Abstract
Rab coupling protein (RCP) aggravates cancer cell metastasis and has been implicated in various cancer patient outcomes. Recently, we showed that RCP induces Slug expression and cancer cell invasion by stabilizing the β1 integrin protein. In the present study, we demonstrated that FAK is implicated in RCP-induced EGFR phosphorylation and ovarian cancer cell invasion with inhibition by curcumin. Ectopic expression of RCP induced FAK phosphorylation, which links β1 integrin with EGFR and participates in a positive regulation loop with EGFR. Interestingly, we observed for the first time that curcumin attenuates RCP-induced ovarian cancer cell invasion by blocking stabilization of β1 integrin and consequently inhibiting FAK and EGFR activation, providing potential biomarkers for ovarian cancer and therapeutic approaches for this deadly disease.
Ovarian cancer: Natural compound suppresses cancer cell invasiveness
Rab coupling protein (RCP)-induced tumor cell migration has been implicated in tumor pathophysiology and patient outcomes. Hoi Young Lee and colleagues at Konyang University in Daejeon, South Korea, have previously shown that RCP promotes ovarian cancer cell invasiveness by stabilizing cell adhesion receptors. In their latest study they find that RCP also increases the levels of two of its protein-binding partners and activates an important mediator of growth factor signaling, Focal Adhesion Kinase (FAK). Interestingly, treating ovarian cancer cells with curcumin, a natural compound extracted from the spice turmeric, not only blocked the effects of RCP on cell adhesion and FAK activation, it also potentiated the inhibitory effects of the chemotherapeutic agent doxorubicin on cell invasiveness. Further research will determine whether curcumin could be used to halt ovarian cancer progression.
Introduction
Ovarian cancer is highly metastatic disease and the fifth leading cause of cancer-related death among women1. Early detection and diagnosis of ovarian cancer might significantly improve the survival rate, but the 5-year survival rate is less than 30–50%2. This deadly disease spreads to various sites, such as the liver, pleura, and lung, and the survival rate of patients is based on their metastatic status3.
Metastasis is a multi-step event that includes epithelial-to-mesenchymal transition (EMT)4. During EMT, the cells lose their epithelial characteristics and acquire a spindle-shaped morphology, initiating invasion and metastasis5. Metastatic tumor cells detach from adjacent cells by expressing reduced amounts of E-cadherin. In addition, these mesenchymal cells show higher expression of mesenchymal markers, including Snail, Slug, Zeb1, and Twist1, than epithelial cells6.
Rab coupling protein (RCP), known as Rab11 family-interacting protein 1 (Rab11FIP1), is located within the 8p11–12 chromosomal region that is frequently amplified in breast cancers7. Accumulating studies have shown that RCP augments cancer tumorigenesis, invasion, and metastasis7–10. Mechanistically, RCP associates with β1 integrin and links this integrin with receptor tyrosine kinases, such as EGFR, at recycling endosomes that magnify signaling to activate Ras and Erk11. In addition, we recently showed that RCP aggravates cancer cell invasion and metastasis by stabilizing β1 integrin and consequently upregulating Slug expression and EMT10.
Growing evidence has shown the potential of natural products to act as cancer therapeutic agents. A naturally occurring component of turmeric, curcumin has been shown to inhibit multiple signaling pathways associated with cancer invasion and metastasis. Notably, curcumin inhibits activation of FAK12, NF-κB13, and STAT-3 (refs. 14,15). Curcumin also attenuates lysophosphatidic acid15- and epidermal growth factor (EGF)16-induced ovarian cancer cell migration.
Focal adhesion kinase (FAK) is a key signaling factor that regulates cancer cell motility. Upon activation by numerous stimuli, including integrin clustering, FAK is associated with various small GTPase proteins (Rho, Rac, Cdc42, and Ras) and Src, leading to alteration in the polymerization or stabilization of actin and microtubule filaments17. Additionally, FAK has been shown to aggravate ovarian and breast cancer progression by regulating phosphatidylinositol 3-kinase and activation of AKT signaling18,19.
Recent studies have demonstrated that integrin endocytosis regulates FAK signaling and that endosomal FAK signaling increases cancer metastasis20. Although it is well documented that RCP-induced β1 integrin signaling is closely associated with ovarian cancer cell progression, the detailed underlying mechanism by which RCP induces ovarian cancer cell invasion remains unclear. In the present study, we showed that FAK is implicated in RCP-induced EGFR phosphorylation, leading to ovarian cancer cell invasion. In addition, we demonstrated that FAK links β1 integrin with EGFR and participates in a positive regulation loop with EGFR. Finally, and more importantly, we showed for the first time that curcumin efficiently inhibits RCP-induced ovarian cancer invasion by blocking RCP-induced stabilization of β1 integrin and consequently inhibiting FAK and EGFR activation.
Materials and methods
Reagents
PF573228, curcumin, cycloheximide (CHX), and G418 were obtained from Sigma-Aldrich (St Louis, MO). Gefitinib was from Selleckchem (Houston, TX). Doxorubicin (DOX) was obtained from Cayman (Ann Arbor, MI). All reagents were of the purest grade available.
Cell culture
Ovarian cancer SKOV-3, OVCAR-3, and PA-1 cells were purchased from American Type Culture Collection (Manassas, VA) and used between the 10th passage and 30th passage. SKOV-3 and OVCAR-3 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and 1% penicillin/streptomycin (HyClone). PA-1 cells were maintained in MEM supplemented with 10% fetal bovine serum. All cells were incubated at 37 °C under 5% CO2 in a humidified incubator.
siRNA and plasmid DNA transfection
SKOV-3, OVCAR-3, and PA-1 cells were transiently transfected with Lipofectamine 3000 or RNAiMAX according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). SKOV-3 cells were stably transfected with RCP (4 μl) by utilizing Lipofectamine 3000 (10 μl) in a six-well plate and selecting for stable transfectants with G418 (400 µl/ml). The empty pEGFP-C3 vector was used as a negative control. siRNAs against FAK (PTK2), β1 Integrin (No. 1 and No. 2), ILK (No. 1 and No. 2), Rab11, and Rab25 were purchased from Sigma-Aldrich. Control scrambled siRNA was from Invitrogen.
Immunoblotting
Proteins were extracted using RIPA lysis buffer (0.5 M Tris, Triton X-100, Na-deoxycholate, 10% SDS, NaCl and 0.5 M EDTA) containing a complete protease inhibitor cocktail tablet (Roche, Indianapolis, IN). Total cell lysates were measured using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL) and were resolved by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Temecula, CA). The membranes were blocked with 5% nonfat dried milk in TBST (Tris-buffered saline with 0.1% Tween-20), incubated for 2 h, and then probed with the indicated primary antibodies overnight at 4 °C. Next, the membranes were washed twice with washing buffer and incubated with secondary antibodies (rabbit, mouse; Thermo Fisher Scientific) for 2 h at room temperature. Then, the immunoreactive bands were visualized by enhanced chemiluminescence (Thermo Fisher Scientific) using ImageQuant 400 (GE Healthcare, Buckinghamshire, UK). Antibody for E-cadherin (610182) was obtained from BD Bioscience (San Jose, CA). Anti-FAK (557), EGFR (03), β1 Integrin (53,711), and GAPDH (25,778) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Texas, CA). Anti-RCP (12,849), p-FAK (3283 S), Slug (9585), and p-EGFR (4407) antibodies were from Cell Signaling Technology (Danvers, MA). Anti-ILK (76,468) was purchased from Abcam (Cambridge, MA).
Immunofluorescence
The cells were fixed with cold methanol prior to being blocked in 1% phosphate-buffered saline (PBS). Immunofluorescence was carried out with anti-p-FAK (16,665, 1:100; Santa Cruz Biotechnology), E-cadherin (7870, 1:100; Santa Cruz Biotechnology), Slug (15,391, 1:100; Santa Cruz Biotechnology), and β1 integrin (53,771, 1:100; Santa Cruz Biotechnology) antibodies overnight. The cells were washed with ice-cold PBS three times and incubated with Cy2-conjugated donkey anti-goat IgG (305-155-003, 1:500; Jackson ImmunoResearch, West Grove, PA), Cy2-conjugated goat anti-rabbit IgG (111-225-144, 1:500; Jackson ImmunoResearch), Cy3-conjugated goat anti-rabbit IgG (111-165-003, 1:500; Jackson ImmunoResearch), and Cy2-conjuated goat anti-mouse (115-225-003, 1:500; Jackson ImmunoResearch) at room temperature. The cells were examined by confocal microscopy (×200 and ×400, LSM710; Carl Zeiss, Jena, Germany).
In vitro invasion assay
In vitro invasion assays were performed in triplicate with an invasion assay kit with Matrigel-coated inserts (BD Bioscience, San Jose, CA), as described previously21. A total of 1 × 106 SKOV-3 and PA-1 cells per well were added to the upper compartment of the invasion chamber with or without pharmacologic inhibitors. To the lower compartment, we added serum-free conditioned medium. After incubation for 18 h at 37 °C, the invaded cells were sequentially fixed, stained with Diff-Quik reagents (Dade Behring Inc., Newark, DE), and quantitated by counting the number of cells in five random high-power fields for each replicate (×200) with a light microscope.
Three-dimensional Matrigel culture
We established three-dimensional (3-D) cultures with Matrigel (BD Biosciences). The SKOV-3 cells were suspended in 2% (v/v) Matrigel and seeded over a layer of polymerized 100% Matrigel at 3 × 104 cells/ml in eight-well chamber slides (Nunc, Littleton, CO). Cell culture medium was changed every second day. Cultures were analyzed after 7 days of cultivation.
Cell viability assay
Serum-starved cells were treated with or without curcumin and DOX for 24 h and then washed with PBS, followed by addition of 5 mg/ml MTT [(3,4,5-dimetylathiazol-2yl)-5-diphenyl-tetrazolium bromide]. After incubation for 2 h, cells were washed with PBS and combined with 100 μl dimethyl sulfoxide. The absorbance was measured at 540 nm using an ELISA plate reader (Bio-Rad Laboratories, Hercules, CA). The results are expressed as the percent decrease in cell viability compared with the control.
Statistical analysis
Data are shown as the mean ± standard deviation (s.d.). Differences between two groups were assessed using Student’s t-test. Differences among three or more groups were evaluated by analysis of variance followed by Bonferroni multiple comparison tests.
Results
FAK is important for RCP-induced ovarian cancer cell EMT
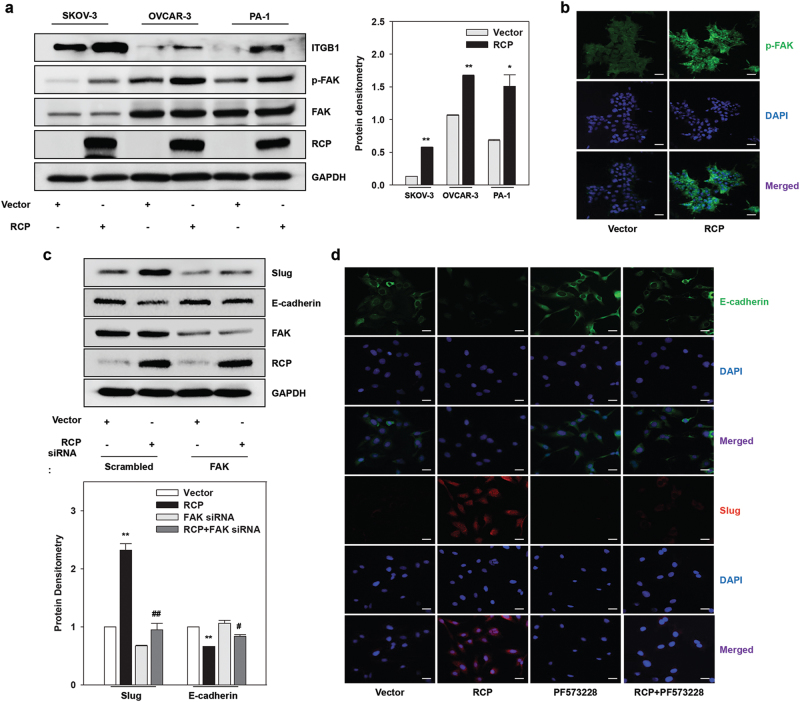
Previously, we showed that RCP induces ovarian cancer cell invasion through stabilizing the β1 integrin protein and inducing Slug expression10. To identify the role of FAK in RCP-induced ovarian cancer invasion, we first determined whether RCP activates FAK in ovarian cancer cells. Indeed, ectopic expression of RCP strongly increased FAK phosphorylation at Tyr397 to create a binding motif for various SH2-domain-containing proteins, including Src (Fig. 1a). Immunofluorescence data showed that RCP induces FAK phosphorylation (Fig. 1b). We next addressed the role of FAK in EMT of RCP-induced ovarian cancer cells. As reported previously10, RCP induced Slug expression (Fig. 1c). However, silencing of FAK expression abolished RCP-induced Slug expression along with recovery of E-cadherin expression that was reduced by RCP (Fig. 1c). Furthermore, treatment of the cells with a pharmacological inhibitor of FAK, PF573228, showed similar results to inhibition of RCP-induced Slug expression (Supplementary Figure 1). Immunofluorescence analysis also demonstrated that inhibition of FAK activation by PF573228 abolishes RCP-induced Slug expression and subsequently increases E-cadherin expression (Fig. 1d). Therefore, these data indicate that FAK is implicated in RCP-induced ovarian cancer cell EMT.
Fig. 1. FAK is important for RCP-induced ovarian cancer cell EMT.
a Ovarian cancer cells were transfected with the indicated vectors for 48 h. Immunoblotting (left). Immunoblot bands were quantified by Image J densitometric analysis and normalized to the control vector (right; mean ± s.d. *P < 0.05, **P < 0.01 vs. the control vector). b PA-1 cells were transfected with the indicated vectors, and the expression of FAK phosphorylation was visualized by immunofluorescence. Original magnification, ×200; scale bar, 20 μm. Representative results are presented from at least three independent experiments with similar results. c SKOV-3 cells were co-transfected with the indicated vectors and siRNAs for 48 h. Immunoblotting (upper). Densitometric analysis (lower; mean ± s.d. **P < 0.01 vs. the control vector, #P < 0.05, ##P < 0.01 vs. RCP overexpression with scrambled siRNA). d SKOV-3 cells were transfected with the indicated vectors for 48 h, serum-starved, and treated with PF573228 (10 μM) for 1 h, and the expression of E-cadherin and Slug was visualized by immunofluorescence. Original magnification, ×200; scale bar, 20 μm. All experiments were repeated three times
FAK is important for ovarian cancer cell invasion
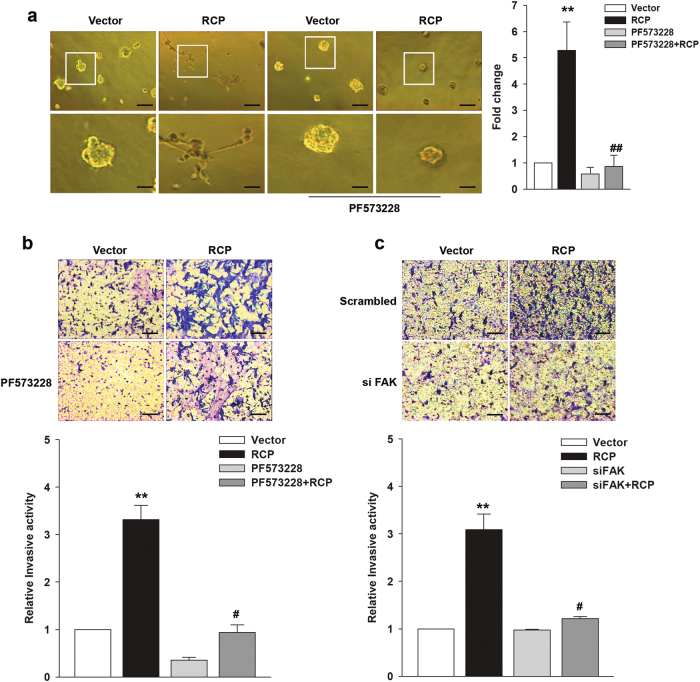
Since RCP is known to induce invasive migration of cancer cells9,10, we next determined the role of FAK in RCP-induced cancer cell invasion. Cells transfected with RCP substantially increased the number of invasive foci on 3-D Matrigel compared those transfected with the control vector (Fig. 2a). However, pretreatment of the cells with PF573228 abolished the RCP-induced increase in invasive foci, indicating that FAK is implicated in RCP-induced morphological changes of ovarian cancer cells. In addition, pretreatment of the cells with PF573228 (Fig. 2b and Supplementary Figure 2a) or transfection of the cells with FAK siRNA (Fig. 2c and Supplementary Figure 2b) significantly inhibited RCP-induced ovarian cancer cell invasion, indicating that FAK plays an indispensable role in RCP-induced ovarian cancer cell invasion.
Fig. 2. FAK is important for ovarian cancer cell invasion.
a Phase contrast images showing the morphology of stably transfected SKOV-3 cells with the indicated vectors followed by treatment with PF573228 (10 μM) for 4 days on Matrigel. Image original magnification ×100; scale bar, 200 μm. Inset shows higher magnification (left). The number of branches was counted and quantified (right); mean ± s.d. **P < 0.01 vs. the control vector, ##P < 0.01 vs. RCP overexpression only. b SKOV-3 cells were transfected with the indicated vectors for 48 h followed by treatment with PF573228 (10 μM) for 1 h. Invasion assay (mean ± s.d. **P < 0.01 vs. the control vector, #P < 0.05 vs. RCP overexpression only). c SKOV-3 cells were co-transfected with the indicated vectors and siRNAs for 48 h prior to invasion assays (mean ± s.d. **P < 0.01 vs. the control vector, #P < 0.05 vs. RCP overexpression with scrambled siRNA). Original magnification, ×200; scale bar, 200 μm. All experiments were repeated three times
FAK links β1 integrin with EGFR
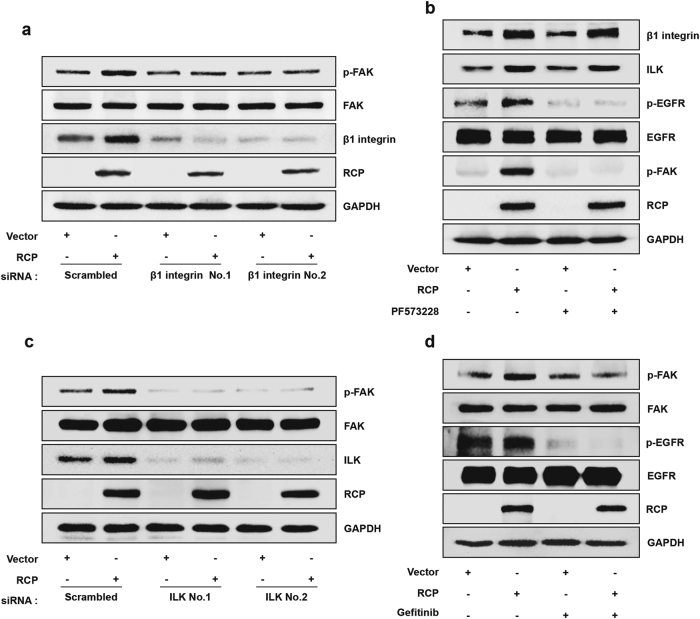
Previous studies noted that FAK is located in the recycling endosome and activated by β1 integrin22. However, the downstream target of FAK required for cancer cell invasion has not been identified. To determine the location of FAK in the RCP-induced signaling cascade, we transfected the cells with two different β1 integrin siRNAs. Silencing of β1 integrin expression strongly reduced RCP-induced FAK phosphorylation (Fig. 3a). Conversely, treatment of the cells with PF573228 did not affect RCP-induced β1 integrin expression (Fig. 3b), indicating that FAK is located downstream of β1 integrin. We observed very similar results with silencing of ILK; transfection of the cells with ILK siRNAs substantially inhibited RCP-induced FAK phosphorylation (Fig. 3c). However, treatment of the cells with PF573228 and a pharmacological inhibitor of EGFR, gefitinib, abrogated RCP-induced EGFR, and FAK phosphorylation, respectively (Fig. 3b, d). These findings indicate that FAK links β1 integrin with EGFR and participates in the positive regulation loop with EGFR in RCP-induced ovarian cancer cell invasion.
Fig. 3. FAK links β1 integrin with EGFR.
a SKOV-3 cells were co-transfected with the indicated vectors and siRNAs for 48 h. b SKOV-3 cells were transfected with the indicated vectors for 48 h, serum-starved, and treated with PF573228 (10 μM) for 1 h. c SKOV-3 cells were co-transfected with the indicated vectors and siRNAs for 48 h. d SKOV-3 cells were transfected with the indicated vectors for 48 h, serum-starved, and treated with gefitinib (1 μM) for 1 h. Immunoblotting. All experiments were repeated three times
Rab11 and Rab25 are required for RCP-induced FAK phosphorylation
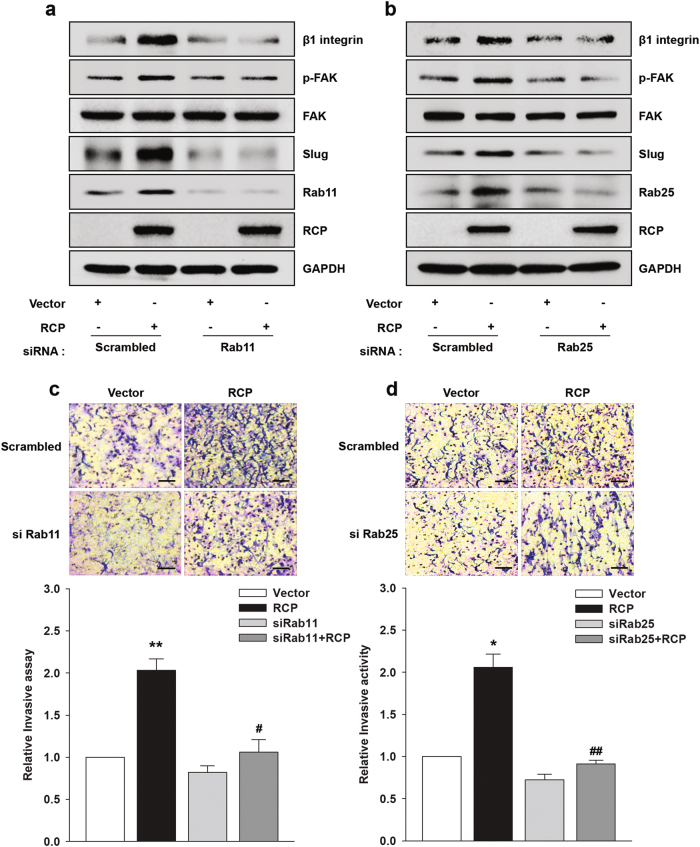
RCP has been shown to interact with Rab11 or Rab25 (refs. 23,24). In addition, Rab11 and Rab25 augment ovarian cancer cell invasion by recycling both integrins and EGFR25,26. Therefore, we decided to investigate whether Rab11 and Rab25 are prerequisite for RCP-induced FAK phosphorylation. Indeed, silencing of Rab11 or Rab25 expression markedly reduced RCP-induced β1 integrin expression and phosphorylation of FAK and EGFR (Fig. 4a, b). In addition, we observed that RCP induces the expression of Rab11 (Fig. 4a) and Rab25 (Fig. 4b). More importantly, Rab11 or Rab25 siRNA completely inhibited RCP-induced ovarian cancer cell invasion (Fig. 4c, d). Therefore, these data indicate that RCP regulates the levels of Rab11 and Rab25 and that both Rab11 and Rab25 are essential for RCP-induced ovarian cancer cell invasion.
Fig. 4. Rab11 and Rab25 are required for RCP-induced FAK phosphorylation.
a, b SKOV-3 cells were co-transfected with the indicated vectors and siRNAs for 48 h. Immunoblotting and c, d invasion assays (mean ± s.d. *P < 0.05, **P < 0.01 vs. the control vector, #P < 0.05, ##P < 0.01 vs. RCP overexpression with scrambled siRNA). Original magnification, ×200; scale bar, 200 μm. All experiments were repeated three times
Curcumin inhibits stabilization of β1 integrin and FAK phosphorylation
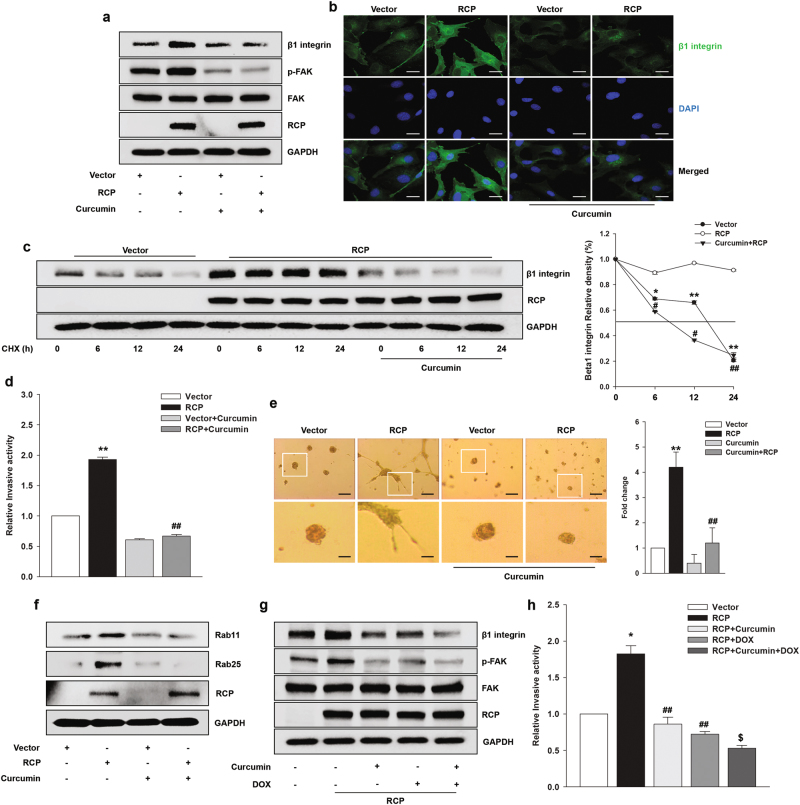
Since curcumin has been shown to inhibit FAK phosphorylation in various cancer cells12,15,27, we next investigated its effect on RCP-induced FAK phosphorylation. Treatment of the cells with 20 μM curcumin (Supplementary Figure 3) effectively inhibited RCP-induced FAK phosphorylation (Fig. 5a). Interestingly, we observed that curcumin also reduces RCP-induced β1 integrin expression. Immunofluorescence analysis confirmed the inhibitory effects of curcumin on RCP-induced β1 integrin expression (Fig. 5b). Since our previous report demonstrated that RCP stabilizes β1 integrin protein10, we next explored whether curcumin affects the stability of β1 integrin. When the cells were treated with CHX to block de novo protein synthesis, RCP increased the half-life of β1 integrin protein (Fig. 5c). However, treatment of the cells with curcumin significantly inhibited the RCP-induced increase in the half-life of β1 integrin. In addition, curcumin efficiently attenuated RCP-induced cancer cell invasion (Fig. 5d) and the number of invasive foci on 3-D Matrigel (Fig. 5e). These data, therefore, indicate that curcumin inhibits RCP-induced cancer cell invasion by reducing the recycling of β1 integrin and subsequent inactivation of FAK and EGFR. To determine the mechanism underlying curcumin-mediated reduction of RCP-induced recycling of β1 integrin, we compared the effects of curcumin on the levels of Rab11 and Rab25 in RCP-overexpressing cells. Interestingly, curcumin dramatically downregulated the expression of Rab11 and Rab25 induced by RCP to the level of the control (Fig. 5f), suggesting that losing partners of RCP reduces the endosome recycling of β1 integrin to the plasma membrane.
Fig. 5. Curcumin inhibits stabilization of β1 integrin protein and FAK phosphorylation.
a SKOV-3 cells were transfected the indicated vectors for 48 h, serum-starved, and treated with curcumin (20 μM) for 2 h. Immunoblotting. b The expression of β1 integrin was visualized by immunofluorescence. Original magnification, ×400; scale bar, 20 μm. c SKOV-3 cells were transfected with the indicated vectors for 48 h, serum-starved, and pretreated with curcumin (20 μM) for 2 h and then incubated with CHX (20 μg/ml) for the indicated times. Immunoblotting (left). Densitometric analysis (right; mean ± s.d. *P < 0.05, **P < 0.01 vs. the control vector, #P < 0.05, ##P < 0.01 vs. RCP overexpression only). d SKOV-3 cells were transfected with the indicated vectors for 48 h followed by treatment with curcumin (20 μM) for 1 h. Invasion assay (mean ± s.d. **P < 0.01 vs. the control vector, ##P < 0.01 vs. RCP overexpression only). e Phase contrast images showing the morphology of stably transfected SKOV-3 cells with the indicated vectors followed by treatment with curcumin (20 μM) for 4 days on Matrigel. Original magnification, ×100; scale bar, 200 μm. Inset shows higher magnification (left). The number of branches was counted and quantified (right); mean ± s.d. **P < 0.01 vs. the control vector, ##P < 0.01 vs. RCP overexpression only. f SKOV-3 cells were transfected with the indicated vectors for 48 h, serum-starved, and treated with curcumin (20 μM) for 1 h. Immunoblotting. g SKOV-3 cells were transfected with the indicated vectors for 48 h, serum-starved, and treated with curcumin (20 μM), DOX (0.001 μM), and curcumin with DOX. Immunoblotting and h invasion assay (mean ± s.d. *P < 0.05 vs. the control vector, ##P < 0.01 vs. RCP overexpression only, $P < 0.05 vs. RCP overexpression and DOX treatment). All experiments were repeated three times
Given that DOX in a PEGylated liposomal nanoencapsulation has been used for recurrent ovarian cancer28, we investigated whether curcumin potentiates the inhibitory effect of DOX on RCP-induced ovarian cancer cell invasion. Intriguingly, we observed increased inhibition of RCP-induced β1 integrin expression (Fig. 5g) and ovarian cancer cell invasion (Fig. 5h) when the cells were treated with both curcumin and DOX at a dose with an over 85% survival rate (Supplementary Figure 4) compared with treatment with single agent, suggesting that DOX potentiates the inhibitory effects of curcumin on RCP-induced β1 integrin expression and FAK phosphorylation as well as the consequent ovarian cancer cell invasion.
Discussion
Herein, we elucidated the essential role of FAK in RCP-induced cancer cell invasion and its inhibition by curcumin. Ectopic expression of RCP induced FAK phosphorylation, which creates a positive feedback loop with EGFR. In addition, FAK acts as a link between the β1 integrin/ILK axis and EGFR, leading to ovarian cancer cell EMT and invasion. Furthermore, our present data demonstrated that both Rab11 and Rab25 are essential for RCP-induced FAK phosphorylation and that curcumin inhibits RCP-induced FAK phosphorylation and ovarian cancer invasion by blocking stabilization of β1 integrin. Finally, we provided evidence that DOX potentiates the inhibitory effect of curcumin on RCP-induced stabilization of β1 integrin and on FAK phosphorylation, leading to attenuation of RCP-induced ovarian cancer cell invasion.
RCP was initially identified as a Rab11/Rab4/Rab25-interacting protein with putative physiological roles in endosomal trafficking and receptor sorting23,29,30. RCP was co-localized with Rab11 and required for recycling of integrin a5β1 at the tips of the long pseudopods31. Furthermore, RCP was proposed to be a key component of an integrin recycling system through binding with Rab25 and Rab11 (ref. 32). Recently, the Rab25 and RCP expression levels were shown to be coordinately regulated with a positive feedback loop33. Consistent with this finding, we demonstrated in the present study that RCP induces the expression of Rab11 and Rab25, suggesting that it amplifies the common signaling of RCP, Rab11 and Rab25 for ovarian cancer cell invasion.
We recently showed that RCP induces ovarian cancer cell EMT through coordinate activation of the β1 integrin/ILK/EGFR signaling axis and Slug expression10. Ectopic expression and silencing of RCP induced and reduced ovarian cancer cell EMT, respectively10. In addition, FAK is located downstream of β1 integrin34 and implicated in ovarian cancer cell metastasis35. Our present study further elucidated the underlying mechanism of RCP-induced ovarian cancer cell invasion and identified the downstream target of FAK. We show that FAK positioned between ILK and EGFR activates EGFR, which in turn phosphorylates FAK, leading to a positive feedback loop in RCP-induced ovarian cancer cell invasion. We note that these results should be verified in physiological conditions.
Curcumin was shown to have anti-cancer and anti-metastatic properties. In addition to reducing expression of various metastasis-related factors36, curcumin was recently shown to inhibit EMT of breast cancer stem cells by suppressing Slug expression37. Furthermore, curcumin was reported to suppress IL-1β-induced β1 integrin expression in human chondrocytes38. Unexpectedly, our present data showed that curcumin hampers RCP-induced recycling of β1 integrin, thereby blocking activation of FAK and the EGFR/Slug signaling axis and ovarian cancer cell invasion. The underlying mechanism of reduced recycling of RCP-induced β1 integrin by curcumin appears to be caused by downregulation of a critical partner of RCP, Rab11 and Rab25 expression, since curcumin strongly reduced the levels of RCP-induced Rab11 and Rab25 expression. Moreover, we provide evidence that a combination of DOX with curcumin enhances the anti-invasive properties by reducing the recycling of β1 integrin and FAK activation in ovarian cancer cells compared with single treatment, providing a potential therapeutic armament for this deadly disease.
Collectively, our results demonstrated that RCP increases ovarian cancer cell invasion through the β1 integrin/ILK/FAK and EGFR signaling pathway and Slug expression and that curcumin efficiently reduces RCP-induced stabilization of β1 integrin protein, leading to attenuation of RCP-induced ovarian cancer cell invasion.
Electronic supplementary material
Acknowledgements
This study was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology [NRF-2016R1D1A1B04931788, NRF-2017R1E1A1A01074091].
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: So Ra Choe, Yu Na Kim.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims inpublished maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s12276-018-0078-1.
Contributor Information
Do Yeun Cho, Phone: +82-42-600-6921, Email: dycho@kyuh.ac.kr.
Hoi Young Lee, Phone: +82-42-600-6413, Email: hoi@konyang.ac.kr.
References
- 1.Ozols RF, et al. Cancer Cell. 2004. Focus on epithelial ovarian cancer; pp. 19–24. [DOI] [PubMed] [Google Scholar]
- 2.Zhou ES, Partridge AH, Syrjala KL, Michaud AL, Recklitis CJ. Evaluation and treatment of insomnia in adult cancer survivorship programs. J. Cancer Surviv. 2017;11:74–79. doi: 10.1007/s11764-016-0564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cormio G, et al. Distant metastases in ovarian carcinoma. Int. Gynecol. Cancer. 2003;13:125–129. doi: 10.1046/j.1525-1438.2003.13054.x. [DOI] [PubMed] [Google Scholar]
- 4.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 5.Vergara D, et al. Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett. 2010;291:59–66. doi: 10.1016/j.canlet.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11:213. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, et al. RCP is a human breast cancer-promoting gene with Ras-activating function. J. Clin. Invest. 2009;119:2171–2183. doi: 10.1172/JCI37622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morello V, et al. beta1 integrin controls EGFR signaling and tumorigenic properties of lung cancer cells. Oncogene. 2011;30:4087–4096. doi: 10.1038/onc.2011.107. [DOI] [PubMed] [Google Scholar]
- 9.Jacquemet G, et al. RCP-driven alpha5beta1 recycling suppresses Rac and promotes RhoA activity via the RacGAP1-IQGAP1 complex. J. Cell Biol. 2013;202:917–935. doi: 10.1083/jcb.201302041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang MH, et al. RCP induces Slug expression and cancer cell invasion by stabilizing beta1 integrin. Oncogene. 2017;36:1102–1111. doi: 10.1038/onc.2016.277. [DOI] [PubMed] [Google Scholar]
- 11.Mills GB, Jurisica I, Yarden Y, Norman JC. Genomic amplicons target vesicle recycling in breast cancer. J. Clin. Invest. 2009;119:2123–2127. doi: 10.1172/JCI40256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leu TH, Su SL, Chuang YC, Maa MC. Direct inhibitory effect of curcumin on Src and focal adhesion kinase activity. Biochem. Pharmacol. 2003;66:2323–2331. doi: 10.1016/j.bcp.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S, et al. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol. Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 14.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J. Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 15.Seo JH, et al. Lysophosphatidic acid induces STAT3 phosphorylation and ovarian cancer cell motility: their inhibition by curcumin. Cancer Lett. 2010;288:50–56. doi: 10.1016/j.canlet.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Ji C, et al. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother. Pharmacol. 2008;62:857–865. doi: 10.1007/s00280-007-0674-6. [DOI] [PubMed] [Google Scholar]
- 17.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 18.Reif S, et al. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J. Biol. Chem. 2003;278:8083–8090. doi: 10.1074/jbc.M212927200. [DOI] [PubMed] [Google Scholar]
- 19.You D, et al. FAK mediates a compensatory survival signal parallel to PI3K-AKT in PTEN-null T-ALL cells. Cell Rep. 2015;10:2055–2068. doi: 10.1016/j.celrep.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alanko J, Ivaska J. Endosomes: emerging platforms for integrin-mediated FAK signalling. Trends Cell Biol. 2016;26:391–398. doi: 10.1016/j.tcb.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Jeong KJ, et al. The Rho/ROCK pathway for lysophosphatidic acid-induced proteolytic enzyme expression and ovarian cancer cell invasion. Oncogene. 2012;31:4279–4289. doi: 10.1038/onc.2011.595. [DOI] [PubMed] [Google Scholar]
- 22.Nader GP, Ezratty EJ, Gundersen GG. FAK, talin and PIPKIgamma regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat. Cell Biol. 2016;18:491–503. doi: 10.1038/ncb3333. [DOI] [PubMed] [Google Scholar]
- 23.Hales CM, et al. Identification and characterization of a family of Rab11-interacting proteins. J. Biol. Chem. 2001;276:39067–39075. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- 24.Lindsay AJ, et al. Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J. Biol. Chem. 2002;277:12190–12199. doi: 10.1074/jbc.M108665200. [DOI] [PubMed] [Google Scholar]
- 25.Cheng KW, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat. Med. 2004;10:1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 26.Tang BL, Ng EL. Rabs and cancer cell motility. Cell Motil. Cytoskeleton. 2009;66:365–370. doi: 10.1002/cm.20376. [DOI] [PubMed] [Google Scholar]
- 27.Chen CC, et al. Curcumin suppresses metastasis via Sp-1, FAK inhibition, and E-Cadherin upregulation in colorectal cancer. Evid. Complement Altern. Med. 2013;2013:541695. doi: 10.1155/2013/541695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelberth SA, Hempel N, Bergkvist M. Development of nanoscale approaches for ovarian cancer therapeutics and diagnostics. Crit. Rev. Oncog. 2014;19:281–315. doi: 10.1615/CritRevOncog.2014011455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyers JM, Prekeris R. Formation of mutually exclusive Rab11 complexes with members of the family of Rab11-interacting proteins regulates Rab11 endocytic targeting and function. J. Biol. Chem. 2002;277:49003–49010. doi: 10.1074/jbc.M205728200. [DOI] [PubMed] [Google Scholar]
- 30.Peden AA, et al. The RCP-Rab11 complex regulates endocytic protein sorting. Mol. Biol. Cell. 2004;15:3530–3541. doi: 10.1091/mbc.e03-12-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caswell PT, et al. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J. Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal R, Jurisica I, Mills GB, Cheng KW. The emerging role of the RAB25 small GTPase in cancer. Traffic. 2009;10:1561–1568. doi: 10.1111/j.1600-0854.2009.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitra S, et al. Rab25 acts as an oncogene in luminal B breast cancer and is causally associated with Snail driven EMT. Oncotarget. 2016;7:40252–40265. doi: 10.18632/oncotarget.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buczek-Thomas JA, Chen N, Hasan T. Integrin-mediated adhesion and signalling in ovarian cancer cells. Cell Signal. 1998;10:55–63. doi: 10.1016/S0898-6568(97)00074-0. [DOI] [PubMed] [Google Scholar]
- 35.Chen CH, et al. MUC20 promotes aggressive phenotypes of epithelial ovarian cancer cells via activation of the integrin beta1 pathway. Gynecol. Oncol. 2016;140:131–137. doi: 10.1016/j.ygyno.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 36.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee S, et al. Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/beta-catenin negative feedback loop. Stem Cell Res. Ther. 2014;5:116. doi: 10.1186/scrt506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shakibaei M, Schulze-Tanzil G, John T, Mobasheri A. Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: an immunomorphological study. Ann. Anat. 2005;187:487–497. doi: 10.1016/j.aanat.2005.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.