Abstract
Objective
The aim of the study is to conduct an initial pilot trial evaluating the feasibility, safety, and efficacy of bupropion for smoking cessation in pregnancy.
Study Design
A randomized, double-blind, parallel-group pilot study of bupropion versus placebo with 50 pregnant smokers was planned. Eligibility criteria were restrictive (e.g., 14–26 weeks’ gestation; no psychiatric conditions or medications) due to the unknown safety, tolerability, and side effect profile of bupropion in pregnancy. Bayesian analyses were planned to provide probability of benefit.
Results
Significant challenges were encountered with regard to trial feasibility. Of 820 women screened, 112 were current smokers, but only 11 women were eligible and consented to participate in the study. Excluded women most often had a psychiatric disorder (23%); were outside the gestational range (14%); or declined to participate (11%).
Conclusions
This initial attempt to evaluate bupropion for smoking cessation during pregnancy will inform future trial methodology. Because of the unknown safety profile, conservative eligibility criteria were used and resulted in a large portion of this high-risk, low-income smoker population being excluded from the trial, raising questions regarding broad applicability, and highlighting the need to balance patient safety and trial feasibility. Large multisite studies will likely be needed to conduct definitive pharmacotherapy studies.
Keywords: pregnancy, smoking, bupropion, pharmacotherapy, smoking cessation
Cigarette smoking during pregnancy is one of the leading preventable causes of low birth weight1 and is associated with multiple other adverse outcomes, such as fetal growth restriction, preterm birth, spontaneous abortions, stillbirth, and potential increased risk of neurodevelopmental disorders.2,3 Despite risks, between 5.2 and 35.7% of the U.S. women smoke through pregnancy, with an average estimate of 14.6%.4 Annual U.S. health care costs because of effects of prenatal smoking on neonatal outcomes were estimated at $263 to 366 million,5 and increased to $593 to 706 million over the first year of life6 in 1995 and 1996 dollars, respectively. Smoking cessation in pregnancy has been estimated to prevent up to 5% of perinatal deaths, 20 to 30% of low birth weight births, and 15% of preterm deliveries.7,8 Quitting even in the last trimester is clinically meaningful, as smoking in the third trimester has the greatest impact on birth weight.9
Numerous trials of brief counseling and pregnancy-related self-help materials have been conducted with modest effects.10,11 Psychosocial interventions are superior to usual care in assisting pregnant women to stop smoking; however, their average estimated quit rates are only around 13% and the interventions are rarely implemented with high fidelity.12 Clinical Practice Guidelines for treating tobacco use and dependence indicate that pharmacotherapy can double smoking quit rates in the general population of smokers, but till date this effective therapy has not been extended to pregnant smokers.12
The lack of clinical trial data to guide obstetricians and other providers has precluded the systematic use of evidence-based pharmacotherapies for smoking cessation, such as nicotine replacement therapy (NRT) and bupropion.13 A few trials of NRT for pregnancy smoking have been attempted with mixed results initially,14,15 and negative findings more recently.16,17 Bupropion may be preferable to NRT in pregnancy, however, because exposure to nicotine can be harmful to the fetus. Bupropion is also associated with reductions of common symptoms of nicotine withdrawal (i.e., weight gain and craving) and it targets symptoms of depression and negative effect which have been found to be very high among pregnant smokers.18
Bupropion is a Pregnancy Category C drug with no known fetal effects.19 Studies of rats and rabbits have suggested limited evidence of teratogenicity and obstetric care providers prescribe this drug when clinically indicated, though prospectively collected data on the safety of bupropion use in human pregnancies is limited. In a prospective, comparative safety study, 136 women who were prescribed bupropion in or before their first trimester for either depression or smoking were compared with 133 women with no medication or other known teratogen exposure. Comparisons indicated a higher rate of spontaneous abortion in the bupropion group (14.7 vs. 4.5%, p ≤ 0.009), but no increase in rates of major malformations, low birth weight or neonatal death.19 Another small, matched, controlled, observational study suggested higher rates of cessation in the bupropion group (n = 10/22, 45%) compared with the control group (n = 3/22, 14%).20 Thus, both risks and benefits of bupropion for pregnant smokers have been identified, but without data from randomized trials firm conclusions cannot be drawn.
The original purpose of this study was to conduct a small randomized, placebo-controlled, double-blind pilot trial comparing bupropion to placebo for smoking cessation in 50 pregnant women to: (1) obtain preliminary safety data for bupropion by assessing maternal side effects and perinatal/neonatal outcomes; (2) determine rates of enrollment and retention, indicating feasibility and clinical acceptability of pharmacological treatment by pregnant smokers; and (3) obtain unbiased treatment effect estimates. Significant challenges affected trial feasibility which precluded obtaining effect estimates and definitive conclusions with regard to safety. This report describes the trial and highlights the challenges, which will inform future trial methodology in this line of research.
Methods
Participants
Pregnant women attending a large university-based urban prenatal clinic were screened for the pilot trial during their first visit to the clinic after study initiation. Data were collected for 16 months from April 2011 to August 2012. In general, the clinic primarily served African-American and Hispanic women, with approximately 98% of women using Medicaid insurance to pay for prenatal expenses.
Eligible participants were as follows: (1) pregnant; (2) ≥ 18 years; (3) between 14 and 26 weeks’ gestation confirmed by ultrasound (upper limit ensures sufficient length of treatment); (4) currently smoking ≥ 1 cigarette per day. Exclusion criteria included the following: (1) abnormal liver function tests; (2) history of or current seizure disorder or closed head injury with loss of consciousness; (3) known hypersensitivity to bupropion; (4) any psychiatric disorder requiring psychotropic medication; (5) current anorexia or bulimia; (6) use of monoamine oxidase (MAO) inhibitors or discontinuation within the last 2 weeks; (7) Major depressive disorder or current suicidal risk; (8) use of any illicit substances in the last 30 days; (9) regular use of alcohol (> 1 drink/week on average); (10) unstable medical problems, such as liver or renal disease, uncontrollable hypertension, and lupus; (11) twins or other multiple gestation; (12) any fetal structural abnormality, previously known or detected at fetal anatomy ultrasound; (13) plans to deliver at a nonclinic-affiliated hospital; (14) inability to communicate with research staff or make study visits because of lack of phone or transportation access; (15) currently taking NRT, bupropion, or varenicline. The number of exclusions was large as investigators as well as the data and safety monitoring board believed that minimizing risks of poor perinatal and neonatal outcomes was paramount in this first randomized controlled trial of sustained-release (SR) bupropion in pregnant smokers, given the mixed animal data and lack of human trial data on safety, tolerability, and side effects.
Procedures
Women were screened using an author-constructed, self-report prenatal questionnaire described later and a qualitative urine cotinine test, followed by a chart review and nurse interview. Current smokers signed a consent form in accord with our approval from the Committee for the Protection of Human Subjects, the Institutional Review Board for the University of Texas Medical School at Houston. After screening and baseline assessments and an ultrasound confirming gestational age, participants were randomly assigned using a permuted block design to receive either bupropion, SR or matching placebo, stratifying on number of cigarettes/day (≤ 10 or >10) and history of preterm birth (yes or no). Bupropion SR was dosed at 150 mg/d for the first 3 days and 300 mg/d thereafter (150 mg twice a day). Placebo appearance, taste, and dosing instructions were identical. Patients and providers were masked to treatment group. Both the groups received four, weekly, 15-minute smoking cessation counseling sessions based on Clinical Practice Guidelines delivered by a research nurse.12 Procedures were developed and monitored via an FDA IND (10769) and an external Data and Safety Monitoring Board.
Participants attended research visits on weeks 1, 2, 3, and 5 of the 8-week trial, with an end-of-treatment (EOT) assessment conducted in study week 9. At each clinic visit, medication compliance and adverse reactions were assessed, and medical charts were reviewed. Medication was dispensed during weeks 1 and 5 clinic visits for the current and subsequent weeks. Follow-up assessments were also scheduled at the end of pregnancy (EOP at 34 weeks’ gestation and 2 weeks postpartum (PP). In cases where the EOT and EOP assessments were less than 2 weeks apart only the EOT assessment was completed.
Measures
The prenatal questionnaire assessed sociodemographics and smoking status. Self-reported smoking status was assessed using a multiple choice question demonstrated previously to improve disclosure of smoking in pregnant samples.21 Approximately half-way through the study, we added a qualitative, dipstick urine cotinine test (Accutest Urine Cotinine Test, cassette format) to verify smoking status (positive or negative; n = 432), with a 200 ng/mL threshold indicating regular smoking. Five percent of women tested (n = 22) claimed on the questionnaire to be nonsmokers but had a dipstick indicating that they were active smokers. Eligibility criteria involving psychiatric conditions, assessed via medical chart review and interview, were critical as bupropion reportedly has the potential to increase neuropsychiatric symptoms.22 Current depression and suicidality were assessed using the Patient Health Questionnaire—9-item version (PHQ-9). The PHQ-9 has acceptable reliability, validity, sensitivity, and specificity for diagnosing depression in primary care settings.23
The primary smoking outcome was self-reported total abstinence in the prior 7 days (7-day point prevalence)24 with saliva cotinine validation at the EOT. Saliva cotinine assays were collected at baseline, EOT, EOP, and PP and analyses were conducted by Salimetrics laboratory, with a 20 ng/mL threshold indicating regular smoking. Exhaled Carbon Monoxide (CO) concentration in parts per million (ppm) was measured at each assessment time point using the EC-50 (Vitalograph, Inc., Lenexa, KS), to indicate recent exposure to tobacco smoke in ppm. Maternal, perinatal, and neonatal outcomes assessed included intrauterine fetal death, spontaneous abortion, placental abruption, preterm birth (< 37 weeks, 0 days), preeclampsia, maternal weight gain, birth weight, umbilical artery pH, gestational age at delivery, fetal growth restriction (birth weight < 10th percentile),25 neonatal intensive care unit (NICU) admission, respiratory complications (per physician notes), other neonatal complications, and infant death.
Statistical Analyses
As this was a pilot study, sample size estimation was based on feasibility and with harm minimization as the guiding factor. Given the limited data on the use of bupropion in pregnancy, it seemed most prudent to initially conduct a smaller trial rather than incur potential risks to larger numbers of women. Our data plan included Bayesian statistical procedures which are more robust in their ability to detect treatment effects with small samples. Because of issues of recruitment feasibility, however, only descriptive statistics are presented.
Results
Sociodemographics
The full sample of 820 pregnant women screened for study eligibility were primarily nonHispanic black, (59.8%) and Hispanic white (27.6%), with a mean age of 26 years (standard deviation [SD] = 5.9). Mean gestational age was 16 weeks (SD = 8.8). The median number of pregnancies (including the current one) was 3 (range = 0–14); median number of living children was 1 (range = 0–9); and nearly a quarter of the sample (21%) reported a history of preterm birth. The majority of women (> 90%) were Medicaid recipients. Overall 14% of the women screened were smokers. The final sample, accounted for below, was 63.6% nonHispanic black; 27.3% nonHispanic white, and; 9.1% Hispanic white. Mean age was 26.9 (SD = 4.3); 18% were married; 45.5% were employed; 80% were multiparous; and 90% used Medicaid insurance. At baseline, the mean CO level was 12 ppm (SD = 12.4) and average number of cigarettes smoked per day was 10.7 (SD = 8.0).
Study Outcomes
Feasibility: Recruitment, Enrollment, and Retention
Fig. 1 indicates the significant difficulty with study recruitment and enrollment. Over a 16-month period of recruitment, 112 women were identified as smokers, of which 33 were not yet 18 years or were outside the gestational window by self-report. Of those initially eligible (n = 79), 86% were not eligible to participate based on the exclusionary criteria set a priori. The most common reason for exclusion was having a psychiatric disorder and/or taking psychiatric medication—over a quarter of the pregnant women smokers met this criteria. Also, many women were outside the gestational age window according to ultrasound. Thirteen percent of women declined to participate because they did not want to take medication during pregnancy or did not wish to participate in research. After 16 months of recruitment, only 11 women were eligible and willing to participate in the study. Five women were randomized to Bupropion and six to placebo. The 2-year pilot trial was not extended as the medication passed its expiration date.
Fig. 1.
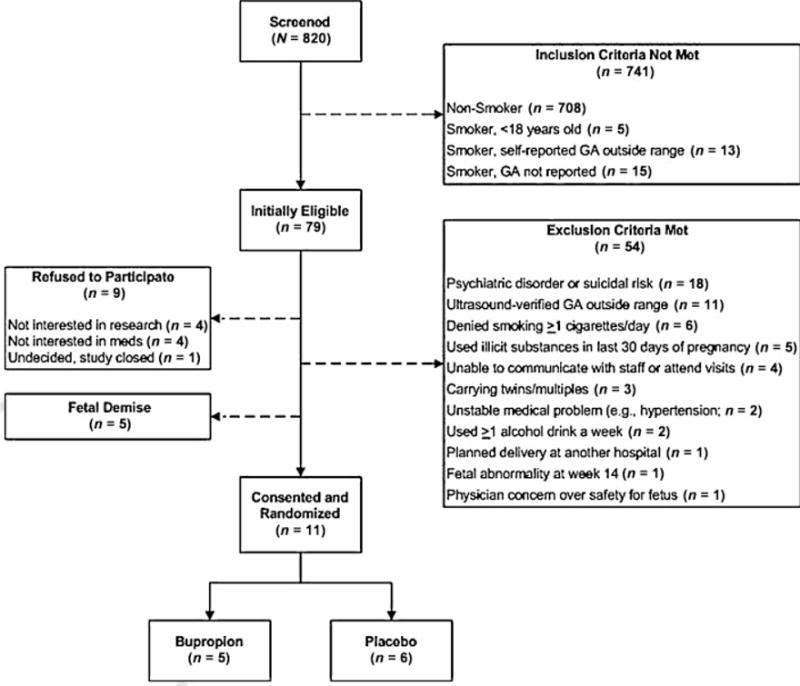
Study recruitment flow chart. GA, gestational age.
Counseling and follow-up attendance were high: One woman in each of the groups missed one counseling session each, and all women completed an EOT assessment. Eight women completed the PP assessment.
Smoking outcomes
At the EOT, two women in the placebo group reported abstinence in the last 7 days and cotinine samples were negative. All other participants reported continued smoking with positive cotinine samples. Four women in each condition reported at least one 24-hour quit attempt during the course of treatment. See, Table 1 for participant information.
Table 1.
Perinatal and smoking outcomes by participant
| End-of-treatment | SAE’s | |||||||
|---|---|---|---|---|---|---|---|---|
| PPT | Condition | GA at delivery (wk/d) | Birth Weight (g) | Birth weight percentile (%) | Smoking status | Saliva cotinine (ng/mL) | CO (ppm) | |
| 1 | Bupropion | 36.6 | 3,000 | 50 | Yes | 25.28 | 2 | Not relateda |
| 2 | Bupropion | ≥ 37b | 3,155 | UNK | Yes | 115.55 | 4 | N/A |
| 3 | Bupropion | 40.2 | 3,120 | 13 | Yes | 242.55 | 10 | N/A |
| 4 | Bupropion | 38.1 | 3,050 | 32 | Yes | 122.40 | 5 | Not relatedc |
| 5 | Bupropion | 41.0 | 3,310 | 17 | Yes | 76.37 | – | N/A |
| 6 | Placebo | 40.5 | 3,860 | 60 | No | 2.45 | 1 | N/A |
| 7 | Placebo | 39.2 | 2,655 | 3 | Yes | 128.11 | 10 | N/A |
| 8 | Placebo | 39.2 | 3,090 | 19 | Yes | 359.25 | 7 | N/A |
| 9 | Placebo | 39.2 | 2,635 | 3 | Yes | 478.22 | 30 | N/A |
| 10 | Placebo | 36.5 | 2,910 | 44 | No | 4.29 | 2 | N/A |
| 11 | Placebo | 39.3 | 2,750 | 5 | Yes | 179.99 | 5 | N/A |
Abbreviations: CO, carbon monoxide level; GA, gestational age; N/A, not applicable; not related, The SAE was determined to be not related to study medication; PPT, participant; SAE’s, serious adverse events; UNK, unknown.
Note: Smoking status refers to 7-day point prevalent smoking abstinence.
Newborn readmission for respiratory failure on day of life 5.
Exact gestational age at delivery was unavailable as participant did not deliver in the study hospital.
Hyperbilirubinemia secondary to ABO incompatibility.
Maternal, perinatal, and neonatal outcomes
Overall women reported few side effects and none chose to discontinue the study or the medication. Two women in the bupropion group reported vomiting; one woman stopped the medication for a few days but was able to resume the regimen. Other side effects in the bupropion group were dry mouth (n = 1) and loss of appetite (n = 1). Agitation (n = 1) and queasiness/nausea (n = 2) were reported by placebo participants. Of the 11 women, 9 reported concerns about possible side effects before beginning the medication, however, at the end of the study all reported they would recommend the medication to others and all but one woman (in the placebo group) would consider taking the medication in the future, indicating high acceptability.
Mean birth weight of infants in the bupropion group was slightly higher, 3,127 g (SD = 119; range, 3,000–3,310 g) than the placebo group, 2,983 g (SD = 462; range, 2,635–3,860 g). Mean length of infants in the bupropion group was 49.1 cm (SD = 1.9; range, 46–51 cm) compared with 43.3 (SD = 11.3; range, 20–48 cm) in the placebo group. Two infant serious adverse events occurred during the study, however, neither was judged to be related to the study medication (see, Table 1).
Discussion
The first, prospective, randomized pilot trial of bupropion with pregnant smokers was attempted at one large, urban clinic over a funded period of 2 years. Study aims to evaluate safety and efficacy were not achieved, yet critical knowledge and experiences were gained that will inform future research in this area. Most notably, there are major concerns with regard to the feasibility of recruitment. A large number of women were screened, the clinic smoking rate was sufficient (14%), but few were enrolled. The majority were excluded due to psychiatric disorders, being outside the gestational window, or refusal to participate. These data highlight the difficulty in conducting trials of psychoactive agents in pregnancy and perhaps call into question the potential for widespread use of bupropion as a treatment for smoking cessation in pregnant women.
As previous cohort studies suggested bupropion may be associated with a higher spontaneous abortion rate (19), we chose conservative eligibility criteria to minimize the likelihood of serious adverse events, both related and unrelated to the medication. We chose to enroll generally healthy women in their second trimester with unremarkable ultrasound results at 14 weeks’ gestation. On the basis of experiences with NRT trials, care was taken to select women less likely to have complications to avoid misattribution of adverse events to the medication. For example, Pollak et al15 were required by the data and safety monitoring board to stop their NRT trial in pregnant women because of higher rates of preterm labor in the NRT condition. Upon further review of these data, study groups appeared to be unbalanced on previous preterm labor at baseline. Stopping the trial was the prudent decision; however, whether or not NRT was the proximal cause of preterm birth remains unclear.
In addition, we excluded women currently taking psychiatric medication, with current major depression, and/or a history of suicide attempts, as the Food and Drug Administration (FDA) has issued a warning that bupropion may increase neuropsychiatric symptoms. A large minority of pregnant smokers were not eligible for the study due to psychiatric symptoms/diagnoses. Previous studies have also documented high rates of depression and psychiatric disorders in other samples of pregnant smokers.18 Future researchers should consider whether to relax the exclusion criteria and include women with current or past psychiatric symptoms or disorders. Bupropion is of course indicated for depression and therefore with close monitoring may incur low risk.
While being cautious in drawing conclusions from such a small sample size, observations of the women who were enrolled suggest that the medication was well tolerated and generally well accepted. Side effects were a concern for almost every woman before the trial and 11% of eligible women declined to participate. Side effect reports from our study women suggested that bupropion may increase risk of vomiting; however, all women were able to resume the medication regimen by adjusting the timing of administration. Two serious adverse events were reported during this trial, and although neither was judged to be related to the medication, increased health risks inherent to a low-income pregnant smoker population are not insignificant and need to be evaluated when considering pharmacotherapy.
While it is tempting to question the clinical utility of a medication that may only be applicable to a small subset of the generally high-risk pregnant smoker population, efficacious treatments are critically needed to reduce consequences (ranging from decreased birth weight to fetal demise) related to tobacco-exposed pregnancies. The potential morbidity, mortality, and economic burden prevented by increasing smoking abstinence (even by small amounts) during pregnancy are not trivial. The cost-to-benefit ratio of including pregnant smokers who might typically be excluded from bupropion medication trials should be carefully weighed against the costs of not intervening to prevent a tobacco-exposed pregnancy, particularly for heavier smokers. Future efforts are needed to determine an adequate balance between safety and feasibility, and large multisite studies will likely be needed to conduct definitive pharmacotherapy trials.
Acknowledgments
This study was supported by the Center for Clinical and Translational Sciences (CCTS) funded by CTSA awarded to the University of Texas Health Science Center at Houston (UL1 RR 024148) and the Larry C. Gilstrap MD Center for Perinatal and Women’s Health Research of the University of Texas Medical School at Houston.
These data, in part, were presented at the 33rd Annual Society for Maternal-Fetal Medicine Meeting, San Francisco, CA, February 13, 2013. The authors acknowledge the staff at the University of Texas-Houston Ostetrics and Gynecology Clinic for their assistance in conducting this study.
Footnotes
Conflicts of Interests
The authors declare that there are no conflicts of interest.
References
- 1.USDHHS. Women and Smoking: A Report of the Surgeon General US Department of Health and Human Services, Centers for Disease Control and Prevention Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2001 [Google Scholar]
- 2.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 3.Polakowski LL, Akinbami LJ, Mendola P. Prenatal smoking cessation and the risk of delivering preterm and small-for-gestational-age newborns. Obstet Gynecol. 2009;114(2 Pt 1):318–325. doi: 10.1097/AOG.0b013e3181ae9e9c. [DOI] [PubMed] [Google Scholar]
- 4.Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM, Centers for Disease Control and Prevention (CDC) Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Summ. 2009;58(4):1–29. [PubMed] [Google Scholar]
- 5.Lightwood JM, Phibbs CS, Glantz SA. Short-term health and economic benefits of smoking cessation: low birth weight. Pediatrics. 1999;104(6):1312–1320. doi: 10.1542/peds.104.6.1312. [DOI] [PubMed] [Google Scholar]
- 6.Miller DP, Villa KF, Hogue SL, Sivapathasundaram D. Birth and first-year costs for mothers and infants attributable to maternal smoking. Nicotine Tob Res. 2001;3(1):25–35. doi: 10.1080/14622200020032079. [DOI] [PubMed] [Google Scholar]
- 7.DHHS. The health benefits of smoking cessation. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1990. [Google Scholar]
- 8.Crawford JT, Tolosa JE, Goldenberg RL. Smoking cessation in pregnancy: why, how, and what next. Clin Obstet Gynecol. 2008;51(2):419–435. doi: 10.1097/GRF.0b013e31816fe9e9. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman E, Gremy I, Lang JM, Cohen AP. Low birthweight at term and the timing of fetal exposure to maternal smoking. Am J Public Health. 1994;84(7):1127–1131. doi: 10.2105/ajph.84.7.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullen PD. Maternal smoking during pregnancy and evidence-based intervention to promote cessation. Prim Care. 1999;26(3):577–589. doi: 10.1016/s0095-4543(05)70118-4. [DOI] [PubMed] [Google Scholar]
- 11.Windsor RA, Boyd NR, Orleans CT. A meta-evaluation of smoking cessation intervention research among pregnant women: improving the science and art. Health Educ Res. 1998;13(3):419–438. doi: 10.1093/her/13.3.419. [DOI] [PubMed] [Google Scholar]
- 12.Fiore MC, Jaen CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- 13.Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;9:CD010078. doi: 10.1002/14651858.CD010078. [DOI] [PubMed] [Google Scholar]
- 14.Oncken C, Dornelas E, Greene J, et al. Nicotine gum for pregnant smokers: a randomized controlled trial. Obstet Gynecol. 2008;112(4):859–867. doi: 10.1097/AOG.0b013e318187e1ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollak KI, Oncken CA, Lipkus IM, et al. Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. Am J Prev Med. 2007;33(4):297–305. doi: 10.1016/j.amepre.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berlin I, Grangé G, Jacob N, Tanguy ML. Nicotine patches in pregnant smokers: randomised, placebo controlled, multicentre trial of efficacy. BMJ. 2014;348:g1622. doi: 10.1136/bmj.g1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman T, Cooper S, Thornton JG, et al. Smoking, Nicotine, and Pregnancy (SNAP) Trial Team A randomized trial of nicotine-replacement therapy patches in pregnancy. N Engl J Med. 2012;366(9):808–818. doi: 10.1056/NEJMoa1109582. [DOI] [PubMed] [Google Scholar]
- 18.Blalock JA, Fouladi RT, Wetter DW, Cinciripini PM. Depression in pregnant women seeking smoking cessation treatment. Addict Behav. 2005;30(6):1195–1208. doi: 10.1016/j.addbeh.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Chun-Fai-Chan B, Koren G, Fayez I, et al. Pregnancy outcome of women exposed to bupropion during pregnancy: a prospective comparative study. Am J Obstet Gynecol. 2005;192(3):932–936. doi: 10.1016/j.ajog.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Chan B, Einarson A, Koren G. Effectiveness of bupropion for smoking cessation during pregnancy. J Addict Dis. 2005;24(2):19–23. doi: 10.1300/J069v24n02_02. [DOI] [PubMed] [Google Scholar]
- 21.Mullen PD, Carbonari JP, Tabak ER, Glenday MC. Improving disclosure of smoking by pregnant women. Am J Obstet Gynecol. 1991;165(2):409–413. doi: 10.1016/0002-9378(91)90105-z. [DOI] [PubMed] [Google Scholar]
- 22.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;1:CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 24.Jorenby DE, Leischow SJ, Nides MA, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340(9):685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- 25.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]


