Abstract
MK801 and ketamine, which are phencyclidine (PCP) derivative N-methyl-d-aspartate receptor (NMDAr) blockers, reportedly enhance the function of 5-hydroxytryptamine (HT)-2A receptors (5-HT2ARs). Both are believed to directly affect the pathogenesis of schizophrenia, as well as hypertension. 5-HT2AR signaling involves the inhibition of Kv conductance. This study investigated the interaction of these drugs with Kv1.5, which plays important roles in 5-HT2AR signaling and in regulating the excitability of the cardiovascular and nervous system, and the potential role of this interaction in the enhancement of the 5-HT2AR-mediated response. Using isometric organ bath experiments with arterial rings and conventional whole-cell patch-clamp recording of Chinese hamster ovary (CHO) cells ectopically overexpressing Kv1.5, we examined the effect of ketamine and MK801 on 5-HT2AR-mediated vasocontraction and Kv1.5 channels. Both ketamine and MK801 potentiated 5-HT2AR-mediated vasocontraction. This potentiation of 5-HT2AR function occurred in a membrane potential-dependent manner, indicating the involvement of ion channel(s). Both ketamine and MK801 rapidly and directly inhibited Kv1.5 channels from the extracellular side independently of NMDArs. The potencies of MK801 in facilitating the 5-HT2AR-mediated response and blocking Kv1.5 were higher than those of ketamine. Our data demonstrated the direct inhibition of Kv1.5 channels by MK801/ketamine and indicated that this inhibition may potentiate the functions of 5-HT2ARs. We suggest that 5-HT2AR-Kv1.5 may serve as a receptor-effector module in response to 5-HT and is a promising target in the pathogenesis of MK801-/ketamine-induced disease states such as hypertension and schizophrenia.
Serotonin: Flipping a cellular switch
The drugs ketamine and MK801, which are derivatives of phencyclidine (PCP, or angel dust), may provide clues to treatment of schizophrenia and hypertension. Both ketamine and MK801 have been reported to induce symptoms of schizophrenia and hypertension, and are used as to study these illnesses. The two drugs are known to affect serotonin receptors, but the mechanism remains unclear. Young Min Bae at Konkuk University School of Medicine, Chungju, South Korea, and colleagues investigated how ketamine and MK801 interact with a type of electrically activated biological switch known as a voltage-gated ion channel to influence serotonin receptors. They found that both ketamine and MK801 blocked the switch and enhanced activity of serotonin receptors, with MK801 having a stronger effect than ketamine. These results may help identify drug targets for treating hypertension and schizophrenia.
Introduction
MK801 and ketamine are derivatives of phencyclidine (PCP), which is also known as angel dust1,2. These PCP-related drugs are well known to block the ionotropic N-methyl-d-aspartate receptor (NMDAr) by non-competitively binding to the internal ionic pore region of NMDAr1–3. These PCP-related NMDAr antagonists have been reported to induce various clinical symptoms, such as psychosis, schizophrenia, and hypertension. However, the mechanisms underlying these symptoms are unclear and controversial4–7. The direct effects of ketamine and PCP on dopamine D2 and serotonin 5-hydroxytryptamine (HT)2 receptors have been suggested to be implicated in the pathogenesis of schizophrenia8–11. In agreement with this, a previous study showed that 5-HT2A receptor (5-HT2AR)-mediated arterial contraction was facilitated by ketamine12, which was suggested to be the mechanism underlying ketamine-induced hypertension. In addition, NMDAr antagonists, including MK801 and ketamine, enhanced the head-twitch response, a 5-HT2R-mediated behavior, in reserpine-treated mice13.
Voltage-gated K+ channel (Kv) currents in arterial smooth muscle cells have been reported to be blocked by ketamine and MK80114,15. However, reports on the effects of MK801 or ketamine on the specific subtype(s) of Kv are not available yet. Because Kv channels such as Kv1.5 in the arterial smooth muscle play a critical role in 5-HT2AR signaling16–18, whether Kv1.5 is blocked by MK801 and ketamine is worth examining. Moreover, Kv1.5 plays critical roles in regulating the membrane excitabilities of atrial cardiomyocytes19,20 and several neuronal and glial cells, such as pituitary neurons and Schwann cells21,22. In this study, we report that MK801 and ketamine facilitated the response of 5-HT2AR activation in a membrane potential (Em)-dependent manner and directly blocked Kv1.5 channels from the extracellular side. From these findings, we suggest that 5-HT2AR-Kv1.5 may play an important role as a receptor-effector module in response to 5-HT. Moreover, 5-HT2AR-Kv1.5 is a promising target of MK801 and ketamine in the pathogenesis of clinical symptoms induced by these PCP derivative NMDAr antagonists.
Materials and methods
Animals and tissue preparation
All experiments were conducted in accordance with the National Institutes of Health guidelines for the care and use of animals. The Institutional Animal Care and Use Committee of Konkuk University approved this study. Mesenteric arterial rings and aorta rings were prepared, as previously described17,23. The carotid arteries of male Sprague-Dawley (SD) rats (10–11 weeks old) were cut to exsanguinate the rats under deep ketamine-xylazine anesthesia or after exposure to 100% carbon dioxide. The branches of the superior mesenteric arteries and thoracic aorta were promptly isolated and placed in physiological saline solution (PSS) containing 136.9 mM NaCl, 5.4 mM KCl, 1.5 mM CaCl2, 1.0 mM MgCl2, 23.8 mM NaHCO3, 1.2 mM NaH2PO4, 0.01 mM ethylenediaminetetraacetic acid (EDTA), and 5.5 mM glucose. The arteries were carefully cleaned of fat and connective tissues under a stereomicroscope and prepared as rings (3.5 mm in length) for tension measurements. The endothelium was mechanically removed with a fine stainless-steel wire. The endothelial removal was confirmed by the absence of relaxation induced by acetylcholine (10 μM) after norepinephrine (NE; 1–10 μM) or 5-HT (1–10 μM)-induced contraction.
Tension measurements
The isometric tension of the arterial rings was measured, as previously described17,23. The arterial rings were mounted vertically on two L-shaped stainless-steel wires in a 3-mL tissue chamber. One wire was attached to a micromanipulator and the other to an isometric force transducer (FT03; Grass, West Warwick, RI, USA). The changes in isometric force were digitally acquired at 1 Hz with a PowerLab data acquisition system (ADInstruments, Colorado Springs, CO, USA). Resting tension was set to 1 g (mesenteric arterial rings) or 2 g (aorta) by the micromanipulator. After equilibration for 60 min under resting tension in a tissue chamber filled with PSS, the rings were sequentially exposed to 70 mM KCl PSS (10 min) and PSS (15 min) thrice for stabilization. High KCl (70 mM) PSS was prepared by replacing NaCl with equimolar KCl in PSS. The bathing solutions were thermostatically controlled at 37 °C and continuously saturated with a mixture of 95% O2 and 5% CO2 to achieve a pH of 7.4.
Cell culture and transfection
Chinese hamster ovary (CHO) cells expressing Kv1.5 derived from the rat brain were used for electrophysiological recordings24,25. Kv1.5 cDNA26 was transferred into the plasmid expression vector pCR3.1 (Invitrogen Corporation, San Diego, CA, USA). CHO cells were transfected with Kv1.5 cDNA using FuGENE™6 reagent (Boehringer Mannheim, Indianapolis, IN, USA). The transfected cells were cultured in Iscove’s modified Dulbecco’s medium (Invitrogen Corporation) supplemented with 10% fetal bovine serum, 2 mM glutamine, 0.1 mM hypoxanthine, 0.01 mM thymidine, and 300 µg/mL G418 (A.G. Scientific, San Diego, CA, USA) in a 95% humidified air-5% CO2 incubator at 37 °C. The cultures were passaged every 4–5 days with a brief trypsin-EDTA treatment and then seeded onto glass coverslips (diameter: 12 mm, Fisher Scientific, Pittsburgh, PA, USA) in a Petri dish. After 12–24 h, the cell-attached coverslips were used for electrophysiological recordings.
Electrophysiology
Kv1.5 currents were recorded from CHO cells using the whole-cell patch-clamp technique27 at room temperature (22–23 °C). Micropipettes fabricated from glass capillary tubing (PG10165-4; World Precision Instruments, Sarasota, FL, USA) with a double-stage vertical puller (PC-10; Narishige, Tokyo) had a tip resistance of 2–3 MΩ when filled with the pipette solution. Whole-cell currents were amplified with an Axopatch 200 B amplifier (Molecular Devices, Sunnyvale, CA, USA), digitized with the Digidata 1440 A (Molecular Devices) at 5 kHz, and low-pass filtered with a four-pole Bessel filter at 2 kHz. Capacitive currents were canceled, and series resistance was compensated at 80% with the amplifier, while leak subtraction was not used. The generation of voltage commands and acquisition of data were controlled with pClamp 10.1 software (Molecular Devices) running on an IBM-compatible Pentium computer. The recording chamber (RC-11, Warner Instrument Corporation, Hamden, CT, USA) was continuously perfused with the bath solution (see below for composition) at a rate of 1 mL/min.
The intracellular pipette solution for whole-cell recordings contained 140 mM KCl, 5 mM NaCl, 5 mM MgATP, 10 mM 4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid (HEPES), and 10 mM 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) and was adjusted to a pH of 7.2 with KOH. The bath solution for whole-cell recordings contained 143 mM NaCl, 5.4 mM KCl, 0.33 mM NaH2PO4, 1.8 mM CaCl2, 0.5 mM MgCl2, 5 mM HEPES, and 11 mM glucose and was adjusted to a pH of 7.35–7.40 with NaOH.
Drugs
All chemicals, including ketamine and MK801, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Data analysis
Origin 8.0 software (Microcal Software, Inc., Northampton, MA, USA) was used for data analysis. The results are shown as the means ± SEM. Paired or independent Student’s t-tests were performed to test for significance as appropriate, and p < 0.05 was regarded as significant. For analysis of the concentration–response curves (CRCs), Student’s t-tests were performed at a given concentration to compare the two groups. When the two groups were not statistically different at any given concentration, the differences in the CRCs were determined to be N.S. (not significant) (Fig. 1c–e, g, and Fig. 2c).
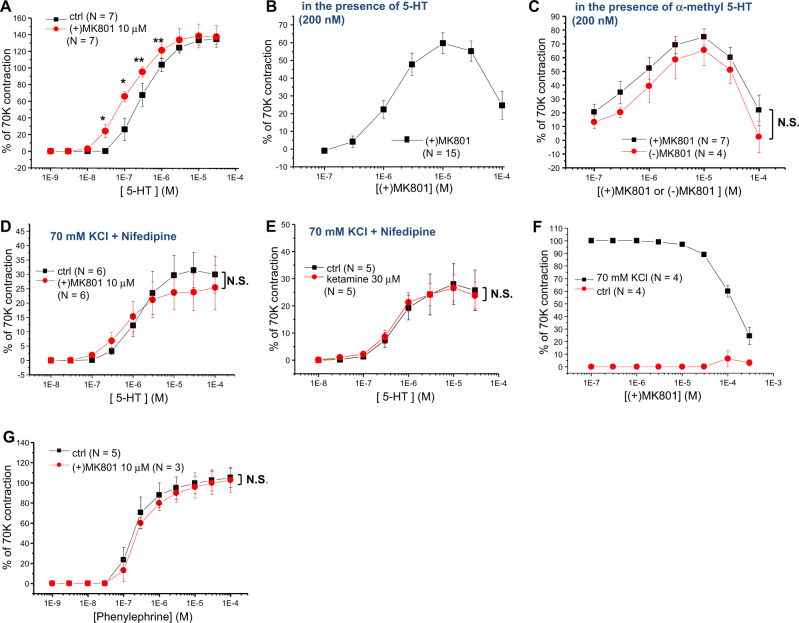
Fig. 1. Facilitation of 5-HT2AR-mediated arterial contractions by MK801 and ketamine.
a Concentration-response curves (CRCs) of 5-HT-induced rat mesenteric arterial contractions in the absence and presence of MK801. b CRC of MK801-induced rat mesenteric arterial contractions in the presence of a physiological concentration (200 nM) of 5-HT. c CRC of (+) and (−) MK801-induced rat mesenteric arterial contractions in the presence of 200 nM of α-methyl 5-HT, a selective 5-HT2AR agonist. d Membrane potential (Em)-independent CRCs of 5-HT-induced rat mesenteric arterial contractions in the absence and presence of MK801 after pretreatment with 70 mM KCl and nifedipine (1 µM). e Em-independent CRCs of 5-HT-induced rat mesenteric arterial contractions in the absence and presence of ketamine after pretreatment with 70 mM KCl and nifedipine (1 µM). f CRC of MK801-induced rat mesenteric arterial contractions in the absence and presence of high (70 mM) KCl. g CRCs of phenylephrine-induced rat aorta contractions in the absence and presence of MK801. N in parentheses indicates the number of animals examined. *p < 0.05; **p < 0.01; N.S. not significant
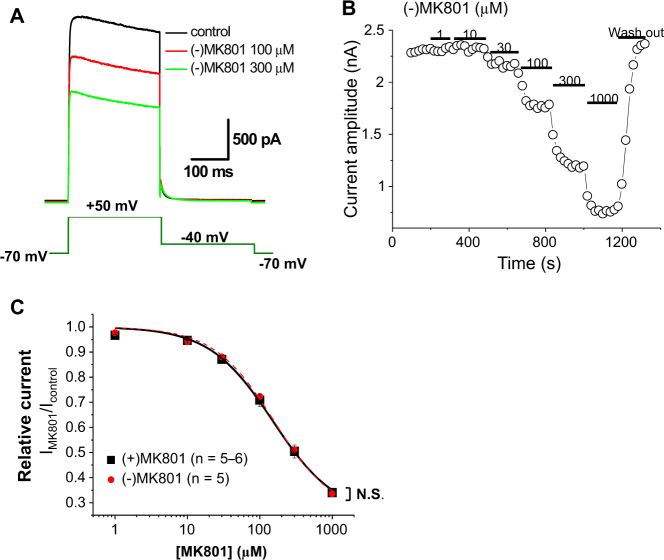
Fig. 2. Inhibition of Kv1.5 currents by MK801 enantiomers recorded from CHO cells overexpressing Kv1.5.
a Representative Kv1.5 current tracings elicited by voltage steps shown in the figure inset in the absence and presence of MK801 (100 and 300 µM). b Kv 1.5 current amplitudes at + 50 mV are plotted against time. The period of application of various concentrations of MK801 is indicated as solid bars. c CRCs of (+) and (−) MK801 enantiomer-induced Kv1.5 inhibition. N in parentheses indicates the number of cells examined. N.S. not significant
Results
Em-dependent facilitation of the 5-HT2AR-mediated arterial contraction induced by MK801 and ketamine
5-HT2ARs have been previously reported to mediate the effect of 5-HT in rat mesenteric arteries17, and ketamine has been shown to facilitate 5-HT2AR-mediated contraction without unmasking other subtypes of 5-HT receptors, such as 5-HT1B or 5-HT2B receptors12. In this study, we examined whether MK801, another PCP derivative, also has similar effects on 5-HT2AR-mediated arterial contraction. The CRC of 5-HT-induced contraction of rat mesenteric arteries was evidently shifted to the left in the presence of MK801 (10 µM, Fig. 1a), indicating that MK801 also facilitated the 5-HT2AR-mediated response in rat mesenteric arteries. Furthermore, in the presence of a physiologically relevant concentration of 5-HT (200 nM, which is close to the resting plasma level of 5-HT), MK801 contracted the mesenteric arterial rings (Fig. 1b) in a concentration-dependent manner. However, MK801 alone, similar to ketamine12, had a negligible effect on arterial contraction in the control condition without 5-HT (Fig. 1f). The CRCs of MK801-induced arterial contractions in the presence of 200 nM α-methyl 5-HT, which is a selective agonist of 5-HT2Rs, were similar between the (+) and (−) MK801 enantiomers (Fig. 1c), although the potencies of these optical isomers in blocking NMDArs are known to be quite different1,2.
To further examine whether this facilitating effect of MK801 on 5-HT2AR-mediated arterial contraction is related to ion channel regulation, we examined the effect of MK801 on Em-independent arterial contraction. The Em-independent arterial contraction was isolated by pretreatment of the arterial rings with both nifedipine (1 µM) and high KCl (70 mM)17. Notably, the left shift of the CRC of the 5-HT2AR-mediated arterial contraction by MK801 was not observed when the Em-dependent 5-HT response was excluded (Fig. 1d). Similarly, the facilitating effect of ketamine12 was not observed when the Em-dependent 5-HT response was excluded (Fig. 1e). These results suggest that the PCP derivatives MK801 and ketamine facilitate the 5-HT2AR-mediated response by regulating ion channels and Em. In support of this Em-dependent facilitation hypothesis, MK801 did not contract the high (70 mM) KCl-precontracted arterial rings (Fig. 1f).
To define whether the facilitating effects of MK801 on arterial contraction were receptor-specific, we further examined the effect of MK801 on the CRCs of phenylephrine-induced vasocontraction. In contrast to the contractions induced by 5-HT, MK801 had negligible effects on the CRCs of phenylephrine-induced vasocontraction (Fig. 1g).
Direct inhibition of Kv1.5 by MK801 and ketamine from the extracellular side
The response of 5-HT2ARs is mediated by a decrease in Kv conductance9,16–18. Noticeably, hallucinogens other than PCP derivatives (such as LSD) have also been reported to modulate Kv channel activity, acting at 5-HT2Rs9. In particular, Kv1.5 has been reported to be primarily responsible for mediating 5-HT2AR activation in the arteries18. If MK801 and ketamine inhibit Kv1.5, these drugs could be hypothesized to augment 5-HT2AR signaling by facilitating 5-HT2AR-mediated Kv1.5 inhibition and Em depolarization. To address this hypothesis, we examined the effect of MK801 on Kv1.5 currents in CHO cells stably overexpressing Kv1.5. MK801 concentration-dependently [IC50 = 156.8 ± 7.9 μM, Hill coefficient = 1.03 ± 0.05 for (−) MK801; IC50 = 157.3 ± 14.0 μM, Hill coefficient = 0.93 ± 0.03 for (+) MK801] inhibited Kv1.5 currents (Fig. 2a–c). The inhibition of Kv1.5 currents upon MK801 application was rapid and reversible (Fig. 2b). The optical isomers of MK801 [(+) and (−) MK801] inhibited Kv1.5 currents with similar potency and efficacy (Fig. 2a, c).
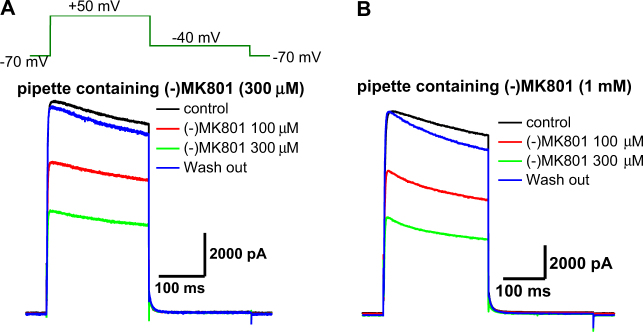
To determine whether MK801 blocks Kv1.5 from the extracellular or intracellular side, we examined the effect of MK801 using a pipette containing MK801. When the Kv1.5 currents were recorded with a pipette containing MK801 (up to 1 mM), the amplitudes of Kv1.5 currents were still comparable to those recorded under the control condition without MK801 in the pipette (Fig. 3). Moreover, Kv1.5 current inhibition by the bath application of MK801 was not affected by MK801 in the pipette (Fig. 2a, c). These results indicate that MK801 inhibited Kv1.5 from the extracellular side.
Fig. 3. Inhibition of Kv1.5 currents by extracellular MK801 recorded with MK801-containing pipettes.
a Representative Kv1.5 current tracings elicited by voltage steps shown in the figure inset with the pipette containing 300 µM MK801 in the absence and presence of various concentrations of MK801 in the bath. b Representative Kv1.5 current tracings with the pipette containing 1 mM MK801 in the absence and presence of various concentrations of MK801 in the bath. Similar results from 3 further independent experiments
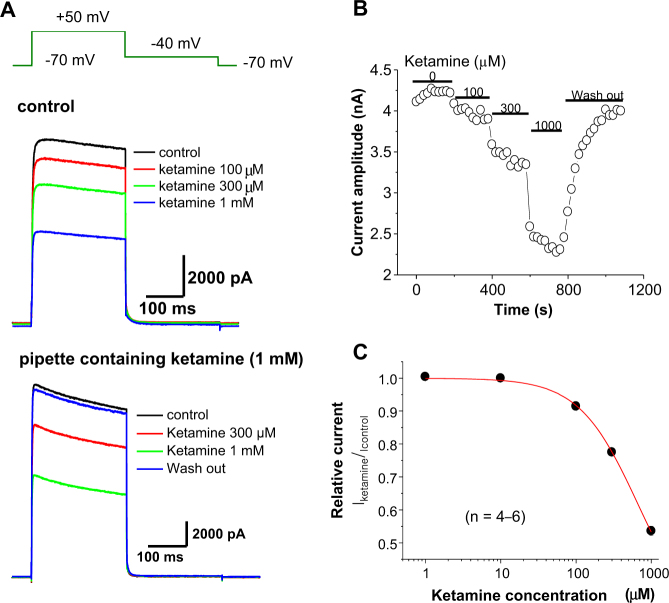
Similarly, ketamine [(+) and (−) racemate] inhibited Kv1.5 from the extracellular side (Fig. 4a). The ketamine-induced Kv1.5 inhibition was also rapid and reversible after wash-out (Fig. 4b). The potency of ketamine in inducing Kv1.5 inhibition was relatively lower than that of MK801 (IC50 = 640.5 ± 33.2 μM, Hill coefficient = 1.12 ± 0.06, Fig. 4a–c).
Fig. 4. Inhibition of Kv1.5 by ketamine.
a (upper) Representative Kv1.5 current tracings elicited by voltage steps shown in the figure inset in the absence and presence of ketamine (100, 300, and 1000 µM); lower, Representative Kv1.5 current tracings recorded with the pipette containing ketamine (1000 µM) in the absence and presence of bath ketamine (100, 300, and 1000 µM). b Kv1.5 current amplitudes at +50 mV are plotted against time. The period of application of various concentrations of ketamine is indicated as solid bars. c CRCs of ketamine-induced Kv1.5 inhibition. N in parentheses indicates the number of cells examined
Discussion
In our previous study, we reported that ketamine facilitated 5-HT2AR-mediated arterial contraction12. The findings of this study further demonstrated that MK801, another PCP derivative NMDAr antagonist, also similarly facilitated 5-HT2AR-mediated arterial contraction. The facilitation of 5-HT2AR-mediated arterial contraction by MK801/ketamine was Em-dependent, indicating that ion channel regulation is critically involved. Accordingly, Kv1.5 inhibition was suggested to play a role in mediating the facilitating effect of MK801/ketamine. To the best of our knowledge, this is the first report to show Em-dependent facilitation of 5-HT2AR-mediated arterial contraction, as well as Kv1.5 inhibition by MK801 and ketamine. We believe that the findings of this study suggest that 5-HT2AR-Kv1.5 may function as an important receptor-effector module and an important target of MK801 and ketamine.
Signaling of 5-HT2ARs and their regulation by MK801 and ketamine
Neither the facilitation of the 5-HT2AR response nor the inhibition of Kv1.5 by MK801/ketamine shown in this study were related to NMDAr regulation based on the following observations: (1) MK801/ketamine inhibited Kv1.5, which was overexpressed in CHO cells. (2) The inhibition of Kv1.5 by MK801 was not stereospecific, although inhibition of NMDArs by MK801 is known to be stereospecific. (3) The facilitation of 5-HT2AR-mediated arterial contraction by MK801 was not stereospecific either. These observations indicate that MK801/ketamine interacted with the 5-HT2AR-Kv1.5 receptor-effector module. In addition, MK801-induced facilitation was receptor-specific; 5-HT2ARs but not α-adrenoceptors were facilitated by MK801 (Fig. 1g).
In the rat aorta and mesenteric arteries, 5-HT2AR activation has been reported to be followed by Src phosphorylation and consequent Kv inhibition17,28. Because Kv inhibition is a downstream effector step in 5-HT2AR-mediated signaling, ~70% of 5-HT2AR-mediated arterial contraction was dependent on Em depolarization17. In this regard, as a mechanism for MK801/ketamine-induced facilitation of 5-HT2ARs, the direct interaction of MK801 and ketamine with 5-HT2ARs8 and consequent facilitation of Src phosphorylation17 could be reasoned to contribute to the MK801 and ketamine-induced facilitation of the 5-HT2AR-mediated response. In agreement with this hypothesis, a previous study reported that PCP derivatives have some direct partial agonist or allosteric activator effects on 5-HT2 and D2 receptors8,11. Alternatively, as shown in this study, the direct inhibition of Kv1.5 by MK801/ketamine could potentiate the Kv1.5 inhibition mediated by 5-HT2AR activation. Notably, the facilitating concentrations of MK801 (maximal at ~10 µM) and ketamine (maximal at ~30 µM) on 5-HT2ARs are relatively lower than the inhibiting concentrations of MK801 and ketamine on Kv1.5 (IC50 = ~150 and ~600 nM, respectively). The native 4-aminopyridine-sensitive Kv currents in the mesenteric arterial smooth muscle cells were also inhibited by MK801 and ketamine with similar potencies as the Kv1.5 current14,15. We interpreted these observations to indicate that low concentrations (around threshold for Kv1.5 inhibition) of MK801 and ketamine make Kv1.5 ready to be blocked by low concentrations of 5-HT, i.e., cooperative actions of threshold levels of 5-HT on 5-HT2ARs and MK801/ketamine on Kv1.5 facilitate 5-HT action. This interpretation likely explains the finding that MK801/ketamine only shifted the CRCs of 5-HT-induced arterial contraction without increasing the maximal efficacy (Fig. 1a). Furthermore, the facilitating actions of MK801 and ketamine notably occurred at their clinical concentrations with physiological concentrations of 5-HT, reinforcing their clinical relevance. At concentrations of MK801 above 30 µM, the facilitating effect on 5-HT2AR-mediated arterial contraction was decreased (Fig. 1b, c), although the inhibitory effect on Kv1.5 became stronger (Figs. 2 and 3). A similar observation was found with ketamine12, which is probably due to some other non-specific effect, such as inhibition of L-type voltage-gated Ca2+ channels or Ca2+-induced Ca2+ release from the sarcoplasmic reticulum29–31. In agreement, MK801 also inhibited the 70-mM KCl-induced arterial contraction at concentrations above 30 µM (Fig. 1f).
Both MK801 and ketamine directly inhibit Kv1.5 from the extracellular side
Although previous studies reported that MK801 and ketamine inhibited the native 4-aminopyridine-sensitive Kv current in dispersed rat mesenteric arterial smooth muscle cells14,15, this is the first study showing the inhibition of Kv1.5 by MK801 and ketamine. The potency of MK801 in inhibiting Kv1.5 was higher than that of ketamine. Accordingly, MK801 facilitated the 5-HT2AR response at concentrations (from 0.3 µM and peak at 10 µM; Fig. 1b) lower than those of ketamine (from 10 µM and peak at 30 µM; Fig. 1a of ref. 12). Upon application, Kv1.5 inhibition by MK801 and ketamine was very rapid and occurred from the extracellular side (Figs. 2–4), indicating that the direct inhibition of Kv1.5 by MK801 and ketamine is important in regulating the electrical excitabilities of atrial myocytes and glial and neuronal cells19–22. In pulmonary arterial smooth muscle cells, Kv1.5 was convincingly demonstrated to be an effector of 5-HT2AR signaling18. Therefore, whether the inhibition of Kv1.5 and related facilitation of the 5-HT2AR-induced response is involved in the pathogenesis of clinical symptoms in MK801/ketamine-induced experimental animal models of schizophrenia, hypertension, and dissociative anesthesia should be considered4,5,7,8,32–34. Notably, serotonergic mechanisms are critically involved in the pathogenesis of psychosis and schizophrenia6,35,36. The precise mechanism underlying the MK801 and ketamine-induced facilitation of 5-HT2ARs and Kv1.5 inhibition and their clinical outcomes warrants future study. In addition, the effects of other Kv subtypes, such as Kv1.1, Kv1.2, and Kv1.6, and their role in the facilitation of 5-HT2Rs need to be evaluated9.
In conclusion, MK801 and ketamine facilitate the 5-HT2AR activation response in an Em-dependent manner, especially at lower, physiologically relevant concentrations of 5-HT, likely by acting on the 5-HT2AR-Kv1.5 receptor-effector module, which is independent of NMDArs.
Acknowledgements
This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2016. It was also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI15C1540), and by a Basic Science Research Program (2015R1C1A1A02036887) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning.
Authors' contributions
H.L., J.G.K., and S.W.P. performed the research. H.L., H.J.N., J.M.K., C.Y.Y., N.S.W., and B.H.C. analyzed the data. B.K., S.I.C., and Y.M.B. designed the research study. Y.M.B. wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: H Lin, JG Kim, SW Park.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
References
- 1.Goodman LS, Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. 10th edn. New York, NY, USA: McGraw-Hill; 2001. [Google Scholar]
- 2.Katzung BG. A Concise Medical Library for Practitioner and Student. Los Altos, CA, USA: Lange Medical Publications; 1982. [Google Scholar]
- 3.Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc. Natl Acad. Sci. USA. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis SJ, Barres C, Jacob HJ, Ohta H, Brody MJ. Cardiovascular effects of the N-methyl-D-aspartate receptor antagonist MK-801 in conscious rats. Hypertension. 1989;13(6 Pt 2):759–765. doi: 10.1161/01.HYP.13.6.759. [DOI] [PubMed] [Google Scholar]
- 5.Loscher W, Fredow G, Ganter M. Comparison of pharmacodynamic effects of the non-competitive NMDA receptor antagonists MK-801 and ketamine in pigs. Eur. J. Pharmacol. 1991;192:377–382. doi: 10.1016/0014-2999(91)90228-I. [DOI] [PubMed] [Google Scholar]
- 6.Winter JC, Doat M, Rabin RA. Potentiation of DOM-induced stimulus control by non-competitive NMDA antagonists–A link between the glutamatergic and serotonergic hypotheses of schizophrenia. Life Sci. 2000;68:337–344. doi: 10.1016/S0024-3205(00)00934-6. [DOI] [PubMed] [Google Scholar]
- 7.Andine P, et al. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J. Pharmacol. Exp. Ther. 1999;290:1393–1408. [PubMed] [Google Scholar]
- 8.Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol. Psychiatry. 2002;7:837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- 9.D’Adamo MC, et al. 5-HT2 receptors-mediated modulation of voltage-gated K + channels and neurophysiopathological correlates. Exp. Brain Res. 2013;230:453–462. doi: 10.1007/s00221-013-3555-8. [DOI] [PubMed] [Google Scholar]
- 10.Ninan I, Kulkarni SK. 5-HT2A receptor antagonists block MK-801-induced stereotypy and hyperlocomotion. Eur. J. Pharmacol. 1998;358:111–116. doi: 10.1016/S0014-2999(98)00591-3. [DOI] [PubMed] [Google Scholar]
- 11.Seeman P, Ko F, Tallerico T. Dopamine receptor contribution to the action of PCP, LSD and ketamine psychotomimetics. Mol. Psychiatry. 2005;10:877–883. doi: 10.1038/sj.mp.4001682. [DOI] [PubMed] [Google Scholar]
- 12.Park SW, et al. Facilitation of serotonin-induced contraction of rat mesenteric artery by ketamine. Korean J. Physiol. Pharmacol. 2016;20:605–611. doi: 10.4196/kjpp.2016.20.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HS, et al. N-Methyl-D-aspartate receptor antagonists enhance the head-twitch response, a 5-hydroxytryptamine2 receptor-mediated behaviour, in reserpine-treated mice. J. Pharm. Pharmacol. 2000;52:717–722. doi: 10.1211/0022357001774390. [DOI] [PubMed] [Google Scholar]
- 14.Kim JM, et al. Blockade of voltage-gated K(+) currents in rat mesenteric arterial smooth muscle cells by MK801. J. Pharmacol. Sci. 2015;127:92–102. doi: 10.1016/j.jphs.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, et al. Ketamine blocks voltage-gated K(+) channels and causes membrane depolarization in rat mesenteric artery myocytes. Pflug. Arch. 2007;454:891–902. doi: 10.1007/s00424-007-0240-4. [DOI] [PubMed] [Google Scholar]
- 16.Bae YM, et al. Serotonin depolarizes the membrane potential in rat mesenteric artery myocytes by decreasing voltage-gated K+ currents. Biochem. Biophys. Res. Commun. 2006;347:468–476. doi: 10.1016/j.bbrc.2006.06.116. [DOI] [PubMed] [Google Scholar]
- 17.Sung DJ, et al. Serotonin contracts the rat mesenteric artery by inhibiting 4-aminopyridine-sensitive Kv channels via the 5-HT2A receptor and Src tyrosine kinase. Exp. Mol. Med. 2013;45:e67. doi: 10.1038/emm.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cogolludo A, et al. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ. Res. 2006;98:931–938. doi: 10.1161/01.RES.0000216858.04599.e1. [DOI] [PubMed] [Google Scholar]
- 19.Brendel J, Peukert S. Blockers of the Kv1.5 channel for the treatment of atrial arrhythmias. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2003;1:273–287. doi: 10.2174/1568016033477441. [DOI] [PubMed] [Google Scholar]
- 20.Ravens U, Wettwer E. Ultra-rapid delayed rectifier channels: molecular basis and therapeutic implications. Cardiovasc. Res. 2011;89:776–785. doi: 10.1093/cvr/cvq398. [DOI] [PubMed] [Google Scholar]
- 21.Attardi B, Takimoto K, Gealy R, Severns C, Levitan ES. Glucocorticoid induced up-regulation of a pituitary K+ channel mRNA in vitro and in vivo. Recept. Channels. 1993;1:287–293. [PubMed] [Google Scholar]
- 22.Horio Y. Potassium channels of glial cells: distribution and function. Jpn J. Pharmacol. 2001;87:1–6. doi: 10.1254/jjp.87.1. [DOI] [PubMed] [Google Scholar]
- 23.Noh HJ, et al. The vasodilatory effect of ketamine is independent of the N-methyl-D-aspartate receptor: lack of functional N-methyl-D-aspartate receptors in rat mesenteric artery smooth muscle. Eur. J. Anaesthesiol. 2009;26:676–682. doi: 10.1097/EJA.0b013e32832a1704. [DOI] [PubMed] [Google Scholar]
- 24.Lee HM, Hahn SJ, Choi BH. Blockade of Kv1.5 by paroxetine, an antidepressant drug. Korean J. Physiol. Pharmacol. 2016;20:75–82. doi: 10.4196/kjpp.2016.20.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HM, Hahn SJ, Choi BH. Blockade of Kv1.5 channels by the antidepressant drug sertraline. Korean J. Physiol. Pharmacol. 2016;20:193–200. doi: 10.4196/kjpp.2016.20.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson R, et al. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990;4:929–939. doi: 10.1016/0896-6273(90)90146-7. [DOI] [PubMed] [Google Scholar]
- 27.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflug. Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 28.Lu R, et al. c-Src tyrosine kinase, a critical component for 5-HT2A receptor-mediated contraction in rat aorta. J. Physiol. 2008;586:3855–3869. doi: 10.1113/jphysiol.2008.153593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akata T, Izumi K, Nakashima M. Mechanisms of direct inhibitory action of ketamine on vascular smooth muscle in mesenteric resistance arteries. Anesthesiology. 2001;95:452–462. doi: 10.1097/00000542-200108000-00030. [DOI] [PubMed] [Google Scholar]
- 30.Hara Y, Chugun A, Nakaya H, Kondo H. Tonic block of the sodium and calcium currents by ketamine in isolated guinea pig ventricular myocytes. J. Vet. Med Sci. 1998;60:479–483. doi: 10.1292/jvms.60.479. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki M, Ito Y, Kuze S, Shibuya N, Momose Y. Effects of ketamine on voltage-dependent Ca2+ currents in single smooth muscle cells from rabbit portal vein. Pharmacology. 1992;45:162–169. doi: 10.1159/000138994. [DOI] [PubMed] [Google Scholar]
- 32.Becker A, et al. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 33.Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125–134. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]
- 34.Rung JP, Carlsson A, Ryden Markinhuhta K, Carlsson ML. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:827–832. doi: 10.1016/j.pnpbp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Busatto GF, Kerwin RW. Perspectives on the role of serotonergic mechanisms in the pharmacology of schizophrenia. J. Psychopharmacol. 1997;11:3–12. doi: 10.1177/026988119701100102. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Maeso J, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]