Abstract
Many forms of psychopathology are tied to a heightened tendency to respond impulsively to strong emotions, and this tendency, in turn, is closely tied to problems with cognitive control. The goal of the present study was to test whether a two-week, six-session cognitive control training program is efficacious in reducing emotion-related impulsivity. Participants (N = 52) reporting elevated scores on an emotion-related impulsivity measure completed cognitive control training targeting working memory and response inhibition. A subset of participants were randomized to a waitlist control group. Impulsivity, emotion regulation, and performance on near and far-transfer cognitive tasks were assessed at baseline and after completion of training. Emotion-related impulsivity declined significantly from pre-training to post-training and at two- week follow-up; improvements were not observed in the waitlist control group. A decrease in brooding rumination and an increase in reappraisal were also observed. Participants showed significant improvements on trained versions of the working memory and inhibition tasks as well as improvements on an inhibition transfer task. In sum, these preliminary findings show that cognitive training appears to be well-tolerated for people with significant emotion-driven impulsivity. Results provide preliminary support for the efficacy of cognitive training interventions as a way to reduce emotion-related impulsivity.
Keywords: impulsivity, emotion, urgency, cognitive control, cognitive training
Impulsive responses to strong emotions are increasingly recognized as a common feature of many diverse forms of psychopathology (Johnson, Carver, & Joormann, 2013). The concept of a distinct emotion-related type of impulsivity began in large part with the publication of Whiteside and colleagues’ (2005) influential model of impulsivity, which differentiated Negative Urgency (the tendency to act impulsively in negative mood states) from other forms of impulsivity. This model has since been extended to include Positive Urgency, or tendencies toward impulsive reactions to positive mood (Cyders et al., 2007). More recently, researchers have suggested that Positive and Negative Urgency may not be truly distinct factors, but instead may be grouped together into a general feature of emotion-related impulsivity (Carver, Johnson, Joormann, Kim, & Nam, 2011).
Various indices of emotion-related impulsivity (ERI) are robustly correlated with a range of symptoms, problematic behaviors, and clinical diagnoses. A recent meta-analysis of more than 40,000 individuals found that compared to other aspects of impulsivity, Urgency was the strongest predictor of every psychopathology or symptom group studied, including anxiety, depression, eating disorders, aggression, borderline personality traits, suicidality and non-suicidal self-injury (Berg, Latzman, Bliwise, & Lilienfeld, 2015). ERI is also elevated in many psychiatric disorders, including bipolar disorder (Muhtadie, Johnson, Carver, Gotlib, & Ketter, 2014) and major depressive disorder in remission (Carver, Johnson, & Joormann, 2013), and several longitudinal studies show that ERI can predict the onset and course of psychopathologies (e.g., Riley, Combs, Jordan, & Smith, 2015; Smith, Guller, & Zapolski, 2013).
A growing body of theory and research has focused on mechanisms that might contribute to ERI. At a conceptual level, multiple theories state that impulsivity, including ERI, might be best understood within the context of two-mode models (Carver, Johnson, & Joormann, 2008). These models describe how the tendency to react impulsively during strong emotions is shaped by the relationship between a bottom-up, reflexive, system (such as automatically initiating responses without deliberation) and a top-down, reflective system (including cognitive control mechanisms). Consistent with two-mode models, empirical evidence indicates that deficits in top-down cognitive control overlap substantially with many behavioral conceptualizations of impulsivity (Sharma, Markon, & Clark, 2014). Cognitive control circuitry implicated in impulsivity is also involved in emotion regulation: for example, successful use of the emotion regulation strategy of reappraisal is linked to engagement of dorsolateral prefrontal cortex (dlPFC; Buhle et al., 2014), while weakened dlPFC activation is linked to impulsivity (Figner et al., 2010). In sum, converging evidence supports a two-mode view of impulsive behavior.
Drawing from this two-mode model, Carver and colleagues developed a factor-analytically derived composite measure of impulsivity, which included a factor specific to impulsive speech and behavior in response to emotion—“Feelings Trigger Action” (FTA). The FTA measure includes items from the Negative and Positive Urgency scales, as well as additional items measuring reflexive responses to emotions (Carver et al., 2011). FTA scores have been associated with many dimensions of psychopathology (Johnson et al., 2013) and diagnoses (Carver et al., 2013). Given its broad applicability to outcomes and its inclusion of items from two well-validated impulsivity scales, the FTA measure was used as the primary outcome variable in this study.
Impulsivity is Related to Cognitive Control Deficits
A growing body of empirical research suggests that different forms of impulsivity can be tied to deficits in cognitive control, including response inhibition (the ability to withhold or cancel a behavioral response; Bari & Robbins, 2013) and working memory (WM; the capacity to briefly store, update, and monitor information; Wesley & Bickel, 2014). Beyond impulsivity, response inhibition and WM deficits are found in many forms of psychopathology (Snyder, Miyake, & Hankin, 2015). Thus, cognitive control deficits in response inhibition and WM are two common factors linking psychopathology and impulsivity.
There is also strong evidence that these same cognitive control deficits are important components of ERI in particular. Both the Negative and Positive Urgency measures have been linked to poor performance on response inhibition tasks in multiple studies (Cyders & Coskunpinar, 2011; Johnson, Tharp, Peckham, Sanchez, & Carver, 2016). In contrast, direct evidence linking ERI to deficits in WM has been mixed. The lack of a clear relationship between these two constructs is surprising, given that WM capacity is thought to be an important component of the ability to use strategies such as reappraisal to regulate emotions (Schmeichel, Volokhov, & Demaree, 2008). Results of one recent study found evidence that weaknesses in WM may indirectly play a role in the expression of ERI, showing that Negative Urgency was related to inhibition deficits only in the context of low WM capacity (Gunn & Finn, 2015). However, WM weaknesses also directly correlated with negative urgency in this study. Taken together, these results indicate a role for both WM and response inhibition as potential mechanisms underlying ERI.
Given the extensive evidence for cognitive control deficits underlying impulsivity, we hypothesized that remediating cognitive deficits would yield changes in ERI. Although researchers have not tested the ability of cognitive training to shift ERI per se, several have considered effects of cognitive training on behaviors relevant to impulse control. Researchers have used modified response inhibition paradigms to train inhibition of disorder-specific cues, with results supporting the efficacy of these interventions in reducing drinking behavior (Houben, Nederkoorn, Wiers, & Jansen, 2011), and high-calorie food consumption (Houben & Jansen, 2011). A smaller number of studies have tested whether training basic inhibitory control, rather than inhibition to specific cues, can help reduce impulsivity-related behaviors. Some evidence supports this hypothesis, with effects of inhibition training on risky decision making on a gambling task (Verbruggen, Adams, & Chambers, 2012), reduced alcohol consumption (Jones et al., 2011), and more efficient emotion regulation at the neural level (Beauchamp, Kahn, & Berkman, 2016). Taken together, these findings suggest the merit of considering general response inhibition training for ERI.
In addition to response inhibition, three lines of work suggest the potential of WM training for reducing ERI. First, at the neural level of analysis, WM plays a role in inhibition itself: a meta-analysis of neuroimaging studies shows that WM resources support successful response inhibition when more complex inhibition tasks are used (Simmonds, Pekar, & Mostofsky, 2008). Similarly, another meta-analysis finds that complex versions of response inhibition tasks recruit many of the same neural regions as WM tasks, including dlPFC (Criaud & Bolinguez, 2013). Second, WM training alone has been found to reduce impulsive choice in adults with stimulant abuse disorders (Bickel, Yi, Landes, Hill, & Baxter, 2011) and to reduce alcohol consumption (Houben, Wiers, & Jansen, 2011). Third, several studies have used WM training to improve emotion regulation in mood disorders. In these studies, cognitive training included an adaptive Paced Auditory Serial Addition Task (PASAT), a computerized auditory WM task. Use of this task has been shown to enhance cognitive control via selective activation of dlPFC (Price, Paul, Schneider, & Siegle, 2013) and to reduce brooding rumination, a maladaptive emotion regulation strategy (Siegle, Ghinassi, & Thase, 2007; Siegle et al., 2014). These studies demonstrate that training basic cognition can enhance control over emotional responses, suggesting that similar training could be helpful for ERI.
In sum, evidence supports a role for response inhibition in influencing ERI, and a role for WM in either supporting inhibition or directly influencing impulsivity and related outcomes. Intriguingly, effects of WM training and inhibition training are relatively domain-specific, with evidence showing that inhibition training does not lead to improvements in WM, and vice versa (Maraver, Bajo, & Gomez-Ariza, 2016). Given these findings, one goal of this study was to conjointly train response inhibition and WM in order to maximize the effects of two hypothetical mechanisms of change.
Aims and Hypotheses
The goal of this study was to test whether a combined cognitive control training intervention comprising both response inhibition and WM could reduce ERI. We hypothesized that the intervention would reduce ERI and improve response inhibition and WM. We also hypothesized that training would lead to “near transfer” (changes in performance on non- adaptive versions of training tasks) and “far transfer” (changes in performance on unrelated WM and inhibition tasks). At a broader level, we hypothesized that training would reduce rumination, and would improve reappraisal, given evidence linking WM capacity with reappraisal ability (Schmeichel et al., 2008). Consistent with the RDoC initiative (Insel et al., 2010), these aims were evaluated in a heterogeneous sample of individuals with high scores on an ERI measure (without regard to specific clinical diagnoses). Symptoms of psychopathology commonly associated with ERI were assessed to characterize the sample.
Method
All procedures were approved by the University Institutional Review Board. Participants were recruited through online advertising and flyers distributed to support groups and clinics for specific populations known to have difficulties with ERI. Additional participants included undergraduate students who received extra credit for participation in the study. Potential participants were directed to a website to complete an initial online consent form and the Feelings Trigger Action (FTA) impulsivity scale (described below). Those who obtained an FTA score of 92 or higher (corresponding to an average response of 3.5 on a 5-point scale, one standard deviation above average scores in a validation sample [Carver et al., 2011]) were invited to complete a phone screen with a member of study staff. Exclusion criteria assessed during this call included age outside the study range (18-65); history of traumatic brain injury, brain tumor, or neurological disorders; acute suicidality, or psychotic symptoms. Participants who appeared eligible after the screening phone call were invited to attend an enrollment session.
At the enrollment session, written informed consent was obtained and participants completed the Wechsler Test of Adult Reading (WTAR) to verify an estimated IQ score equal to or greater than 70 (all participants met this criterion). Participants also completed a brief interview to assess history of mental health treatment and self-reported diagnoses. Eligible participants were then randomized to the waitlist or no-waitlist condition. For those not randomized to the waitlist, the enrollment session included all baseline measures described below. Those randomized to the waitlist completed a limited set of questionnaires at the enrollment session before returning to complete baseline measures two weeks later. All measures were administered via computer, with the exception of the Digit Span and WTAR. Table 1 lists all measures, reliability of self-report scales (Cronbach’s α), and their administration timeline.
Table 1.
Timeline of Study Measures.
| Measure | Timeline and Baseline Reliability | |||||
|---|---|---|---|---|---|---|
| Waitlist Measures | Enrollment/Baseline | Baseline α for self-rated scales | Training | Post-Training | 2-Week Follow-Up | |
| WTAR | x | |||||
| Feelings Trigger Action | x | .86 | x | x | ||
| PASAT | x | x | ||||
| Go/No-Go | x | x | ||||
| Adaptive PASAT | x | |||||
| Adaptive Go/No-Go | x | |||||
| Antisaccade | x | x | ||||
| Digit Span | x | x | ||||
| ERQ-Reappraisal | x | x | .90 | x | ||
| RRS-Brooding | x | .71 | x | x | ||
| MASQ-SF | x | x | .78 (Gen Distress Anxiety) .87 (Anx Arousal) .94 (Gen Distress Depression) .94 (Anhedonic Depression) |
x | ||
| BSL-23 | x | x | .90 | x | ||
| ABUSI | x | .94 | x | |||
Note: ABUSI=Alexian Brothers Urge to Self-Injure scale; BSL-23= Borderline Symptom Inventory; ERQ=Emotion Regulation Questionnaire; MASQ-SF = Mood and Anxiety Symptoms Questionnaire-Short Form; PASAT = Paced Auditory Serial Addition Task; RRS=Ruminative Responses Scale; WTAR = Wechsler Test of Adult Reading.
After completion of baseline measures, participants were scheduled for cognitive training. Training included six in-lab sessions, each including the adaptive PASAT WM task and adaptive Go/No-Go response inhibition task, in random order. Each training session lasted about 35 minutes. Sessions were scheduled based on participant availability, with the requirement that sessions were completed within two weeks. After the six training sessions, a post-training session was conducted that contained most of the same measures as the baseline session (see Table 1). Participants completed online questionnaires two weeks after the post-training session.
Measures
Wechsler Test of Adult Reading (WTAR; Wechsler, 2001)
The WTAR is a brief measure used to estimate intellectual functioning. Previous studies have found that the WTAR estimates of IQ are correlated between 0.73 and 0.75 with full-scale IQ scores (Wechsler, 2001).
Feelings Trigger Action scale (FTA; Carver et al., 2011)
The FTA scale was the primary screening measure for impulsivity and the chief outcome measure. The scale includes items rated from 1 to 5 including Positive Urgency (Cyders et al., 2007; 7 items), Negative Urgency (Whiteside et al., 2005; 12 items), and Reflexive Reactions to Feelings (Carver et al., 2011; 7 items). Analyses presented for the FTA are based on average response to the 26 items. As noted above, scores have been related to psychopathology and behavioral outcomes.
Ruminative Response Scale - Brooding subscale (RRS; Treynor, Gonzalez, & Nolen-Hoeksema, 2003)
The RRS Brooding Scale measures the tendency to repetitively think about negative affect in an unconstructive manner. This subscale has been identified as the RRS subscale of most relevance to psychopathology; in previous studies, the Brooding subscale has shown acceptable internal consistency (α= 0.77) and test-retest reliability (r = 0.62 over one year) (Treynor et al., 2003). Brooding was assessed at baseline, post-training, and follow-up.
Emotion Regulation Questionnaire Reappraisal Subscale (ERQ; Gross & John, 2003)
The ERQ Reappraisal scale consists of 6 items that have been widely used and well- validated. Initial validation of this scale showed good internal consistency (α = 0.79;) and test- retest reliability (r = 0.69 over three months; Gross & John, 2003). This scale was administered at baseline and at follow-up (rather than at the post-training session), to allow for sufficient time to test whether emotion regulation strategies changed following the full “dose” of the training intervention.
Borderline Symptom List-Short Form (BSL-23; Bohus et al., 2009)
The BSL-23 assesses current (past week) symptoms of borderline personality disorder (BPD), rated on a 0 (“Not At All”) to 4 (“Very Strong”) scale. It displays good psychometric properties (internal consistency: α = 0.94-0.97) and is sensitive to change in symptoms during treatment (Bohus et al., 2009). Symptoms of BPD have been strongly associated with Negative Urgency (Berg et al., 2015). As in some previous studies (e.g., Bohus et al., 2009), the BSL-23 was paired with a Visual Analogue Scale assessing global functioning on a scale of 0 to 100, as well as questions assessing the frequency of impulsive behaviors associated with BPD, including substance use and NSSI, rated on a 1 (“Not at All”) to 5 (“Daily or More Often”) scale for the past week.
Mood, Anxiety, and Stress Questionnaire-Short Form (MASQ-SF; Watson et al., 1995)
The MASQ-SF is a 62-item abbreviated version of the original MASQ, based on the tripartite model of depression and anxiety (Watson et al., 1995). This well-validated scale assesses current (past week) symptoms of depression and anxiety. It includes subscales of General Distress-Depression symptoms and General Distress-Anxiety symptoms, Anxious Arousal (e.g., physiological symptoms of anxiety), and Anhedonic Depression (e.g., lack of positive affect). Previous studies have linked ERI to anxiety and depression (Berg et al., 2015).
Alexian Brothers Urge to Self-Injure scale (ABUSI; Washburn, Juzwin, Styer, & Aldridge, 2010)
The ABUSI contains five items evaluating frequency and intensity of urges to engage in NSSI; initial validation work suggests that this scale has good reliability (α = .92 to 0.96) and validity, correlating with other established measures of suicidal ideation and self-harm (Washburn et al., 2010). ERI has been shown to predict the onset of NSSI (Riley et al., 2015).
Treatment expectancy and credibility
Participants completed items to assess beliefs about the rationality and perceived helpfulness of the cognitive training. Before the first training session, participants rated their expectations of cognitive training with three items on a 1 “(Not at All”) to 5 (“A lot”) scale, including the extent to which participants thought “these tasks will help to improve your self-control?,” “these tasks will help you to regulate emotions?,” and “How confident in your ability to do these tasks do you feel right now?”. After the training session, participants rated the perceived difficulty of tasks and the amount of effort they took to complete.
Measures of Near Transfer
Paced Auditory Serial Attention Task (PASAT; Gronwall, 1977; Siegle et al., 2007)
The PASAT, a WM task that has been validated in previous cognitive training studies, was used as a measure of near transfer of WM effects (Siegle et al., 2007; Siegle et al., 2014). Participants listened to numbers presented one at a time and were instructed to add each number to the previous number and then enter the correct answer by clicking the corresponding number. With each trial, participants added the most recent two numbers together. Numbers were presented every three seconds during one three-minute block. This version is non-adaptive, consistent with previous cognitive control studies that have used the PASAT as a measure of both training and near transfer (e.g., Hoorelbeke & Koster, 2017). Accuracy was evaluated based on the proportion of correct answers out of the total of 60 trials.
Go/No-Go task
The Go/No-go task is a commonly-used response inhibition task. On each trial, participants are instructed to press a button in response to a certain letter (the “Go” stimulus) and to withhold responses to another letter (the “No-go” stimulus). Because most trials are “go” trials, the task measures the tendency to withhold an automatic response. The Go/No- Go task was used as a measure of near transfer for response inhibition. The task was programmed in E-Prime (version 2.0) and included three blocks, each consisting of 70 “go” trials” and 30 “no-go” trials in random order. As in previous training studies, we used a “complex” Go/No-Go task that used varied letters as the “Go” and “No-Go” cues (T, X, O, E, I, S, A, M, and H), with the “No-Go” letter changing with each new block (similar to Chavan and colleagues (2015). Stimuli were presented for 200ms; on Go trials, responses were considered correct if participants responded within 1500. Feedback (“Right!” or “XXX”) was displayed for 1000ms following each trial. Each trial was separated with a fixation cross, with a duration jittered to randomly range from 500 to 1500ms in 100ms intervals. Accuracy was evaluated based on percentage of false alarms (“Go” responses to “No-Go” cues) and the reaction time to “Go” cues, consistent with other cognitive training research. Sensitivity (d’) and percentage of correct hits (correct “Go” responses) were also calculated. To calculate d’, the false alarm rate for no-go trials and hit rate for “go” trials were z-transformed, and the false alarm rate was subtracted from the hit rate (cf. Stanislaw & Todorov, 1999).
Measures of Far Transfer
Antisaccade task (Unsworth, Schrock, & Engle, 2004)
The antisaccade task, a well-validated measure of response inhibition and a correlate of positive urgency (Johnson et al., 2016), was included as a measure of “far transfer” of inhibition training effects. This version of the task consisted of a 10-trial prosaccade practice block and a 40-trial antisaccade block. On each antisaccade trial, a cue is flashed on one side of the screen for 100ms, and participants are instructed to look at the opposite side of the screen to identify a letter displayed for 100ms. Trials were separated by a fixation cross with a jittered ITI of 200 to 2200ms. Trials were excluded if reaction times were greater than three standard deviations from the mean, resulting in elimination of 1.45% of antisaccade trials pretraining. Remaining trials were again subject to the same procedure, resulting in removal of an additional 0.47% of trials. At post-training, 1.25% of trials were removed on the first pass of cleaning and 0.67% on the second pass. Five participants were excluded from analyses because they achieved less than 50% accuracy on the prosaccade block at pre or post-training.
Digits Forward and Digits Backwards Task (Wechsler et al., 2008)
The Digits Forward and Backwards subtests from the Wechsler Adult Intelligence Scale-4th Edition were administered as a measure of short-term memory and WM capacity. This task is conceptually similar to the PASAT in that it involves briefly storing verbally-presented numbers in short-term memory. Several previous studies using versions of the digit span have reported working memory transfer effects following training (Elgamal, McKinnon, Ramakrishnan, Joffe, & MacQueen, 2007; Klingberg et al., 2005); deficits in digit span performance are also frequently observed in many clinical syndromes associated with emotion-relevant impulsivity (e.g., Bourne et al., 2013; Snyder, 2013). This task was included as a measure of “far transfer” of WM training effects.
Tasks Administered During Training
Adaptive PASAT (Siegle et al., 2007)
The adaptive PASAT is identical to the baseline PASAT, with the exception being that in the adaptive version, the inter-stimulus interval increases or decreases across trials based on participant performance. The inter-stimulus interval (ISI) begins at 3000 msc and increases by 100 msc after four incorrect responses, or decreases by 100 msc after four correct responses. Training consisted of three 5-minute blocks, for a total of 15 minutes. The DV for the adaptive PASAT was the median ISI per training day.
Adaptive Go/No-Go
The adaptive Go/No-Go task is identical to the Go/No-Go task given at baseline, with the exception that the response time window for Go responses varied based on performance, with an increasing response “deadline” for incorrect trials and decreasing deadline for correct trials (Benikos et al., 2013a; 2013b). The goal of these adaptations is to keep task difficulty at a moderate level, considered optimal for training (Benikos et al., 2013b). Responses to Go trials were initially marked as accurate if the participant responded within 300ms of stimulus onset. After each correct “go” response, the deadline for accurate responses was decreased by 25ms; following an incorrect Go response (no response, or a response outside of the deadline), the deadline was increased by 25ms. The minimum deadline possible was 50ms and the maximum was 1000ms. At each training session, participants completed three 5-minute blocks, with a different No-Go stimulus for each block. At the end of each block, a feedback screen showed the false alarm rate for that block. The dependent variable for the Go/No-Go task was the average false alarm rate per training day over all three blocks.
Analysis Plan
Before conducting analyses, all study variables were graphed and checked for normality. To test relationships between variables at baseline and across training, chi-square tests and Pearson correlations were used for dichotomous and continuous variables, respectively. Repeated measures ANOVA were used to test change in FTA scores during the waitlist, performance on adaptive training tasks during training, and change in performance on the non-adaptive versions of the training task; where sphericity was violated in these analyses, Greenhouse-Geisser corrected statistics are presented. Paired t-tests were used to test within-subjects change in other study variables from pre to post-training, with Cohen’s dz used to index effect size.
Results
The FTA scale was administered to 926 adults; of those who completed the survey, 221 were potentially eligible on the basis of their high score on the FTA measure, and 110 of these provided contact information and were screened by phone (see Figure 1). 63 were found to be eligible during the brief phone screening and were scheduled for randomization into the main study. Eleven of these participants did not attend their enrollment session, resulting in a final sample of 52 participants. Table 2 shows relevant demographic and clinical variables for the 52 participants, as well as the subsample of 39 individuals who completed the study.
Figure 1. CONSORT Diagram of Study Enrollment.
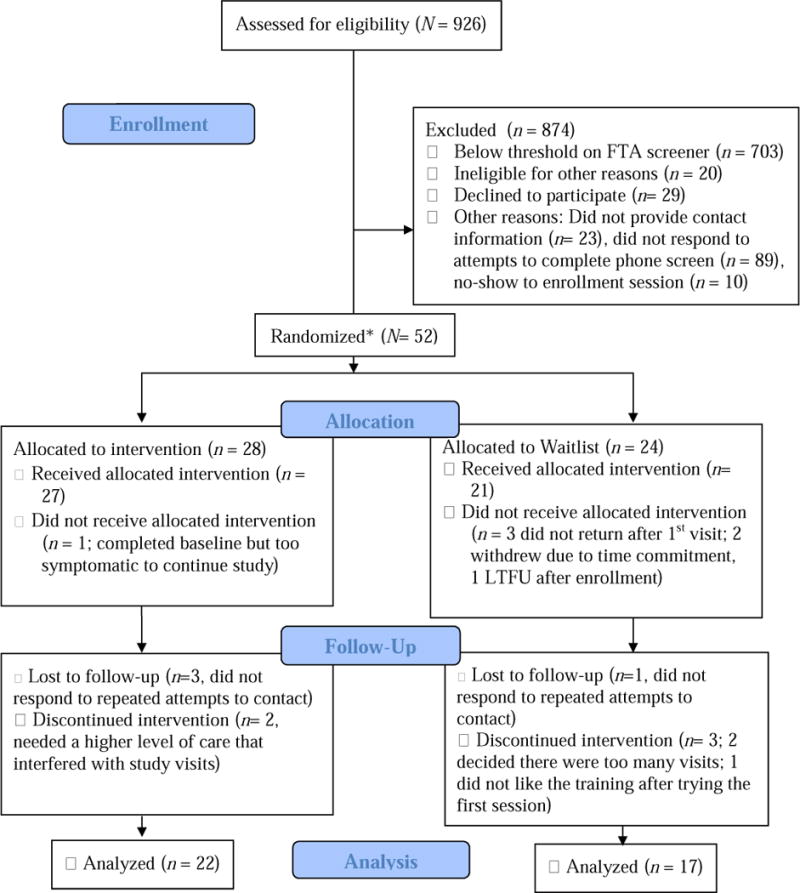
Note. *The first 10 participants were automatically enrolled in the pilot phase; the remainder were randomly assigned. “Received Intervention” indicates that participant completed at least one full training session. Not all participants completed the target dose of 6 sessions (see Results).
Table 2.
Baseline Sample Characteristics: Demographic and Clinical Variables.
| Full Sample N = 52 Mean (SD) or % |
Study Completers n = 39 Mean (SD) or % |
|
|---|---|---|
| Age | 32.17 (14.67) | 31.97 (14.82) |
| Years of education | 15.27 (1.44) | 15.46 (1.34) |
| Percent female | 67.3 | 69.2 |
| Race | ||
| Caucasian | 57.7 | 64.1 |
| African American | 1.9 | 0 |
| Asian American/Pacific Islander | 21.1 | 20.5 |
| More than one race | 19.2 | 15.4 |
| Percent Hispanic/Latino/a | 11.5 | 12.8 |
| Current undergraduate student | 36.5 | 41 |
| Full-time work or full-time student | 73.1 | 74.4 |
| Visited mental health professional past month | 50 | 48.7 |
| Taking psychiatric medication(s) | 40.4 | 35.9 |
| History of mental health treatment | 82.7 | 79.5 |
| History of psychiatric hospitalization | 25 | 17.9 |
| Ever dropped out of school/dropped class due to symptoms | 44.2 | 41 |
| Ever left a job due to symptoms | 29.4 | 26.3 |
| WTAR Standard Score | 111.35 (12.04) | 112.77 (11.94) |
| Treatment Expectancy #1: Self-Control | 3.09 (.80) | 3.08 (0.78) |
| Treatment Expectancy #2: Emo. Regulation | 2.89 (.95) | 2.95 (0.9) |
| Borderline Symptom List-23 (BSL-23) | .50 (.46) | .40 (.40) |
| BSL-23 Overall Functioning | 61.13 (23.36) | 64.54 (23.99) |
| BSL % NSSI Past Week | .04 | .06 |
| BSL % Used Drugs in Past Week | 34.8 | 32.4 |
| MASQ General Distress-Depression | 28.89 (11.17) | 27.03 (10.94) |
| MASQ General Distress-Anxiety | 24.38 (7.02) | 23.35 (6.89) |
| MASQ Anhedonic Depression | 63.43 (16.95) | 61.19 (16.77) |
| MASQ Anxious Arousal | 25.92 (9.27) | 24.17 (8.22) |
| ABUSI | 2.34 (3.91) | 2.03 (3.50) |
Note: WTAR = Wechsler Test of Adult Reading; MASQ= Mood and Anxiety Symptom Questionnaire; ABUSI=Alexian Brothers Urge to Self-Injure Scale. For full sample, n = 47 for Treatment Expectancy #1, BSL-23, STAB, MASQ,. & ABUSI; n = 46 for Treatment Expectancy #2 and BSL-Overall Functioning. For Completers, n = 37 for ABUSI, BSL, and MASQ.
Baseline values for key study variables are presented in Table 3. FTA scores were significantly correlated with brooding rumination scores at baseline, r = .52, p < .01. FTA scores were not significantly correlated with any other baseline variables. By design, FTA scores were constricted to the upper range of this scale; therefore, correlations comparing the FTA to other measures should be interpreted cautiously given the limited strength of these analyses. FTA scores did not differ between men and women, t(44) = 1.62, p = .11, nor between those taking psychiatric medication vs. those who were not, t(44) = 1.66, p = .11.
Table 3.
Change in Impulsivity, Cognitive Control, and Symptoms (n = 39).
| Measure | Pre-Training Mean (SD) or % | Post-Training Mean (SD) or % | t | p | Cohen’s dz |
|---|---|---|---|---|---|
| Feelings Trigger Action | 3.95 (.46) | 3.61 (.58) | 4.04 | < .001 | .66 |
| PASAT % Accurate Responses |
57.37 (22.14) | 87.31 (15.45) | −10.22 | < .001 | 1.66 |
| Go/No-Go % False Alarms |
12.23 (10.63) | 17.69 (20.37) | −2.03 | .05 | .33 |
| Digit Span Forward | 6.9 (1.12) | 7.1 (1.07) | −1.28 | .21 | .20 |
| Digit Span Backward | 5.10 (1.19) | 5.31 (1.28) | −1.09 | .28 | .17 |
| Antisaccade % Errors | 35.26 (15.63) | 29.57 (14.91) | 2.31 | .03 | .37 |
| RRS-Brooding | 2.81 (.66) | 2.49 (.66) | 2.97 | <.01 | .49 |
| ERQ-Reappraisal* | 4.07 (1.25) | 4.72 (.93) | −2.96 | <.01 | .52 |
Note: n = 37 for FTA and Brooding; n = 33 for Reappraisal; n = 29 for Antisaccade. PASAT = Paced Auditory Serial Addition Task (non-adaptive), RRS = Ruminative Responses Scale, ERQ = Emotion Regulation Questionnaire.
collected at two-week follow-up.
Most participants reported a history of mental health treatment, and more than 40% reported a history of work or school-related disability related to mental illness. Undergraduate students (n = 19) did not differ from community participants (n = 33) on any measure, including MASQ scales (ps > .24); the BSL behavioral supplement (ps > .16); self-injury urges, t(45) = - .37, p = .71; substance use, ps > .60, nor baseline FTA scores, t(44) = 1.63, p = .11. As Table 2 shows, rates of current symptoms on any specific scale were low; given the restricted range, further analyses of symptoms are not reported.
As shown in Figure 1, some (25%) participants dropped out of the study, resulting in 39 completers. Participants who completed the study attended an average of 5.49 days of training (SD = 0.92 days). 92.3% of participants completed the baseline measures and at least one cognitive training session, allowing for an analysis of factors influencing dropout. Non- completers did not differ from completers on demographic variables (ps > .10), FTA score (p = .88), use of psychiatric medications (p = .25), performance on cognitive tasks (ps > .14), substance use (ps > .23), or Anhedonic Depression scores, p = .08. Non-completers, however, were more likely to endorse a history of psychiatric hospitalizations (χ2(1) = 4.14, p = .04), and they obtained higher scores on MASQ scales of General Distress-Depression, t(45) = 2.31, p = .03, Anxious Arousal, t(45) = 2.65, p = .01, and General Distress-Anxiety, t(45) = 2.00, p = .05); the BSL-23, t(44) = 3.20, p = .003, and reported lower quality of life, t(20.54) = 2.82, p = .01.
Pilot Phase and Waitlist Analyses
To pilot test treatment feasibility and acceptability, the first ten participants were automatically enrolled in the non-waitlist condition. Seven of these ten participants successfully completed the study; one did not attend their enrollment session. One dropped out after three training sessions but returned to complete the post-treatment questionnaires, and one was lost to follow-up after two training sessions. Based on the treatment expectancy and credibility measure, participants (n = 9) found the training to be moderately challenging (M = 3.33, SD = 0.50), used a high amount of effort to complete the tasks (M = 4.44, SD = 0.73), and felt relatively confident in their ability to complete the training tasks (M = 3.44, SD = 1.13). Based on this feedback, the pilot phase was concluded and all following participants were randomly assigned.
Twenty-eight participants were enrolled to the non-waitlist condition and 24 were enrolled to the waitlist. Individuals on the waitlist began the active training phase an average of 36.5 days (SD = 24.25) after they were screened with the FTA measure as compared to a 24.25- day (SD = 21.06) average time in the non-waitlist condition. Thus, individuals allocated to the waitlist had an average additional wait time of 12.32 days, in comparison to the average 14.63 (SD = 2.3) days that non-waitlist participants spent on the training tasks. To evaluate whether ERI changed during the waitlist period in comparison to those who received the cognitive training program, a 2 (Time: pre/post waitlist as compared to pre/post training for the non-waitlist) × 2 (Condition: waitlist, training) repeated measures ANOVA was conducted with FTA score as the DV (Figure 2). In this analysis, the “pre” timepoint was the FTA screening score. Results showed a significant main effect of Time, F(1, 39) = 23.79, p < .001, partial η2= .38, qualified by a Time × Condition interaction, F(1, 39) = 11.74, p = .001, partial η2= .23. Follow up dependent t-tests showed that FTA scores did not significantly change during the waitlist period, t(18) = 1.16, p = .26, but scores declined significantly from screening to post-training in the training group, t(22) = 5.50, p < .001. Given that the waitlist period was not associated with significant changes in ERI, individuals on the waitlist were combined with the remainder of the sample for all analyses of pre- and post-training measures.
Figure 2. Change in Feelings Trigger Action scores in the Waitlist and Intervention Conditions.
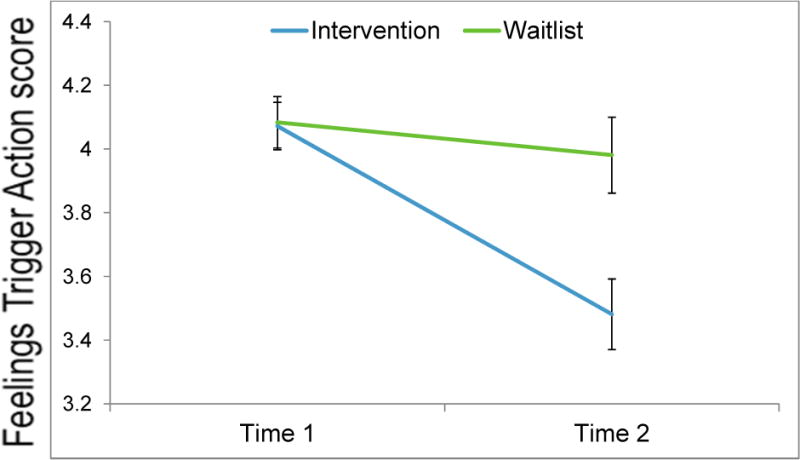
Note. Time 1: Screening; Time 2: Post-Waitlist (waitlist), Post-Training (Intervention). n = 19 (waitlist), n =22 (intervention). Error bars = +/- 1 SE.
Analyses of Hypotheses
Change in performance on adaptive training tasks
Repeated-measures ANOVAs with training day as the repeated measure were computed for the adaptive PASAT and Go/No-Go tasks, to test whether performance improved on the performance-adaptive training tasks. For these analyses, the first five days of training were used (n = 32), as only 25 participants completed six training sessions (results were parallel when analyses were restricted to participants who completed all days). Accuracy on the adaptive PASAT significantly improved during training, F(1.94, 64.13) = 51.03, p < .001, partial η2= 0.61. Similarly, false alarm rate on the adaptive Go/No-Go decreased significantly across training, F(2.29, 73.23) = 5.30, p < .01, partial η2 = 0.14.
Change in emotion-related impulsivity
As shown in Table 3, a paired t-test of FTA scores showed a significant decrease from baseline to post-training. To test the hypothesis that decreases in impulsivity would persist after the training period had ended, an ANOVA including the 30 individuals who completed the FTA at baseline, post-training and follow-up showed a significant main effect of time, F(2, 58) = 13.88, p < .001, partial η2= 0.32; post-hoc contrasts showed that FTA scores significantly decreased from post-training to the follow-up in this group, F(1, 29) = 4.14, p = .05.
Pearson correlations were used to test whether treatment expectancy or change in performance on cognitive control tasks were related to the observed change in FTA scores (post- pre) during training. Change in FTA score was not significantly correlated with change in non- adaptive PASAT accuracy, r(36) = −.06, p = .73, nor to change in non-adaptive Go/No-Go accuracy, r(36) = −.13, p = .46. Greater belief that the training would improve self-control (r = - .30, p = .08) or emotion regulation (r = −.27, p = .11) was not significantly correlated with a larger decrease in FTA scores.
Change in performance on near transfer tasks
A 2 (Time: pre, post) by 2 (Task: non-adaptive PASAT, non-adaptive Go/No-Go) ANOVA with task accuracy as the dependent variable was used to test whether performance improved on non-adaptive versions of the two cognitive training tests from pre- to post- intervention. Results showed a significant main effect of Time, F(1, 38) = 43.14, p < .001, partial η2= 0.53; a significant main effect of Task, F(1, 38) = 16.95, p < .001, partial η2 = 0.31; and a significant Time × Task interaction, F(1, 38) = 70.6, p < .001, partial η2= 0.65. As shown in Table 3, post-hoc paired t-tests showed that performance on the non-adaptive PASAT significantly improved, while accuracy on the non-adaptive Go/No-Go actually declined slightly (an increased false alarm rate)., Other Go/No-Go performance indices revealed no evidence of proximal transfer effects: average reaction times to Go trials decreased significantly, t(38) = 6.85, p < .001, Cohen’s dz = 1.10, butthere were no significant training-related changes in hit rate (p = .51) nor changes in sensitivity (d’, p = 0.99).
Change in performance on far transfer tasks
Paired t-tests showed that participants did not show significant improvement on WM performance (longest digit span recalled) from pre- to post-training of the Digit Span task (see Table 3). Improvements on the PASAT were not significantly correlated with improvements on the Forward Digit Span, r(38) = −.07, p = .68 or the Reverse Digit Span, r(38) = .21, p = .21. However, as Table 3 shows, participants did show improved accuracy on the antisaccade task, an index of transfer of response inhibition, from pre- to post-training. Improvements on the antisaccade task were not significantly correlated with improvements on the Go/NoGo task, r(28) = .20, p = .31.
Change in emotion regulation
As hypothesized, brooding rumination decreased significantly from baseline to post-training (Table 3) and continued to decrease from post- training to the follow-up, dependent t(30) = 2.24, p = .03, Cohen’s dz = .40. Lower brooding scores at post-training were marginally but not significantly correlated with improvements on the PASAT, r = −.27, p = .09 and were not correlated with changes in Go/No-Go accuracy, r = −.22, p = .18. Reappraisal significantly increased from pre-training to follow-up (Table 3). Follow-up reappraisal scores were not significantly correlated with improvements on the PASAT, r = .07, p = .71, nor on the Go/NoGo, r = −.02, p = .92.
Discussion
Emotion-related impulsivity (ERI) is robustly tied to many forms of psychopathology and to other behavioral problems such as aggression and substance use. To the best of our knowledge, this study provides the first test of a cognitive training intervention as a way to target ERI. Results provided initial evidence that cognitive control training consisting of WM and response inhibition training was feasible and efficacious in reducing ERI, and that the training was associated with improvements in emotion regulation. This preliminary study represents the first step towards developing an intervention for ERI that could eventually be implemented in samples with greater clinical severity.
In terms of feasibility, we found that highly impulsive people would take part in a frequent and relatively demanding in-person cognitive training program involving six sessions over two weeks. Most participants completed at least four sessions and many completed all six sessions, showing that this intensive program was well-tolerated. Of concern, however, people who endorsed more symptoms of depression, anxiety, and borderline personality traits were less likely to complete the intervention, even though other symptom and cognitive factors did not predict attrition. Integration of training with motivational support may be necessary to prevent attrition for those with high levels of these symptoms.
Beyond feasibility, participants who completed the intervention reported a significant decrease in ERI. In contrast, ERI scores did not significantly decline during the waitlist period, which suggests that some component of the cognitive training intervention influenced the observed decrease in impulsivity. The absence of an active control condition limits our ability to decipher which aspect of training might have contributed to the decrease in ERI. The possibility that demand characteristics influenced outcome cannot be excluded, given that participants were recruited on the basis of self-rated problems in ERI and were aware of the study’s focus on impulsivity. Belief that training would improve self-control was marginally but not significantly correlated with change in impulsivity, suggesting that attitudes about the intervention could be responsible for some degree of change. These findings are consistent with other studies showing a relationship between motivation and training outcome (e.g., Maraver et al., 2016).
Although our sample was recruited from the community, most participants reported having received a mental health diagnosis, and more than 40% reported that their symptoms had led to a school or work-related disability. Nonetheless, symptom rates for any given condition were low, and it will be important to understand how findings can be applied in clinical settings. Beyond the present study, experimental tests of novel interventions to reduce ERI are ongoing (for example, Weiss et al., 2015), but there are no targeted treatments for ERI currently available to patients. Psychosocial treatments provide strategies for managing impulsive responses to emotion, including components of Dialectical Behavior Therapy. The present findings suggest that targeted cognitive training could be a feasible component of interventions. Future studies could test cognitive training alongside more established psychosocial interventions for managing emotions.
In addition to changes in ERI, participants who completed the training demonstrated significant improvements in two domains of emotion regulation: a decrease in brooding rumination and an increase in reappraisal. The decrease in rumination replicates findings that have shown similar effects of PASAT training in clinically depressed and non-clinical samples (Hoorelbeke, Koster, Demeyer, Loeys, & Vanderhasselt, 2016; Hoorelbeke & Koster, 2017; Siegle et al., 2007; 2014). The present study extends these findings to individuals with high ERI.
Participants also reported a significant increase in use of reappraisal, an emotion regulation strategy that is robustly associated with clinical and health benefits (e.g., Gross & John, 2003). Of note, one recent cognitive training study found no evidence of change in reappraisal following training on the PASAT alone (Hoorelbeke et al., 2016). Taken together with the present finding, it is possible that the additional training in response inhibition provided in the current study is important to increased use of reappraisal. Inhibition has been identified as an important mechanism underlying cognitive reappraisal (Ochsner et al., 2004); moreover, there is evidence that inhibition training leads to more efficient neural activation during emotion regulation tasks (Beauchamp et al., 2016). More studies are needed to examine how inhibition and WM training might separately or jointly contribute to reappraisal improvement. As the current study assessed reappraisal at follow-up but not at the end of the training protocol, another unanswered question is the rate at which reappraisal changes in the context of cognitive training. Future studies could include more frequent assessment of emotion regulation to parse this process.
Another key goal of the study was to examine potential cognitive mechanisms underlying change in ERI. Participants who completed the training demonstrated significant improvements on the training tasks, and a significant and large improvement in WM performance on a near- transfer task (the non-adaptive PASAT), consistent with prior findings. Nonetheless, the intervention was not associated with significantly improved WM performance on a far transfer task (Digit Span). This could reflect a ceiling effect on the 8-digit Digit Span task, as participants at baseline remembered a mean of nearly seven digits (Forward span) and more than 5 digits (Backwards). Alternatively, it is possible that participants improved on the PASAT due to factors other than WM. In particular, the PASAT is a more stressful task than the digit span task, and has even been used to induce acute stress (Brown et al., 2002)—thus PASAT improvements may reflect an improved ability to complete a stressful task rather than WM improvement. Future studies would do well to include a WM task that more closely mirrors the type of memory training practiced in the PASAT, such as an N-back or similar task, to assess WM transfer effects. In sum, people who struggle with ERI improved their ability to perform a demanding cognitive task that taps WM resources, but it is unclear whether this training generalizes to broader gains in WM.
On the Go/No-Go measure of response inhibition, participants showed an unexpected increase in inhibition failures (false alarms), despite showing a decrease in false alarms on the adaptive version used during training. It is possible that participants’ increased false alarm rate on the Go/No-Go task was affected by fatigue, as this task was the last to be administered during the post-training session. Alternatively, the increased error rate could reflect difficulty habituating to a non-performance adaptive version of this task after two weeks of training with performance-adaptive measures that calibrated response time-deadlines to participants’ performance. Regarding far transfer of response inhibition, there was clear evidence of improved inhibition on the antisaccade task. Although one might wonder if this reflected practice effects, performance on the antisaccade task has been shown to be relatively stable over time (Klein & Fischer, 2005). In sum, findings regarding response inhibition indicated that despite a lack of gains on the nonadaptive Go/No-Go task, highly impulsive individuals improved their ability to inhibit responses on both an adaptive training task and on a conceptually similar transfer task.
Despite improvements on measures of impulsivity, emotion regulation, and cognitive control, several limitations are apparent. One concern is our limited evidence that the improvements in cognitive control explain the observed declines in impulsivity. That is, because we relied on a waitlist control rather than a control that involved sham training, expectancies and other variables could help explain the shift in impulsivity. Indeed, shifts in cognitive control did not robustly predict declines in impulsivity, and we were underpowered to identify mediators of change through multivariate models or to compare the wait list control to the active treatment. Testing mechanisms of change was also thwarted by the relatively restricted range of initial FTA scores. Another limitation was the lack of ability to consider psychopathology outcomes. Previous studies of cognitive control training have successfully integrated training programs into clinical settings (e.g., Siegle et al., 2014), so one future goal is to test the efficacy of training programs for emotion-relevant impulsivity in treatment-seeking samples. Studies conducted in clinical settings could also assess the degree to which exposure to specific treatment modalities, such as cognitive therapy, may moderate the effects of cognitive training on ERI and emotion regulation outcomes. Finally, for these future studies, researchers could consider using different stimuli for assessments as compared to training, as using the same stimuli at training and assessment sessions could have inadvertently resulted in additional training in the present study.
Notwithstanding limitations, the positive findings here warrant future investigation. In such work, a goal would be to more directly disentangle how working memory training and inhibition training independently impact impulsivity (compared to a combined approach), whether working memory gains can moderate the effects of inhibition training, and how behavioral shifts correspond to changes in putative neural mechanisms of impulsivity and cognitive control. Each of these future goals could benefit from the inclusion of a more active control condition, such as sham training. Future studies could also consider testing alternative mechanisms, within the domain of cognitive control or more broadly, that might support observed change in impulsivity in the training paradigm. An untested hypothesis is that practicing response inhibition and WM strategies allowed some participants to better control their responses to emotion in daily life and that this improved control over emotional reactivity is the active ingredient driving change in emotion-relevant impulsivity. This is a hypothesis for future studies as we did not test cognitive control during heightened emotional arousal, either in the lab or daily life, as part of this study. Another question for future study is to evaluate whether the gains in this study can be replicated in real-life environments. Beyond delivery within a clinical setting, many participants asked whether they could take the intervention home at the end of the study. Thus, studies could test if cognitive training for impulsivity could be integrated into internet-delivered interventions.
In sum, these findings suggest that cognitive control training may be efficacious in reducing ERI. ERI was reduced after a brief cognitive training intervention aimed at enhancing cognitive control, and people who struggle with ERI showed improvements in two aspects of emotion regulation after just two weeks of practice with a cognitive training program. Future research is needed to better understand the mechanisms and potential clinical utility of cognitive training for impulsivity.
Highlights.
People with high emotion-related impulsivity completed cognitive training.
Emotion-related impulsivity significantly decreased during cognitive training.
Impulsivity did not significantly change in a waitlist control condition.
Transfer effects were observed on a response inhibition task.
Transfer effects were not observed on a working memory task.
Acknowledgments
We wish to acknowledge Kiana Modavi, James Madole, and Jordan Tharp for their assistance with data collection.
This work was supported by a National Institute of Mental Health Institutional Training Grant Fellowship awarded to Andrew Peckham (T32-MH089919) and by a Dissertation Research Award from the Society for a Science of Clinical Psychology awarded to Andrew Peckham.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- Bari A, Robbins TW. Inhibition and impulsivity: Behavioral and neural basis of response control. Progress in Neurobiology. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Beauchamp KG, Kahn LE, Berkman ET. Does inhibitory control training transfer?: Behavioral and neural effects on an untrained emotion regulation task. Social Cognitive and Affective Neuroscience. 2016;11:1374–1382. doi: 10.1093/scan/nsw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benikos N, Johnstone SJ, Roodenrys SJ. Short-term training in the Go/Nogo task: Behavioural and neural changes depend on task demands. International Journal of Psychophysiology. 2013a;87:301–312. doi: 10.1016/j.ijpsycho.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Benikos N, Johnstone SJ, Roodenrys SJ. Varying task difficulty in the Go/Nogo task: The effects of inhibitory control, arousal, and perceived effort on ERP components. International Journal of Psychophysiology. 2013b;87:262–272. doi: 10.1016/j.ijpsycho.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Berg JM, Latzman RD, Bliwise NG, Lilienfeld SO. Parsing the heterogeneity of impulsivity: A meta-analytic review of the behavioral implications of the UPPS for psychopathology. Psychological Assessment. 2015;27:1129–1146. doi: 10.1037/pas0000111.. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remembering the future: Working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohus M, Kleindienst N, Limberger MF, Stieglitz RD, Domsalla M, Chapman AL, Wolf M. The short version of the Borderline Symptom List (BSL-23): Development and initial data on psychometric properties. Psychopathology. 2009;42:32–39. doi: 10.1159/000173701. [DOI] [PubMed] [Google Scholar]
- Bourne C, Aydemir O, Balanzá-Martínez V, Bora E, Brissos S, Cavanagh JTO, Goodwin GM. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: An individual patient data meta-analysis. Acta Psychiatrica Scandinavica. 2013;128:149–162. doi: 10.1111/acps.12133. https://doi.org/10.1111/acps.12133. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111:180–185. doi: 10.1037/0021-843X.111.1.180. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Ochsner KN. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex. 2014;24:1–10. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Major depressive disorder and impulsive reactivity to emotion: Toward a dual-process view of depression. British Journal of Clinical Psychology. 2013;52:285–299. doi: 10.1111/bjc.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J, Kim Y, Nam JY. Serotonin transporter polymorphism interacts with childhood adversity to predict aspects of impulsivity. Psychological Science. 2011;22:589–595. doi: 10.1177/0956797611404085. [DOI] [PubMed] [Google Scholar]
- Chavan CF, Mouthon M, Draganski B, van der Zwaag W, Spierer L. Differential patterns of functional and structural plasticity within and between inferior frontal gyri support training-induced improvements in inhibitory control proficiency. Human Brain Mapping. 2015;36:2527–2543. doi: 10.1002/hbm.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M, Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience and Biobehavioral Reviews. 2013;37(1):11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A. Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clinical Psychology Review. 2011;31:965–82. doi: 10.1016/j.cpr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C. Integration of impulsivity and positive mood to predict risky behavior: development and validation of a measure of positive urgency. Psychological Assessment. 2007;19:107–118. doi: 10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- Elgamal S, McKinnon MC, Ramakrishnan K, Joffe RT, MacQueen G. Successful computer-assisted cognitive remediation therapy in patients with unipolar depression: A proof of principle study. Psychological Medicine. 2007;37(9):1229–1238. doi: 10.1017/S0033291707001110. https://doi.org/10.1017/S0033291707001110. [DOI] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nature Neuroscience. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- Gronwall DMA. Paced auditory serial addition task: A measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gunn RL, Finn PR. Applying a dual process model of self-regulation: The association between executive working memory capacity, negative urgency, and negative mood induction on pre-potent response inhibition. Personality and Individual Differences. 2015;75:210–215. doi: 10.1016/j.paid.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorelbeke K, Koster EHW. Internet-delivered cognitive control training as a preventive intervention for remitted depressed patients: Evidence from a double-blind randomized controlled trial study. Journal of Consulting and Clinical Psychology. 2017;85:135–146. doi: 10.1037/ccp0000128. [DOI] [PubMed] [Google Scholar]
- Hoorelbeke K, Koster EHW, Demeyer I, Loeys T, Vanderhasselt MA. Effects of cognitive control training on the dynamics of (mal)adaptive emotion regulation in daily life. Emotion. 2013;13:1689–1699. doi: 10.1017/CBO9781107415324.004. [DOI] [PubMed] [Google Scholar]
- Houben K, Jansen A. Training inhibitory control: a recipe for resisting sweet temptations. Appetite. 2011;56:345–349. doi: 10.1016/j.appet.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Houben K, Nederkoorn C, Wiers RW, Jansen A. Resisting temptation: Decreasing alcohol-related affect and drinking behavior by training response inhibition. Drug and Alcohol Dependence. 2011;116:132–136. doi: 10.1016/j.drugalcdep.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW, Jansen A. Getting a grip on drinking behavior: training working memory to reduce alcohol abuse. Psychological Science. 2011;22:968–975. doi: 10.1177/0956797611412392. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvie M, Heinssen R, Pine DS, Quinn K, Wang P. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. The American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. https://doi.org/10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Carver CS, Joormann J. Impulsive responses to emotion as a transdiagnostic vulnerability to internalizing and externalizing symptoms. Journal of Affective Disorders. 2013;150:872–878. doi: 10.1016/j.jad.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Tharp J, Peckham AD, Sanchez AH, Carver CS. Positive urgency is related to difficulty inhibiting prepotent responses. Emotion. 2016;16:750–759. doi: 10.1037/emo0000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Guerrieri R, Fernie G, Cole J, Goudie A, Field M. The effects of priming restrained versus disinhibited behaviour on alcohol-seeking in social drinkers. Drug and Alcohol Dependence. 2011;113:55–61. doi: 10.1016/j.drugalcdep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Klein C, Fischer B. Instrumental and test-retest reliability of saccadic measures. Biological Psychology. 2005;68:201–213. doi: 10.1016/j.biopsycho.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlström K, Westerberg H. Computerized training of working memory in children with ADHD - A randomized, controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. https://doi.org/10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Maraver MJ, Bajo MT, Gomez-Ariza CJ. Training on working memory and inhibitory control in young adults. Frontiers in Human Neuroscience. 2016;10:588. doi: 10.3389/fnhum.2016.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhtadie L, Johnson SL, Carver CS, Gotlib IH, Ketter TA. A profile approach to impulsivity in bipolar disorder: The key role of strong emotions. Acta Psychiatrica Scandinavica. 2014;129:100–108. doi: 10.1111/acps.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Price RB, Paul B, Schneider W, Siegle GJ. Neural correlates of three neurocognitive intervention strategies: A preliminary step towards personalized treatment for psychological disorders. Cognitive Therapy and Research. 2013;37:657–672. doi: 10.1007/s10608-012-9508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EN, Combs JL, Jordan CE, Smith GT. Negative Urgency and lack of perseverance: Identification of differential pathways of onset and maintenance risk in the longitudinal prediction of non-suicidal self-injury. Behavior Therapy. 2015;46:439–448. doi: 10.1016/j.beth.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel BJ, Volokhov RN, Demaree HA. Working memory capacity and the self-regulation of emotional expression and experience. Journal of Personality and Social Psychology. 2008;95:1526–40. doi: 10.1037/a0013345. [DOI] [PubMed] [Google Scholar]
- Sharma L, Markon KE, Clark LA. Toward a theory of distinct types of “impulsive” behaviors: A meta-analysis of self-report and behavioral measures. Psychological Bulletin. 2014;140:374–408. doi: 10.1037/a0034418. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Ghinassi F, Thase ME. Neurobehavioral therapies in the 21st century: Summary of an emerging field and an extended example of cognitive control training for depression. Cognitive Therapy and Research. 2007;31:235–262. doi: 10.1007/s10608-006-9118-6. [DOI] [Google Scholar]
- Siegle GJ, Price RB, Jones NP, Ghinassi F, Painter T, Thase ME. You gotta work at it: Pupillary indices of task focus are prognostic for response to a neurocognitive intervention for rumination in depression. Clinical Psychological Science. 2014;2:455–471. doi: 10.1177/2167702614536160. [DOI] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologica. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Guller L, Zapolski TCB. A comparison of two models of Urgency: Urgency predicts both rash action and depression in youth. Clinical Psychological Science. 2013;1:266–275. doi: 10.1037/a0030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological Bulletin. 2013;139:81–732. doi: 10.1037/a0028727. https://doi.org/10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Frontiers in Psychology. 2015;6:328. doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers. 1999;31:137–149. doi: 10.3758/BF03207704. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27:247–259. doi: 10.1023/A:1023910315561. [DOI] [Google Scholar]
- Unsworth N, Schrock JC, Engle RW. Working memory capacity and the antisaccade task: individual differences in voluntary saccade control. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:1302–21. doi: 10.1037/0278-7393.30.6.1302. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Adams RC, Chambers CD. Proactive motor control reduces monetary risk taking in gambling. Psychological Science. 2012;23:805–815. doi: 10.1177/0956797611434538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn JJ, Juzwin KR, Styer DM, Aldridge D. Measuring the urge to self- injure: Preliminary data from a clinical sample. Psychiatry Research. 2010;178:540–544. doi: 10.1016/j.psychres.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037/0021-843X.104.1.3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Weiss NH, Tull MT, Davis LT, Searcy J, Williams I, Gratz KL. A preliminary experimental investigation of emotion dysregulation and impulsivity in risky behaviours. Behaviour Change. 2015;32:127–142. doi: 10.1017/bec.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Bickel WK. Remember the future II: Meta-analyses and functional overlap of working memory and delay discounting. Biological Psychiatry. 2014;75:435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behavior scale: A four factor model of impulsivity. European Journal of Personality. 2005;19:559–574. doi: 10.1002/per.556. [DOI] [Google Scholar]


