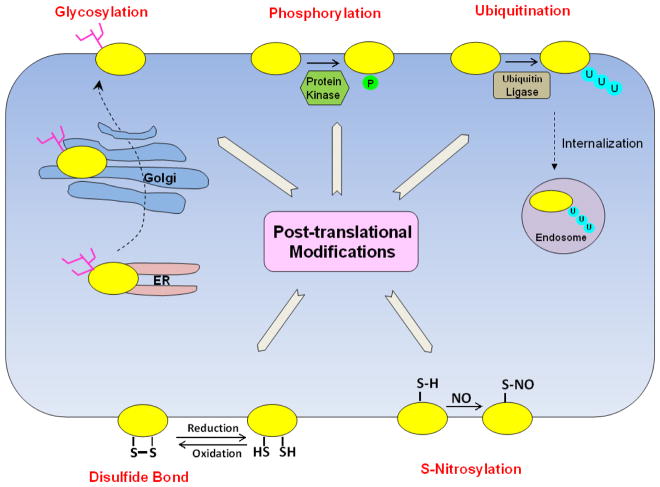
Abstract
Drug transporters encoded by solute carrier (SLC) family are distributed in multiple organs including kidney, liver, placenta, brain, and intestine, where they mediate the absorption, distribution, and excretion of a diverse array of environmental toxins and clinically important drugs. Alterations in the expression and function of these transporters play important roles in intra- and inter-individual variability of the therapeutic efficacy and the toxicity of many drugs. Consequently, the activity of these transporters must be highly regulated so as to carry out their normal functions. While it is clear that the regulation of these transporters tightly depends on genetic mechanisms, many studies have demonstrated that these transporters are the target of various post-translational modifications. This review article summarizes the recent advances in identifying the posttranslational modifications underlying the regulation of the drug transporters of SLC family. Such mechanisms are pivotal not only in physiological conditions, but also in diseases.
Keywords: Membrane transporter, Drug transporter, Posttranslational modification, regulation
Graphical Abstract
1. Introduction
The major physiological functions of membrane transporters are to facilitate the transfer of nutrients or endogenous necessities across the cell membrane, such as endogenous metabolites and signaling molecules. However, the specificity of some transporters is not strictly constrained to their physiological substrates in that exogenous drugs that bear similar structural features can also be recognized and transported. These transporters are thus referred to the term of “drug transporters”. The solute carrier (SLC) family of drug transporters includes but is not limited to organic anion transporters (OAT), organic cation transporters (OCT), organic zwitterion/cation transporters (OCTN), organic anion transporting polypeptides (OATP), monocarboxylate transporters (MCT), nucleoside transporters (CNT/ENT), bile acid transporters (NTCP/ASBT), and multidrug and toxin extrusion transporters (MATE). These transporters are distributed in multiple organs including kidney, liver, placenta, brain, and intestine, and translocate substrates through either secondary or tertiary mechanisms, which require the movement of a co-substrate (such as an ion) or are indirectly linked to the hydrolysis of ATP. The basic properties of these transporters such as their substrate specificities, tissue and membrane localizations, and transport mechanisms have been described in several excellent review articles [1–8].
The SLC family of drug transporters plays critical roles in the handling of common drugs, environmental toxins, signaling molecules, and nutrients [9–13]. Because of their wide range of substrate recognition, co-administered drugs may compete for the same transporters, causing serious side effects through drug-drug interaction, and therefore affecting the pharmacokinetics and pharmacodynamics of the drug profile. In recognizing these facts, the International Transporter Consortium in conjunction with the United States Food and Drug Administration (FDA) issued guidance/recommendations for the assessment of transporter-mediated drug-drug interactions during drug development [14, 15]. Among these transporters are the organic anion transporting polypeptide 1B1 and 1B3 (OATP1B1/OATP1B3, SLCO1B1/SLCO1B3), organic cation transporter 2 (OCT2, SLC22A2), and organic anion transporters 1 and 3 (OAT1/OAT3, SLC22A6/SLC22A8), and multidrug and toxin extrusion transporters (MATEs, SLC47A).
Alterations in the expression and function of SLC family of drug transporters have been observed with several disease states, which can have a significant impact on drug disposition and therefore affect drug efficacy and toxicity. For example, Na+ taurocholate cotransporting polypeptide (NTCP), organic anion transporting polypeptide (OATP) 1B1, and OATP1B3 are the major transporters responsible for bile acids uptake on the sinusoidal membrane in liver [16]. In patients with progressive familial intrahepatic cholestasis, the protein level of NTCP and both the mRNA and protein levels of OATP1B1 and OATP1B3 were found to be reduced in their liver samples [17]. Similarly, in liver biopsies of patients with primary sclerosing cholangitis, a chronic cholestatic liver disease, the level of OATP1B1 mRNA was decreased nearly by half [18]. The decreased level of expression and function of rOat1/3 have been reported in rats with chronic renal failure [19, 20] and acute renal failure [21, 22]. In a rat model of bilateral ureteral obstruction (BUO), a disease that blocks the urine to pass from kidney to bladder, the function and expression of renal rOat1/3 were also decreased [23]. OCT family and MATE family are two SLC transporter subfamilies that work in concert to play pivotal roles in the clinical profile of metformin for type II diabetic patients. Genetic polymorphisms of OCT1 [24], OCT3 [25] and MATE1 [26] in the liver are associated with the altered uptake and pharmacological action of metformin, while the genetic variation of OCT2 [27] and MATE1 [28] MATE2-K [29] in the kidney is directly related to variable metformin clearance and therapeutic response.
Given the critical roles the drug transporters of SLC family play in determining the effects of therapeutics and toxic chemicals, understanding the molecular and cellular mechanisms underlying the regulation of these transporters is physiologically and clinically important.
2. Regulation of SLC Family of Drug Transporters
The activity of drug transporters must be delicately controlled in order to carry out their normal activity. Like other proteins, drug transporters can be regulated at multiple levels from gene to protein, including transcriptional regulation, post-transcriptional regulation, translational regulation, and post-translational regulation. The regulations at the levels of transcription, post-transcription, and translation happen within hours to days, and are therefore called long-term or chronic regulation. Long-term regulation usually occurs when the body undergoes massive change, for example, during growth or the development of disease. In contrast, post-translational regulation happens within minutes to hours, and is therefore called short-term or acute regulation. Short-term regulation often takes place when the body has to deal with rapidly changing amounts of substrates in the case of variable intake of drugs, fluids, ions, meals, and metabolism processes.
Post-translational modification is a process where the amino acid side chains in a target protein are modified by conjugating new functional group(s) through reversible or irreversible biochemical reactions. The common modifications include glycosylation, phosphorylation, ubiquitination, sumoylation, sulfation, methylation, acetylation, nitrosylation, palmitoylation and hydroxylation [30]. Post-translational modification affects the folding, conformation, distribution, trafficking, stability, and activity of the proteins and therefore contributes significantly to the structural complexity and functional diversity of the proteins beyond the coding capacity of the genome. Over the last decade, our laboratory and other laboratories have discovered several mechanisms underlying the post-translational regulation of SLC family of drug transporters. In the following, we will focus on these discoveries that are pivotal not only in physiological conditions, but also in pathophysiological states.
2.1 Phosphorylation
Phosphorylation is defined as the reversible addition of a negatively charged phosphate group to a protein substrate, typically to a serine, threonine, or tyrosine residue. The presence of this heavily charged group is important for changing the hydrophobicity and electric charge of a protein region and, therefore, it can result in a change in the protein conformation, cellular localization, or interactions with other proteins. The phosphorylation state of a target protein is dynamically controlled by protein kinases and protein phosphatases, which act in an exact opposite fashion to remove phosphate, making phosphorylation a reversible process [31].
The functional effects of phosphorylation were observed with many drug transporters. For example, treatment of the mouse organic anion transporter 1 (mOat1)-expressing cells with phosphatase inhibitor okadaic acid promotes serine/threonine phosphorylation of the transporter and inhibits mOat1-mediated transport of para-aminohippurate (PAH), a prototypical organic anion. Activation of protein kinase C (PKC) enhances the phosphorylation of the rat organic cation transporter 1 (rOct1), and substitutions of PKC-sites on rOct1 with alanine suppressed PKC-induced stimulation of rOct1 transport activity [32, 33]. Another member of the OCT family human organic cation transporter 2 (hOCT2) was subject to tyrosine kinase-induced increase in phosphorylation and transport activity. PKC activation or treatment with phosphatase inhibitor okadaic acid also results in an increased phosphorylation and a functional inhibition of organic anion-transporting polypeptides OATP2B1, OATP1B3, and rOatp1a1, which correlated with an accelerated internalization of these transporters from cell surface. The phosphorylation and transport activity of sodium taurocholate cotransporting polypeptide (rNtcp) was also influenced by protein kinase A (PKA), PKC, and hyperosmolarity. Similarly, interleukin-1β promoted degradation of apical sodium-dependent bile acid transporter rAsbt through JNK-regulated phosphorylation. PKA-regulated phosphorylation of monocarboxylate transporter rMct1 and serum- and glucocorticoid-inducible kinase 1 (SGK1)-regulated phosphorylation of peptide transporter PEPT2 both exerted functional consequences on these transporters. The details on the functional regulation of these transporters by phosphorylation can be found in Table 1.
Table 1.
Post-translational modifications (PTM) of SLC drug transporters
| Type of PTM | Transporter | Subfamily | Details | Ref. |
|---|---|---|---|---|
| Phosphorylation | Organic anion Transporter (OAT) | mOat1 | Treatment with phosphatase inhibitor okadaic acid promoted serine/threonine phosphorylation of mOat1 and inhibited mOat1 transport activity. | [66] |
| Organic cation Transporter (OCT) | rOct1 | The substrate affinity of rOct1 was increased by PKC-dependent phosphorylation. Substitutions of single (Ser286, Ser292, Thr296, Ser328, and Thr550) or of all five PKC-sites on rOct1 with alanine suppressed PKC-induced stimulation of rOct1 transport activity. | [32, 33] | |
| hOCT2 | Tyrosine kinase inhibitors (TKIs) inhibited the Src family kinase Yes1, and consequently impaired hOCT2 tyrosine phosphorylation and function. Tyr241, Tyr362 and Tyr377 were three phosphorylation sites; with Tyr362 had the most dramatic effect. | [67] | ||
| Organic anion-transporting polypeptide (OATP) | rOatp1a1 | Incubation with phosphatase inhibitors okadaic acid and calyculin A reduced transporter activity because of phosphorylation on Ser634 and Ser635. The constitutive phosphorylated form of rOatp1a1 showed increased internalization rate compared with the nonphosphorylatable form. | [68–70] | |
| OATP1B3 | Treatment with PKC activator PMA decreased OATP1B3-induced substrate accumulation in human hepatocytes by direct phosphorylation of the transporter. | [71] | ||
| OATP2B1 | PKC activation resulted in increased phosphorylation of OATP2B1 and a reduced transport activity, which was due to a clathrin-dependent internalization of the transporter, followed by lysosomal degradation. | [72] | ||
| Sodium taurocholate cotransporting polypeptide (NTCP) | rNtcp | The cyclic adenosine monophosphate (cAMP) stimulated rNtcp transport activity via dephosphorylation through inhibiting Ca2+/calmodulin-dependent protein phosphatase (PP2B) and reducing translocation of rNtcp to the plasma membrane. Mutation of Ser226 impaired rNtcp phosphorylation, reduced transport activity and prevented translocation of the mutant to the plasma membrane. | [73–75] | |
| Mutation of two phosphorylation sites (Thr225 and Ser226) surrounding the dileucine motif of rNtcp inhibited PKC-induced endocytosis. | [76] | |||
| Hyperosmolarity induced a retrieval of rNtcp from the basolateral membrane of rat liver, which is accompanied by an increased rNtcp serine phosphorylation. | [77] | |||
| Apical sodium-dependent bile acid transporter (ASBT) | rAsbt | rAsbt degradation was increased by Interleukin-1β (IL-1β) due to JNK-regulated phosphorylation on Ser335 and Thr339. | [54] | |
| Monocarboxylate transporter (MCT) | rMct1 | The cyclic adenosine monophosphate (cAMP) induced dephosphorylation and internalization of rMct1 from the plasma membrane into early endosomes, which also caused a reduction of Vmax. | [78] | |
| Peptide transporter (PEPT) | PEPT2 | SGK1 enhanced PEPT2 function and surface expression through phosphorylation on Ser185. Disruption of this site abolished the kinase-mediated regulation on transporter. | [79] | |
| Glycosylation | Organic anion Transporter (OAT) | mOat1 hOAT1 hOAT4 |
Treatment with N-linked glycosylation inhibitor, tunicamycin, or mutagenesis of all glycosylation sites impaired the targeting of OATs to the plasma membrane and inhibited OAT transport activity. | [39, 40, 80] |
| hOAT4 | Expressing of hOAT4 in cells defective in processing of oligosaccharides from mannose-rich type to complex type decreased the affinity of hOAT4 for its substrates. | [40] | ||
| Organic cation Transporter (OCT) | rbOct2 | N-glycosylation happened at Asn71, Asn96, and Asn112, and mutation of the latter two impaired transporter function and diminished the maximum transport rate Vmax. However, the two glycosylation sites functioned differently, with Asn96 important for transporter turnover number and Asn112 responsible for plasma membrane targeting. | [81] | |
| OCTN2 | OCTN2 was physiologically glycosylated at Asn57, Asn64, and Asn91, and mutation of the glycosylation sites lead to a reduced expression level, an impaired transport function and a decreased maximum transport activity. Tunicamycin blocked OCTN2 glycosylation, but it didn’t impair its maturation to the plasma membrane. A splice variant of OCTN2 (OCTN2VT) with the insertion of 24 amino acids in the first extracellular loop was identified in human kidney, which showed accumulation in the endoplasmic reticulum (ER) with poor N-glycosylation and no transport activity. In addition, mutations (P46S and R83L) in patients with systemic primary carnitine deficiency (CDSP) were found to be related with the impaired glycosylation and maturation of OCTN2 to the plasma membrane. | [82, 83] | ||
| Organic anion-transporting polypeptide (OATP) | rOatp1a1 | Treatment with tunicamycin decreased rOatp1a1 molecular weight from 72 to 62 kDa and abolished transport activity. Lee et al. reported that mutations of four glycosylation sites (Asn62, Asn124, Asn135, and Asn492) revealed a cumulative negative effect on its function, leading to total loss of transport activity when all glycosylation sites were simultaneously removed, which was due to the reduced rOatp1a1 surface expression. However, according to Wang et al. ‘s report they agreed on three of the glycosylation sites, but argued that Asn62 may not be the glycosylation site, as it was proposed within a transmembrane segment. | [84, 85] | |
| OATP1A2 OATP1B1 |
Mutation of glycosylation sites significantly reduced protein level on cell surface as well as loss of transport activity, which was due to the impaired targeting of OATP to the plasma membrane. Specifically, Asn135 was the glycosylation site on OATP1A2, while Asn134, Asn503 and Asn516 were the glycosylation sites of OATP1B1 and disruption of these glycosylation sites would affect the stability of OATP1B1. | [86, 87] | ||
| Equilibrative nucleoside transporter (ENT) | hENT1 | Glycosylation happened at Asn48. Mutation of this site retained hENT1 function and its capacity for inhibitor (NBMPR; 6-[(4-nitrobenzyl)thio]-9-β-D-ribofuranosyl purine)-sensitive thymidine transport, but decreased the affinity of the mutant for the inhibitor NBMPR due to an increased rate of dissociation and a decreased rate of association. | [88, 89] | |
| hENT2 | Asn48 and Asn57 were two glycosylation sites, which was required for efficient targeting of hENT2 to the plasma membrane. However, mutation of the glycosylation sites had no effect on its transport affinity with substrate, but had a decreased maximum transport velocity Vmax. | [90] | ||
| Concentrative nucleoside cotransporter (CNT) | rCnt1 | Glycosylation happened at C terminus Asn605 and Asn643, but not at the predicted glycosylation site at N terminus, or between putative transmembrane domain 4 and 5. | [91] | |
| hCNT3 | Deglycosylation with endoglycosidase H (Endo H) and peptide N-glycosidase F (PNGase F) shifted the mobility of hCNT3 from 66 kDa to 58 kDa. | [92] | ||
| Peptide transporter (PEPT) | Murine Pept1 |
Six asparagine residues (Asn50, Asn406, Asn439, Asn510, Asn515, and Asn532) were found to be the N-glycosylation sites with five of them locating in the large extracellular loop between transmembrane domain 9 and 10. Treatment with tunicamycin or mutation of these glycosylation sites did not alter membrane protein abundance but changed the transport kinetics, in which mutation at Asn50 is the most prominent with a twofold decreased affinity for glycyl-sarcosine (Gly-Sar) but a 2.5-fold increase in the maximal inward currents. | [93] | |
| Apical sodium -dependent bile acid transporter (ASBT) | hASBT | Inhibition of glycosylation by tunicamycin significantly decreased hASBT function and showed an unglycosylated hASBT band around 30 kDa. In addition, Treatment with Endo H or PNGase F glycosidases indicated that the upper 41kDa band of hASBT represented a mature N-acetylglucosamine rich glycoprotein and the lower 35-kDa band to be a mannose-rich core glycoprotein. Glycosylation happened at Asn10 and mutation of this glycosylation site did not affect its targeting to plasma membrane. However, mature glycosylation significantly increased ASBT half-life on the plasma membrane. | [94, 95] | |
| Ubiquitination | Organic anion Transporter (OAT) | rOat1 hOAT1 hOAT3 |
Treatment of OAT-expressing cells or rat kidney slides with protein kinase C activator, PMA, increased OAT ubiquitination, while PKC inhibitor staurosporin blocked OAT ubiquitination. | [51, 96] |
| hOAT1 hOAT3 hOAT4 |
Overexpression of Nedd4-2 increased OAT ubiquitination and decreased OAT activity. siRNA knockdown of endogenous Nedd4-2 abolished PKC-stimulated hOAT1/hOAT3 ubiquitination, reversed PKC-induced decrease of OAT activity. Knockdown of endogenous Nedd4-2 also abolished sgk2-stimulated hOAT4 activity. | [96–98] | ||
| hOAT1 | LC-MS/MS detected Lys48-linked polyubiquitin peptides conjugated to OAT. Ubiquitin mutant Ub-K48R prevents the formation of polyubiquitin chains, abolishes PKC-stimulated hOAT1 ubiquitination, internalization and PKC-induced decrease in hOAT1 expression at the cell expression. | [51] | ||
| Mutagenesis of Lys297, Lys303 and Lys315 abolished PKC-stimulated hOAT1 ubiquitination. | [52] | |||
| Overexpression of Nedd4-1 increased hOAT1 ubiquitination and decreased hOAT1 transport activity. | [97] | |||
| Organic anion-transporting polypeptide (OATP) | rOatp1a1 | Hepatocyte growth factor reversed the down-regulation of the rOatp1a1 protein level in cholestasis by suppressing the ubiquitination of the transporter. | [53] | |
| Peptide transporter (PEPT) | rbPept1, rbPept2 | Nedd4-2 decreased transport activity of rabbit Pept1 and Pept2 in Xenopus laevis oocytes, whereas overexpression of USP18 stimulated their transport activities. | [57] | |
| Apical sodium-dependent bile acid transporter (ASBT) | rAsbt | Interleukin-1β induced a down-regulation of rAsbt, which was accompanied by an increase in rAsbt ubiquitination and a reduction in rAsbt half-life. | [54] | |
| mAsbt | Ileal mAsbt protein levels were down-regulated by bile acids through ubiquitin-dependent lysosome or proteasome protein degradation. | [55] | ||
| hASBT | Resveratrol (RSV), a major constituent of red wine, could inhibit hASBT protein expression and function without changing its affinity with its substrates. RSV treatment increased hASBT ubiquitination and the lysosome and proteasome inhibitor could reverse the inhibition effect by RSV. | [99] | ||
| Monocarboxylate transporter (MCT) | rMct1 | Activation of the canonical Wnt/β-catenin pathway in RBE4 cells via nuclear β-catenin signaling with LiCl increased the protein expression of monocarboxylic acid transporter rMct1, which resulted from a decreased rMct1 ubiquitination. | [56] | |
| Disulfide bond | Organic cation Transporter (OCT) | rOct1 | Replacement of the cysteine residues in the large extracellular loop of rOct1 by serine residues prevented oligomerization, which did not influence substrate affinities but impaired membrane targeting. | [58] |
| hOCT2 | hOCT2 formed oligomers both in vitro and in vivo through the Cys51 and Cys143 of the large extracellular loop, which was important for correct folding, oligomerization, and plasma membrane insertion of hOCT2. | [59] | ||
| S-nitrosylation | taurocholate cotransporting polypeptide (NTCP) | hNTCP rNtcp |
Nitric oxide (NO) could inhibit hNTCP function by decreasing its amount at the plasma membrane through S-nitrosylation of cysteine thiols on hNTCP, the effect of which did not change its affinity with substrates. The data obtained in vitro were confirmed in an experimental model of cholestasis, in which the administration of a-Tocopherol or NO donors reduced rNtcp expression and liver injury in bile duct obstructed rats. Another study also confirmed S-nitrosylated Cys 96 of rNtcp was responsible for NO-mediated inhibition of taurocholate uptake. | [60–62] |
| Monocarboxylate transporter (MCT) | hMCT | S-nitroso-L-cysteine (L-CysNO) and D-CysNO, which are nitrosylating agent used to initiate intracellular S-nitrosylation, could inhibit cellular pyruvate uptake, which was linked to S-nitrosylation of thiol groups on hMCT. | [100] |
2.2 Glycosylation
Glycosylation is a modification that involves the addition of oligosaccharides to secretory and membrane proteins. There are two main types of glycosylation, with sugar moiety added on to NH2 group of asparagine (N-linked) and on to the OH group of serine/threonine (O-Linked) [34–36]. N-linked glycosylation initiates co-translationally at rough endoplasmic reticulum and further processes in the Golgi apparatus, while O-linked glycosylation occurs post-translationally in the Golgi apparatus [37, 38]. Glycosylation is one of the most common forms of posttranslational protein modification and rapidly emerges as a fundamental mechanism not only controlling the proper folding of nascent transporter proteins but also their subcellular localization, and function.
Studies from our laboratory showed that members of organic anion transporter (OAT) family are heavily glycosylated under normal condition. When all of the potential glycosylation sites localized in the large extracellular loop between transmembrane domains 1 and 2 of OAT were simultaneously removed by mutagenesis approach, the transporter was then trapped in an intracellular compartment, suggesting that glycosylation is important for the targeting of the transporter to the plasma membrane [39, 40]. Pelis, et al also demonstrated that N-glycosylation happened at another member of the SLC transporter subfamily OCT, specifically rabbit Oct2, at asparagine (Asn) 71, 96, and 112. Interestingly, these glycosylation sites played differential roles in Oct function: with Asn112 being responsible for plasma membrane targeting and with Asn96 being important for transporter turnover number. Glycosylation has been shown to play various functional roles in other drug transporters of SLC family including organic cation/carnitine transporter OCTN2, organic anion-transporting polypeptides rOatp1a1, OATP1A2, OATP1B1, equilibrative nucleoside transporters hENT1 and hENT2, concentrative nucleoside cotransporters rCnt1 and hCNT3, Peptide transporter mPept1, and Apical sodium-dependent bile acid transporter hASBT. Please refer to Table 1 for details.
2.3 Ubiquitination
Another type of post-translational modification named ubiquitination gets much attention recently as more and more evidence revealed its importance in controlling the trafficking of proteins at the plasma membrane. Modification of receptors and channels by ubiquitin conjugation, which can be recognized by the components of plasma membrane internalization and endosomal sorting machinery, has been demonstrated as the major regulatory mechanism of internalization, intracellular sorting, and turnover of many membrane proteins [41, 42].
Ubiquitin is a small, globular protein of 76 amino acids that act as basic unit in the process of ubiquitination and can be covalently conjugated to the lysine residues of the substrate proteins. Ubiquitination is mediated by the coordinated steps of three enzymes. First, the ubiquitin-activating enzyme E1 catalyzes the formation of a thioester bond between its active cysteine and glycine (Gly76) at the COOH terminus of ubiquitin. Secondly, the activated ubiquitin is transferred to an ubiquitin-conjugating enzyme E2 forming the similar thioester bond. The last step is the most important step, in which an E3 ubiquitin-protein ligase binds to the substrate and forms an isopeptide bond between the Gly76 of ubiquitin and the ε-amine of lysine residue on the target substrate [43]. Modification of substrate proteins by single ubiquitin moiety is called monoubiquitination, whereas modification of substrate proteins by multiple moieties of monoubiquitin on distinct lysine residues is known as multiple monoubiquitination. An ubiquitin molecule itself has seven lysine residues (Lys6, 11, 27, 29, 33, 48, and 63), all of which may conjugate through an isopeptide bond with the C-terminus of another ubiquitin to form a polyubiquitin chain. There are only a few E1 enzymes exist (1 in yeast and 10 in human) and a little bit more E2 conjugating enzymes (11 in yeast and at least 100 in human), but hundreds of E3 enzymes (54 in yeast and 1,000 in human genome) [42, 44]. As E3 ligase is responsible for substrate recognition, such an abundance of E3 guarantees the specificity of target recognition in the ubiquitin-ligase system [45]. Ubiquitination, similarly to phosphorylation, is a reversible modification and, in mammals, approximately 100 deubiquitination enzymes (DUBs) function to depolymerize and remove ubiquitin adducts [46]. Recent evidences have shown that proteins that are ubiquitinated in the plasma membrane are internalized into early endosomes, where these proteins are then deubiquitinated by deubiquitinating enzymes and recycle back to the plasma membrane. Alternatively, these ubiquitinated proteins can also interact with the endosomal sorting complexes required for transport machinery and are sorted to late endosomes, and ultimately, to the lysosomes for degradation [47, 48]. Thus, the balance between ubiquitination (mediated by E3 ligases) and deubiquitination (mediated by DUBs) regulates the abundance of membrane proteins on plasma membrane.
The investigation from our laboratory on organic anion transporters OAT in cultured cells demonstrated that OAT constitutively internalizes from and recycles back to cell surface. The rate of OAT internalization is equal to the rate of OAT recycling being ~ 10% per 5 min [49]. PKC activation accelerates the rate of OAT internalization from cell surface to EEA1-positive early endosomes, without changes in the rate of OAT recycling. As a result, the amount of OAT at the cell surface is reduced and OAT transport activity is decreased [49, 50]. Prolonged PKC activation results in the degradation of OAT in both proteasome and lysosome [50]. A critical step preceding OAT internalization is the ubiquitination of the transporter. Mass spectroscopy analysis revealed that the ubiquitination of OAT occurs through the conjugation of a lysine 48-linked polyubiquitin chain to the transporter [51]. Three important ubiquitin-accepting lysine residues Lys297, Lys303, and Lys315 were identified within the large intracellular loop between transmembrane domains 6 and 7 of hOAT1 [52]. These lysine residues play a synergistic role in PKC-regulated hOAT1 ubiquitination, as mutating any one of the three lysines prevented the ubiquitin conjugation to the other two lysines [52]. Our unpublished results also indicated that lysine 48-linked polyubiquitin chain plays an important role in the long-term PKC regulation of hOAT1 stability.
Ubiquitination of other drug transporters has also been reported. Iwakiri, et al. reported that the protein expression and function of organic anion-transporting polypeptide (rOatp1a1), which is responsible for the uptake of bile salts into hepatocytes, decreased in cholestatic humans and rats. The ameliorative effects of hepatocyte growth factor (HGF) in cholestasis was, at least in part, due to its reversal of the down-regulation of the rOatp1a1 protein level by suppressing the ubiquitination of the transporter [53]. Xia, et al. showed that the expression of liver-specific apical sodium-dependent bile acid transporter (ASBT) is regulated by the ubiquitin proteasome pathway [54]. Interleukin-1β induced a down-regulation of rAsbt, which was accompanied by an increase in rAsbt polyubiquitin conjugates and a reduced rAsbt half-life. Interestingly, this process was enhanced through JNK-regulated serine/threonine phosphorylation of rAsbt protein at both Ser-335 and Thr-339 [55]. Liu, et al revealed that activation of the canonical Wnt/β-catenin pathway in RBE4 cells via nuclear β-catenin signaling with LiCl increased the protein expression of monocarboxylic acid transporter rMct1, which resulted from a reduced rMct1 trafficking from the plasma membrane via the endosomal/lysosomal pathway and was facilitated by a decreased rMct1 ubiquitination [56]. Warsi, et al demonstrated that transport activity of rabbit peptide transporters Pept1 and Pept2 was decreased in Xenopus laevis oocytes injected with cRNA encoding the E3 ubiquitin ligase Nedd4-2, whereas overexpression of USP18 (Ubiquitin-like specific protease 18), an enzyme cleaving ubiquitin from target proteins, stimulated the transport activity of rbPept1 and rbPept2 [57].
2.4 Disulfide Bonds
Disulfide bonds or disulfide bridges in proteins are usually formed between the thiol groups of cysteine residues by the process of oxidative folding, and can be reversibly reduced and re-oxidized. Disulfide bonds play critical role in stabilizing protein oligomerization, trafficking, and activity. Keller, et al showed that the tertiary structure of the large extracellular loop of rat organic anion transporter 1(rOct1), which was stabilized by disulfide bonds, was required for oligomerization and membrane insertion of rOct1 and had an effect on the affinity of organic cations. When the six cysteine residues in the large extracellular loop of rOct1 were replaced by serines or when the disulfide bridge(s) in the loop were reduced by dithiothreitol, a redox reagent, oligomerization was abolished [58]. Brast et al. demonstrated that human organic anion transporter 2 (hOCT2) formed oligomers both in the HEK293 expression system and in native human kidneys. The cysteines of the large extracellular loop were important to enable correct folding, oligomeric assembly, and plasma membrane insertion of hOCT2. Mutation of the first and the last cysteines of the loop at positions 51 and 143 abolished oligomer formation [59].
2.5 S-Nitrosylation
S-Nitrosylation is the covalent incorporation of a nitric oxide moiety into cysteine thiols of a protein substrate. In a human hepatoma cell line stably expressing hNTCP, a transporter responsible for the taurocholate (TC) uptake across the sinusoidal membrane of hepatocytes, Schonhoff, et al. discovered that nitric oxide (NO) donors sodium nitroprusside and S-nitrosocysteine decreased the amount of hNTCP at the plasma membrane, and inhibited TC uptake, which was accompanied by the S-nitrosylation of hNTCP. Dithiothreitol, a redox reagent, reversed NO-mediated inhibition of TC uptake and S-nitrosylation of hNTCP [60]. The same research group also demonstrated that replacing cysteine 96 with alanine (C96A) rendered rNtcp insensitive to NO-mediated inhibition of TC uptake. rNtcp/C96A was not S-nitrosylated by NO, suggesting that Cys96 is important in regulating rNtcp function in response to elevated levels of NO [61]. Gonzalez, et al. examined the cytoprotective effect of α-Tocopherol against glycochenodeoxycholate-induced cell death in hepatocytes. α-Tocopherol and/or NO donors (DETA-NONOate or CSNO, and V-PYRRO/NO) were administered to glycochenodeoxycholic acid (GCDCA)-treated cultured human hepatocytes or to bile duct obstructed rats. They found that α-Tocopherol and NO donors increased NTCP S-nitrosylation, and reduced TC uptake in hepatocytes. α-Tocopherol and V-PYRRO/NO also reduced liver injury and rNtcp expression in obstructed rats [62]. Therefore, NO-dependent post-translational modifications of NTCP by α-Tocopherol and NO donors reduced the uptake of toxic bile acids by hepatocytes.
3. Conclusion and future perspectives
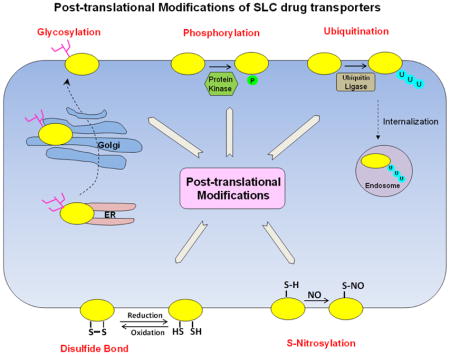
In this review, we attempted to summarize and updated recent advances from our laboratory as well as from others in uncovering the loops and layers of regulation of drug transporters from SLC family by posttranslational modifications (Table 1 and Figure 1), such as phosphorylation, glycosylation, ubiquitination, disulfide bonds, and S-nitrosylation. Up to now, most of these posttranslational modifications have been investigated in isolation. Therefore, it is imperative that we next look at a bigger picture and address an intriguing question: whether there is a crosstalk among these posttranslational modifications. Functionally, crosstalk may occur within the same protein (cis crosstalk) or between posttranslational modifications on two different proteins (trans crosstalk). Evidences suggest that different types of posttranslational modifications may work together to accomplish a specific biological function. For example, phosphorylation has been implicated frequently in crosstalk with and precedes ubiquitination in directing protein degradation[63]. Also, ubiquitination is known to either agonize or antagonize other types of posttranslational modifications such as sumoylation [64]. In addition, the interplay between palmitoylation and phosphorylation or between palmitoylation and ubiquitination has also been observed [65]. Another interesting area that needs much attention is the potential drug-induced post-translational modifications of the drug transporters. For example, the anti-cancer drugs that target different kinases such as tyrosine kinase inhibitor Imatinib or the anti-cancer drugs that target ubiquitin-proteasome system such as proteasome inhibitor Bortezomib, may lead to possible alteration of post-translational modifications of the drug transporters. Hence, posttranslational modifications represent an important set of mechanisms to regulate drug transporters, and the importance and complexity of its code remain a major challenge for our complete understanding of their roles in transporter-mediated drug disposition.
Fig. 1. Simplified model for posttranslational modifications of drug transporters.
 Membrane transporter, U: ubiquitin, P: phosphate group, NO: nitro oxide, ER: endoplasmic reticulum, Tree-like structure: carbohydrates
Membrane transporter, U: ubiquitin, P: phosphate group, NO: nitro oxide, ER: endoplasmic reticulum, Tree-like structure: carbohydrates
Acknowledgments
This work was supported by grants (to Dr. Guofeng You) from National Institute of General Medical Sciences (R01-GM079123 and R01-GM097000).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nigam SK, Bush KT, Martovetsky G, Ahn SY, Liu HC, Richard E, Bhatnagar V, Wu W. The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev. 2015;95:83–123. doi: 10.1152/physrev.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol Aspects Med. 2013;34:413–435. doi: 10.1016/j.mam.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DE, Clemencon B, Hediger MA. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol Aspects Med. 2013;34:323–336. doi: 10.1016/j.mam.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halestrap AP. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life. 2012;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 6.Young JD, Yao SY, Baldwin JM, Cass CE, Baldwin SA. The human concentrative, equilibrative nucleoside transporter families, SLC28 and SLC29. Mol Aspects Med. 2013;34:529–547. doi: 10.1016/j.mam.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Doring B, Lutteke T, Geyer J, Petzinger E. The SLC10 carrier family: transport functions and molecular structure. Curr Top Membr. 2012;70:105–168. doi: 10.1016/B978-0-12-394316-3.00004-1. [DOI] [PubMed] [Google Scholar]
- 8.Yonezawa A, Inui K. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br J Pharmacol. 2011;164:1817–1825. doi: 10.1111/j.1476-5381.2011.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Sweet DH. Renal organic anion transporters (SLC22 family): expression, regulation, roles in toxicity, and impact on injury and disease. AAPS J. 2013;15:53–69. doi: 10.1208/s12248-012-9413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol. 2009;76:481–490. doi: 10.1124/mol.109.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelis RM, Wright SH. Renal transport of organic anions and cations. Compr Physiol. 2011;1:1795–1835. doi: 10.1002/cphy.c100084. [DOI] [PubMed] [Google Scholar]
- 12.Terada T, Inui K. Gene expression and regulation of drug transporters in the intestine and kidney. Biochem Pharmacol. 2007;73:440–449. doi: 10.1016/j.bcp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 13.You G. Structure, function, and regulation of renal organic anion transporters. Med Res Rev. 2002;22:602–616. doi: 10.1002/med.10019. [DOI] [PubMed] [Google Scholar]
- 14.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillgren KM, Keppler D, Zur AA, Giacomini KM, Stieger B, Cass CE, Zhang L. Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94:52–63. doi: 10.1038/clpt.2013.74. [DOI] [PubMed] [Google Scholar]
- 16.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keitel V, Burdelski M, Warskulat U, Kuhlkamp T, Keppler D, Haussinger D, Kubitz R. Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology. 2005;41:1160–1172. doi: 10.1002/hep.20682. [DOI] [PubMed] [Google Scholar]
- 18.Oswald M, Kullak-Ublick GA, Paumgartner G, Beuers U. Expression of hepatic transporters OATP-C and MRP2 in primary sclerosing cholangitis. Liver. 2001;21:247–253. doi: 10.1034/j.1600-0676.2001.021004247.x. [DOI] [PubMed] [Google Scholar]
- 19.Monica Torres A, Mac Laughlin M, Muller A, Brandoni A, Anzai N, Endou H. Altered renal elimination of organic anions in rats with chronic renal failure. Biochim Biophys Acta. 2005;1740:29–37. doi: 10.1016/j.bbadis.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Naud J, Michaud J, Beauchemin S, Hebert MJ, Roger M, Lefrancois S, Leblond FA, Pichette V. Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab Dispos. 2011;39:1363–1369. doi: 10.1124/dmd.111.039115. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzaki T, Watanabe H, Yoshitome K, Morisaki T, Hamada A, Nonoguchi H, Kohda Y, Tomita K, Inui K, Saito H. Downregulation of organic anion transporters in rat kidney under ischemia/reperfusion-induced acute [corrected] renal failure. Kidney Int. 2007;71:539–547. doi: 10.1038/sj.ki.5002104. [DOI] [PubMed] [Google Scholar]
- 22.Schneider R, Sauvant C, Betz B, Otremba M, Fischer D, Holzinger H, Wanner C, Galle J, Gekle M. Downregulation of organic anion transporters OAT1 and OAT3 correlates with impaired secretion of para-aminohippurate after ischemic acute renal failure in rats. Am J Physiol Renal Physiol. 2007;292:F1599–1605. doi: 10.1152/ajprenal.00473.2006. [DOI] [PubMed] [Google Scholar]
- 23.Villar SR, Brandoni A, Anzai N, Endou H, Torres AM. Altered expression of rat renal cortical OAT1 and OAT3 in response to bilateral ureteral obstruction. Kidney Int. 2005;68:2704–2713. doi: 10.1111/j.1523-1755.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Takizawa M, Chen E, Schlessinger A, Segenthelar J, Choi JH, Sali A, Kubo M, Nakamura S, Iwamoto Y, Iwasaki N, Giacomini KM. Genetic polymorphisms in organic cation transporter 1 (OCT1) in Chinese and Japanese populations exhibit altered function. J Pharmacol Exp Ther. 2010;335:42–50. doi: 10.1124/jpet.110.170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Pawlikowski B, Schlessinger A, More SS, Stryke D, Johns SJ, Portman MA, Chen E, Ferrin TE, Sali A, Giacomini KM. Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenet Genomics. 2010;20:687–699. doi: 10.1097/FPC.0b013e32833fe789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Interaction between polymorphisms in the OCT1 and MATE1 transporter and metformin response. Pharmacogenet Genomics. 2010;20:38–44. doi: 10.1097/FPC.0b013e328333bb11. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Li S, Brown C, Cheatham S, Castro RA, Leabman MK, Urban TJ, Chen L, Yee SW, Choi JH, Huang Y, Brett CM, Burchard EG, Giacomini KM. Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet Genomics. 2009;19:497–504. doi: 10.1097/FPC.0b013e32832cc7e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer zu Schwabedissen HE, Verstuyft C, Kroemer HK, Becquemont L, Kim RB. Human multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter: functional characterization, interaction with OCT2 (SLC22A2), and single nucleotide polymorphisms. Am J Physiol Renal Physiol. 2010;298:F997–F1005. doi: 10.1152/ajprenal.00431.2009. [DOI] [PubMed] [Google Scholar]
- 29.Choi JH, Yee SW, Ramirez AH, Morrissey KM, Jang GH, Joski PJ, Mefford JA, Hesselson SE, Schlessinger A, Jenkins G, Castro RA, Johns SJ, Stryke D, Sali A, Ferrin TE, Witte JS, Kwok PY, Roden DM, Wilke RA, McCarty CA, Davis RL, Giacomini KM. A Common 5′-UTR Variant in MATE2-K Is Associated With Poor Response to Metformin. Clinical Pharmacology & Therapeutics. 2011;90:674–684. doi: 10.1038/clpt.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karve TM, Cheema AK. Small changes huge impact: the role of protein posttranslational modifications in cellular homeostasis and disease. J Amino Acids. 2011;2011:207691. doi: 10.4061/2011/207691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barford D. Molecular mechanisms of the protein serine/threonine phosphatases. Trends Biochem Sci. 1996;21:407–412. doi: 10.1016/s0968-0004(96)10060-8. [DOI] [PubMed] [Google Scholar]
- 32.Mehrens T, Lelleck S, Cetinkaya I, Knollmann M, Hohage H, Gorboulev V, Boknik P, Koepsell H, Schlatter E. The affinity of the organic cation transporter rOCT1 is increased by protein kinase C-dependent phosphorylation. J Am Soc Nephrol. 2000;11:1216–1224. doi: 10.1681/ASN.V1171216. [DOI] [PubMed] [Google Scholar]
- 33.Ciarimboli G, Koepsell H, Iordanova M, Gorboulev V, Durner B, Lang D, Edemir B, Schroter R, Van Le T, Schlatter E. Individual PKC-phosphorylation sites in organic cation transporter 1 determine substrate selectivity and transport regulation. J Am Soc Nephrol. 2005;16:1562–1570. doi: 10.1681/ASN.2004040256. [DOI] [PubMed] [Google Scholar]
- 34.Aebi M. N-linked protein glycosylation in the ER. Biochimica et biophysica acta. 2013;1833:2430–2437. doi: 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz F, Aebi M. Mechanisms and principles of N-linked protein glycosylation. Current opinion in structural biology. 2011;21:576–582. doi: 10.1016/j.sbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Hang HC, Bertozzi CR. The chemistry and biology of mucin-type O-linked glycosylation. Bioorganic & medicinal chemistry. 2005;13:5021–5034. doi: 10.1016/j.bmc.2005.04.085. [DOI] [PubMed] [Google Scholar]
- 37.Creighton TE. Proteins: Structures and molecular properties. 2. W. H. Freeman; New York: 1992. [Google Scholar]
- 38.Starr CM, Hanover JA. Glycosylation of nuclear pore protein p62. Reticulocyte lysate catalyzes O-linked N-acetylglucosamine addition in vitro. J Biol Chem. 1990;265:6868–6873. [PubMed] [Google Scholar]
- 39.Tanaka K, Xu W, Zhou F, You G. Role of glycosylation in the organic anion transporter OAT1. J Biol Chem. 2004;279:14961–14966. doi: 10.1074/jbc.M400197200. [DOI] [PubMed] [Google Scholar]
- 40.Zhou F, Xu W, Hong M, Pan Z, Sinko PJ, Ma J, You G. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol Pharmacol. 2005;67:868–876. doi: 10.1124/mol.104.007583. [DOI] [PubMed] [Google Scholar]
- 41.Miranda M, Sorkin A. Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms. Mol Interv. 2007;7:157–167. doi: 10.1124/mi.7.3.7. [DOI] [PubMed] [Google Scholar]
- 42.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev. 2006;86:669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- 43.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 44.Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat Rev Mol Cell Biol. 2005;6:610–621. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- 45.Laedermann CJ, Decosterd I, Abriel H. Ubiquitylation of voltage-gated sodium channels. Handb Exp Pharmacol. 221:231–250. doi: 10.1007/978-3-642-41588-3_11. [DOI] [PubMed] [Google Scholar]
- 46.Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol. 2011;12:439–452. doi: 10.1038/nrm3143. [DOI] [PubMed] [Google Scholar]
- 47.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 48.Bonifacino JS, Weissman AM. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q, Hong M, Duan P, Pan Z, Ma J, You G. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J Biol Chem. 2008;283:32570–32579. doi: 10.1074/jbc.M800298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Q, Suh W, Pan Z, You G. Short-term and long-term effects of protein kinase C on the trafficking and stability of human organic anion transporter 3. Int J Biochem Mol Biol. 2012;3:242–249. [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Q, Li S, Patterson C, You G. Lysine 48-linked polyubiquitination of organic anion transporter-1 is essential for its protein kinase C-regulated endocytosis. Mol Pharmacol. 2012;83:217–224. doi: 10.1124/mol.112.082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li S, Zhang Q, You G. Three ubiquitination sites of organic anion transporter-1 synergistically mediate protein kinase C-dependent endocytosis of the transporter. Mol Pharmacol. 2013;84:139–146. doi: 10.1124/mol.113.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwakiri T, Okumura M, Matsunaga N, Ichihara E, Shiotsuki S, Nagata M, Kumagai Y, Kai H, Arimori K. Hepatocyte growth factor increases uptake of estradiol 17beta-D-glucuronide and Oatp1 protein level in rat hepatocytes. Eur J Pharmacol. 2008;580:19–26. doi: 10.1016/j.ejphar.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 54.Xia X, Roundtree M, Merikhi A, Lu X, Shentu S, Lesage G. Degradation of the apical sodium-dependent bile acid transporter by the ubiquitin-proteasome pathway in cholangiocytes. J Biol Chem. 2004;279:44931–44937. doi: 10.1074/jbc.M400969200. [DOI] [PubMed] [Google Scholar]
- 55.Miyata M, Yamakawa H, Hayashi K, Kuribayashi H, Yamazoe Y, Yoshinari K. Ileal apical sodium-dependent bile acid transporter protein levels are down-regulated through ubiquitin-dependent protein degradation induced by bile acids. Eur J Pharmacol. 2013;714:507–514. doi: 10.1016/j.ejphar.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Sneve M, Haroldson TA, Smith JP, Drewes LR. Regulation of Monocarboxylic Acid Transporter 1 Trafficking by the Canonical Wnt/beta-Catenin Pathway in Rat Brain Endothelial Cells Requires Cross-talk with Notch Signaling. J Biol Chem. 2016;291:8059–8069. doi: 10.1074/jbc.M115.710277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warsi J, Hosseinzadeh Z, Elvira B, Pelzl L, Shumilina E, Zhang DE, Lang KS, Lang PA, Lang F. USP18 Sensitivity of Peptide Transporters PEPT1 and PEPT2. PLoS One. 2015;10:e0129365. doi: 10.1371/journal.pone.0129365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller T, Egenberger B, Gorboulev V, Bernhard F, Uzelac Z, Gorbunov D, Wirth C, Koppatz S, Dotsch V, Hunte C, Sitte HH, Koepsell H. The large extracellular loop of organic cation transporter 1 influences substrate affinity and is pivotal for oligomerization. J Biol Chem. 2011;286:37874–37886. doi: 10.1074/jbc.M111.289330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brast S, Grabner A, Sucic S, Sitte HH, Hermann E, Pavenstadt H, Schlatter E, Ciarimboli G. The cysteines of the extracellular loop are crucial for trafficking of human organic cation transporter 2 to the plasma membrane and are involved in oligomerization. FASEB J. 2012;26:976–986. doi: 10.1096/fj.11-180679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schonhoff CM, Ramasamy U, Anwer MS. Nitric oxide-mediated inhibition of taurocholate uptake involves S-nitrosylation of NTCP. Am J Physiol Gastrointest Liver Physiol. 2011;300:G364–370. doi: 10.1152/ajpgi.00170.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramasamy U, Anwer MS, Schonhoff CM. Cysteine 96 of Ntcp is responsible for NO-mediated inhibition of taurocholate uptake. Am J Physiol Gastrointest Liver Physiol. 2013;305:G513–519. doi: 10.1152/ajpgi.00089.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzalez R, Cruz A, Ferrin G, Lopez-Cillero P, Fernandez-Rodriguez R, Briceno J, Gomez MA, Rufian S, de Mata ML, Martinez-Ruiz A, Marin JJ, Muntane J. Nitric oxide mimics transcriptional and post-translational regulation during alpha-tocopherol cytoprotection against glycochenodeoxycholate-induced cell death in hepatocytes. J Hepatol. 2010;55:133–144. doi: 10.1016/j.jhep.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 63.Swaney DL, Beltrao P, Starita L, Guo A, Rush J, Fields S, Krogan NJ, Villen J. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat Methods. 2013;10:676–682. doi: 10.1038/nmeth.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gareau JR, Lima CD. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol. 2010;11:861–871. doi: 10.1038/nrm3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blaskovic S, Blanc M, van der Goot FG. What does S-palmitoylation do to membrane proteins? FEBS Journal. 2013;280:2766–2774. doi: 10.1111/febs.12263. [DOI] [PubMed] [Google Scholar]
- 66.You G, Kuze K, Kohanski RA, Amsler K, Henderson S. Regulation of mOAT-mediated organic anion transport by okadaic acid and protein kinase C in LLC-PK(1) cells. J Biol Chem. 2000;275:10278–10284. doi: 10.1074/jbc.275.14.10278. [DOI] [PubMed] [Google Scholar]
- 67.Sprowl JA, Ong SS, Gibson AA, Hu S, Du G, Lin W, Li L, Bharill S, Ness RA, Stecula A, Offer SM, Diasio RB, Nies AT, Schwab M, Cavaletti G, Schlatter E, Ciarimboli G, Schellens JH, Isacoff EY, Sali A, Chen T, Baker SD, Sparreboom A, Pabla N. A phosphotyrosine switch regulates organic cation transporters. Nat Commun. 2016;7:10880. doi: 10.1038/ncomms10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glavy JS, Wu SM, Wang PJ, Orr GA, Wolkoff AW. Down-regulation by extracellular ATP of rat hepatocyte organic anion transport is mediated by serine phosphorylation of oatp1. J Biol Chem. 2000;275:1479–1484. doi: 10.1074/jbc.275.2.1479. [DOI] [PubMed] [Google Scholar]
- 69.Xiao Y, Nieves E, Angeletti RH, Orr GA, Wolkoff AW. Rat organic anion transporting protein 1A1 (Oatp1a1): purification and phosphopeptide assignment. Biochemistry. 2006;45:3357–3369. doi: 10.1021/bi052437v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi JH, Murray JW, Wolkoff AW. PDZK1 binding and serine phosphorylation regulate subcellular trafficking of organic anion transport protein 1a1. Am J Physiol Gastrointest Liver Physiol. 2011;300:G384–393. doi: 10.1152/ajpgi.00500.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Powell J, Farasyn T, Kock K, Meng X, Pahwa S, Brouwer KL, Yue W. Novel mechanism of impaired function of organic anion-transporting polypeptide 1B3 in human hepatocytes: post-translational regulation of OATP1B3 by protein kinase C activation. Drug Metab Dispos. 2014;42:1964–1970. doi: 10.1124/dmd.114.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kock K, Koenen A, Giese B, Fraunholz M, May K, Siegmund W, Hammer E, Volker U, Jedlitschky G, Kroemer HK, Grube M. Rapid modulation of the organic anion transporting polypeptide 2B1 (OATP2B1, SLCO2B1) function by protein kinase C-mediated internalization. J Biol Chem. 2010;285:11336–11347. doi: 10.1074/jbc.M109.056457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukhopadhyay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. Sodium taurocholate cotransporting polypeptide is a serine, threonine phosphoprotein and is dephosphorylated by cyclic adenosine monophosphate. Hepatology. 1998;28:1629–1636. doi: 10.1002/hep.510280624. [DOI] [PubMed] [Google Scholar]
- 74.Webster CR, Blanch C, Anwer MS. Role of PP2B in cAMP-induced dephosphorylation and translocation of NTCP. Am J Physiol Gastrointest Liver Physiol. 2002;283:G44–50. doi: 10.1152/ajpgi.00530.2001. [DOI] [PubMed] [Google Scholar]
- 75.Anwer MS, Gillin H, Mukhopadhyay S, Balasubramaniyan N, Suchy FJ, Ananthanarayanan M. Dephosphorylation of Ser-226 facilitates plasma membrane retention of Ntcp. J Biol Chem. 2005;280:33687–33692. doi: 10.1074/jbc.M502151200. [DOI] [PubMed] [Google Scholar]
- 76.Stross C, Kluge S, Weissenberger K, Winands E, Haussinger D, Kubitz R. A dileucine motif is involved in plasma membrane expression and endocytosis of rat sodium taurocholate cotransporting polypeptide (Ntcp) Am J Physiol Gastrointest Liver Physiol. 2013;305:G722–730. doi: 10.1152/ajpgi.00056.2013. [DOI] [PubMed] [Google Scholar]
- 77.Sommerfeld A, Mayer PG, Cantore M, Haussinger D. Regulation of plasma membrane localization of the Na+-taurocholate cotransporting polypeptide (Ntcp) by hyperosmolarity and tauroursodeoxycholate. J Biol Chem. 2015;290:24237–24254. doi: 10.1074/jbc.M115.666883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith JP, Uhernik AL, Li L, Liu Z, Drewes LR. Regulation of Mct1 by cAMP-dependent internalization in rat brain endothelial cells. Brain Res. 2012;1480:1–11. doi: 10.1016/j.brainres.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boehmer C, Palmada M, Klaus F, Jeyaraj S, Lindner R, Laufer J, Daniel H, Lang F. The peptide transporter PEPT2 is targeted by the protein kinase SGK1 and the scaffold protein NHERF2. Cell Physiol Biochem. 2008;22:705–714. doi: 10.1159/000185554. [DOI] [PubMed] [Google Scholar]
- 80.Kuze K, Graves P, Leahy A, Wilson P, Stuhlmann H, You G. Heterologous expression and functional characterization of a mouse renal organic anion transporter in mammalian cells. J Biol Chem. 1999;274:1519–1524. doi: 10.1074/jbc.274.3.1519. [DOI] [PubMed] [Google Scholar]
- 81.Pelis RM, Suhre WM, Wright SH. Functional influence of N-glycosylation in OCT2-mediated tetraethylammonium transport. Am J Physiol Renal Physiol. 2006;290:F1118–1126. doi: 10.1152/ajprenal.00462.2005. [DOI] [PubMed] [Google Scholar]
- 82.Maekawa S, Mori D, Nishiya T, Takikawa O, Horinouchi T, Nishimoto A, Kajita E, Miwa S. OCTN2VT, a splice variant of OCTN2, does not transport carnitine because of the retention in the endoplasmic reticulum caused by insertion of 24 amino acids in the first extracellular loop of OCTN2. Biochim Biophys Acta. 2007;1773:1000–1006. doi: 10.1016/j.bbamcr.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 83.Filippo CA, Ardon O, Longo N. Glycosylation of the OCTN2 carnitine transporter: study of natural mutations identified in patients with primary carnitine deficiency. Biochim Biophys Acta. 2011;1812:312–320. doi: 10.1016/j.bbadis.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee TK, Koh AS, Cui Z, Pierce RH, Ballatori N. N-glycosylation controls functional activity of Oatp1, an organic anion transporter. Am J Physiol Gastrointest Liver Physiol. 2003;285:G371–381. doi: 10.1152/ajpgi.00358.2002. [DOI] [PubMed] [Google Scholar]
- 85.Wang P, Hata S, Xiao Y, Murray JW, Wolkoff AW. Topological assessment of oatp1a1: a 12-transmembrane domain integral membrane protein with three N-linked carbohydrate chains. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1052–1059. doi: 10.1152/ajpgi.00584.2007. [DOI] [PubMed] [Google Scholar]
- 86.Yao J, Hong W, Huang J, Zhan K, Huang H, Hong M. N-Glycosylation dictates proper processing of organic anion transporting polypeptide 1B1. PLoS One. 2012;7:e52563. doi: 10.1371/journal.pone.0052563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, Leake BF, Kim RB. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem. 2005;280:9610–9617. doi: 10.1074/jbc.M411092200. [DOI] [PubMed] [Google Scholar]
- 88.Vickers MF, Mani RS, Sundaram M, Hogue DL, Young JD, Baldwin SA, Cass CE. Functional production and reconstitution of the human equilibrative nucleoside transporter (hENT1) in Saccharomyces cerevisiae. Interaction of inhibitors of nucleoside transport with recombinant hENT1 and a glycosylation-defective derivative (hENT1/N48Q) Biochem J. 1999;339(Pt 1):21–32. [PMC free article] [PubMed] [Google Scholar]
- 89.Sundaram M, Yao SY, Ingram JC, Berry ZA, Abidi F, Cass CE, Baldwin SA, Young JD. Topology of a human equilibrative, nitrobenzylthioinosine (NBMPR)-sensitive nucleoside transporter (hENT1) implicated in the cellular uptake of adenosine and anti-cancer drugs. J Biol Chem. 2001;276:45270–45275. doi: 10.1074/jbc.M107169200. [DOI] [PubMed] [Google Scholar]
- 90.Ward JL, Leung GP, Toan SV, Tse CM. Functional analysis of site-directed glycosylation mutants of the human equilibrative nucleoside transporter-2. Arch Biochem Biophys. 2003;411:19–26. doi: 10.1016/s0003-9861(02)00718-x. [DOI] [PubMed] [Google Scholar]
- 91.Hamilton SR, Yao SY, Ingram JC, Hadden DA, Ritzel MW, Gallagher MP, Henderson PJ, Cass CE, Young JD, Baldwin SA. Subcellular distribution and membrane topology of the mammalian concentrative Na+-nucleoside cotransporter rCNT1. J Biol Chem. 2001;276:27981–27988. doi: 10.1074/jbc.M100518200. [DOI] [PubMed] [Google Scholar]
- 92.Toan SV, To KK, Leung GP, de Souza MO, Ward JL, Tse CM. Genomic organization and functional characterization of the human concentrative nucleoside transporter-3 isoform (hCNT3) expressed in mammalian cells. Pflugers Arch. 2003;447:195–204. doi: 10.1007/s00424-003-1166-0. [DOI] [PubMed] [Google Scholar]
- 93.Stelzl T, Baranov T, Geillinger KE, Kottra G, Daniel H. Effect of N-glycosylation on the transport activity of the peptide transporter PEPT1. Am J Physiol Gastrointest Liver Physiol. 2016;310:G128–141. doi: 10.1152/ajpgi.00350.2015. [DOI] [PubMed] [Google Scholar]
- 94.Muthusamy S, Malhotra P, Hosameddin M, Dudeja AK, Borthakur S, Saksena S, Gill RK, Dudeja PK, Alrefai WA. N-glycosylation is essential for ileal ASBT function and protection against proteases. Am J Physiol Cell Physiol. 2015;308:C964–971. doi: 10.1152/ajpcell.00023.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang EY, Phelps MA, Banerjee A, Khantwal CM, Chang C, Helsper F, Swaan PW. Topology scanning and putative three-dimensional structure of the extracellular binding domains of the apical sodium-dependent bile acid transporter (SLC10A2) Biochemistry. 2004;43:11380–11392. doi: 10.1021/bi049270a. [DOI] [PubMed] [Google Scholar]
- 96.Xu D, Wang H, You G. An Essential Role of Nedd4-2 in the Ubiquitination, Expression, and Function of Organic Anion Transporter-3. Mol Pharm. 2015 doi: 10.1021/acs.molpharmaceut.5b00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu D, Wang H, Zhang Q, You G. Nedd4-2 but not Nedd4-1 is Critical for Protein Kinase C-Regulated Ubiquitination, Expression and Transport Activity of Human Organic Anion Transporter 1. Am J Physiol Renal Physiol. 2016 doi: 10.1152/ajprenal.00522.2015. ajprenal 00522 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang H, Xu D, Toh MF, Pao AC, You G. Serum- and glucocorticoid-inducible kinase SGK2 regulates human organic anion transporters 4 via ubiquitin ligase Nedd4-2. Biochem Pharmacol. 2015;102:120–129. doi: 10.1016/j.bcp.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chothe PP, Swaan PW. Resveratrol promotes degradation of the human bile acid transporter ASBT (SLC10A2) Biochem J. 2014;459:301–312. doi: 10.1042/BJ20131428. [DOI] [PubMed] [Google Scholar]
- 100.Diers AR, Broniowska KA, Chang CF, Hill RB, Hogg N. S-Nitrosation of monocarboxylate transporter 1: inhibition of pyruvate-fueled respiration and proliferation of breast cancer cells. Free Radic Biol Med. 2014;69:229–238. doi: 10.1016/j.freeradbiomed.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]