Abstract
Background
Intestinal colonization by MDR/XDR gram-negative bacteria leads to an increased risk of subsequent bloodstream infections (BSI) in patients receiving chemotherapy as a treatment for hematologic malignancies.
Objectives
The objective of this study was to evaluate the efficacy of oral colistin in eradicating the intestinal carriage of MDR/XDR Gram-negative bacteria in patients with hematological malignancies.
Methods
In a tertiary hematology center, adult patients with intestinal colonization by MDR/XDR Gram-negative bacteria were included in a randomized controlled trial (RCT) during a period from November 2016 to October 2017. Patients were treated with oral colistin for 14 days or observed with the primary outcome set as decolonization on day 21 post-treatment. Secondary outcomes included treatment safety and changes in MICs of isolated microorganisms. ClinicalTrials.gov Identifier: NCT02966457.
Results
Short-time positive effect (61.3% vs 32.3%; OR 3.32; 95% CI 1.17–9.44; p=0.0241) was demonstrated on the day 14 of colistin treatment, without any statistical difference on day 21 post-treatment. The incidence of BSI in decolonization group was lower in the first 30 days after the intervention (3.2% vs. 12.9%), but overall in the 90-day observation period, it did not show any advantages comparing to control group (log-rank test; p=0.4721). No serious adverse effects or increase in resistance to colistin was observed.
Conclusions
This study suggests that in hematological patients the strategy of selective intestinal decolonization by colistin may be beneficial to decrease the rate of MDR/XDR Gram-negative intestinal colonization and the risk of BSI in the short-term period, having no long-term sustainable effects.
Keywords: Multidrug-resistant bacteria, Selective oral decolonization, Polymyxins, Hematology, Neutropenia
Introduction
MDR/XDR (multidrug-resistant/extensively drug-resistant) gram-negative bacteria have emerged as the most dangerous cause of bloodstream infection (BSI) in hospitalized patients, especially in immunocompromised hosts. It was shown earlier, that intestinal colonization with extended-spectrum β-lactamases (ESBL)-producing or carbapenem-resistant Enterobacteriaceae spp., carbapenem-resistant A. baumannii and P. aeruginosa might be a prolonged condition in certain populations of patients.1,2 It is especially dangerous in patients with hematological malignancies and HSCT, during the chemotherapy-induced neutropenia, when mucosal colonization by MDR/XDR pathogens is considered as a risk factor for subsequent infectious complication.3–6 It was also demonstrated previously, that the inadequacy of empirical antibacterial therapy and the isolation of carbapenem-resistant A. baumannii or P. aeruginosa were among the most significant risk factors for mortality in adult patients with BSI in the pre-engraftment period after hematopoietic stem cells transplantation (HSCT).7
There were numerous studies published on decolonization strategies in patients with different primary conditions,8–12 but due to the broad preventive use of antibiotics and profound neutropenia, the problem of choice of strategy of intestinal decolonization of MDR/XDR Gram-negative bacteria is primarily important in hematology. Earlier decolonization regimens have been studied for Staphylococcus aureus, but there is a noticeable lack of data on the regimens to decolonize Gram-negative carriage nowadays.13,14 To the investigator’s knowledge, no randomized clinical trial has been performed to study the efficacy and safety of selective intestinal decolonization by Colistimethate sodium (colistin) in high-risk adult patients with hematological malignancies. It is important to mention that in a condition of high incidence of carbapenem-resistant Gram-negative bacteria colistin remains a single therapeutic option in a number of cases. Colistin, being a non-absorbable antibiotic may have certain importance as a decolonizing agent, especially in case of Gram-negative carbapenem-resistant colonization. Gram-negative We have estimated that possible decolonization of MDR/XDR gram-negative bacteria in hematological patients could be beneficial for the patients by reducing the risk of infection and for the community by reducing the risk of transmission. The aim of the proposed study is to assess the efficacy and safety of selective intestinal decolonization of MDR/XDR gram-negative bacteria with oral administration of Colistimethate sodium in adult patients with hematological malignancies.
Methods
Trial design and setting
This was a non-blind parallel assignment controlled trial with balanced (1:1) randomization. The primary purpose of this Phase 4 trial was the prevention of BSI caused by XDR/MDR Gram-negative bacteria in patients with hematological malignancies by decreasing the intestinal colonization level through selective intestinal decolonization. The trial protocol was approved by the local institutional review board (IRB) and Ethical Committee (Protocol №11) of the Republican center for hematology and bone marrow transplantation (Minsk, Belarus) and has been registered with the US National Institute of Health (NIH) and the National Library of Medicine (NLM): A Study of Decolonization in Patients with Haematological Malignancies (DEHAM); ClinicalTrials.gov Identifier: NCT02966457.
Republican center for hematology and bone marrow transplantation is a tertiary national clinical and research center for adult patients situated in Minsk, Republic of Belarus. Clinical departments are based in the 9th clinical hospital of Minsk, which is one of the largest teaching hospitals in Belarus performing more than a hundred HSCT every year. This center has 150 beds including intensive care unit for patients with various hematological diseases and patients undergoing HSCT, as well as an out-patient clinic. Center also includes: microbiology laboratory, laboratory of bone marrow separation and freezing, laboratory of cellular biotechnology, HLA-typing laboratory and clinical diagnostics laboratory.
Participants
Participants were enrolled in the study during the period from November 2016 to October 2017. Patients with hematological malignancies aged ≥18 years with a positive rectal swab for MDR/XDR Gram-negative microorganism and the ability to provide informed consent were eligible. MDR/XDR classification of Gram-negative bacteria was performed according to Magiorakos et al. was used in the study.15
Active screening of patients admitted to hospital with hematological malignancies, primarily to receive a course of chemotherapy, was performed by way of rectal swabs during the study period. Patients with signs or symptoms of active bacterial, viral, fungal or protozoal infection were excluded from the study. Among other exclusion criteria used: pregnant or nursing women, use of antibacterial therapy in previous 10 days; contraindication to the use of the study drug (including known hypersensitivity); enrollment in another study, or in the present study for a previous episode; psychiatric disorder or no ability to understand or to follow the protocol directions; resistance of the primarily isolated colonizing microorganism to polymyxin antibiotics (MIC ≥ 0.5 mg/L). No standard antibacterial prophylaxis was used during the study period in the included patients. In all cases measures of contact, precautions were established to prevent the spread of XDR/MDR microorganisms. Prophylaxis against Pneumocystis jirovecii with trimethoprim-sulfamethoxazole was administered to all patients in the study with absolute neutrophil count (ANC) < 100 cells/mm3. Prophylaxis of infections caused by herpes simplex viruses (HSV) was performed by acyclovir only in patients with high clinical risk of HSV reactivation. Real-time quantitative polymerase chain reaction (PCR) was used for monitoring CMV and EBV DNA levels in high risk patients weekly.
Interventions
Patients randomized to the treatment arm received selective intestinal decolonization with colistin in a dose of 2 mln I.U. 4 times per day PO for 14 days. Patients in the control group were observed during the study period without any interventions while they received their standard treatment for hematological malignancies (“watch and wait” strategy).
Outcomes
Patients were assessed at baseline, on the last day of treatment (day 14) and on day 21 after the end of treatment. At each visit, rectal swabs were performed by inserting the swab immediately in culture media. The pre-defined primary outcome of the study was the detection of intestinal MDR/XDR Gram-negative bacteria carriage by a rectal swab during day 21 post-treatment (rate of eradication of MDR/XDR Gram-negative bacteria at day 21 post-treatment). Safety of the study regimen (incidence and intensity of possible adverse effects) and change in colistin MICs between baseline and the final visit were taken as secondary outcomes.
Microbiological procedures
Microbial cultures were isolated and grown on different manufactured culture media. Identification and antimicrobial susceptibility testing were performed using a bioMerieux VITEK 2 automatic system and commercial panels, and the ESBL-phenotype was determined using a VITEK 2 ESBL Test System. Additional antimicrobial susceptibility in carbapenem-resistant isolates (resistance to imipenem, meropenem, and doripenem) was confirmed by E-tests and disc-diffusion assays. The minimum inhibitory concentration (MIC) breakpoints used for susceptibility testing were based on current EUCAST guidelines,16 with commercially available Mueller–Hinton agar and antimicrobial discs used in disc-diffusion method (bioMerieux).
According to previously published studies, colistin resistance of Gram-negative bacteria may be underestimated by Phoenix100/Vitek2 systems, potentially leading to inappropriate colistin administration. It is also recommended to retest the isolates with MIC to colistin at susceptibility breakpoint (2 mg/L).17 Keeping these arguments in mind, we have decided to estimate as a susceptible to colistin only isolates with MIC < 0.5 mg/l.
Sample size and power calculation
Based on our clinical experience, we assumed that 25% of patients would clear the MDR/XDR Gram-negative intestinal colonization spontaneously within the period of study and hypothesized, that a decolonization regimen would be clinically effective if able to clear colonization in a 60% of patients. Using a two-sided Alpha of 0.05 and a power of the study of 80%, with an enrollment ratio of 1 and a dichotomous endpoint, we calculated a minimal sample size of 60 patients.
Randomization
Randomization was performed by computerized randomization program (ALEA) in the proof assistant Coq v. 8.3., which is validated for use in randomized clinical trials. The block size randomly varies between 4, 6 and 8.
Blinding
Due to the decision of IRB and Ethical Committee, blinding in the planned study was not considered appropriate from the ethical positions, so the study protocol did not include it.
Statistical methods
Based on the study design, the intention-to-treat analysis was performed, while none of the patients were excluded in the process of the trial and the study characteristics were analyzed according to the randomization scheme. Due to the ongoing chemotherapy treatment for a primary hematological disease, there were no cases of data missing or exclusion of the patients in the process of the trial due to loss to follow-up. All of the patients in the study were included in the monitoring of adverse effects of the decolonization regimen. The distribution of the variables was determined by the Shapiro-Wilk test. Differences in MDR/XDR Gram-negatives carriage between the study groups were analyzed by methods of non-parametric statistics for categorical variables (Chi-squared or Fisher’s exact tests). Univariate logistic regression was used to determine the odds ratio for the presence of MDR/XDR Gram-negative intestinal colonization in the treatment and control group. The probability of development of BSI after the decolonization was estimated using the Kaplan–Meier method and compared with log-rank test. Day count in Kaplan-Meier probability test started from day 21 post-treatment and included 90 days of observation. Data processing and analysis were performed using MedCalc Statistical Software v. 18 (MedCalc Software bvba, Ostend, Belgium) and SPSS v. 21.0 (IBM Co., Armonk, NY, USA), and results were regarded statistically significant when p<0.05.
Results
Participant flow and recruitment
The study flowchart according to CONSORT Statement18 is shown in Figure 1.
Figure 1.
Study flow diagram of the randomized controlled trial.
Therefore, among the main causes of exclusion from the study before a randomization procedure were: absence of MDR/XDR Gram-negative intestinal colonization on baseline screening and the use of antibacterial therapy in previous ten days. After the baseline assessment, there were 62 patients included in the parallel allocated groups in a balance of 1:1.
Baseline data
After the randomization procedure, two study groups were showing similar baseline clinical and demographic characteristics (Table 1).
Table 1.
Demographical and clinical baseline characteristics of patients by group in the study (randomized patients).
| Baseline characteristics | Decolonization group (n=31), % | Observation group (n=31), % |
|---|---|---|
|
| ||
| Age (years, median, interquartile range) | 49 (39–63) | 49 (35–63) |
|
| ||
| Sex (female) | 15 (48.4) | 16 (51.6) |
|
| ||
| BMI (kg/m2, median, interquartile range) | 25.5 (24.1–28.4) | 25.8 (24.2–27.6) |
|
| ||
| Primary disease: | ||
| Acute myeloid leukemia | 17 (54.8) | 16 (51.6) |
| Multiple myeloma | 7 (22.6) | 9 (29.0) |
| Hodgkin’s lymphoma | 1 (3.2) | - |
| Chronic lymphocytic leukemia | 3 (9.7) | 4 (12.9) |
| Myelodysplastic syndrome | 1 (3.2) | 1 (3.2) |
| Acute lymphocytic leukemia | 2 (6.5) | 1 (3.2) |
|
| ||
| Primary disease stage: | ||
| Progression | 19 (61.3) | 17 (54.8) |
| Remission | 12 (38.7) | 14 (45.2) |
|
| ||
| Level of neutropenia on day 1 of trial: | ||
| <100 cells/mm3 | 15 (48.4) | 18 (58.1) |
| 100–500 cells/mm3 | 5 (16.2) | 3 (9.7) |
| >500 cells/mm3 | 11 (35.5) | 10 (32.3) |
|
| ||
| MDR/XDR resistant species at baseline rectal swab: | ||
| K. pneumoniae | 16 (51.6) | 13 (41.9) |
| E. coli | 4 (13.0) | 8 (25.8) |
| A. baumannii | 5 (16.1) | 5 (16.1) |
| P. aeruginosa | 6 (19.4) | 5 (16.1) |
|
| ||
| Carbapenem resistant species at baseline rectal swab: | ||
| K. pneumoniae | 8(25.8) | 7(22.6) |
| E. coli | 2(6.5) | 1(3.2) |
| A. baumannii | 5(16.1) | 4(12.9) |
| P. aeruginosa | 3(9.7) | 4(12.9) |
|
| ||
| Infections caused by colonizing microorganism in previous 6 months: | 4 (12.9) | 3 (9.7) |
The median age of all of the participants in the study was 49 years (interquartile interval 36–63 years); 31/62 (50%) were female. More patients in the control group were colonized by MDR/XDR E. coli (8/31 versus 4/31), while the decolonization group had more patients with MDR/XDR K. pneumoniae colonization at baseline (16/31 vs. 13/31). Overall, K. pneumoniae was the most frequent intestinal colonizer in adult hematological patients in the study, detected in 29/62 (46.8%) patients. All of the selected microorganisms at baseline showed susceptibility to colistin (with MIC<0.5 mg/l). Among the patients included, in the absence of chemotherapy, recovery of the peripheral neutrophil count over 500 cells/mm3 estimated on the last day of decolonization regimen (day 14) was observed in 12 (38.7%) in decolonization group and in 15 (48.4%) in a control group.
Outcomes and estimation
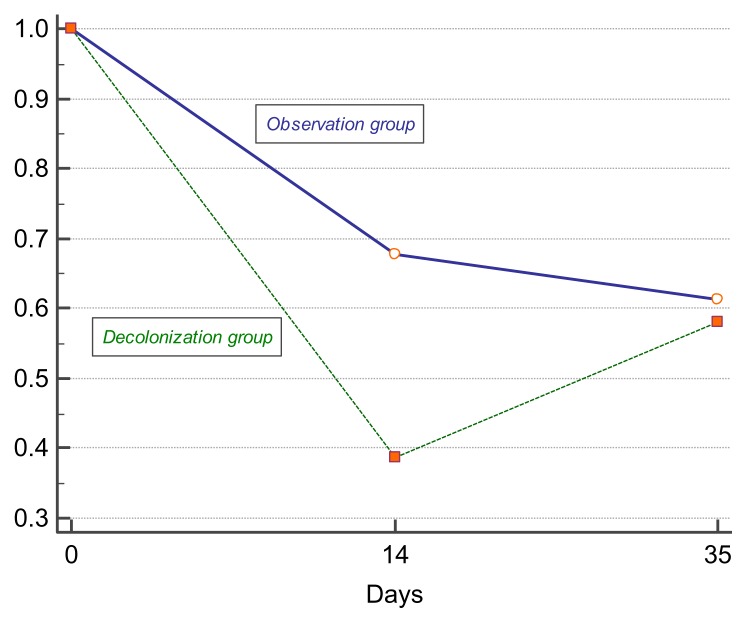
In the primary outcome analysis, 19 of 31 patients (61.3%) in the treatment group and 10 of 31 (32.3%) in the control group have shown negative rectal swab for MDR/XDR Gram-negative bacteria on the last day of oral decolonization regimen (day 14). Although, later on day 21 post-treatment the numbers of intestinal colonization by the same pathogens remained to some extent similar, with 13 of 31 patients (41.9%) showing decolonization effect in the treatment group and 12 of 31 (38.7%) – in the control group. The observed changes may indicate that this procedure of selective oral decolonization by colistin had only a short-time effect, with no long-lasting microbiological benefits.
Based on the results of univariate statistical analysis using Chi-squared test and logistic regression, there was a favourable microbiological effect of oral decolonization by colistin on intestinal MDR/XDR Gram-negative bacteria in the conducted study (OR 3.32; 95% CI 1.17–9.44; p=0.0241) on the last day of treatment (day 14). Although, on day 21 post-treatment there was already no statistical significance shown in the treatment and control groups (OR 1.14; 95% CI 0.41–3.16; p=0.7958). As an additional characteristic of the efficacy of oral decolonization of MDR/XDR Gram-negative bacteria in patients with hematological malignancies, the number needed to treat (NNT) was analyzed for the last day of treatment (NNT 3.44; 95% CI 1.89–18.99; p=0.0241), showing the short-time effect of treatment. Figure 2 displays the evolution of MDR/XDR carriage over time in the colistin oral decolonization group and observation control and shows the short-time effect of decolonization in the study.
Figure 2.
Evolution of rectal carriage of XDR/MDR Gram-negative bacteria over time with regard to decolonization with oral colistin in the study.
Estimation of risk for development of bloodstream infection
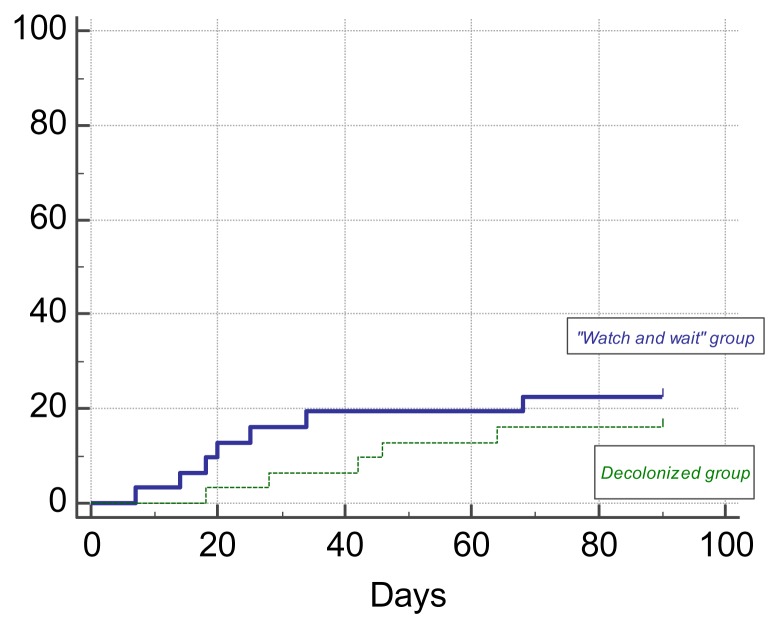
Additionally, to get an understanding of the possible clinical effect of decolonization in MDR/XDR colonized hematological patients the incidence of bloodstream infections (BSI) was monitored for 90 days in both decolonized and control groups. All of the patients included in the study were continuing to receive chemotherapy and have follow-up visits for their primary hematological disease while being monitored clinically and microbiologically for possible infectious complications. Totally, there were 5/31 (16.13%) cases of BSI observed in decolonization group and 7/31 (22.58%) cases in the control group. Due to adequately prescribed empiric antibiotic treatment, no adverse clinical outcomes in the study groups was reported up-to-date. The incidence of BSI in decolonization group was lower in the first 30 days after the intervention compared to control group (3.2% vs. 12.9%), but overall in the 90-day observation period, it did not show any advantages comparing to control group (log-rank test; p=0.4721). It is important to add, that during the first 14 days after the intervention none of the decolonized patients had a documented BSI, while during the later period BSIs occurred in both groups.
Probability graph for subsequent bloodstream infections in patients with intestinal colonization by MDR/XDR Gram-negative bacteria with regard to decolonization by oral colistin is shown in Figure 3.
Figure 3.
Probability of development of bloodstream infection in patients with intestinal colonization by MDR/XDR Gram-negative bacteria with regard to decolonization by oral colistin in the 90-day period after the intervention.
Adverse effects
No increase in resistance to colistin above MIC of 0.5 mg/l was observed in any of isolates during the study and follow-up period. Among the registered events, there were only 6 cases of liquid stool without any systemic effects or signs of infection occurring in 4 patients in decolonization arm and 2 patients in control arm of the study (Fisher’s exact test; 12.9% vs. 6.45%; P =0 .06713). None of the patients in the study had to stop treatment prematurely due to serious adverse effects of the treatment. This may be explained by low intestinal absorption of colistin, leading to potentially minimal numbers of systemic effects of the drug.
Discussion
This randomized, controlled trial of an oral colistin decolonization regimen of MDR/XDR Gram-negative bacteria in adult patients with hematological malignancies demonstrated a significant temporary suppression of rectal colonization rate on the last day of treatment (day 14), with no sustained effect at 21 days after the treatment. Observation of the incidence of BSI in the studied groups during a 90-days period has additionally shown the short-time protective effect of decolonization on the risk of BSI up to first 30 days after the treatment. It may be explained in a quantitative decrease of MDR/XDR colonizing bacteria in the gut, what may have some protective effect during chemotherapy-induced mucositis. To our knowledge, we report the first randomized, controlled trial examining a decolonization strategy with colistin for carriers of MDR/XDR Gram-negative bacteria in a group of adult patients with hematological malignancies, including patients with chemotherapy-induced neutropenia.
The possibilities for eradicating the colonizing microorganisms in various groups of patients were studied earlier in different settings. For example, Huttner et al. have shown the temporary decolonizing effect of oral colistin on ESBL-producing Enterobacteriaceae spp. rectal carriage in patients with various comorbidities, what may correspond with results of our study.9 Additionally, Saidel-Odes et al. have demonstrated in the study, that colistin-based regimen could be a suitable decolonization therapy for selected patients colonized with carbapenem-resistant K. pneumoniae, such as transplant recipients or immunocompromised patients pending chemotherapy.8 Oral gentamicin was also reported as a possible decolonizing agent in an HSCT setting.12 It is important to mention, that one of the most effective directions of research in the studied area should be based on investigation of changes in intestinal microbiota composition, leading to expansion and domination of certain bacteria, with a future possibilities to establish the risk factors for domination of MDR/XDR microorganisms and potential preventive strategies, including decolonization regimens.19,20 In future studies it may be suggested, that not only rectal swabs should be studied in hematological and HSCT patients populations, but a pharyngeal carriage and skin colonization by MDR/XDR Gram-negatives, what may lead to important practical recommendations.21
Limitations and generalizability
One of the most important limitations of the conducted study is an absence of blinding procedure due to ethical reasons, especially in high-risk patients with hematological malignancies. The other important issue is that rectal swabs may be inadequate to detect resistant pathogens present in small amounts and stool cultures may be an inappropriate way to monitor gut colonization.22 In some of the cases, we were not able to differentiate the exogenous and endogenous rebound of colonization, what may have been controlled by genotyping techniques.
Finally, this study was conducted in one clinical center, meaning that the external validity of this trial may be limited.
Recommendations
Due to the fact, that intestinal colonization by MDR/XDR Gram-negatives is an independent risk factor for adverse clinical outcome in hematological patients with neutropenia, even a temporary suppression of MDR/XDR Gram-negatives intestinal carriage may result in a clinical benefit during the period of profound chemotherapy-induced neutropenia. Thus, a strategy of early detection and selective suppression of highly-resistant microorganisms in such patients during prolonged periods of immunosuppression could result in a reduction in the incidence of subsequent bloodstream infections in a short period. A large multicentre trial would be needed to test this hypothesis.
Conclusions
We observed a temporary suppression of MDR/XDR Gram-negative bacteria carriage during oral antibiotic treatment by colistin at the end of decolonization regimen. The study, though, did not demonstrate an effect of the used decolonization regimen on rectal MDR/XDR Gram-negative bacteria carriage 21 days after the end of treatment. Therefore, in high risk hematological patients with chemotherapy-induced neutropenia, the strategy of selective intestinal decolonization with colistin may be beneficial to decrease the short-term probability of developing bloodstream infections up to 30 days from the end of treatment with low incidence of the adverse effects and minimal risk of increase in colistin drug resistance in Gram-negative colonizing bacteria.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Birgand G, Armand-Lefevre L, Lolom I, Ruppe E, Andremont A, Lucet JC. Duration of colonization by extended-spectrum β-lactamase-producing Enterobacteriaceaeafter hospital discharge. Am J Infect Control. 2013;41(5):443–7. doi: 10.1016/j.ajic.2012.05.015. https://doi.org/10.1016/j.ajic.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Löhr IH, Rettedal S, Natås OB, Naseer U, Oymar K, Sundsfjord A. Long-termfaecal carriage in infants and intra-household transmission of CTX-M-15-producingKlebsiella pneumoniae following a nosocomial outbreak. J Antimicrob Chemother. 2013 May;68(5):1043–8. doi: 10.1093/jac/dks502. https://doi.org/10.1093/jac/dks502. [DOI] [PubMed] [Google Scholar]
- 3.Schwaber MJ, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA. 2008;300(24):2911–3. doi: 10.1001/jama.2008.896. https://doi.org/10.1001/jama.2008.896. [DOI] [PubMed] [Google Scholar]
- 4.Yu VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von Gottberg A, Goossens H, Wagener MM, Benedi VJ International Klebseilla Study Group. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis. 2007;13(7):986–93. doi: 10.3201/eid1307.070187. https://doi.org/10.3201/eid1307.070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denis B, Lafaurie M, Donay JL, Fontaine JP, Oksenhendler E, Raffoux E, Hennequin C, Allez M, Socie G, Maziers N, Porcher R, Molina JM. Prevalence, risk factors, and impact on clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia: a five-year study. Int J Infect Dis. 2015;39:1–6. doi: 10.1016/j.ijid.2015.07.010. https://doi.org/10.1016/j.ijid.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52(4):e56–93. doi: 10.1093/cid/cir073. https://doi.org/10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 7.Stoma I, Karpov I, Milanovich N, Uss A, Iskrov I. Risk factors for mortality in patients with bloodstream infections during the pre-engraftment period after hematopoietic stem cell transplantation. Blood Res. 2016;51(2):102–6. doi: 10.5045/br.2016.51.2.102. https://doi.org/10.5045/br.2016.51.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saidel-Odes L, Polachek H, Peled N, Riesenberg K, Schlaeffer F, Trabelsi Y, Eskira S, Yousef B, Smolykov R, Codish S, Borer A. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol. 2012 Jan;33(1):14–9. doi: 10.1086/663206. https://doi.org/10.1086/663206. [DOI] [PubMed] [Google Scholar]
- 9.Huttner B, Haustein T, Uçkay I, Renzi G, Stewardson A, Schaerrer D, Agostinho A, Andremont A, Schrenzel J, Pittet D, Harbarth S. Decolonization of intestinal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae with oral colistin and neomycin: a randomized, double-blind, placebo-controlled trial. J Antimicrob Chemother. 2013;68(10):2375–82. doi: 10.1093/jac/dkt174. https://doi.org/10.1093/jac/dkt174. [DOI] [PubMed] [Google Scholar]
- 10.de Jonge E, Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J, Kesecioglu J. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003 Sep 27;362(9389):1011–6. doi: 10.1016/S0140-6736(03)14409-1. https://doi.org/10.1016/S0140-6736(03)14409-1. [DOI] [PubMed] [Google Scholar]
- 11.Rieg S, Küpper MF, de With K, Serr A, Bohnert JA, Kern WV. Intestinal decolonization of Enterobacteriaceae producing extended-spectrum β-lactamases (ESBL): a retrospective observational study in patients at risk for infection and a brief review of the literature. BMC Infect Dis. 2015 Oct 28;15:475. doi: 10.1186/s12879-015-1225-0. https://doi.org/10.1186/s12879-015-1225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuckerman T, Benyamini N, Sprecher H, Fineman R, Finkelstein R, Rowe JM, Oren I. SCT in patients with carbapenem resistant Klebsiella pneumoniae: a single center experience with oral gentamicin for the eradication of carrier state. Bone Marrow Transplant. 2011 Sep;46(9):1226–30. doi: 10.1038/bmt.2010.279. https://doi.org/10.1038/bmt.2010.279. [DOI] [PubMed] [Google Scholar]
- 13.Mody L, Kauffman CA, McNeil SA, Galecki AT, Bradley SF. Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2003 Dec 1;37(11):1467–74. doi: 10.1086/379325. https://doi.org/10.1086/379325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weintrob A, Bebu I, Agan B, Diem A, Johnson E, Lalani T, Wang X, Bavaro M, Ellis M, Mende K, Crum-Cianflone N. Randomized, Double-Blind, Placebo-Controlled Study on Decolonization Procedures for Methicillin-Resistant Staphylococcus aureus (MRSA) among HIV-Infected Adults. PLoS One. 2015;10(5):e0128071. doi: 10.1371/journal.pone.0128071. eCollection 2015. https://doi.org/10.1371/journal.pone.0128071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012 Mar;18(3):268–81. doi: 10.1111/j.1469-0691.2011.03570.x. https://doi.org/10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 16.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 7.1. 2017. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf.
- 17.Chew KL, La MV, Lin RTP, Teo JWP. Colistin and Polymyxin B Susceptibility Testing for Carbapenem-Resistant and mcr-Positive Enterobacteriaceae: Comparison of Sensititre, MicroScan, Vitek 2, and Etest with Broth Microdilution. J Clin Microbiol. 2017 Sep;55(9):2609–2616. doi: 10.1128/JCM.00268-17. https://doi.org/10.1128/JCM.00268-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. https://doi.org/10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, Lee YJ, Dubin KA, Socci ND, Viale A, Perales MA, Jenq RR, van den Brink MR, Pamer EG. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(7):905–14. doi: 10.1093/cid/cis580. https://doi.org/10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taur Y, Pamer EG. The intestinal microbiota and susceptibility to infection in immunocompromised patients. Curr Opin Infect Dis. 2013;26(4):332–7. doi: 10.1097/QCO.0b013e3283630dd3. Review. https://doi.org/10.1097/QCO.0b013e3283630dd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tschudin-Sutter S, Frei R, Dangel M, Stranden A, Widmer AF. Sites of colonization with extended-spectrum β-lactamases (ESBL)-producing enterobacteriaceae: the rationale for screening. Infect Control Hosp Epidemiol. 2012 Nov;33(11):1170–1. doi: 10.1086/668027. https://doi.org/10.1086/668027. [DOI] [PubMed] [Google Scholar]
- 22.D’Agata EM, Gautam S, Green WK, Tang YW. High rate of false-negative results of the rectal swab culture method in detection of gastrointestinal colonization with vancomycin-resistant enterococci. Clin Infect Dis. 2002 Jan 15;34(2):167–72. doi: 10.1086/338234. https://doi.org/10.1086/338234. [DOI] [PubMed] [Google Scholar]