behavior. The stage of change categories have strong predictive validity for reducing health risk behaviors over 24 months [51].
2.9.7. Intervention engagement
Facebook data will be extracted from the private groups we develop on Facebook using the Facebook API. These data will include the content of the posts and comments, the activity of each participant (e.g., number, content, and time of all comments), and interactions among participants.
2.10. Data analysis
2.10.1. Feasibility
Feasibility analyses will examine four areas: 1) Recruitment Efforts: length of time to recruit and enroll 225 participants in the pilot trial; and demographic, smoking, and drinking characteristics of those eligible and who enroll; (2) Usability: mean responses to Likert scale items of the usability measure, with any sections with lower than median values indicating changes should be made before future investigations take place; (3) Implementation of biochemical verification of tobacco abstinence and (4) Attrition: We will compare attrition rates to previous Internet smoking cessation trials (e.g., [52,53]).
2.10.2. Preliminary efficacy
To test for the effects of treatment condition on smoking abstinence at 3 through 12 months, we will estimate and test a generalized estimating equation (GEE) (PROC GENMOD in SAS version 9.4; SAS Institute, Cary, NC). This model account for dependence of responses within individuals attributable to repeated measures. Abstinence status will be examined as 7-day point prevalence at each of the assessment points. Time, in the form of the month of assessment, will be included in the model as a random effect and the test of the treatment condition by time interaction will directly test the preliminary efficacy of the intervention. The independent variables to be included for the purposed of covariate adjustment are based on the known relationships of the variables to smoking behavior. They include TSP versus TSP+ALC, plus variables that are related to abstinence (e.g., stage of change, cigarettes per day at baseline), differ by condition at baseline, or predict attrition.
The GEE procedure allows for the derivation of effect estimates from all available data using maximum likelihood estimation. Prior to analysis we will examine the data to see if there is any evidence that the missing data are not missing at random. This will be accomplished by comparing measures from those who completed the protocol against those who did not.
Parallel methods of modeling the secondary measures will be used by selecting the appropriate link and distribution function for each outcome. Days of smoking, number of cigarettes smoked and the drinking measures will be modeled as count variables. The presence of a quit attempt and any other dichotomous measures will be modeled as binary variables.
2.10.3. Secondary outcomes
For secondary outcomes we will estimate and test mixed effects logistic and multinomial regression models for longitudinal ordinal response data to model secondary outcomes for tobacco use and alcohol use across time (3, 6, 12 months): 1) reduction of cigarettes by 50% or more (y/n), 2) tobacco or alcohol quit attempt (y/n; 2 models), 3) readiness to quit tobacco and alcohol (precontemplation, contemplation, preparation, action/maintenance; 2 models); and 4) commitment to abstinence (no goal, intermediary goal, complete abstinence). In parallel, mixed effects linear regression analyses will model continuous outcomes over time. Independent variables in all models will be treatment condition, abstinence status, and covariates identified as relevant to smoking characteristics in the literature.
2.10.4. Engagement and patterns of social interaction
We will determine patterns of engagement, type of posts that are most “engaging” to participants, and relate engagement to smoking and drinking outcomes using three main analyses: (1) Patterns of engagement: Total number of “likes” and comments to Facebook groups over the 3 month intervention period will be tallied for each group (TSP, TSP+ALC), and the non-parametric Kruskal-Wallis ANOVA will compare likes and comments in each intervention and include covariates of baseline characteristics (readiness to quit tobacco/alcohol, daily/non-daily smoking status, gender) and group membership; a social network analysis will examine the interaction patterns among different types of users with which participants engage in each intervention; a content analysis will evaluate the content of comments within each group and observe differences among the two groups; (2) Success of specific posts: During the intervention design phase, posts were been coded based on content (e.g., US Public Health Service/NIAAA Guidelines, Motivational Interviewing, 5Rs, CBT skills, TTM processes of change) and, in the TSP+ALC intervention, by substance (alcohol, tobacco, both). ANOVA will then be used to examine likes and comments by post type within each group (TSP, TSP+ALC); (3) Engagement and outcomes: We will compare TSP and TSP+ALC groups on the relationship between intervention engagement (Facebook comments) and primary tobacco and alcohol outcomes (abstinence, heavy episodic drinking days) at the end of the 3-month intervention period. Logistic regression will be used to analyze tobacco abstinence, and linear regression for days of HED at 3 months, controlling for any baseline differences in treatment groups. Kruskal-Wallis tests will evaluate the effect of incentive condition (personal, altruistic, no incentive) on comments to the Facebook group, in both the full sample and only those who made at least one comment to the group to address the likely preponderance of zero values for those who do not comment at all.
3. Discussion
Given the extent to which young adults combine tobacco use and HED, and the relative lack of treatments available that address both health risk behaviors using digital tools or social media, this study is expected to expand the knowledge base regarding how best to design and implement social media interventions targeting polysubstance use. Use of both cigarettes and alcohol is extremely common among young people [47,54–56], associated with greater likelihood of a dependence diagnosis for either substance in adulthood [57,58], and increases the risk for certain cancers (e.g., mouth, throat, esophagus, upper aerodigestive tract) [59–61]. Further, co-use makes it more difficult to quit either substance [62–66]. Given the public health threat caused by combined tobacco use and HED among young adults, engaging and effective interventions targeting both substances are greatly needed.
Addressing tobacco and other substance use simultaneously can lead to better outcomes than addressing each substance separately. Smoking cessation interventions delivered in the context of alcohol or drug treatment do not impede recovery [67], and may actually reduce alcohol and other substance use by adults [68] as well as teens [69]. Smoking cessation is associated with better long-term alcohol and drug treatment outcomes [70]. School-based prevention interventions such as those based on the TTM [71] and Project Toward No Drug Abuse [72] have demonstrated that targeting multiple substances simultaneously can have positive outcomes. An understudied area related to combined tobacco use and HED intervention is how to best design and disseminate secondary prevention interventions for maximum engagement among the potentially hard-to-reach population of young adults who exhibit both health risk behaviors.
Extending an integrated intervention to a digital environment could maximize reach and utility for young adults. Our previous work has examined Facebook as a tool to recruit young adult smokers [13,73] and engage them in smoking cessation intervention [16], and has shown that a Facebook-based intervention can yield smoking cessation rates as high as those of other online interventions [11]. Yet quit rates can be substantially improved in order to maximize potential public health benefits. Further, no intervention on Facebook has addressed multiple substance use, and formative work (focus groups, usability-testing) is needed to ensure that an integrated intervention will be received well by the target population.
A key question related to both the design of an intervention targeting two substances, as well as the delivery in a social media context, is related to how exactly content will address both alcohol and tobacco. To address this question, our group conducted focus groups to address how these substances are combined in the target populations, and how young adults feel about the use and changing each substance. This work supported design of an intervention that included some social media posts that addressed one substance only, some that address both substances (e.g., “What do you think is the relationship between tobacco and alcohol”), and some posts that addressed the combining of the two substances (e.g., “Parties can be a big trigger for smoking and drinking. Is this true for you? How do you think that is?”). A key goal of usability-testing of this type of intervention can (and should) include analysis of which type of posts are the most engaging (e.g., get the most likes or comments), and which have the best feedback from participants. Social media data are well-suited to this type of analysis, and usability-testing allows for refinement of intervention content by substance as well as other features that may be related to engagement (image/text).
Design considerations required us to make choices between multiple options in developing the intervention and randomized trial. First, we chose to implement the interventions in both arms of the study through Facebook rather than through another social media platform. Facebook remains by far the most widely used form of social media by young adults in the United States compared to Twitter, Pinterest, LinkedIn, and Instagram; 76% of online young adults used Facebook daily in 2016 [74], up from 70% in 2015. Given that social media is increasingly accessed via mobile technology, and its use among those online does not differ by income, it is a particularly good option to deliver interventions to diverse groups of young adults who smoke and exhibit hazardous drinking throughout the US.
Second, in this pilot trial, we chose to compare TSP+ALC to TSP targeting only tobacco, instead of using a less active control condition (e.g., treatment as usual). We are currently conducting a trial comparing TSP to a website referral control, with promising initial results [12]. This trial is testing an active control (TSP) that addresses tobacco alone to determine whether an intervention combining both substances (TSP+ALC) might result in better smoking cessation outcomes than treating tobacco alone. If TSP+ALC results in better tobacco or alcohol outcomes than TSP, we plan to conduct an additional study to test TSP+ALC on tobacco and alcohol outcomes against a control condition that more closely approximates standard treatment (e.g., a website referral).
In the study design, we also carefully considered how best to protect the privacy of participants. Privacy is an important consideration in any intervention administered through social media. Social media, by nature, is a public forum for interaction, and there is potential for unintended sharing of information. In this trial, all intervention components will be administered entirely through private groups that will not be visible beyond the participants in the groups. It is possible that participants who are placed in a group together may know each other outside of the social media context, and this will not be prevented to maximize external validity of the project and for ease of dissemination. Any participants wishing to be placed in a group separate from someone in their real life social networks will be honored at the group assignment process. Participants will be given detailed information about the intervention in the consent process, including notice that all groups are private, and that all information reported in groups will be visible only to those invited to participate in the groups. The exchange of substance use is increasingly being conducted through social media [75], and there is great potential to use this space to help people make positive life changes with the support of an intimate support network. Yet research conducted on this platform must take appropriate steps to maximize privacy.
The study methods do have some limitations. First, while there is near saturation of Internet use among young adults, not all young adults who drink or use tobacco actively use Facebook. Although widely used, Facebook may not remain the most viable social media tool to reach young people for health behavior change. Further, attrition in Internet smoking cessation trials can be high (up to 75% for online intervention studies) [52,53,76–78], however the drop-out rate in the TSP feasibility study was only 16% at 6 months. We chose a sample size that would allow us to examine differences on the primary and secondary outcomes given expected control variables, assuming attrition of 30%. The trial is not powered to detect effects on secondary outcomes, and a larger trial will likely need to be conducted to determine the most effective way to intervene through social media on tobacco use and HED.
Results are designed to inform best practices for digital-delivery of intervention for smoking cessation among those who also exhibit HED. Our primary outcome is smoking cessation, yet we proposed to measure drinking outcomes and expect greater reduced drinking in those receiving TSP+ALC compared to those receiving TSP. Should the results fail to show differences on drinking outcomes between groups, we will look to engagement and usability (e.g, liability, perceived helpfulness), to inform our interpretation of the results. Subsequent studies will be directly informed by outcomes for both smoking and HED.
To our knowledge this is the first trial to test the efficacy of implementing a Facebook-delivered intervention targeting smoking and heavy drinking among young adults. Particularly novel components of this trial include the delivery of substance use treatment over social media, the application of a social media intervention to multiple substances (tobacco and alcohol), and the testing of an intervention tailored to readiness to quit smoking, combining automated messages (study posts) and counselor-delivered intervention (The Dr. Is In Sessions). Many digital health tools only use one or two of these features, and combining all three strengthens the potential effect of our intervention. Additionally, we propose to use social media analytics to evaluate relationships among user activity, substance use profiles, and tobacco and alcohol outcomes from the intervention. Our combined Facebook and secure survey data will allow for an unprecedented examination of the relationship between communication patterns and health behavior, informing digital health tools across populations and health risk behaviors.
References
- 1.Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29(3):162–171. [PMC free article] [PubMed] [Google Scholar]
- 2.Toll BA, et al. A randomized trial for hazardous drinking and smoking cessation for callers to a quitline. J Consult Clin Psychol. 2015;83(3):445–454. doi: 10.1037/a0038183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ames SC, et al. Integrated smoking cessation and binge drinking intervention for young adults: a pilot investigation. Ann Behav Med. 2010;40(3):343–349. doi: 10.1007/s12160-010-9222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ames SC, et al. Integrated smoking cessation and binge drinking intervention for young adults: a pilot efficacy trial. Addict Behav. 2014;39(5):848–853. doi: 10.1016/j.addbeh.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Witkiewitz K, et al. Development and evaluation of a mobile intervention for heavy drinking and smoking among college students. Psychol Addict Behav. 2014;28(3):639–650. doi: 10.1037/a0034747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenwood S, Perrin A, Duggan M. Social Media Update. 2016 (2016 [cited 2016 November 29]; Available from) http://www.pewinternet.org/2016/11/11/social-media-update-2016/
- 7.Cheung YT, et al. Online social support for the prevention of smoking relapse: a content analysis of the WhatsApp and Facebook social groups. Telemed J E Health. 2017 Jun;23(6):507–516. doi: 10.1089/tmj.2016.0176. [DOI] [PubMed] [Google Scholar]
- 8.Strekalova YA, Damiani RE. Message design and audience engagement with tobacco prevention posts on social media. J Cancer Educ. 2016 Nov;10:1–5. doi: 10.1007/s13187-016-1135-x. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, et al. Harnessing Facebook for smoking reduction and cessation interventions: Facebook user engagement and social support predict smoking reduction. J Med Internet Res. 2017;19(5):e168. doi: 10.2196/jmir.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baskerville NB, et al. Effect of a digital social media campaign on young adult smoking cessation. Nicotine Tob Res. 2016 Mar;18(3):351–360. doi: 10.1093/ntr/ntv119. [DOI] [PubMed] [Google Scholar]
- 11.Ramo DE, et al. Feasibility and quit rates of the tobacco status project: a Facebook smoking cessation intervention for young adults. J Med Internet Res. 2015;17(12):e291. doi: 10.2196/jmir.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramo DE, et al. The tobacco status project (TSP): study protocol for a randomized controlled trial of a Facebook smoking cessation intervention for young adults. BMC Public Health. 2015;15(1):897. doi: 10.1186/s12889-015-2217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramo DE, et al. Facebook recruitment of young adult smokers for a cessation trial: methods, metrics, and lessons learned. Internet Interv. 2014;1(2):58–64. doi: 10.1016/j.invent.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sargent JD, Mott LA, Stevens M. Predictors of smoking cessation in adolescents. Arch Pediatr Adolesc Med. 1998;152(4) doi: 10.1001/archpedi.152.4.388. [DOI] [PubMed] [Google Scholar]
- 15.Ping Q, Yang C, Ramo DE. Engagement in a Facebook smoking cessation intervention for young adults: Effects of motivation and monetary incentive. Paper presented at the Society for Research on Nicotine and Tobacco Annual Meeting; Philadelphia, PA. 2015. [Google Scholar]
- 16.Thrul J, Klein AB, Ramo DE. Smoking cessation intervention on Facebook: which content generates the best engagement? J Med Internet Res. 2015;17(11):e244. doi: 10.2196/jmir.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qualtrics, Qualtrics. UT: Provo: 2017. [Google Scholar]
- 18.Fiore MC, et al. Treating tobacco use and dependence: 2008 update, Clinical Practice Guideline. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: 2008. [Google Scholar]
- 19.Prochaska JO, DiClemente CC. Stages and processes of self-change for smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter MJ, et al. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72:371–381. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- 21.Carpernter M, Hughes JR, Keely J. Effect of smoking reduction on later cessation: a pilot experimental study. Nicotine Tob Res. 2003;5:155–162. doi: 10.1080/146222003100007385. [DOI] [PubMed] [Google Scholar]
- 22.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2. Guilford; New York, NY: 2002. [Google Scholar]
- 23.Hall SM, et al. Using extended cognitive behavioral treatment and medication to treat dependent smokers. Am J Public Health. 2011;101(12):2349–2356. doi: 10.2105/AJPH.2010.300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thrul J, Belohlavek A, Hambrick D, Kaur M, Ramo DE. Conducting online focus groups on Facebook to inform health behavior change interventions: Two case studies and lessons learned. Internet Interv. 2017 Sep;9:106–111. doi: 10.1016/j.invent.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute on Alcohol Abuse and Alcoholism, editor. Rethinking Drinking: Alcohol Use and Your Health. US Department of Health and Human Services; Rockville, MD: 2010. [Google Scholar]
- 26.US Department of Health and Human Services, editor. National Institute on Alcohol Abuse and Alcoholism, Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide. NIAAA; Rockville, MD: 2011. [Google Scholar]
- 27.Bush TM, et al. The impact of a free nicotine patch starter kit on quit rates in a state quit line. Nicotine Tob Res. 2008;10(9):1511–1516. doi: 10.1080/14622200802323167. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis MJ, et al. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health. 1987;77(11):1435–1438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 30.Etter JF, Perneger TV, Ronchi A. Collecting saliva samples by mail. Am J Epidemiol. 1998;147(2):141–146. doi: 10.1093/oxfordjournals.aje.a009426. [DOI] [PubMed] [Google Scholar]
- 31.Brown RA, et al. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12(2):101–112. [Google Scholar]
- 32.Sobell LC, et al. The reliability of the alcohol timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- 33.Prochaska JO, et al. Multiple risk expert systems interventions: impact of simultaneous stage-matched expert system interventions for smoking, high-fat diet, and sun exposure in a population of parents. Health Psychol. 2004;23(5):503–516. doi: 10.1037/0278-6133.23.5.503. [DOI] [PubMed] [Google Scholar]
- 34.DiClemente CC, et al. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol. 1991;59(2):295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- 35.Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. J Consult Clin Psychol. 1990;58(2):175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- 36.Heatherton TF, et al. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 37.Fagerstrom K. Determinants of tobacco use and renaming the FTND to the Fagerstrom test for cigarette dependence. Nicotine Tob Res. 2012;14(1):75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 38.National Institute in Alcohol Abuse and Alcoholism. Drinking Levels Defined. [cited 2015 April 4]; Available from, 2015. http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- 39.Heather N, Smailes D, Cassidy P. Development of a readiness ruler for use with alcohol brief interventions. Drug Alcohol Depend. 2008;98(3):235–240. doi: 10.1016/j.drugalcdep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Maisto SA, et al. A comparison of the concurrent and predictive validity of three measures of readiness to change alcohol use in a clinical sample of adolescents. Psychol Assess. 2011;23(4):983–994. doi: 10.1037/a0024136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubinsky AD, et al. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin Exp Res. 2013;37(8):1380–1390. doi: 10.1111/acer.12092. [DOI] [PubMed] [Google Scholar]
- 42.Chiolero A, et al. Clustering of risk behaviors with cigarette consumption: a population-based survey. Prev Med. 2006;42(5):348–353. doi: 10.1016/j.ypmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Ramo DE, Hall SM, Prochaska JJ. Reliability and validity of self-reported smoking in an anonymous online survey with young adults. Health Psychol. 2011;30(6):693–701. doi: 10.1037/a0023443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall SM, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808–1814. doi: 10.2105/AJPH.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schane RE, Glantz SA, Ling PM. Nondaily and social smoking: an increasingly prevalent pattern. Arch Intern Med. 2009;169(19):1742–1744. doi: 10.1001/archinternmed.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lisha NE, et al. Prevalence and correlates of social smoking in young adults: comparisons of behavioral and self-identified definitions. Nicotine Tob Res. 2015;17(9):1076–1084. doi: 10.1093/ntr/ntu242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKee SA, et al. Survey of subjective effects of smoking while drinking among college students. Nicotine Tob Res. 2004;6(1):111–117. doi: 10.1080/14622200310001656939. [DOI] [PubMed] [Google Scholar]
- 48.Gubner NR, et al. Young adults report increased pleasure from smoking cigarettes when drinking alcohol but not when using marijuana. Addict Res Theory. 2017:1–6. doi: 10.1080/16066359.2017.1311877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 50.Cocco KM, Carey KB. Psychometric properties of the drug abuse screening test in psychiatric outpatients. Psychol Assess. 1998;10:408–414. [Google Scholar]
- 51.Johnson SS, et al. Transtheoretical model-based multiple behavior intervention for weight management: effectiveness on a population basis. Prev Med. 2008;46(3):238–246. doi: 10.1016/j.ypmed.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKay HG, et al. Comparing two web-based smoking cessation programs: randomized controlled trial. J Med Internet Res. 2008;10(5):e40. doi: 10.2196/jmir.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swartz LH, et al. A randomised control study of a fully automated internet based smoking cessation programme. Tob Control. 2006;15(1):7–12. doi: 10.1136/tc.2003.006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoek J, et al. Social smokers’ management of conflicted identities. Tob Control. 2013;22(4):261–265. doi: 10.1136/tobaccocontrol-2011-050176. [DOI] [PubMed] [Google Scholar]
- 55.Nichter M, et al. Gendered dimensions of smoking among college students. J Adolesc Res. 2006;21(3):215–243. [Google Scholar]
- 56.Stromberg P, Nichter M, Nichter M. Taking play seriously: low-level smoking among college students. Cult Med Psychiatry. 2007;31(1):1–24. doi: 10.1007/s11013-006-9042-y. [DOI] [PubMed] [Google Scholar]
- 57.Quek LH, et al. Concurrent and simultaneous polydrug use: latent class analysis of an Australian nationally representative sample of young adults. Front Public Health. 2013;1:61. doi: 10.3389/fpubh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moss HB, Chen CM, Yi HY. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug Alcohol Depend. 2014 Mar 1;136:51–62. doi: 10.1016/j.drugalcdep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 59.American Cancer Society. Alcohol use and cancer. [cited 2015 May 7]; Available from: http://www.cancer.org/Cancer/CancerCauses/DietandPhysicalActivity/alcohol-use-and-cancer.
- 60.Pelucchi C, et al. Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol Res Health. 2006;29(3):193–198. [PMC free article] [PubMed] [Google Scholar]
- 61.Lee CH, et al. Independent and combined effects of alcohol intake, tobacco smoking and betel quid chewing on the risk of esophageal cancer in Taiwan. Int J Cancer. 2005;113(3):475–482. doi: 10.1002/ijc.20619. [DOI] [PubMed] [Google Scholar]
- 62.Murray RP, et al. Level of involvement with alcohol and success at smoking cessation in the lung health study. J Stud Alcohol. 1995;56(1):74–82. doi: 10.15288/jsa.1995.56.74. [DOI] [PubMed] [Google Scholar]
- 63.Hymowitz N, et al. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;2(Suppl 6):S57–62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walsh RA, et al. Smoking cessation interventions in Australian drug treatment agencies: a national survey of attitudes and practices. Drug Alcohol Rev. 2005;24(3):235–244. doi: 10.1080/09595230500170282. [DOI] [PubMed] [Google Scholar]
- 65.Duffy SA, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol Biomark Prev. 2006;15(11):2203–2208. doi: 10.1158/1055-9965.EPI-05-0880. [DOI] [PubMed] [Google Scholar]
- 66.Jaszyna-Gasior M, Schroeder JR, Moolchan ET. Alcohol use and tobacco abstinence among adolescents in cessation treatment: preliminary findings. Addict Behav. 2007;32(3):617–621. doi: 10.1016/j.addbeh.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Kalman D. Smoking cessation treatment for substance misusers in early recovery: a review of the literature and recommendations for practice. Subst Use Misuse. 1998;33:2021–2047. doi: 10.3109/10826089809069815. [DOI] [PubMed] [Google Scholar]
- 68.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 69.Myers MG, Prochaska JJ. Does smoking intervention influence adolescent substance use disorder treatment outcomes? Subst Abus. 2008;29(2):81–88. doi: 10.1080/08897070802093361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Satre DD, Kohn CS, Weisner C. Cigarette smoking and long-term alcohol and drug treatment outcomes: a telephone follow-up at five years. Am J Addict. 2007;16(1):32–37. doi: 10.1080/10550490601077825. [DOI] [PubMed] [Google Scholar]
- 71.Evers KE, et al. Results of a transtheoretical model-based alcohol, tobacco and other drug intervention in middle schools. Addict Behav. 2012;37(9):1009–1018. doi: 10.1016/j.addbeh.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Sussman S, et al. One-year outcomes of a drug abuse prevention program for older teens and emerging adults: evaluating a motivational interviewing booster component. Health Psychol. 2012;31(4):476–485. doi: 10.1037/a0025756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramo DE, Prochaska JJ. Broad reach and targeted recruitment using Facebook for an online survey of young adult substance use. J Med Internet Res. 2012;14(1):e28. doi: 10.2196/jmir.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duggan M, et al. Social Media Update 2014. Pew Research Center; Washington, DC: 2015. [Google Scholar]
- 75.Costello CR, Ramo DE. Social media and substance use: what should we be recommending to teens and their parents? J Adolesc Health. 2017;60(6):629–630. doi: 10.1016/j.jadohealth.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 76.Cobb NK, et al. Initial evaluation of a real-world internet smoking cessation system. Nicotine Tob Res. 2005;7(2):207–216. doi: 10.1080/14622200500055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dallery J, Glenn IM, Raiff BR. An internet-based abstinence reinforcement treatment for cigarette smoking. Drug Alcohol Depend. 2007;86(2–3):230–238. doi: 10.1016/j.drugalcdep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 78.Stoddard J, et al. Smoking cessation research via the internet: a feasibility study. J Health Commun. 2005;10(1):27–41. doi: 10.1080/10810730590904562. [DOI] [PubMed] [Google Scholar]