Abstract
The extracellular matrix is a biologically critical entity that has historically been poorly understood. Here we discuss how new tools for characterizing matrix composition and function enable us to design and deliver advanced matrices in vitro, to optimize regeneration, and in vivo, within a variety of tissues and organs.
Essential Functions of Matrix
Cell biology is experiencing an evolving paradigm shift. The central dogma of biology states that DNA is transcribed into RNA, which in turn is translated into a resulting protein product. These intra-cellular events have been the focal point of much of the work in molecular and developmental biology. But while we were paying attention to the events inside of cells and on cell surfaces, we were missing a big part of the picture: the extracellular matrix (ECM). We are just beginning to understand the profound impact of the ECM on stem cell differentiation and tissue repair and regeneration. Here we discuss some of the evidence supporting the role for matrix in tissue regeneration. We also discuss how new tools for investigating matrix composition and function will enable us to design more advanced matrices to aid stem cell-based regeneration in a wide range of tissues and organs.
Adherent cells, along with their close neighbors, secrete a variety of matrix molecules that can drive stemness, differentiation, survival, and motility. Our ability to exploit ECM to drive tissue renewal will depend on our understanding of both the quantitative composition and the biological function of matrix elements, both individually and as combinations. We have already observed that administration of tissue-homologous extracellular matrices can drive connective tissue regeneration in vivo, sometimes with improved outcomes as compared with regeneration using non-homologous matrices (Lawson et al., 2016; Weber et al., 2014). As we learn more about organ-specific matrix composition and functionality, we may be able to rationally modify matrices to drive desired regenerative outcomes in vitro and in vivo. Eventually, we may even generate completely synthetic matrices that can act as agents for directing tissue restoration in humans.
Matrix in Regenerative Medicine
Relationship of Matrix and Stem Cells
It is interesting to note that many surface markers of stem and progenitor cells are actually matrix binding proteins. For example, CD49a–f markers, which are expressed on basal epithelial progenitor cells as well as tumor stem cells, correspond to integrin α subunits 1–6 (Naba et al., 2016). CD29, which is expressed by neural stem cells and is lost upon differentiation to neurons, is the integrin β1. Similarly, Lgr5 is a receptor for R-spondin molecules in the matrix and is expressed by intestinal stem cells as well as colorectal adenomas (Naba et al., 2016).
Specific matrix glycoproteins are also known to impact stemness. Laminin-421, consisting of α4, β2, and γ1 chains, is known to influence hematopoietic stem and progenitor cell cycling and homing in the bone marrow (Susek et al., 2018). The hematopoietic stem cell niche contains a cluster of glycoproteins and small collagens, including fibronectin, tenascin-C, and collagens IV and VI. The mechanical properties of matrix are also important for driving or preventing stem cell differentiation. For example, many laboratories have shown that increased stiffness can drive fibroblasts toward a myofibroblastic phenotype, while soft substrates (<100 kPa in stiffness) help maintain ESC and iPSC pluripotency.
Matrix for Repair of Connective Tissues
For all connective tissues, it is the tissue-specific fibrillar collagens, elastins, and hydrated glycosaminoglycans that provide their mechanical, load-bearing properties, which far exceed those provided by the tissue-specific cells themselves (Table 1). Hence, it is not surprising that acellular, processed collagenous matrices can be efficacious in repairing various load-bearing tissues. For example, porcine intestinal submucosa and decellularized human or porcine skin have been utilized for reconstruction of connective tissues in hundreds of thousands of patients (Rastegarpour et al., 2016). Connective tissues that have been replaced include fascia, ligament, trachea, esophagus, blood vessel, and bladder. Because many matrix-based products have been approved through a Food and Drug Administration 510(k) process (Weber et al., 2014), we have limited published clinical trial information regarding their capacity to regenerate connective tissues in situ. However, some published data points to inefficient (and sometimes failed) repair, when a non-homologous matrix is utilized to replace a given tissue (Rastegarpour et al., 2016).
Table 1.
Roles of Extracellular Matrix Constituents
| ECM Constituents | Function | Role | Examples |
|---|---|---|---|
| Fibrillar collagens | resist tensile and shearing forces | primarily mechanical | collagen I and III |
| Elastin | provide recoil and tissue shape memory | primarily mechanical | n/a |
| Proteoglycans | resist compressive forces, provide some recoil | primarily mechanical | dermatan, heparan, chondroitin sulfates |
| Small leucine-rich repeat proteins (SLRPs) | matrix fiber assembly | cell- and matrix-interactive | asporin, byglycan, lumican |
| Non-fibrillar collagens | basement membrane and cartilage | cell- and matrix-interactive | collagens II and IV |
| Smaller collagens | bind cells to other matrix molecules | cell- and matrix-interactive | collagens V, VI, and XII |
| Basement membrane glycoproteins | bind cells to substrate | cell- and matrix-interactive | laminin, nidogen, fibronectin |
| Growth factor binding proteins | modulate bioavailability | cell- and matrix-interactive | TGFβ and IGF |
Primarily mechanical roles are typically widely distributed and less idiotypic. Cell- and matrix-interactive roles are typically more cell idiotypic.
Tissue-Specific Matrix May Lead to More Efficient Regeneration
Non-homologous Matrix for Repair
The repair of large abdominal hernias sometimes requires augmentation of the abdominal fascia. Both acellular skin matrix (human) and acellular intestinal submucosa ECM (porcine) have been studied in this setting (Rastegarpour et al., 2016). When these materials are used for fascial repair, up to one-third of hernia patients suffer complications. Mechanical failure of the implanted ECM, due primarily to inadequate repopulation of the foreign matrix material, is the cause of most of the clinical complications. Since abdominal fascia is derived from skeletal muscle, it is perhaps not surprising that ECM derived from either dermis or small intestine does not provide the correct biological signals for proper host cell repopulation. Similarly, porcine intestinal submucosa has been studied in the vascular system as a carotid artery patch (Weber et al., 2014). But xenogeneic ECM can undergo slow dilatation and mechanical failure over a period of months in the arterial setting, sometimes accompanied by substantial ECM destruction by host macrophages.
Vascular Matrix in Arterial Regeneration
In contrast, implanting a homologous, human vascular matrix into the arterial system may meet with better outcomes. Recent tissue engineering techniques have generated macroscopic blood vessels that are many centimeters in length. The protocol first involves culturing differentiated human vascular smooth muscle cells in vitro (Lawson et al., 2016). Decellularization of these engineered vessels results in an acellular ECM, which is likely idiotypic for arterial smooth muscle cells. Following implantation, within 3–12 months the acellular vessels become repopulated with host cells that initially express the monocyte marker CD68+, but which are negative for other leukocyte antigens and may represent a local mesenchymal progenitor. After 12 months, cells repopulating the vessel wall uniformly express smooth muscle α-actin, and the luminal surface repopulates with CD31+ endothelial-like cells. Hence, the human arterial ECM becomes repopulated with tissue-appropriate vascular cells in vivo, likely under the influence of arterial-specific matrix elements residing within the smooth muscle cell-derived construct. Importantly, these vessels do not appear to suffer from high rates of mechanical failure, implying that tissue-appropriate ECM may be superior for long-term function as compared with ECM derived from non-homologous sources.
Myocardial Matrix
Myocardium is another example of a connective tissue that may be restored by the application of organ-specific matrix (Wassenaar et al., 2016). ECM isolated from decellularized adult myocardium contains a mixture of cardiac-specific basement membrane proteins and glycoproteins, and it can be processed into a hydrogel that is suitable for injection. When injected into infarcted rat myocardium 1 week after injury, cardiac ECM hydrogel results in decreased myocyte apoptosis, enhanced neovascularization, and diminished fibrosis as compared with saline injections. Furthermore, there is increased expression of primitive myocardial markers including GATA4, Nkx2.5, MEF2d, and myocardin in the vicinity of the injected cardiac matrix. These results imply that cardiac-idiotypic ECM aids in recruitment of more primitive cells that may promote repair. Currently, this cardiac matrix is undergoing phase I studies in patients who have previously suffered a myocardial infarction (NCT02305602).
Decellularized Tracheas
While connective tissue matrix can be a potent tool for regeneration, success is not universal. Decellularized human airways that were used for surgical reconstruction of tracheas have failed in multiple patients, leading to significant morbidity and mortality. These clinical failures may be attributed to insufficient consideration of the crucial functional criteria for large airway replacements: suitable mechanical characteristics and sufficient cell host infiltration to protect the airway from infection (see http://www.sciencemag.org/news/2016/03/karolinska-institute-fires-fallen-star-surgeon-paolo-macchiarini). Despite using a “tissue appropriate” matrix, the failure of investigators to consider other key functional aspects of the tracheal connective tissue led to poor clinical outcomes.
New Tools for Driving Matrix Science
Quantitative ECM Proteomics
The fundamental reason that our under-standing of ECM has lagged behind our understanding of intracellular processes has been our lack of tools for reliable quantification of ECM components. Conventional ECM isolation methods that use only detergents or chaotropes result in insoluble protein pellets, which contain most of the cross-linked matrix, but which are typically excluded from proteomic analysis. Vigorous solubilization protocols that utilize detergents, urea, cyanogen bromide, and quantitative trypsinization can now successfully solubilize most ECM components (Johnson et al., 2016). Furthermore, synthetic homeotypic peptides that are specific to one matrix protein and can be synthesized in bacteria using 13C can now provide quantitative standards for Liquid chromatography tandem mass spectrometry. Such 13C homeotypic peptides are often consensus sequences across species, making these reagents especially powerful tools. By using such next-generation proteomics approaches, it is now possible to objectively quantify the retention of native ECM components in various ECM compositions and decellularized matrices. Now we are able to determine the fidelity of ECM matrices compared to their native tissue counterparts. This type of next-generation proteomics methodology will transform our understanding of the bioactivity of tissue and organ matrix both in vivo and in vitro.
Emerging Matrisome Database
Recently, investigators have introduced a web interface dubbed “the Matrisome Project” that hosts novel databases of ECM components (http://matrisomeproject.mit.edu). This database—also known as MatrisomeDB—compiles in silico and experimental results and datasets, including proteomics and gene expression information, into a single unified system. The in silico definition of the Matrisome relies on the interrogation of protein databases including UniProt, InterPro, and SMART and then relates these proteins to genes using NCBI Entrez Gene. As these databases mature, our understanding of the correlations between ECM constituents and bioactivity or disease states will undoubtedly improve. Thereafter, testing mechanistic hypotheses regarding matrix components will be possible.
Implications for the Future of Regenerative Medicine
Matrix Generated from Solid Organs
The previous 10 years have witnessed a proliferation of techniques for the decellularization of solid organs, including the heart, kidney, liver, lung, etc. Techniques for performing decellularization and for assessing the adequacy of cell removal and ECM preservation have likewise proliferated, making inter-laboratory comparisons difficult. Because we are only just beginning to gather quantitative information on these cross-linked ECM mixtures, our level of understanding regarding solid organ ECM still remains at an early stage.
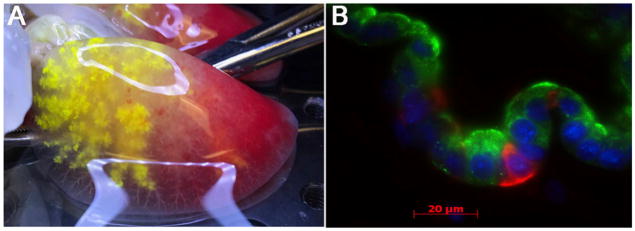
Careful decellularization of solid organs can produce a matrix that is hospitable for repopulation with homologous, and sometimes non-homologous, cells (Li et al., 2016). As an example, despite relatively harsh decellularization using the detergent SDS, acellular liver matrix retains some 24 relatively liver-specific ECM proteins, including asporin, dermatopontin, fibulin, lumican, and collagens V, VI, and XIV. These idiotypic ECM elements, either individually or in combination, provide enhanced adhesion and survival of cultured hepatocytes compared with that provided by non-liver-derived materials. Similar outcomes have been observed using mixed populations of respiratory epithelium to repopulate acellular lung matrices. Repopulation of rodent lung matrices with an array of epithelial cell types leads to region-specific cell adhesion as well as cellular organization (Figure 1), implying that the acellular matrix may retain “zip codes” that inform adhesion (Petersen et al., 2010). These observations mean that endogenous organ ECM will be important as regenerative medicine strategies—either cell therapies or whole-organ engineering—continue to evolve in the coming years.
Figure 1. Acellular Organ Matrix Maintains Structure and Drives Cell Adhesion.
(A) Acellular matrix from adult rat lung shows alveolar barrier integrity, with retention of yellow micro-particles that are injected into the airway.
(B) Mixed neonatal rat pulmonary epithelium that is seeded onto an acellular lung scaffold organizes to form airway structures containing cells expressing clara cell secretary protein (green) as well as keratin5, a basal cell marker (red).
Functional Proteomics
Fully understanding the regenerative power of the ECM depends on not only our understanding of its quantitative composition, but also its biological functionality. The evolving field of functional proteomics has previously been primarily applied to intracellular protein interactions, though some of these techniques will be powerful for the study of ECM (Wasik and Schiller, 2017). Affinity-purification and cross-linking mass spectrometry (AP-MS and XL-MS, respectively) will enable us to study the interactions of matrix molecules more precisely. Indeed, XL-MS can cross-link and identify closely apposed ECM proteins in situ, thereby providing high-fidelity information on protein-protein interactions occurring within the ECM. In situ cross-linking approaches, followed by matrix isolation and purification, may allow us to characterize the biological functions of collections of matrix molecules, rather than the effects of single proteins. The evolution of ECM proteomics strategies, combined with high-throughput assays for cellular behavior, may pave the way for “tailor-made” ECMs possessing the desired regenerative properties. Hence, an encyclopedic understanding of the biological and mechanical roles of the several hundred existing matrix elements may be within our grasp in the next couple of decades. Understanding the regenerative roles of individual and clustered groups of ECM molecules may then allow synthetic chemistry, 3D printing, and other technologies to make designer ECMs that can direct stem cell fate and tissue renewal.
Conclusions
Aided by new tools in quantitative proteomics, decellularization, and the analysis of large datasets, we are rapidly learning about the crucial nature of the ECM. It is anticipated that we will continue to see an increased use of matrix in regenerative medicine to drive stem cell differentiation and to reconstitute increasingly complex tissues and organs. It is clear that the role for ECM extends beyond mechanics, and that local ECM environments are highly cell specific, with tremendous biological instruction capacity for adherent cells. Therefore, stem cell biology and regenerative medicine will advance more rapidly if we deepen our understanding of the matrix cues that support cellular, tissue, and organ function.
Acknowledgments
This work was supported by R01 HL127386 and by CT Innovations 15-YMB-YALE-07, both to L.E.N.. L.E.N. is a Nicholas M. Greene Professor and Vice-Chair.
Footnotes
DECLARATION OF INTERESTS
L.E.N. is a founder and shareholder in Humacyte, Inc., which is a regenerative medicine company. Humacyte produces engineered blood vessels from allogeneic smooth muscle cells for vascular surgery. L.E.N.’s spouse has equity in Humacyte, and L.E.N. serves on Humacyte’s Board of Directors. L.E.N. is an inventor on patents that are licensed to Humacyte and produce royalties for L.E.N.; patent numbers and associated patents include the following: 19,657,265, Tubular tissue-engineered constructs; 29,650,603, Tissue-engineered constructs; 39,556,414, Tissue-engineered constructs; 49,127,242, Tissue and organ graft bioreactor and method of operation; 58,198,245, Compositions and methods for soft tissue augmentation; 67,993,567, Method and system for aligning fibers during electrospinning; 77,943,378, Tissue engineering; 87,498,332, Therapy for cerebral vasospasm; 96,962,814, Decellularized tissue engineered constructs and tissues; and 106,537,567, Tissue-engineered tubular construct having circumferentially oriented smooth muscle cells. L.E.N. has received an unrestricted research gift to support research in her laboratory at Yale. Humacyte did not fund these studies, and Humacyte did not influence the conduct, description, or interpretation of the findings in this report.
References
- Johnson TD, Hill RC, Dzieciatkowska M, Nigam V, Behfar A, Christman KL, Hansen KC. Quantification of decellularized human myocardial matrix: A comparison of six patients. Proteomics Clin Appl. 2016;10:75–83. doi: 10.1002/prca.201500048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JH, Glickman MH, Ilzecki M, Jakimowicz T, Jaroszynski A, Peden EK, Pilgrim AJ, Prichard HL, Guziewicz M, Przywara S, et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. 2016;387:2026–2034. doi: 10.1016/S0140-6736(16)00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Uygun BE, Geerts S, Ozer S, Scalf M, Gilpin SE, Ott HC, Yarmush ML, Smith LM, Welham NV, Frey BL. Proteomic analysis of naturally-sourced biological scaffolds. Biomaterials. 2016;75:37–46. doi: 10.1016/j.biomaterials.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastegarpour A, Cheung M, Vardhan M, Ibrahim MM, Butler CE, Levinson H. Surgical mesh for ventral incisional hernia repairs: Understanding mesh design. Plast Surg (Oakv) 2016;24:41–50. doi: 10.4172/plastic-surgery.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek KH, Korpos E, Huppert J, Wu C, Savelyeva I, Rosenbauer F, Muller-Tidow C, Koschmieder S, Sorokin L. Bone marrow laminins influence hematopoietic stem and progenitor cell cycling and homing to the bone marrow. Matrix Biology. 2018 doi: 10.1016/j.matbio.2018.01.007. Published online January 19, 2018. https://doi.org/10.1016/j.matbio.2018.01.007. [DOI] [PMC free article] [PubMed]
- Wasik AA, Schiller HB. Functional proteomics of cellular mechanosensing mechanisms. Semin Cell Dev Biol. 2017;71:118–128. doi: 10.1016/j.semcdb.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Wassenaar JW, Gaetani R, Garcia JJ, Braden RL, Luo CG, Huang D, DeMaria AN, Omens JH, Christman KL. Evidence for mechanisms underlying the functional benefits of a myocardial matrix hydrogel for post-MI treatment. J Am Coll Cardiol. 2016;67:1074–1086. doi: 10.1016/j.jacc.2015.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SS, Annenberg AJ, Wright CB, Braverman TS, Mesh CL. Early pseudoaneurysm degeneration in biologic extracellular matrix patch for carotid repair. J Vasc Surg. 2014;59:1116–1118. doi: 10.1016/j.jvs.2013.05.012. [DOI] [PubMed] [Google Scholar]