Abstract
Background
Whilst the dangers of ‘legal highs’ have been widely publicised in the media, very few cases of the neurological syndrome associated with the inhalation of nitrous oxide (N2O) have been reported. Here we set out to raise awareness of subacute degeneration of the spinal cord arising from recreational N2O use so that formal surveillance programs and public health interventions can be designed.
Methods
Case series documenting the clinical and investigational features of ten consecutive cases of subacute degeneration of the spinal cord presenting to a hospital with a tertiary neurosciences service in East London.
Results
Sensory disturbance in the lower (± upper) limbs was the commonest presenting feature, along with gait abnormalities and sensory ataxia. MRI imaging of the spine showed the characteristic features of dorsal column hyperintensity on T2 weighted sequences. Serum B12 levels may be normal because subacute degeneration of the spinal cord in this situation is triggered by functional rather than absolute B12 deficiency.
Discussion
A high index of suspicion is required to prompt appropriate investigation, make the diagnosis and commence treatment early. This is the largest reported series of patients with subacute degeneration of the spinal cord induced by recreational use of N2O. However, the number of patients admitted to hospital likely represents the ‘tip of the iceberg’, with many less severe presentations remaining undetected. After raising awareness, attention should focus on measuring the extent of the problem, the groups affected, and devising ways to prevent potentially long-term neurological damage.
Keywords: Subacute degeneration of the spinal cord, Myelopathy, Hydroxocobalamin, Vitamin B12
Introduction
Nitrous oxide (N2O or laughing gas) has been used in clinical practice as a dissociative anaesthetic for over 170 years, but its recreational use has become widespread, facilitated by legal over-the-counter availability. N2O abuse is rapidly rising throughout the USA and UK, and has become the seventh most commonly used recreational drug according to the Global Drug Survey 2016 [1].
Several case reports have documented the potential for N2O to cause damage to the nervous system through interference with vitamin B12 metabolism, leading to megaloblastic anaemia and subacute degeneration of the spinal cord [2–5] which itself can be irreversible [6]. Here, we report the largest series of patients with neurological complications from N2O abuse, in an effort to raise awareness and prompt appropriate surveillance, so that an adequate public health response may be designed and implemented.
Methods
All adult patients presenting between 1st of November 2016 and 1st May 2017 to the Emergency Department of the Royal London Hospital, with a history of N2O use and symptoms suggestive of subacute degeneration of the spinal cord were included. Two patients had a pre-existing diagnosis of subacute degeneration of the spinal cord and re-presented during this period (cases 8 and 10). Blood tests were performed to rule out alternative causes of myelopathy in most patients, including virology, anti-nuclear antibodies, anti-neutrophil cytoplasmic antibodies, anti-cardiolipin antibodies, aquaporin 4 antibodies and paraneoplastic antibodies. Nutritional deficiencies of copper and folate were also routinely tested. Cerebrospinal fluid examination was performed in four patients. All patients were seen and examined by an attending consultant neurologist and imaging reported by a consultant neuroradiologist. Where serum vitamin B12 level was normal, serum methylmalonic acid (MMA) was also measured. Where possible, patients were followed up in the outpatient department. Due to difficulties contacting patients (change of address, GP or phone number), not all patients were available to provide consent, and therefore all cases have been fully anonymised.
Results
Demographics
There were approximately 150,000 attendances to the Emergency Department over the 6-month study period. Table 1 summarises the clinical features of the ten study cases included in this report. The median age of patients was 22 years (range 17–26), three were women and seven were men. Seven patients were of Bangladeshi descent, one Asian, one mixed White-Caribbean and one was White. Eight were current smokers, six drank alcohol more than twice per week, and three used other drugs recreationally (two used cocaine and one marijuana). On average, patients used N2O around two-three times per week, and the number of N2O canisters consumed ranged between 75 and 2000 per week.
Table 1.
Clinical and investigational findings in 10 patients with N2O-induced subacute degeneration of the spinal cord
| Case | Number of reported N2O canisters per week | UL sensory | LL sensory | UL distal motor power | LL distal motor power | UL reflexes | LL reflexes | Gait ataxia | Bladder/bowel | Other | Vitamin B12 level (ng/L) | MMA level (μmol/L) | MRI at presentation | Follow-up MRI* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 100 | PP/JPS/LT loss | Vib loss | IV | IV | + | +++ C | Y | Nil | Myoclonic jerks, Uhtoff’s | 138 | 0.7 | C2–7 dorsal column hyperintensity and enhancement | Persistent dorsal column hyperintensity, enhancement resolved |
| 2 | 1000 | JPS/Vib loss | Vib loss | V | V | ++ | ++ | Y | Nil | Lhermitte’s, PA | 169 | ND | C1–7 dorsal column hyperintensity, cord atrophy | ND |
| 3 | 300 | HA/PP loss | HA PP, Vib loss | V | V | + | +++ C | Y | Constipation | PA | 196 | 2.36 | C1–6 dorsal column hyperintensity | ND |
| 4 | 192 | JPS/Vib loss | JPS/Vib loss | IV | IV | (+) | (+) | N | Nil | PA | > 2000*** | ND | C1–7 dorsal column hyperintensity | ND |
| 5 | 500 | Normal | PP/JPS loss | V | IV | 0 | 0 | Y | Nil | Nil | 186 | 3.45 | C2–6 dorsal column hyperintensity | ND |
| 6 | 600 | Normal | HA PP/JPS/Vib loss | IV | IV | ++ | +++ | Y | Nil | Nil | 109 | 110 | ND | ND |
| 7 | 75 | JPS loss | PP/JPS/Vib/LT loss | V | V | ++ | ++ | Y | Nil | PA | 321*** | 0.16 | C3–5 dorsal column hyperintensity | Complete resolution |
| 8** | 180 | Normal | LT loss | V | V | ++ | 0 | N | Nil | Nil | 229 | 14 | C2–6 dorsal column hyperintensity | Persistent dorsal column hyperintensity |
| 9 | 700 | Normal | HA PP/JPS/Vib loss | V | IV | ++ | 0 | Y | Nil | Nil | 169 | 0.61 | C1–7 dorsal column hyperintensity | ND |
| 10** | 1500-2000 | PP/JPS/Vib loss | PP/JPS/Vib loss | V | V | + | + | Y | Nil | PA | 226 | 7.8 | C4–7 dorsal column hyperintensity | Complete resolution |
Power assessments according to MRC power score. Reflexes 0 = absent, (+) with reinforcement, + = diminished, ++ = normal, +++ = brisk, C = with clonus, UL = upper limbs, LL = lower limbs. MMA = methylmalonic acid. B12 reference range 191-900 ng/L, MMA 0.0-0.29umol/L
HA hyperaesthesia, LT light touch sensation, PP pin prick sensation, JPS joint position sensation, Vib vibration sensation, PA pseudoathetosis. ND not done/not available
* Follow-up MRI was performed at 5 months for case 1, 8 months case 7, 16 months case 8 and 27 months case 10
** both patients re-presented following a previous diagnosis of subacute degeneration of the spinal cord
*** Already on B12 replacement
Clinical features
Altered sensation in the limbs was the predominant presenting feature (seven had symptoms in the upper limbs and all ten had symptoms in the lower limbs). Strength was well preserved in most patients. Additional clinical features were gait ataxia (n = 8), falls (n = 3), Romberg’s sign (n = 6), pseudoathetosis (n = 5), Lhermitte’s phenomenon (n = 1), Uhtoff’s phenomenon (n = 1), and segmental myoclonus (n = 1).
Investigations
Median haemoglobin level was 148 (range 117–170 g/L). All patients had normal mean cell volume (median 92.4; range 89.8–94.8 fL). Four patients had low vitamin B12 levels (median 191; range 109–2000 ng/L). MMA was measured in eight patients (median 2.9; range 0.16–110 μmol/L) and was not taken in patients whose B12 level was below normal or elevated as a result of replacement. MMA was elevated in seven of the eight patients. Four of these could be deemed clinically relevant/revealing, with an associated normal B12 level. Three were ‘complimentary’, with an associated low B12 level. The MMA level was normal in one patient whose vitamin B12 level was low (case 7), but it transpired that they had been aware of the risks of myelopathy and had been concurrently taking oral B12 1000 μg once a day as prophylaxis. Despite this they had nonetheless developed subacute degeneration of the spinal cord.
A CSF examination was performed in four patients. White cell counts were < 1 in all cases. Protein was raised in two patients at 0.5 and 0.7 g/L (normal ≤ 0.4 g/L). Glucose was within normal limits in all. Unmatched oligoclonal bands were demonstrated in one patient, the significance of which is unclear but would suggest a degree of intrathecal immunological response.
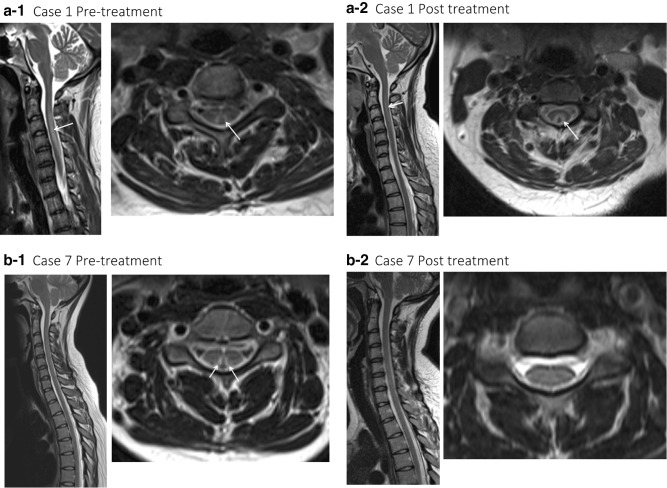
MRI of the spinal cord was performed in nine patients and showed T2 signal change affecting the dorsal columns of the cervical spine, consistent with subacute degeneration of the spinal cord (see Fig. 1). In one patient, pathological enhancement was detected initially. Follow-up MRI was performed in four patients after an average of 14 months (range 5–27 months) from presentation. In two patients the signal change persisted and in the other two it had resolved. In the cases where MRI signal change had resolved (cases 7 and 10), both received treatment for a minimum of 4 months. Case 7 had abstained from N20, had been taking oral B12 prior to presenting, and was asymptomatic following treatment. Case 10 continued to use N20 once a fortnight and experienced persistent paraesthesia in the feet. The patient also had poor diabetic control, and nerve conduction studies demonstrated mixed axonal and demyelinating features consistent with diabetic neuropathy.
Fig. 1.
Pre- and post-treatment sagittal and axial T2 weighted MRI imaging of the cervical cord in N2O-induced subacute degeneration of the spinal cord. a-1: Sagittal T2 weighted sequence demonstrating extensive cord signal change from the C2 to the C7 level within the dorsal aspect of the cord confirmed on the axial sequences, with enhancement (not shown). a-2: Taken 5 months following showing persistent abnormal T2 signal change within the dorsal columns on this sagittal T2 weighted sequence; however, the abnormal enhancement had resolved (not shown). b-1: Sagittal T2 weighted sequence demonstrating cord signal change from C3 to C5 within the dorsal aspect of the cord confirmed on the axial sequences, without enhancement, and resolution over time (b-2)
Management
All patients received hydroxocobalamin injections, 1 mg intramuscularly on alternate days, for a median of 2 weeks. Four patients were lost to follow-up. Of the remaining six patients, two recovered without residual symptoms, three continued to have paraesthesia in the feet and one continued to have paraesthesia, gait ataxia, and proprioceptive sensory loss to the ankles (case 10 above).
Discussion
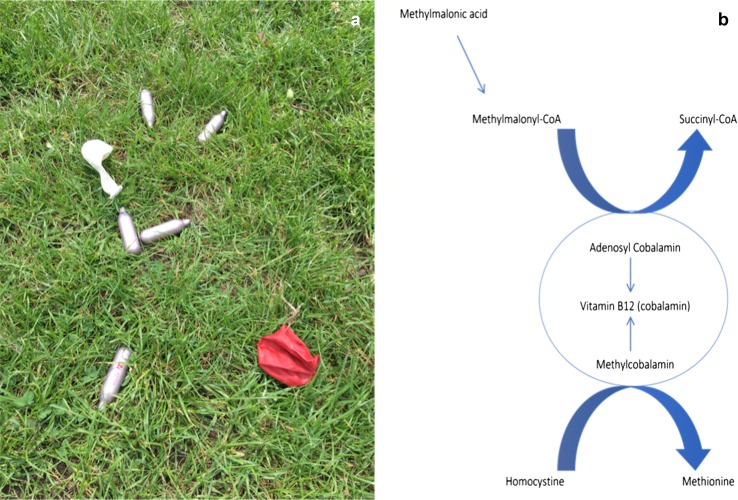
Recreational use of N2O is largely through use of whipped cream chargers or ‘whippets’ bought from ‘head shops’ or online (see Fig. 2a). Whippets fit onto a dispenser, which is attached to a balloon that enables gas inhalation. Inhalation results in almost immediate psychotropic effects including euphoria, giggling, distortion of sound and mild hallucinations, peaking after about 20 s before rapidly diminishing. Users often feel entirely normal within 2 min following inhalation. Side effects of dissociation, blurred vision, acute ataxia, nausea and headache have been reported [7].
Fig. 2.
a Discarded nitrous oxide canisters and balloons in East London. b Metabolic pathway of vitamin B12 involved in pathogenesis of N2O-induced subacute degeneration of the spinal cord. Vitamin B12 is a cofactor in the conversion of methylmalonyl-CoA to succinyl-CoA and homocystine to methionine. Non-functioning B12 leads to accumulation of methylmalonic acid and homocystine, which can be tested in the patient sera when B12 levels appear normal, suggesting a ‘functional; B12 disorder. Figure created using Microsoft Word
Recreational use of N2O in the UK has recently increased [7]. According to the Global Drug Survey, which collected data from over 100,000 drug users in 20 countries, N2O is the seventh most popular recreational drug worldwide, and the fourth most commonly used by UK nightclub attendees, with 48% admitting to using the drug [1]. Since the Psychoactive Substances Act came into effect in the UK in May 2016, it has become illegal to supply N2O for recreational consumption. Two recent high-profile cases of failed prosecutions for N2O supply have forced the Crown Prosecution Service to consider the implications for future cases [8]. Nonetheless, whippets are still being sold at a price of approximately £30 ($40) for 96 canisters.
Previous reports have been published of patients presenting to hospital with acute pain and administration of medicinal N2O excessively, with subsequent development of subacute degeneration of the spinal cord [9]. However, routine use in anaesthesia or dental procedures only carries a risk of subacute degeneration of the spinal cord in patients with low or low/normal serum vitamin B12 [2, 6].
The deleterious effects of N2O overuse are secondary to its interference with the action of vitamin B12. N2O causes oxidation of cobalt ions in vitamin B12, leading to its inactivation (see Fig. 2b). This results in reduced recycling of homocystine to methionine, preventing methylation of myelin proteins, thereby causing demyelination.
Identification of subacute degeneration of the spinal cord secondary to N2O abuse requires a high index of suspicion and a thorough history, supported by the clinical examination, laboratory data and radiological findings. A normal vitamin B12 level does not rule out the possibility of N2O-induced subacute degeneration of the spinal cord, given that functional B12 deficiency can occur in the presence of normal serum vitamin B12 levels. In such cases, detecting raised MMA and homocystine, which rely on normally functioning vitamin B12 for their metabolism, can give crucial clues to the diagnosis. Sagittal MRI imaging demonstrates a classic appearance of T2 hyperintensity in the dorsal spinal cord. Contrast enhancement is uncommon.
The management of patients includes educating them about the risks of N2O and vitamin B12 replacement using high-dose intramuscular hydroxocobalamin (1 mg on alternate days until no further neurological improvement, followed by 1 mg every 2 months) [10]. Neurological recovery may be incomplete, particularly when patients continue to use N2O [6].
Our series revealed that N2O-related subacute degeneration of the spinal cord tended to occur in young people. While most were smokers, unexpectedly, and not in keeping with the habits of other drug users [11], the majority did not drink alcohol or take other recreational drugs. However, this may also reflect the culture of the population local to our hospital. The finding that 70% of patients were of Bangladeshi origin emphasises the importance of considering recreational drug use even when religious and/or cultural assumptions may dissuade clinicians from this line of enquiry. The high Bangladeshi proportion also raises questions whether ethnicity-related metabolic, nutritional or genetic predispositions influence functional B12 metabolism and predispose to neurological damage. Anecdotally, many N2O users were not aware of the harmful effects of the drug and believed that because it was not previously illegal to consume, it was also safe to use.
The true scale of the problem is hard to gauge, but may only be improved through collaborative working by health professionals in neurology, public health and emergency medicine. Better recognition and accurate coding at the point of access to care will help ascertain the incidence and prevalence of neurological damage related to N2O, and plan an effective public health response.
Author contributions
All authors provided substantial contributions to the conception or design of the work, analysis, interpretation of data and necessary revisions. All authors have confirmed final approval for this version to be published and are accountable for all aspects of the work.
Compliance with ethical standards
Conflicts of interest
None of the authors have any conflict of interest to disclose.
Financial disclosure
No authors have any financial disclosures.
Transparency declaration
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Data sharing
No additional data available.
Ethical statement
This is a retrospective study describing the presentation and management of clinical cases. Individual patient consent has proved a significant issue in this case series in that many were not contactable to consent (contact details changed, no next of kin, no GP information). Such are the risks to young people using nitrous oxide, we felt that fully anonymising all information would allow for presentation of important clinical data whilst being impossible to identify any individual case.
References
- 1.Winstock A, Barratt M, Ferris J, Maie L (2017) Global overview and highlights. Global Drugs Survey, London, UK. Retrieved from https://www.globaldrugsurvey.com/wp-content/-themes/globaldrugsurvey/results/GDS2017_key-findings-report_final.pdf. Accessed 26 Feb 2018
- 2.Thompson AG, Leite MI, Lunn MP, Bennett DLH. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. 2015;15:207–209. doi: 10.1136/practneurol-2014-001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng J, O’Grady G, Pettit T, Frith R. Nitrous oxide use in first-year students at Auckland University. Lancet. 2003;361:1349–1350. doi: 10.1016/S0140-6736(03)13045-0. [DOI] [PubMed] [Google Scholar]
- 4.Morris N, Lynch K, Greenberg SA. Severe motor neuropathy or neuronopathy due to nitrous oxide toxicity after correction of vitamin B12 deficiency. Muscle Nerve. 2015;51:614–616. doi: 10.1002/mus.24482. [DOI] [PubMed] [Google Scholar]
- 5.Layzer R. Myeloneuropathy after prolonged exposure to nitrous oxide. Lancet. 1978;312:1227–1230. doi: 10.1016/S0140-6736(78)92101-3. [DOI] [PubMed] [Google Scholar]
- 6.Vasconcelos OM, Poehm EH, McCarter RJ, Campbell WW, Quezado ZMN. Potential outcome factors in subacute combined degeneration: review of observational studies. J Gen Intern Med. 2006;21:1063–1068. doi: 10.1111/j.1525-1497.2006.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Home Office (2017) Nitrous Oxide|FRANK. http://www.talktofrank.com/drug/nitrous-oxide. Accessed 17th April 2017
- 8.Rawlingson K (2017) Laughing gas still illegal despite court decisions, UK government says. The Guardian
- 9.Doran M, Rassam SS, Jones LM, Underhill S. Toxicity after intermittent inhalation of nitrous oxide for analgesia. BMJ. 2004;328:1364–1365. doi: 10.1136/bmj.328.7452.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joint Formulary Committee (2017) British National Formulary, 74th edn. BMJ Group and Pharmaceutical Press, London
- 11.UK National Statistics (2017) The health social care information centre. Statistics on drugs misuse. NHS Digital, London, UK. Retrieved from https://www.gov.uk/government/statistics/statistics-on-drug-misuse-england-2017. Accessed 26 Feb 2018