Abstract
Melatonin is a molecule that can enhance the resistance of plants to abiotic stress. It can alleviate the damage of heavy metal ions, and other chemical substances, changes in temperature and humidity, oxidative stress in higher plants, and enhance resistance of plants to abiotic stress. The transformation of N-Acetyl-5-hydroxy tryptamin into melatonin requires the involvement of methyltransferase. In this study, a methyltransferase gene ZjOMT has been cloned from Zoysia japonica. The gene was induced by aluminum (Al) stress in the leaves and roots of Zoysia japonica, and was up-regulated by 20.86- and 31.18-folds, respectively. The expression of ZjOMT in Escherichia coli increased the content of melatonin by about 8-fold in the recombinant strain compared with that of the empty vector strain. Al resistance test showed that the resistance of recombinant strain BL21-pET32-ZjOMT to Al was significantly higher than that of the empty vector strain BL21-pET32. The survival rate of the recombinant strain expressing ZjOMT was about 100-fold higher than that of the empty vector strain when treated with 0.35 mM Al. These findings suggest that the heterologous expression of ZjOMT improved the resistance of E. coli to Al by increasing the content of melatonin.
Introduction
Melatonin (N-acetyl-5-methoxytryptamine) is an important molecule that belongs to the class of indoles, and is widely used in animals and plants [1]. There were several studies on melatonin mainly in mammals. More in-depth research of melatonin in plants demonstrated its role in physiological regulation, and enhances plant resistance. It has been found that melatonin plays a key role in the defense system of Arabidopsis thaliana [2]. Melatonin can alleviate the damage of heavy metal ions, and other chemical substances, changes in temperature and humidity, oxidative stress in higher plants, and the provide resistance to plants in adverse environments [3,4,5]. For example, melatonin can reduce oxygen under high temperature stress in the production rate of cucumber and H2O2 content, effectively remove reactive oxygen species (ROS). It can also increase the inhibition of cell membrane permeability and MDA content, improve the antioxidant enzyme activity in cucumber leaves (such as SOD, POD, CAT) and the content of soluble protein. So the resistance of cucumber to high temperature stress is improved. Melatonin can also significantly reduce chlorophyll degradation of apple leaves under drought stress, improve leaf photosynthetic efficiency, alleviate oxidative damage, and effectively delay the leaf senescence [6]. Melatonin has the ability to resist ultraviolet stress in algae and higher plants [7]. It can also protect the integrity of the photosynthesis system and increase the chlorophyll content [8]. Exogenous melatonin treatment enhances the ability of plants to resist adversity, such as the significant reduction of germination rate of cucumber seeds under low temperature stress. Melatonin treatment can alleviate this phenomenon, improving the seed germination rate and seedling dry and fresh weight under stress conditions [9]. Exogenous melatonin can also relieve the cold stress of Camellia sinensis [10]. Exogenous addition of 1 μM melatonin effectively alleviates the inhibitory effects of salt stress on the growth of Hubei crabapple, by effectively alleviating chlorophyll degradation, increasing leaf photosynthetic rate and reducing oxidative damage [11,12]. Several studies have demonstrated that exogenous melatonin can significantly improve the survival rate of pea plant in high copper content soil. Studies reported that melatonin can improve the ability of plants to repair soil that is contaminated with heavy metals. The proper concentration of melatonin can effectively slow down Al induced toxicity in soybean [13].
The synthetic precursor of melatonin is tryptophan [14,15]. The production of melatonin from tryptophan involves a four-step continuous enzymatic reaction. Six enzymes are involved in this process including tryptophan decarboxylase (TDC), tryptophan hydroxylase (TPH), tryptamine 5-hydroxylase (T5H), serotonin N-acetyltransferase (SNAT), N-acetylserotonin methyltransferase (ASMT) and caffeic acid O-methyltransferase (COMT). The SNAT and ASMT/COMT are involved in the final two steps of melatonin synthesis [16]. The ASMT and COMT belong to O-methyltransferase family [17]. The COMT of Arabidopsis and rice also catalyzes the production of melatonin by the activity of ASMT [18,19]. Overexpression of MzASMT1, an apple ASMT in Arabidopsis thaliana increased the content of melatonin by 2–4 times [20]. Adverse stress conditions can promote the plant itself to synthesize melatonin. The expression of ASMT in rice could be induced by aging and some stress factors [17]. The melatonin contents in plants under different environmental conditions, especially in stress conditions such as drought, high temperature, oxidative stress, etc. demonstrated great differences [8,21]. The content of melatonin was increased significantly when Aloe vera was cultured at 25°C and then transferred to 4°C, and this suggested that melatonin might be involved in the resistance of plants under low temperature stress conditions [8]. High temperature may induce an increase in melatonin content in Ulva, and exogenous melatonin treatment could improve the resistance of macroalgae to high temperature stress [22]. Light has great effect on the content of endogenous melatonin in plants. The content of melatonin in roots and leaves of outdoor hyacinth that is planted under natural sunlight was 3 to 2.5 times that of the indoor light with relatively weak light intensity [23]. The concentration of melatonin in roots was significantly increased by 50μM Al treatment in soybean [13].
Zoysiagrass (Zoysia japonica Steud.) is commonly found in temperate climate regions and widely used for lawns [24]. Zoysiagrass is the most cold-tolerant grass among the warm-season turfgrasses and is widely cultivated in the world [25]. It has developed stolons and rhizomes, hard and thick subleathery leaves, and the lawn that was built has good elasticity, shear resistance, tolerance, salinity, high temperature and drought resistant properties. Zoysiagrass is also an Al resistant plant of turfgrass. It can also grow well in the acidic soil with high Al content in the south. It is more resistant to Al than many other grasses. We identified an annotated O-methyltransferase gene in the early stage of Al stress in Zoysiagrass transcriptome analysis, which was significantly up-regulated under Al stress conditions. In order to evaluate whether this gene plays a role in melatonin biosynthesis or it is helpful for Al resistance. The study of prokaryotic expression was performed. In this study, the gene was cloned, and a recombinant vector was constructed and expressed in Escherichia coli. The gene expression product and E. coli resistance were detected to explore the role of ZjOMT gene in melatonin synthesis and Al resistance and augment the research theory of melatonin synthesis for applying in the production practically.
Materials and methods
Plant materials and treatments
Using 400 μM AlCl3 solution to irrigate (each pot soil weighing 2 kg) and when the artificial climate indoor pot (at 28°C, 16 h light/ 8 h dark) was cultured for 1 year, Zoysia japonica varieties "Company" was grown up to 20 cm. Twenty plants per pot were planted and similarly three pots were repeated. The plants were treated for 10 consecutive days, and the roots and leaves of each basin were collected, respectively. Total RNA was extracted from leaf and root of Zoysiagrass using TRIzol reagent according to the manufacturer’s instructions (Tiangen, China). The first strand cDNA was synthesized using the Superscript™ III RNase H-Reverse Transcriptase kit (Invitrogen, USA).
Isolation and sequence analysis of ZjOMT
Full-length cDNA sequence of ZjOMT was amplified using gene-specific primers ZjOMT-F: GGCACTGGGCCTGGACCAAGGATT and ZjOMT-R: CGCAGACTTAATATATACGAAGAAAC which were designed according to the Zoysia Genome Database (http://zoysia.kazusa.or.jp/). The PCR product was cloned into the pGEM-T (Promega, USA) and subsequently sequenced.
CDS was predicted by DNAMAN 7 software (Lynnon Corporation). The domain of the deduced ZjOMT protein was analyzed using Conserved Domain Database (CDD) (https://www.ncbi.nlm.nih.gov/cdd). The theoretical pI and molecular weight were predicted by the ProtParam online software (http://web.expasy.org/protparam).
Expression pattern of ZjOMT in Al treated Z. japonica
The cDNA of Leaves and roots of the Al treated Zoysiagrass was used as template, q-ZjOMT-F: TGGTCGCTGATAGCCAAATC and q-ZjOMT-R: GCCGTCACCATTGATACCTT as primers. Zjactin was used as the reference gene. Primers were as follows: q-Zjactin-F: GTGCTTGACTCTGGTGATGGT q-Zjactin-R: GAACCACCAATCCAGACGCTG. qRT-PCR was performed on a Roche LightCycler 480 Real Time PCR System (Roche, Switzerland) in a final volume of 20 μl that contains 2 μl of a 1/10 diluted cDNA template, 10 μl of the 2× SYBR Premix Ex Taq (Takara, Japan) and 1.5 μl (5 mM) of gene-specific forward and reverse primers. Three biological replications and three parallel reactions were performed.
Induction and expression of ZjOMT protein
Specific primers of additional enzyme cutting sites and 3 protective bases were designed according to the ZjOMT CDS sequences: ZjOMT-EcoRI-F: CCGGAATTCATGGCGCTTAGACTCTTAGCGGAAG (EcoR I restriction site underlined) and ZjOMT-HindIII-R: CCCAAGCTTTGGGTAGATCTCAATAACTGAAATGGG (Hind III restriction site underlined). PCR amplification was conducted using the following protocol: pre-denaturation for 5 min at 94°C, 30 cycles at 94°C for 30 s, 58°C for 30 s, 72°C for 30 s, followed by 1 cycle at 72°C for 10 min. Then PCR products and pET32a plasmids were used for double enzyme digestion using EcoR I and Hind III, respectively. The enzyme cut products were separated by agarose gel electrophoresis and after purification of the target fragment, T4 DNA ligase was used to connect the enzyme. The positive clones were obtained after transformation of the linked products. The recombinant plasmid of pET32-ZjOMT was obtained by PCR and sequencing. The recombinant plasmid was reformed using the E. coli BL21 DE3 PlysS expression strain. The pET32a transformed strains were used as control. Positive plaques were obtained after plaque detection by PCR and sequencing. BL21-pET32-ZjOMT recombinant strain and BL21-pET32a empty vector strain were cultivated by oscillating in 50 ml LB medium for 16 h at 37°C, respectively. 1 ml bacterial culture was transferred to 100 ml LB medium (including 0.2 mM IPTG), and then centrifuged to collect bacteria after 4 h. After ultrasonic breakage, SDS-PAGE gel electrophoresis was performed.
N-acetyl-5-methoxytryptamine content test assay
BL21-pET32-ZjOMT recombinant strain and BL21-pET32a empty vector strain were cultivated in the LB medium by shaking culture at 37°C for 16 h, respectively. Then, 1 ml bacteria solution of the 2 strains were transferred to 10 ml fresh LB medium (1 mM IPTG), respectively. Induction and culturing was performed for 6 h, and then centrifuged at 8000 rpm for 5 min. 10μl supernatant samples were collected. Melatonin content was detected by the plant melatonin ELISA detection kit (R&D Systems, USA).
Assay of Al stress tolerance of E. coli transformants
BL21-pET32-ZjOMT recombinant strain and BL21-pET32a empty vector strain were cultivated by oscillating in 50 ml LB medium at 37°C and cultured for 16 h. The LB medium was diluted by 10, 102, 103 and 104 times, respectively. The bacterial solution of 5μl at different concentrations was added to solid 2×GM medium (with 0.2 mM IPTG) containing 0.4 mM AlCl3 and no Al(control),respectively. They were cultured at 37°C for 96 h. Then the growth of the colonies was observed.
Recombinant E. coli growth curve under Al stress: The 20μl of remaining bacterial solution of the above strains was taken, and inoculated into the 48 whole enzyme labelled plates containing 980μl LB liquid medium (including 350 μM AlCl3 and 0.2 mM IPTG). The growth curve was detected in the TECAN infinite M200 PRO enzyme labyrinth (TECAN, Austria) after sealing the membrane. The incubation temperature was set at 37°c, and the detection wavelength was 600 nm. The amplitude of the linear vibration was 6 mm, and the readings were taken every 1 h. Each strain was set up for 6 repetitions, and the growth curve was drawn after culturing for 18 h.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS 19.0) with Student’s t test in a two-tailed manner. Statistical significance was set at P < 0.01.
Results
Cloning and sequence analysis of ZjOMT
To investigate the molecular function of ZjOMT, we obtained its CDS with a length of 1080 bp (Table 1). This gene encodes a protein containing 359 amino acids with a predicted theoretical isoelectric point and molecular weight of 5.52 and 39.026 kDa, respectively.
Table 1. Nucleic acid and amino acid sequences of ZjOMTPosition.
| Sequence | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | atg | gcg | ctt | aga | ctc | tta | gcg | gaa | gtg | agt | cca | cag | gac | ttg | ctt | gta | gct | ctt | acc | gag |
| 1 | M | A | L | R | L | L | A | E | V | S | P | Q | D | L | L | V | A | L | T | E |
| 61 | ttt | cac | aac | cac | atg | ata | ggt | tat | gtc | aag | tca | atg | gcc | ctc | aag | tgc | gcc | gtg | gat | ctt |
| 21 | F | H | N | H | M | I | G | Y | V | K | S | M | A | L | K | C | A | V | D | L |
| 121 | ggc | atc | ccc | gac | gtt | atc | cac | cgc | cgc | ggc | ggt | gca | gcc | acc | ctt | gct | gac | att | gta | act |
| 41 | G | I | P | D | V | I | H | R | R | G | G | A | A | T | L | A | D | I | V | T |
| 181 | gac | act | gcg | gtg | cat | cca | gcc | aag | atc | tcg | gac | ctc | cag | cgc | gtg | atg | gag | ctg | ctt | agc |
| 61 | D | T | A | V | H | P | A | K | I | S | D | L | Q | R | V | M | E | L | L | S |
| 241 | tct | tca | ggt | atg | ttc | agt | act | ggg | gaa | gac | agc | aac | ggt | gct | gtc | atg | tac | agg | tta | aca |
| 81 | S | S | G | M | F | S | T | G | E | D | S | N | G | A | V | M | Y | R | L | T |
| 301 | act | ccg | ggc | cgc | ttc | tta | gtg | ggc | gag | cgc | aat | ctc | tct | ccc | atg | gta | ccg | ttc | ctg | gtg |
| 101 | T | P | G | R | F | L | V | G | E | R | N | L | S | P | M | V | P | F | L | V |
| 361 | agc | cct | ctc | gtc | gtc | tcc | tca | ttc | ttc | agc | atg | agc | gac | tgg | ctt | agg | tgc | gag | ccg | gcg |
| 121 | S | P | L | V | V | S | S | F | F | S | M | S | D | W | L | R | C | E | P | A |
| 421 | gtc | agc | ggc | tct | cta | ttt | gag | ctg | tca | cat | ggc | tgc | cca | cag | tgg | gag | atg | gcg | agc | aag |
| 141 | V | S | G | S | L | F | E | L | S | H | G | C | P | Q | W | E | M | A | S | K |
| 481 | gat | gcc | acg | ttc | aat | aga | ata | ctg | aat | ggc | tcc | atg | gtc | gct | gat | agc | caa | atc | ttc | ctt |
| 161 | D | A | T | F | N | R | I | L | N | G | S | M | V | A | D | S | Q | I | F | L |
| 541 | gaa | gtc | gtc | att | cta | gac | aag | ggc | cat | atc | ttc | cgc | ggg | ttg | aag | tca | ctg | gtt | gat | gtg |
| 181 | E | V | V | I | L | D | K | G | H | I | F | R | G | L | K | S | L | V | D | V |
| 601 | ggt | gga | ggc | cgc | ggc | gcg | gct | gcg | cag | gtc | ctc | gcg | tcc | tcg | ttt | cca | cgc | atc | aag | tgc |
| 201 | G | G | G | R | G | A | A | A | Q | V | L | A | S | S | F | P | R | I | K | C |
| 661 | act | gtc | cta | gac | ctt | ccc | cat | gtg | att | aat | caa | ggt | atc | aat | ggt | gac | ggc | aat | ctg | caa |
| 221 | T | V | L | D | L | P | H | V | I | N | Q | G | I | N | G | D | G | N | L | Q |
| 721 | ttc | gtc | gct | ggc | gac | atg | ttt | gag | tcc | att | cca | cct | gct | gat | gcc | gtc | tta | ctc | aag | aat |
| 241 | F | V | A | G | D | M | F | E | S | I | P | P | A | D | A | V | L | L | K | N |
| 781 | att | ttg | cat | gac | tgg | gct | gat | gat | gat | tgc | atc | aag | att | cta | aaa | cgt | tgc | aag | gaa | gca |
| 261 | I | L | H | D | W | A | D | D | D | C | I | K | I | L | K | R | C | K | E | A |
| 841 | atc | cct | gct | aga | aat | gat | ggg | gga | aaa | gtg | ata | atc | ata | gat | atg | gtg | aga | ggg | tcg | ctc |
| 281 | I | P | A | R | N | D | G | G | K | V | I | I | I | D | M | V | R | G | S | L |
| 901 | cag | gga | aac | aca | aaa | atc | agt | gag | atg | gaa | gcc | tca | cag | aac | ctg | ttc | atg | atg | tcc | atc |
| 301 | Q | G | N | T | K | I | S | E | M | E | A | S | Q | N | L | F | M | M | S | I |
| 961 | aat | ggg | gtg | gaa | cga | ggg | gaa | agt | gaa | tgg | aag | aag | tta | ttt | tcc | gct | gca | gga | ttc | agt |
| 321 | N | G | V | E | R | G | E | S | E | W | K | K | L | F | S | A | A | G | F | S |
| 1021 | gac | aac | tac | aag | atc | atg | caa | ata | ttg | ggt | ccc | att | tca | gtt | att | gag | atc | tac | cca | taa |
| 341 | D | N | Y | K | I | M | Q | I | L | G | P | I | S | V | I | E | I | Y | P | * |
*Nucleic acid sequence is consisting of triplet lowercase letters, while amino sequence is consisting of single uppercase letters.
The ZjOMT amino acid sequence was used to search and analyze the conserved domains in CDD. The gene contains dimerization domain and S-adenosyl methionine binding site that belongs to Ado-Met methyltransferase (Fig 1).
Fig 1. Conserved domains of ZjOMT.
Expression analysis of ZjOMT in Al treated Z. japonica
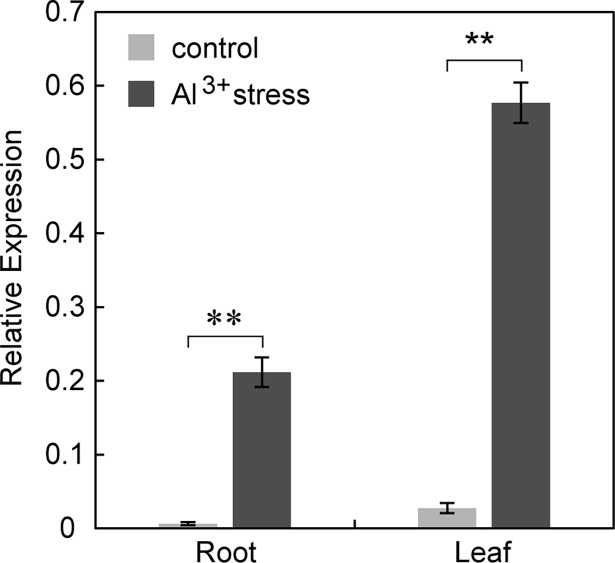
The expression of ZjOMT in Zoysia leaf was about 3-folds higher than that in root. After AlCl3 stress conditions, at 400 μM per kg of soil was treated for 10 d. The expression of ZjOMT in leaves and roots of Zoysiagrass was induced by Al stress and increased by 20.86-fold and 31.18-fold, respectively (Fig 2). The difference is significant (P<0.01).
Fig 2. Expression of ZjOMT in Al treated Z. japonica.
Light grey indicated without Al control, while the dark grey indicated 400 μM Al stress. “**” indicated the difference of P<0.01 in t test.
Induction of fusion protein in recombinant E. coli cells
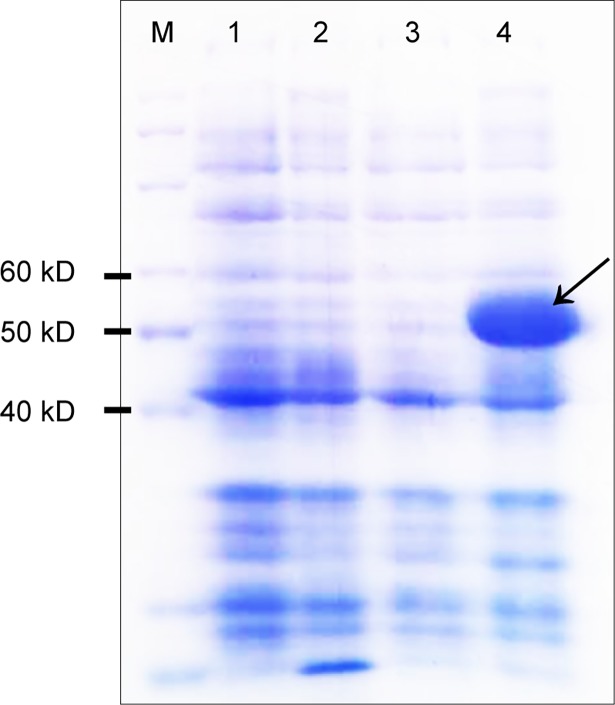
The coding region of ZjOMT gene was amplified with two primers, designed and cloned into the pET-32a(+) plasmid to construct pET32-ZjOMT expression vector to analyze the expression of fusion protein. Results showed that the ZjOMT protein was expressed in Bl21 DE3 PlysS E. coli strain. The total protein of recombinant strains was analyzed by SDS-PAGE. A band of about 58 kD fusion protein was clearly observed in gels, indicating that this vector works normally and ZjOMT protein was expressed in the transformed E. coli BL21 cells after 3 h induction using 0.2 mM IPTG (Fig 3).
Fig 3. Total protein by SDS-PAGE electrophoresis of BL21-pET32a empty vector strain and BL21-pET32-ZjOMT recombinant strain.
Lane 1 showed whole-cell proteins of uninduced empty vector strain of E. coli BL21-pET32a; Lane 2 showed whole-cell proteins of induced empty vector strain of E. coli BL21-pET32a; Lane 3 showed whole-cell proteins of uninduced recombinant strain E. coli BL21-pET32-ZjOMT1; Lane 4 showed whole-cell proteins of induced recombinant strain E. coli BL21-pET32-ZjOMT1; Lane M is protein marker.
ZjOMT overexpression enhanced N-acetyl-5-methoxytryptamine content of E. coli
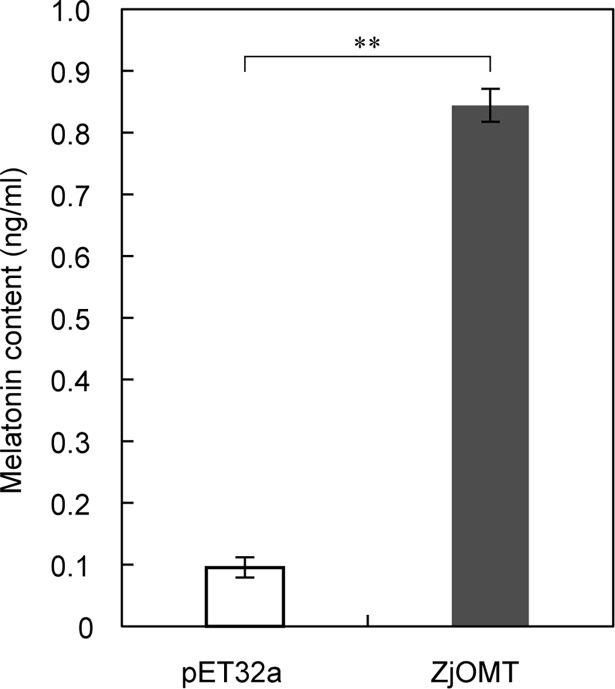
The recombinant strain of BL21-pET32-ZjOMT was induced by 1 mm IPTG for 6 h. The content of melatonin in the bacterial liquid supernatant was significantly higher than that of the empty vector strain, and was about 8-fold that of the empty vector strain (Fig 4). This showed that the ZjOMT gene was involved in the production of melatonin.
Fig 4. Production of melatonin by BL21-pET32-ZjOMT and BL21-pET32a empty vector strains induced by IPTG.
The column chart was presented as mean±SD, white indicated an empty vector strain, the dark grey indicated ZjOMT recombinant strain, “**” indicated the difference of P<0.01 in t test.
ZjOMT overexpression enhanced E. coli growth under Al stress
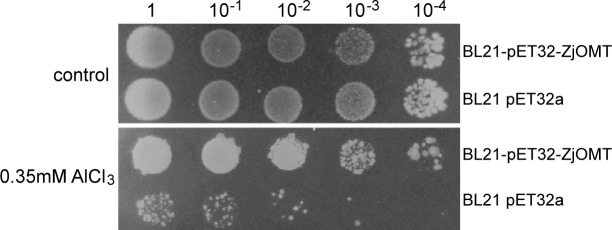
To evaluate the effect of ZjOMT gene induced expression on host bacterial stress resistance, BL21-pET32-ZjOMT recombinant strain and BL21-pET32a empty vector E. coli were treated with Al stress. The growth of the empty vector strain was severely inhibited under Al stress, the survival rate of 100-times-diluted empty vector strain was similar to that of 10000-times-diluted recombinant strain, so the survival rate was about 1/100 of the recombinant strain (Fig 5).
Fig 5. Growth of BL21 -pET32a and BL21-pET32-ZjOMT under Al stress.
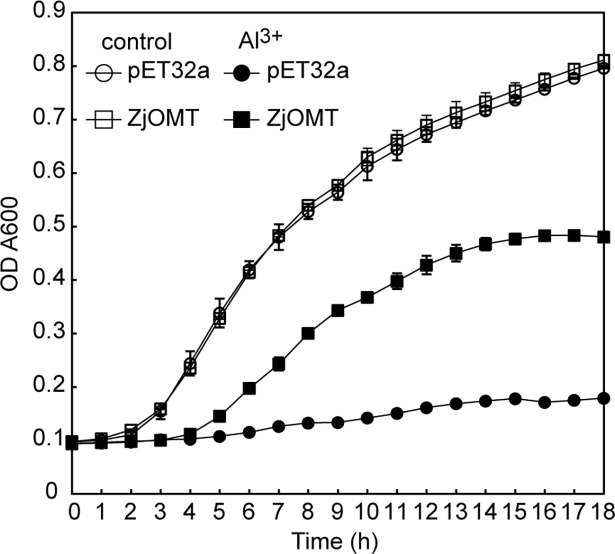
In the control medium without Al, the growth rate of BL21-pET32-ZjOMT recombinant strain and the empty vector strain remained similar. In the medium containing 350 μm AlCl3, the growth rate of the recombinant strain of BL21-pET32-ZjOMT was significantly higher than that of empty vector strain. The reproduction of the empty vector strain was obviously inhibited by Al, and the final concentration of the bacteria was about 1/3 that of the recombinant strain (Fig 6). These results indicated that the expression of ZjOMT gene significantly increased the resistance of E. coli to Al toxicity.
Fig 6. Growth curve of BL21 pET32a and BL21-pET32-ZjOMT under Al stress.
Discussion
Melatonin plays an important role as antioxidant in plants [8,26,27]. Al stress produces oxygen for living. Antioxidation may be an important way for melatonin to resist Al. Melatonin in soybean reduces the toxicity of Al by regulating the antioxidant system [13]. Heterologous expression of ZjOMT in E. coli increased the content of melatonin and enhanced the resistance of E. coli to Al. Heterologous expression of ZjOMT may also reduce the toxicity of Al to E. coli by removing the ROS through melatonin. Therefore, ZjOMT may not only enhance the resistance of Al, but also increase the resistance to other factors such as drought, salt, heavy metal, cold and other adversities of reactive oxygen damage. For example, melatonin can improve the plants’ salt tolerance and promote its growth [28].
In addition to the antioxidative regulation of plant resistance, melatonin also regulates plant resistance by alleviating the expression of related genes. For example, endogenous melatonin of Arabidopsis can induce stress under high temperatures, resulting in the continuously increased content, while exogenous melatonin pretreatment can improve plant tolerance to high temperatures, where high temperature environment and the expression of exogenous melatonin induces the transcription factor HSFA1. This in turn activates the downstream gene, providing high tolerance to high temperatures in Arabidopsis [29]. Melatonin acts as not only a pineal hormone, but also an important signaling substance of functioning in animals [30], but also as a signaling substance in plants to regulate plant resistance. Melatonin acts as not only an antioxidant, but also regulates the resistance of plants by regulating the expression of other genes in the process of resistance.
The proper concentration of melatonin can effectively slow down the Al-induced phytotoxicity in soybean [13]. High melatonin content inhibited plant growth. Melatonin was used to treat the stem segments of Polygonum cuspidatum in vitro, and 3 μM and 6 μM melatonin demonstrated significant effects on the growth of the established stem segments of Polygonum cuspidatum and seedling growth of Polygonum cuspidatum. When the concentration was 6 μM, the height of the seedlings was increased by 87.5% and the leaf area was increased by 67.5%. However, when the concentration reached 24 μM, the growth of the seedlings was inhibited. The growth rate of Scutellaria amoena callus was more than 1 time higher than that of the control by 0.1 μM exogenous melatonin treatment, while 100.0 μM inhibited the proliferation of callus and differentiation of adventitious buds [31]. It was considered that the content of melatonin also has a dose effect in the resistance to Al stress of Zoysiagrass, and only an appropriate amount can better play the role of resistance. Further research on these points is warranted.
COMT is a multifunctional enzyme with ASMT activity. Rice COMT catalyzes the transformation of serotonin to melatonin, while rice ASMT1 showed no obvious conversion of serotonin to melatonin in E. coli [32]. There was a great difference between the types of catalytic substrates and the activity of enzymes in the O-methyltransferase family, and the ability to catalyze the production of melatonin was different. The expression of ZjOMT in E. coli significantly increased the production of melatonin, indicating that it has a considerable catalytic activity in the conversion of serotonin to melatonin.
Conclusions
In this study, we showed that overexpression of ZjOMT increases the content of melatonin and the resistance to Al stress in E. coli. Therefore, our findings reveal that ZjOMT can be a useful target of genetic engineering for improving the tolerance of plants to Al stress.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 31601989 and No. 31672477). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kolár J, Machácková I. Melatonin in higher plants: occurrence and possible functions. J Pineal Res. 2005; 39(4): 333–341. doi: 10.1111/j.1600-079X.2005.00276.x [DOI] [PubMed] [Google Scholar]
- 2.Weeda S, Zhang N, Zhao X, Ndip G, Guo Y, Buck GA, et al. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE. 2014, 9(3): e93462 doi: 10.1371/journal.pone.0093462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, et al. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J Exp Bot. 2011; 63(2): 577–597. doi: 10.1093/jxb/err256 [DOI] [PubMed] [Google Scholar]
- 4.Janas KM, Posmyk MM. Melatonin, an underestimated natural substance with great potential for agricultural application. Acta Physiol Plant. 2013; 35(12): 3285–3292. https://doi.org/10.1007/s11738-013-1372-0 PMID: 5661470 [Google Scholar]
- 5.Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot. 2014; 66(3): 695–707. doi: 10.1093/jxb/eru392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang P, Sun X, Li C, Wei Z, Liang D, Ma F. Long-term exogenous application of melatonin delays drought- induced leaf senescence in apple. J Pineal Res. 2013; 54(3): 292–302. doi: 10.1111/jpi.12017 [DOI] [PubMed] [Google Scholar]
- 7.Lazar D, Murch S J, Beilby MJ, Al Khazaaly S. Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal Behav. 2013; 8(3): e23279 doi: 10.4161/psb.23279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR. Significance of melatonin in antioxidative defense system: reactions and products. Biol Signals Recept. 2000;9(3–4): 137–159. doi: 10.1159/000014635 [DOI] [PubMed] [Google Scholar]
- 9.Malgorzata M, Posmyk M, Bałabusta M, Wieczorek M, Sliwinska E, Janas KM. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J Pineal Res. 2009; (46): 214–223. doi: 10.1111/j.1600-079X.2008.00652.x [DOI] [PubMed] [Google Scholar]
- 10.Li X, Wei J P, Scott E R, Liu JW, Guo S, Li Y, et al. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules. 2018; 23(1): 165 doi: 10.3390/molecules23010165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Wang P, Wei Z, Liang D, Liu C, Yin L, et al. The mitigation effects of exogenous melatonin on salinity-inducedstressin Malus hupehensis. J Pineal Res. 2012; 53(3): 298–306. doi: 10.1111/j.1600-079X.2012.00999.x [DOI] [PubMed] [Google Scholar]
- 12.Tan DX, Manchester LC, Helton P, Reiter RJ. Phytoremediative capacity of plants enriched with melatonin. Plant Signal Behav. 2007; 2(6): 514–516. https://doi.org/10.4161/psb.2.6.4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Zeng B, Mao Y, Kong X, Wang X, Yang Y, et al. Melatonin alleviates aluminium toxicity through modulating antioxidative enzymes and enhancing organic acid anion exudation in soybean. Funct Plant Biol. 2017; 44(10): 961–968. https://doi.org/10.1071/FP17003 [DOI] [PubMed] [Google Scholar]
- 14.Facchini PJ, Huber-Allanach KL, Tari LW. Plant aromatic L-amino acid decarboxylases: evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry. 2000; 54 (2): 121–138. https://doi.org/10.1016/S0031-9422(00)00050-9 [DOI] [PubMed] [Google Scholar]
- 15.Van Tassel DL O'neill SD. Putative regulatory molecules in plants: evaluating melatonin. J Pineal Res. 2001; 31(1): 1–7. https://doi.org/10.1034/j.1600-079X.2001.310101.x [DOI] [PubMed] [Google Scholar]
- 16.Back K, Tan DX, Reiter RJ. Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J Pineal Res. 2016; 61(4): 426–437. doi: 10.1111/jpi.12364 [DOI] [PubMed] [Google Scholar]
- 17.Kang K, Kong K, Park S, Natsagdorj U, Kim YS, Back K. Molecular cloning of a plant N-acetylserotonin methyltransferase and its expression characteristics in rice. J Pineal Res. 2011; 50(3): 304–309. doi: 10.1111/j.1600-079X.2010.00841.x [DOI] [PubMed] [Google Scholar]
- 18.Byeon Y, Lee HY, Lee K, Back K. Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J Pineal Res. 2014; 57(2): 219–227. doi: 10.1111/jpi.12160 [DOI] [PubMed] [Google Scholar]
- 19.Byeon Y, Choi GH, Lee HY, Back K. Melatonin biosynthesis requires N-acetylserotonin methylTransferase activity of caffeic acid O-methyltransferase in rice. J Exp Bot. 2015; 66(21): 6917–6925. doi: 10.1093/jxb/erv396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuo BX, Zheng XD, He PL, Wang L, Lei Q, Feng C, et al. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J Pineal Res. 2014; 57: 408–417. doi: 10.1111/jpi.12180 [DOI] [PubMed] [Google Scholar]
- 21.Zhang HJ, Zhang NA, Yang RC, Wang L, Sun QQ, Li DB, et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pineal Res. 2014; 57(3): 269–279. doi: 10.1111/jpi.12167 [DOI] [PubMed] [Google Scholar]
- 22.Tal O, Haim A, Harel O, Gerchman Y. Melatonin as an antioxidant and its semi-lunar rhythm in green macroalga Ulva sp. J Exp Bot. 2011; 62(6): 1903–1910. doi: 10.1093/jxb/erq378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan DX, Manchester LC, Di Mascio P, Martinez GR, Prado FM, Reiter RJ. Novel rhythms of N1-acetyl-N2-formyl -5-methoxykynuramine and its precursor melatonin in water hyacinth: importance for phytoremediation. FASEB J. 2017; 21(8): 1724–1729. doi: 10.1096/fj.06-7745com [DOI] [PubMed] [Google Scholar]
- 24.Ahn JH, Kim JS, Kim S, Soh HY, Shin H, Jang H, et al. De novo transcriptome analysis to identify anthocyanin biosynthesis genes responsible for tissue-specific pigmentation in Zoysiagrass (Zoysia japonica Steud.). PLoS ONE. 2017; 10 (4): e0124497 doi: 10.1371/journal.pone.0124497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xuan J, Song Y, Zhang H, Liu J, Guo Z, Hua Y. Comparative proteomic analysis of the stolon cold stress response between the C4 perennial grass species Zoysia japonica and Zoysia metrella. PLoS ONE. 2013; 8 (9): e75705 doi: 10.1371/journal.pone.0075705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiter RJ, Tan DX. Melatonin: an antioxidant in edible plants. Ann N Y Acad Sci. 2002; 957: 341–344. https://doi.org/10.1111/j.1749-6632.2002.tb02938.x [DOI] [PubMed] [Google Scholar]
- 27.Nawaz MA, Huang Y, Bie Z, Ahmed W, Reiter RJ, Niu M, et al. Melatonin: current status and future perspectives in plant science. Front Plant Sci. 2002; 6(1230). doi: 10.3389/fpls.2015.01230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang C, Zheng G, Li W, Wang Y, Hu B, Wang H,et al. Melatonin delays leaf senescence and enhances salt stress tolerance in Rice. J Pineal Res. 2015; 59(1): 91–101. doi: 10.1111/jpi.12243 [DOI] [PubMed] [Google Scholar]
- 29.Shi H, Tan D, Reiter RJ, Ye T, Yang F, Chan Z. Melatonin induces class A1 heat-shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J Pineal Res. 2015; 58(3): 335–342. doi: 10.1111/jpi.12219 [DOI] [PubMed] [Google Scholar]
- 30.Gall CV, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002; 309(1): 151–162. doi: 10.1007/s00441-002-0581-4 [DOI] [PubMed] [Google Scholar]
- 31.Zhang LJ, Jia JF. Effect of Melatonin on the proliferation and differentiation of calli of Scutellaria amoena. North Hortic. (in Chinese) 2013; (8): 106–109. [Google Scholar]
- 32.Byeon Y, Back K. Melatonin production in Escherichia coli by dual expression of serotonin N-acetyltransferase and caffeic acid O-methyltransferase. Appl Microbiol Biotechnol. 2016; 100(15): 6683–6691. doi: 10.1007/s00253-016-7458-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.