Abstract
Systemic lupus erythematosus is a debilitating autoimmune disease in which autoantibodies and autoreactive T cells destroy kidneys and other organs. Disease is clinically and genetically heterogeneous, suggesting that underlying mechanisms vary between patients. We previously used an autoantibody transgenic mouse reporter system to examine the effect of different autoimmune backgrounds on B cell tolerance, failure of which is a fundamental defect in lupus. We identified a defect consistent with reversible anergy induced by endotoxin stimulation of B cells from Ig transgenic New Zealand Black (NZB) mice. Herein we report that the tolerance defect is revealed by TLR7 and TLR9 as well as TLR4 ligands, with additive effect, and is partially reversed by Mek inhibition. Gene expression analysis reveals significant differences in transcription of multiple TLR pathway genes and ptpn22 in stimulated NZB compared to B6 B cells. Additionally, the defect is detected in Ig transgenic NZB F1 hybrid strains (NZBxNZW)F1 and (B6xNZB)F1. These results implicate an inherited defect wherein NZB anergic B cells maintain coordinated TLR/BCR signaling that permits autoantibody production. Agents targeting these pathways may have therapeutic benefit in the subset of lupus patients that manifest similar defects in B cell regulation.
Keywords: anergy, autoimmunity, animal models, B cells, gene expression
Introduction
Systemic lupus erythematosus (SLE) is one of the most debilitating autoinflammatory diseases, due in part to renal failure and accelerated atherosclerosis. The fundamental defect involves escape of autoreactive B and T cells from normal regulation, with production of autoantibodies (autoIg) that deposit in multiple organs. Disease can often be controlled using broad immunosuppressants, but these are nonspecific and fraught with complications. Rational design of urgently needed mechanism-based interventions requires better understanding of disease initiation and pathogenesis.
Discovery systems must consider the phenotypic heterogeneity of SLE. Manifestations can vary widely due to the complex genetic and environmental susceptibility that underlies lupus [1]. Multiple disease-associated loci and genes have been identified, with different constellations expressed in different families and patients. In our search for genetic modifiers of tolerance checkpoints and related biologic pathways that may provide therapeutic targets tailored to subsets of SLE patients, we developed a mouse Ig transgenic (Tg) reporter system within the context of four classic inbred lupus strains: New Zealand Black (NZB), BXSB/MpJ (BXSB), MRL/MpJ (MRL), and (NZBxNZW)F1 (BWF1). Each strain develops spontaneous lupus with nephritis due to multiple susceptibility loci, and is genetically distinct. Thus, they collectively model the genetic heterogeneity of human SLE. In the reporter system, each strain carries an Ig Tg that is crossreactive with DNA and laminin, a protein expressed in kidney basement membranes. In non-autoimmune C57BL/6 (B6) mice, B cells expressing the autoIg Tg are stringently regulated by deletion, editing, and anergy [2, 3], mechanisms that also regulate autoreactive B cells in man [4–6], whereas expression of the autoIg Tg in autoimmune strains revealed strain-specific tolerance defects [7]. Thus, this multistrain Ig Tg reporter system is a suitable platform for systematic, mechanistic dissection of autoimmune strain influences on B cell tolerance, and on autoIg production triggered by superimposed environmental factors.
The most striking tolerance deviation was observed in NZB mice, which develop severe autoimmune hemolytic anemia and late onset nephritis. NZB carry major susceptibility genes that contribute to fulminant nephritis in F1 hybrids [8]. Ig Tg NZB mice produce autoIg Tg B cells that are regulated by deletion, editing, and anergy, similar to their Tg B6 counterparts [7]. Whereas murine models generally display B cell hyperactivity, NZB Tg B cells are unique in their production of high levels of Tg autoIg after in vitro stimulation with lipopolysaccharide (LPS), a ligand for Toll-like receptor (TLR) 4 [7]. LPS also induces Tg autoIg production in vivo in a subset of Tg NZB mice. These findings are consistent with results of Wither and colleagues using a different NZB autoIg Tg model [9], suggesting that abnormal TLR ligand-reversible B cell anergy is a key defect that contributes to autoimmunity in NZB.
In contrast to results from Ig Tg NZB, initial studies using B cells from Ig Tg BWF1 mice (mean age 3.4 months) showed only low levels of Tg autoIg after LPS stimulation [7]. This was surprising in that BWF1 develop autoimmune hemolytic anemia of comparable severity and onset as NZB, and develop anti-dsDNA/anti-chromatin IgG and severe nephritis [10], suggesting that BWF1 and NZB have similar defects in regulation. To better understand B cell regulation in F1 hybrids relative to parental NZB, we explored additional Tg BWF1 mice of an older age range, as well as Tg (B6xNZB)F1 hybrids. Herein we report that the reversible anergy phenotype is present in both NZB F1 hybrids. Moreover, autoIg secretion by NZB Tg B cells can be induced by TLR7 and TLR9, as well as by TLR4, ligands, with additive effect. Altered expression of TLR signaling components in NZB B cells and partial reversal of the phenotype by blockade of MAPK signaling suggest that the inherited NZB defect eases the brake on BCR/TLR signaling that normally maintains anergy in healthy B cells.
Results and Discussion
Reversible anergy is detected in multiple NZB F1 hybrids
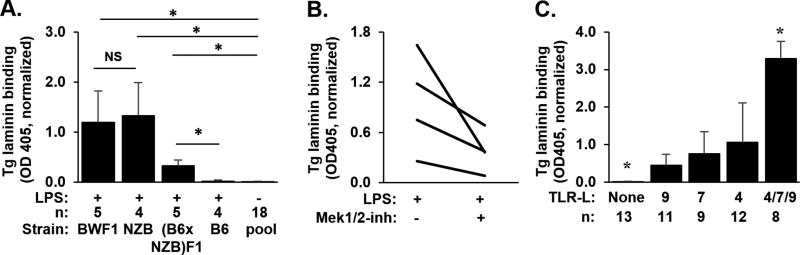
To determine the heritability of the NZB B cell defect, we assessed B cells from two strains of Ig Tg NZB F1 progeny. In a cohort of 6–10 month old Tg BWF1 mice, older than those studied in our initial report [7], we detect the LPS-exposed defect: Tg autoIg are present at high levels in LPS-stimulated B cell supernatants (Figure 1A). Like the parental Ig Tg NZB strain previously described [7], traditional indicators of a tolerance phenotype are present in this older cohort: Tg anti-laminin autoIg is not detected in serum, and B cell numbers or frequency are decreased in spleen and bone marrow compared to the non-Tg BWF1; endogenous (IgMb) heavy chains are highly expressed, despite presence of the productively rearranged Ig Tg, suggesting editing; and, surface Ig (IgM/IgD) expression is significantly decreased, consistent with anergy (Table 1). The Tg BWF1 contrast with the previously reported parental Tg NZB in surface IgLambda expression, in that this parameter does not differ between Tg and non-Tg BWF1 (Table 1), whereas in the NZB strain a significantly higher proportion of B cells expressed lambda in Tg vs non-Tg mice [7].
Figure 1. A–C: TLR ligand-stimulated autoantibody production.
(A) 1×106 B cells from Ig Tg mice were cultured with (+) or without (−) LPS stimulation. Anti-laminin Tg Ig is shown as mean +/− S.D. of OD405 on antigen-coated minus diluent-coated wells of duplicate samples, normalized to A10C antibody OD to control for interassay variability. *p<0.05 by nonparametric multiple comparison Wilcoxon Each Pair test, 4–5 mice per group, except unstimulated sample results pooled from all subjects (n=18). Ages in months as mean (range) are: Tg B6, 5.5 (3–8); Tg (B6xNZB)F1, 3.7 (3.5–4); Tg BWF1, 8.4 (6–10); and Tg NZB, 8.5 (8–9), from 1–2 experiments per strain. (B) Tg NZB (0.5×106 cultured cells, age 11–16 months from 2 experiments); Each line reflects paired samples from a single mouse +/− Mek1/2 inhibitor U0126; p=0.0625, Wilcoxon Signed Rank test; and, (C) Tg NZB (age 5–14 mon, 0.5×106 B cells per well), n=8–13 mice per group from 4 experiments, *p<0.05 vs each other group, Wilcoxon Each Pair test.
Table 1.
B cell phenotype in spleen and bone marrow (NZBxNZW)F1 and (B6xNZB)F1 mice.
| (NZBxNZW)F1
|
(B6xNZB)F1
|
|||
|---|---|---|---|---|
| Spleen | Tg+ | non-Tg | Tg+ | non-Tg |
| B cells, millions | 5.8 ± 2.4* | 26.7 ± 7.6 | 4.2 ± 1.0* | 29.6 ± 5.9 |
| (8) | (5) | (5) | (4) | |
| Reduction in B cells vs non-Tg | 78% | 86% | ||
| %Tg+ IgMa (of B cells) | 84.7 ± 4.3 | N/A | 68.2 ± 6.6 | N/A |
| (8) | (5) | |||
| % Endogenous IgMb (of B cells) | 24.0 ± 10.0* | 83.8 ± 5.3 | 32.1 ± 3.0* | 88.0 ± 2.7 |
| (8) | (5) | (5) | (4) | |
| % lambda+, of B cells | 3.2 ± 0.9 | 2.9 ± 0.5 | 7.8 ± 1.5* | 3.3 ± 0.7 |
| (8) | (5) | (5) | (4) | |
| Surface Ig MFI (of B cells)a | 0.8 ± 0.1* | 1.0 ± 0.1 | 0.7 ± 0.1* | 1.0 ± 0.0 |
| (3) | (3) | (5) | (4) | |
| Bone Marrow | ||||
|
| ||||
| % B cells, of lymphocytes | 20.3 ± 5.0* | 36.2 ± 8.4 | 21.4 ± 5.9* | 37.7 ± 4.6 |
| (8) | (5) | (5) | (4) | |
| Surface Ig MFI (of B cells)a | 0.4 ± 0.0* | 1.0 ± 0.2 | 0.3 ± 0.1 | 1.0 ± 0.1 |
| (3) | (3) | (2) | (3) | |
p<0.05 vs non-Tg
MFI = mean fluorescence intensity, IgM+IgD, normalized to concurrently assayed non-Tg cells.
Because Tg BWF1 mice inherit potent autoimmune modulators from both NZB and NZW, we assessed whether the NZB contribution was sufficient to overcome tolerance in B cells from F1 mice generated by crossing NZB with B6, in which Tg B cells are stringently regulated and fail to produce Tg autoIg in response to LPS [7]. TLR4-stimulated B cells from Tg (B6xNZB)F1 mice produce significantly more Tg autoIg than B cells from Tg B6 mice (Figure 1A), a defect already apparent in B cells from young adult (B6xNZB)F1 mice (aged 3.5–4 months). Therefore, the defect in B cell tolerance unmasked by TLR4 stimulation likely can be attributed to NZB loci in both F1 progeny. As with older Tg BWF1 mice, Tg anti-laminin autoIg is not detected in serum (mean OD405 = 0.0 ± 0.0) and other tolerance pathways appear intact in young adult Tg (B6xNZB)F1 mice despite detection of the defect (Table 1). The tolerance phenotype of Tg (B6xNZB)F1 mice is similar to that previously described for the parental Tg B6 line [2, 3].
Preservation of the TLR4-exposed defect in distinct NZB F1 hybrids suggests that dominant NZB susceptibility genes are responsible. Known major NZB loci that link to Ig production and nephritis include Nba2 on chromosome 1, which also links to increased expression of TLR adaptor Unc93b1 [11, 12], and Sle2c1 on chromosome 4, a major contributor to breaches in B cell tolerance [13–15]. A chromosome 13 locus has been linked to hyperreponsiveness to TLR3, but not TLR7 or 9 [16]. Ultimately, backcross of the autoIg Tg to B6 congenic lines carrying NZB-derived loci may better pinpoint the genetic basis of the reversible anergy defect.
The NZB B cell defect is blunted by Ras/MAPK pathway inhibition
In multiple models, B cell anergy is maintained by alterations in coordinated BCR/TLR signaling compared to signaling in naïve B cells, such that TLR ligands fail to induce autoIg secretion [17–20]. Known mechanisms center on altered activation or function of MAPK pathways[21], and particularly ERK activity, which may be critical for Blimp-1 activation and Ig secretion [22]. To assess ERK activation in Ig Tg NZB B cells, we used fluorescently-labeled probes and observed ERK phosphorylation and p-ERK nuclear translocation after costimulation with LPS and BCR crosslinking (not shown). We then used the selective Mek1/2 inhibitor U0126 to determine whether blockade of MAPK activation modifies the Tg NZB defect. Coincubation of Tg NZB B cells with LPS and low concentrations of U0126 partially correct the defect, reducing Tg autoIg levels (Figure 1B) while preserving cell viability (not shown). This suggests that defective tolerance in NZB mice may be due in part to abnormally preserved signaling in MAPK pathways in NZB anergic B cells.
Multiple TLRs expose the NZB tolerance defect
To determine whether the defect is specific to TLR4 stimulation, we cultured Tg NZB B cells with ligands for TLR7 and TLR9, as well as TLR4, separately and in combination. Each TLR ligand induced Tg autoIg production (Figure 1C), with additive effect.
Because the tolerance defect is exposed by multiple TLR ligands, we compared expression levels of genes in TLR signaling pathways for TLR-stimulated wildtype NZB vs B6 splenic B cells, and identified several genes that show differential expression (Table 2). This includes significant overexpression of TLR4 in NZB B cells, a finding that may explain accentuation of autoIg production by LPS-stimulated Tg NZB and F1 B cells. However, this alone cannot fully explain the high level autoIg secretion because the phenotype is also induced by TLR7 and TLR9 ligands. Protein tyrosine phosphatase non-receptor type 22 (ptpn22), variants of which are linked to human autoimmunity, is overexpressed 4-fold in TLR-stimulated NZB B cells. Ptpn22 encodes hematopoietic cell tyrosine phosphatase Lyp (human) or PEP (mouse), a key regulator of BCR and TLR proximal signaling. Several PTPN22 risk alleles link to alterations in BCR signaling, expanded anergic subsets, and autoimmunity in humans [23–25]. Lyp/PEP inhibits BCR signaling and binds and activates TRAF3 [26], which is also significantly overexpressed (1.6-fold) in NZB vs B6 stimulated B cells. TRAF3 has unique negative as well as positive regulatory functions, including regulation of TLR signals, and is activated by TLR4, 7, and 9 [27], each of which elicits the NZB B cell defect. Excess ptpn22 activity may inhibit BCR signaling, raising tolerance and activation thresholds, while simultaneously enhancing TLR signaling; both actions are likely to alter central and peripheral B cell tolerance [28].
Table II.
TLR pathway genes differentially expressed in stimulated NZB vs B6 B cells
| Genea | Ratio NZB:B6 |
|---|---|
| Ptpn22 | 4.0 |
| Tlr8 | 3.1 |
| Tlr4 | 2.5 |
| Nfkbie | 2.0 |
| IL6 | 0.5 |
Shown are differences ≥ 2.0-fold in either direction
Other genes with expression differences between stimulated NZB and B6 B cells ranging from 1.5 < 2.0 fold in either direction (ratio NZB:B6 shown): Cd14 (1.9), Fn1 (0.6), Il11 (1.8), Irak2 (1.5), Ly96 (1.7), Map2k3 (1.5), Map3k7 (1.6), MyD88 (1.8), Tbc1d10c (1.95), Tirap (1.8), Tlr2 (1.7), Traf3 (1.6)
Genes investigated showing no difference (<1.5-fold): Becn1, c-Cbl, Cbl-b, Ccl11*, Ccl20*, Chuk, Cxcl10, Egr2, Ifnb1*, Ikbkb, Il4*, Irak1, Irak3, Irak4, Jun, Lbp*, Nfkb1, Nfkb2, Nfkbia, Nfkbib, Rel, Rela, Sftpa1*, Tlr1, Tlr5*, Tlr9, Traf6; *expression in these cells was too low to accurately measure.
Others have shown extrinsic regulation of some anergic cell subsets, with suppression of autoIg secretion by TLR-stimulated mononuclear cell-derived cytokines (IL6, sCD40L, TNFa) [29–31]. Our data in NZB point to an intrinsic B cell defect in this strain, and preliminary studies (not shown) suggest that addition of B-depleted splenic cell subsets or splenocyte cultures fail to suppress TLR-induced autoIg. Lack of in vitro suppression may relate to abnormally restored BCR/TLR signaling in anergic cells, particularly because receptors for cytokines implicated in the non-autoimmune setting can signal through ptpn22-regulated TRAF3.
We cannot rule out a concurrent defect in the central compartment. Defective central deletion was previously postulated for NZB based on evidence from the 4C8 Ig Tg model; anti-red blood cell B cells are controlled primarily by deletion in 4C8 Tg B6, whereas loss of tolerance and autoimmune hemolytic anemia develop in 4C8 Tg NZB[32, 33]. It is noteworthy that aberrant TLR signaling and the autoimmunity-linked R620W ptpn22 variant are associated defective central B cell tolerance in man [28, 34, 35]. It is possible that altered BCR and TLR signaling in NZB not only facilitates reversal of anergy but also permits a subset of autoreactive early B cells to escape deletion.
Concluding remarks
We determined that a B cell regulatory defect previously observed in the NZB lupus strain is dominant and heritable to multiple F1 crosses, and can be exposed by multiple TLR ligands signaling from the cell surface and/or endosome. Gene expression analysis and the unmasking of autoreactivity by TLR ligands in what otherwise appears to be effective tolerance in these mice (via deletion, editing, and anergy) implicate aberrant genetic modulation of B cell-intrinsic TLR signaling-related pathways. A Mek1/2 inhibitor decreases NZB Tg autoIg production in response to TLR ligand, thus partially correcting the defect. Because lupus is a clinically and genetically heterogeneous disease in mice and man, it is anticipated that a subset of lupus patients manifests similar B cell regulatory defects. TLRs, endosomal signaling, and ptpn22 are considered promising therapeutic targets [36], such that intervention tailored to these pathways may offer the possibility of rapid clinical translation to this subset of patients.
Materials and Methods
Mice
All studies were approved by the Animal Care and Use Committee of Duke University and the Durham VAMC. Characterization of the LamH IgMa+ autoIg Tg was previously described [2, 3, 7]. Tg BWF1 mice were generated by breeding Tg NZB females×NZW/LacJ (NZW) males (Jackson Labs), and Ig Tg (B6xNZB)F1 by breeding B6 females (Jackson Labs) with Tg NZB males. Mice ages ranged from 3–16 months.
B cell isolation and culture
B cells were purified and cultured for 7–8 days as described [7], stimulated with either 50 µg/mL LPS (TLR4 agonist, Sigma), 2 µg/mL resiquimod (R848, TLR7 agonist, Sigma), 1 µg/mL ODN 1668 CpG oligos (CpG, TLR9 agonist, Invivogen), 0.5 µM U0126 (MEK1/2 inhibitor, Calbiochem), combinations of these, or medium alone. Cell viability was determined using trypan blue.
Autoantibody measurement by ELISA
Serum (1:20 dilution) and cell culture supernatants (undiluted) were assayed for Ig Tg anti-laminin autoIg as described [7]. All Tg anti-laminin ELISAs were standardized to co-plated IgMa anti-laminin control antibody f413A10C [37] to correct for interassay variability. Measurements above the linear range occurred in a subset of LPS-stimulated Tg+ NZB B cell cultures (Figure 1A) and TLR 4/7/9 stimulated Tg+ NZB cultures (Figure 1C), which were accommodated using a rank sum statistical analysis as described.
Flow cytometry
Splenocytes or bone marrow cells were incubated with antibodies against Tg IgMa and other surface markers and data acquired and analyzed as described [3, 7].
Gene expression analysis by qPCR
Purified B cells from non-transgenic NZB and B6 mice were cultured overnight with TLR4, TLR7, and TLR9 stimulation, harvested into RLT buffer (Qiagen), homogenized using Qiashredder (Qiagen), and RNA isolated using the RNEasy mini kit (Qiagen). cDNA was generated using the High Capacity cDNA reverse transcription kit with oligo d(T) primer (Applied Biosystems) and assayed using the TLR m96 array (BioRad). Fold change was calculated using the ddCT method, standardized to GAPDH. Additionally, cDNA was assayed in duplicate using TaqMan Gene Expression Assay systems (Applied Biosystems) to quantify expression of Tbc1d10c, GPR97, Ptpn22, Traf3, c-Cbl, Egr2, Cbl-b, standardized to succinate dehydrogenase A.
Immunofluorescence microscopy
Purified B cells were incubated in an 8-well chamber slide (ThermoFisher) for 1 hr, then stimulated 30 min with diluent or TLR ligand (LPS or CpG oligo) plus anti-mouse IgM+IgG-Alexafluor 488. Fixed cells were stained with rabbit anti-p-ERK (Cell Signaling) followed by goat anti-rabbit-Alexafluor 555 (Invitrogen), and DAPI nuclear stain (Sigma), and imaged on a Zeiss 710 confocal microscope at 630x, using consistent capture settings.
Supplementary Material
Acknowledgments
This work was supported by grant NIDDK R01DK047424. We thank Lauren Howard of the Duke Department of Biostatistics and Bioinformatics for statistical guidance; Brian Brockway, Melissa Weston Boor, and Qihua Fan for technical support; Elizabeth Finch, PhD, for help with fluorescence imaging analysis; the Duke Cancer Institute Flow Cytometry Shared Resource; and, the Duke Light Microscopy Core Facility.
Abbreviations
- autoIg
autoantibody/autoantibodies
- TLR
toll like receptor
- NZB
New Zealand Black
- B6
C57BL/6J
- BCR
B cell receptor
- Tg
transgene
- Ig
immunoglobulin
- BWF1
(NZB x NZW)F1
Footnotes
Conflict of Interest
The authors declare no commercial or financial conflict of interest.
References
- 1.Tsokos GC. Systemic lupus erythematosus. New Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph EH, Congdon KL, Sackey FN, Fitzsimons MM, Foster MH. Humoral autoimmunity to basement membrane antigens is regulated in C57BL/6 and MRL/MpJ mice transgenic for anti-laminin Ig receptors. J Immunol. 2002;168:5943–5953. doi: 10.4049/jimmunol.168.11.5943. [DOI] [PubMed] [Google Scholar]
- 3.Brady GF, Congdon KL, Clark AG, Sackey FN, Rudolph EH, Radic MZ, Foster MH. Kappa editing rescues autoreactive B cells destined for deletion in mice transgenic for a dual specific anti-laminin Ig. J Immunol. 2004;172:5313–5321. doi: 10.4049/jimmunol.172.9.5313. [DOI] [PubMed] [Google Scholar]
- 4.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 5.Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, Mathias M, et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang J, Ota T, Kelly M, Strauch P, Freed BM, Torres RM, Nemazee D, Pelanda R. Receptor editing and genetic variability in human autoreactive B cells. J Exp Med. 2016;213:93–108. doi: 10.1084/jem.20151039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark AG, Fan Q, Brady GF, Mackin KM, Coffman ED, Weston ML, Foster MH. Regulation of basement membrane-reactive B cells in BXSB, (NZBxNZW)F1, NZB, and MRL/lpr lupus mice. Autoimmunity. 2013;46:188–204. doi: 10.3109/08916934.2012.746671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morel L. Genetics of SLE: evidence from mouse models. Nat Rev Rheumatol. 2010;6:348–357. doi: 10.1038/nrrheum.2010.63. [DOI] [PubMed] [Google Scholar]
- 9.Chang N-H, Cheung Y-H, Loh C, Pau E, Roy V, Cai Y-C, Wither J. B cell activating factor (BAFF) and T cells cooperate to breach B cell tolerance in lupus-prone New Zealand Black (NZB) mice. PLoS One. 2010;5:e11691. doi: 10.1371/journal.pone.0011691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn BH, Shulman LE. Autoantibodies and nephritis in the white strain (NZW) of New Zealand mice. Arth Rheum. 1969;12:355–364. doi: 10.1002/art.1780120403. [DOI] [PubMed] [Google Scholar]
- 11.Drake CG, Rozzo SJ, Hirschfeld HF, Smarnworawong NP, Palmer E, Kotzin BL. Analysis of the New Zealand Black contribution to lupus-like renal disease. Multiple genes that operate in a threshold manner. J Immunol. 1995;154:2441–2447. [PubMed] [Google Scholar]
- 12.Panchanathan R, Liu H, Choubey D. Expression of murine Unc93b1 is up-regulated by interferon and estrogen signaling: implications for sex bias in the development of autoimmunity. Int Immunol. 2013;25:521–529. doi: 10.1093/intimm/dxt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Duan B, Croker BP, Wakeland EK, Morel L. Genetic dissection of the murine lupus susceptibility locus Sle2: contributions to increased peritoneal B-1a cells and lupus nephritis map to different loci. J Immunol. 2005;175:936–943. doi: 10.4049/jimmunol.175.2.936. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Li L, Kumar KR, Xie C, Lightfoot S, Zhou XJ, Kearney JF, Weigert M, Mohan C. Lupus susceptibility genes may breach tolerance to DNA by impairing receptor editing of nuclear antigen-reactive B cells. J Immunol. 2007;179:1340–1352. doi: 10.4049/jimmunol.179.2.1340. [DOI] [PubMed] [Google Scholar]
- 15.Zeumer L, Sang A, Niu H, Morel L. Murine lupus susceptibility locus Sle2 activates DNA-reactive B cells through two sub-loci with distinct phenotypes. Genes and immunity. 2011;12:199–207. doi: 10.1038/gene.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loh C, Pau E, Chang NH, Wither JE. An intrinsic B-cell defect supports autoimmunity in New Zealand black chromosome 13 congenic mice. Eur J Immunol. 2011;41:527–536. doi: 10.1002/eji.201040983. [DOI] [PubMed] [Google Scholar]
- 17.Rui L, Vinuesa C, Blasioli J, Goodnow C. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 18.Rui L, Healy JI, Blasioli J, Goodnow CC. ERK signaling is a molecular switch integrating opposing inputs from B cell receptor and T cell cytokines to control TLR4-driven plasma cell differentiation. J Immunol. 2006;177:5337–5346. doi: 10.4049/jimmunol.177.8.5337. [DOI] [PubMed] [Google Scholar]
- 19.O'Neill SK, Veselits ML, Zhang M, Labno C, Cao Y, Finnegan A, Uccellini M, et al. Endocytic sequestration of the B cell antigen receptor and toll-like receptor 9 in anergic cells. Proc Natl Acad Sci. 2009;106:6262–6267. doi: 10.1073/pnas.0812922106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SR, Rutan JA, Monteith AJ, Jones SZ, Kang SA, Krum KN, Kilmon MA, et al. Receptor cross-talk spatially restricts p-ERK during TLR4 stimulation of autoreactive B cells. J Immunol. 2012;189:3859–3868. doi: 10.4049/jimmunol.1200940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teodorovic LS, Babolin C, Rowland SL, Greaves SA, Baldwin DP, Torres RM, Pelanda R. Activation of Ras overcomes B-cell tolerance to promote differentiation of autoreactive B cells and production of autoantibodies. Proc Natl Acad Sci. 2014;111:E2797–2806. doi: 10.1073/pnas.1402159111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasuda T, Kometani K, Takahashi N, Imai Y, Aiba Y, Kurosaki T. ERKs induce expression of the transcriptional repressor Blimp-1 and subsequent plasma cell differentiation. Sci Signal. 2011;4:ra25. doi: 10.1126/scisignal.2001592. [DOI] [PubMed] [Google Scholar]
- 23.Arechiga AF, Habib T, He Y, Zhang X, Zhang ZY, Funk A, Buckner JH. Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J Immunol. 2009;182:3343–3347. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habib T, Funk A, Rieck M, Brahmandam A, Dai X, Panigrahi AK, Luning Prak ET, et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J Immunol. 2012;188:487–496. doi: 10.4049/jimmunol.1102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanford SM, Bottini N. PTPN22: the archetypal non-HLA autoimmunity gene. Nat Rev Rheumatol. 2014 doi: 10.1038/nrrheum.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Shaked I, Stanford SM, Zhou W, Curtsinger JM, Mikulski Z, Shaheen ZR, et al. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity. 2013;39:111–122. doi: 10.1016/j.immuni.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hacker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev Immunol. 2011;11:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 28.Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G, Massad C, Price C, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilmon MA, Rutan JA, Clarke SH, Vilen BJ. Low-affinity, Smith antigen-specific B cells are tolerized by dendritic cells and macrophages. J Immunol. 2005;175:37–41. doi: 10.4049/jimmunol.175.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilmon MA, Wagner NJ, Garland AL, Lin L, Aviszus K, Wysocki LJ, Vilen BJ. Macrophages prevent the differentiation of autoreactive B cells by secreting CD40 ligand and interleukin-6. Blood. 2007;110:1595–1602. doi: 10.1182/blood-2006-12-061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert MR, Wagner NJ, Jones SZ, Wisz AB, Roques JR, Krum KN, Lee SR, et al. Autoreactive preplasma cells break tolerance in the absence of regulation by dendritic cells and macrophages. J Immunol. 2012;189:711–720. doi: 10.4049/jimmunol.1102973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto M, Murakami M, Shimizu A, Ozaki S, Tsubata T, Kumagai S, Honjo T. A transgenic model of autoimmune hemolytic anemia. J Exp Med. 1992;175:71–79. doi: 10.1084/jem.175.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami M, Tsubata T, Okamoto M, Shimizu A, Kumagai S, Imura H, Honjo T. Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature. 1992;357:77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- 34.Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von Bernuth H, Zhang SY, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann N Y Acad Sci. 2011;1246:1–10. doi: 10.1111/j.1749-6632.2011.06347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzsimons MM, Chen H, Foster MH. Diverse endogenous light chains contribute to basement membrane reactivity in nonautoimmune mice transgenic for an anti-laminin Ig heavy chain. Immunogenetics. 2000;51:20–29. doi: 10.1007/s002510050004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.