Abstract
Many patients diagnosed with opioid dependence do not adequately respond to pharmacologic, psychosocial, or combination treatment, highlighting the importance of novel treatment strategies for this population. The current study examined the efficacy of a novel behavioral treatment focusing on internal cues for drug use (Cognitive Behavioral Therapy for Interoceptive Cues; CBT-IC) relative to an active comparison condition, Individual Drug Counseling (IDC), when added to methadone maintenance treatment (MMT) among those who had not responded to MMT. Participants (N=78) were randomly assigned to receive 15 sessions of CBT-IC or IDC as an adjunct to ongoing MMT and counseling. Oral toxicology screens were the primary outcome. Results indicated no treatment differences between CBT-IC and IDC and a small, significant reduction of self-reported drug use, but no change on toxicology screens. Tests of potential moderators, including sex, anxiety sensitivity, and coping motives for drug use, did not yield significant interactions. Among opioid-dependent out-patients who have not responded to MMT and counseling, the addition of IDC or CBT-IC did not result in additive outcome benefits. These results highlight the need for more potent treatment strategies for opioid dependence, particularly among those who do not fully respond to frontline treatment.
Keywords: anxiety sensitivity, cognitive behavioral therapy, coping motives, interoceptive exposure, opioid dependence, treatment outcome
Opioid agonist therapies and a number of psychosocial treatment approaches (e.g., cognitive-behavioral therapy) demonstrate efficacy in reducing illicit drug use among opioid-dependent patients (for review, see Dutra et al. 2008; Veilleux et al. 2010). However, many patients receiving treatment for opioid dependence continue to engage in regular use of other illicit substances (van den Brink & Haasen 2006). Among patients enrolled in methadone maintenance treatment programs, 50% report use of other drugs and alcohol (Brands et al. 2004; Nurco et al. 1988; San et al. 1990; Stizer et al. 1992; Woody et al. 1990), and many also meet criteria for alcohol, cannabis, stimulant, or sedative dependence (Strain 2002). Moreover, relapse rates following treatment for opioid dependence are high, with estimates suggesting that less than 25% of opioid-dependent individuals remain abstinent after ending methadone maintenance treatment (Dekimpe et al. 1998). The chronic relapsing nature of opioid dependence has been shown to drastically increase premature mortality (Hser et al. 2001; Jimenez-Trevino et al. 2011), highlighting the need for improved treatment strategies for this population.
Although cognitive-behavioral interventions such as contingency management, relapse prevention, and other focused interventions offer reliable benefit across drug use disorders, these benefits tend to be lower among those with drug dependence characterized by polysubstance use (Dutra et al. 2008). Also, the addition of psychosocial interventions (e.g., enhanced drug counseling, short-term interpersonal therapy, as well as cognitive-behavioral interventions) to agonist maintenance treatments, which generally already include a limited psychosocial counseling component, often does not yield additive benefits (see Amato et al. 2011). For example, a recent Cochrane review indicated that, as a whole, the addition of psychosocial interventions had no effect on treatment retention, rates of abstinence, treatment compliance, or psychiatric symptoms when added to agonist therapy in this manner (Amato et al. 2011). However, psychosocial treatments added to methadone maintenance are extremely heterogeneous with few large-scale randomized controlled trials, indicating that such studies are needed before definitive conclusions can be drawn (Veilleux et al. 2010).
Research on the chronic nature of drug dependence has stressed the importance of environmental contextual cues in drug craving (Rohsenow & Monti 1999). Some treatments, based on exposure to external drug use cues, have demonstrated promising results; however, findings have been somewhat inconsistent, with external cue exposure treatments often failing to show any benefit for reducing drug use behavior (for a review, see Conklin & Tiffany 2002). A possible explanation for this may be the limited ability of external cue procedures carried out in the context of a treatment center to generalize to natural settings (O’Brien et al. 1990; Conklin & Tiffany 2002).
Moreover, research has shown that drug craving may be associated with interoceptive as well as external cues (Marlatt & Gordon 1980; O’Connell & Martin 1987; Siegel 2005; Wikler 1965). In particular, both induced and naturally occurring negative mood states have been associated with increased drug craving (Childress et al. 1994; Robbins et al. 2000; Sherman et al. 1989) and use (Ouimette et al. 2010). Therefore, a treatment that addresses tolerance of internal cues for drug use may provide a promising approach for helping patients to respond appropriately to craving cues in a way that generalizes beyond the clinic setting (Otto, Powers & Fischmann 2005).
Cognitive Behavioral Therapy for Internal Cues (CBT-IC) is a novel cognitive-behavioral treatment for drug dependence focusing on interoceptive cues (Pollack et al. 2002). CBT-IC is a 15-session intervention (12 weekly sessions plus three booster sessions) emphasizing stepwise exposure, with rehearsal of adaptive behavioral responses to emotional and somatic cues associated with drug craving. In an initial examination of CBT-IC for illicit drug use among patients receiving methadone maintenance treatment, Pollack and colleagues (2002) found that this treatment approach was associated with greater reductions in illicit drug use among women, but not men, compared to a matching number of sessions of intensified treatment with their current outpatient drug counselor. This sex difference may be accounted for by differences in the relationship between affect and drug use in men and women. Research suggests that women may react with stronger craving to negative mood induction (Monti et al. 1995; Perkins et al. 2013; Rubonis et al. 1994), and women presenting for treatment of opioid dependence often report poorer health status, poorer health-related quality of life, and higher rates of co-occurring mental health disorders (Domingo-Salvany et al. 2010; Shand et al. 2011; Williamson et al. 2009). In addition, evidence suggests that women score higher on measures of anxiety sensitivity, indicating that they may be differentially sensitive to biological provocation of anxious states (Zvolensky, Eifert & Lejuez 2001). Women also endorse using licit and illicit drugs to regulate negative mood at a higher rate than men (Hearon et al. 2011; McHugh et al. 2013a; Stewart et al. 1997; Zywiak et al. 1996). Taken together, these results indicate that women may face more psychosocial stressors in daily life, have greater sensitivity to both physical and emotion distress, and be more likely to engage in drug use as a means of coping with these difficulties.
Since the publication of our pilot study (Pollack et al. 2002), the focus on interoceptive cue exposure for drug use disorders has received encouragement from additional pilot studies, including those examining cue exposure for opioid dependence (Tull et al. 2007) and distress tolerance treatments for substance use disorders (Bornovalova et al. 2012) and nicotine dependence (Brown et al. 2008). These studies provide additional evidence that modifying responses to internal cues (emotional or withdrawal related) is a promising avenue for expanding upon cognitive-behavioral interventions for drug use disorders.
To follow up on these promising findings, and to specifically examine potential differential response to CBT-IC among women and men, we conducted a randomized trial of the efficacy of CBT-IC relative to an active comparison treatment, Individual Drug Counseling (IDC; Mercer & Woody 1999). In addition, we focused our study exclusively on individuals who were failing to respond to current counseling and agonist (methadone maintenance) therapy, providing an estimate of the efficacy of a novel treatment strategy relative to an intensification of available counseling approaches. IDC focuses on identifying and avoiding cues for drug use, while making use of available 12-step treatment resources. In contrast, CBT-IC focuses on enhancing resilience to internal cues using exposure to interoceptive cues for drug use with rehearsal of adaptive responses, provided against a backdrop of other cognitive and coping interventions (Otto, O’Cleirigh & Pollack 2007).
Our study plan included examination of possible moderators of outcome, such as drug use motives and anxiety sensitivity. As specified by Kraemer et al. (2002), these variables meet criteria to be evaluated as moderators by being present before treatment, presumably uncorrelated with the treatments under study, and hypothesized to interact with the treatments under study. We hypothesized that CBT-IC would significantly reduce drug use as assessed by both self-report and biological measures in women but not men. We further hypothesized that those using drugs to cope, and those with elevated fears of somatic sensations of arousal (i.e., those with higher anxiety sensitivity), would respond better to CBT-IC (Otto et al. 2005).
METHODS
Participants
Participants meeting Diagnostic and Statistical Manual for Mental Disorders, 4th ed. (DSM-IV; American Psychiatric Association 1995) criteria for opioid dependence were recruited from two urban methadone maintenance treatment facilities between June 2005 and July 2011. Those who had failed to respond to the standard course of treatment provided by these facilities, treatment as usual (TAU; methadone maintenance plus group counseling provided by clinic substance abuse counselors on a weekly basis), were offered the opportunity to participate in a study that included the addition of one of two psychosocial treatments to TAU. Attendance at group treatment provided by the facilities was mandatory; therefore, all participants maintained the minimum required attendance in TAU throughout the study. Failure to respond to TAU was defined by: (1) the failure to achieve “take-home” status (a supply of methadone to be self-administered for one-week periods) for methadone dosing (participants had to have been at the clinic for at least four months before they were eligible to participate); (2) positive test results on at least two toxicology screens for illicit drugs during the two months prior to recruitment (screens were to be conducted by the treatment facility approximately twice per month); and (3) never having achieved two consecutive toxicology screens free of illicit substances since entering the current treatment episode.
Participants were recruited through posted study flyers or were referred to study staff by their substance abuse counselor. Additional eligibility criteria included current use of illicit substances (verified by oral toxicology screen), stable dose of methadone for at least two weeks prior to enrollment, and the presence of a current chronic stressor or affective disorder. Stressors included non- or limited-employment (defined as less than 20 hours per week of employment). Exclusion criteria included significant unstable medical illness, uncontrolled bipolar disorder, psychotic disorder, use of medication affecting methadone metabolism, and inability to complete informed consent procedures.
A total of 133 individuals provided informed consent (see CONSORT diagram, Figure 1). Of this total, 78 individuals remained interested and eligible for the study after completing screening assessments and were randomized to CBT-IC (n = 41) or to IDC (n = 37). These treatment conditions were provided in addition to any services patients were receiving as adjuncts to methadone maintenance; participants were compensated for completion of assessments and toxicology swabs associated with the study. All study procedures were approved by the Institutional Review Boards at Boston University and Massachusetts General Hospital.
FIGURE 1.
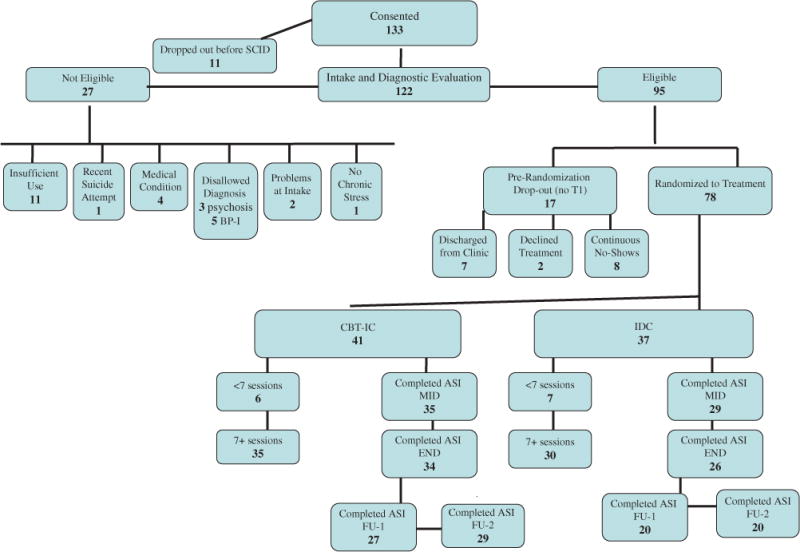
Consort Diagram Illustrating Derivation of Final Sample
Procedures
Participants were assessed for current Axis I disorders, including substance use, mood, and anxiety disorders, at a screening visit using the Structured Clinical Interview for DSM-IV (SCID; First et al. 1996). Those entering the protocol then completed a baseline assessment and were randomized to a treatment condition (CBT-IC vs. IDC). Treatment in both conditions included 12 weekly meetings with a study therapist followed by three booster sessions staggered across the next two months. Assessments occurred throughout the baseline, treatment, and booster phases and included both objective and clinician-rated instruments. Independent evaluators blind to study condition conducted all assessments.
Measures
The primary outcome measure was the percentage of oral toxicology swabs that were positive for any illicit substance. Participants completed these swabs at each assessment point as well as at each study therapy session. Interviewer-administered and self-report measures were used to complement these biological measures. Toxicology swabs were supervised by study staff and used oral specimen collection (Intercept®) to screen for opiates, methadone, cocaine, benzodiazepines, amphetamines, THC, and barbiturates.
Addiction Severity Index
The Addiction Severity Index (ASI; McLellan et al. 1980) is a clinician-administered interview designed to evaluate treatment outcome in substance-abusing populations across a number of domains, including health, employment, social functioning, and drug use behavior (McLellan et al. 1980). The ASI has demonstrated high inter-rater reliability (r =.89) and test-retest reliability (r =.92). This instrument was administered at baseline, mid-treatment, end of treatment, and each follow-up assessment and the Drug Use Composite score was used as an index of current substance use severity.
Drug Use Motives Questionnaire
The Drug Use Motives Questionnaire (DUMQ; Mueser et al. 1995) was used to assess reasons for use. It was adapted from the Drinking Motives Questionnaire (Cooper et al. 1992), which is a 15-item self-report measure assessing reasons for drinking across three dimensions: social, coping, and pleasure enhancement. The drug use adaptation of this measure has been used to assess reasons for drug use among patients with psychosis (Baker et al. 2005) and depression (Kay-Lambkin et al. 2011).
Anxiety Sensitivity Index
Anxiety sensitivity was measured using the Anxiety Sensitivity Index (Peterson & Reiss 1992), a 16-item questionnaire designed to assess one’s tendency to respond fearfully to anxiety-related symptoms. Data on the reliability and validity of the Anxiety Sensitivity Index scales have been favorable (Reiss et al. 1986). The Anxiety Sensitivity Index has been used as a measure of distress intolerance (McHugh & Otto 2011), and is linked to coping motives for drug use (e.g., Hearon et al. 2011; Johnson et al. 2010; Stewart et al. 1997), as well as other, maladaptive avoidance reactions among substance-abusing populations (Lejuez et al. 2008).
Study Therapists and Treatment Conditions
A total of 13 masters- or doctoral-level clinicians provided treatment in this trial. Study therapists provided interventions in both conditions. Sessions were audiotaped and the fidelity of interventions was assessed by a doctoral-level rater. Therapists were provided with weekly supervision by the first author (MWO).
CBT-IC has been described in detail in previous publications (Pollack et al. 2002; Otto et al. 2007). CBT-IC was conducted in 12 weekly, one-hour sessions followed by three booster sessions scheduled at two weeks, one month, and two months following completion of the protocol. Treatment focused on using exposure to emotional and somatic cues to help patients accept and tolerate these negative emotional states and craving sensations, and replace drug-use responses to these emotions and sensations with alternative, adaptive behaviors. This core treatment focus was embedded within four main components: (1) psychoeducation; (2) exposure to interoceptive cues for drug use with rehearsal of adaptive responses; (3) cognitive restructuring; and (4) somatic coping skills. Psychoeducation included a review of the patients’ behavioral patterns associated with craving and drug use as well as a rationale for incorporating an alternative response to these cues. Exposure to interoceptive cues involved repeated exposure to mostly emotional cues that the patient identified as increasing his/her drug craving, and focused on reducing conditioned drug craving or drug use by having patients sit with the emotional state followed by practice of a pre-identified alternative response. Cognitive restructuring was used as both an independent strategy and in conjunction with exposure procedures to help modify maladaptive cognitions regarding drug use. Somatic coping skills included muscle relaxation procedures and breathing retraining to aid patients in reducing the intensity of physical sensations associated with withdrawal.
IDC was adapted from the manual used in the National Institute on Drug Abuse Cocaine Collaborative Study authored by Mercer and Woody (1999), which has been adapted for use with other illicit drugs, including opioids (e.g., Weiss et al. 2011). This manual was chosen as an active comparison condition, given its efficacy in the Cocaine Collaborative Study when compared to group counseling alone, psychodynamic psychotherapy, and cognitive therapy for cocaine dependence (Crits-Christoph et al. 1999). The focus of IDC is helping patients to achieve and maintain abstinence through restructuring of behavioral patterns such that drug triggers are identified and avoided. This approach views addiction as a disease that damages multiple areas of an individual’s functioning and is consistent with the 12-step philosophy, including attendance at self-help groups such as Narcotics Anonymous. The timing and frequency of sessions were matched to CBT-IC.
Data Analysis
Both self-report and objective measures of drug use were utilized; the primary outcome was the proportion of positive toxicology screens for each participant collected over three time periods: baseline, treatment, and eight weeks of follow-up. Given that this sample was characterized by the use of multiple substances of abuse and that the treatment was not targeted to any individual substance, we examined whether toxicology screens were positive for any illicit or non-prescribed drug. The drug use composite score of the ASI was examined as a secondary outcome. Separate repeated measures ANOVAs were used to examine the main and interaction effects of time, condition, and sex on these outcomes. When examining toxicology outcomes, proportion of screens positive for illicit substances during the screening, treatment, and final follow-up periods were entered into the model with total number of swabs included as a covariate. For the ASI, drug use composite scores at baseline, termination, and final follow-up were included in the model. Post-hoc comparisons utilized Bonferroni correction to address inflation of alpha.
Linear regression analyses were used to test our hypotheses that coping motives and anxiety sensitivity would moderate the association between treatment condition and drug outcomes. DUMQ coping motives subscale score at baseline, treatment condition, and the coping motive by treatment interaction term (reflecting moderation, if significant) were entered into the model. Baseline ASI drug composite score was entered as a covariate. Finally, given that CBT-IC is targeted at increasing tolerance to interoceptive cues, we used a linear regression analysis to examine changes in anxiety sensitivity over the course of treatment, examining possible differing effects between treatment condition and sex.
Basic assumptions for all analyses were examined, with only one exception noted. Toxicology screen data were skewed in distribution: however, our sample size was sufficient so that effects of skewness should be trivial (see Games 1984), and these data were not transformed prior to analyses.
RESULTS
Baseline Characteristics
The sample included 78 adults (35 women) with a mean age of 42.3 years (SD = 9.9). Patients self-reported race and ethnicity. The majority of the sample was non-Hispanic (90%), with 68% reporting race as Caucasian, and the remaining 32% reporting race as Black/African American. The sample endorsed high levels of comorbidity, with 91% meeting criteria for at least one Axis I mood or anxiety disorder, and 85.9% meeting criteria for a secondary substance use disorder in addition to opioid dependence. Table 1 provides a breakdown of demographics, drug use history, and psychiatric diagnoses by both sex and treatment condition. These characteristics did not differ by study condition.
TABLE 1.
Baseline Drug Use and Psychiatric Characteristics of the Sample
| Women | Men | |||
|---|---|---|---|---|
| CBT-IC | IDC | CBT-IC | IDC | |
| Sample size | 18 | 17 | 23 20 | |
| Age (years) | 45 (10) | 44 (10) | 40 (10) | 41 (10) |
| Duration of heroin addiction (years) | 13 (8) | 13 (9) | 15 (10) | 16 (12) |
| % with at least 1 secondary SA disorder | 67% | 94% | 91% | 90% |
| Additional substance use disorders (%): | ||||
| Cocaine Use Disorder | 56% | 71% | 48% | 75% |
| Sedative Use Disorder | 11% | 24% | 26% | 25% |
| Cannabis Use Disorder | 33% | 18% | 26% | 35% |
| Amphetamine Use Disorder | 0% | 6% | 0% | 0% |
| Mood Disorder (%) | 72% | 59% | 48% | 60% |
| Anxiety Disorder (%) | 89% | 82% | 91% | 70% |
Note: CBT-IC = Cognitive Behavioral Therapy for Internal Cues; IDC = Individual Drug Counseling.
Patient Attrition
Eighteen of the sample of 78 (23%) were defined as non-completers based on a failure to complete at least 12 of 15 sessions. Completers and non-completers were not significantly different with respect to sex, race, ethnicity, or total years, but non-completers were significantly younger than completers (mean difference = −6.0 years, t (76) = −2.33, p < .05; see McHugh et al. 2013b for further details).
Non-completers did not differ by treatment, with 22% in the CBT-IC condition and 24% in the IDC condition failing to complete treatment (Fisher exact test p = 1.00).
Treatment Integrity
Therapy sessions were rated by a doctoral-level clinician. For evaluation of CBT-IC, ratings were made using a CBT-IC Adherence Rating Guide designed specifically for this study. This measure consists of five overall structure ratings based on general components of each session. Ratings of adherence to IDC were made using the Adherence/Competence Scale for Individual Drug Counseling (IDC) for Cocaine Dependence (Mercer et al. 1995), assessing five main domains of IDC (including monitoring drug use behaviors, encouraging abstinence, encouraging 12-step participation, relapse prevention, educating the client, and miscellaneous), and summarized for this study as an overall IDC counselor performance score. Finally, tapes from IDC sessions were rated for the presence of proscribed interventions from the CBT-IC condition (e.g., use of a CBT-IC model for treatment or specific cognitive-restructuring, problem-solving, or interoceptive exposure interventions). Sessions to be rated were selected at random from the available sessions for each of the study therapists. For CBT-IC, 24 sessions were evaluated and provided an overall average adherence score of 25.7 from a possible score of 34, indicating that, on average, 76% of session content goals were addressed in CBT sessions. For the IDC sessions, 17 sessions were evaluated and provided a mean quality score of 5.0 from a possible score of 7 (reflecting an overall rating of “good quality”). Importantly, no proscribed interventions (CBT-IC interventions) occurred during the 17 evaluated sessions of IDC.
Treatment Outcome
The treatment groups did not differ on the proportion of positive toxicology screens at baseline (81.3% in CBT-IC and 79.1% in IDC; t (76) = .34, p =.74), nor did they differ in the baseline ASI Drug Use Composite scores (CBT-IC, M = .23, SD = .09; IDC, M = .21, SD = .12 t (75) = .85, p = .40).
Examining our primary outcome measure, proportion of positive toxicology swabs, a repeated-measures ANOVA revealed no significant main effects of time (F (2, 116) = 1.19, p = .31, partial η2 = .02), condition (F (1, 58) = .41, p = .53, partial η2 = .007) or sex (F (1, 58) = .07, p = .80, partial η2 = .001), nor significant interactions of sex by time (F (2, 116) = .90, p = .41, partial η2 = .02), condition by time (F (2, 116) = .07, p = .93, partial η2 = .001), or their three-way interaction (F (2, 116) = .34, p = .72, partial η2 = .006). We also examined the potential for sleeper effects, given evidence in the literature for delayed efficacy for CBT interventions (Carroll et al. 1994; Carroll et al. 2006; Rawson et al. 2002). An exploratory review of pairwise comparisons across time revealed that patients had a significant decrease in proportion of positive toxicology swabs between the treatment period and followup period (p = .04). This occurred after a non-insignificant increase in positive toxicology swabs between the baseline and treatment periods (p = .94), indicating that patients slightly increased use during treatment followed by a significant reduction from this highest point of use during follow-up (see Figure 2).
FIGURE 2.
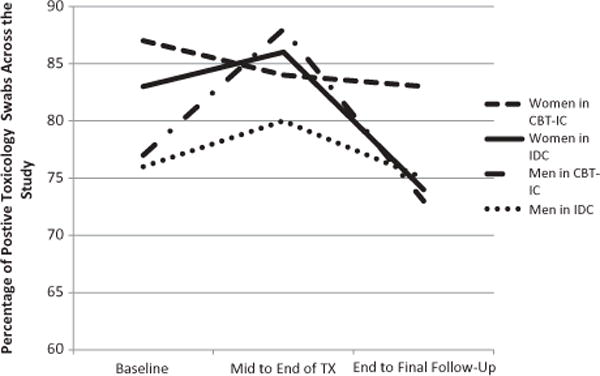
Percentage of Positive Toxicology Screens across the Study by Sex and Treatment Condition
When the same model was examined looking at ASI drug composite scores at baseline, termination, and final follow-up, results revealed a significant main effect of time (F (2, 84) = 67.59, p < .01, partial η2 = .15); however, no significant effects emerged for condition (F (1, 42) = .02, p = .89, partial η2 = .00), sex (F (1, 42) = .75, p = .39, partial η2 = .02), sex by time (F (2, 84) = .04, p = .96, partial η2 = .001), condition by time (F (2, 84) = .44, p = .65, partial η2 = .01), nor their three-way inter-action (F (2, 84) = .41, p = .67, partial η2 = .01). Pairwise comparisons revealed a significant reduction in ASI scores between baseline and treatment termination (p < .01) that was maintained at final follow-up.
Moderators of Treatment Outcome
Multiple regression analyses were used to examine the predictive influence of anxiety sensitivity on our selected outcome variables. In the first model, the Anxiety Sensitivity Index score (β = .30; t = 2.25; p = .03) and the covariate (ASI drug use severity at baseline: β = .33: t = 2.41; p = .02) significantly predicted ASI drug use severity at termination, but the interaction term (reflecting moderation) did not predict significantly. An identical model was examined predicting ASI at follow-up; in this model, only ASI at baseline emerged as a significant individual predictor (β = .38; t = 2.34; p = .03). In a similar regression analysis examining toxicology screen results, Anxiety Sensitivity Index score approached significance as a predictor (β = −.29; t = −2.01; p = .051), with non-significant results for the covariate, baseline ASI drug composite (β = −.12; t = −.82; p = .42).
In the regression examining moderational effects of coping motives on toxicology outcomes, there was no evidence for a moderational effect of either variables and only the covariate (baseline ASI drug use severity) was significantly associated with toxicology outcomes.
Changes in Anxiety Sensitivity Across Treatment
To examine whether our treatment changed anxiety sensitivity scores across treatment, change scores from baseline to termination for the Anxiety Sensitivity Index was calculated. Multiple regression was used to predict changes in this moderator with treatment condition, sex, and their interaction included as predictors. Results indicate no significant effects for treatment condition (β = −.10; t = −.22; p = .83), sex (β = −.09; t = −.57; p = .57), or their interaction (β = .19; t = .39; p = .70) in predicting changes in anxiety sensitivity.
DISCUSSION
In a previous study, CBT-IC offered significant benefit over ongoing counseling treatment only for women (Pollack et al. 2002). This finding fits well with the available literature on differences between the sexes in both drug use motives and sensitivity to internal cues (for review, see Otto et al. 2005). However, in the current study we found no evidence for the superiority of CBT-IC over the active comparison condition, IDC, for either women or men. The failure to detect differences between treatments was not due to both intervention groups doing well; although there were subtle signs of benefit for specific contrasts at followup for the objective measure, and self-report of decreased use, the primary analysis of change in objective data on illicit drug use over time failed to support the efficacy of the interventions. This mirrors recent findings in prescription opioid dependence that found no benefit of adding counseling (IDC) to buprenophine-naloxone maintenance (Weiss et al. 2011).
There are several potential explanations for the failure of this trial to replicate findings from our pilot study. One difference between the pilot study and the current trial concerns the comparison condition. Instead of relying on counseling treatment provided by current therapists in the clinic as done in the pilot study, treatment in the comparison condition in the current trial was provided by study therapists in addition to the ongoing counseling provided as part of the methadone maintenance treatment program. Thus, in the current study, we were attempting to detect outcome benefits relative to another active treatment strategy among patients who had not initially responded to treatment.
Indeed, all patients enrolled in the trial had been unable to achieve an illicit drug-free state despite at least four months of combined counseling and agonist therapy. Most of these patients (52%) had been using more than one illicit drug for over 10 years, and all patients had been enrolled in the clinic for at least three months. Hence, the failure of benefit of CBT-IC in this study reflects a lack of efficacy in a sample that has not responded to a frontline treatment for chronic opioid dependence (methadone maintenance therapy plus counseling).
Also, despite the finding that anxiety sensitivity was a significant predictor of drug use across the treatment period, we did not find the expected interactions between this sensitivity to internal cues and our treatment designed to address these sensitivities. This outcome reflects a failure to change one of the specific instrumental outcomes under study. Given ample evidence for the efficacy of interoceptive exposure for reducing anxiety sensitivity and aiding medication discontinuation in other populations (Otto et al. 2010; Smits et al. 2008), there may be factors unique to this population that prevented therapeutic learning from occurring. Our therapist adherence data indicated adequate differentiation of the two treatment conditions and that, in general, component interventions for CBT-IC were provided to patients. Nonetheless, supervision indicated that helping patients attend to therapeutic content, recall therapeutic interventions between sessions, and engage in homework outside of the session was a challenge. In particular, the presence of ongoing illicit drug use in addition to methadone treatment may have interfered with the acquisition and/or consolidation of therapeutic learning hypothesized to be the active mechanism of this type of treatment approach. This is consistent with evidence for fear-conditioning deficits among this population of patients (Basden 2010). If this is the case, the question of whether CBT-IC has greater efficacy when used in individuals who have achieved a period of abstinence and/or who are not currently receiving opioid agonist treatment remains an interesting but untested issue. The application of this treatment to a population that is not characterized by such learning deficits may achieve benefits that were not detected in this chronic, treatment-refractory population.
There are several study limitations. In this study, we used both biological and self-report outcomes to attempt to minimize the limitations of measuring illicit drug use. The discrepant results between self-report and biological measures may be attributable to a reduction in use or a reduction in functional interference related to use, which is captured by the ASI, but not drug screens. Nonetheless, the absence of an effect in the primary outcome measure (drug screens) suggested that the impact of the treatments on outcomes was minimal. Second, a number of participants (23%) did not receive a full dose of treatment (i.e., non-completers); however, this did not differ by treatment condition. Third, we did not find evidence of a moderational effect of sex that was observed in a pilot trial of this treatment. Although it is possible that a small effect was present but could not be detected with this moderate sample size, the absence of a treatment by sex effect is consistent with the literature on sex differences in treatment outcome for substance use disorders (see Greenfield et al. 2007). Finally, despite efforts to retain participants, a number of participants were lost to follow-up. This is not unexpected in this population, which is characterized by high rates of homelessness and incarceration; however, it is possible that the results would have differed if these participants had completed all follow-up assessments.
In this treatment-refractory, opioid-dependent sample, the addition of a novel cognitive-behavioral treatment (CBT-IC) or Individual Drug Counseling (IDC) did not differ from each other and did not result in reliable benefits over time, with significant changes on self-report but not objective measures of illicit drug use. These results, considered along with evidence for high rates of relapse following discontinuation of opioid agonist therapies (Dekimpe et al. 1998; Weiss et al. 2011), highlight the need for the development of new treatment strategies for addressing ongoing illicit drug use during opioid agonist treatment.
References
- Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Systematic Review. 2011;10:CD004147. doi: 10.1002/14651858.CD004147.pub4. [DOI] [PubMed] [Google Scholar]
- Baker A, Bucci S, Lewin TJ, Richmond R, Carr VJ. Comparisons between psychosis samples with different patterns of substance use recruited for clinical and epidemiological studies. Psychiatry Research. 2005;134(3):241–250. doi: 10.1016/j.psychres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Basden SLD. Doctoral thesis. Boston University; Boston, MA: 2010. Acquisition and extinction in a substance dependent sample: A de novo fear conditioning study. [Google Scholar]
- Bornovalova MA, Gratz KL, Daughters SB, Hunt ED, Lejuez CW. Initial RCT of a distress tolerance treatment for individuals with substance use disorders. Drug Alcohol Dependence. 2012;122(1–2):70–76. doi: 10.1016/j.drugalcdep.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands B, Blake J, Sproule B, Gourlay D, Busto U. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug and Alcohol Dependence. 2004;73(2):199–207. doi: 10.1016/j.drugalcdep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, et al. Distress tolerance treatment for earlylapse smokers: Rationale, program description, and preliminary findings. Behavior Modification. 2008;32(3):302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, Rounsaville BJ. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. Journal of Consulting and Clinical Psychology. 2006;7(5):955–966. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin FH. One year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: Delayed emergence of psychotherapy effects. Archives of General Psychiatry. 1994;51(12):989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, McLellan AT, MacRae J, Natale M, O’Brien CP. Can induced moods trigger drug-related responses in opiate abuse patients? Journal of Substance Abuse Treatment. 1994;11(1):17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97(2):155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, Skinner MB, Windle M. Development and validation of a three dimensional measure of drinking motives. Psychological Assessment. 1992;4:123–132. [Google Scholar]
- Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Muenz LR, Thase ME, Weiss RD, Gastfriend DR, et al. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Archives of General Psychiatry. 1999;56(6):493–502. doi: 10.1001/archpsyc.56.6.493. [DOI] [PubMed] [Google Scholar]
- Dekimpe MG, Van de Gucht LM, Hanssens DM, Keiko I. Long-run abstinence after treatment for narcotics abuse: What are the odds? Management Science. 1998;44:1478–1492. [Google Scholar]
- Domingo-Salvany A, Brugal MT, Barrio G, Gonzalez-Saiz F, Bravo MJ, de la Fuente L. Gender differences in health related quality of life of young heroin users. Health and Quality of Life Outcomes. 2010;8:145. doi: 10.1186/1477-7525-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Power MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165(2):179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Games PA. Data transformations, power, and skew: A rebuttal to Levine and Dunlap. Psychological Bulletin. 1984;95:345–347. [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, et al. Substance abuse treatment entry, retention, and outcome in women: A review of the literature. Drug and Alcohol Abuse. 2007;86(1):1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearon BA, Calkins AW, Halperin DM, McHugh RK, Murray HW, Otto MW. Anxiety sensitivity and illicit sedative use among opiate-dependent women and men. American Journal of Drug and Alcohol Abuse. 2011;37(1):43–47. doi: 10.3109/00952990.2010.535581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Archives of General Psychiatry. 2001;58(5):503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- Jimenez-Trevino L, Saiz PA, Garcia-Portilla MP, Diaz-Mesa EM, Sanchez-Lasheras F, et al. A 25-year follow-up of patients admitted to methadone maintenance for the first time: Mortality and gender differences. Addictive Behaviors. 2011;36(12):1184–1190. doi: 10.1016/j.addbeh.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Johnson K, Mullin JL, Marshall EC, Bonn-Miller MO, Zvolensky M. Exploring the mediational role of coping motives for marijuana use in terms of the relation between anxiety sensitivity and marijuana dependence. American Journal of Addiction. 2010;19(3):277–82. doi: 10.1111/j.1521-0391.2010.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay-Lambkin FJ, Baker AL, Kelly B, Lewin TJ. Clinician-assisted computerized versus therapist-delivered treatment for depressive and addictive disorders: S randomized controlled trial. The Medical Journal of Australia. 2011;195(3):44–50. doi: 10.5694/j.1326-5377.2011.tb03265.x. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Zvolensky MJ, Daughters SB, Bornovalova MA, Paulson A, Tull MT, Ettinger K, Otto MW. Anxiety sensitivity: A unique predictor of dropout among inner-city heroin and crack/cocaine users in residential substance use treatment. Behavior Research and Therapy. 2008;46(7):811–818. doi: 10.1016/j.brat.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Determinants of relapse: Implications for the maintenance of behavior change. In: Davidson P, editor. Behavioral Medicine: Changing Health Life Styles. New York: Brunner/Mazel; 1980. [Google Scholar]
- McHugh RK, Devito EE, Dodd D, Carroll KM, Potter JS, et al. Gender differences in a clinical trial for prescription opioid dependence. Journal of Substance Abuse Treatment. 2013a;45(1):38–43. doi: 10.1016/j.jsat.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Murray HW, Hearon BA, Pratt EM, Pollack MH, Safren SA, Otto MW. Predictors of dropout from psychosocial treatment in opioid-dependent outpatients. American Journal on Addictions. 2013b;22(1):18–22. doi: 10.1111/j.1521-0391.2013.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Otto MW. Domain-general and domain-specific strategies for the assessment of distress intolerance. Psychology of Addictive Behaviors. 2011;25(4):745–749. doi: 10.1037/a0025094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, O’Brien CP, Woody GE. An improved evaluation instrument for substance abuse patients: The Addition Severity Index. Journal of Nervous and Mental Diseases. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Mercer DE, Woody GE. Individual Drug Counseling. Washington, DC: U.S. Government Printing Office; 1999. (NIH Pub. No. 99-4380). [Google Scholar]
- Monti PM, Rohsenow DJ, Colby SM, Abrams DB. Smoking among alcoholics during and after treatment: Implications for models, treatment strategies, and policy. In: Ferig JB, editor. Alcohol and Tobacco: From Basic Science to Policy. Bethesda: National Institute of Alcohol Abuse and Addiction; 1995. [Google Scholar]
- Mueser KT, Nishith P, Tracy JI, DeGirolamo J, Molinaro M. Expectations and motives for substance use in schizophrenia. Schizophrenia Bulletin. 1995;21(3):367–378. doi: 10.1093/schbul/21.3.367. [DOI] [PubMed] [Google Scholar]
- Nurco DN, Kinlock TW, Hanlon TE, Ball JC. Nonnarcotic drug use over an addiction career—a study of heroin addicts in Baltimore and New York City. Comprehensive Psychiatry. 1988;29(5):450–459. doi: 10.1016/0010-440x(88)90060-0. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systematic cue exposure with standard treatment in recovering drug dependent patients. Addictive Behaviors. 1990;15(4):355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- O’Connell KA, Martin EJ. Highly tempting situations associated with abstinence, temporary lapse, and relapse among participants in smoking cessation programs. Journal of Consulting and Clinical Psychology. 1987;55(3):367–371. doi: 10.1037//0022-006x.55.3.367. [DOI] [PubMed] [Google Scholar]
- Otto MW, McHugh RK, Simon NM, Farach FJ, Worthington JJ, Pollack MH. Efficacy of CBT for benzodiazepine discontinuation in patients with panic disorder: Further evaluation. Behavior Research and Therapy. 2010;48(8):720–727. doi: 10.1016/j.brat.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, O’Cleirigh CM, Pollack MH. Attending to emotional cues for drug use: Bridging the gap between clinic and home behavior. NIDA Science and Practice Perspectives. 2007 Apr;:48–55. doi: 10.1151/spp073248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Powers MB, Fischmann D. Emotional exposure in the treatment of substance use disorders: Conceptual model, evidence, and future directions. Clinical Psychology Review. 2005;25(6):824–39. doi: 10.1016/j.cpr.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Ouimette P, Read JP, Wade M, Tirone V. Modeling associations between posttraumatic stress symptoms and substance use. Addictive Behaviors. 2010;35(1):64–67. doi: 10.1016/j.addbeh.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. Negative mood effects on craving to smoke in women versus men. Addictive Behaviors. 2013;38(2):1527–1531. doi: 10.1016/j.addbeh.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RA, Reiss S. Anxiety Sensitivity Index Revised Test Manual. Palos Heights, IL: International Diagnostic Systems; 1992. [Google Scholar]
- Pollack MH, Penava SA, Bolton E, Worthington JJ, Allen GL, Farach FJ, Otto MW. A novel cognitive-behavioral approach for treatment-resistant drug dependence. Journal of Substance Abuse Treatment. 2002;23(4):335–342. doi: 10.1016/s0740-5472(02)00298-2. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann MJ, Shoptaw S, Farabee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance for cocaine dependence. Archives of General Psychiatry. 2002;59(9):817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky M, McNally RJ. Anxiety sensitivity, anxiety frequency, and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24(1):1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, Cornish JW, O’Brien CP. Mood state and recent cocaine use are not associated with levels of cocaine cue reactivity. Drug and Alcohol Dependence. 2000;59(1):33–42. doi: 10.1016/s0376-8716(99)00103-9. [DOI] [PubMed] [Google Scholar]
- Rohsenow JH, Monti PM. Does urge to drink predict relapse after treatment? Alcohol Research and Health. 1999;23(3):225–232. [PMC free article] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DT, Gulliver SB, Sirota AD. Alcohol cue reactivity and mood induction in male and female alcoholics. Journal of Studies on Alcohol. 1994;55(4):487–494. doi: 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- San L, Cami J, Peri JM, Mata R, Porta M. Efficacy of clonidine, guanfacine and methadone in the rapid detoxification of heroin addicts: A controlled trial. British Journal of Addiction. 1990;85(1):141–147. doi: 10.1111/j.1360-0443.1990.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Shand FL, Degenhardt L, Slade T, Nelson EC. Sex differences amongst dependent heroin users: Histories, clinical characteristics and predictors of other substance dependence. Addictive Behaviors. 2011;36(1–2):27–36. doi: 10.1016/j.addbeh.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman JE, Zinser MC, Sideroff SI, Baker TB. Subjective dimensions of heroin urges: Influence of heroin-related and affectively negative stimuli. Addictive Behaviors. 1989;14(6):611–623. doi: 10.1016/0306-4603(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Siegel S. Two views of the addiction elephant: A comment on McSweeney, Murphy, and Kowal. Experimental and Clinical Psychopharmacology. 2005;13(3):190–193. doi: 10.1037/1064-1297.13.3.190. [DOI] [PubMed] [Google Scholar]
- Smits JA, Berry AC, Tart CD, Powers MB. The efficacy of cognitive-behavioral interventions for reducing anxiety sensitivity: A meta-analytic review. Behavior Research and Therapy. 2008;46(9):1047–54. doi: 10.1016/j.brat.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Karp J, Pihl RO, Peterson RA. Anxiety sensitivity and self-reported reasons for drug use. Journal of Substance Abuse. 1997;9:223–240. doi: 10.1016/s0899-3289(97)90018-3. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Iguchi MY, Felch LJ. Contingent take-home incentive: Effects on drug use of methadone maintenance patients. Journal of Consulting and Clinical Psychology. 1992;60(6):927–934. doi: 10.1037//0022-006x.60.6.927. [DOI] [PubMed] [Google Scholar]
- Strain EC. Assessment and treatment of comorbid psychiatric disorders in opioid-dependent patients. The Clinical Journal of Pain. 2002;18:14–27. doi: 10.1097/00002508-200207001-00003. [DOI] [PubMed] [Google Scholar]
- Tull MT, Schulzinger D, Schmidt NB, Zvolensky MJ, Lejuez CW. Development and initial examination of a brief intervention for heightened anxiety sensitivity among heroin users. Behavior Modification. 2007;31(2):220–242. doi: 10.1177/0145445506297020. [DOI] [PubMed] [Google Scholar]
- van den Brink W, Haasen C. Evidenced-based treatment of opioid-dependent patients. Canadian Journal of Psychiatry. 2006;51(10):635–646. doi: 10.1177/070674370605101003. [DOI] [PubMed] [Google Scholar]
- Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ. A review of opioid dependence treatment: Pharmacological and psychosocial interventions to treat opioid addiction. Clinical Psychology Review. 2010;30(2):155–166. doi: 10.1016/j.cpr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Archives of General Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Conditioning factors in opiate addiction and relapse. In: Wilner DI, Kassenbaum GG, editors. Narcotics. New York: McGraw-Hill; 1965. pp. 85–100. [Google Scholar]
- Williamson A, Darke S, Ross J, Teeson M. Changes and predictors of change in the physical health status of heroin users over 24 months. Addiction. 2009;104(3):465–470. doi: 10.1111/j.1360-0443.2008.02475.x. [DOI] [PubMed] [Google Scholar]
- Woody GE, McLellan AT, Luborsky L, O’Brien CP. Psychotherapy and counseling for methadone-maintained opiate addicts: Results of research studies. NIDA Research Monograph. 1990;104:9–23. [PubMed] [Google Scholar]
- Zvolensky MJ, Eifert GH, Lejuez CW. Emotional control during recurrent 20% carbon dioxide-enriched air induction: Relation to individual difference variables. Emotion. 2001;1(2):148–165. doi: 10.1037/1528-3542.1.2.148. [DOI] [PubMed] [Google Scholar]
- Zywiak W, Connors GJ, Maisto S, Westerberg VS. Section IIA. Replication and extension of Marlatt’s taxonomy: Relapse research and the reasons for drinking questionnaire: A factor analysis of Marlatt’s relapse taxonomy. Addiction. 1996;91:s121–s130. [PubMed] [Google Scholar]


