Abstract
In addition to using glutamate as a neurotransmitter at central synapses, many primary sensory neurons release glutamate from peripheral terminals. Primary sensory neurons with cell bodies in dorsal root or trigeminal ganglia produce glutaminase, the synthetic enzyme for glutamate, and transport the enzyme in mitochondria to peripheral terminals. Vesicular glutamate transporters fill neurotransmitter vesicles with glutamate and they are shipped to peripheral terminals. Intense noxious stimuli or tissue damage causes glutamate to be released from peripheral afferent nerve terminals and augmented release occurs during acute and chronic inflammation. The site of action for glutamate can be at the autologous or nearby nerve terminals. Peripheral nerve terminals contain both ionotropic and metabotropic excitatory amino acid receptors (EAARs) and activation of these receptors can lower the activation threshold and increase the excitability of primary afferents. Antagonism of EAARs can reduce excitability of activated afferents and produce antinociception in many animal models of acute and chronic pain. Glutamate injected into human skin and muscle causes acute pain. Trauma in humans, such as arthritis, myalgia, and tendonitis, elevates glutamate levels in affected tissues. There is evidence that EAAR antagonism at peripheral sites can provide relief in some chronic pain sufferers.
Keywords: Excitatory amino acid receptors, Glutaminase, Inflammation, Sensory nerves, Vesicular glutamate transporters
1. Introduction
Peripheral afferent nerve terminals provide sensory innervation to skin, joint, fascia, muscle, bone, and viscera. In the role as sensory terminals, they transduce mechanical, thermal, and chemical stimuli to electrochemical information that is transmitted to the spinal cord and brainstem (Woolf & Ma, 2007). The current review will focus on the glutamatergic role of the peripheral sensory terminal based upon two important neuroscience concepts from the last half of the 20th century. Firstly, L-glutamate is a major excitatory neurotransmitter of the vertebrate nervous system including primary afferents (Johnson, 1972a,b), and secondly, some peripheral sensory terminals have efferent functions (Jancso et al., 1967). Although there is evidence for the role of glutamate in visceral peripheral afferents (McRoberts et al., 2001; Ghosh et al., 2007; Lindström et al., 2008), the focus of this review will concentrate on evidence of glutamate release from and influence on somatic peripheral afferents.
2. Primary afferents and efferent function
Primary afferent neurons are nerve cells that convey peripheral sensory information to the spinal cord and brainstem (Fig. 1). They possess a cell body located in the dorsal root ganglion (DRG) or trigeminal ganglion (TG) and an axonal fiber that projects from the periphery to the spinal cord or brainstem (Woolf & Ma, 2007). Primary afferent neurons can be classified into two broad functional categories. In one category, neurons convey proprioceptive, vibratory, or discriminative touch sensations and have axons that are associated with peripheral cellular receptors, e.g., muscle spindles, Meissners’s corpuscles, and Pacian corpuscles. The DRG and TG cell bodies typically are large in diameter, the ‘A’ subtype, and have large, heavily myelinated, fast conducting axons, Aα and Aβ fibers. The second category is composed of neurons that transmit innocuous thermal or noxious information and have fibers distributed as free nerve endings in peripheral tissues. The DRG and TG cell bodies can range in diameter from large to small, the ‘B’ subtype, and have myelinated to unmyelinated axons, Aβ, Aδ, and C fibers.
Fig. 1.
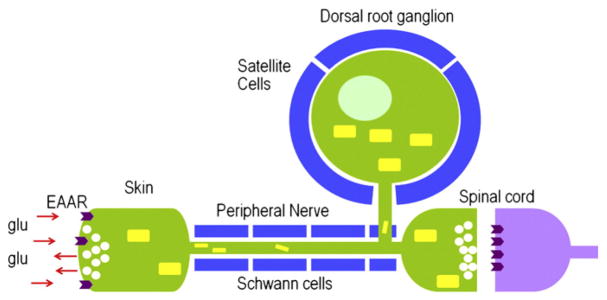
Primary afferent neuron. Nociceptive and thermally responsive non-nociceptive neurons have free nerve endings distributed in target tissue, e.g., skin. The neuronal soma resides in the dorsal root ganglion or trigeminal ganglion. A primary sensory neuron is a pseudo-unipolar cell with a single axon projecting from the periphery to the spinal cord or brainstem. TG and DRG neurons store neurogenic substances, such as substance P, calcitonin gene-related peptide, and glutamate, in vesicles (white circles) for release in the periphery and spinal cord. Glutaminase, the synthetic enzyme for glutamate, is produced in the cell body, translocated to mitochondria (yellow rectangles), and shipped to nerve terminals. Glutamate, therefore, can be synthesized for neurotransmission at peripheral and spinal nerve endings. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Pain-sensing, primary afferent neurons are termed nociceptors and are classified into two general types. Cutaneous ‘Aδ’ and ‘Aβ’ nociceptors have lightly to heavily myelinated fibers and are responsible for rapid, acute pain sensation, whereas ‘C’ nociceptors have unmyelinated fibers and produce a delayed, ‘aching’ pain (Fang et al., 2005; Willis, 2007). In addition to sensory responsiveness and conductance, these neurons release substances into the periphery to cause neurogenic inflammation. Neuropeptides, such as substance P (SP) and calcitonin gene-related peptide (CGRP), have proinflammatory actions such as vasodilation, plasma extravasation, and stimulation of immune and resident tissue cells (O’Connor et al., 2004). In addition, peripheral nerve terminals release glutamate causing sensitization of surrounding afferent terminals and local tissues (Skerry & Genever, 2001; Carozzi et al., 2008a). The ability of peripheral nerve terminals to release glutamate after specific types of stimulation intimates that DRG neurons are glutamatergic and participate in a peripheral glutamine metabolic cycle (Miller et al., 2002).
3. Glutamate metabolism in primary afferent neurons
3.1. Glutamate
Both the central and peripheral nervous systems (CNS and PNS) have a glutamine cycle for the production and degradation of glutamate as a neurotransmitter (Fig. 2; Miller et al., 2002; McKenna, 2007). A series of studies demonstrates a high concentration of glutamate in DRG, dorsal roots, and peripheral nerve (Porcellati and Thompson, 1957; Graham et al., 1965; Graham et al., 1967; Wheeler & Boyarsky, 1968; Duggan & Johnston, 1970a,b; Johnson & Aprison, 1970a,b; Johnson, 1972b; Santini & Berl, 1972; Roberts et al., 1973; Osborne et al., 1974; Roberts & Keen, 1974a; Johnson, 1977). Using immunohistochemistry, glutamate-immunoreactivity (ir) has been demonstrated in rat DRG and TG neurons ranging from 30 to 70% of the total percentage of cells (Wanaka et al., 1987; Battaglia & Rustioni, 1988; Kai-Kai, 1989; Kai-Kai & Howe, 1991; Azerad et al., 1992; Keast & Stephensen, 2000).
Fig. 2.
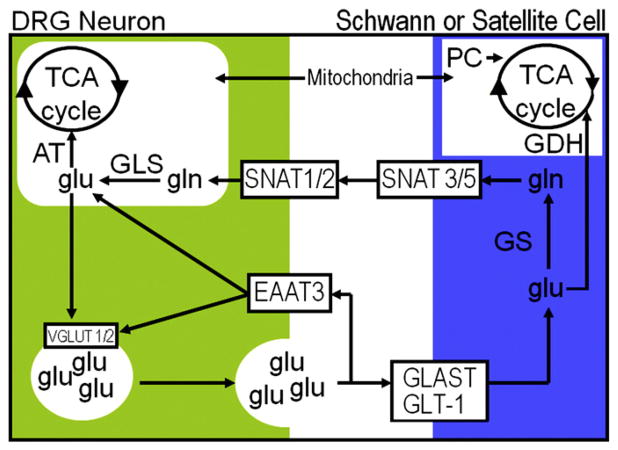
Glutamine cycle in peripheral nervous system. Glutamate (glu) can be taken up by neurons or glia. In neurons, glu is taken up by excitatory amino acid transporter 3 (EAAT). Schwann or satellite cells take up glu via glutamate–aspartate transporter (GLAST) and glutamate transporter 1 (GLT-1) for conversion to glutamine (gln) via glutamine synthetase (GS). Sodium-coupled neutral amino acid transporters (SNAT) transport gln back to neurons for conversion to glu by glutaminase (GLS) and packaging into vesicles by vesicular glutamate transporters (VGLUT). Peripheral glia have glutamate dehydrogenase (GDH) for adding or removing glu from the glutamine cycle. GDH is a bidirectional enzyme for the conversion of 2-oxoglutarate [2-OG] to glu. When 2-OG is removed from the tricarboxylic acid cycle (TCA), pyruvate carboxylase (PC) adds to the glial TCA cycle by converting pyruvate to oxaloacetate. Within neurons, the glutamine cycle interacts with the TCA cycle via aspartate aminotransferase (AT). AT is a bidirectional enzyme for conversion of aspartate and 2-OG to oxaloacetate and glu.
Most studies have focused on the use of glutamate as a central neurotransmitter for primary afferents, but evidence for the transport of glutamate in peripheral nerve also has been obtained. Free glutamate in the sensory portion of peripheral nerve immediately distal to the DRG (“distal sensory root”) is elevated over ventral roots compared to total free amino acids (Johnson & Aprison, 1970a). Following peripheral nerve cut, there is an increase in free glutamate (Porcellati & Thompson, 1957; reviewed in Johnson, 1977) consistent with anterograde movement of glutamate toward the periphery. Crushing the peripheral and central processes of acutely isolated rat DRGs causes accumulation of glutamate-ir proximal to the neuronal cell body (Keast & Stephensen, 2000). Ultrastructural examination of the primate medial articular nerve indicates that ~25% of the axons are glutamate-immunoreactive (IR; Westlund et al., 1992). Most of these axons are unmyelinated (C) and lightly myelinated (Aδ) fibers. Within 4 h of knee joint inflammation with kaolin and carrageenan, the number of glutamate-IR axons increases to over 60%, primarily in Aδ fibers (Westlund et al., 1992). Glutamate-IR nerve fibers are rare to infrequent in non-inflamed skin (Nordlind et al., 1993), but, during adjuvant-induced arthritis (AIA), are elevated in the dermis and epidermis (Miller & Kriebel, 2003).
3.2. Glutaminase
Glutamate is produced from the hydrolytic deamidation of glutamine by phosphate-activated glutaminase (GLS; EC 3.5.1.2; Kvamme, 1998). GLS is a mitochondrial enzyme that requires inorganic phosphate for activation, but also is regulated by its end products, glutamate and ammonia, as well as other intracellular components, 2-oxoglutarate, calcium, fatty acids, and fatty acyl-coenzyme A derivatives (Fig. 3; Kvamme & Torgner, 1975; Kvamme & Olsen, 1979; Kvamme & Lenda, 1982; Kvamme et al., 1983). GLS mRNA and protein from CNS is the same as kidney GLS with two mRNAs (Fig. 4) and two isoenzyme peptides (Holcomb et al., 2000; Miller et al., 2011). For example, rat and cat spinal cord show two GLS mRNAs, 6 kb and 3.4 kb, and cDNA in situ hybridization demonstrates their presence in DRG neurons (Fig. 4; Srinivasan & Miller, 1992, 1994). Both GLS mRNAs produce a 72 kDa GLS precursor that is processed into 68 and 66 kDa peptides (Holcomb et al., 2000). All DRG neurons are labeled for GLS-ir with antiserum that recognizes both the 68 and 66 kDa peptides with small to medium diameter neurons exhibiting more GLS-ir than large diameter neurons (Miller et al., 1993; Hoffman et al., 2010). The 68 and 66 kDa GLS peptides both have enzymatic activity, but combine in heteromeric aggregates in a 3:1 ratio respectively to form active GLS enzymes (Srinivasan et al., 1995; Holcomb et al., 2000). Both GLS peptides have been identified in rat DRG and are elevated in amount during chronic peripheral inflammation (Hoffman et al., 2011a, 2011b; Miller et al., 2011). After initiation of AIA, increased GLS protein is found first in the cytoplasm followed by increased mitochondrial GLS (Miller et al., 2010a).
Fig. 3.
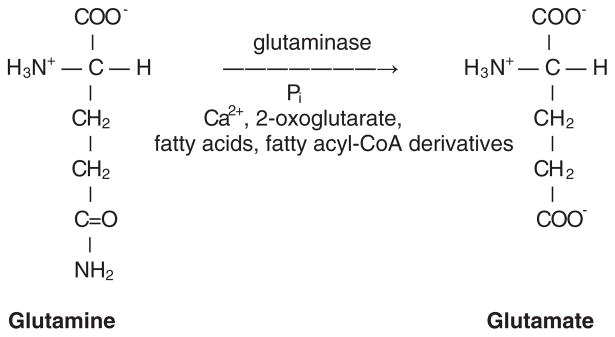
Phosphate activated glutaminase. Glutamate is produced from the hydrolytic deamidation of glutamine by phosphate-activated glutaminase (GLS; EC 3.5.1.2). GLS is a mitochondrial enzyme that requires inorganic phosphate (Pi) for activation, but also is regulated by its end products, glutamate and ammonia, as well as other intracellular components, 2-oxoglutarate, calcium (Ca2+), fatty acids, and fatty acyl-coenzyme A derivatives.
Fig. 4.
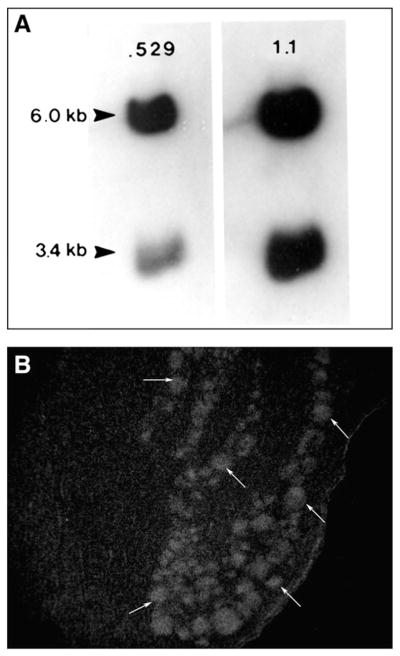
Cat spinal cord glutaminase mRNA Northern blot and dorsal root ganglion in situ hybridization. Northern blot analysis of cat spinal cord shows two glutaminase mRNAs of 6.0 and 3.4 kb similar to what has been described for rat brain. Northern blot was first evaluated using a 35S-529bp cDNA and then stripped and reprobed with a 35S-1.1 kb cDNA (GLS cDNA vectors supplied by C. Banner, NIH). Using 35S-529bp cDNA, all neuronal profiles (arrows) are labeled with in situ hybridization under stringent conditions.
GLS enzyme activity also has been detected in the DRG and in dorsal roots, sciatic nerve and trigeminal nerve (Graham & Aprison, 1969; McDougal et al., 1981; Hassel et al., 2003; Miller et al., 2011). GLS enzyme activity in trigeminal nerve is comparable to GLS activity in CNS white matter axons (Hassel et al., 2003). GLS, therefore, is produced in sensory neuronal cell bodies and transported both centrally and peripherally in dorsal roots and peripheral nerves (McDougal et al., 1981; Zhang et al., 2010). Although GLS occurs in all DRG neurons, exogenous nerve growth factor (NGF) increases GLS expression in small to medium diameter DRG neurons during development (Miller et al., 1999a) and embryonic NGF deprivation decreases GLS in the DRG (McDougal et al., 1981). NGF, however, does not appear to be required for basal GLS expression in adult DRG neurons (Hoffman et al., 2011b). AIA induces increased GLS expression in most DRG neurons within 4 days, but is restricted to small to medium diameter neurons at longer time points (Hoffman et al., 2011a; Miller et al., 2011). Some of the elevated GLS is transported peripherally, since sciatic nerve and skin dermal nerves show elevated GLS-ir during AIA (Miller et al., 1999b; Zhang et al., 2010).
3.3. Glutamate and glutamine transporters
DRG neurons import both glutamate and glutamine into the cell body and axons using excitatory amino acid (EAAT) and sodium-coupled neutral amino acid (SNAT) transporters, respectively (Fig. 2). EAAT3 (EAAC1) predominates as the neuronal glutamate transporter in the DRG (Tao et al., 2004; Carozzi et al., 2008b). Nearly half the neurons (mostly small diameter) are labeled with EAAC1-ir (Tao et al., 2004; Carozzi et al., 2008b) and EAAC1-ir also is located in the peripheral nerve (Carozzi et al., 2008b). Both SNAT 1 and 2 are expressed by DRG neurons (Miller et al., 2005) and high (Km = 2.06×10−5 M) and low affinity (Km = 1.13×10−3 M) transport systems for glutamate have been described in DRG (Roberts & Keen, 1974b). Whereas most exogenously supplied glutamate enters peripheral glia and is converted to glutamine, some glutamate appears to enter the sensory neuronal cells bodies (~22% after a 1 h incubation), dorsal roots, and peripheral nerve (Wheeler & Boyarsky, 1968, 1971; Roberts & Keen, 1974c; Duce & Keen, 1983). Evaluation by proton MR spectroscopy of injected glutamate (1 M, 100 μl) into the masseter muscle indicates that the glutamate signal decays mono-exponentially with a halflife of 108 s (Gambarota et al., 2005). It is unknown at present if this clearance of glutamate involves peripheral afferents and related Schwann cells or if other mechanisms are involved, e.g., myocytes and vasculature (Gambarota et al., 2005).
Glutamine is the major precursor for the production of glutamate in DRG neurons and is transported by SNAT 1 and 2 (Fig. 2). Exogenously administered glutamine is transported into neurons and rapidly is converted to glutamate (>50% in 10 min; Duce and Keen, 1983). Using 3H-glutamine, small diameter DRG neurons in rat accumulate considerably more glutamine (6×) than large diameter neurons (Duce & Keen, 1983). In mouse, large diameter neurons appear to lack uptake, whereas small to medium diameter neurons have high affinity uptake for 3H-glutamine (Sommer et al., 1985). Differences in uptake may be due to the type or amount of SNAT in DRG subpopulations. For example, SNAT 1 is found primarily in small to medium diameter neurons and SNAT 2 is located in large diameter neurons (Miller et al., 2005; Miller, unpublished data). SNAT 1 has a preference for glutamine transport (K0.5 = 0.3 mM), whereas SNAT 2 may transport a number of neutral amino acids, e.g., proline, alanine, and glutamine (Mackenzie & Erickson, 2004) which may account for the production of glutamate following incubation in proline (Johnson, 1975). A high affinity transport system for glutamine also occurs in DRG axons in dorsal roots, but has yet to be described in peripheral nerve (Roberts & Keen, 1974c).
3.4. Tricarboxylic acid cycle
In addition to the glutamine cycle, primary sensory neurons also produce glutamate via interactions with the neuronal tricarboxylic acid (TCA) cycle (Fig. 2). Neurons transport glucose (Uldry & Thorens, 2004) and 40–60% of labeled glucose is converted to glutamate in 15–60 min in cat and rat DRG (Minchin & Beart, 1975; Johnson, 1976). Incubation of isolated rat DRG with [14C]-glucose followed by autoradiography shows that primary sensory neurons preferentially are labeled compared to satellite cells (Minchin & Beart, 1975). Interaction of the neuronal glutamine and TCA cycles may come via aspartate aminotransferase (EC 2.6.1.1; AAT; glutamate oxalacetic transaminase; Fig. 2). Aspartate is localized to DRG and TG neurons and, after incubation of isolated cat DRG, labeled aspartate is rapidly converted into glutamate (Johnson, 1974; Schmidt & Wolf, 1984, 1986; Okhotin et al., 1993; Keast & Stephensen, 2000). AAT activity is found in DRG, dorsal roots, and peripheral nerve (Graham & Aprison, 1969; Johnson, 1972b; Okhotin et al., 1993; Hassel et al., 2003), whereas AAT-ir predominantly occurs in small to medium diameter DRG neurons (Inagaki et al., 1987).
3.5. N-acetyl-aspartyl-glutamate
It also has been suggested that neurotransmitter glutamate in DRG neurons comes from the degradation of N-acetyl-aspartyl-glutamate (NAAG) or that NAAG is a neurotransmitter released by primary afferents (Cangro et al., 1987). NAAG-ir is located in many DRG neurons and dorsal roots (Cangro et al., 1987; Kowalski et al., 1987; Ory-Lavollee et al., 1987). Although NAAG has not been reported to be released from or have action on peripheral afferents, NAAG is located in peripheral nerve (Ory-Lavollee et al., 1987) and N-acetylated α-linked acidic dipeptidase (NAALADase; glutamate carboxypeptidase II: GCPII) a peptidase that cleaves NAAG to glutamate and N-acetyl-aspartate, is found primarily in the non-myelinating Schwann cells of peripheral nerve (Berger et al., 1995; Carozzi et al., 2008a, 2008b).
3.6. Vesicular glutamate transporters
Once synthesized in or taken up into neurons, glutamate is packaged into neurotransmitter vesicles via vesicular glutamate transporters (VGLUTs; Fig. 2). A vacuolar H+-ATPase establishes a proton electrochemical gradient across the vesicular membrane and VGLUTs use this gradient to exchange H+ for glutamate (Ozkan & Ueda, 1998). VGLUTs have 10 transmembrane regions, are highly specific with low affinity (Km = 1–3 mM) for L-glutamate, and rely on chloride for maximum transport (Ozkan & Ueda, 1998; Shigeri et al., 2004). There are three isoforms of VGLUT (1, 2, and 3) and these appear to be definitive markers for glutamatergic neurons (Takamori, 2006). VGLUT 1 and 2 mRNA is present in DRG and VGLUT 1- and 2-ir is localized to DRG neuronal cell bodies (Oliveira et al., 2003; Landry et al., 2004; Morris et al., 2005; Atoji & Islam, 2009). Medium to large sized neurons contain VGLUT1 and small to medium sized cells with CGRP or IB4 contain VGLUT2 (Oliveira et al., 2003; Landry et al., 2004; Brumovsky et al, 2007). VGLUT 2-ir is greater in peripheral nerve than dorsal root and VGLUT 2-IR nerve fibers are abundant in the dermal plexus and intraepidermal nerves (Brumovsky et al., 2007; Ibitokun & Miller, 2010b). During chronic peripheral inflammation of the rat hindlimb, VGLUT 2-ir is elevated in the sciatic nerve compared to normal nerve (Zhang et al., 2010) indicating an increased transport of vesicular glutamate to the peripheral nerve terminal. Upon reaching the peripheral nerve terminal, increased glutamate release from primary afferents could occur due to elevated numbers of glutamate-containing vesicles.
4. Release of glutamate from peripheral afferents
4.1. Stimulated release
The previous section indicated that the primary afferent cell body is a neuron that produces glutamate via particular biochemical pathways and that glutamate is packaged in synaptic vesicles via VGLUTs. Furthermore, evidence indicates that similar biochemical events occur in the peripheral axon, that glutamate is transported in peripheral nerve, and that there is mechanism for release, i.e., VGLUTs and vesicular release proteins (Fig. 5; Averill et al., 2004; Ibitokun & Miller, 2010a, b). Isolated frog sciatic nerves preincubated with 14C-glutamate have a 200% rise in glutamate release above resting levels when electrically stimulated. This does not occur with other amino acids and is blocked by sodium azide. One interpretation is that glutamate discharge from sciatic nerve occurs via vesicular release (Wheeler et al., 1966). Since this early study, several investigations have demonstrated glutamate release from primary afferent nerve terminals and a number of stimuli, such as natural and electrical stimulation, chemical activation, and inflammation, evoke glutamate release from peripheral nerve trunks, skin, joints, and dental pulp (Weinreich & Hammerschlag, 1975; Bledsoe, et al., 1980, 1989; Jackson et al., 1993; Omote et al., 1998; deGroot et al., 2000; Lawand et al., 2000; McNearney et al., 2000, 2004).
Fig. 5.
Peripheral glutamate mechanisms. Nociceptive free nerve endings store glutamate in neurotransmitter vesicles (white circles) and release glutamate (glu) into peripheral tissue following noxious stimulation (leftward going red arrows). Glu released from the same or a nearby terminal can interact with excitatory amino acid receptors (EAAR; chevrons) to activate or sensitize the terminal. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Pulsed water and potassium depolarization causes glutamate efflux in isolated skin of Xenopus laevis, presumably from primary afferents (Bledsoe, et al., 1980, 1989). Potassium depolarization of peripheral nerve terminals or TPRV1 activation of primary afferents by capsaicin evokes the release of glutamate from bovine dental pulp which has a rich innervation of C-fibers (Jackson et al., 1993). TRPV1 activation of afferents in rat skin by subcutaneous (s.c.) capsaicin injection or capsaicin cream skin application causes glutamate levels to elevate in the subcutaneous glabrous skin of the rat hindpaw. This effect can be blocked by capsazepine, TRPV1 antagonist, desensitization of primary afferents by capsaicin pretreatment, or morphine inhibition of peripheral terminals (Jin et al., 2006, 2009). TRPV1 receptors are the noxious heat detectors on primary afferents and 50 °C thermal stimulation of rat skin also produces subcutaneous glutamate release (Jin et al., 2006). Antidromic electrical stimulation of sciatic nerve or capsaicin application to sciatic nerve for activating C fibers causes glutamate release in the subcutaneous skin (deGroot et al., 2000; Jin et al., 2006). Introduction of glutamate (10, 50 mg/kg, monosodium salt) systemically raises interstitial glutamate concentrations in the rat masseter muscle from 24 μM to 63 μM. (Cairns et al., 2007). During hypertonic saline injection into human calf muscles, glutamate microdialysate levels nearly double in concentration (26 → 50 μM; Tegeder et al., 2002). Intramuscular injection of botulinum neurotoxin type A (BoNTA; 5 U/10 μl) into the female rat temporalis muscle causes an acceleration in the decline of interstitial glutamate compared to PBS over a 3 h period (Gazerani et al., 2010a). Furthermore, BoNTA decreases SNAP-25 content in the temporalis muscles and BoNTA’s effect on interstitial glutamate concentration may be due to inhibition of vesicular release of glutamate and/or other neuroactive substances from muscle primary afferents (Gazerani et al., 2010a).
4.2. Release during inflammation
Inflammation and/or tissue damage causes elevated glutamate release from primary afferents (Fig. 5) leading to increased activation of excitatory amino acid receptors in peripheral tissue (described in subsequent sections). For example, intraplantar (i.pl.) injection of formalin causes activation of TRPA1 receptors on primary afferents followed by sensitization of afferents by inflammatory mediators (Bautista et al., 2006; McNamara et al., 2007). Following formalin i.pl. injection, glutamate levels increase subcutaneously by nearly 200% in the rat glabrous hindpaw. Levels increase within minutes of formalin injection and remain elevated for 3 h (Omote et al., 1998). Skin injury by barrier disruption of the epidermis causes glutamate elevation at the epidermal basal layer possibly from sensory afferents in the dermal plexus (Fuziwara et al., 2003). Experimental osteoarthritis in rats and rabbits by anterior cruciate ligament transection causes glutamate concentrations in knee joint dialysates to increase for several weeks. In rats, glutamate levels increase over 90% by twenty weeks of osteoarthritis (Jean et al., 2005). In rabbits, glutamate levels steadily rise until, after thirty weeks of osteoarthritis, dialysates are elevated over 200% compared to control (Jean et al., 2008). Intra-articular injection of parecoxib, cyclooxygenase-2 inhibitor, or hyaluronic acid (100 μg, 1×/week, 5 weeks) reduces glutamate levels in osteoarthritic knees (Jean et al., 2006, 2007).
Glutamate levels also are altered acutely after knee inflammation. Within 4 h of kaolin and carrageenan inflammation, the number of glutamate-IR axons in the monkey medial articular nerve increases from 25% to over 60%. This increase occurs primarily in unmyelinated (C) to lightly myelinated (Aδ) axons (Westlund et al., 1992). In similar knee joint inflammation in rat, glutamate concentration doubles in synovial fluid within minutes and remains elevated for over 2 h. Administration of lidocaine to the knee joint prevents elevation indicating that joint nerve terminals may be major contributors of the increased glutamate concentration induced by inflammation (Lawand et al., 2000). Inflammation-induced glutamate elevation has been confirmed in humans during chronic arthritis. Synovial glutamate concentrations from synovitis patients are over fifty times the concentration from non-arthritic control autopsy collections (McNearney et al., 2000, 2004).
5. Localization of excitatory amino acid receptors
5.1. Excitatory amino acid receptors
Release of glutamate in the periphery would warrant the presence of EAARs for biological function (Fig. 5). DRG neuronal cell bodies synthesize a number of EAARs (Sato et al., 1993; Petralia et al, 1994a, b; Ohishi et al., 1995a,b; Li et al., 1996; Kinoshita et al., 1998; Walker et al., 2001; Marvizón et al., 2002) and one site of action for glutamate could be the peripheral primary afferent terminal. EAARs have been localized to primary afferents and peripheral tissues with immunohistochemistry and have been evaluated with electrophysiology, behavior, and cellular assays. This section will focus on anatomical localization with immunohistochemistry and peripheral effects will be examined later.
5.2. Receptor subtypes
EAARs are classified into two major categories: ionotropic receptors and metabotropic receptors. There are three ionotropic receptors: N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate (KA). [Note: NMDA and AMPA are specific agonists for their respective receptors, however, kainate binds to both KA and AMPA receptors (Huettner, 1990; Lodge, 2009; Fenwick & Oswald, 2010)]. Ionotropic EAARs are heterotetramers allowing for a variety of receptor subtypes. NMDA receptors (NMDAR) are composed of NR1 and NR2 subunits with at least eight NR1 variants produced from alternative splicing of NR1 RNA. The ion channel of the NMDAR is blocked by magnesium by a voltage-dependent mechanism, but upon depolarization Mg2+ leaves the channel and the ion channel conducts Ca2+ and Na+. AMPA receptors (AMPAR) consist of GluR1–4 subunits and are permeable to Na+ (and Ca2+ when GluR2 absent) when the nerve membrane depolarizes. There are five subunits (KA1–2, GluR5–7) for kainate receptors (KAR) and these receptors are permeable to Na+. Metabotropic glutamate receptors (mGluR) are G-protein coupled receptors and are categorized into three groups: Groups I, II, and III. Group I (mGluR1, 5) are located postsynaptically, are coupled to Gq to stimulate phospholipases, and modulate excitatory actions of glutamate. Groups II (mGluR2, 3) and III (mGluR4, 6–8) are located presynaptically, are coupled to Gαi to inhibit adenylate cyclase, and modulate inhibitory actions of glutamate.
5.3. Ionotropic receptor axonal localization
First indication that EAARs are transported peripherally in sensory axons came from studies of vagal afferents (Lewis et al., 1987; Cincotta et al., 1989). Subsequent to these investigations, transport of EAARs in somatic afferents has been demonstrated. Following rat sciatic nerve ligation for 48 h, NR1-ir accumulates proximal to the ligature indicating peripheral transport of NR1 from DRG to sciatic nerve (Liu et al., 1994). Ultrastructural analysis of NR1- (NMDAR), GluR1- (AMPAR), and GluR5–7- (KAR) ir in rat sural and plantar nerves indicates that both myelinated and unmyelinated axons have ionotropic EAAR-ir (Coggeshall & Carlton, 1998). In rat sural nerve, 48% of myelinated and 21% of unmyelinated axons have NR1-ir, whereas 56% of myelinated and 30% of unmyelinated appear labeled in plantar nerve. GluR1-ir occurs in 28% of myelinated and 8% of unmyelinated axons in sural nerve and, in plantar nerve, 66% of myelinated and 17% of unmyelinated. For GluR5–7-ir, 11% of myelinated and 7% of unmyelinated sural axons and 64% and 44% of plantar axons are labeled (Coggeshall & Carlton, 1998). NR1-, NR2B-, GluR1-, GluR2/3-, and GluR5–7-ir occur in unmyelinated nerve fibers beneath the dermal–epidermal junction in both rat glabrous and hairy and human hairy skin (Carlton et al., 1995; Coggeshall & Carlton, 1998; Kinkelin et al., 2000; Gazerani et al., 2010b). In rat, 5–30 unmyelinated (C) axonal fibers occur in bundles below the dermal–epidermal junction and EAAR-ir occurs in a subpopulation (17–21%) of these axons (Coggeshall & Carlton, 1998a). GluR1-ir accumulates with round clear vesicles in these unmyelinated axons indicating AMPAR transport with putative glutamate containing neurotransmitter vesicles (Carlton et al, 1995). Many muscle afferents in the female rat temporalis muscle have NR2B-ir (~70%) and approximately half of these contain SP or CGRP (Gazerani et al., 2010a). In rat facial skin, over 50% of cutaneous afferents in rat contain NR2B-ir (Gazerani et al., 2010b). In human hairy skin, ~20–25% of unmyelinated axons below the dermal–epidermal junction contain EAAR-ir, as well as some nonmyelinating Schwann cells (Kinkelin et al., 2000). Sensory nerves in human tendon appear to contain NR1 in areas with high levels of acetylcholinesterase (Alfredson et al., 2001a).
AIA, induced with complete Freund’s adjuvant (CFA), in rats causes the number of peripheral axons with EAAR-ir to increase (Carlton & Coggeshall, 1999; Du et al., 2003, 2006). Increased numbers of EAAR-IR axons occur within two days of inflammation, remain elevated for seven days, but return to normal numbers by day fourteen. Cutaneous digital nerves in rat hindpaw have 48%, 22% and 27% of the unmyelinated axons labeled for NR1, GluR1, and GluR5–7, respectively, under control (contralateral) conditions. After two days of AIA, these percentages change to 61%, 43% and 48%. The proportions of thinly myelinated axons with EAAR-ir also change from 43%, 42% and 28% for NR1, GluR1, and GluR5–7, respectively to 61%, 61% and 43% (Carlton & Coggeshall, 1999). KARs (GluR5–7) comprise 28% of unmyelinated axons in digital nerves from naive control rats and this proportion increases to 40% at two days AIA (Du et al., 2006). In a similar study, digital nerves from control naive rats have NR1 in 47% of unmyelinated axons, but this percentage elevates to 64% and 69% after two to seven days AIA (Du et al., 2003). Phosphorylation of the NR1 subunit at the serine-896 site by kinases causes the NMDAR to be unblocked by Mg2+ under resting membrane potential conditions (Chen & Huang, 1992; Xiong et al., 1998; Lan et al., 2001). Following peripheral stimulation with noxious heat, there is a rapid phosphorylation of NR1 in the spinal cord leading to sensitization of spinal cord neurons and excessive pain (Brenner et al., 2004). Some NR1 is phosphorylated in nerve fibers of the dermal plexus in naive rats, indicating that activation of NMDAR may not be voltage dependent in some peripheral afferents (Miller & Aluwalia, unpublished observations).
5.4. Metabotropic receptor axonal localization
Metabotropic glutamate receptors are produced in many DRG neurons, can be co-expressed, and are altered in amount during nerve injury (Li et al., 1996; Carlton et al, 2001; Walker et al., 2001; Hudson et al., 2002; Carlton & Hargett, 2007). Transection or ligation of rat sciatic nerves causes accumulation of mGluR5-ir indicating transport from the DRG cell body to the periphery (Hudson et al., 2002). Ultrastructural analysis of rat digital nerves indicates that ~30% of unmyelinated axons contain mGluRα- and mGluR 2/3-ir, 22% of myelinated axons have mGluRα-ir, and 30% of myelinated axons have mGluR 2/3-ir (Zhou et al., 2001). Following transection of sciatic nerve and neuropathy by partial nerve section, mGluR5-ir increases in the lesioned nerve. Furthermore, neuropathy by L5 spinal ligation causes elevated mGluR5-ir in the L5 spinal nerve and in the undamaged L4 spinal nerve. These alterations occur in myelinated (Aδ) fibers, whereas unmyelinated fibers appear not to change (Hudson et al., 2002). In rat skin, mGluR5 is localized to dermal nerve bundles and intraepidermal nerve fibers (Walker et al., 2001).
5.5. Peripheral localization
Based on glutamate and glutamate receptor studies, it would be likely that glutamate, released by noxious stimuli from sensory afferents, would interact with EAARs on autologous or nearby terminals (Fig. 5). Evidence for this interpretation will be explored further in the next section. In addition, glutamate receptors, ionotropic and metabotropic, are located on some peripheral tissues and glutamate release from primary afferents could influence their function. Examples of peripheral tissues with EAARs include stomach, lung, lymphocytes, uterus, heart, osteoclasts, osteocytes, osteoblasts, synoviocytes, and keratinocytes (Tsai & Wu, 2005; Said, 2005; Haas & Schauenstein, 2005; Spencer et al., 2005; Gill & Pulido, 2005; Ghosh et al., 2007; Flood et al., 2007; McNearney et al., 2010). In the human and rodent skin, NR1 (NMDAR) is present in keratinocyes with a high expression in stratum granulosum (Fuziwara et al., 2003; Fischer et al., 2004a,b; Miller & Aluwalia, unpublished data). These NMDARs appear to be related to maintenance of the cutaneous barrier (Fuziwara et al., 2003) and, during skin disease, NR1 levels decrease in the upper epidermal cells (Fischer et al., 2004b). There is a high degree of phosphorylation of NR1 (serine 896) in rat keratinocytes primarily in stratum spinosum (Miller & Aluwalia, unpublished observations). This suggests that the action of endogenous ligands, e.g., glutamate, may have more affect in the middle of the epidermis than outer epidermis where NR1 is concentrated.
6. Peripheral effects of glutamate: electrophysiology (Table 1)
Table 1.
Peripheral effects of glutamate: electrophysiology.
| Drug | Dose | Route | Reference |
|---|---|---|---|
| Agonist | |||
| L-glutamate | 100–300 μM; ED50 = 136 μM | Superfusion — rat tail | Ault & Hildebrand, 1993a |
| Kainate | 10–300 μM; EC50=63 μM | Superfusion — rat tail | Ault & Hildebrand, 1993b |
| Domoate | 0.1–10 μM; EC50=1 μM | Superfusion — rat tail | Ault & Hildebrand, 1993b |
| AMPA | 0.1–1.0 mM | Superfusion — rat tail | Ault & Hildebrand, 1993b |
| Quisqualate | 0.1–1.0 mM | Superfusion — rat tail | Ault & Hildebrand, 1993b |
| Glutamate | 100–1000 μM; max. response=300 μM | Superfusion — rat hindpaw | Du et al., 2001 |
| NMDA | 0.01–3.0 mM | Superfusion — rat hindpaw | Du et al., 2003 |
| Kainate | 0.01–3 mM | Superfusion — rat hindpaw | Du et al., 2006 |
| Glutamate | 1.0 M, 10–100 μl | Masseter injection — rat | Cairns et al., 2001a, 2002a; 2003a, 2003b; Gambarota et al., 2005 |
| Glutamate | 0.5 M, 10 μl | TMJ injection — rat | Cairns et al., 2001b; 2002b; Lam et al., 2009b |
| NMDA | 0.5–1600 mM, 10 μl | Masseter injection — rat | Cairns et al., 2003a; Dong et al., 2007, 2009 |
| Glutamate | 0.5 M, 10 μl | TMJ injection — rat | Cairns et al., 2001b; Lam et al., 2009a,b |
| Glutamate | 0.01–1.0 M, 10 μl | s.c. injection — face — rat | Gazerani et al., 2010b |
| Antagonist | |||
| DNQX | 0.1–30 μM | Superfusion — rat tail | Ault & Hildebrand, 1993c |
| CNQX | 0.1–100 μM | Superfusion — rat tail | Ault & Hildebrand, 1993c |
| Kynurenate | 1–300 μM | Superfusion — rat tail | Ault & Hildebrand, 1993c |
| DL-AP4 | 3–300 μM | Superfusion — rat tail | Ault & Hildebrand, 1993c |
| L-AP4 | 3–300 μM | Superfusion — rat tail | Ault & Hildebrand, 1993c |
| MK 801 | 0.03 mM | Superfusion — rat hindpaw | Du et al., 2003 |
| CNQX | 0.1 mM | Superfusion — rat hindpaw | Du et al., 2006 |
| LY382884 | 0.1 mM | Superfusion — rat hindpaw | Du et al., 2006 |
| Kynurenate | 0.1 M, 10 μl | Masseter injection — rat | Cairns et al., 2002a, 2003b |
| APV | 1–100 mM, 10 μl | Masseter injection — rat | Cairns et al., 2003b; Hakim et al., 2011 |
| Ketamine | 1–20 mM, 10 μl | Masseter injection — rat | Cairns et al., 2003b |
| Dextromethorphan | 1–40 mM, 10 μl | Masseter injection — rat | Cairns et al., 2003b |
| Ifenprodil | 100 mM, 10 μl | Masseter injection — rat | Cairns et al., 2003b |
| AP5 | 10 mM (200 μg/100 μl) | i.pl. — cat | Chen et al., 1999a |
| CNQX | 357 mM (8.3 μg/100 μl) | i.pl. — cat | Chen et al., 1999a |
| AP5 | 10 mM, 50 μl | i.pl. — rat | You et al., 2002 |
| MK 801 | 2 mM, 50 μl | i.pl. — rat | You et al., 2002 |
| CHPG | 20 mM (100 nmol/5 μl) | i.pl. — rat | Walker et al., 2001 |
| MPEP | 20 mM (100 nmol/5 μl) | i.pl. — rat | Walker et al., 2001 |
6.1. Ventral root potentials
The electrophysiological actions of glutamate and EAAR agonists first were studied indirectly by recording the ventral root potentials in an isolated spinal cord-tail preparation in neonatal rat. Nociceptive afferents in neonatal rat tail skin are activated by L-glutamate (ED50 = 136 μM) to produce nociceptive reflexes, but not D-glutamate or other L-amino acids (Ault & Hildebrand, 1993a,b,c). Peripheral application of kainate (10–300 μM) and domoate (0.1–10 μM), KAR agonists, produce ventral root responses that are comparable to bradykinin (0.1–10 μM) and capsaicin (0.3–10 μM), but quickly diminish when applied continuously (Ault & Hildebrand, 1993b). Kainate’s actions can be inhibited by application of DNQX (10–100 μM), a KAR antagonist. AMPA (0.1–1 mM) and quisqualate (0.1–1 mM), mixed AMPAR/mGluR agonist, also activate peripheral afferents, but at lower potency than kainate. NMDA (1 mM) is ineffective in this preparation (Ault & Hildebrand, 1993b). Capsaicin’s effects can be diminished by peripheral application of EAAR antagonists indicating interaction between EAARs and TRPV1 (Ault & Hildebrand, 1993c). DNQX (0.1–30 μM) and CNQX (0.1–100 μM), AMPAR antagonists, block nociceptive activity induced by capsaicin and AP-5 (100 μM), NMDAR antagonist, reduces nociceptive potentials. DL-AP4 (3–300 μM), non-selective EAAR antagonist, kynurenic acid (1–300 μM), non-selective AMPAR/NMDAR antagonist, and L-AP4 (3–300 μM), group III mGluR agonist, also inhibit nociceptive potentials as well as non-nociceptive responses (Ault & Hildebrand, 1993c).
6.2. In vitro skin–nerve recording
The effects of glutamate and EAARs have been studied more directly with an in vitro glabrous skin–nerve preparation, i.e., rat hindpaw skin with intact medial and lateral plantar nerves (Du et al., 2001, 2003, 2006). Application of L-glutamate to the skin in ascending concentration causes excitation of both Aδ and C fibers, but not Aβ fibers. There is a bell-curve dose response for Aδ and C fibers with a maximal response at 300 μM (range: 10–1000 μM). Glutamate typically causes an increase in activity within 30 s of application and its effect lasts longer than the application period (2 min). Only 43% of Aδ fibers respond to ascending concentrations of glutamate, but 60% respond to the 300 μM application. For C fibers, 67–68% of these respond to ascending concentrations and 300 μM of glutamate. The application of 300 μM glutamate sensitizes most Aδ (90%) and C (92%) fibers to heat stimulation. This sensitization includes a decrease in the threshold for stimulation: 44.1 to 41.7 °C for Aδ fibers and 42.3 to 40.5 °C for C fibers. Furthermore, the fibers show increases in discharge rate and “mean peak instantaneous frequency.” The mechanical threshold for afferent fibers, however, is not altered following glutamate application (Du et al., 2001).
The role of the NMDA receptor also has been evaluated in the in vitro glabrous skin–nerve preparation (Du et al., 2003). An ascending concentration of NMDA (0.01–3.0 mM) excites nearly half of the normal C nociceptors (48%), while over three quarters (80%) are activated from inflamed skin. This activation occurs in a dose dependent fashion. Using 1.0 mM NMDA, C fibers are activated and sensitized in both normal and inflamed skin. A second application of NMDA causes an increase in discharge rate in normal (0.12 to 0.25 impulses/s) and inflamed (0.22 to 0.34 impulses/s) nociceptors. These increases are prevented when MK-801 (0.03 mM), NMDAR antagonist, is co-applied and decreases the activity of inflamed C fibers to near normal levels (0.16 impulses/s). The second application of NMDA also increases the number of activated fibers in normal (100%) and inflamed (88%) rats and MK-801 (0.03 mM) blocks this increase, 55% in control skin and 12.5% in inflamed skin. The NMDAR on primary afferents also is involved in heat sensitization. After NMDA application, the mean threshold for heat is reduced to 40.1 °C (design: heat stimulus, NMDA application, 2nd heat stimulus). The mean discharge rate also increases from 1.6 to 2.2 impulses/s. NMDA decreases the mean heat threshold in inflamed skin to 39.1 °C. Discharge rates in inflamed skin after a second heat stimulus are not altered by NMDA (Du et al., 2003).
The in vitro glabrous skin–nerve preparation has been used to study the interaction of TRPV1 and group II mGluR receptors on peripheral afferents (Carlton et al., 2009). Capsaicin (0.05%) application to the receptive field causes excitation in C fibers (C mechanoheat) and co-administration of (2R,4R)-4-aminopyrrolidine-2,4-dicarbox-ylate (APDC; 1.0 μM), group II mGluR agonist, attenuates the capsaicin-evoked response. The APDC effect exposes two phases in the capsaicin-elicited activity. When capsaicin is co-applied with APDC an initial phase of activity still occurs, but a later phase of excitation during a 2 min application is diminished. Administration of capsaicin, APDC, and (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl)propanoic acid (LY341495; 1.0 μM), group II mGluR antagonist, produces robust excitation of C fibers (Carlton et al., 2009). The interaction of the group II mGluRs and TRPV1s may occur by modulation of the cAMP/PKA pathway (Carlton et al., 2009). Application of forskolin (5 μM) sensitizes the C fibers (C mechanoheat) by reducing the thermal threshold from 43 °C to 39 °C. Co-administration of forskolin with H89 (10 μM), PKA inhibitor, or APDC (0.5 μm) blocks the sensitization of C fibers (Carlton et al., 2009). There also is an interaction between SP and glutamate on Aδ and C fibers (Zhang et al., 2006). Co-admininstration s.c. of SP (1 μmol/l, 10 μl) with glutamate (10 μmol/l) increases Aδ and C fiber mean discharge from ~5 impulses/minute to ~18 impulses/minute and activates over 80% of fibers from ~30% with glutamate alone (Zhang et al., 2006).
6.3. Additional in vitro studies
Other in vitro studies give further evidence for glutamate’s activation/sensitization of primary afferents and KARs have been explored as one of the ionotropic EAARs producing these responses (Davies et al., 1979; Evans, 1980, 1985; Agrawal & Evans, 1986; Hawkins et al., 1991; Du et al., 2006). A number of studies in rats, toads, and frogs show that glutamate and kainate depolarize primary afferents in dorsal roots (Davies et al., 1979; Evans, 1980, 1985; Agrawal & Evans, 1986; Hawkins et al., 1991; Pook et al., 1993). Isolated dorsal roots from neonatal rats are depolarized selectively by L-glutamate and kainate and blocked by CNQX and NBQX. Quisqualate is less active than kainate and NMDA has no effect (Agrawal & Evans, 1986; Pook et al., 1993). Kainate also depolarizes afferents in adult dorsal roots and peripheral nerves (Agrawal & Evans, 1986). The in vitro rat glabrous skin–nerve preparation (described above) has been used to further explore the actions of kainate in normal and inflamed skin (Du et al., 2006). In these studies, inflammation is created by i.pl. injection of CFA (25 μl) and animals are evaluated at 48 h post-injection. An ascending concentration series (0.01–3 mM) of kainate excites most C fiber nociceptors (89%) in a dose dependent manner from naive rats (Fig. 6). Fibers are excited during the 2 min of application with many having an irregular firing pattern after removal of kainate. Following 48 h of inflammation with CFA, C fiber nociceptors have elevated background activity, but kainate still excites most fibers (75%) in a dose dependent fashion (Fig. 6). At 1 mM, the peak discharge in control rats is 0.10 impulses/s, whereas it is 0.22 impulses/s in inflamed rats. When comparing normal to inflamed C fibers, kainate concentrations (0.01–3 mM) produce a 116–500% increase in activity (Fig. 6; Du et al., 2006). Inflammatory mediators, such as glutamate, produce sensitization of the peripheral terminal of sensory afferents (Woolf & Ma, 2007). Application of kainate (0.01–3 mM) to both control and inflamed C fibers demonstrates that fibers in both states can be sensitized via the KAR (Du et al., 2006). After a five minute interval between 0.3 mM kainate applications, C fibers from controls and inflamed rats have enhanced responses above the initial response, 54% and 24%, respectively. In controls, concurrent application of CNQX (0.1 mM) or LY382884 (0.1 mM), KAR antagonist, with the second application of kainate (0.3 mM) blocks the enhanced response. In inflamed tissue, concomitant application of CNQX (0.1 mM) decreases the C fiber response by 43%, whereas LY382884 has no effect (Du et al., 2006). In addition to stimulation of ongoing activity, kainate also has effects on thermal and mechanical elicited responses. In normal skin, 0.3 mM kainate decreases the activation threshold to a heat stimulus (41.2 to 38.7 °C) and increases by 51% the firing rate (1.8 to 2.7 impulses/s) of C fibers (design: heat stimulus, kainate application, 2nd kainate application, 2nd heat stimulus). C fibers from inflamed skin already have reduced heat thresholds (39.99 °C) and kainate does not alter the threshold, but does increase the firing rate to the heat stimulus by 24% (1.5 to 1.9 impulses/s). When given concurrently with the second application of kainate, CNQX and LY382884 in normal skin not only antagonize kainate’s effects to heat activation, but further decrease responses by 28% and 37%, respectively. In inflamed skin, CNQX and LY382884 antagonize kainate’s effects on heat sensitization bringing responses comparable to responses of the initial heat stimulus (Du et al., 2006).
Fig. 6.
Dose response relationship of nociceptors to kainate using the in vitro rat glabrous skin–nerve preparation. Ascending concentrations of kainate excite most C fiber nociceptors in normal (naive) rats. In these studies, inflammation is created by injection (i.pl.) of CFA (25 μl) and animals evaluated at 48 h post-injection. An ascending concentration series (0.01–3 mM) of kainate excites most C fiber nociceptors (89%) in a dose dependent manner from naive rats (open circles). Following 48 h of adjuvant induced arthritis, C fiber nociceptors have elevated background activity, but kainate excites most fibers (75%; black circles) in a dose dependent fashion. Used with permission from author; Du et al., Neuroscience, 2006.
6.4. In vivo single unit recording
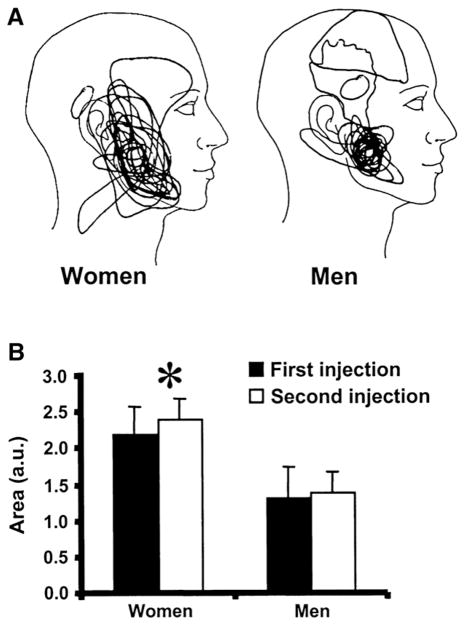
The role of glutamate and EAARs on regulating the electrical activity (single unit) of primary afferents also has been studied in vivo (Cairns et al., 2001a, 2002a, 2003a, 2003b; Gambarota et al., 2005; Tian et al., 2005; Dong et al., 2007, 2009; Lam et al., 2009a; Gazerani et al., 2010a,b; Hakim et al., 2011; Luo et al., 2010). In vivo injection of 1.0 M (100 μl) glutamate into the rat masseter muscle evokes trigeminal nerve fiber activity with a half-life of 76 s similar to the half-life (108 s) of glutamate clearance from the muscle (Gambarota et al., 2005). Injection of 0.5 M (10 μl) glutamate into the rat masseter evokes activity in almost all Aδ nociceptive afferents (86%) that project to the spinal trigeminal nucleus (caudalis or upper cervical segments; Cairns et al., 2001a). Afferent activity is induced with a latency of 3–10 s and duration of 10–1800 s. Glutamate produces the largest activity in afferents with the slowest conduction velocities (2–5 m/s). In female rats, the peak response and overall afferent discharge to glutamate (382 spikes/min; 494 spikes×min) is much greater than in males (42 spikes/min; 43 spikes×min; Cairns et al., 2001a). Injection of 0.1 M (10 μl) glutamate into the rat masseter muscle evokes activity of 40% of muscle afferents, whereas 1.0 M glutamate (10 μl) causes activation of all afferents along with increases in rate, duration, and overall discharge (afferent conduction velocity: 2.7–45.7 m/s; Cairns et al., 2002a). Co-administration with kynurenate (0.1 M, 10 μl), EAAR antagonist, blocks or diminishes overall discharge and discharge duration, but does not alter increases in rate of discharge (Cairns et al., 2002a). In afferents that project to caudal brainstem, overall discharge induced by glutamate (1.0 M, 10 μl) is greater in females compared to males (Cairns et al., 2002a, 2003a). Injection of 1.0 M (10 μl) glutamate in the masseter also decreases (48%) the mechanical threshold (von Frey anesthesiometer) to activate muscle afferents for over 30 min. No difference occurs between sexes for the glutamate induced alteration in mechanical threshold (Cairns et al., 2002a). The duration of lidocaine block (37 mM, 10 μl) of afferent activity is shortened in rats receiving masseter injection of glutamate compared to isotonic saline injection (Cairns et al., 2003a). Kynurenate (0.1 M, 10 μl) blocks the decrease in mechanical threshold and the decreased duration of lidocaine block (Cairns et al., 2002a, 2003a). Intramuscular injection of glutamate (1.0 M, 10μl) into the female rat temporalis muscle causes sensitization of muscle nociceptors and this effect is attenuated by BoNTA (Gazerani et al., 2010a).
The NMDAR participates in glutamate induced activation of masseter muscle afferents (Cairns et al., 2003b; Dong et al., 2007, 2009). For example, when NMDA (0.5–1600 mM, 10 μl) is injected into the rat masseter muscle, an increase occurs in muscle afferent discharge in a dose related manner (Cairns et al., 2003b; Dong et al., 2007, 2009). The cumulative discharge of all afferents is greater in females compared to males at 160 mM NMDA and is greater for females versus males at 160 and 1600 mM in slowly conducting afferents (Dong et al., 2007). In addition, female rats with estrogen levels of >60 pg/ml have a greater dose response (leftward shift) to NMDA for slow-conducting afferent activity than males and females with low estrogen levels of <60 pg/ml (Dong et al., 2007). When all afferent activity is compared after 0.5 M NMDA (10 μl) injection, female rats with estrogen levels >120 pg/ml or ovariectomized rats with 282 pg/ml (5.0 μg/day) have greater activity compared to females with <60 pg/ml estrogen or ovariectomized rats with 132 pg/ml (0.5 μg/day). Likewise, ovariectomized rats with high estrogen replacement (5.0 μg/day) have greater activity after 0.5 M NMDA (10 μl) injection compared to ovariectomized rats with low estrogen replacement (0.5 μg/day; Dong et al., 2007).
Following an initial excitation with glutamate (0.5 M, 10 μl), co-administration of NMDAR antagonists, 2-amino-5-phosphonvalerate (APV, 1–100 mM), ketamine (1–20 mM), and dextromethorphan (1–40 mM), dose dependently decreases the afferent activity caused by a second application of glutamate (0.5 M, 10 μl; Cairns et al., 2003b). In addition, co-application of APV and ketamine decrease evoked afferent activity from a second application of hypertonic saline (10 μl; Cairns et al., 2003b). Co-injection of ketamine (1–20 mM) and ifenprodil (100 mM), a non-competitive NR2B antagonist, decrease afferent activity after a second injection of NMDA (0.5 M, 10 μl; Dong et al., 2007). A systemic dose of monosodium glutamate (50 mg/kg) reduces the mechanical threshold of masseter Aδ fibers and preadministration of ketamine (1 mg/kg) prevents this decrease in threshold (Cairns et al., 2007). Diclofenac (0.1 mg/ml, 10 μl), a prostaglandin synthesis inhibitor, attenuates the cumulative nociceptor discharge and mechanical threshold when co-administered with a second injection of NMDA (0.5 M, 10 μl) with or without prostaglandin E2 (0.1 mg/ml) into the masseter (Dong et al., 2009). The dose response to a second injection of NMDA (50–1600 mM, 10 μl) is decreased (rightward shift) in the presence of diclofenac (0.1 mg/ml, 10 μl; EC50 = 141 mM NMDA alone; EC50 = 539 mM NMDA+diclofenac) indicating a competitive action of the drug (Dong et al., 2009). Diclofenac (0.1 mg/ml, 10 μl) also attenuates TNFα-induced (1 μg, 10 μl) mechanical sensitization of masseter nociceptors, whereas APV (10–50 mM, 10 μl) is ineffective (Hakim et al., 2011).
The NMDAR also influences activity of temporalis muscle afferents (Dong et al., 2006, 2009; Gazerani et al., 2010a). Co-administration of APV (20 mM, 10 μl) with glutamate (1.0 M) into the female rat temporalis muscle blocks glutamate-induced sensitization of muscle nociceptors (Gazerani et al., 2010a). Injection of NMDA (50–1600 mM, 10 μl) into the temporalis muscle in rats produces an increase in afferent discharge only at the highest dose (Dong et al., 2006). Unlike the masseter, there is no difference between sexes in response to NMDA injection into the temporalis. For fibers that respond to injection of 0.5 M NMDA (10 μl), a second injection (30 min) of the same dose causes an afferent discharge response half as large as the first. Co-administration of ketamine (10–20 mM, 10 μl) with the second NMDA injection (0.5 M, 10 μl) suppresses the NMDA-evoked afferent discharge (Dong et al., 2006). Similar to the response in the masseter, diclofenac (0.1 mg/ml) decreases the nociceptor discharge when co-administered with a second injection of NMDA (0.5 M, 10 μl; Dong et al., 2009).
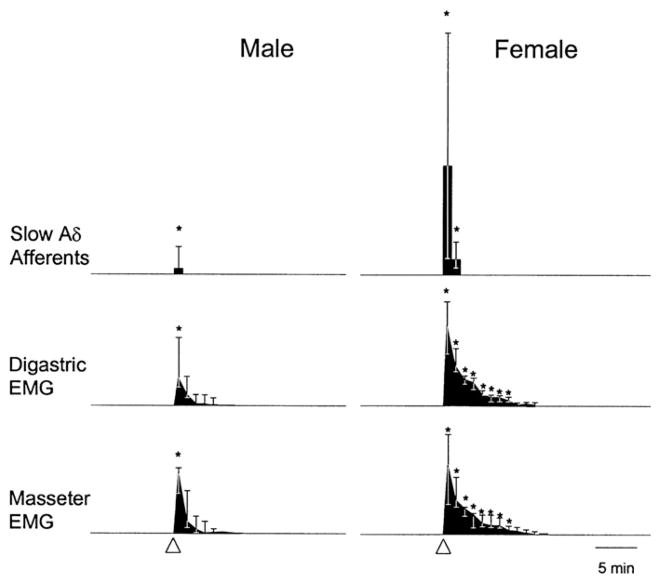
Injection of 0.5 M glutamate (10 μl) into the rat temporomandibular joint (TMJ) causes activation of half to three quarters of TMJ afferents (Cairns et al., 2001b; Lam et al., 2009a). Many afferents are in the slow Aδ range (~6.5 m/s) and all project to the caudal spinal medulla or upper cervical spinal segments (Cairns et al., 2001b,c). A robust, prolonged action potential discharge (30–1800 s, 103 spikes/min) is evoked in slow (<10 m/s) Aδ afferents, but a brief discharge (5–20 s; 10 spikes/min) in fast (>10 m/s) Aδ afferents. Sex differences occur with glutamate evoking greater discharges in slow Aδ afferents from females than from males regardless of the estrous cycle stage (Fig. 7; Cairns et al., 2001b). In a separate set of experiments, 0.5 M glutamate (10 μl) injection into the TMJ activates both Aδ (39%) and C (60%) deep, mechanosensitive afferents (43% total; Lam et al., 2009a). The mechanical activation threshold (MAT) is reduced from 33 g to 20 g in about half of the afferents (Aδ — 48%; C — 40%). The MAT is not reduced in half of the glutamate sensitive afferents and some afferents (44%) with no discharge in response to glutamate have a reduced MAT. Interaction between EAARs (glutamate, 0.5 M, 10 μl) and TRPV1 (capsaicin, 1%, 10 μl) also occurs in some TMJ primary afferents. Four groups of glutamate/capsaicin responsive afferents from TMJ can be identified: 1. glutamate and capsaicin sensitive (15%); 2. glutamate and capsaicin insensitive (18%); 3. glutamate sensitive and capsaicin insensitive (30%); 4. glutamate insensitive and capsaicin sensitive (37%). After an initial glutamate injection (0.5 M, 10 μl) into the TMJ, capsaicin (1%, 10 μl) activates some afferents (33%) with increases in response magnitude and peak frequency, but no changes in response latency or duration. Glutamate, however, does not change the activity or the MAT of afferents following a preadministration of capsaicin (Lam et al., 2009a).
Fig. 7.
Comparison of differences in the responses in male and female rats to the injection of 0.5 M glutamate into the temporomandibular joint capsule. Glutamate injection in female rats causes a larger median response and longer afferent discharge in slow Aδ afferent fibers than in male rats. EMG activity in digastric and masseter muscles caused by glutamate injection in the temporomandibular joint capsule is greater in female rats than in male rats.
Am Physiol Soc, used with permission; Cairns et al., J Neurophysiol, 2001b.
Injection of glutamate (0.01–1.0 M; 10 μl) into the facial cutaneous field of TG Aδ neurons decreases the mechanical threshold (von Frey) for 10 min with female rats more sensitive (EC50 = 16 mM) than males (EC50 = 73 mM). APV attenuates the decrease in threshold and the effect is greater in males than females (Gazerani et al., 2010a). Antidromic electrical stimulation of rat T9 nerve causes increased spontaneous activity in Aβ, Aδ and C fibers of T10 nerve, an effect that can be blocked in the T10 cutaneous field by MK-801 and DNQX (0.1 mM, 10 μl; Fig. 8; Cao et al., 2007). Injection of glutamate s.c. (0.3 mM, 10 μl) into cutaneous field of T9–12 primary afferents increases the excitation of most Aδ (73–78%) and C (81%) fibers (Tian et al., 2005; Luo et al., 2010). Morphine (1.0 mM) blocks glutamate-induced activity and naloxone inhibits morphine’s effect (Tian et al., 2005). Octreotide (20 μM), somatostatin analogue, attenuates the glutamate-evoked activities of Aδ and C fibers, an effect that is blocked by cyclo-somatostatin (128 μM), somatostatin receptor antagonist (Luo et al., 2010).
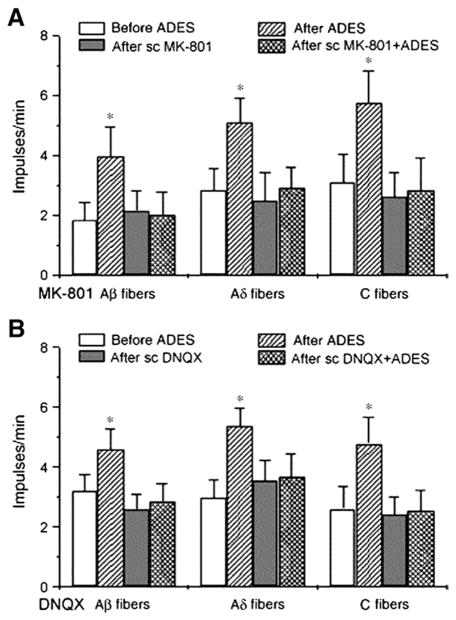
Fig. 8.
Antidromic electrical stimulation (ADES) of T9 dorsal rami causes increased activity in T10 isolated cutaneous branches of Aβ, Aδ, and C fibers (after ADES). Subcutaneous (sc) infusion of MK-801, NMDAR antagonist, into the region of T10 cutaneous branches brings T9 ADES evoked activity to control levels in T10 Aβ, Aδ and C fibers (after sc MK-801+ADES).
Used with permission from author; Cao et al, Brain Res Bull, 2007.
6.5. In vivo extracellular recording of dorsal horn neurons
Nociceptive primary afferents terminate in the spinal trigeminal or spinal dorsal horn where glutamate is released to activate dorsal horn neurons (Millan, 1999). For the trigeminal system, most spinal trigeminal, dorsal horn neurons (86%) with a cutaneous receptive field over the TMJ are activated when 0.5 M glutamate (10 μl) is injected into the TMJ (Lam et al., 2009b). Dorsal horn neurons are activated within 5 s of glutamate injection and the effect lasts for ~2 min. Glutamate also causes a reduction in MAT within 10–20 min in many neurons (57% TMJ MAT; 64% cutaneous MAT) and an expansion of the cutaneous receptive field in almost all (93%) dorsal horn neurons. Evaluation of the peripheral effect of glutamate on capsaicin stimulation also can be observed in dorsal horn electrical activity. After glutamate injection (0.5 M, 10 μl) in the TMJ, capsaicin (1%, 10 μl) activates almost all (92%) neurons and increases the response magnitude, peak frequency, and response duration while decreasing the response latency. The second injection with capsaicin (1%, 10 μl) does not affect the MAT from TMJ, but does reduce the cutaneous MAT. This preadministration effect of glutamate is similar to pretreatment with capsaicin followed by a second application of capsaicin. Cutaneous field expansion is significantly less when the TMJ is pretreated with capsaicin followed by glutamate injection. This may illustrate a desensitization of the afferents due to strong activation of the TRPV1 receptor by capsaicin (Lam et al., 2009b).
The role of ionotropic and metabotropic glutamate receptors on peripheral afferents also has been investigated with single unit recording of feline and rat dorsal horn neurons (Chen et al., 1999a; Wang et al., 2000; Walker et al., 2001; You et al., 2002). Injection i.pl. (20 μl) of 0.1–10 mM 2-amino-5-phosphonopentanoic acid (AP5) and 0.01–1.0 mM DNQX dose dependently reduces C-fiber evoked responses in most wide-dynamic range (WDR) neurons (Wang et al., 2000). This attenuation occurs within 1 min of application and lasts for 10 min. When injected i.pl. after 3 h of carrageenan inflammation, AP5 and DNQX again dose-dependently reduce C-fiber evoked responses (Wang et al., 2000).
Injection of bee venom (BV; Apis mellifera; 0.2 mg/50 μl) into the cat hindpaw causes a local inflammatory response (Chen et al., 1999a). Activated peripheral afferents carry this ‘inflammatory’ information to the spinal dorsal horn causing an increase in firing in WDR neurons for over 1 h. i.pl. pretreatment (10 min) with AP5 (10 mM; 200 μg/100 μl), NMDAR antagonist, or CNQX (357 mM; 8.3 μg/100 μl) blocks the BV-induced activity in WDR neurons. Post-treatment (10 min) with AP5 (10 mM; 200 μg/100 μl) attenuates BV evoked activity, but DNQX (4 mM; 100 μg/100 μl) does not. This may indicate that peripheral NMDA receptors are involved in the initiation and maintenance of persistent activity in WDR neurons, whereas AMPA/KA receptors may be involved only in induction of activity (Chen et al., 1999a).
BV s.c. injection (0.2 mg/ml) in the rat hindpaw also causes increases in WDR activity for over an hour (You et al., 2002). Pre-injection s.c. (10 min) of AP5 (10 mM, 50 μl) or MK 801 (2 mM, 50 μl) reduces BV-induced WDR activity. Post-administration (10 min) of MK 801 (2 mM, 50 μl) into the BV inflamed field also reduces activity of WDR neurons, whereas post-treatment with AP5 (10 mM, 50 μl) has no effect on WDR activity. CNQX (5 mM, 50 μl) has no effect on WDR activity administered before or after BV injection (You et al., 2002). WDR neuronal activity has been used to study the stimulation of mGluR5 receptors on rat primary afferents (Walker et al., 2001). i.pl. injection of (S)-4-carboxy-phenylglycine (CHPG; 20 mM, 100 nmol/5 μl), mGluR5 agonist, into the peripheral receptive field increases the frequency and duration of firing in WDR spinal dorsal horn neurons. Co-application of 2-methyl-6-(phenylethynyl)-pyridine (MPEP; 20 mM, 100 nmol/5 μl), mGluR5 antagonist, blocks CHPG induced activity (Walker et al., 2001).
7. Peripheral effects of glutamate: biophysical
A large body of biophysical evidence demonstrates glutamate’s numerous effects in the periphery. In the spinal cord, presynaptic regulation of glutamate release from primary afferent fibers involves activation of EAARs by glutamate (Kerchner et al., 2001; Huettner et al., 2002; Lee et al., 2002; Bardoni et al., 2004; Park et al., 2004) and a similar phenomenon may occur at the peripheral terminal (Table 2).
Table 2.
Peripheral effects of glutamate: biophysical.
| Drug | Dose | Route | Reference |
|---|---|---|---|
| Agonist | |||
| L-glutamate | 0.6 mM-2.0 M (0.03–100 μmol/50 μl) | i.pl. — rat | Liu et al., 2002; Aumeerally et al., 2004 |
| Glutamate | 1–3 mM | Superfusion of bovine dental pulp | Jackson & Hargreaves, 1999 |
| NMDA | 100–300 μM | Superfusion of bovine dental pulp | Jackson & Hargreaves, 1999 |
| AMPA | 0.01–10 nM; EC50 = 0.27 nM | Superfusion of bovine dental pulp | Jackson & Hargreaves, 1999 |
| Kainate | 1–100 μM; EC50 = 3.2 μM | Superfusion of bovine dental pulp | Jackson & Hargreaves, 1999 |
| Glutamate | 0.015–1.5 M (0.3– 30 μmol/20 μl); ED50 = 0.5 μM | i.pl. — mouse | Beirith et al., 2002, 2003 |
| Glutamate | 1.0 M, 10 μl | Injection — masseter | Cairns et al., 2002a, 2003a |
| Glutamate | 100 mM (300 μg/20 μl) | i.pl. — rat | Lin et al., 2009 |
| NMDA | 50–250 mM (10– 50 μmol/200 μl) | i.pl. — rat | Wang et al., 1997 |
| NMDA | 250 mM (25 μmol/100 μl) | i.pl. — rat | Wang et al., 1999 |
| Antagonist | |||
| MK801 | 1 mM, 50 μl | i.pl. — rat | Jin et al., 2009 |
| NBQX | 5 mM, 50 μl | i.pl. — rat | Jin et al., 2009 |
| CPCCOEtC | 5 mM, 50 μl | i.pl. — rat | Jin et al., 2009 |
| MK 801 | 20, 200 μM (1, 10 nmol/50 μl) | i.pl. — rat | Liu et al., 2002 |
| CNQX | 200 μM (10 nmol/50 μl) | i.pl. — rat | Liu et al., 2002 |
| CNQX | 30 μM | Superfusion of bovine dental pulp | Jackson & Hargreaves, 1999 |
| NBQX | 3.6–6.0 μmol/paw | i.pl. — mouse | Beirith et al., 2002 |
| DON | 400 mM (10 μmol/25 μl) | i.pl. — rat | Hoffman & Miller, 2010 |
| Iodowillardiine | 5–20 mg/kg | i.v. — rat | Andreou et al., 2009 |
| UBP 302 | 50 mg/kg | i.v. — rat | Andreou et al., 2009 |
| Kynurenate | 100 mM, 10 μl | Injection — masseter | Cairns et al., 2003a |
| MK 801 | 0.3 mg/kg/50 μl | Injection — masseter | Ro et al., 2004 |
| MK 801 | 4–22 mM (25– 150 μg/20 μl) | i.pl. — rat | Wang et al., 1997 |
| MK 801 | 0.1, 0.3 mg/kg | Injection — intramuscular | Ro et al., 2004, 2007 |
7.1. Interaction with TRPV1
Capsaicin induced release of glutamate is regulated by EAARs in peripheral tissue (Jin et al., 2009). Injection of i.pl. capsaicin (3 mM, 50 μl), TRPV1 agonist, into the rat hindpaw causes a 300% increase in interstitial glutamate that is suppressed by preadministration (30 min) of capsazepine (30 mg/kg, s.c.). Co-injection of MK801 (1 mM) or NBQX (5 mM) also blocks capsaicin-induced (3 mM, 50 μl) glutamate release. Antagonism of mGluR1 with 7-(hydroxyimino) cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEtC; 5 mM) decreases the glutamate release in response to co-injection with capsaicin (3 mM, 50 μl), whereas (2S,3S,4S)-2-methyl-2-(carboxycyclopropyl)glycine (MCCG; 5 mM, 50 μl), group II mGluR antagonist, and (R,S)-α-methylserine-O-phosphate (MSOP; 5 mM, 50 μl), group III mGluR antagonist, have no influence. The inhibitory effects of both ionotropic and group I metabotropic EAAR antagonists last for 2–3 h post-administration (Jin et al., 2009).
7.2. Adenosine release
Glutamate regulates the release of adenosine in the periphery from primary afferent nerve terminals (Liu et al., 2002; Aumeerally et al., 2004). Adenosine is presumed to inhibit further release of glutamate and/or other neuroactive substances, such as substance P, from primary afferents. I.pl. injection of L-glutamate (0.6 mM – 2.0 M; 0.03–100 μmol/50 μl) into the rat hindpaw promotes subcutaneous release of adenosine in a dose-dependent manner (Liu et al., 2002). The response is rapid and transitory, occurring within 10 min following injection. Co-application of MK-801 (20, 200 μM; 1, 10 nmol/50 μl) or CNQX (200 μM; 10 nmol/50 μl) block the glutamate (20 mM; 1 μmol/50 μl) evoked release of adenosine. Pretreatment with capsaicin (3 days s.c.: 30 mg/kg, 50 mg/kg, 70 mg/kg) to desensitize primary afferents inhibits glutamate’s (20 mM; 1 μmol/50 μl) ability to release adenosine, whereas 6-hydroxydopamine (3 days i.p.: 75 mg/kg) is ineffective. These results indicate that glutamate’s effect is on primary afferent terminals and not sympathetic terminals. Co-administration of MK801 (20, 200 μM; 1, 10 nmol) or CNQX (200 μM; 10 nmol) are ineffective in reducing adenosine release in response to i.pl. injection (50 μl) of 5% formalin (Liu et al., 2002). When injected separately, formalin (1.5%, 50 μl) and glutamate (20 mM; 1 μmol/50 μl) produce similar increases in adenosine levels (Aumeerally et al., 2004).
7.3. CGRP release and nitric oxide production
Glutamate influences the peripheral production and release of vasoactive substances such as CGRP and nitric oxide (Jackson and Hargreaves, 1999; Beirith et al., 2002). Superfusion of bovine dental pulp with glutamate (1–3 mM) and NMDA (100–300 μM) produces inconsistent release of CGRP, but AMPA (0.01–10 nM) and kainate (1–100 μM) cause CGRP release in a dose-dependent fashion (Jackson and Hargreaves, 1999). AMPA is more potent (EC50 = 0.27 nM) than kainate (EC50 = 3.2 μM), but kainate has greater efficacy for causing CGRP release (167% vs. 52%). Pretreatment with CNQX (30 μM) blocks the effect of AMPA (1 nM) and kainate (10 μM) release of CGRP (Jackson and Hargreaves, 1999). I.pl. injection of glutamate (0.015–1.5 M; 0.3–30 μmol/20 μl) into the mouse paw dose dependently causes the production of nitric oxide (Beirith et al., 2002).
7.4. Edema and vasodilation
The hallmarks of inflammation are redness (rubor), swelling or edema (tumor), pain (dolor), and warmth (calor). During neurogenic inflammation, CGRP and SP are released from primary afferents and cause vasodilation (redness) and plasma extravasation (swelling), respectively, in local tissues and glutamate conceivably could contribute to these processes. Several investigations have found no or little evidence to support glutamate’s participation in vasodilation or plasma extravasation. Administration of glutamate to rat knee joint, subcutaneous skin, or TMJ does not cause noticeable redness, edema, or heat beyond vehicle controls (Coggeshall et al., 1997; Lawand et al., 1997; Cairns et al., 1998; Fiorentino et al., 1999). Intra-articular injection of MK-801 (0.3–1.5 mM, 40 μl), AP7 (0.2 mM, 100 μl), CNQX (0.1 mM, 100 μl), or NBQX (0.25–2.5 mM, 40 μl) does not decrease knee joint edema produced by carrageenan or kaolin/carrageenan injection (Lawand et al., 1997; Zhang et al., 2003). Swelling of rat hindpaw by i.pl. spider or bee venom is not diminished by pretreatment with MK-801, AP5, CNQX (Zanchet & Cury, 2003), group I mGluR antagonist, (RS)-1-Aminoindan-1,5-dicarboxylic acid (AIDA), or group II or III mGluR agonists, APDC and L-AP4 (Chen et al., 2010). Edema in rat hindpaw caused by carrageenan is not diminished by intravenous (i.v.) administration of HA-966, NMDAR antagonist, alone or in conjunction with niflumic acid, nonsteroidal anti-inflammatory drug (NSAID; Buritova et al., 1996).
In contrast, glutamate (0.015–1.5 M; 0.3–30 μmol/20 μl) injection (i.pl.) induces dose dependent redness and swelling in mouse skin (Beirith et al., 2002, 2003). This effect lasts for at least 40 min with an ED50 of 0.025 M (0.5 μmol/20 μl; Beirith et al., 2002). Pretreatment (30 min) with Chicago (pontamine) sky blue 6B (100 μg/kg, i.p.), inhibitor of vesicular glutamate uptake (Roseth et al., 1995), reduces glutamate-induced edema (Beirith et al., 2002). I.pl. pretreatment with MK 801 (0.01–1.0 μmol/paw) fails to affect glutamate-induced (30 μmol/20 μl) edema, but there is a modest reduction (17%) with i. pl. NBQX (3.6–6.0 μmol/paw). The glutamate-induced paw edema appears to have a nitric oxide mechanism. Pre- and concurrent i.p. treatment with Lω-N-nitro-arginine (NOARG), nitric oxide synthase inhibitor (0.1–1.1 mmol/kg), reduces glutamate-induced (30 μmol/20 μl) edema and this is reversed by pretreatment with L-arginine (3.4 mmol/kg, i.p.), but not D-arginine (Beirith et al., 2002). I.pl. co-injection of S-nitroso-N-acetyl-D,L-penicillamine (SNAP, nitric oxide donor, 0.1–1.0 μmol/20 μl) with glutamate (0.3, 10.0 μmol/20 μl) potentiates paw edema (Beirith et al., 2002). Tachykinin receptors have a role in glutamate-induced edema. Co-administration of the NK2 (tachykinin) receptor antagonist, SR 48968, dose dependently (0.05–0.5 nmol) decreases glutamate induced edema (36%; Beirith et al., 2003). Other antagonists have no effect: FK 888, NK1 receptor antagonist; SR 142801, NK3 receptor antagonist; CGRP8–37, CGRP receptor antagonist; bradykinin B1 receptor antagonist, des-Arg9-[Leu8]-BK; B2 receptor antagonist, HOE 140. Destruction of C fibers by neonatal capsaicin treatment inhibits (30%) glutamate induced edema (Beirith et al., 2003). Furthermore, the local administration of the glutaminase inhibitor, 6-diazo-5-oxo-L-norleucine (DON; 400 mM; 10 μmol/25 μl), modestly decreases swelling in the rat hindpaw during carrageenan induced inflammation (Hoffman & Miller, 2010).
Dilation of the middle meningeal artery via electrical stimulation is blocked by iodowillardiine (5–20 mg/kg, i.v.), KAR GluR5 antagonist (Andreou et al., 2009). Iodowillardiine’s effect is blocked by pretreatment with (S)-1-(2-Amino-2-carboxyethyl)-3-(2-carboxy-benzyl)pyrimidine-2,4-dione (UBP 302; 50 mg/kg, i.v.), GluR5 agonist. Neither GluR5 agonists nor antagonists have influence on baseline vessel diameter and iodowillardiine has no effect on CGRP-induced vasodilation (Andreou et al., 2009). In a similar study, NMDAR antagonists, ketamine and MK801, inhibit dural vessel dilation induced by electrical and capsaicin stimulation and ketamine attenuates CGRP-induced dilation (Chan et al., 2010). AMPAR antagonist, GYKI52466, diminishes the response to CGRP. KAR antagonism with LY466195 is unable to attenuate dural dilatation with electrical, capsaicin, or CGRP stimulation. In contrast, GYKI52466 only attenuates the vasodilation to exogenous α-CGRP, while LY466195 does not affect the vasodilator responses to endogenous or exogenous CGRP (Chan et al., 2010).
MK-801 is effective when given systemically (0.5 mg/kg) in reducing mustard oil (MO) induced plasma extravasation in the TMJ, but is ineffective when administered locally (Yu et al., 1996). Based on T2 weighted magnetic resonance imaging, increased extracellular water percentage and edema volume occurs in the rat masseter muscle following injection of glutamate (1.0 M, 10 μl) into the rat masseter muscle (Cairns et al., 2002a, 2003b). Co-injection of kynurenate (100 mM, 10 μl) with glutamate has no effect on edema volume, but does decrease the percentage of peak extracellular water (Cairns et al., 2003b). Injection of glutamate (1.0 M, 10 μl) into the masseter also produces elevated masseter blood flow that is attenuated by co-administration with kynurenate (100 mM, 10 μl; Cairns et al., 2003b). MO injection into the rat masseter muscle induces inflammatory edema that is attenuated with local injection of MK-801 (0.3 mg/kg/50 μl), but does not attenuate MO-induced edema in the rat biceps muscle (Ro, 2003).
7.5. Spinal and Trigeminal Fos
Stimulation of primary afferents induces Fos-ir in spinal and trigeminal dorsal horn neurons (Coggeshall, 2005). Activation and antagonism of glutamate’s peripheral actions, therefore, can be evaluated indirectly by examining Fos-ir in the dorsal horn. Glutamate (100 mM; 300 μg/20 μl) i.pl. injection causes an increase in Fos-IR neurons in superficial dorsal horn, an action that can be inhibited by i. pl. honokiol and magnolol (10 mg/kg, i.p.; Lin et al., 2009). NMDA (50–250 mM; 10–50 μmol/200 μl) i.pl. in the rat hindpaw causes an increase in Fos-ir in superficial and deep laminae of the spinal dorsal in a dose dependent manner (Wang et al., 1997). NMDA’s (250 mM; 25 μmol/100 μl) effect on Fos expression can be blocked peripherally by co-administration of the nitric oxide synthase inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME; 10–80 μmol/20 μl; Wang et al., 1999). Peripheral glutamate antagonism also can be used to diminish Fos-ir following noxious stimulation. Pretreatment and concurrent i. pl. administration of DON (400 mM; 10 μmol/25 μl), GLS inhibitor, with carrageenan reduces the number of Fos-IR neurons in rat superficial dorsal horn laminae by over 50%, but not in deeper laminae (Hoffman & Miller, 2010). MK-801 (4–22 mM; 25–150 μg/20 μl) decreases, in a dose-dependent fashion, Fos-ir following 0.5% formalin (100 μl) injection into the rat hindpaw (Wang et al., 1997). Hypertonic saline infusion or 20% MO injection into the rat masseter muscle causes an increase in Fos-ir in the superficial dorsal horn of the caudal spinal trigeminal nucleus. Intramuscular pretreatment with MK-801 (0.1, 0.3 mg/kg) or NBQX (1–100 nmol/10 μl) blocks or reduces this increase in Fos-ir (Ro et al., 2004, 2007; Chun et al., 2008). Some EAAR antagonists are administered peripherally, e.g., intraper-itoneal (i.p.), i.v., or s.c., with the assumption that antagonism acts centrally, but some effects could be on the peripheral terminals of primary afferents. For example, after MO injection in the masseter, i.v. administration of MK-801 reduces Fos-ir in the spinal trigeminal nucleus (Ro et al., 2004). MK-801 (i.p., i.v.) reduces Fos-ir in the rat and cat caudal spinal trigeminal nuclei following experimental tooth (molar) movement, formalin injection in whisker pad, MO injection in TMJ, and electrical stimulation of the superior sagittal sinus (Bereiter & Bereiter, 2000; Classey et al., 2001; Otahara et al., 2003; Hattori et al., 2004). Increases in Fos-ir by carrageenan or formalin injection in the rat hindpaw can be diminished i.v. or s.c. HA-966 (Chapman et al., 1995, 1996; Buritova et al., 1996, 2003).
8. Peripheral effects of glutamate: animal behavior
Glutamate, released from nociceptors or exogenously applied, produces nociceptive actions in animals and painful responses in humans. Altered nociceptive behavior often is described in terms of response to mechanical and/or thermal stimulation. Increased response to a noxious mechanical or thermal stimulus is termed hyperalgesia, whereas a nociceptive response to a non-noxious stimulus is termed allodynia (Table 3).
Table 3.
Peripheral effects of glutamate: animal behavior.
| Drug | Dose | Route | Reference |
|---|---|---|---|
| Mechanical hyperalgesia/allodynia | |||
| Agonist | |||
| KA | Mice: 20 mg/kg, 100 μl; rats: 7 mg/kg, 500–800 μl | i.p. | Giovengo et al., 1999 |
| L-glutamate | 100 pg/paw; ED50 = 0.7 pg/paw | i.pl. | Follenfant & Nakamura-Craig, 1992 |
| Glutamate | 0.1–10 mM (1–100 nmol/10 μl) | i.pl. | Walker et al., 2001 |
| Glutamate | 30 nmol/paw | i.pl. | Zanchet & Cury, 2003 |
| L-glutamate | 30 nM (3 pmol/100 μl) | i.pl. | Leem et al., 2001 |
| Glutamate | 0.01–0.3 mM | i.pl. | Carlton et al., 1995; Coggeshall et al., 1997 |
| KA | 0.005–1.0 mM, 20 μl | i.pl. | Du et al., 2006 |
| Glutamate, NMDA, AMPA, CHPG, DHPG | 0.1–10 mM (1–100 nmol/10 μl) | i.pl. | Walker et al., 2001 |
| NMDA, AMPA, KA | 0.001–5 mM, 20 μl | i.pl. | Zhou et al., 1996 |
| Glutamate | 0.1–10.0 mM, 20 μl | Tail — injection | Carlton et al., 1998 |
| S-DHPG | 0.01–1.0 mM, 20 μl | i.pl. | Zhou et al., 2001 |
| Antagonist | |||
| AP-7 | 240 mg/kg | s.c. | Follenfant & Nakamura-Craig, 1992 |
| MK 801 | 100 pmol/paw | i.pl. | Zanchet & Cury, 2003 |
| AP5 | 40 pmol/paw | i.pl. | Zanchet & Cury, 2003 |
| CNQX | 100 nmol/paw | i.pl. | Zanchet & Cury, 2003 |
| CNQX | 1 mg/kg | i.p. — mice | Giovengo et al., 1999 |
| CPP | 10 mg/kg | i.p. — mice | Giovengo et al., 1999 |
| CNQX | 1–100 μM, 20 μl | i.pl. | Zhou et al., 1996 |
| MK 801 | 1–10 μM, 20 μl | i.pl. | Zhou et al., 1996 |
| MK 801 | 0.01 mM, 20 μl | Tail — injection | Carlton et al., 1998 |
| CNQX | 0.3 mM, 20 μl | Tail — injection | Carlton et al., 1998 |
| MPEP | 0.3–3 mM (0.03–.03 μmol/10 μl) | i.pl. | Walker et al., 2001 |
| AIDA | 1.0 mM, 20 μl | i.pl. | Zhou et al., 2001 |
| Thermal hyperalgesia | |||
| Agonist | |||
| L-glutamate | 0.6 mM (30 nmol/50 μl) | i.pl. | Jackson et al., 1995; Peana et al., 2004 |
| Glutamate, NMDA, or AMPA | 100 μM–100 mM, 50 μl | i.pl. | Jin et al., 2009 |
| S-DHPG | 1–100 mM, 50 μl | i.pl. | Jin et al., 2009 |
| Glutamate | 100 mM (300 μg/20 μl) | i.pl. | Lin et al., 2009 |
| NMDA | 50 mM (150 μg/20 μl) | i.pl. | Lin et al., 2009 |
| CHPG | 250 μM (1 μg/20 μl) | i.pl. — mice | Lin et al., 2009 |
| Glutamate | 5.0 mM, 20 μl | Tail, s.c. | Carlton et al., 1998 |
| RS-DHPG, S-DHPG | 1–5 mM (10–50 nmol/10 μl) | i.pl. — mice | Bhave et al., 2001 |
| Antagonist | |||
| CNQX | 2 mM (100 nmol/50 μl) | i.pl. | Jackson et al., 1995 |
| MK-801 | 0.01–0.1 mM, 20 μl | Tail, s.c. | Carlton et al., 1998 |
| MPEP | 3 mM (30 nmol/10 μl) | i.pl. | Bhave et al., 2001 |
| CPCCOEt, LY367385 | 10 mM (100 nmol/10 μl) | i.pl. | Bhave et al., 2001 |
| Motor activity | |||
| Agonist | |||
| L-glutamate | 0.01–10 mM | Tail — superfusion | Ault & Hildebrand, 1993a |
| Domoate | 0.1–10 μM; EC50 = 1 μM | Tail — superfusion | Ault & Hildebrand, 1993b |
| KA | 10–300 μM; EC50 = 63 μM | Tail — superfusion | Ault & Hildebrand, 1993b |
| Quisqualate | 0.1–1.0 mM; EC50 = 511 μM | Tail — superfusion | Ault & Hildebrand, 1993b |
| AMPA | 0.1–1.0 mM | Tail — superfusion | Ault & Hildebrand, 1993b |
| Glutamate | 10–500 mM (0.1–5.0 μmol/10 μl) | Rat TMJ — injection | Cairns et al., 1998, 2001b |
| NMDA, KA, AMPA | 10–100 mM (0.1–1.0 μmol/10 μl) | Rat TMJ — injection | Cairns et al., 1998 |
| Antagonists | |||
| AP5 | 10 mM, 50 μl | Muscle — injection | You et al., 2002 |
| CNQX | 5 mM, 50 μl | Muscle — injection | You et al., 2002 |
| APV, CNQX | 50 mM (0.5 μmol/10 μl) | Rat TMJ — injection | Cairns et al., 1998 |
| MK-801 | 0.001–0.1 M, 10 μl | Rat TMJ — injection | Lam et al., 2005 |
| Nocifensive responses | |||
| Agonists | |||
| Glutamate | 0.5 mM, 20 μl | Tail — injection | Carlton et al., 1998 |
| Glutamate | 100 mM (300 μg/20 μl) | i.pl. — mice | Lin et al., 2009 |
| NMDA | 50 mM (150 μg/20 μl) | i.pl. — mice | Lin et al., 2009 |
| Glutamate | 0.015–3.0 M (0.3–60 μmol/20 μl); ED50 = 0.13 M | i.pl. — mice | Beirith et al., 2002, 2003 |
| Glutamate | 30 μmol/paw | i.pl. — mice | Quintao et al., 2010 |
| Antagonists | |||
| MK 801 | 0.5–50 mM (0.01–1.0 μmol/20 μl); ID50 = 3 mM | i.pl. — mice | Beirith et al., 2002 |
| NBQX | 0.18–1.8 M (3.6–36.0 μmol/20 μl); ID50 = 1.7 M | i.pl. — mice | Beirith et al., 2002 |
| GAMS | 5–150 mM (0.1–3.0 μmol/20 μl) | i.pl. — mice | Beirith et al., 2003 |
| E4CPG | 50–500 mM; (1–10 μmol/20 μl) | i.pl. — mice | Beirith et al., 2002 |
| NA-3,4,-DCM | 2.8–85.2 μmol/kg, i.p.; 28.4–284.1 μmol/kg, oral | i.p., oral — mice | Quintao et al., 2010 |
8.1. Glutaminase deficiency
GLS deficient mice, heterozygous GLS1+/− (GLS1−/− mice die after birth), have GLS1 levels and activity that are half of wild-type (WT) mice (Masson et al., 2006). Compared to WT, heterozygous GLS1 mice show reduced mechanical (tail pressure, von Frey) and thermal (radiant heat, hot plate) nociceptive responses (Kayser et al., 2008). In both phases of the formalin test, GLS1+/− mice have diminished nociceptive responses, i.e., lick, flick, flinch, compared to WT. Although the peripheral and central afferent endings have not been evaluated in GLS1+/− mice, the ‘low glutamatergic tone’ is most likely occurring at both peripheral and central locations leading to diminished nociceptive responses (Kayser et al., 2008).
8.2. Mechanical hyperalgesia/allodynia
I.p. KA administration (mice: 20 mg/kg, 100 μl; rats: 7 mg/kg, 500–800 μl) produces a persistent hyperalgesia in mice and rats (Giovengo et al., 1999). Whereas, thermal hyperalgesia occurs within 6 h, mechanical hyperalgesia (von Frey) does not develop until 48 h and remains for 7 weeks. No difference in responses (abdominal stretch assay) with chemical stimulation, i.e., acetic acid (i.p.), occurs between KA or vehicle injected mice. KA injected s.c. also causes thermal and mechanical hyperalgesia, but not when injected intrathecally. Pretreatment of mice with CNQX (1 mg/kg) prevents the KA induced hyperalgesia when administered i.p., but not when given intrathecally. I.p. pretreatment of 3-(2-carboxypiperazine-4-yl) propyl-1-phosphonic acid (CPP; 10 mg/kg), NMDAR antagonist, delays onset of hyperalgesia for 48 h, but intrathecal (i.t.) pretreatment has no effect. These routes of administration indicate that KA’s effect is most likely at a peripheral site, i.e., primary afferents (Giovengo et al., 1999).
Injection of i.pl. glutamate in the rat hindpaw causes mechanical hyperalgesia and allodynia in Randall–Selitto, plantar anesthesi-ometer, and von Frey filament tests (Carlton et al., 1995; Coggeshall et al., 1997; Follenfant & Nakamura-Craig, 1992; Leem et al., 2001; Zanchet & Cury, 2003). L-glutamate causes mechanical hyperalgesia (ED50 = 0.7 pg/paw, Randall–Selitto), but not D-glutamate (100 pg/paw; Follenfant & Nakamura-Craig, 1992). Using a smaller i.pl. volume, another study confirms the dose dependency of mechanical hyperalgesia induced by glutamate (0.1–10 mM; 1–100 nmol/10 μl; Randall–Selitto; Walker et al., 2001). Mechanical hyperalgesia after a single large dose of L-glutamate (100 pg/paw) lasts for up to 8 days (Follenfant & Nakamura-Craig, 1992). In addition, a second challenge of a subthreshold dose of glutamate (0.1 fg/paw) at 11 days produces mechanical hyperalgesia lasting 24 h. Daily subthreshold doses of glutamate (0.1, 1.0 fg/paw, 2× daily) for 4 days causes hyperalgesia on day 5 that lasts until day 10. Glutamate-induced (100 pg/paw) hyperalgesia is blocked by AP-7 (240 mg/kg, s.c.), NMDAR antagonist, but not DNQX (80 μg/kg, s.c.; Follenfant & Nakamura-Craig, 1992). Glutamate i.pl. injection (30 nmol/paw) produces mechanical hyperalgesia (Randall–Selitto) that is maximal at 1 h and this effect is attenuated by concurrent administration of MK-801 (100 pmol/paw), AP5 (40 pmol/paw), or CNQX (100 nmol/paw; Zanchet & Cury, 2003). A single i.pl. injection of L-glutamate (30 nM; 3 pmol/100 μl), but not D-glutamate, produces mechanical hyperalgesia (plantar anesthesi-ometer) that increases over time (2 h; Leem et al., 2001). Glutamate (0.01–0.3 mM) i.pl. causes a dose dependent mechanical hyperalgesia (39.2, 47 mN von Frey), but at 1 mM there is little to no mechanical response due possibly to desensitization (Carlton et al., 1995; Coggeshall et al., 1997). I.pl. injection of 0.1 mM L-glutamate causes mechanical allodynia using innocuous stimulation (12.5 mN von Frey) and mechanical hyperalgesia with noxious stimuli (47 mN). Mechanical allodynia occurs at 25 min, whereas mechanical hyperalgesia occurs by 5 min and lasts until 20 min (Carlton et al., 1995). Pretreatment with capsazepine (120 μmol/kg, i.v.), TRPV1 antagonist, however, has no effect on ip.l. glutamate-induced (30 nmol/paw) hyperalgesia (Randall & Selitto; Zanchet & Cury, 2003).
Further dissection of glutamate’s effect on mechanical hyperalgesia in rat paw comes from studies using EAAR agonists (Zhou et al., 1996; Walker et al., 2001; Du et al., 2006). For example, i.pl. KA (0.005–1.0 mM, 20 μl) injection causes a dose-dependent reduction in mechanical threshold (21–160 mN von Frey) with 1.0 mM kainate producing a maximal response (Du et al., 2006). I.pl. injection of NMDA and AMPA produce mechanical hyperalgesia with equal potency, but with lower potency compared to glutamate (0.1–10 mM; 1–100 nmol/10 μl; Randall–Selitto; Walker et al., 2001). I.pl. administration of NMDA, AMPA, or KA (0.001–5 mM, 20 μl) produces dose dependent mechanical hyperalgesia (39.2 mN von Frey) within 20 min (Zhou et al., 1996). The maximal effect occurs at 1 mM for each agonist with a sharp decrease in response at 5 mM possibly indicating desensitization. Co-application of CNQX (1–100 μM, 20 μl) dose dependently blocks the 1.0 mM AMPA and KA induced mechanical hyperalgesia. AMPA’s effect is blocked maximally with 10 μM CNQX, whereas KA’s effect is blocked with 100 μM CNQX. NMDA’s effect is inhibited by MK-801 (1–10 μM, 20 μl) and the maximal result occurs at 10 μM. CNQX has no effect on NDMA induced behavior and MK-801 has no influence on AMPA or KA induced behavior. I.pl. injection of 1.0 mM (20 μl) NMDA, AMPA, or KA produces robust mechanical hyperalgesia (18.8–39.2 von Frey) for more than 1 h and mild mechanical allodynia (6.7–10.4 mN von Frey) for 30–40 min. Co-injection with 10 or 100 μM (20 μl) CNQX blocks the effects of AMPA and KA, whereas 10 μM (20 μl) MK-801 blocks NMDA effects (Zhou et al., 1996). The effects of I.pl. NMDA (0.05–1 mM, 20 μl) are dose dependent with a decrease in mechanical threshold (21–160 mN von Frey) within 30 min (Du et al., 2003).
Glutamate injected into the rat tail also produces mechanical hyperalgesia (39.2 mN von Frey) that is dose dependent (0.1–10.0 mM, 20 μl; Carlton et al., 1998). There is a lack of response at the highest dose, 10.0 mM, possibly due to densitization. The maximal responsive dose, 5 mM, shows mechanical allodynia and hyperalgesia for 40 min with a wide-range of mechanical stimulation (6.7–39.2 mN von Frey). Co-injection (20 μl) with 0.01 mM MK-801 or 0.3 mM CNQX blocks the mechanical responses to glutamate. Co-administration with SP (0.01 mM, 20 μl) augments the mechanical hyperalgesia (18.8–39.2 mN) compared to SP (0.01 mM) or glutamate 5 mM alone (Carlton et al., 1998).
Group I metabotropic glutamate receptor agonists also produce mechanical hyperalgesia when injected into the rat hindpaw (Walker et al., 2001; Zhou et al., 2001). Glutamate, CHPG, and 3,5-dihydrox-yphenylglycine (DHPG), group I mGluR agonist, all produce mechanical hyperalgesia (Randall–Selitto test) in a dose dependent manner (0.1–10 mM; 1–100 nmol/10 μl; potency: glutamate>CHPG=DHPG; Walker et al., 2001). Co-administration of 2-methyl-6-(phenylethynyl)-pyridine (MPEP; 0.3–3 mM; 0.03–0.3 mmol/10 μl), mGluR5 antagonist, with glutamate, CHPG, or DHPG (20 mM; 0.1 μmol/10 μl) causes a dose-dependent inhibition of mechanical hyperalgesia. The mechanical hyperalgesia created by i.pl. DHPG (10 mM; 0.1 μmol/10 μl) is not affected by co-injection with (S)-4-carboxy-phenylglycine (4-CPG; 0.3–3 mM; 0.03–0.3 mmol/10 μl; mGluR1 antagonist). L-AP4 (0.1–10 mM; 1–100 nmol/10 μl), group III mGluR agonist, and LY314582 (0.1–10 mM; 1–100 nmol/10 μl) group II mGluR agonist, do not produce alterations paw withdrawal (Walker et al., 2001). I.pl. injection of (S)-3,5-dihydroxyphenylglycine (S-DHPG), mGluR1/5 agonist, dose dependently (0.01–1.0 mM, 20 μl) reduces the mechanical threshold (21–160 mN von Frey; Zhou et al., 2001). S-DHPG (0.1 mM, 20 μl) induced decrease in mechanical threshold starts within 10 min, lasts for at least 1 h, and is blocked by co-injection with 1.0 mM AIDA, mGluR1 antagonist (Zhou et al., 2001).
8.3. Thermal hyperalgesia
As mentioned previously, i.p. KA administration (mice: 20 mg/kg; rats: 7 mg/kg) produces persistent hyperalgesia in mice and rats (Giovengo et al., 1999). Thermal hyperalgesia occurs within 6 h and persists for 2–12 weeks. Withdrawal latencies decrease in both heat (hot plate, 49° and 53 °C water bath, tail flick) and cold (−10 °C water bath) assays. Pretreatment of mice with i.p. CNQX prevents the KA induced hyperalgesia and CPP delays onset of hyperalgesia for 48 h. Intrathecal administration of KA, CNQX, or CPP has no effect indicating that KA’s effect is most likely at the peripheral primary afferent terminal (Giovengo et al., 1999).
Glutamate injection in the rat hindpaw or tail causes thermal hyperalgesia in thermal plantar, hot water bath, and tailflick tests (Jackson et al., 1995; Carlton et al., 1998; Peana et al., 2004; Jin et al., 2009). I.pl. L-glutamate (0.6 mM; 30 nmol/50 μl), but not D-glutamate, produces thermal hyperalgesia (thermal plantar) within 5 min (Jackson et al., 1995; Peana et al., 2004). Interestingly, CNQX (2 mM; 100 nmol/50 μl) also reduces paw withdrawal latencies in normal rats (Jackson et al., 1995). I.pl. application of glutamate, NMDA, or AMPA dose-dependently (0.1–100 mM, 50 μl) produces thermal hyperalgesia (thermal plantar) in the rat hindpaw within 15 min lasting for 3–4 h (Jin et al., 2009). I.pl. glutamate (10 mM; 300 μg/20 μl) and NMDA (5 mM; 150 μg/20 μl) cause thermal hyper-algesia (water bath, 47 °C) that starts within 5 min lasting for over 2 h (Lin et al., 2009). This thermal hyperalgesia can be blocked by i.pl. honokiol and magnolol (0.05–1.0 μg/paw; Lin et al., 2009). Glutamate injection (5.0 mM, 20 μl) into the tail causes thermal hyperalgesia (water bath, 44 °C) for 10–30 min and MK-801 co-administration (0.01–0.1 mM) blocks the glutamate effect (Carlton et al., 1998).
There are conflicting results in regard to the role of mGluRs in producing thermal hyperalgesia in normal rodents. For example, i.pl. or tail injection of S-DHPG (0.1–10 mM, 20 μl) does not cause thermal hyperalgesia (thermal plantar or water bath, 44 °C) in rats (Zhou et al., 2001), whereas, in a second study, i.pl. injection of S-DHPG (1–100 mM, 50 μl) causes long lasting (15 min–6 h) thermal hyperalgesia (thermal plantar; Jin et al., 2009). In mice, injection (i.pl.) of RS-DHPG and S-DHPG causes a dose-dependent (1–5 mM; 10–50 nmol/10 μl) thermal hyperalgesia (thermal plantar) starting within 15 min and lasting for over 2 h (Bhave et al., 2001). Preadministration i.pl. of MPEP (3 mM; 30 nmol/10 μl), CPCCOEt (10 mM; 100 nmol/10 μl), or S-+-a-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385; 10 mM; 100 nmol/10 μl), noncompetitive mGluR1 antagonist, blocks or decreases DHPG and glutamate induced thermal hyperalgesia. None of the group I mGluR antagonists have any effect on baseline thermal sensitivity. Also, i.pl. preadministration of APV (5 mM; 50 nmol/10 μl) or R,S-α-methyl-4-phosphonophenylglycine (MPPG; 50 mM; 500 nmol/10 μl), group II/III mGluR antagonist, has no effect on DHPG induced hyperalgesia (Bhave et al., 2001). Injection i.pl. of CHPG (250 μM; 1 μg/20 μl) also induces thermal hyperalgesia (water bath, 47 °C) in mice and this effect is blocked by honokiol (0.1–1.0 μg/paw) and magnolol (0.05–0.5 μg/paw; Lin et al., 2009). Group II and III mGluR agonists, e.g., L-AP4 (1–100 mM, 50 μl), APDC (11 μM; 20 ng/10 μl), and (2S,1′S,2′S)-2-(carboxycyclopropyl) glycine (L-CCG-I; 1–100 mM, 50 μl), appear to not influence thermal sensitivity in rodents (Yang & Gereau, 2002; Jin et al., 2009). Modulation of thermal hyperalgesia by group II mGluRs may occur by modulation of cAMP/PKA pathways. For example, i.pl. injection of forskolin (10 μM) in rats causes of thermal hyperalgesia, but co-administration of ADPC (0.5 μM) prevents sensitization to heat (Carlton et al., 2009).
8.4. Motor activity
Nociceptive behavior is dependent on the motor system and recordings of the ventral root reflex or electromyographic (EMG) responses provide an understanding of activation of peripheral primary afferent EAARs. For example, L-glutamate (0.01–10 mM), exposed to tail skin, evokes nociceptive ventral root reflexes in an isolated neonatal rat spinal cord-tail preparation (Ault & Hildebrand, 1993a). Domoate (0.1–10 μM; EC50 = 1 μM) and KA (10–300 μM; EC50 = 63 μM) are potent activators of the ventral root reflex, comparable to capsaicin or bradykinin (Ault & Hildebrand, 1993b). Quisqualate (0.1–1.0 mM; EC50 = 511 μM) and AMPA (0.1–1.0 mM) are of lower potency than KA, and NMDA (0.1, 1.0 mM) and L-aspartate (10 mM) are inactive (Ault & Hildebrand, 1993b). In addition, antagonism of EAARs can decrease the EMG activity evoked from innocuous and noxious stimuli (You et al., 2002). Injection of AP5 (10 mM, 50 μl), but not CNQX (5 mM, 50 μl) into the rat gastrocnemius soleus muscle decreases the single motor unit EMG responses to innocuous pressure (1.72→0.60 spikes/s) and noxious pinch (10.95→3.46 spikes/s) of the muscle (You et al., 2002). Preinjection of MK-801 (0.001–0.1 M, 10 μl) or APV (0.05 M) into the TMJ prior to capsaicin (1%, 10 μl) reduces digastric and masseter muscle EMG activity (Lam et al., 2005).
Injection of glutamate (250 mM; 2.5 μmol/10 μl or 500 μM, 10 μl) and other ionotropic EAAR agonists (50 mM; 0.5 μmol/10 μl), i.e., NMDA, KA, and AMPA, into the rat TMJ produces increases in ipsilateral digastric and masseter muscle EMG activities (Fig. 7; Cairns et al., 1998, 2001b,c). The latency to response in muscle activity is faster with NMDA and AMPA (4.2 s) than glutamate (9.3 s) or KA (12.6 s; Cairns et al., 1998). Dose response studies with glutamate (10–500 mM; 0.1–5.0 μmol/10 μl) and EAAR agonists (10–100 mM; 0.1–1.0 μmol/10 μl) demonstrate a steep response relationship for the ipsilateral digastric, whereas the response of the masseter is less pronounced (Cairns et al., 1998). The contralateral digastric and masseter muscles also exhibit a dose response to glutamate and EAAR agonist stimulation in the TMJ, but with much less EMG activity compared to ipsilateral muscles. I.v. administration of glutamate (50 mM; 5 μmol/100 μl) or EAAR agonists (NMDA, KA, and AMPA; 5 mM; 0.5 μmol/100 μl) does not promote EMG in muscles. Repeated injection (30 min intervals) of the TMJ with glutamate (250 mM; 2.5 μmol/10 μl), NMDA (50 mM; 0.5 μmol/10 μl), or AMPA (50 mM; 0.5 μmol/10 μl) yields similar EMG responses in the ipsilateral digastric and masseter muscles, but repeated KA (50 mM; 0.5 μmol/10 μl) administration causes decreased activity, possibly illustrating desensitization. APV (50 mM; 0.5 μmol/10 μl) and CNQX (50 mM; 0.5 μmol/10 μl) reduce EMG activity when co-administered with glutamate (250 mM; 2.5 μmol/10 μl) into the TMJ. APV (50 mM; 0.5 μmol/10 μl), but not CNQX, reduces NMDA (50 mM; 0.5 μmol/10 μl) induced activity and CNQX (50 mM; 0.5 μmol/10 μl), but not APV, reduces AMPA (50 mM; 0.5 μmol/10 μl) induced activity into the TMJ (Cairns et al., 1998). Injection (10 μl) of α,β-methylene adenosine 5′-triphosphate (10 mM), P2X agonist, into the TMJ increases EMG activity in the digastric and masseter muscles and this can be attenuated by pre-injection (10 min) of APV (0.5 μM, 10 μl), NMDA antagonist (Watanabe et al., 2010).
There is a sex difference in the TMJ activation of EMG activity where the dose response to glutamate (0.25–1.0 M, 10 μl) is greater in female rats (all estrous stages) compared to male rats (Cairns et al., 2001b, 2002b). In addition, glutamate-evoked (0.5 M, 10 μl) activity in the EMG of digastric and masseter muscles is longer in female (8 min) compared to male rats (1 min; Cairns et al., 2001b; Fig. 7). Injection of 1.0 M (10 μl) glutamate into the TMJ evokes both a larger peak and duration of EMG activity in digastric and masseter muscles in female than male rats (Cairns et al., 2002b). Furthermore, TMJ injection (10 μl) of 0.25 M glutamate causes greater masseter EMG activity in female than male rats. Gonadectomy in female rats decreases the digastric EMG activity evoked by 1.0 M glutamate (10 μl) injected into the TMJ compared to intact females, an effect that is reversed with estrogen treatment. Gonadectomy in male rats does not alter glutamate-evoked (1.0 M, 10 μl, TMJ) digastric activity compared to intact males, but estrogen treatment raises digastric EMG activity to a level comparable to females (Cairns et al., 2002b). Preadministration (30 min) of glutamate (0.01–1.0 M, 10 μl) into the TMJ followed by MO (20%, 10 μl) elicits a larger EMG response in the digastric muscle compared to MO alone. Pre-injection (60 or 120 min) of 0.25 M glutamate (10 μl) followed by MO continues to evoke a greater EMG response compared to MO alone. There is, however, no difference in the magnitude of EMG activity evoked by MO with or without pre-application of glutamate in males compared to female rats (Cairns et al., 2002b).
8.5. Nocifensive responses
In addition to hyperalgesia demonstrated by evoked nociceptive tests, glutamate and EAARs appear to have a role in nocifensive responses or spontaneous pain (Carlton et al., 1998; Beirith et al., 2002, 2003; Lin et al., 2009; Quintao et al., 2010). Spontaneous tail flicks occur when 5.0 mM glutamate and 0.01 mM SP are co-administered (20 μl, tail injection), but not when administered singly (Carlton et al., 1998). Following i.pl. injection of glutamate (100 mM; 300 μg/20 μl) or NMDA (50 mM; 150 μg/20 μl), mice undergo licking the injected foot, a behavior that is reduced by i.pl. honiokiol and magnolol (0.05–1.0 μg/paw; Lin et al., 2009). Furthermore, i.pl. glutamate (0.015–3.0 M; 0.3–60 μmol/20 μl) dose dependently induces paw licking in mice (Beirith et al., 2002). This behavior occurs within the first 15 min, but decreases in the following 15 min and is absent by 40 min. The ED50 for glutamate-induced nocifensive licking is 0.13 M (2.6 μmol/20 μl) and the maximal effect occurs at 1.5 M (30 μmol/20 μl). Pretreatment (30 min) with i.pl. MK 801 (0.5–50 mM; 0.01–1.0 μmol/20 μl, ID50 = 3 mM; 0.06 μmol/20 μl) or NBQX (0.18–1.8 M; 3.6–36.0 μmol/20 μl; ID50 = 1.7 M; 33.9 μmol/20 μl) dose dependently inhibits i.pl. glutamate-induced (1.5 M; 30 μmol/20 μl) nocifensive behavior. I.pl. pretreatment with γ-D-glutamylaminomethyl sulfonic acid (GAMS; kainate antagonist [Zhou et al., 1993]; 5–150 mM; 0.1–3.0 μmol/20 μl), (RS)-α-ethyl-4-carboxyphenylglycine (E4CPG; group I–II mGluR antagonist; 50–500 mM; 1–10 μmol/20 μl), and NOARG (nitric oxide synthase inhibitor; 25–70 mM; 0.5–1.4 μmol/20 μl) also decrease the nociception induced by the i.pl. glutamate (1.5 M; 30 μmol/20 μl). Co-administration of SNAP (nitric oxide donor, 5–50 mM; 0.1–1.0 μmol/20 μl) potentiates nocifensive behavior induced by i.pl. glutamate (15–500 mM; 0.3 or 10.0 μmol/20 μl; Beirith et al., 2002). Glutamate-induced (30 μmol/paw) paw licking is reduced by systemic administration (2.8–85.2 μmol/kg, i.p.; 28.4–284.1 μmol/kg, oral) of N-antipy-rine-3,4-dichloromaleimide (NA-3,4,-DCM), possibly via interaction with peripheral NMDARs or group I mGluRs (Quintao et al., 2010). I.pl. glutamate-induced (1.5 M; 30 μmol/20 μl) licking in mice is inhibited or reduced dose dependently is by co-administration of SR48968 (0.05–0.5 nmol) and FK 888 (0.25–1.0 nmol), selective NK2R and NK1R tachykinin antagonists, respectively (Beirith et al., 2003). SR142801 (0.05–1.0 nmol/20 μl) and CGRP 8–37 (1 nmol/20 μl), NK3R and CGRPR antagonists, have no effect on glutamate-induced (30 μmol/20 μl) licking. Co-administration of des-Arg9-[Leu8]-bradykinin (0.2–0.8 nmol/20 μl), B1 antagonist, reduces hindpaw licking, but not the B2 receptor antagonist HOE 140 (1.0–4.0 nmol/20 μl). In addition, destruction of C fiber afferents by neonatal capsaicin treatment inhibits glutamate induced licking (Beirith et al., 2003).
9. Acute and chronic pain
Inflammatory pain involves numerous chemical agents that act directly as transducers or sensitizers on primary afferent terminal receptors or indirectly activate primary afferents by initiating an inflammatory cascade (Woolf & Ma, 2007). During inflammation, peripheral or primary sensitization of primary afferent fibers occurs with a lowering of nociceptive threshold and increased excitability. Central or secondary sensitization can occur at primary afferent synapses with elevated glutamate production/release and increased sensitivity to glutamate in postsynaptic neurons. Sensitization can be studied in animals by evaluating responses to thermal, mechanical, or chemical stimuli and by assessing nocifensive behavior. As mentioned previously, increased response to a noxious mechanical or thermal stimulus is termed hyperalgesia, whereas a nociceptive response to a non-noxious stimulus is termed allodynia. Ongoing inflammation or tissue damage stimulates alterations in primary afferent neurons by novel, elevated, or diminished production of select proteins (Woolf & Ma, 2007). These alterations may enhance or attempt to modulate ongoing primary and central sensitization.
Damage to or dysfunction of a peripheral nerve, i.e., neuropathy, leads to neuropathic pain, a condition that typically is a chronic situation. Neuropathic pain may be initiated at ectopic areas along axons and may involve ephaptic coupling of axons. Alterations of primary afferent neurons also occur during neuropathies leading to abnormal generation action potentials in peripheral fibers and central sensitization in postsynaptic neurons. In subsequent sections, the role of the peripheral glutamatergic system during acute and chronic inflammation and chronic neuropathy will be reviewed.
10. Pain during acute inflammation: skin (Table 4)
Table 4.
Pain during acute inflammation: skin.
| Drug | Dose | Route | Reference |
|---|---|---|---|
| Capsaicin | |||
| Antagonists | |||
| MK-801 | 0.1–1.0 mM, 50 μl | i.pl. | Jin et al., 2009 |
| CNQX | 1–5 mM, 50 μl | i.pl. | Jin et al., 2009 |
| CPCCOEt | 5 mM, 50 μl | i.pl. | Jin et al., 2009 |
| MPEP | 30 mM, 50 μl | i.pl. | Jin et al., 2009 |
| Carrageenan | |||
| Antagonists | |||
| MK-801 | 200 μM (10 nmol/50 μl) | i.pl. | Jackson et al., 1995 |
| CNQX | 2 mM (100 nmol/50 μl) | i.pl. | Jackson et al., 1995 |
| MPEP | 3–30 mM (0.03–0.3 μmol/10 μl) | i.pl. | Walker et al., 2001 |
| NA-3,4,-DCM | 0.85–8.52 μmol/kg | i.p. | Quintao et al., 2010 |
| Prostaglandin and interleukins | |||
| Agonists | |||
| APDC | 11 μM (20 ng/10 μl) | i.pl. | Yang & Gereau, 2002 |
| APDC | 46 μM (200 ng/25 μl) | s.c., orofacial | Ahn et al., 2005; Jung et al., 2006 |
| DCG4 | 39 μM (200 ng/25 μl) | s.c., orofacial | Ahn et al., 2005 |
| Antagonists | |||
| AP-7 | 240 mg/kg | s.c. | Follenfant & Nakamura-Craig, 1992 |
| DNQX | 80 pg/kg | s.c. | Follenfant & Nakamura-Craig, 1992 |
| LY341495 | 57 nM (0.2 ng/10 μl) | i.pl. | Yang & Gereau, 2002 |
| DNQX | 1 mM, 20 μl | s.c., orofacial | Ahn et al., 2004 |
| CPCCOEt | 0.65 or 6.5 mM (4 or 40 μg/25 μl) | s.c., orofacial | Ahn et al., 2005 |
| LY367385 | 1.9 or 19 mM (10 or 100 μg/25 μl) | s.c., orofacial | Ahn et al., 2005 |
| MPEP | 1.2 or 12 mM (7 or 70 μg/25 μl) | s.c., orofacial | Ahn et al., 2005 |
| SIB1893 | 2 or 20 mM (10 or 100 μg/25 μl) | s.c., orofacial | Ahn et al., 2005 |
| Endothelin-1 (ET-1) | |||
| Antagonists | |||
| MK-801 | 100 μM, 10 μl | i.pl. | Khodorova et al., 2009 |
| D-AP-5 | 5 mM, 10 μl | i.pl. | Khodorova et al., 2009 |
| Bee (Apis mellifera) venom | |||
| Agonists | |||
| APDC | 2–100 μM (0.1–5 nmol/50 μl) | i.pl. | Chen et al., 2010 |
| Antagonists | |||
| MK 801 | 0.01 mg/kg | i.p. | Chen & Chen, 2000 |
| AIDA | 10–500 μM (0.5–25 nmol/50 μl) | i.pl. | Chen et al., 2010 |
| LY341495 | 4 μM (0.2 nmol/50 μl) | i.pl. | Chen et al., 2010 |
| L-AP4 | 2–200 μM (0.1–10 nmol/50 μl) | i.pl. | Chen et al., 2010 |
| MSOP | 200 μM (10 nmol/50 μl) | i.pl. | Chen et al., 2010 |
| AP-5 | 10 mM, 50 μl | Muscle, injection | You et al., 2002 |
| Phoneutria nigriventer spider venom | |||
| Antagonists | |||
| MK-801 | 1 μM (100 pmol/100 μl) | i.pl. | Zanchet & Cury, 2003 |
| CNQX | 1 mM (100 nmol/100 μl) | i.pl. | Zanchet & Cury, 2003 |
| AP5 | 0.4 μM (40 pmol/100 μl) | i.pl. | Zanchet & Cury, 2003 |
| Formalin i.pl. | |||
| Agonists | |||
| S-DHPG | 1.0 μm, 20 μl | Zhou et al., 2001 | |
| Antagonists | |||
| MK-801 | 0.1–100 μM, 30 μl | i.pl. | Davidson et al., 1997 |
| CNQX | 1–1000 μM, 30 μl | i.pl. | Davidson et al., 1997 |
| Dextrorphan | 0.1–10 μM, 40 μl | i.pl. | Davidson & Carlton, 1998 |
| Memantine, ketamine | 1–10 μM, 40 μl | i.pl. | Davidson & Carlton, 1998 |
| AIDA | 0.4–40 μM, 20 μl | i.pl. | Zhou et al., 2001 |
| MPEP | 3 mM (30 nmol/10 μl) | i.pl. — mice | Bhave et al., 2001 |
| CPCCOEt | 10 mM (100 nmol/10 μl) | i.pl. — mice | Bhave et al., 2001 |
| NA-3,4,-DCM | 14–140 mM (0.28–2.8 μmol/20 μl) | i.pl. — mice | Quintao et al., 2010 |
10.1. Capsaicin
Capsaicin, TRPV1 agonist, produces thermal hyperalgesia when injected into the rat hindpaw (Turner et al., 2003; Carlton et al., 2009; Jin et al., 2009). Co-administration i.pl. of MK-801 (0.1–1.0 mM) or CNQX (1–5 mM) decreases capsaicin-induced (3.0 mM, 50 μl) thermal hyper-algesia (thermal plantar) in a dose dependent manner (Jin et al., 2009). This effect occurs within 15 min and lasts for more than 6 h. Co-treatment of mGluR1 or mGluR5 antagonists, CPCCOEt (5 mM) or MPEP (30 mM), dose dependently decreases capsaicin induced thermal hyperalgesia, but co-application with group II or III antagonists, MCCG (5 mM) or MSOP (5 mM) does not. The effect of CPCCOEt and MPEP occurs within 15 min and lasts for over 5 h (Jin et al., 2009). I.pl. preadministration (5 min) of (2S,4R)-4-methyl glutamic acid (SYM 2081; 1.2 or 12 mM; 10 or 100 μg/50 μl), KAR agonist/desensitizer, does not attenuate mechanical (von Frey, 149 mN) or thermal (thermal plantar) hyperalgesia due to capsaicin (10 μg/10 μl; Turner et al., 2003). I.p. preadministration (30 min) of SYM 2081 (10–100 mg/kg) is effective by 5 min in reducing thermal and mechanical hyperalgesia following i.pl. capsaicin, whereas i.t. pretreatment (5 min; 1–100 μg/5 μl) is effective only in reducing mechanical hyperalgesia (Turner et al., 2003). Nocifensive behaviors evoked by i.pl. injection of capsaicin (0.1%, 20 μl) are reduced by preadministration of the group II agonist, APDC (0.1 μM, 30 μl; Carlton et al., 2009). Addition of LY341495 (1.0 μM, 30 μl), group II antagonist, blocks the effects of APDC (Carlton et al., 2009) (Table 4).
10.2. Carrageenan
Carrageenan, a proinflammatory agent, injected into the rat hindpaw causes thermal and mechanical hyperalgesia (Jackson et al., 1995; Wang et al., 2000; Walker et al., 2001; Quintao et al., 2010). Injection i.pl. (20 μl) of AP5 or 0.01–1.0 mM DNQX after 3 h of carrageenan inflammation dose dependently reduces C-fiber evoked responses in WDR neurons (Wang et al., 2000). The effect of AP5 or DNQX occurs within 1 min following injection, peaks at 6 min, and for over 10 min (Wang et al., 2000). Injection i.pl. of MK-801 (200 μM; 10 nmol/50 μl) or CNQX (2 mM; 100 nmol/50 μl) after 2.5 h of carrageenan (2 mg/paw) inflammation moderately reduces thermal hyperalgesia (thermal plantar; Jackson et al., 1995). The MK801 effect is stereospecific with the (+)MK-801 enantiomer effective, but not (−)MK-801 (Jackson et al., 1995). Preadministration i.pl. (30 min) with MPEP (3–30 mM; 0.03–0.3 mmol/10 μl) reduces carrageenan (1%, 250 μl) induced mechanical hyperalgesia (Randall–Selitto test) at 1 and 3 h of acute inflammation (Walker et al., 2001). Administration of NA-3,4,-DCM (0.85–8.52 μmol/kg, i.p.) reduces mechanical hyperalgesia (electronic anesthesiometer) induced by carrageenan (300 μg/50 μl) up to 48 h in mice (Quintao et al., 2010). The analgesic effect of NA-3,4,-DCM may occur via interaction with peripheral NMDARs or group I mGluRs (Quintao et al., 2010).
10.3. Prostaglandins and interleukins
During inflammation, prostaglandins and interleukins are produced and interact with their respective receptors located on the peripheral terminals of primary afferents (Woolf & Ma, 2007). S.c. administration of prostaglandins and interleukins, therefore, is used to mimic an inflammatory response and cause peripheral sensitization. For example, i.pl. injection of prostaglandin E2 in rats and mice causes thermal and mechanical hyperalgesia for 1–2 h (Follenfant & Nakamura-Craig, 1992; Yang & Gereau, 2002). Mechanical hyperalgesia (Randall–Selitto test) is reduced in PGE2 (100 ng/100 μl) injected rat hindpaw by AP-7 (240 mg/kg, s.c.) and DNQX (80 pg/kg, s.c.; Follenfant & Nakamura-Craig, 1999). I.pl. co-administration of APDC (11 μM; 20 ng/10 μl) reduces thermal responses (thermal plantar) to baseline levels during PGE2 (100 ng/10 μl) induced inflammation in mice. Co-treatment i.pl. with (2s)-2-amino-2-[(1s,2s)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495; 57 nM; 0.2 ng/10 μl) blocks the analgesic effects of APDC (Yang & Gereau, 2002).
Injection of interleukin-1β (IL-1β; 0.1–10 pg/25 μl) into the rat orofacial area (vibrissa pad) dose dependently produces allodynia (air-puff threshold; Ahn et al., 2004, 2005). Pretreatment of the vibrissa pad with ionotropic and metabotropic EAAR antagonists diminishes IL-1β-induced (10 pg/25 μl) allodynia for up to 3 h (Ahn et al., 2004, 2005; Jung et al., 2006). Pretreatment (10 min) with DNQX (1 mM, 20 μl), but not AP5 (10 mM, 20 μl), attenuates the IL-1β-induced (10 pg/25 μl) allodynia (Ahn et al., 2004). IL-1β-induced (10 pg/25 μl) allodynia also is diminished by pretreatment (10 min) with mGluR1 antagonists, CPCCOEt (0.65 or 6.5 mM; 4, 40 μg/25 μl) or LY367385 (1.9 or 19 mM; 10, 100 μg/25 μl) and mGluR5 antagonists, MPEP (1.2 or 12 mM; 7, 70 μg/25 μl) or 2-methyl-6-(2-phenylethenyl)pyridine (SIB1893; 2 or 20 mM; 10, 100 μg/25 μl; Ahn et al., 2005). Post-injection (30 min) of CPCCOEt (0.65 or 6.5 mM; 4, 40 μg/25 μl) or MPEP (1.2 or 12 mM; 7, 70 μg/25 μl) following IL-1β injection (10 pg/25 μl) has no effect on allodynia (Jung et al., 2006). Pre-administration (10 min) of group II mGluR agonists, APDC (46 μM; 200 ng/25 μl) or (2S, 2′R, 3′R)-2-(2′, 3′-dicarboxycyclopropyl)glycine (DCG4; 39 μM; 200 ng/25 μl), decreases IL-1β allodynia, and the effect of APDC is blocked by co-administration of the group II mGluR antagonist, LY341495 (2.3 μM; 20 ng/25 μl; Ahn et al., 2005). Post-administration (30 min) of APDC (46 μM; 200 ng/25 μl) blocks IL-1β allodynia starting within 30 min and lasting for 2 h (Jung et al., 2006).
10.4. Endothelin
Endothelin-1 (ET-1) injection i.pl. (10 μM, 10 μl) produces mechanical allodynia (von Frey, 0.07–60 g) in rats (Khodorova et al., 2009). Pre-and concomitant administration of 100 μM MK-801 and 5 mM D-AP5 (10 μl) strongly attenuates ET-1 induced mechanical allodynia for 90 min. Nocifensive behavior, i.e., paw flinching, induced by ET-1 (100 μM) is not affected by D-AP5 (5 mM, 10 μl; Khodorova et al., 2009).
10.5. Bee and spider venom
The venom from bee stings and spider bites elicit an inflammatory response in skin (Chen et al., 1999b, 2010; Chen & Chen, 2000; You et al., 2002; Zanchet & Cury, 2003). Injection i.pl. of bee venom (BV; A. mellifera) in rats causes acute (1–2 h) nocifensive responses (flinching, licking, or lifting paw) along with a longer (72–96 h) period of mechanical and thermal hyperalgesia (Chen et al., 1999b). Pre-treatment with MK-801 (0.01 mg/kg, i.p.) attenuates bee venom (BV; 0.2 mg/50 μl)-induced nocifensive responses and thermal hyperalgesia (Chen & Chen, 2000). Pre-treatment i.pl. with AIDA (10–500 μM; 0.5–25 nmol/50 μl) dose dependently reduces BV-induced (0.2 mg/50 μl) nocifensive responses, i.e., persistent spontaneous paw flinching reflexes, but not mechanical hyperalgesia (von Frey, 2–40 g; Chen et al., 2010). Preapplication i.pl. with APDC (2–100 μM; 0.1–5 nmol/50 μl) produces a dose dependent inhibition of nocifensive responses and mechanical hyperalgesia, an effect that can be blocked by LY341495 (4 μM; 0.2 nmol/50 μl). Preadministration i.pl. of L-AP4 (2–200 μM; 0.1–10 nmol/50 μl) causes a dose related inhibition of nocifensive responses, but not mechanical hyperalgesia. The group III mGluR antagonist, MSOP (200 μM; 10 nmol/50 μl), blocks the analgesic effects of L-AP4. LY341495 and MSOP alone have no effect on BV-induced nocifensive behavior or mechanical responses (Chen et al., 2010). Post-treatment (1 h) of the rat gastrocnemius soleus muscle injected with AP5 (10 mM, 50 μl), but not CNQX (5 mM, 50 μl), decreases allodynia and mechanical hyperalgesia induced by BV (0.2 mg/50 μl) injected into the muscle (You et al., 2002).
Phoneutria nigriventer spider venom (0.01–10 μg/100 μl) causes intense pain followed by edema and erythema (Zanchet & Cury, 2003). Concomitant i.pl. administration of MK-801 (1 μM; 100 pmol/100 μl) and CNQX (1 mM; 100 nmol/100 μl) reduces, whereas AP5 (0.4 μM; 40 pmol/100 μl) inhibits, the mechanical hyperalgesia (Randall–Selitto test) for more than 4 h produced by i.pl. P. nigriventer venom (1 μg/100 μl). Co-treatment of MK-801 (1 μM; 100 pmol/100 μl) or CNQX (1 mM; 100 nmol/100 μl) with pGlu-Ala-Asp-Pro-Asn-Lys-Phe-Tyr-Pro(spiro-g-lactam)Leu-Trp-NH2 (GR82334; 10 μmol/100 μl), NK1 tachykinin antagonist, inhibits the mechanical hyperalgesia caused by P. nigriventer venom (Zanchet & Cury, 2003).
10.6. Formalin
Formalin injection i.pl. causes a biphasic behavioral response based on two generalized mechanisms of action. During phase 1, formalin directly activates the TRPA1 receptors on primary afferents, while phase 2 behavior is due to a response to numerous inflammatory mediators (McNamara et al., 2007). The NMDAR has been investigated as having a key role in phase 2 (Davidson et al, 1997; Davidson & Carlton, 1998; McRoberts et al., 2011). Selective knockdown of NR1 in mouse DRG reduces the phase 2 behavior induced by formalin (5%, 20 μl; McRoberts et al., 2011). Pretreatment i.pl. of 1.0 μM MK-801 (30 μl) or 10 μM CNQX (30 μl) diminishes phase 2 licking and lifting nocifensive behavior induced by 5% formalin (15 μl), but not flinching (Davidson et al, 1997). Pretreatment with MK-801 is more effective than CNQX in reducing nocifensive behavior. Post-treatment i.pl. (8 min) with MK-801 (0.1–100 μM, 30 μl) or CNQX (1–1000 μM, 30 μl) has no effect overall on phase 2 licking and lifting behavior, although MK-801 (1.0 μM, 30 μl) and CNQX (1.0 μM, 1.0 mM, 30 μl) briefly attenuate flinching behavior at specific time points (Davidson et al., 1997). Preapplication i.pl. (40 μl) of NMDAR antagonists, dextrorphan (0.1–10 μM), memantine (1–10 μM) or ketamine (1–10 μM), produces a decrease in formalin-induced (5%, 20 μl) phase 2 nocifensive licking and lifting in a dose-related fashion (Davidson & Carlton, 1998). Memantine (10 μM, 40 μl) decreases licking and lifting during phase 1 at 5 min and phase 2 at 15–30 min. Dextrorphan (5 μM, 40 μl) and ketamine (10 μM, 40 μl) reduce licking and licking between 15–25 min and 20–25 min, respectively. Flinching behavior is not reduced by any of the antagonists used (Davidson & Carlton, 1998).
Group I, mGluRs also have a role in formalin induced behavior (Bhave et al., 2001; Zhou et al., 2001; Quintao et al., 2010). I.pl. preapplication AIDA (0.4–40 μM, 20 μl) causes a dose dependent decrease in formalin (2%, 20 μl) phase 2 licking and lifting and, at 40 μM, a decrease in flinching behavior (Zhou et al., 2001). In a temporal evaluation, AIDA (40 μM, 20 μl) reduces licking and lifting in phase 2 at 20–25 min and flinching at 25–30 min, effects that are blocked by co-administration of 1.0 μM S-DHPG (20 μl; Zhou et al., 2001). In mice, preadministration of MPEP (30 nmol/10 μl) or CPCCOEt (100 nmol/10 μl) diminishes formalin-induced (2%, 10 μl) phase 2 licking and lifting whether administered singly or in combination. Injection i.pl. of MPEP (30 nmol/10 μl) or CPCCOEt (100 nmol/10 μl) during phase 1 also reduces formalin induced licking and lifting during phase 2 (Bhave et al., 2001). Co-injection i. pl. of NA-3,4,-DCM (0.28–2.8 μmol/20 μl) with formalin (2.5%, 20 μl) dose dependently inhibits (ID50 = 0.84 pmol/20 μl) nociceptive behavior in both phases in mice (Quintao et al., 2010).
11. Pain during acute inflammation: joint and muscle
Acute inflammation of the rat knee joint causes mechanical and thermal hyperalgesia in the hindpaw and altered weight-bearing (Sluka & Westlund, 1993; Sluka et al., 1994; Lawand et al., 1997; Min et al., 2001; Zhang et al., 2003, 2009). Post-administration (3 h; 100 μl) of AP7 (0.2 mM), CNQX (0.1 mM) or ketamine (0.1 mM) into the knee synovial cavity reduces paw mechanical (von Frey, 30–100 mN) and thermal (thermal plantar) hyperalgesia during kaolin/carrageen (3%, 100 μl) induced inflammation (Lawand et al., 1997). Mechanical hyperalgesia is blocked and thermal hyperalgesia is attenuated for 1–2 h (4–5 h post induction) with all three antagonists (Lawand et al., 1997). Preinjection of MK-801 (0.3–1.5 mM, 40 μl) and NBQX (0.25–2.5 mM, 40 μl) into the knee joint immediately prior to carrageenan (2%, 40 μl) dose dependently attenuates decreases in weight load bearing for 4–24 h (Zhang et al., 2003). There is no effect at 72 h, but weight load bearing approaches normal levels in all inflamed animals at this time point. Post-treatment (5 h) with MK-801 (0.75, 1.5 mM, 40 μl) or NBQX (0.625, 2.5 mM, 40 μl) into the knee joint does not alter decreases in weight load bearing caused by carrageenan (2%, 40 μl; Zhang et al., 2003). Preapplication of AP5 (0.1–0.3 mg/40 μl) dose dependently reduces carrageenan-induced (2%, 40 μl) alterations in weight load bearing for 4–8 h (Zhang et al., 2009). Some interaction of NMDARs with peripheral opiate receptors occurs in this model since post-administration (5 h) of naloxone (0.2–2.0 mg/40 μl) blocks AP5 pretreatment (Zhang et al., 2009). Further evidence for an EAAR–opioid receptor interaction occurs from investigation of tibiotarsal joint inflammation (Mecs et al., 2009). Carrageenan (300 μg/20 μl) into the rat tibiotarsal joint causes mechanical hyperalgesia (von Frey, 0.064–110 g) in the hindpaw. Post-treatment with kynurenic acid (8–106 mM; 30–400 μg/20 μl) dose dependently (ED30 = 54 mM; 204 μg/20 μl; ED50 = 87 mM; 330 μg/20 μl) decreases mechanical hyperalgesia. Co-administration of kynurenic acid (8–106 mM; 30–400 μg/20 μl) with endomorphin-1 (30–200 μg/20 μl) in a 1:1 dose ratio shows an additive effect (kynurenic acid ED30 = 38 mM; 145 μg/20 μl; ED50 = 58 mM; 220 μg/20 μl) in decreasing carrageenan-induced hyperalgesia (Mecs et al., 2009) (Table 5).
Table 5.
Pain during acute inflammation: joint and muscle.
| Drug | Dose | Route | Reference |
|---|---|---|---|
| Antagonist | |||
| AP7 | 0.2 mM, 100 μl | Intra-articular — knee | Lawand et al., 1997 |
| CNQX, ketamine | 0.1 mM, 100 μl | Intra- articular — knee | Lawand et al., 1997 |
| MK-801 | 0.3–1.5 mM, 40 μl | Intra- articular — knee | Zhang et al., 2003 |
| NBQX | 0.25–2.5 mM, 40 μl | Intra- articular — knee | Zhang et al., 2003 |
| AP5 | 13–38 mM (0.1–0.3 mg/40 μl) | Intra- articular — knee | Zhang et al., 2009 |
| Kynurenic acid | 8–106 mM (30–400 μg/20 μl); ED30 = 54 mM; 204 μg/20 μl; ED50 = 87 mM; 330 μg/20 μl | Intra- articular — tibiotarsal | Mecs et al., 2009 |
| MK-801 | 0.3 mg/kg/50 μl | Intramuscular | Ro, 2003 |
MO (20%, 30 μl) injected into the masseter or biceps muscle of lightly anesthetized rats causes nocifensive behavior, i.e., ipsilateral hind paw shaking (Ro, 2003; Chun et al., 2008). Preinjection (5 min) of masseter with MK-801 (0.3 mg/kg/50 μl) or NBQX (1–100 nmol/10 μl) reduces the number and magnitude of paw shakes induced by MO for 30–180 min, but preadministration in biceps muscle does not have a significant effect (Ro, 2003; Table 5).
12. Pain during chronic inflammation
Injection of CFA initiates an acute inflammatory response that develops into AIA, a chronic arthritis-like inflammation. Glutamate release from and the EAARs on primary afferents influence the development and maintenance of nociceptive behaviors during chronic inflammation (Leem et al., 2001; Walker et al., 2001; Du et al., 2003, 2006; Miller et al., 2010b). When administered during AIA, i. pl. injection of GLS inhibitors produces potent, long-lasting analgesia (Miller et al., 2010b). Injection of DON, N-ethylmaleimide, bromothymol blue, and palmitoyl-CoA attenuates mechanical (von Frey; plantar anesthesiometer) and thermal (plantar thermal) hyperalgesia (Miller et al., 2010b and unpublished observations). DON’s effect is dose dependent (0.8–800 mM; 0.02–20 μmol/25 μl) and lasts for up to 96 h after one application (Miller et al., 2010b). DON irreversibly binds to the glutamine site on GLS and causes permanent inactivation. Peripheral terminal replenishment of GLS comes from the DRG neuronal cell body many centimeters distant and is accomplished over several days. These studies, therefore, indicate that production and release of glutamate from primary afferents continues to be important for maintaining chronic inflammatory pain (Table 6).
Table 6.
Pain during chronic inflammation.
| Drug | Dose | Route | Reference |
|---|---|---|---|
| CFA inflammation | |||
| Agonist | |||
| NMDA | 0.01–0.5 mM, 20 μl | i.pl. | Du et al., 2003 |
| Kainate | 1 mM, 20 μl | i.pl. | Du et al., 2006 |
| Antagonist | |||
| DON | 0.8–800 mM (0.02–20 μmol/25 μl) | i.pl. | Miller et al., 2010b |
| MK 801 | 0.5 mM, 20 μl | i.pl. | Du et al., 2003 |
| CNQX | 0.1 mM, 20 μl | i.pl. | Du et al., 2006 |
| MK 801 | 10 nM (1 pmol/100 μl) | i.pl. | Leem et al., 2001 |
| MPEP | 10–30 mM (100–300 nmol/10 μl) | i.pl. | Walker et al., 2001 |
| Formalin | |||
| Agonist | |||
| Glutamate, NMDA, AMPA, or kainate | 20 mM; (1 μmol/50 μl) | i.pl. | Aumeerally et al., 2004 |
Other studies show the importance of EAARs in chronic pain (Leem et al., 2001; Walker et al., 2001; Du et al., 2003, 2006). During AIA (100 μl CFA; 5 day), i.pl. injection of NMDA (0.01–05 mM, 20 μl) or kainate (1 mM, 20 μl) accentuates CFA induced mechanical hyper-algesia/allodynia (von Frey, 21–160 mN; Du et al., 2003, 2006). This enhancement is dose dependent for NMDA (Du et al., 2003). After 2 days of AIA (35 μl CFA), i.pl. administration of 0.5 mM MK-801 (20 μl) brings mechanical responses to near normal levels within 30 min and lasting for 30 min (Du et al., 2003). Injection i.pl. of 0.1 mM CNQX (20 μl) causes mechanical responses to return to near normal levels within 45 min and lasting for 45 min (Du et al., 2006). I.pl. application of MK-801 (10 nM; 1 pmol/100 μl) at day 2 AIA (150 μl CFA) reduces mechanical hyperalgesia (plantar asthesiometer) at 15 min post-administration, but i.pl. treatment with CNQX (10–100 nM; 1–10 pmol/100 μl) has no effect (Leem et al., 2001). Application i.pl. of MPEP (10–30 mM; 100–300 nmol/10 μl) reduces mechanical hyperalgesia (Randall–Selitto test) at 1–3 h post-administration on 1 day after CFA injection (2.5 μl; Walker et al., 2001) (Table 6).
A preconditioning with formalin also provides a chronic model that involves peripheral EAARs (Sawynok & Liu, 2003; Aumeerally et al., 2004). Formalin (2.5%, 50 μl) injected into the dorsal contralateral paw conditions the ipsilateral paw for a dorsal injection of formalin (1.5%, 50 μl) three–four days later (Sawynok & Liu, 2003). Co-injection (20 mM; 1 μmol/50 μl) of glutamate, NMDA, AMPA, or kainate with the 2nd injection of formalin enhances phase 1 and 2 flinching behavior. This behavior is augmented further by co-injection of 8-cyclopentyl-theophylline (CPT; 150 nmol/50 μl), adenosine A1R antagonist, with glutamate, AMPA, and kainate, but not NMDA (Aumeerally et al., 2004).
13. Pain during chronic neuropathy
Several animal models of neuropathy are used and peripheral EAARs may have a role in the induction and/or maintenance of neuropathic pain (Aley & Levine, 2002). Systemic ketamine, memantin, NMDA antagonist, and 2S,4R-4-methylglutamate, KAR antagonist, are effective in reducing mechanical and thermal hyperalgesia and spontaneous pain in the sciatic nerve chronic constriction injury, freeze injury, and L5–6 spinal nerve ligation (Carlton & Hargett, 1995; Qian et al., 1996; Sutton et al., 1999; Ta et al., 2000; Simmons et al., 2002). Some effects of EAAR antagonists may be due to interaction with peripheral EAARs on primary afferents (Jang et al., 2004). In a rat neuropathic model, where the L5 spinal nerve transection is preceded (6 days) by an L5 dorsal rhizotomy, mechanical hyperalgesia/allodynia (von Frey, 0.35–12.5 g) occurs in the ipsilateral hindpaw for over 40 days. Pretreatment i.pl. with MK-801 (20 nmol/30 μl; 15 min), but not NBQX (100 nmol/30 μl), attenuates altered mechanical thresholds for up to 4 days. Preapplication i.pl. with α-methyl-4-carboxyphenylglycine (MCPG; 50 nmol/30 μl), non-selective mGluR antagonist, produces no effect on mechanical hyperalgesia. Preinjection with DL-AP3 (70 nmol/30 μl), group I mGluR antagonist, however, reduces mechanical hyperalgesia for 4 days. APDC (20 nmol/30 μl) maintains normal mechanical thresholds for one day when administered i.pl. before nerve transection. When applied i. pl. at day 10 following nerve transaction, MK-801 (20 nmol/30 μl) attenuates mechanical hyperalgesia from 45 to 75 min post-treatment. NBQX (100 nmol/30 μl), AP3 (70 nmol/30 μl), or APDC (20 nmol/30 μl) do not have an effect on mechanical hyperalgesia when given at day 10 after nerve transection (Jang et al., 2004).
14. Glutamate and human pain
This review has illustrated that inflammatory animal models cause increased levels of glutamate in peripheral tissues and that peripheral glutamate, interacting with numerous EAAR receptors, produces nociceptive behaviors. Other questions remain for human studies. Are glutamate levels elevated in peripheral tissues in patients with chronic painful conditions? Does peripheral glutamate cause pain in humans? Can inhibition of the peripheral glutamatergic system produce pain relief?
14.1. Glutamate levels during tissue trauma
Glutamate levels are elevated in synovial fluid collected from patients with chronic joint inflammation compared to synovial fluid collected at autopsy (3–15 h) from individuals who did not experience joint trauma. Chronic joint inflammation includes patients with rheumatoid arthritis (RA), osteoarthritis (OA), juvenile arthritis, gout, pseudogout, Reiter’s syndrome, psoriatic arthritis, infectious arthropathy, joint trauma, and systemic lupus erythematosus. Retrospective study shows that these patients “had sought medical attention for painful, swollen joints and the chart confirmed the joint to be painful, swollen, erythematosus, and warm to touch.” Glutamate and aspartate are elevated 54- and 28-fold, respectively, in all arthritic patients compared to controls. Patients with RA, OA, and gout comprise the largest populations and, when evaluated within the inflamed groups, OA patients had lower levels of glutamate and aspartate compared to RA and gout patients. Glutamate and aspartate levels were 81% and 55% of levels in RA and 73% and 61% of levels in gout. There is no correlation of glutamate or aspartate levels with white blood cell (WBC) counts from synovial fluid indicating that WBCs are not the source of elevated levels and/or that increases in glutamate and aspartate levels are not “strictly associated with inflammation variables as defined” by WBCs. It may be, therefore, that other inflammatory mediators are responsible for elevated glutamate and aspartate concentrations (McNearney et al., 2000).
Overuse injuries appear to cause glutamate levels to rise in sore or damaged muscles and tendons. For example, eccentric muscle contraction occurs when a muscle contracts as it lengthens. This leads to potential muscle damage and delayed muscle soreness. During calf muscle soreness due to eccentric contractions 24 h earlier, glutamate dialysate concentrations increase by ~30% (125 μM) compared to the non-exercised contralateral calf muscle (Tegeder et al., 2002). Interstitial glutamate levels at rest are higher in female patients with trapezius myalgia than normal females. Both groups have increased glutamate levels during exercise of the trapezius, but myalgia patients appear to have a greater percentage increase (Rosendal et al., 2004). Patellar tendinosis (jumper’s knee) is an overuse syndrome leading to chronic patellar tendon pain. Glutamate concentrations are 5–6 fold higher in patients with patellar tendinosis (250–300 μmol/l) compared to normal individuals (Alfredson et al., 2001a, b; Alfredson & Lorentzon, 2002). Glutamate- and NR1-ir are elevated and co-localized in nerve fibers of affected tendons in patellar tendinosis, whereas, glutamate-ir or glutamate/NR1 colocalization do not occur in nerve fibers from control patients (Schizas et al., 2010). Other chronic tendonitis conditions of the tendocalcaneus (Achilles) and extensor carpi radialis brevis (ECRB) tendons also show elevated glutamate and NR1-ir levels (Alfredson et al., 2000, 2001a). Patients with ECRB tendonitis (tennis elbow) have 3 fold higher glutamate concentrations (215 μmol/l) in the affected tendon despite a lack of inflammatory markers in the tissue (Alfredson et al., 2000).
14.2. Pain caused by glutamate (Table 7)
Table 7.
Glutamate and human pain.
| Drug | Dose | Route | Reference |
|---|---|---|---|
| Agonist | |||
| Glutamate | 1–100 mM, 100 μl | s.c., face | Gazerani et al., 2006 |
| Glutamate | 1.0 M, 200 μl | Intramuscular | Cairns et al., 2001a, 2003a, 2003b, 2006; Wang et al., 2004, 2010; Svensson et al., 2003, 2005, 2008; Castrillon et al., 2008 |
| Glutamate | 0.5 ml, 1 M | Intratendoninous | Gibson et al., 2009 |
| Monosodium glutamate | 75 or 150 mg/kg | Oral | Baad-Hansen et al., 2010 |
| Antagonist | |||
| Ketamine | 10 mM, 200 μl | Intramuscular | Cairns et al., 2003a, 2003b, 2006 |
| Ketamine | 0.83 mg/ml, 6 ml | s.c. | Warncke et al., 1997 |
| Ketamine gel (Ketalar®) | 20 mg/ml, 1 ml | Topical | Pöyhiä & Vainio, 2006 |
| Ketamine gel | 0.093 mg/kg–9.33 mg/kg | Topical | Gammaitoni et al., 2000 |
| Ketamine gel | 5 mg/ml | Topical | Quan et al., 2003 |
| Ketamine | 0.5%, 5 ml | Topical | Lynch et al., 2003 |
| Ketamine/amitriptyline cream | 1% ketamine/2% amitriptyline, 4 ml | Topical | Lynch et al., 2005a, b |
| DON | 480 mg/m2 (10- minute infusion daily for 3 days) | i.v. | Kovach et al., 1981 |
| DON | 450 mg/m2 (15- min infusion 2× weekly) | i.v. | Sullivan et al, 1988 |
Subcutaneous injection of glutamate (1–100 mM, 100 μl) into the forehead of men and women produces dose dependent pain, sensitization, elevated skin temperature, and increased blood flow (Gazerani et al., 2006). Pain lasts 5–10 min and is considered to be “moderate” in intensity. At 100 mM glutamate, women experience greater pain than men, as well as a quicker response to maximal pain, longer duration of pain, and more overall pain using a visual analog scale (VAS). Face-chart pain (drawing) is larger and quality of pain (pain rating indices: sensory, affective, evaluative and miscellaneous) is greater in women vs. men (Fig. 9; Cairns et al., 2001a). Glutamate at the highest concentration (100 mM, 100 μl) causes secondary hyperalgesia (von Frey, 34 g) and the area of hyperalgesia was larger in women compared to men. Injection of 100 mM glutamate reduces pressure pain thresholds (handheld pressure algometer), but no sex differences occur. Skin temperature (infrared thermography) is elevated by ~1 °C with 100 mM glutamate in both women and men. Glutamate increases dose dependently the subcutaneous blood flow (laser Doppler) in men and women with a 700–900% increase with 100 mM glutamate (Gazerani et al., 2006).
Fig. 9.
A. Following injection of 1.0 M glutamate into the masseter muscle, women and men drew their perceived areas of pain. Subjects describe a deep, aching pain that spread to the temporomandibular joint and teeth. Women had a larger area of perceived pain on drawings than men. B. The area of perceived pain was measured and illustrated in the histogram. Women had a larger area of perceived pain than men after a single or two injections of glutamate.
Am Physiol Soc, used with permission; Cairns et al., J Neurophysiol, 2001a.
Injection of glutamate into normal human muscles causes dose dependent elevations in pain perceptions (deep, sharp, pressing, aching pain) spreading to other tissues, e.g., TMJ, teeth, temple, and lasting for 5–10 min (Cairns et al., 2001a,b, 2003a, 2003b; Svensson et al., 2003; Wang et al., 2004; Svensson et al., 2005, 2008; Arendt-Nielsen et al., 2008; Gibson et al., 2009). When injected with glutamate, the human masseter muscle appears to be more sensitive to glutamate than the splenius muscle (Svensson et al., 2005). Human males injected with 1.0 M glutamate in the masseter (200 μl) have higher perceived pain intensity and lower pressure pain thresholds than when glutamate is injected in the splenius (400 μl; Svensson et al., 2005). When injected into the masseter muscle, 1.0 M (200 μl) glutamate produces pain responses to pressure comparable to hypertonic saline (200 μl) in human males (Cairns et al., 2003b). Co-injection of 10 mM (200 μl) ketamine attenuates glutamate induced pain responses, but has no effect on hypertonic saline induced pain. On VAS, ketamine reduces the peak response to glutamate as well as the overall amount of pain (Cairns et al., 2003b). Injection of 1.0 M (200 μl) glutamate into the masseter muscle of males produces an increase in EMG amplitude in the ipsilateral masseter and sternocleidomastoid muscles evoked by the jaw stretch reflex (Wang et al., 2004). Sensitization in the masseter by glutamate can be observed with a second glutamate (1.0 M; 200 μl) injection. In males, an initial injection of 0.1 M glutamate causes a decrease in pain pressure threshold that is further reduced (~50% of control) by a second injection (Svensson et al., 2003). Co-injection of 10 mM ketamine (200 μl) with the second injection of glutamate (1.0 M; 200 μl) attenuates the pain pressure response as well as overall pain, peak pain, pain duration, and pain distribution (Cairns et al., 2006). Injection of 1.0 M (200 μl) glutamate in the masseter of males followed by injection of capsaicin (100 μg/ml, 200 μl) causes increases in EMG activity, jaw opening, and biting force plus elevations in perceived pain distribution, pain intensity, and overall amount of pain compared to injection of saline followed by capsaicin (Wang et al., 2010). Desensitization of TRPV1 receptors on primary afferents by a pretreatment of capsaicin diminishes pain perceptions evoked by glutamate (Arendt-Nielsen et al., 2008; Wang et al., 2010). Following NGF pretreatment (5 μg/200 μl; 24 h) in the masseter in males, 1.0 M (200 μl) glutamate injection causes increases in the area of perceived pain and lowers the pain threshold to pressure (Svensson et al., 2008). Oral administration of monosodium glutamate (75 or 150 mg/kg) produces tenderness in the masseter muscles and zygomatic region along with headache in normal males. No changes, however, occur in pain pressure threshold or pain tolerance levels (Baad-Hansen et al., 2010).
As in rats, there is a sex difference in masseter muscle nociception (Cairns et al., 2001a, 2003a, 2003b; Svensson et al., 2003; Castrillon et al., 2008). Pain distribution, peak pain, and overall pain, but not pain pressure threshold is larger in females than in males following one or two injections of 1.0 M (200 μl) glutamate (Cairns et al., 2001a, 2003a, 2003b; Svensson et al., 2003). Jaw-stretch reflex responses are larger in females than males under control conditions (Cairns et al., 2003b). Following 1.0 M (200 μl) glutamate injection into the masseter, however, the reflex response is facilitated in males, but not in females (Cairns et al., 2003b). Myofascial temporomandibular disorder (TMD), a disorder prevalent to women, may have a glutamatergic component (Castrillon et al., 2008). Healthy control females injected with 1.0 M (200 μl) glutamate in the masseter have similar pain distributions and perceptions to women with TMD. Psycho-social features, i.e., coping strategies, and pressure pain thresholds/tolerance are different between the two groups, most likely reflecting the differences between acute and chronic pain (Castrillon et al., 2008).
14.3. Pain relief (Table 7)
Ketamine i.v. has been widely studied for analgesic properties in postoperative, neuropathic, fibromyalgia, and migraine pain (Ben-Ari et al., 2007; Chizh, 2007; Vikelis & Mitsikostas, 2007). As in animal studies, most interpretations concerning ketamine analgesia invoke a spinal mechanism of action, but, based on studies reviewed in this article, some analgesia most likely occurs by decreased activity in the peripheral terminals of primary afferents.
Topical ketamine has been used to study or relieve human pain (Warncke et al., 1997; Lynch et al., 2003, 2005a,b; Quan et al., 2003; Pöyhiä & Vainio, 2006). For example, experimental burn injury on both legs (calves) induces mechanical and thermal hyperalgesia that can be altered by local ketamine (Warncke et al., 1997). Pretreatment (20 min) with local injection of ketamine (0.83 mg/ml, 6 ml) attenuates thermal hyperalgesia, i.e., heat pain detection threshold and heat threshold, and mechanical hyperalgesia, i.e., tactile pain detection threshold, for up to 90 min postinjury (Warncke et al., 1997). After one week following injury, saline (0.9%, 6 ml) injected into the injury site produces hyperalgesia in the control leg, but does not in the ketamine treated leg, indicating a long lasting effect of peripheral ketamine (Warncke et al., 1997). Intradermal capsaicin (250 μg/25 μl) injection into the forearm produces intense pain for over 5 min and mechanical hyperalgesia (von Frey, 164 g) for over 1 h (Pöyhiä & Vainio, 2006). Ketamine gel (Ketalar®; 20 mg/ml, 1 ml) pretreatment (10 min) reduces both pain perception (VAS) and mechanical hyperalgesia (Pöyhiä & Vainio, 2006).
Several case studies or clinical trials have evaluated the topical use of ketamine in neuropathic pain (Gammaitoni et al., 2000; Quan et al., 2003; Lynch et al., 2003, 2005a,b). In five patients with neuropathy (n=3 complex regional pain syndrome, n=1 radiculopathy, n=1 post-herpetic neuralgia), topical ketamine gel (0.093–9.33 mg/kg) provided pain relief based on a numerical analog scale (Gammaitoni et al., 2000). In a study of subjects with post-herpetic neuralgia (n=13 men; n=10 women), topical ketamine gel (5 mg/ml) was effective in providing pain reduction in nearly two thirds (65%) of the patients (Quan et al., 2003). In a pilot study of neuropathic pain (n=8 men; n=12 women), topical (5 ml) ketamine (0.5%), amitriptyline (1%), or combined ketamine/amitriptyline were studied in a randomized 2 day, double blind, placebo control, 4 way cross-over trial (Lynch et al., 2003). Neuropathies included post-herpetic neuralgia (n=9), diabetic neuropathy (n=4), and post-surgical/trauma neuropathy (n=7) and all patients had allodynia and/or hyperalgesia. There was no pain relief compared to placebo after 2 days for any treatment using several measures of pain (VAS, McGill Pain Questionnaire, Likert scale). In a seven day open label trial, 11 patients reported pain relief from days 3 to 7 using the combined ketamine/amitriptyline cream (Lynch et al., 2003). An extension of this pilot study was performed in twenty one subjects for six months using combined 1% ketamine/2% amitriptyline (4 ml; Lynch et al., 2005a). Patients had neuropathies of various types: post-herpetic neuralgia (n=3), diabetic neuropathy (n=1), and post-surgical/trauma neuropathy (n=16). An 11-point numerical scale for pain intensity and a 5-point satisfaction scale were used to evaluate effectiveness. After six months, subjects reported long-term pain reduction of 34–50% and nearly all (89%) rated satisfaction from good to excellent (Lynch et al., 2005a). A larger, double-blind, randomized, placebo-controlled study evaluated the efficacy of topical ketamine (1%), amitriptyline (2%), or combined ketamine/amitriptyline (4 ml) or neuropathic pain. Patients had neuropathies that included: post-herpetic neuralgia (n=14), diabetic neuropathy (n=20), and post-surgical/trauma neuropathy (n=58). Average daily pain intensity was evaluated with an 11-point numerical pain rating scale, McGill Pain Questionnaire, patient satisfaction, and allodynia and hyperalgesia testing. Although a reduction in pain scores occurred over a three week period there was no difference between patients with placebo treatment and patients with any of the topical applications (Lynch et al., 2005b).
Inhibition of the biosynthesis of glutamate in the peripheral primary afferent terminal also may provide analgesia in patients with pain. Anecdotal reports of pain relief have been reported following treatment with DON in clinical cancer trials. A woman “reported marked diminution in pain from a destructive lesion in the lumbar spine due to a fibrosarcoma” following DON administration by a 10-minute infusion daily for 3 days (total doses: 480 mg/m2). Pain relief was long-lasting (6 weeks) despite progression of the disease (Kovach, et al., 1981). In a pediatric study, a 14-year-old male with a vertebral tumor reported decrease in pain after 450 mg/m2 of DON (15-min infusion 2× weekly every 2 weeks; Sullivan et al, 1988). These patients had tumor invasion of spinal vertebrae, a highly painful condition and one where adequate pain relief is difficult to provide. DON’s effect may have occurred at the sensitized peripheral terminal in these patients. Our understanding of bone pain from cancer and pain from intervertebral disc inflammation has grown in recent years (Mantyh & Hunt, 2004; Takahashi et al., 2008). Sensitization of bone and disc peripheral terminals occurs in an avascular environment and may require or involve fewer sensitizing agents than inflammation of the skin and joints. DON efficacy in these clinical cases could be due to GLS inhibition and decreased glutamate production in peripheral terminals.
15. Summary
The current review has focused on the glutamatergic nature of peripheral terminals of primary afferents. These neurons are sensory transducers and transmitters, but they also interact with the peripheral environment by releasing and reacting to glutamate under various physiological and pathophysiological conditions. Intense noxious stimuli or inflammatory conditions cause primary afferent terminals to release glutamate into peripheral tissues. Many peripheral primary afferents have EAARs and respond to glutamate by reducing threshold and increasing excitability. Considering our current understanding of glutamatergic peripheral terminals, we envision several challenges in this field for the future. For example, there appears to be an interaction of EAARs and TRPV1 at the peripheral terminal, but it is unclear what intracellular events participate in this interaction. Interaction of EAARs and other membrane bound proteins such as sodium channels and bradykinin receptors still needs exploration. Likewise, glutamate interacts with a number of EAARs on peripheral terminals, but interaction between EAARs and the intracellular pathways required for interaction is largely unknown. Furthermore, it is unclear what complement of EAARs occurs in various subtypes of DRG neurons. Clarification of this issue might help in understanding interactions at the peripheral terminal. Further investigation of the roles of EAARs and the release of glutamate and other substances, e.g., SP, is needed for a better appreciation of neurogenic inflammation. Lastly, can our understanding of glutamate and the peripheral afferent terminal be translated to pain relief for pain sufferers? Results from animal studies show analgesic benefit by regulation of the peripheral glutamatergic system and clinical studies show potential for relieving some aspects of chronic pain. A more detailed understanding of glutamate metabolism and peripheral EAARs could provide new targets or therapies for sufferers of acute and chronic pain.
Acknowledgments
The authors thank the excellent technical support of Bharathi Srnivasan, Kristy Edwards, Brent Richards, Zijia Zhang, Jeff McCosh, Sheila Pete, and Sumit Aluwahlia.
Supported by NIH grants NS27213 and AR047410 (KEM).
Abbreviations
- LY367385
S-+-a-amino-4-carboxy-2-methylbenzeneacetic acid
- UBP 302
(S)-1-(2-Amino-2-carboxyethyl)-3-(2-carboxybenzyl)pyrimidine-2,4-dione
- LY341495
(2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl)propanoic acid
- APV
2-amino-5-phosphonvalerate
- DCG4
(2S, 2′R, 3′R)-2-(2′, 3′-dicarboxycyclo-propyl)glycine
- MCCG
(2S,3S,4S)-2-methyl-2-(carboxycyclopropyl)glycine
- SIB1893
2-methyl-6-(2-phenylethenyl)pyridine
- DON
6-diazo-5-oxo-L-norleucine
- CPCCOEtC
7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester
- CPT
8-cyclopentyl-theophylline
- DHPG
3,5-dihydroxyphenylglycine
- MPEP
2-methyl-6-(phenylethynyl)-pyridine
- CPP
3-(2-carboxypiperazine-4-yl)propyl-1-phosphonic acid
- 4-CPG
(S)-4-carboxy-phenylglycine
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- E4CPG
(RS)-α-ethyl-4-carboxyphenylglycine
- L-AP4
L-(1)-2-Amino-4-phosphonobutyric acid
- L-NAME
Nω-nitro-L-arginine methyl ester
- APDC
(2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate
- AIDA
(RS)-1-aminoindan-1, 5-dicarboxylic acid
- MSOP
(RS)-a-methylserine-O-phosphate
- MPPG
R,S-α-methyl-4-phosphonophenylglycine
- L-CCG-I
(2S,1′S,2′S)-2-(carboxycyclopropyl) glycine
- S-DHPG
(S)-3,5-dihydroxyphenylglycine
- CHPG
(S)-4-carboxy-phenylglycine
- AIA
adjuvant-induced arthritis
- AMPAR
AMPA receptors
- AAT
aspartate aminotransferase
- BV
bee venom
- BoNTA
botulinum neurotoxin type A
- CGRP
calcitonin gene-related peptide
- CNS
central nervous system
- CFA
complete Freud’s adjuvant
- DRG
dorsal root ganglion
- EMG
electromyographic
- ET-1
endothelin-1
- EAAR
excitatory amino acid receptor
- ECRB
extensor carpi radialis brevis
- GAMS
γ-D-glutamylaminomethyl sulfonic acid
- GLS
glutaminase
- EAAT
excitatory amino acid transporter
- IR
immunoreactive
- ir
immunoreactivity
- IL-1β
interleukin-1β
- i.p.
intraperitoneal
- i.pl.
intraplantar
- i.v.
intravenous
- KA
kainate
- KAR
kainate receptors
- MAT
mechanical activation threshold
- mGluR
metabotropic glutamate receptors
- MO
mustard oil
- NA-3,4,-DCM
N-antipyrine-3, 4-dichloromaleimide
- NAAG
N-acetyl-aspartyl-glutamate
- NMDAR
NMDA receptors
- NOARG
Lω-N-nitro-arginine
- NGF
nerve growth factor
- NMDA
N-methyl-D-aspartate
- OA
osteoarthritis
- PNS
peripheral nervous system
- SNAP
S-nitroso-N-acetyl-D,L-penicillamine
- SNAT
sodium-coupled neutral amino acid transport
- s.c.
subcutaneous
- SP
substance P
- TMJ
temporomandibular joint
- TCA
tricarboxylic acid
- TG
trigeminal ganglion
- TNFα
tumor necrosis factor-α
- VGLUT
vesicular glutamate transporter
- VAS
visual analog scale
- WBC
white blood cell
- WDR
wide-dynamic range
- WT
wild-type.
Footnotes
Some studies using drug administration describe the application of the drug in terms of mol/μl or weight/μl. These have been converted to molarity when ever possible as well as whenever reporting the original units.
References
- Agrawal SG, Evans RH. The primary afferent depolarizing action of kainate in the rat. Br J Pharmacol. 1986;87(2):345–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn DK, Jung CY, Lee HJ, Choi HS, Ju JS, Bae YC. Peripheral glutamate receptors participate in interleukin-1beta-induced mechanical allodynia in the orofacial area of rats. Neurosci Lett. 2004;357(3):203–206. doi: 10.1016/j.neulet.2003.12.097. [DOI] [PubMed] [Google Scholar]
- Ahn DK, Kim KH, Jung CY, Choi HS, Lim EJ, Youn DH, et al. Role of peripheral group I and II metabotropic glutamate receptors in IL-1beta-induced mechanical allodynia in the orofacial area of conscious rats. Pain. 2005;118(1–2):53–60. doi: 10.1016/j.pain.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Different peripheral mechanisms mediate enhanced nociception in metabolic/toxic and traumatic painful peripheral neuropathies in the rat. Neuroscience. 2002;111(2):389–397. doi: 10.1016/s0306-4522(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Alfredson H, Lorentzon R. Chronic tendon pain: no signs of chemical inflammation but high concentrations of the neurotransmitter glutamate. Implications for treatment? Curr Drug Targets. 2002;3(1):43–54. doi: 10.2174/1389450023348028. [DOI] [PubMed] [Google Scholar]
- Alfredson H, Ljung BO, Thorsen K, Lorentzon R. In vivo investigation of ECRB tendons with microdialysis technique—no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand. 2000;71(5):475–479. doi: 10.1080/000164700317381162. [DOI] [PubMed] [Google Scholar]
- Alfredson H, Forsgren S, Thorsen K, Fahlstrom M, Johansson H, Lorentzon R. Glutamate NMDAR1 receptors localised to nerves in human Achilles tendons. Implications for treatment? Knee Surg Sports Traumatol Arthrosc. 2001a;9(2):123–126. doi: 10.1007/s001670000188. [DOI] [PubMed] [Google Scholar]
- Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. J Orthop Res. 2001b;19(5):881–886. doi: 10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- Andreou AP, Holland PR, Goadsby PJ. Activation of iGluR5 kainate receptors inhibits neurogenic dural vasodilatation in an animal model of trigeminovascular activation. Br J Pharmacol. 2009;157(3):464–473. doi: 10.1111/j.1476-5381.2009.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Svensson P, Sessle BJ, Cairns BE, Wang K. Interactions between glutamate and capsaicin in inducing muscle pain and sensitization in humans. Eur J Pain. 2008;12(5):661–670. doi: 10.1016/j.ejpain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atoji Y, Islam MR. Localization of vesicular glutamate transporter 2 mRNA in the dorsal root ganglion of the pigeon (Columba livia) Anat Histol Embryol. 2009;38(6):475–478. doi: 10.1111/j.1439-0264.2009.00978.x. [DOI] [PubMed] [Google Scholar]
- Ault B, Hildebrand LM. Activation of nociceptive reflexes by peripheral kainate receptors. J Pharmacol Exp Ther. 1993a;265(2):927–932. [PubMed] [Google Scholar]
- Ault B, Hildebrand LM. Effects of excitatory amino acid receptor antagonists on a capsaicin-evoked nociceptive reflex: a comparison with morphine, clonidine and baclofen. Pain. 1993b;52(3):341–349. doi: 10.1016/0304-3959(93)90168-O. [DOI] [PubMed] [Google Scholar]
- Ault B, Hildebrand LM. L-glutamate activates peripheral nociceptors. Agents Actions. 1993c;39(Spec No):C142–144. doi: 10.1007/BF01972747. [DOI] [PubMed] [Google Scholar]
- Aumeerally N, Allen G, Sawynok J. Glutamate-evoked release of adenosine and regulation of peripheral nociception. Neuroscience. 2004;127(1):1–11. doi: 10.1016/j.neuroscience.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Averill S, Robson LG, Jeromin A, Priestley JV. Neuronal calcium sensor-1 is expressed by dorsal root ganglion cells, is axonally transported to central and peripheral terminals, and is concentrated at nodes. Neuroscience. 2004;123(2):419–427. doi: 10.1016/j.neuroscience.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Azerad J, Boucher Y, Pollin B. Demonstration of glutamate in primary sensory trigeminal neurons innervating dental pulp in rats. CR Acad Sci III. 1992;314(10):469–475. [PubMed] [Google Scholar]
- Baad-Hansen L, Cairns BE, Ernberg M, Svensson P. Effect of systemic monosodium glutamate (MSG) on headache and pericranial muscle sensitivity. Cephalalgia. 2010;30(1):68–76. doi: 10.1111/j.1468-2982.2009.01881.x. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J Neurosci. 2004;24(11):2774–2781. doi: 10.1523/JNEUROSCI.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Rustioni A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J Comp Neurol. 1988;277(2):302–312. doi: 10.1002/cne.902770210. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Beirith A, Santos AR, Calixto JB. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 2002;924(2):219–228. doi: 10.1016/s0006-8993(01)03240-1. [DOI] [PubMed] [Google Scholar]
- Beirith A, Santos AR, Calixto JB. The role of neuropeptides and capsaicin-sensitive fibres in glutamate-induced nociception and paw oedema in mice. Brain Res. 2003;969(1–2):110–116. doi: 10.1016/s0006-8993(03)02286-8. [DOI] [PubMed] [Google Scholar]
- Ben-Ari A, Lewis M, Davidson E. Chronic administration of ketamine for analgesia. J Pain Palliat Care Pharmacother. 2007;21(1):7–14. [PubMed] [Google Scholar]
- Bereiter DA, Bereiter DF. Morphine and NMDA receptor antagonism reduce c-fos expression in spinal trigeminal nucleus produced by acute injury to the TMJ region. Pain. 2000;85(1–2):65–77. doi: 10.1016/s0304-3959(99)00246-8. [DOI] [PubMed] [Google Scholar]
- Berger UV, Carter RE, McKee M, Coyle JT. N-acetylated alpha-linked acidic dipeptidase is expressed by non-myelinating Schwann cells in the peripheral nervous system. J Neurocytol. 1995;24(2):99–109. doi: 10.1007/BF01181553. [DOI] [PubMed] [Google Scholar]
- Bhave G, Karim F, Carlton SM, Gereau RWt. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4(4):417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Bobbin RP, Thalmann R, Thalmann I. Stimulus-induced release of endogenous amino acids from skins containing the lateral-line organ in Xenopus laevis. Exp Brain Res. 1980;40(1):97–101. doi: 10.1007/BF00236667. [DOI] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, McLaren JD, Meyer JR. Potassium-induced release of endogenous glutamate and two as yet unidentified substances from the lateral line of Xenopus laevis. Brain Res. 1989;493(1):113–122. doi: 10.1016/0006-8993(89)91005-6. [DOI] [PubMed] [Google Scholar]
- Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci. 2004;20(2):375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Watanabe M, Hokfelt T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 2007;147(2):469–490. doi: 10.1016/j.neuroscience.2007.02.068. [DOI] [PubMed] [Google Scholar]
- Buritova J, Chapman V, Prisca Honor P, Besson J. Interactions between NMDA- and prostaglandin receptor-mediated events in a model of inflammatory nociception. Eur J Pharmacol. 1996;303:91–100. doi: 10.1016/0014-2999(96)00074-x. [DOI] [PubMed] [Google Scholar]
- Buritova J, Le Guen S, Fournie-Zaluski MC, Roques BP, Besson JM. Antinociceptive effects of RB101(S), a complete inhibitor of enkephalin-catabolizing enzymes, are enhanced by (+)-HA966, a functional NMDA receptor antagonist: a c-Fos study in the rat spinal cord. Eur J Pain. 2003;7(3):241–249. doi: 10.1016/S1090-3801(02)00122-2. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Sessle BJ, Hu JW. Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J Neurosci. 1998;18(19):8056–8064. doi: 10.1523/JNEUROSCI.18-19-08056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2001a;86(2):782–791. doi: 10.1152/jn.2001.86.2.782. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Sessle BJ, Hu JW. Characteristics of glutamate-evoked temporomandibular joint afferent activity in the rat. J Neurophysiol. 2001b;85(6):2446–2454. doi: 10.1152/jn.2001.85.6.2446. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Sessle BJ, Hu JW. Temporomandibular-evoked jaw muscle reflex: role of brain stem NMDA and non-NMDA receptors. NeuroReport. 2001c;12(9):1875–1878. doi: 10.1097/00001756-200107030-00022. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Gambarota G, Svensson P, Arendt-Nielsen L, Berde CB. Glutamate-induced sensitization of rat masseter muscle fibers. Neuroscience. 2002a;109(2):389–399. doi: 10.1016/s0306-4522(01)00489-4. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Sim Y, Bereiter DA, Sessle BJ, Hu JW. Influence of sex on reflex jaw muscle activity evoked from the rat temporomandibular joint. Brain Res. 2002b;957(2):338–344. doi: 10.1016/s0006-8993(02)03671-5. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Gambarota G, Dunning PS, Mulkern RV, Berde CB. Activation of peripheral excitatory amino acid receptors decreases the duration of local anesthesia. Anesthesiology. 2003a;98(2):521–529. doi: 10.1097/00000542-200302000-00035. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, et al. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2003b;90(4):2098–2105. doi: 10.1152/jn.00353.2003. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Castrillon E, Hupfeld S, Sessle BJ, et al. Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res. 2006;169(4):467–472. doi: 10.1007/s00221-005-0158-z. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Dong X, Mann MK, Svensson P, Sessle BJ, Arendt-Nielsen L, et al. Systemic administration of monosodium glutamate elevates intramuscular glutamate levels and sensitizes rat masseter muscle afferent fibers. Pain. 2007;132(1–2):33–41. doi: 10.1016/j.pain.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangro CB, Namboodiri MA, Sklar LA, Corigliano-Murphy A, Neale JH. Immunohistochemistry and biosynthesis of N-acetylaspartylglutamate in spinal sensory ganglia. J Neurochem. 1987;49(5):1579–1588. doi: 10.1111/j.1471-4159.1987.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Cao DY, You HJ, Zhao Y, Guo Y, Wang HS, Arendt-Nielsen L, et al. Involvement of peripheral ionotropic glutamate receptors in activation of cutaneous branches of spinal dorsal rami following antidromic electrical stimulation of adjacent afferent nerves in rats. Brain Res Bull. 2007;72(1):10–17. doi: 10.1016/j.brainresbull.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Coggeshall RE. Inflammation-induced changes in peripheral glutamate receptor populations. Brain Res. 1999;820(1–2):63–70. doi: 10.1016/s0006-8993(98)01328-6. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL. Treatment with the NMDA antagonist memantine attenuates nociceptive responses to mechanical stimulation in neuropathic rats. Neurosci Lett. 1995;198(2):115–118. doi: 10.1016/0304-3940(95)11980-b. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol. 2007;501(5):780–789. doi: 10.1002/cne.21285. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;197(1):25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Coggeshall RE. Evidence for the interaction of glutamate and NK1 receptors in the periphery. Brain Res. 1998;790(1–2):160–169. doi: 10.1016/s0006-8993(97)01471-6. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience. 2001;105(4):957–969. doi: 10.1016/s0306-4522(01)00238-x. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Zhou S. Group II metabotropic glutamate receptor activation on peripheral nociceptors modulates TRPV1 function. Brain Res. 2009;1248:86–95. doi: 10.1016/j.brainres.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carozzi V, Marmiroli P, Cavaletti G. Focus on the role of glutamate in the pathology of the peripheral nervous system. CNS Neurol Disord Drug Targets. 2008a;7(4):348–360. doi: 10.2174/187152708786441876. [DOI] [PubMed] [Google Scholar]
- Carozzi VA, Canta A, Oggioni N, Ceresa C, Marmiroli P, Konvalinka J, et al. Expression and distribution of ‘high affinity’ glutamate transporters GLT1, GLAST, EAAC1 and of GCPII in the rat peripheral nervous system. J Anat. 2008b;213(5):539–546. doi: 10.1111/j.1469-7580.2008.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon EE, Cairns BE, Ernberg M, Wang K, Sessle B, Arendt-Nielsen L, et al. Glutamate-evoked jaw muscle pain as a model of persistent myofascial TMD pain? Arch Oral Biol. 2008;53(7):666–676. doi: 10.1016/j.archoralbio.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KY, Gupta S, de Vries R, Danser AH, Villalon CM, Munoz-Islas E, et al. Effects of ionotropic glutamate receptor antagonists on rat dural artery diameter in an intravital microscopy model. Br J Pharmacol. 2010;160(6):1316–1325. doi: 10.1111/j.1476-5381.2010.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman V, Honore P, Buritova J, Besson JM. The contribution of NMDA receptor activation to spinal c-Fos expression in a model of inflammatory pain. Br J Pharmacol. 1995;116(1):1628–1634. doi: 10.1111/j.1476-5381.1995.tb16383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman V, Buritova J, Honore P, Besson JM. Physiological contributions of neurokinin 1 receptor activation, and interactions with NMDA receptors, to inflammatory-evoked spinal c-Fos expression. J Neurophysiol. 1996;76(3):1817–1827. doi: 10.1152/jn.1996.76.3.1817. [DOI] [PubMed] [Google Scholar]
- Chen HS, Chen J. Secondary heat, but not mechanical, hyperalgesia induced by subcutaneous injection of bee venom in the conscious rat: effect of systemic MK-801, a non-competitive NMDA receptor antagonist. Eur J Pain. 2000;4(4):389–401. doi: 10.1053/eujp.2000.0197. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356(6369):521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Chen J, Li H, Luo C, Li Z, Zheng J. Involvement of peripheral NMDA and non-NMDA receptors in development of persistent firing of spinal wide-dynamic-range neurons induced by subcutaneous bee venom injection in the cat. Brain Res. 1999a;844(1–2):98–105. doi: 10.1016/s0006-8993(99)01841-7. [DOI] [PubMed] [Google Scholar]
- Chen J, Luo C, Li H, Chen H. Primary hyperalgesia to mechanical and heat stimuli following subcutaneous bee venom injection into the plantar surface of hindpaw in the conscious rat: a comparative study with the formalin test. Pain. 1999b;83(1):67–76. doi: 10.1016/s0304-3959(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Chen HS, Qu F, He X, Kang SM, Liao D, Lu SJ. Differential roles of peripheral metabotropic glutamate receptors in bee venom-induced nociception and inflammation in conscious rats. J Pain. 2010;11(4):321–329. doi: 10.1016/j.jpain.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Chizh BA. Low dose ketamine: a therapeutic and research tool to explore N-methyl-D-aspartate (NMDA) receptor-mediated plasticity in pain pathways. J Psychopharmacol. 2007;21(3):259–271. doi: 10.1177/0269881105062484. [DOI] [PubMed] [Google Scholar]
- Chun Y, Frank D, Lee JS, Zhang Y, Auh Q, Ro JY. Peripheral AMPA receptors contribute to muscle nociception and c-fos activation. Neurosci Res. 2008;62(2):97–104. doi: 10.1016/j.neures.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cincotta M, Beart PM, Summers RJ, Lodge D. Bidirectional transport of NMDA receptor and ionophore in the vagus nerve. Eur J Pharmacol. 1989;160(1):167–171. doi: 10.1016/0014-2999(89)90668-7. [DOI] [PubMed] [Google Scholar]
- Classey JD, Knight YE, Goadsby PJ. The NMDA receptor antagonist MK-801 reduces Fos-like immunoreactivity within the trigeminocervical complex following superior sagittal sinus stimulation in the cat. Brain Res. 2001;907(1–2):117–124. doi: 10.1016/s0006-8993(01)02550-1. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. Fos, nociception and the dorsal horn. Prog Neurobiol. 2005;77(5):299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Carlton SM. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Comp Neurol. 1998;391(1):78–86. doi: 10.1002/(sici)1096-9861(19980202)391:1<78::aid-cne7>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Zhou S, Carlton SM. Opioid receptors on peripheral sensory axons. Brain Res. 1997;764(1–2):126–132. doi: 10.1016/s0006-8993(97)00446-0. [DOI] [PubMed] [Google Scholar]
- Davidson EM, Carlton SM. Intraplantar injection of dextrorphan, ketamine or memantine attenuates formalin-induced behaviors. Brain Res. 1998;785(1):136–142. doi: 10.1016/s0006-8993(97)01396-6. [DOI] [PubMed] [Google Scholar]
- Davidson EM, Coggeshall RE, Carlton SM. Peripheral NMDA and non-NMDA glutamate receptors contribute to nociceptive behaviors in the rat formalin test. NeuroReport. 1997;8(4):941–946. doi: 10.1097/00001756-199703030-00025. [DOI] [PubMed] [Google Scholar]
- Davies J, Evans RH, Francis AA, Watkins JC. Excitatory amino acid receptors and synaptic excitation in the mammalian central nervous system. J Physiol Paris. 1979;75(6):641–654. [PubMed] [Google Scholar]
- deGroot J, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. NeuroReport. 2000;11(3):497–502. doi: 10.1097/00001756-200002280-00014. [DOI] [PubMed] [Google Scholar]
- Dong XD, Mann MK, Sessle BJ, Arendt-Nielsen L, Svensson P, Cairns BE. Sensitivity of rat temporalis muscle afferent fibers to peripheral N-methyl-D-aspartate receptor activation. Neuroscience. 2006;141(2):939–945. doi: 10.1016/j.neuroscience.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Dong XD, Mann MK, Kumar U, Svensson P, Arendt-Nielsen L, Hu JW, et al. Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. Neuroscience. 2007;146(2):822–832. doi: 10.1016/j.neuroscience.2007.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XD, Svensson P, Cairns BE. The analgesic action of topical diclofenac may be mediated through peripheral NMDA receptor antagonism. Pain. 2009;147(1):36–45. doi: 10.1016/j.pain.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain. 2001;89(2–3):187–198. doi: 10.1016/s0304-3959(00)00362-6. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118(2):547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou S, Carlton SM. Kainate-induced excitation and sensitization of nociceptors in normal and inflamed rat glabrous skin. Neuroscience. 2006;137(3):999–1013. doi: 10.1016/j.neuroscience.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Duce IR, Keen P. Selective uptake of [3H]glutamine and [3H]glutamate into neurons and satellite cells of dorsal root ganglia in vitro. Neuroscience. 1983;8(4):861–866. doi: 10.1016/0306-4522(83)90016-7. [DOI] [PubMed] [Google Scholar]
- Duggan AW, Johnston GA. Glutamate and related amino acids in cat spinal roots, dorsal root ganglia and peripheral nerves. J Neurochem. 1970a;17(8):1205–1208. doi: 10.1111/j.1471-4159.1970.tb03369.x. [DOI] [PubMed] [Google Scholar]
- Duggan AW, Johnston GA. Glutamate and related amino-acids in cat, dog, and rat spinal roots. Comp Gen Pharmacol. 1970b;1(1):127–128. doi: 10.1016/0010-4035(70)90017-0. [DOI] [PubMed] [Google Scholar]
- Evans RH. Evidence supporting the indirect depolarization of primary afferent terminals in the frog by excitatory amino acids. J Physiol. 1980;298:25–35. doi: 10.1113/jphysiol.1980.sp013064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RH. Kainate sensitivity of C-fibres in rat dorsal roots. J Physiol. 1985;358:42. [Google Scholar]
- Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol. 2005;565(Pt 3):927–943. doi: 10.1113/jphysiol.2005.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick MK, Oswald RE. On the mechanisms of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor binding to glutamate and kainate. J Biol Chem. 2010;285(16):12334–12343. doi: 10.1074/jbc.M109.086371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino PM, Cairns BE, Hu JW. Development of inflammation after application of mustard oil or glutamate to the rat temporomandibular joint. Arch Oral Biol. 1999;44(1):27–32. doi: 10.1016/s0003-9969(98)00095-8. [DOI] [PubMed] [Google Scholar]
- Fischer M, Glanz D, William T, Klapperstuck T, Wohlrab J, Marsch W. N-methyl-D-aspartate receptors influence the intracellular calcium concentration of keratinocytes. Exp Dermatol. 2004a;13(8):512–519. doi: 10.1111/j.0906-6705.2004.00200.x. [DOI] [PubMed] [Google Scholar]
- Fischer M, William T, Helmbold P, Wohlrab J, Marsch W. Expression of epidermal N-methyl-D-aspartate receptors (NMDAR1) depends on formation of the granular layer—analysis in diseases with parakeratotic cornification. Arch Dermatol Res. 2004b;296(4):157–162. doi: 10.1007/s00403-004-0505-0. [DOI] [PubMed] [Google Scholar]
- Flood S, Parri R, Williams A, Duance V, Mason D. Modulation of interleukin-6 and matrix metalloproteinase 2 expression in human fibroblast-like synoviocytes by functional ionotropic glutamate receptors. Arthritis Rheum. 2007;56(8):2523–2534. doi: 10.1002/art.22829. [DOI] [PubMed] [Google Scholar]
- Follenfant RL, Nakamura-Craig M. Glutamate induces hyperalgesia in the rat paw. Br J Pharmacol. 1992;106:49. [Google Scholar]
- Fuziwara S, Inoue K, Denda M. NMDA-type glutamate receptor is associated with cutaneous barrier homeostasis. J Invest Dermatol. 2003;120(6):1023–1029. doi: 10.1046/j.1523-1747.2003.12238.x. [DOI] [PubMed] [Google Scholar]
- Gambarota G, Philippens M, Cairns BE, Dong XD, Renema WK, Heerschap A. MRS assessment of glutamate clearance in a novel masticatory muscle pain model. NMR Biomed. 2005;18(6):345–351. doi: 10.1002/nbm.962. [DOI] [PubMed] [Google Scholar]
- Gammaitoni A, Gallagher RM, Welz-Bosna M. Topical ketamine gel: possible role in treating neuropathic pain. Pain Med. 2000;1(1):97–100. doi: 10.1046/j.1526-4637.2000.00006.x. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Wang K, Cairns BE, Svensson P, Arendt-Nielsen L. Effects of subcutaneous administration of glutamate on pain, sensitization and vasomotor responses in healthy men and women. Pain. 2006;124(3):338–348. doi: 10.1016/j.pain.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Au S, Dong X, Kumar U, Arendt-Nielsen L, Cairns BE. Botulinum neurotoxin type A (BoNTA) decreases the mechanical sensitivity of nociceptors and inhibits neurogenic vasodilation in a craniofacial muscle targeted for migraine prophylaxis. Pain. 2010a;151(3):606–616. doi: 10.1016/j.pain.2010.07.029. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Dong X, Wang M, Kumar U, Cairns BE. Sensitization of rat facial cutaneous mechanoreceptors by activation of peripheral N-methyl-D-aspartate receptors. Brain Res. 2010b;1319:70–82. doi: 10.1016/j.brainres.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Ghosh C, Storey-Workley M, Usip S, Hafemeister J, Miller KE, Papka RE. Glutamate and metabotropic glutamate receptors associated with innervation of the uterine cervix during pregnancy: receptor antagonism inhibits c-Fos expression in rat lumbosacral spinal cord at parturition. J Neurosci Res. 2007;85(6):1318–1335. doi: 10.1002/jnr.21225. [DOI] [PubMed] [Google Scholar]
- Gibson W, Arendt-Nielsen L, Sessle BJ, Graven-Nielsen T. Glutamate and capsaicin-induced pain, hyperalgesia and modulatory interactions in human tendon tissue. Exp Brain Res. 2009;142(2):173–182. doi: 10.1007/s00221-008-1683-3. [DOI] [PubMed] [Google Scholar]
- Gill S, Pulido O. Glutamate receptors in peripheral tissues: distribution and implications for toxicology. In: Gill S, Pulido O, editors. Glutamate receptors in peripheral tissue: excitatory transmission outside the CNS. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 3–26. [Google Scholar]
- Giovengo SL, Kitto KF, Kurtz HJ, Velazquez RA, Larson AA. Parenterally administered kainic acid induces a persistent hyperalgesia in the mouse and rat. Pain. 1999;83(2):347–358. doi: 10.1016/s0304-3959(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Graham LT, Aprison MH. Distribution of some enzymes associated with the metabolism of glutamate, aspartate, gamma-aminobutyrate and glutamine in cat spinal cord. J Neurochem. 1969;16(4):559–566. doi: 10.1111/j.1471-4159.1969.tb06855.x. [DOI] [PubMed] [Google Scholar]
- Graham LT, Jr, Werman R, Aprison MH. Microdetermination of glutamate in single cat spinal roots. Life Sci. 1965;4(10):1085–1090. doi: 10.1016/0024-3205(65)90228-6. [DOI] [PubMed] [Google Scholar]
- Graham LT, Jr, Shank RP, Werman R, Aprison MH. Distribution of some synaptic transmitter suspects in cat spinal cord: glutamic acid, aspartic acid, gamma-aminobutyric acid, glycine and glutamine. J Neurochem. 1967;14(4):465–472. doi: 10.1111/j.1471-4159.1967.tb09545.x. [DOI] [PubMed] [Google Scholar]
- Hakim AW, Dong XD, Cairns BE. TNF{alpha} mechanically sensitizes masseter muscle nociceptors by increasing prostaglandin E2 levels. J Neurophysiol. 2011;105(1):154–161. doi: 10.1152/jn.00730.2010. [DOI] [PubMed] [Google Scholar]
- Haas HS, Schauenstein K. Neuroexcitatory signaling in immune tissues. In: Gill S, Pulido O, editors. Glutamate receptors in peripheral tissue: excitatory transmission outside the CNS. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 191–196. [Google Scholar]
- Hassel B, Boldingh KA, Narvesen C, Iversen EG, Skrede KK. Glutamate transport, glutamine synthetase and phosphate-activated glutaminase in rat CNS white matter. A quantitative study. J Neurochem. 2003;87(1):230–237. doi: 10.1046/j.1471-4159.2003.01984.x. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Watanabe M, Iwabe T, Tanaka E, Nishi M, Aoyama J, et al. Administration of MK-801 decreases c-Fos expression in the trigeminal sensory nuclear complex but increases it in the midbrain during experimental movement of rat molars. Brain Res. 2004;1021(2):183–191. doi: 10.1016/j.brainres.2004.06.048. [DOI] [PubMed] [Google Scholar]
- Hawkins NS, Hearn J, Evans RH. Comparison of the capsaicin- and amino acid-sensitivity of dorsal root C fibres in the rat and the toad. Comp Biochem Physiol C. 1991;99(3):513–516. doi: 10.1016/0742-8413(91)90279-3. [DOI] [PubMed] [Google Scholar]
- Hoffman EM, Miller KE. Peripheral inhibition of glutaminase reduces carrageenan-induced Fos expression in the superficial dorsal horn of the rat. Neurosci Lett. 2010;472(3):157–160. doi: 10.1016/j.neulet.2010.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EM, Schechter R, Miller KE. Fixative composition alters distributions of immunoreactivity for glutaminase and two markers of nociceptive neurons, Nav1.8 and TRPV1, in the rat dorsal root ganglion. J Histochem Cytochem. 2010;58(4):329–344. doi: 10.1369/jhc.2009.954008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EM, Edwards KM, Miller KE. Glutaminase immunoreactivity changes in rat dorsal root ganglion during unilateral adjuvant-induced arthritis. 2011a in revision. Unpublished manuscript. [Google Scholar]
- Hoffman EM, Zhang Z, Anderson MB, Edwards KM, Schechter R, Miller KE. Nav1.8 and glutaminase expression changes in dorsal root ganglion neuron subpopulations as a potential mechanism of hypoalgesia during autoimmune nerve growth factor deprivation in the adult rat. 2011b Unpublished manuscript. [Google Scholar]
- Holcomb T, Taylor L, Trohkimoinen J, Curthoys NP. Isolation, characterization and expression of a human brain mitochondrial glutaminase cDNA. Brain Res Mol Brain Res. 2000;76(1):56–63. doi: 10.1016/s0169-328x(99)00331-9. [DOI] [PubMed] [Google Scholar]
- Hudson LJ, Bevan S, McNair K, Gentry C, Fox A, Kuhn R, et al. Metabotropic glutamate receptor 5 upregulation in A-fibers after spinal nerve injury: 2-methyl-6-(phenylethynyl)-pyridine (MPEP) reverses the induced thermal hyperalgesia. J Neurosci. 2002;22(7):2660–2668. doi: 10.1523/JNEUROSCI.22-07-02660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5(3):255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Huettner JE, Kerchner GA, Zhuo M. Glutamate and the presynaptic control of spinal sensory transmission. Neuroscientist. 2002;8(2):89–92. doi: 10.1177/107385840200800204. [DOI] [PubMed] [Google Scholar]
- Ibitokun O, Miller KE. Corneal Afferents Contain Synaptic Vesicle Proteins: Synaptophysin I and Synaptophysin II. ARVO. 2010a 1974. [Google Scholar]
- Ibitokun O, Miller KE. Corneal Afferents Contain Vesicular Glutamate Transporter 2 (VGLUT2) IASPPM 279 2010b [Google Scholar]
- Inagaki N, Kamisaki Y, Kiyama H, Horio Y, Tohyama M, Wada H. Immunocytochemical localizations of cytosolic and mitochondrial glutamic oxaloacetic transaminase isozymes in rat primary sensory neurons as a marker for the glutamate neuronal system. Brain Res. 1987;402(1):197–200. doi: 10.1016/0006-8993(87)91068-7. [DOI] [PubMed] [Google Scholar]
- Jackson DL, Hargreaves KM. Activation of excitatory amino acid receptors in bovine dental pulp evokes the release of iCGRP. J Dent Res. 1999;78(1):54–60. doi: 10.1177/00220345990780010801. [DOI] [PubMed] [Google Scholar]
- Jackson DL, Aanonsen LM, Richardson JD, Geier H, Hargreaves KM. An evaluation of the effects of excitatory amino acids in bovine dental pulp. Proc Soc Neurosci. 1993;19:996. [Google Scholar]
- Jackson DL, Graff CB, Richardson JD, Hargreaves KM. Glutamate participates in the peripheral modulation of thermal hyperalgesia in rats. Eur J Pharmacol. 1995;284(3):321–325. doi: 10.1016/0014-2999(95)00449-u. [DOI] [PubMed] [Google Scholar]
- Jancso N, Jancso-Gabor A, Szolcsanyi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH, Kim DW, Sang Nam T, Se Paik K, Leem JW. Peripheral glutamate receptors contribute to mechanical hyperalgesia in a neuropathic pain model of the rat. Neuroscience. 2004;128(1):169–176. doi: 10.1016/j.neuroscience.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Jean YH, Wen ZH, Chang YC, Huang GS, Lee HS, Hsieh SP, et al. Increased concentrations of neuro-excitatory amino acids in rat anterior cruciate ligament-transected knee joint dialysates: a microdialysis study. J Orthop Res. 2005;23(3):569–575. doi: 10.1016/j.orthres.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Jean YH, Wen ZH, Chang YC, Lee HS, Hsieh SP, Wu CT, et al. Hyaluronic acid attenuates osteoarthritis development in the anterior cruciate ligament-transected knee: association with excitatory amino acid release in the joint dialysate. J Orthop Res. 2006;24(5):1052–1061. doi: 10.1002/jor.20123. [DOI] [PubMed] [Google Scholar]
- Jean YH, Wen ZH, Chang YC, Hsieh SP, Tang CC, Wang YH, et al. Intra-articular injection of the cyclooxygenase-2 inhibitor parecoxib attenuates osteoarthritis progression in anterior cruciate ligament-transected knee in rats: role of excitatory amino acids. Osteoarthritis Cartilage. 2007;15(6):638–645. doi: 10.1016/j.joca.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Jean YH, Wen ZH, Chang YC, Hsieh SP, Lin JD, Tang CC, et al. Increase in excitatory amino acid concentration and transporters expression in osteoarthritic knees of anterior cruciate ligament transected rabbits. Osteoarthritis Cartilage. 2008;16(12):1442–1449. doi: 10.1016/j.joca.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Jin YH, Nishioka H, Wakabayashi K, Fujita T, Yonehara N. Effect of morphine on the release of excitatory amino acids in the rat hind instep: pain is modulated by the interaction between the peripheral opioid and glutamate systems. Neuroscience. 2006;138(4):1329–1339. doi: 10.1016/j.neuroscience.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Jin YH, Yamaki F, Takemura M, Koike Y, Furuyama A, Yonehara N. Capsaicin-induced glutamate release is implicated in nociceptive processing through activation of ionotropic glutamate receptors and group I metabotropic glutamate receptor in primary afferent fibers. J Pharmacol Sci. 2009;109(2):233–241. doi: 10.1254/jphs.08262fp. [DOI] [PubMed] [Google Scholar]
- Johnson JL. Compartmentation of [U–14C]proline metabolism in the dorsal root ganglion: contrasts with the ventral spinal cord gray and cerebral cortex. Brain Res. 1975;96(1):192–196. doi: 10.1016/0006-8993(75)90596-x. [DOI] [PubMed] [Google Scholar]
- Johnson JL. Glutamic acid as a synaptic transmitter in the nervous system. A review. Brain Res. 1972a;37(1):1–19. doi: 10.1016/0006-8993(72)90343-5. [DOI] [PubMed] [Google Scholar]
- Johnson JL. An analysis of the activities of 3 key enzymes concerned with the interconversion of α-ketoglutarate and glutamate: correlations with free glutamate levels in 20 specific regions of the nervous system. Brain Res. 1972b;45(1):205–215. doi: 10.1016/0006-8993(72)90227-2. [DOI] [PubMed] [Google Scholar]
- Johnson J. Aspartic acid as a precursor for glutamic acid and glycine. Brain Res. 1974;67(2):358–362. doi: 10.1016/0006-8993(74)90289-3. [DOI] [PubMed] [Google Scholar]
- Johnson JL. A comparative analysis of compartmentation of metabolism in the dorsal root ganglion and ventral spinal cord gray using [U–14C]glucose, [2–14C] glucose, [6–14C]glucose, [3, 4–14C]glucose, NaH14CO3, and [2–14C]pyruvate. Brain Res. 1976;101(3):523–532. doi: 10.1016/0006-8993(76)90475-3. [DOI] [PubMed] [Google Scholar]
- Johnson JL. Glutamic acid as a synaptic transmitter candidate in the dorsal sensory neuron: reconsiderations. Life Sci. 1977;20(10):1637–1644. doi: 10.1016/0024-3205(77)90336-8. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Aprison MH. The distribution of glutamate and total free amino acids in thirteen specific regions of the cat central nervous system. Brain Res. 1970a;26:141–148. [Google Scholar]
- Johnson JL, Aprison MH. The distribution of glutamic acid, a transmitter candidate, and other amino acids in the dorsal sensory neuron of the cat. Brain Res. 1970b;24(2):285–292. doi: 10.1016/0006-8993(70)90107-1. [DOI] [PubMed] [Google Scholar]
- Jung CY, Lee SY, Choi HS, Lim EJ, Lee MK, Yang GY, et al. Participation of peripheral group I and II metabotropic glutamate receptors in the development or maintenance of IL-1beta-induced mechanical allodynia in the orofacial area of conscious rats. Neurosci Lett. 2006;409(3):173–178. doi: 10.1016/j.neulet.2006.09.043. [DOI] [PubMed] [Google Scholar]
- Kai-Kai MA. Cytochemistry of the trigeminal and dorsal root ganglia and spinal cord of the rat. Comp Biochem Physiol A Comp Physiol. 1989;93(1):183–193. doi: 10.1016/0300-9629(89)90206-5. [DOI] [PubMed] [Google Scholar]
- Kai-Kai MA, Howe R. Glutamate-immunoreactivity in the trigeminal and dorsal root ganglia, and intraspinal neurons and fibres in the dorsal horn of the rat. Histochem J. 1991;23(4):171–179. doi: 10.1007/BF01046588. [DOI] [PubMed] [Google Scholar]
- Kayser V, Bourgoin S, Melfort M, Hen R, Rayport S, Hamon MD, et al. Impaired nociceptive responses in mutant mice with low glutamatergic tone. Proc Soc Neurosci. 2008;38:856.14. [Google Scholar]
- Keast JR, Stephensen TM. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J Comp Neurol. 2000;424(4):577–587. [PubMed] [Google Scholar]
- Kerchner GA, Wilding TJ, Li P, Zhuo M, Huettner JE. Presynaptic kainate receptors regulate spinal sensory transmission. J Neurosci. 2001;21(1):59–66. doi: 10.1523/JNEUROSCI.21-01-00059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorova A, Richter J, Vasko MR, Strichartz G. Early and late contributions of glutamate and CGRP to mechanical sensitization by endothelin-1. J Pain. 2009;10(7):740–749. doi: 10.1016/j.jpain.2009.01.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkelin I, Brocker EB, Koltzenburg M, Carlton SM. Localization of ionotropic glutamate receptors in peripheral axons of human skin. Neurosci Lett. 2000;283(2):149–152. doi: 10.1016/s0304-3940(00)00944-7. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Shigemoto R, Ohishi H, van der Putten H, Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR7a and mGluR7b, in the central nervous system of the adult rat and mouse: a light and electron microscopic study. J Comp Neurol. 1998;393(3):332–352. [PubMed] [Google Scholar]
- Kovach JS, Eagan RT, Powis G, Rubin J, Creagan ET, Moertel CG. Phase I and pharmacokinetic studies of DON. Cancer Treat Rep. 1981;65(11–12):1031–1036. [PubMed] [Google Scholar]
- Kowalski MM, Cassidy M, Namboodiri MA, Neale JH. Cellular localization of N-acetylaspartylglutamate in amphibian retina and spinal sensory ganglia. Brain Res. 1987;406(1–2):397–401. doi: 10.1016/0006-8993(87)90814-6. [DOI] [PubMed] [Google Scholar]
- Kvamme E. Synthesis of glutamate and its regulation. Prog Brain Res. 1998;116:73–85. doi: 10.1016/s0079-6123(08)60431-8. [DOI] [PubMed] [Google Scholar]
- Kvamme E, Lenda K. Regulation of glutaminase by exogenous glutamate, ammonia and 2-oxoglutarate in synaptosomal enriched preparation from rat brain. Neurochem Res. 1982;7(6):667–678. doi: 10.1007/BF00965520. [DOI] [PubMed] [Google Scholar]
- Kvamme E, Olsen BE. Evidence for two species of mammalian phosphate-activated glutaminase having different regulatory properties. FEBS Lett. 1979;107(1):33–36. doi: 10.1016/0014-5793(79)80456-1. [DOI] [PubMed] [Google Scholar]
- Kvamme E, Torgner IA. Regulatory effects of fatty acyl-coenzyme A derivatives on phosphate-activated pig brain and kidney glutaminase in vitro. Biochem J. 1975;149(1):83–91. doi: 10.1042/bj1490083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvamme E, Svenneby G, Torgner IA. Calcium stimulation of glutamine hydrolysis in synaptosomes from rat brain. Neurochem Res. 1983;8(1):25–38. doi: 10.1007/BF00965651. [DOI] [PubMed] [Google Scholar]
- Lam DK, Sessle BJ, Cairns BE, Hu JW. Peripheral NMDA receptor modulation of jaw muscle electromyographic activity induced by capsaicin injection into the temporomandibular joint of rats. Brain Res. 2005;1046(1–2):68–76. doi: 10.1016/j.brainres.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Lam DK, Sessle BJ, Hu JW. Glutamate and capsaicin effects on trigeminal nociception I: activation and peripheral sensitization of deep craniofacial nociceptive afferents. Brain Res. 2009a;1251:130–139. doi: 10.1016/j.brainres.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DK, Sessle BJ, Hu JW. Glutamate and capsaicin effects on trigeminal nociception II: activation and central sensitization in brainstem neurons with deep craniofacial afferent input. Brain Res. 2009b;1253:48–59. doi: 10.1016/j.brainres.2008.11.056. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, et al. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4(4):382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Landry M, Bouali-Benazzouz R, El Mestikawy S, Ravassard P, Nagy F. Expression of vesicular glutamate transporters in rat lumbar spinal cord, with a note on dorsal root ganglia. J Comp Neurol. 2004;468(3):380–394. doi: 10.1002/cne.10988. [DOI] [PubMed] [Google Scholar]
- Lawand NB, Willis WD, Westlund KN. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur J Pharmacol. 1997;324(2–3):169–177. doi: 10.1016/s0014-2999(97)00072-1. [DOI] [PubMed] [Google Scholar]
- Lawand NB, McNearney T, Westlund KN. Amino acid release into the knee joint: key role in nociception and inflammation. Pain. 2000;86(1–2):69–74. doi: 10.1016/s0304-3959(99)00311-5. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Bardoni R, Tong CK, Engelman HS, Joseph DJ, Magherini PC, et al. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron. 2002;35(1):135–146. doi: 10.1016/s0896-6273(02)00729-8. [DOI] [PubMed] [Google Scholar]
- Leem JW, Hwang JH, Hwang SJ, Park H, Kim MK, Choi Y. The role of peripheral N-methyl-D-aspartate receptors in Freund’s complete adjuvant induced mechanical hyperalgesia in rats. Neurosci Lett. 2001;297(3):155–158. doi: 10.1016/s0304-3940(00)01662-1. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Cincotta M, Verberne AJ, Jarrott B, Lodge D, Beart PM. Receptor autoradiography with [3H]L-glutamate reveals the presence and axonal transport of glutamate receptors in vagal afferent neurones of the rat. Eur J Pharmacol. 1987;144(3):413–415. doi: 10.1016/0014-2999(87)90399-2. [DOI] [PubMed] [Google Scholar]
- Li JL, Ohishi H, Kaneko T, Shigemoto R, Neki A, Nakanishi S, et al. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR7, in ganglion neurons of the rat; with special reference to the presence in glutamatergic ganglion neurons. Neurosci Lett. 1996;204(1–2):9–12. doi: 10.1016/0304-3940(95)12299-0. [DOI] [PubMed] [Google Scholar]
- Lin YR, Chen HH, Lin YC, Ko CH, Chan MH. Antinociceptive actions of honokiol and magnolol on glutamatergic and inflammatory pain. J Biomed Sci. 2009;16:94. doi: 10.1186/1423-0127-16-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström E, Brusberg M, Hughes PA, Martin CM, Brierley SM, Phillis BD, et al. Involvement of metabotropic glutamate 5 receptor in visceral pain. Pain. 2008;137(2):295–305. doi: 10.1016/j.pain.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Sheng M, Jan LY, Jan YN, Basbaum AI. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc Natl Acad Sci USA. 1994;91(18):8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ, White TD, Sawynok J. Intraplantar injection of glutamate evokes peripheral adenosine release in the rat hind paw: involvement of peripheral ionotropic glutamate receptors and capsaicin-sensitive sensory afferents. J Neurochem. 2002;80(4):562–570. doi: 10.1046/j.0022-3042.2001.00721.x. [DOI] [PubMed] [Google Scholar]
- Lodge D. The history of the pharmacology and cloning of ionotropic glutamate receptors and the development of idiosyncratic nomenclature. Neuropharmacology. 2009;56(1):6–21. doi: 10.1016/j.neuropharm.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Luo R, Guo Y, Cao DY, Pickar JG, Li L, Wang J, et al. Local effects of octreotide on glutamate-evoked activation of Adelta and C afferent fibers in rat hairy skin. Brain Res. 2010;1322:50–58. doi: 10.1016/j.brainres.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Lynch M, Clark A, Sawynok J. A pilot study examining topical amitriptyline, ketamine, and a combination of both in the treatment of neuropathic pain. Clin J Pain. 2003;19(5):323–328. doi: 10.1097/00002508-200309000-00007. [DOI] [PubMed] [Google Scholar]
- Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical 2% amitriptyline and 1% ketamine in neuropathic pain syndromes: a randomized, double-blind, placebo-controlled trial. Anesthesiology. 2005a;103(1):140–146. doi: 10.1097/00000542-200507000-00021. [DOI] [PubMed] [Google Scholar]
- Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical amitriptyline and ketamine in neuropathic pain syndromes: an open-label study. J Pain. 2005b;6(10):644–649. doi: 10.1016/j.jpain.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pfluegers Arch. 2004;447(5):784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Hunt SP. Mechanisms that generate and maintain bone cancer pain. Novartis Found Symp. 2004;260:221–238. [PubMed] [Google Scholar]
- Marvizón JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, et al. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446(4):325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- Masson J, Darmon M, Conjard A, Chuhma N, Ropert N, Thoby-Brisson M, et al. Mice lacking brain/kidney phosphate-activated glutaminase have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci. 2006;26(17):4660–4671. doi: 10.1523/JNEUROSCI.4241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal DB, Yu MJ, Gorin PD, Johnson EM. Transported enzymes in sciatic nerve and sensory ganglia of rats exposed to maternal antibodies against nerve growth factor. J Neurochem. 1981;36(5):1847–1852. doi: 10.1111/j.1471-4159.1981.tb00439.x. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate–glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85(15):3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104(33):13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNearney T, Speegle D, Lawand N, Lisse J, Westlund KN. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J Rheumatol. 2000;27(3):739–745. [PMC free article] [PubMed] [Google Scholar]
- McNearney T, Baethge BA, Cao S, Alam R, Lisse JR, Westlund KN. Excitatory amino acids, TNF-alpha, and chemokine levels in synovial fluids of patients with active arthropathies. Clin Exp Immunol. 2004;137(3):621–627. doi: 10.1111/j.1365-2249.2004.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNearney TA, Ma Y, Chen Y, Taglialatela G, Yin H, Zhang WR, et al. A peripheral neuroimmune link: glutamate agonists upregulate NMDA NR1 receptor mRNA and protein, vimentin, TNF-alpha, and RANTES in cultured human synoviocytes. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R584–R598. doi: 10.1152/ajpregu.00452.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, et al. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120(7):1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Ennes HS, Marvizon JC, Fanselow MS, Mayer EA, Vissel B. Selective knockdown of NMDA receptors in primary afferent neurons decreases pain during phase 2 of the formalin test. Neuroscience. 2011;172:474–482. doi: 10.1016/j.neuroscience.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecs L, Tuboly G, Nagy E, Benedek G, Horvath G. The peripheral antinociceptive effects of endomorphin-1 and kynurenic acid in the rat inflamed joint model. Anesth Analg. 2009;109(4):1297–1304. doi: 10.1213/ane.0b013e3181b21c5e. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The inductionof pain: an integrative review. Prog Neurobiol. 1999;57(1):1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Miller KE, Kriebel RM. Intraepidermal Glutamate-Immunoreactive Nerve Fibers in Rat Skin Following Inflammation. IBRO abstract 2003 [Google Scholar]
- Miller KE, Douglas VD, Kaneko T. Glutaminase immunoreactive neurons in the rat dorsal root ganglion contain calcitonin gene-related peptide (CGRP) Neurosci Lett. 1993;160(1):113–116. doi: 10.1016/0304-3940(93)90926-c. [DOI] [PubMed] [Google Scholar]
- Miller KE, Akesson E, Seiger A. Nerve growth factor-induced stimulation of dorsal root ganglion/spinal cord co-grafts in oculo: enhanced survival and growth of CGRP-immunoreactive sensory neurons. Cell Tissue Res. 1999a;298(2):243–253. doi: 10.1007/s004419900097. [DOI] [PubMed] [Google Scholar]
- Miller KE, Kriebel RM, Chandler MJ, Ross CD, Foreman RD. Glutamate- and glutaminase-immunoreactive nerve fibers in rat skin following peripheral inflammation. Proc Soc Neurosci. 1999b;685 [Google Scholar]
- Miller KE, Richards BA, Kriebel RM. Glutamine-, glutamine synthetase-, glutamate dehydrogenase- and pyruvate carboxylase-immunoreactivities in the rat dorsal root ganglion and peripheral nerve. Brain Res. 2002;945(2):202–211. doi: 10.1016/s0006-8993(02)02802-0. [DOI] [PubMed] [Google Scholar]
- Miller KE, Kriebel RM, Edwards KM, Bartley E, Varoqui H, Erickson JD, et al. Localization of glutamine (SNAT1) and neutral amino acid transporters (ASCT1, ASCT2) in rat DRG and spinal dorsal horn. Proc Soc Neurosci. 2005:749.13. [Google Scholar]
- Miller KE, Edwards KE, Balbás J, Richards AB, Benton R, Schechter R, Kriebel RM. Glutaminase immunoreactivity and enzyme activity is increased in the rat dorsal root ganglion following inflammation. 2011 doi: 10.1155/2012/414697. Unpublished manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Reed S, Zhang Z, Schechter R. Increased cytosolic and mitochondrial glutaminase in rat DRG during peripheral inflammation. Proc Soc Neurosci. 2010a;40:78.11. [Google Scholar]
- Miller KE, Herzog B, Sutharshan M. 6-Diazo-5-Oxo-L-Norleucine: A Glutaminase Inhibitor with Local Analgesic Properties. IASP. 2010b:4193. [Google Scholar]
- Min SS, Han JS, Kim YI, Na HS, Yoon YW, Hong SK, et al. A novel method for convenient assessment of arthritic pain in voluntarily walking rats. Neurosci Lett. 2001;308(2):95–98. doi: 10.1016/s0304-3940(01)01983-8. [DOI] [PubMed] [Google Scholar]
- Minchin MC, Beart PM. Compartmentation of amino acid metabolism in the rat dorsal root ganglion; a metabolic and autoradiographic study. Brain Res. 1975;83(3):437–449. doi: 10.1016/0006-8993(75)90835-5. [DOI] [PubMed] [Google Scholar]
- Morris JL, Konig P, Shimizu T, Jobling P, Gibbins IL. Most peptide-containing sensory neurons lack proteins for exocytotic release and vesicular transport of glutamate. J Comp Neurol. 2005;483(1):1–16. doi: 10.1002/cne.20399. [DOI] [PubMed] [Google Scholar]
- Nordlind K, Johansson O, Lidén S, Hökfelt T. Glutamate- and aspartate-like immunoreactivities in human normal and inflamed skin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64(2):75–82. doi: 10.1007/BF02915098. [DOI] [PubMed] [Google Scholar]
- O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201(2):167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol. 1995a;360(4):555–570. doi: 10.1002/cne.903600402. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Nomura S, Ding YQ, Shigemoto R, Wada E, Kinoshita A, et al. Presynaptic localization of a metabotropic glutamate receptor, mGluR7, in the primary afferent neurons: an immunohistochemical study in the rat. Neurosci Lett. 1995b;202(1–2):85–88. doi: 10.1016/0304-3940(95)12207-9. [DOI] [PubMed] [Google Scholar]
- Okhotin VE, Kalinichenko SG, Pigolkin Yu I, Motavkin PA. Localization of aspartate aminotransferase in structures of a human sensory neuron. Neurosci Behav Physiol. 1993;23(4):364–370. doi: 10.1007/BF01183031. [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, et al. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50(2):117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res. 1998;787(1):161–164. doi: 10.1016/s0006-8993(97)01568-0. [DOI] [PubMed] [Google Scholar]
- Ory-Lavollee L, Blakely RD, Coyle JT. Neurochemical and immunocytochemical studies on the distribution of N-acetyl-aspartylglutamate and N-acetyl-aspartate in rat spinal cord and some peripheral nervous tissues. J Neurochem. 1987;48(3):895–899. doi: 10.1111/j.1471-4159.1987.tb05601.x. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Wu PH, Neuhoff V. Free amino acids and related compounds in the dorsal root ganglia and spinal cord of the rat as determined by the micro dansylation procedure. Brain Res. 1974;74(1):175–181. doi: 10.1016/0006-8993(74)90122-x. [DOI] [PubMed] [Google Scholar]
- Otahara N, Ikeda T, Sakoda S, Shiba R, Nishimori T. Involvement of NMDA receptors in Zif/268 expression in the trigeminal nucleus caudalis following formalin injection into the rat whisker pad. Brain Res Bull. 2003;62(1):63–70. doi: 10.1016/j.brainresbull.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Ozkan ED, Ueda T. Glutamate transport and storage in synaptic vesicles. Jpn J Pharmacol. 1998;77(1):1–10. doi: 10.1254/jjp.77.1. [DOI] [PubMed] [Google Scholar]
- Park YK, Galik J, Ryu PD, Randic M. Activation of presynaptic group I metabotropic glutamate receptors enhances glutamate release in the rat spinal cord substantia gelatinosa. Neurosci Lett. 2004;361(1–3):220–224. doi: 10.1016/j.neulet.2003.12.075. [DOI] [PubMed] [Google Scholar]
- Peana AT, De Montis MG, Sechi S, Sircana G, D’Aquila PS, Pippia P. Effects of (−)-linalool in the acute hyperalgesia induced by carrageenan, L-glutamate and prostaglandin E2. Eur J Pharmacol. 2004;497(3):279–284. doi: 10.1016/j.ejphar.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci. 1994a;14(10):6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol. 1994b;349(1):85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- Pook P, Brugger F, Hawkins NS, Clark KC, Watkins JC, Evans RH. A comparison of the actions of agonists and antagonists at non-NMDA receptors of C fibres and motoneurones of the immature rat spinal cord in vitro. Br J Pharmacol. 1993;108(1):179–184. doi: 10.1111/j.1476-5381.1993.tb13459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcellati G, Thompson RH. The effect of nerve section on the free amino acids of nervous tissue. J Neurochem. 1957;1(4):340–347. doi: 10.1111/j.1471-4159.1957.tb12091.x. [DOI] [PubMed] [Google Scholar]
- Pöyhiä R, Vainio A. Topically administered ketamine reduces capsaicin-evoked mechanical hyperalgesia. Clin J Pain. 2006;22(1):32–36. doi: 10.1097/01.ajp.0000149800.39240.95. [DOI] [PubMed] [Google Scholar]
- Qian J, Brown SD, Carlton SM. Systemic ketamine attenuates nociceptive behaviors in a rat model of peripheral neuropathy. Brain Res. 1996;715(1–2):51–62. doi: 10.1016/0006-8993(95)01452-7. [DOI] [PubMed] [Google Scholar]
- Quan D, Wellish M, Gilden D. Topical ketamine treatment of postherpetic neuralgia. Neurology. 2003;60(8):1391–1392. doi: 10.1212/01.wnl.0000055848.00032.39. [DOI] [PubMed] [Google Scholar]
- Quintao NL, da Silva GF, Antonialli CS, de Campos-Buzzi F, Correa R, Filho VC. N-antipyrine-3, 4-dichloromaleimide, an effective cyclic imide for the treatment of chronic pain: the role of the glutamatergic system. Anesth Analg. 2010;110(3):942–950. doi: 10.1213/ANE.0b013e3181cbd7f6. [DOI] [PubMed] [Google Scholar]
- Ro JY. Contribution of peripheral NMDA receptors in craniofacial muscle nociception and edema formation. Brain Res. 2003;979(1–2):78–84. doi: 10.1016/s0006-8993(03)02873-7. [DOI] [PubMed] [Google Scholar]
- Ro JY, Capra NF, Masri R. Contribution of peripheral N-methyl-D-aspartate receptors to c-fos expression in the trigeminal spinal nucleus following acute masseteric inflammation. Neuroscience. 2004;123(1):213–219. doi: 10.1016/s0306-4522(03)00465-2. [DOI] [PubMed] [Google Scholar]
- Ro JY, Capra NF, Lee JS, Masri R, Chun YH. Hypertonic saline-induced muscle nociception and c-fos activation are partially mediated by peripheral NMDA receptors. Eur J Pain. 2007;11(4):398–405. doi: 10.1016/j.ejpain.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Keen P. Effect of dorsal root section on amino acids of rat spinal cord. Brain Res. 1974a;74(2):333–337. doi: 10.1016/0006-8993(74)90587-3. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Keen P. High-affinity uptake system for glutamine in rat dorsal roots but not in nerve-endings. Brain Res. 1974b;67(2):252–257. doi: 10.1016/0006-8993(74)90288-1. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Keen P. (14C)glutamate uptake and compartmentation in glia of rat dorsal sensory ganglion. J Neurochem. 1974c;23(1):201–209. doi: 10.1111/j.1471-4159.1974.tb06935.x. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Keen P, Mitchell JF. The distribution and axonal transport of free amino acids and related compounds in the dorsal sensory neuron of the rat, as determined by the dansyl reaction. J Neurochem. 1973;21(1):199–209. doi: 10.1111/j.1471-4159.1973.tb04239.x. [DOI] [PubMed] [Google Scholar]
- Rosendal L, Larsson B, Kristiansen J, Peolsson M, Sogaard K, Kjaer M, et al. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain. 2004;112(3):324–334. doi: 10.1016/j.pain.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Roseth S, Fykse EM, Fonnum F. Uptake of L-glutamate into rat brain synaptic vesicles: effect of inhibitors that bind specifically to the glutamate transporter. J Neurochem. 1995;65(1):96–103. doi: 10.1046/j.1471-4159.1995.65010096.x. [DOI] [PubMed] [Google Scholar]
- Said SI. Glutamate toxicity in lung and airway disease. In: Gill S, Pulido O, editors. Glutamate receptors in peripheral tissue: excitatory transmission outside the CNS. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 191–196. [Google Scholar]
- Santini M, Berl S. Effects of reserpine and monoamine oxidase inhibition on the levels of amino acids in sensory ganglia, sympathetic ganglia and spinal cord. Brain Res. 1972;47(1):167–176. doi: 10.1016/0006-8993(72)90260-0. [DOI] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. NeuroReport. 1993;4(11):1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69(5):313–340. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Schizas N, Lian Ø, Frihagen F, Engebretsen L, Bahr R, Ackermann PW. Coexistence of up-regulated NMDA receptor 1 and glutamate on nerves, vessels and transformed tenocytes in tendinopathy. Scand J Med Sci Sports. 2010;20(2):208–215. doi: 10.1111/j.1600-0838.2009.00913.x. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Wolf G. Histochemical localization of aspartate aminotransferase activity in the hippocampal formation and in peripheral ganglia of the rat with special reference to the glutamate transmitter metabolism. J Hirnforsch. 1984;25(5):505–510. [PubMed] [Google Scholar]
- Schmidt W, Wolf G. Aspartate aminotransferase activity high affinity (3H) glutamate/(3H) aspartate uptake in rat nervous tissue in postnatal development. Acta Histochem Suppl. 1986;33:281–288. [PubMed] [Google Scholar]
- Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev. 2004;45(3):250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Simmons RM, Webster AA, Kalra AB, Iyengar S. Group II mGluR receptor agonists are effective in persistent and neuropathic pain models in rats. Pharmacol Biochem Behav. 2002;73(2):419–427. doi: 10.1016/s0091-3057(02)00849-3. [DOI] [PubMed] [Google Scholar]
- Skerry TM, Genever PG. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci. 2001;22(4):174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Westlund KN. Centrally administered non-NMDA but not NMDA receptor antagonists block peripheral knee joint inflammation. Pain. 1993;55(2):217–225. doi: 10.1016/0304-3959(93)90150-N. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Jordan HH, Westlund KN. Reduction in joint swelling and hyperalgesia following post-treatment with a non-NMDA glutamate receptor antagonist. Pain. 1994;59(1):95–100. doi: 10.1016/0304-3959(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Sommer EW, Kazimierczak J, Droz B. Neuronal subpopulations in the dorsal root ganglion of the mouse as characterized by combination of ultrastruc-tural and cytochemical features. Brain Res. 1985;346(2):310–326. doi: 10.1016/0006-8993(85)90865-0. [DOI] [PubMed] [Google Scholar]
- Spencer GJ, Hitchcock IS, Genever PG. Glutamate: teaching old bones new tricks — implications for skeletal biology. In: Gill S, Pulido O, editors. Glutamate receptors in peripheral tissue: excitatory transmission outside the CNS. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 197–209. [Google Scholar]
- Srinivasan B, Miller KE. Phosphate-activated glutaminase mRNA expression in rat spinal cord. Proc Soc Neurosci. 1992;18(1149) [Google Scholar]
- Srinivasan B, Miller KE. Enzymes associated with the glutamine cycle in the cat CNS: mRNA analysis. Proc Soc Neurosci. 1994;20:732. [Google Scholar]
- Srinivasan M, Kalousek F, Curthoys NP. In vitro characterization of the mitochondrial processing and the potential function of the 68-kDa subunit of renal glutaminase. J Biol Chem. 1995;270(3):1185–1190. doi: 10.1074/jbc.270.3.1185. [DOI] [PubMed] [Google Scholar]
- Sullivan MP, Nelson JA, Feldman S, Van Nguyen B. Pharmacokinetic and phase I study of intravenous DON (6-diazo-5-oxo-L-norleucine) in children. Cancer Chemother Pharmacol. 1988;21(1):78–84. doi: 10.1007/BF00262746. [DOI] [PubMed] [Google Scholar]
- Sutton JL, Maccecchini ML, Kajander KC. The kainate receptor antagonist 2S,4R-4-methylglutamate attenuates mechanical allodynia and thermal hyper-algesia in a rat model of nerve injury. Neuroscience. 1999;91(1):283–292. doi: 10.1016/s0306-4522(98)00621-6. [DOI] [PubMed] [Google Scholar]
- Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T, Arendt-Nielsen L, et al. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain. 2003;101(3):221–227. doi: 10.1016/S0304-3959(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Svensson P, Wang K, Arendt-Nielsen L, Cairns BE, Sessle BJ. Pain effects of glutamate injections into human jaw or neck muscles. J Orofac Pain. 2005;19(2):109–118. [PubMed] [Google Scholar]
- Svensson P, Wang K, Arendt-Nielsen L, Cairns BE. Effects of NGF-inducedmuscle sensitization on proprioception and nociception. Exp Brain Res. 2008;189(1):1–10. doi: 10.1007/s00221-008-1399-4. [DOI] [PubMed] [Google Scholar]
- Ta LE, Dionne RA, Fricton JR, Hodges JS, Kajander KC. SYM-2081 a kainate receptor antagonist reduces allodynia and hyperalgesia in a freeze injury model of neuropathic pain. Brain Res. 2000;858(1):106–120. doi: 10.1016/s0006-8993(99)02437-3. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Aoki Y, Ohtori S. Resolving discogenic pain. Eur Spine J. 2008;17(Suppl 4):428–431. doi: 10.1007/s00586-008-0752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci Res. 2006;55(4):343–351. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Tao F, Liaw WJ, Zhang B, Yaster M, Rothstein JD, Johns RA, et al. Evidence of neuronal excitatory amino acid carrier 1 expression in rat dorsal root ganglion neurons and their central terminals. Neuroscience. 2004;123(4):1045–1051. doi: 10.1016/j.neuroscience.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Tegeder L, Zimmermann J, Meller ST, Geisslinger G. Release of algesic substances in human experimental muscle pain. Inflamm Res. 2002;51(8):393–402. doi: 10.1007/pl00000320. [DOI] [PubMed] [Google Scholar]
- Tian YL, Guo Y, Cao DY, Zhang Q, Wang HS, Zhao Y. Local application of morphine suppresses glutamate-evoked activities of C and Adelta afferent fibers in rat hairy skin. Brain Res. 2005;1059(1):28–34. doi: 10.1016/j.brainres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Wu J. Glutamate receptors in the stomach and their implications. In: Gill S, Pulido O, editors. Glutamate receptors in peripheral tissue: excitatory transmission outside the CNS. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 179–190. [Google Scholar]
- Turner MS, Hamamoto DT, Hodges JS, Maccecchini ML, Simone DA. SYM 2081, an agonist that desensitizes kainate receptors, attenuates capsaicin and inflammatory hyperalgesia. Brain Res. 2003;973(2):252–264. doi: 10.1016/s0006-8993(03)02525-3. [DOI] [PubMed] [Google Scholar]
- Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447(5):480–489. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- Vikelis M, Mitsikostas DD. The role of glutamate and its receptors in migraine. CNS Neurol Disord Drug Targets. 2007;6(4):251–257. doi: 10.2174/187152707781387279. [DOI] [PubMed] [Google Scholar]
- Walker K, Reeve A, Bowes M, Winter J, Wotherspoon G, Davis A, et al. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology. 2001;40(1):10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]
- Wanaka A, Shiotani Y, Kiyama H, Matsuyama T, Kamada T, Shiosaka S, et al. Glutamate-like immunoreactive structures in primary sensory neurons in the rat detected by a specific antiserum against glutamate. Exp Brain Res. 1987;65(3):691–694. doi: 10.1007/BF00235995. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu RJ, Zhang RX, Qiao JT. Peripheral NMDA receptors contribute to activation of nociceptors: a c-fos expression study in rats. Neurosci Lett. 1997;221(2–3):101–104. doi: 10.1016/s0304-3940(96)13299-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Nie H, Zhang RX, Qiao JT. Peripheral nitric oxide contributes to both formalin- and NMDA-induced activation of nociceptors: an immunocyto-chemical study in rats. J Neurosci Res. 1999;57(6):824–829. [PubMed] [Google Scholar]
- Wang C, Wang Y, Zhao Z. Peripheral NMDA and non-NMDA receptors contribute to nociception: an electrophysiological study. Brain Res Bull. 2000;52(1):31–34. doi: 10.1016/s0361-9230(99)00277-4. [DOI] [PubMed] [Google Scholar]
- Wang K, Sessle BJ, Svensson P, Arendt-Nielsen L. Glutamate evoked neck and jaw muscle pain facilitate the human jaw stretch reflex. Clin Neurophysiol. 2004;115(6):1288–1295. doi: 10.1016/j.clinph.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Wang K, Svensson P, Sessle BJ, Cairns BE, Arendt-Nielsen L. Interactions of glutamate and capsaicin-evoked muscle pain on jaw motor functions of men. Clin Neurophysiol. 2010;121(6):950–956. doi: 10.1016/j.clinph.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Warncke T, Jørum E, Stubhaug A. Local treatment with the N-methyl-D-aspartate receptor antagonist ketamine, inhibit development of secondary hyperalgesia in man by a peripheral action. Neurosci Lett. 1997;227(1):1–4. doi: 10.1016/s0304-3940(97)00263-2. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Tsuboi Y, Sessle BJ, Iwata K, Hu JW. P2X and NMDA receptor involvement in temporomandibular joint-evoked reflex activity in rat jaw muscles. Brain Res. 2010;1346:83–91. doi: 10.1016/j.brainres.2010.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D, Hammerschlag R. Nerve impulse-enhanced release of amino acids from non-synaptic regions of peripheral and central nerve trunks of bullfrog. Brain Res. 1975;84(1):137–142. doi: 10.1016/0006-8993(75)90807-0. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Sun YC, Sluka KA, Dougherty PM, Sorkin LS, Willis WD. Neural changes in acute arthritis in monkeys. II. Increased glutamate immunoreactivity in the medial articular nerve. Brain Res Brain Res Rev. 1992;17(1):15–27. doi: 10.1016/0165-0173(92)90003-5. [DOI] [PubMed] [Google Scholar]
- Wheeler DD, Boyarsky LL. Influx of glutamic acid in peripheral nerve—characteristics in influx. J Neurochem. 1968;15(9):1019–1031. doi: 10.1111/j.1471-4159.1968.tb11645.x. [DOI] [PubMed] [Google Scholar]
- Wheeler DD, Boyarsky LL. Influx of glutamic acid in peripheral nerve. Energy, ionic, and PH dependence. J Neurobiol. 1971;2(2):181–190. doi: 10.1002/neu.480020210. [DOI] [PubMed] [Google Scholar]
- Wheeler DD, Boyarsky LL, Brooks WH. The release of amino acids from nerve during stimulation. J Cell Physiol. 1966;67(1):141–147. doi: 10.1002/jcp.1040670116. [DOI] [PubMed] [Google Scholar]
- Willis WD., Jr The somatosensory system, with emphasis on structures important for pain. Brain Res Rev. 2007;55(2):297–313. doi: 10.1016/j.brainresrev.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors—noxious stimulus detectors. Neuron. 2007;55(3):353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Raouf R, Lu WY, Wang LY, Orser BA, Dudek EM, et al. Regulation of N-methyl-D-aspartate receptor function by constitutively active protein kinase C. Mol Pharmacol. 1998;54(6):1055–1063. [PubMed] [Google Scholar]
- Yang D, Gereau RWt. Peripheral group II metabotropic glutamate receptors (mGluR2/3) regulate prostaglandin E2-mediated sensitization of capsa-icin responses and thermal nociception. J Neurosci. 2002;22(15):6388–6393. doi: 10.1523/JNEUROSCI.22-15-06388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You HJ, Chen J, Morch CD, Arendt-Nielsen L. Differential effect of peripheral glutamate (NMDA, non-NMDA) receptor antagonists on bee venom-induced spontaneous nociception and sensitization. Brain Res Bull. 2002;58(6):561–567. doi: 10.1016/s0361-9230(02)00806-7. [DOI] [PubMed] [Google Scholar]
- Yu XM, Sessle BJ, Haas DA, Izzo A, Vernon H, Hu JW. Involvement of NMDA receptor mechanisms in jaw electromyographic activity and plasma extravasation induced by inflammatory irritant application to temporomandibular joint region of rats. Pain. 1996;68(1):169–178. doi: 10.1016/S0304-3959(96)03181-8. [DOI] [PubMed] [Google Scholar]
- Zanchet EM, Cury Y. Peripheral tackykinin and excitatory amino acid receptors mediate hyperalgesia induced by Phoneutria nigriventer venom. Eur J Pharmacol. 2003;467(1–3):111–118. doi: 10.1016/s0014-2999(03)01604-2. [DOI] [PubMed] [Google Scholar]
- Zhang GH, Yoon YW, Lee KS, Min SS, Hong SK, Park JY, et al. The glutamatergic N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors in the joint contribute to the induction, but not maintenance, of arthritic pain in rats. Neurosci Lett. 2003;351(3):177–180. doi: 10.1016/j.neulet.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhao Y, Guo Y, Cao DY, Tian YL, Yao FR, et al. Electrophysiological evidence for the interaction of substance P and glutamate on Adelta and C afferent fibre activity in rat hairy skin. Clin Exp Pharmacol Physiol. 2006;33(12):1128–1133. doi: 10.1111/j.1440-1681.2006.04504.x. [DOI] [PubMed] [Google Scholar]
- Zhang GH, Min SS, Seol GH, Lee KS, Han H, Kim YI, et al. Inhibition of the N-methyl-D-aspartate receptor unmasks the antinociception of endogenous opioids in the periphery. Pain. 2009;143(3):233–237. doi: 10.1016/j.pain.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang T, Miller KE. Accumulation of glutaminase, VGLUT2, and substance P in sciatic nerve following adjuvant-induced arthritis in rat. Proc Soc Neurosci. 2010;40:586.10. [Google Scholar]
- Zhou S, Hammerland LG, Parks TN. Gamma-D-glutamylaminomethyl sulfonic acid (GAMS) distinguishes kainic acid- from AMPA-induced responses in Xenopus oocytes expressing chick brain glutamate receptors. Neuropharmacology. 1993;32(8):767–775. doi: 10.1016/0028-3908(93)90185-6. [DOI] [PubMed] [Google Scholar]
- Zhou S, Bonasera L, Carlton SM. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. NeuroReport. 1996;7(4):895–900. doi: 10.1097/00001756-199603220-00012. [DOI] [PubMed] [Google Scholar]
- Zhou S, Komak S, Du J, Carlton SM. Metabotropic glutamate 1alpha receptors on peripheral primary afferent fibers: their role in nociception. Brain Res. 2001;913(1):18–26. doi: 10.1016/s0006-8993(01)02747-0. [DOI] [PubMed] [Google Scholar]