Abstract
Vitamin D, traditionally known as an essential nutrient, is a precursor of a potent steroid hormone that regulates a broad spectrum of physiological processes. In addition to its classical roles in bone metabolism, epidemiological, preclinical, and cellular research during the last decades, it revealed that vitamin D may play a key role in the prevention and treatment of many extra-skeletal diseases such as cancer. Vitamin D, as a prohormone, undergoes two-step metabolism in liver and kidney to produce a biologically active metabolite, calcitriol, which binds to the vitamin D receptor (VDR) for the regulation of expression of diverse genes. In addition, recent studies have revealed that vitamin D can also be metabolized and activated through a CYP11A1-driven non-canonical metabolic pathway. Numerous anticancer properties of vitamin D have been proposed, with diverse effects on cancer development and progression. However, accumulating data suggest that the metabolism and functions of vitamin D are dysregulated in many types of cancer, conferring resistance to the antitumorigenic effects of vitamin D and thereby contributing to the development and progression of cancer. Thus, understanding dysregulated vitamin D metabolism and function in cancer will be critical for the development of promising new strategies for successful vitamin D-based cancer therapy.
Cancer: The significance of vitamin D metabolism
Altered vitamin D metabolism in cancer patients might help tumors progress, and understanding this effect could guide new anti-cancer therapies. Sang-Min Jeon and Eun-Ae Shin at Ajou University in South Korea review research on links between cancer and vitamin D metabolism. Vitamin D is best known for its role in building and maintaining healthy bones. Many epidemiological studies, however, suggest vitamin D deficiency is also involved in diseases affecting tissues other than bone. Sunlight promotes vitamin D formation, and low exposure to sunlight was found to be associated with increased incidence of cancer more than 80 years ago. Vitamin D deficiency has since been implicated in at least 18 types of cancer. The authors summarize the metabolic processes underlying the anti-cancer effects of vitamin D, and discuss ways to improve vitamin D-based therapies for cancer.
Introduction
Vitamin D is a fat-soluble vitamin obtainable from the diet, as well as a seco-steroidal prohormone produced in the skin by ultraviolet B (UVB, 290–320 nm) from sunlight. Vitamin D, as a precursor of a potent steroid hormone, undergoes two-step metabolism in the liver and kidney to synthesize a biologically active form, calcitriol, which binds to the vitamin D receptor (VDR) to enable its diverse physiological functions1,2. The classical role of vitamin D is to regulate metabolism of calcium and phosphate, which is essential for bone remodeling. However, extensive research over the past decades has suggested that low sunlight exposure and vitamin D deficiency are also associated with the increased risk of many other extra-skeletal diseases such as cancer3–6.
The first observation of an inverse correlation between sunlight exposure and overall cancer incidence and mortality in North America was published almost 80 years ago7,8. Later, in 1980 and 1992, the first epidemiological studies linking low sunlight exposure and high risk of colon and prostate cancers were reported, respectively, which suggested that vitamin D as a surrogate for sunlight exposure may protect against colon and prostate cancer risk9,10. Since then, many epidemiological studies have supported and extended the UVB–vitamin D–cancer hypothesis in 18 different types of cancers11. The hypothesis has been further supported by studies showing the direct association between vitamin D and cancer risk. Several lines of population-based studies revealed an inverse correlation between serum 25-hydroxyvitamin D (25(OH)D) levels and high risk of colon12, breast13, prostate14,15, gastric, and other cancers16. Moreover, there are strong evidences from several cell culture and animal studies to support the antitumorigenic effects of vitamin D6,17,18. As such, it is now becoming evident that deficiency of vitamin D can contribute to the development and progression of many types of cancers, and thus maintenance of sufficient serum vitamin D levels could be beneficial for prevention and treatment of cancer.
Because the numerous epidemiological and experimental data indicate the beneficial role of vitamin D in the prevention and treatment of several types of cancers, clinical use of calcitriol or vitamin D analogs has been investigated17. However, hypercalcemia, the major toxic effect of vitamin D, has strongly limited its clinical applications19,20. Moreover, accumulating data suggest that cancer cells employ several mechanisms that reduce cellular calcitriol levels, as well as diminish its function to protect themselves from the antitumorigenic effects of vitamin D17,18. Thus, understanding how vitamin D metabolism and signaling are dysregulated in cancer will help develop efficient therapeutic strategies to overcome such limitations of using vitamin D or its analogs for clinical purpose.
In this review, we provide an overview of vitamin D metabolic pathways and summarize antitumorigenic functions and mechanisms of vitamin D. In addition, we discuss how vitamin D metabolism and function are dysregulated in cancer to promote resistance to the antitumorigenic effect of vitamin D. Finally, we discuss future directions to overcome the limitations of and improve vitamin D-based cancer therapy.
Vitamin D metabolism
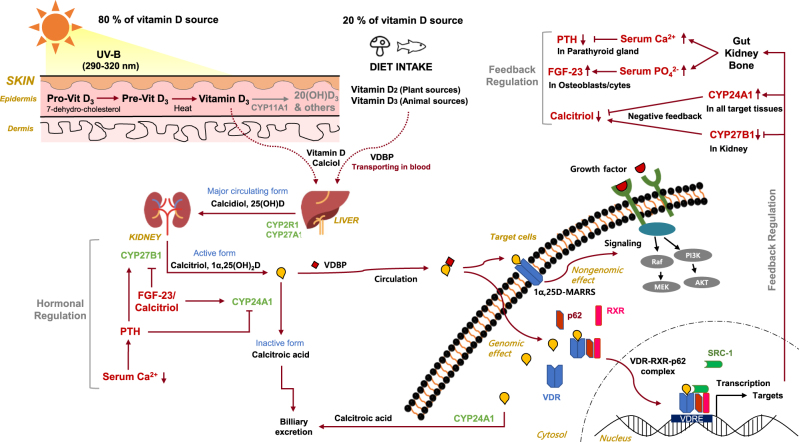
Vitamin D is a prohormone that needs to be metabolized to biologically active products that bind to their cognate nuclear receptors for regulation of diverse physiological processes2. In this section, the classical and alternative vitamin D metabolic pathways, and hormonal regulation of vitamin D metabolism, are discussed and summarized in Figs. 1 and 2.
Fig. 1.
Overview of vitamin D metabolism
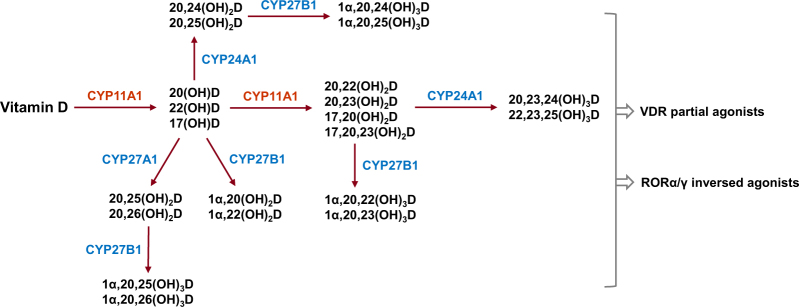
Fig. 2.
The metabolites generated from alternative vitamin D metabolic pathway
Classical metabolic pathway
There are two major isoforms of vitamin D, vitamin D2 (ergocalciferol), and vitamin D3 (cholecalciferol)21,22. Vitamin D2 is synthesized from ergosterol by UVB radiation in plants, yeasts, and fungi and can be ingested in a diet containing plant source foods, such as mushrooms. Vitamin D3 is synthesized from 7-dehydrocholesterol by UVB radiation in the epidermis of skin and can be also ingested in a diet of animal source foods, such as cod liver oil. Vitamin D (both vitamin D2 and D3, calciol) originating from either the diet or the skin binds to vitamin D-binding protein (VDBP) in circulation and is first delivered to the liver.
In the liver, vitamin D is metabolized by vitamin D 25-hydroxylase (CYP2R1 and CYP27A1) to 25(OH)D (calcidiol), which is the major circulating form of vitamin D in serum23,24. 25(OH)D is further metabolized by 25(OH)D 1α-hydroxylase (CYP27B1) mainly in the proximal tubule of the kidney to 1α,25-dihydroxyvitamin D [1α,25(OH)2D, calcitriol], which is the most biologically active form of vitamin D23,24. Calcitriol then enters the circulation and, after binding to VDBP, is delivered target tissues such as intestine, bone, and kidney, where vitamin D is known to regulate absorption, mobilization, and reabsorption, respectively, of calcium and phosphate21. After being produced, the levels of both calcidiol and calcitriol are tightly regulated by 25(OH)D 24-hydroxylase (CYP24A1), which is the primary vitamin D inactivating enzyme catalyzing hydroxylation at C-24 and C-23 of both calcidiol and calcitriol23,24. The 24-hydroxylase pathway produces biologically inactive biliary excreted calcitroic acid, whereas the less known 23-hydroxylase pathway produces 1,25–26,23 lactone, whose relative activity by CYP24A1 catalysis shows species difference23,24. The importance of CYP24A1-driven calcitriol inactivation was underscored by the finding of impaired intramembranous bone mineralization and hypercalcemia in Cyp24a1 knockout mice, leading to perinatal death in 50% of the mice25,26. Interestingly, the defect was normalized in Cyp24a1 and VDR double knockout mice, suggesting that increased calcitriol levels, but not the absence of 24- or 23-hydroxylated vitamin D metabolites, were responsible for the defective phenotype25.
In target tissues, calcitriol binds to VDR, a member of the nuclear receptor family of ligand-activated transcription factors and which induces both genomic and non-genomic regulation of downstream targets involving diverse biological functions2,27,28. In the genomic pathway, calcitriol binds to cytosolic VDR, which promotes phosphorylation of VDR, heterodimerizaion with retinoid-X receptor (RXR), and then nuclear translocation of the complex29. The calcitriol–VDR–RXR complex binds to vitamin D response element (VDRE) in the promoter region of its target genes and recruits transcriptional coactivators or co-repressors to regulate mRNA expression of target genes and thus regulating a variety of their functions, including calcium and phosphate metabolism. Interestingly, a recent study showed that autophagy adaptor protein p62/SQSTM1 plays a key role in heterodimerization and recruitment of the VDR–RXR complex to target genes by directly binding to VDR and RXR in hepatic stellate cells30. In the non-genomic pathway, calcitriol binds to membrane bound VDR, which is identified as 1,25D-membrane-associated, rapid response steroid-binding protein (1,25D-MARRS); this interaction then induces acute changes in cell signaling pathways, including calcium and mitogen-activated protein kinase (MAPK) signaling, through direct protein–protein interaction with intracellular signaling molecules involved in certain phenotypic functions27,31. To elicit the full spectrum of biological functions of calcitriol, both genomic and non-genomic pathways should be investigated.
Alternative metabolic pathway
Recently, an alternative pathway of vitamin D metabolism via CYP11A1, also known as a cytochrome P450 side chain cleavage (P450scc) enzyme, has been reported32–34. Originally, CYP11A1 was known to catalyze the first rate-limiting step of steroidogenesis in steroidogenic organs by inducing hydroxylation of cholesterol at C-22 and C-20 followed by cleavage of the bond between C-20 and C-22 to generate pregnenolone, the common precursor of steroid hormones35. However, CYP11A1 is emerging as a new vitamin D-metabolizing enzyme by the finding of the expression of CYP11A1 in peripheral tissues such as skin and gastrointestinal (GI) tract, and that vitamin D is an alternative substrate to cholesterol for the enzyme36,37. CYP11A1-mediated metabolism of vitamin D involves sequential hydroxylation, predominantly at C-20 or C-22, without the cleavage of the side chain. The main vitamin D metabolites resulting from a single hydroxylation by CYP11A1 include 20(OH)D, 22(OH)D, and 17(OH)D32. These are further hydroxylated by CYP11A1 to generate 20,23(OH)2D, 20,22(OH)2D, 17,20(OH)2D, and 17,20,23(OH)3D. Moreover, the major product of this pathway, 20(OH)D, can also serve as a substrate for CYP27A1 and CYP24A1, with CYP27A1 hydroxylating it at C-25 or C-26, and CYP24A1 hydroxylating it at C-24 or C-25. These products can be further hydroxylated at C-1α by CYP27B1 generating corresponding trihydroxy-vitamin D metabolites, except for 17,20,23(OH)3D, which is the final vitamin D metabolite generated by the CYP11A1-driven metabolic pathway. Altogether, it has been estimated that this alternative metabolic pathway can produce more than 21 hydroxymetabolites of vitamin D (Fig. 2)32.
The products of CYP11A1, such as 20(OH)D and its hydroxymetabolites, induce anti-proliferation, differentiation, and anti-inflammation in skin cells comparable or better than that of calcitriol38,39. Moreover, these metabolites enhance defense mechanisms against UVB-induced DNA damage and oxidative stress and, importantly, elicit anticancer properties in a cell line-dependent manner37. Interestingly, the products of CYP11A1, such as 20(OH)D and 20,23(OH)D, have been shown to function as partial or biased agonists of VDR32. For example, these metabolites do not induce calcemic effects or the expression of CYP24A1 in pharmacological concentrations that activate VDR, which can normally be seen in response to calcitriol treatment.
In addition to VDR, α and γ isoforms of retinoid-related orphan receptors (RORα, RORγ), members of the nuclear receptor family of ligand-dependent transcription factors, have been shown to function as novel receptors for CYP11A1-derived vitamin D metabolites such as 20(OH)D and 20,23(OH)D32,40,41. RORs play key roles in the regulation of many physiological processes including immune and metabolic pathways and are implicated in many pathologies such as cancer, autoimmune diseases, and metabolic syndrome41. Interestingly, the CYP11A1-derived vitamin D metabolites act as inverse agonists of RORα and RORγ, which inhibits their transcriptional activity. For example, 20(OH)D and 20,23(OH)D can suppress the transcription of RORα and RORγ target genes such as Bmal1 and G6Pase, respectively40. Thus, the pleiotropic and diverse effects of vitamin D could be attributed not only to the effects of the calcitriol–VDR pathway, but also to those of CYP11A1-derived vitamin D metabolites–VDR or –RORα/γ pathways. Further work should be conducted to define their specific contributions to the broad spectrum of vitamin D’s effects in health and diseases.
Hormonal regulation of classical vitamin D metabolism
Interestingly, calcitriol, as a hormone, tightly regulates vitamin D metabolism in a negative feedback mechanism42,43. Importantly, the vitamin D inactivating enzyme, CYP24A1, is among the strong transcriptional targets of the calcitriol–VDR–RXR complex. The promoter region of CYP24A1 contains two VDREs approximately 150 and 250-bp upstream of the transcriptional start site, which drives strong induction of CYP24A1 by calcitriol44. In addition, calcitriol can also induce CYP24A1 expression by recruiting histone H4 acetyltransferases and RNA polymerase II to a site approximately 50- to 70-kb downstream of the human CYP24A1 gene45. Thus, the levels of calcidiol and calcitriol can be tightly regulated by calcitriol-driven expression of CYP24A1 in the kidney. Moreover, calcitriol also inhibits the transcription of CYP27B1 in the kidney through complex mechanisms involving epigenetic modifications of its promoter region46.
In addition to the negative feedback regulation by calcitriol, vitamin D metabolism is also regulated by two hormones, parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF-23), both of which play major roles in maintaining calcium and phosphate homeostasis47–49. PTH is secreted by the parathyroid gland in response to low serum calcium levels, as sensed by calcium-sensing receptors (CaSRs) expressed on the surface of parathyroid cells48. PTH stimulates renal expression of CYP27B1 by mechanisms involving increased cAMP-dependent transcription or upregulation of nuclear orphan receptor NR4A2-dependent transcription, leading to the increase in calcitriol production50,51. Although increased calcitriol can induce its own degradation by inducing the expression of CYP24A1, PTH can sustain calcitriol levels by inducing the degradation of CYP24A1 mRNA through activation of the cAMP–PKA pathway in the kidney52,53. The resulting high calcium levels by sustained calcitriol induction can negatively regulate the secretion of PTH through binding to CaSRs in the parathyroid gland as a negative feedback mechanism54. FGF-23 is secreted by osteoblasts and osteocytes in response to both high serum phosphate and calcitriol levels47. FGF-23 facilitates the excretion of phosphate by inhibiting the expression of sodium–phosphate cotransporter 2 (NPT2) located at the apical membranes of proximal renal tubules through binding to FGF receptor–Klotho complexes in cell membranes. In addition, FGF-23 reduces serum calcitriol levels by inhibiting the expression of CYP27B1, whereas stimulating the expression of CYP24A1 in the kidney, although the mechanisms remain to be elucidated55–57.
Anticancer properties of Vitamin D
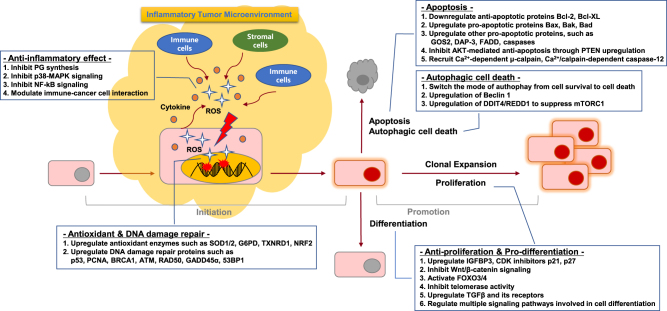
As the beneficial effects of vitamin D in cancer prevention and treatment have been observed in epidemiological and preclinical studies, diverse mechanisms have been proposed to explain its anticancer effects. Accumulating data suggest that vitamin D can regulate the entire process of tumorigenesis, from initiation to metastasis and cell–microenvironment interactions17. These mechanisms include regulation of cell behaviors such as proliferation, differentiation, apoptosis, autophagy, and epithelial–mesenchymal transition (EMT), and modulation of cell–microenvironment interactions such as angiogenesis, antioxidants, inflammation, and the immune system. As the role of vitamin D in cancer has been extensively reviewed in other studies6,17, here, we focus on its versatile roles in tumor initiation and promotion stages, which are summarized in Fig. 3.
Fig. 3.
Anticancer properties of vitamin D
Initiation stage: role in anti-inflammation, antioxidant defense and DNA damage repair
Tumor initiation is a process that introduces irreversible genetic mutations in normal cells, consequently inducing transformation. A body of data supports that vitamin D plays a key role in preventing the initiation stage by exerting anti-inflammatory and antioxidant defenses and DNA damage repair processes6,17.
Anti-inflammation
Chronic inflammation is a low-grade, prolonged inflammatory response resulting in progressive destruction and regeneration of tissues by reactive oxygen species (ROS) and cytokines secreted at the site of inflammation. It is now well-accepted that chronic inflammation is one of the main contributors to the initiation of tumorigenesis58. Accumulating data suggest that vitamin D exerts anti-inflammatory effects via at least four mechanisms.
First, calcitriol inhibits the prostaglandin (PG) pathway involved in pro-inflammatory responses through inhibition of the expression of cyclooxygenase-2 (COX-2) and PG receptors, and degradation of PGs59. In prostate cancer cells, calcitriol reduces the expression levels of COX-2 and that of PG receptors EP2 and FP, whereas it increases the expression of 15-hydroxyprostaglandin-dehydrogenase (15-PGDH), a NAD+-dependent enzyme responsible for the degradation of PGE259,60. In addition, the decrease of COX-2 mRNA expression and PGE2 production has also been reported in calcitriol-treated breast cancer cells61. Consistently, the expression of VDR is inversely correlated with that of COX-2 in malignant breast cell lines and ovarian cancer tissues62,63, supporting the role of the calcitriol–VDR axis in suppressing the expression of COX-2 and production of PGs.
Second, vitamin D can suppress the p38 MAPK-mediated pro-inflammatory signaling pathway. In both normal prostate epithelial cells and prostate cancer cells, calcitriol inhibits the production of pro-inflammatory cytokines such as IL-6 by inducing the expression of MAPK phosphatase-5 (MKP-5), which prevents the phosphorylation and activation of p38 MAPK64. In addition to prostate cells, calcitriol also inhibited Lipopolysaccharides (LPS)-induced production of interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α through the induction of MKP-1 in human monocytes and murine macrophages65.
Third, calcitriol can also inhibit the nuclear factor kappa B (NFκB) signaling pathway through several mechanisms. Calcitriol suppresses the phosphorylation of both AKT and its downstream target I kappa Bα (IκBα) in macrophages through upregulation of thioesterase superfamily member 4 (THEM4), an AKT modulator protein leading to the inhibition of NFκB and COX-2 expression66. In fibroblasts, calcitriol augmented the protein stability of IκBα, and also induced the binding of VDR to IκBα kinase (IKK), preventing its phosphorylation and activation and thereby inhibiting the nuclear translocation of the p65 subunit of NFκB67,68.
Fourth, vitamin D can regulate the interaction between immune and cancer cells to suppress the production of pro-inflammatory cytokines. Co-culture experiments using peripheral blood mononuclear cells (PBMCs) and colon cancer cells revealed that vitamin D treatment significantly decreased the production of pro-inflammatory cytokines such as TNF-α, IL-6 and, to a lesser extent, IL-10, supporting the anti-inflammatory effects of vitamin D in tumor microenvironment69.
Antioxidant defense and DNA damage repair
ROS play a key role in many aspects of tumorigenesis by promoting DNA mutation, cell proliferation, and cell death that also provokes pro-inflammatory responses. Thus, maintaining antioxidant defense systems should be a critical step in preventing tumor development. Accumulating data suggest that vitamin D can protect from oxidative stress-induced DNA damage by promoting antioxidant defenses70,71. It was shown in mice that DNA damage induced by oxidative stress was elevated in colon epithelial cells of VDR-knockout mice72. Moreover, the treatment of rats with calcitriol markedly reduced the levels of malondialdehyde, the end product of lipid peroxidation causing DNA damage73. In line with this, daily supplementation of vitamin D in humans reduced oxidative DNA damage suggesting that vitamin D may protect against oxidative stress-induced DNA damage in humans74.
Vitamin D-mediated protection from ROS-induced DNA damage can be attributed to its role in inducing the expression of numerous enzymes involved in ROS detoxification. Calcitriol induced the expression of superoxide dismutase 1 (SOD1) and 2 (SOD2) in prostate epithelial cells (PECs) and in androgen-sensitive prostate cancer cells (LNCaP), respectively75,76. In addition, calcitriol induced the expression of thioredoxin reductase 1 (TXNRD1), which reduces thioredoxin for its antioxidant function in prostate and breast cancer cells75,77 and glucose-6-phosphate dehydrogenase (G6PD), which produces NADPH for glutathione (GSH) regeneration in prostate and ovarian cancer cells78,79. Moreover, NF-E2-related factor-2 (NRF2), a transcription factor that increases the expression of a diverse array of antioxidant enzymes was shown to be regulated by vitamin D through either increase in its expression, nuclear translocation, or decrease in KEAP1-mediated degradation80–82. As NRF2 is known as the master regulator of the expression of antioxidant enzymes, this can be a potential mechanism by which vitamin D can induce antioxidant enzymes and exert antioxidant defenses83.
In addition to preventing DNA damage by augmenting antioxidant capacity, vitamin D can also directly regulate DNA damage repair processes71. Studies showed that vitamin D increases the expression of genes involved in DNA damage repair such as p53, proliferating cell nuclear antigen (PCNA), and breast cancer 1 (BRCA1) in breast cancer cells77 and ataxia-telangiectasia mutated (ATM) and recombinant DNA repair protein (RAD50) in PECs84, and growth arrest and DNA damage-inducible α (GADD45α) in SCC and ovarian cancer cells85,86. Vitamin D can also prevent the degradation of p53-binding protein 1 (53BP1) mediated by cysteine proteinase Cathepsin L, a lysosomal endopeptidase, in breast cancer87. Thus, vitamin D could be critical for preventing genetic mutations at the tumor initiation stage by inducing anti-inflammatory, antioxidant, and DNA damage repair functions.
Promotion stage: role in cell proliferation/differentiation and apoptosis/autophagy
Even in cells with established genetic mutations during the initiation stage, vitamin D still elicits anticancer properties by blocking the tumor promotion stage through inhibition of cell proliferation, induction of cell differentiation, and cell death.
Cell proliferation and differentiation
Calcitriol was shown to elicit anti-proliferation and pro-differentiation properties both in normal and malignant cells88. In addition to calcitriol, novel vitamin D metabolites produced by CYP11A1 such as 20(OH)D3, 20(OH)D2, 1,20(OH)2D3, and 20,23(OH)2D3, were also shown to inhibit cell proliferation and induce cell differentiation through VDR, which is comparable to those of calcitriol, but with less calcemic effects32,39. The anti-proliferative property of vitamin D is mediated by multiple mechanisms including the regulation of growth factors, cell cycle, and signaling pathways. Vitamin D increases the expression of insulin-like growth factor (IGF)-binding protein 3 and the cyclin-dependent kinase (CDK) inhibitors, p21 and p27, whereas inhibiting the expression of CDK2, leading to inhibition of IGF-1- and IGF-2-stimulated cell proliferation and cell cycle progression88. In addition, calcitriol inhibits the Wnt/β-catenin signaling pathway by decreasing the formation of transcription factor 4-β-catenin, (TCF4-b)–catenin complexes or increasing the expression of the Wnt antagonist, Dickkopf-1 (DKK-1)89,90. Moreover, vitamin D also activates transcription factors forkhead box O3/4 (FoxO3/4), which trigger transcription of target genes involved in cell cycle arrest and anti-proliferation, by inducing their deacetylation and dephosphorylation in neuroblastoma cells91. Vitamin D was also shown to inhibit telomerase activity by reducing the expression of telomerase reverse transcriptase (TERT) via microRNA-49892 and to induce the expression of transforming growth factor β (TGFβ), as well as its receptors, leading to inhibition of cell growth93,94. The induction of differentiation by vitamin D is associated with the anti-proliferative properties and regulation of diverse intracellular signaling pathways, such as phosphatidylinositol 3 kinase/AKT, MAPK, NF-kB, and Ca2+ signaling88.
Apoptosis and autophagy
Vitamin D-induced apoptosis is mainly mediated by both downregulation of the anti-apoptotic proteins Bcl-2 and Bcl-XL, and upregulation of pro-apoptotic proteins Bax, Bak, and Bad95. In addition, induction of apoptosis by upregulation of other pro-apoptotic proteins such as G0-G1 switch 2 (GOS2), death-associated protein (DAP-3), Fas-associated death domain (FADD), and caspases was reported77,96. Vitamin D can also inhibit the AKT-mediated anti-apoptotic signaling pathway by increasing phosphatase and tensin homolog (PTEN) expression97. Moreover, vitamin D can also initiate apoptotic events by recruiting Ca2+-dependent apoptotic effectors such as Ca2+-dependent μ-calpain and Ca2+/calpain-dependent caspase-1298.
Autophagy is a catabolic process that plays a critical role in both cell survival and apoptosis-independent cell death. Interestingly, accumulating data suggest that vitamin D can switch the mode of autophagy from cell survival to cell death in cancer cells. Vitamin D was shown to elicit cytotoxic or cytostatic autophagy, which sensitized radiotherapy commonly inducing cytoprotective autophagy99,100. Vitamin D-induced autophagic cell death can be attributed to the upregulation of beclin 1, an autophagy-related gene101. In addition, CDK inhibitors may be involved in vitamin D-mediated autophagic cell death because this was enhanced by loss of p19 or attenuated by loss of p27102. Moreover, vitamin D can also induce autophagy by inducing the expression of DNA damage-inducible transcript 4 (DDIT4), also known as regulated in development and DNA damage response 1 (REDD1), an inhibitor of mechanistic target of rapamycin complex 1 (mTORC1), which is known to suppress autophagy103,104.
Dysregulation of vitamin D activity in cancer
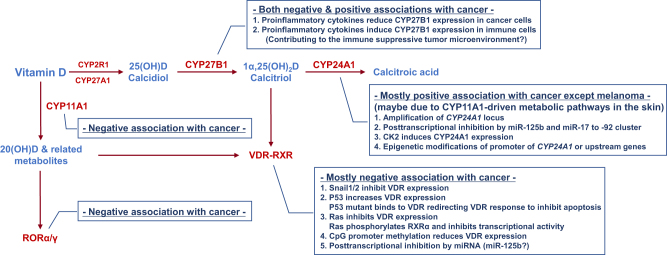
Classically, it has been considered that the calcitriol producing enzyme CYP27B1 is expressed in the kidney, and that VDR and CYP24A1, which mediate calcitriol function and degradation, are expressed in certain target tissues such as intestine, bone, and kidney. However, accumulating data suggest that these proteins are widely expressed in many tissues other than the kidney or the target tissues105,106 suggesting that most cells could be targets of vitamin D and that calcitriol levels could be locally regulated to exert fine-tuned tissue-specific functions. Importantly, this local regulation of vitamin D activity is dysregulated in many cancer cells, which contributes to the resistance to vitamin D-based cancer therapy3,17,18,43. In this section, our current understandings about the dysregulation of vitamin D metabolism and function in cancer are discussed and summarized in Fig. 4.
Fig. 4.
Dysregulation of vitamin D metabolism in cancer
VDR/RXRα
VDR is widely expressed in most cell types, but the expression is progressively reduced during dedifferentiation and tumor progression in many cancer types. Comparing VDR expression levels in normal, benign, and malignant tissues of skin, breast, ovarian, and prostate revealed a negative correlation between VDR expression and tumor malignancy107–112. Importantly, high VDR expression is significantly associated with reduced risk of lethal prostate cancer progression and cancer death112 and also nuclear VDR expression is associated with better overall survival in lung cancer patients113. Consistently, a recent study showed that reduced VDR protein expression is observed in urothelial bladder cancer and is associated with poor prognosis of patients114. These observations suggest that VDR expression may be useful as a valuable early diagnosis biomarker for high-risk populations. Furthermore, vitamin D could be critical in preventing cancer progression and thus cancer cells may actively counteract the tumor-suppressive effects of vitamin D by developing multiple mechanisms to abrogate VDR expression, as well as its activity.
First, snail family transcriptional repressor (Snail) that is overexpressed in several cancers and involved in EMT, tumor invasion, and metastasis was shown to inhibit VDR expression in cancer115. It was shown that Snail1 and Snail2 can bind to E-boxes in the proximal promoter region of the VDR gene to recruit co-repressors that inhibit the transcription of VDR in colon and breast cancer cells116,117. Second, tumor-suppressor p53, which is lost or mutated in almost half of all tumors, can increase the transcription of VDR118. Interestingly, it was shown that cancer-associated p53 mutant cells can also regulate VDR responses by directly binding to VDR and redirecting the VDR-mediated transcriptional program to protect cancer cells from apoptosis119. Third, constitutively active mutations of the Ras oncogene found in many cancers suppress VDR expression in tumors. The expression of K-Ras mutants in human colon cancer cells and H-Ras mutants in mouse colon and rat intestinal epithelial cells inhibit calcitriol-dependent VDR activation by suppressing VDR transcription120. In addition to reducing VDR expression, H-Ras and K-Ras mutations expressed in keratinocytes and PEC lines, respectively, suppressed VDR transcriptional activity by inducing phosphorylation of RXRα, which impairs the recruitment of co-activator SRC-1 to RXRα121,122. Forth, epigenetic silencing of VDR has been reported in cancer. CpG island methylation in the VDR promoter region was associated with reduced expression of VDR in colon and breast cancer cells123,124. Moreover, DNA methyltransferase (DNMT) inhibitor induced VDR expression and enhanced the anti-proliferative effect of calcitriol in breast cancer cells123. Finally, the involvement of microRNA (miRNA) was reported to control VDR expression in cancer125,126.
CYP27B1
As calcidiol is the major circulating form of vitamin D, cells that express CYP27B1 may increase the local concentration of calcitriol further than do cells only dependent on the systemic calcitriol produced in the kidney. Similar to VDR, the expression of CYP27B1 is inversely correlated with the progression of tumors of lung, prostate, colon, parathyroid, and skin108,127–132, suggesting that local production of calcitriol in those tissues could be important for cancer prevention. Interestingly, a recent study showed that pro-inflammatory cytokines such as IL-6 and TNF-α downregulated the expression of CYP27B1 in colon cancer cells133, suggesting that the pro-inflammatory tumor microenvironment could be a contributing factor for decreased CYP27B1 levels during tumor progression. However, the molecular mechanisms responsible for the progressive reduction of CYP27B1 expression during cancer progression are largely unknown. In contrast to the negative association in those cancer types, a positive association was reported in thyroid cancer134 and conflicting results were also reported for breast110,135 and renal cancers136,137. Moreover, in contrast to lung cancer cells, CYP27B1 expression in alveolar macrophages from lung cancer patients showed positive association with cancer progression138. This could be explained in part by the finding that pro-inflammatory cytokines such as IFN-γ and TNF-α, and agonists of Toll-like receptors (TLRs) upregulated the expression of CYP27B1 in monocytes, macrophages, and dendritic cells139,140 suggesting that the pro-inflammatory tumor microenvironment could be a contributing factor for increased CYP27B1 expression in immune cells, which is opposite to the finding in colon cancer cells133 mentioned above. This change in CYP27B1 expression and vitamin D metabolism in immune cells may contribute to the immune-suppressive tumor microenvironment.
CYP24A1
Given that CYP24A1 is an enzyme that degrades calcidiol and calcitriol, it is likely that cancer cells may upregulate CYP24A1 expression to reduce local concentrations of calcitriol, which is similar to the reduction of CYP27B1 in some cancers as discussed above. Indeed, CYP24A1 has been found to be amplified in breast cancer and proposed as an oncogene141. Consistently, CYP24A1 expression is correlated with the advanced stages of colon, prostate, breast, and lung cancers, inducing resistance to vitamin D-based therapy110,129,142–146. In addition, overexpression of CYP24A1 has been also reported in many other cancer types including ovarian, cervical, squamous cell, and basal cell carcinoma147–149. Moreover, CYP24A1 expression is associated with poor prognosis in lung, colon, and esophageal cancer145,150,151. These findings suggest that cancer cells can evade the anticancer effects of calcitriol by inducing the expression of CYP24A1, which reduces the local concentration of calcitriol. Supporting the oncogenic role of CYP24A1, the inhibition of CYP24A1 suppressed tumor growth and potentiated the antitumorigenic effects of calcitriol in breast and lung cancers, suggesting that CYP24A1 could be a promising therapeutic target152–154. In contrast to the positive association in those cancer types, some conflicting data have been reported for prostate cancer146,155 and even an inverse correlation between CYP24A1 expression and tumor progression has been reported in melanoma156, which, here, is discussed later.
Although CYP24A1 expression is induced by calcitriol–VDR activation via negative feedback regulation, the high level of CYP24A1 expression observed in cancer cells is unlikely to be mediated by VDR activation because, as mentioned above, VDR expression and activity are downregulated in most cancer. This suggests that the overexpression of CYP24A1 in many cancer cells may not be the result of normal physiological processes regulated by calcitriol–VDR-dependent mechanisms. Currently, at least four mechanisms responsible for the induction of CYP24A1 in cancer have been proposed. First, as mentioned above, overexpression of CYP24A1 in breast cancer is associated with the amplification of chromosomal locus 20q13.2–20q13.3 containing the CYP24A1 gene, which has been also observed in other cancers, including colon malignancies141,157. Interestingly, the amplification of CYP24A1 was detected only in malignant but not benign colon tumors, suggesting that CYP24A1 overexpression, and thereby inactivation of calcitriol, could be critical for tumors to progress to the malignant status158. Second, post-transcriptional regulation by miRNAs is associated with the CYP24A1 overexpression in cancer. MiR-125b, which is frequently downregulated in many cancers, was shown to bind to the 3′-UTR of CYP24A1 mRNA and thereby inhibit its expression159. In addition, CYP24A1 expression levels were inversely correlated to miR-125b levels in breast cancer tissues159, suggesting that low levels of miR-125b may account for CYP24A1 overexpression in cancer. Moreover, a recent study showed that the miR-17 to -92 cluster also regulates CYP24A1 expression in lung cancer cells160. These authors showed that the inhibitor miR-92a, which reduces levels of the miR-17 to -92 primary transcripts, diminishes the level of CYP24A1 expression in p53-depleted lung cancer cells160. Third, the serine/threonine protein kinase casein kinase 2 (CK2) signaling pathway induces CYP24A1 overexpression in prostate cancers161. Interestingly, overexpression of CK2 has been observed in many cancers, including prostate cancer, and correlates with poor clinical outcomes162. Finally, epigenetic regulation involving the CYP24A1 promoter region contributes to the altered expression of CYP24A1 in cancers. It has been shown that the expression of CYP24A1 is inversely correlated with the methylation of the CYP24A1 promoter in lung and prostate cancer155,163. Consistently, the inhibition of DNMT or histone deacetylase (HDAC) increased the expression of CYP24A1 in lung and colon cancer163. Interestingly, however, the induction of CYP24A1 by the inhibition of epigenetic changes in colon cancer could be an indirect effect via regulation of genes operating upstream of CYP24A1 suggesting the complexity of epigenetic regulation of CYP24A1 expression in cancer164.
CYP11A1 and RORα/γ
The finding of unexpected expression patterns of CYP24A1 in melanoma proposed that, in context of the dominant CYP11A1-driven alternative vitamin D metabolism pathway in the skin, CYP24A1 can produce biologically active tumor-suppressive vitamin D metabolites rather than degrading calcitriol39,156,165. Interestingly, 20(OH)D produced by CYP11A1 can be hydroxylated by CYP24A1 to 20,24(OH)2D and 20,25(OH)2D, which are more potent in suppressing melanoma growth than calcitriol and 20(OH)D165. Consistently, the expression of RORα and RORγ, the receptors of the 20-hydroxylated vitamin D metabolites, are negatively correlated with melanoma progression and positively correlated with prognosis166. Supporting this tumor-suppressive role of CYP11A1-RORα/γ pathway in the skin, a recent study showed that CYP11A1 is also significantly downregulated in many other cancer types, including colon, kidney, liver, lung, prostate, and uterine corpus endometrial carcinoma167. In addition, the expression of CYP11A1 is also reduced in prostate cancer bone metastases168. Consistently, the expression of RORα and RORγ are positively associated with prognosis in patients with breast, lung, or liver cancer169–172. This association between the CYP11A1-RORα/γ pathway and tumor progression suggests that activation of the alternative vitamin D metabolism pathway could be a novel preventive, as well as therapeutic, strategy for cancer.
Vitamin D-based cancer therapy: future directions
Although randomized clinical trial data are still lacking, several epidemiological, clinical, preclinical, and in vitro experimental data strongly suggest that the activation of vitamin D signaling could be a promising strategy for prevention, as well as treatment of many types of cancer. As such, several therapeutic interventions targeting dysregulated vitamin D metabolism or activity have been investigated and developed for cancer therapy17. However, there are some potential limitations of vitamin D-based cancer therapy, which should be taken into consideration to design better therapeutic strategies.
One potential caveat of systemic activation of vitamin D signaling would be the high risk of hypercalcemia, which can result in serious detrimental health effects19,20. To minimize the hypercalcemic effect, many efforts are currently being directed to develop biased agonists of VDR that have little effect on inducing hypercalcemia while retaining anticancer activities comparable to those of calcitriol173–175. To date, nearly 1500 vitamin D analogs have been tested for such effects, but only a few among those compounds have been approved for further evaluation in clinical trials in patients with leukemia, breast, prostate, and colon cancers17. Moreover, the metabolites produced from the CYP11A1-driven alternative vitamin D metabolism pathway have been shown to be a biased agonist of VDR with less calcemic effect while retaining anti-proliferative properties in cancer comparable to those of calcitriol32,39. As the alternative vitamin D metabolism pathway via CYP11A1 is just beginning to be understood, its role in cancer and the relative contributions of VDR and ROR are largely unknown. Thus, intensive research on the alternative vitamin D metabolism pathway and successful application of this pathway for cancer therapy is warranted in the future. In addition, increasing local concentrations of calcitriol in cancer cells would be another strategy to avoid the hypercalcemic effect of calcitriol. As a calcitriol degrading enzyme, CYP24A1 is frequently overexpressed in many cancers; the inhibition of CYP24A1 can increase local concentrations of calcitriol in cancer cells176. Indeed, recent studies showed that inhibition of CYP24A1 by genetic knockdown or pharmacological inhibition greatly sensitized the anticancer effect of calcitriol176. So far, several CYP24A1-specific inhibitors have been developed for clinical purposes and it remains to be seen whether any of these will be used for cancer therapy with suitable clinical effectiveness and safety176.
Additionally, because the expression levels of CYP27B1, VDR, CYP11A1, and RORα/γ in cancer cells progressively decline during cancer progression as discussed earlier, the effectiveness of vitamin D-based therapy may be limited to only early stages, but not late stages, of cancer. Thus, identifying and developing novel diagnostic markers predicting the effectiveness of vitamin D-based cancer therapy may be indispensable for the successful application of such a strategy. Moreover, improved understanding of the mechanisms by which cancer progression reduces the expression of those enzymes will be a critical step for successful vitamin D-based cancer therapy. Currently, as discussed above, the mechanisms that regulate the expression and/or activity of metabolic enzymes involved in CYP27B1-VDR and CYP11A1-RORα/γ pathways are poorly understood. Recent advances in understanding cancer metabolism suggest that many oncogenic signaling pathways converge on metabolic pathways by regulating the expression and/or activity of metabolic enzymes177. Accordingly, these oncogenic signaling pathways are highly likely to regulate the expression and/or activity of enzymes involved in vitamin D metabolism and function. Unraveling such intricate networks involving the oncogenic and vitamin D metabolism pathways will contribute to the understanding of dysregulated vitamin D metabolism and function in cancer and also provide promising new opportunities for cancer therapy.
Acknowledgements
This work was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2017R1C1B2003162), the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (no. HA17C0033), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (No. HI17C0640) to S.-M.J.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang R, Naughton DP. Vitamin D in health and disease: current perspectives. Nutr. J. 2010;9:65. doi: 10.1186/1475-2891-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouillon R, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr. Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikle DD. Extraskeletal actions of vitamin D. Ann. N. Y Acad. Sci. 2016;1376:29–52. doi: 10.1111/nyas.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, et al. Vitamin D and chronic diseases. Aging Dis. 2017;8:346–353. doi: 10.14336/AD.2016.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 7.Bertino JR. Landmark study: the relation of solar radiation to cancer mortality in North America. Cancer Res. 2016;76:185. doi: 10.1158/0008-5472.CAN-15-3169. [DOI] [PubMed] [Google Scholar]
- 8.Apperly FL. The relation of solar radiation to cancer mortality in North America. Cancer Res. 1941;1:191. doi: 10.1158/0008-5472.CAN-15-3169. [DOI] [PubMed] [Google Scholar]
- 9.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J. Epidemiol. 1980;9:227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 10.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–2869. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Grant WB, Mohr SB. Ecological studies of ultraviolet B, vitamin D and cancer since 2000. Ann. Epidemiol. 2009;19:446–454. doi: 10.1016/j.annepidem.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Garland CF, et al. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–1178. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 13.Engel P, et al. Serum 25(OH) vitamin D and risk of breast cancer: a nested case-control study from the French E3N cohort. Cancer Epidemiol. Biomark. Prev. 2010;19:2341–2350. doi: 10.1158/1055-9965.EPI-10-0264. [DOI] [PubMed] [Google Scholar]
- 14.Tretli S, Hernes E, Berg JP, Hestvik UE, Robsahm TE. Association between serum 25(OH)D and death from prostate cancer. Br. J. Cancer. 2009;100:450–454. doi: 10.1038/sj.bjc.6604865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland) Cancer Causes Control. 2000;11:847–852. doi: 10.1023/a:1008923802001. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 17.Giammanco M, et al. Vitamin D in cancer chemoprevention. Pharm. Biol. 2015;53:1399–1434. doi: 10.3109/13880209.2014.988274. [DOI] [PubMed] [Google Scholar]
- 18.Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441:61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Trump DL, Johnson CS. Vitamin D in combination cancer treatment. J. Cancer. 2010;1:101–107. doi: 10.7150/jca.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta RG, Peng X, Alimirah F, Murillo G, Mehta R. Vitamin D and breast cancer: emerging concepts. Cancer Lett. 2013;334:95–100. doi: 10.1016/j.canlet.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 21.Heaney RP. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008;3:1535–1541. doi: 10.2215/CJN.01160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Japelt RB, Jakobsen J. Vitamin D in plants: a review of occurrence, analysis, and biosynthesis. Front Plant Sci. 2013;4:136. doi: 10.3389/fpls.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014;55:13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys. Acta. 2011;1814:186–199. doi: 10.1016/j.bbapap.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 25.St-Arnaud R, et al. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- 26.Masuda S, et al. Altered pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology. 2005;146:825–834. doi: 10.1210/en.2004-1116. [DOI] [PubMed] [Google Scholar]
- 27.Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best. Pract. Res Clin. Endocrinol. Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Haussler MR, et al. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 29.Pike JW, Meyer MB. Fundamentals of vitamin D hormone-regulated gene expression. J. Steroid Biochem Mol. Biol. 2014;144(Pt A):5–11. doi: 10.1016/j.jsbmb.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duran A, et al. p62/SQSTM1 by binding to vitamin D receptor inhibits hepatic stellate cell activity, fibrosis, and liver cancer. Cancer Cell. 2016;30:595–609. doi: 10.1016/j.ccell.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemere I, Safford SE, Rohe B, DeSouza MM, Farach-Carson MC. Identification and characterization of 1,25D3-membrane-associated rapid response, steroid (1,25D3-MARRS) binding protein. J. Steroid Biochem. Mol. Biol. 2004;89-90:281–285. doi: 10.1016/j.jsbmb.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 32.Slominski AT, et al. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORalpha and RORgamma. J. Steroid Biochem. Mol. Biol. 2017;173:42–56. doi: 10.1016/j.jsbmb.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slominski A, et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc. Natl. Acad. Sci. USA. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slominski AT, et al. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014;144(Pt A):28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tongkao-On W, et al. CYP11A1 in skin: an alternative route to photoprotection by vitamin D compounds. J. Steroid Biochem. Mol. Biol. 2015;148:72–78. doi: 10.1016/j.jsbmb.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Slominski, A. T. et al On the role of classical and novel forms of vitamin D in melanoma progression and management. J. Steroid Biochem. Mol. Biol. 10.1016/j.jsbmb.2017.06.013 (2017). [DOI] [PMC free article] [PubMed]
- 39.Slominski AT, et al. Vitamin D signaling and melanoma: role of vitamin D and its receptors in melanoma progression and management. Lab Invest. 2017;97:706–724. doi: 10.1038/labinvest.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slominski AT, et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–2789. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook DN, Kang HS, Jetten AM. Retinoic acid-related orphan receptors (RORs): regulatory functions in immunity, development, circadian rhythm, and metabolism. Nucl. Recept. Res. 2015;2:101185. doi: 10.11131/2015/101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch. Biochem Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zierold C, Darwish HM, DeLuca HF. Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter. J. Biol. Chem. 1995;270:1675–1678. doi: 10.1074/jbc.270.4.1675. [DOI] [PubMed] [Google Scholar]
- 45.Meyer MB, Goetsch PD, Pike JW. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3. J. Biol. Chem. 2010;285:15599–15610. doi: 10.1074/jbc.M110.119958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim MS, Fujiki R, Kitagawa H, Kato S. 1Alpha,25(OH)2D3-induced DNA methylation suppresses the human CYP27B1 gene. Mol. Cell Endocrinol. 2007;265-266:168–173. doi: 10.1016/j.mce.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 2012;92:131–155. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khundmiri SJ, Murray RD, Lederer E. PTH and vitamin D. Compr. Physiol. 2016;6:561–601. doi: 10.1002/cphy.c140071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 2010;61:91–104. doi: 10.1146/annurev.med.051308.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zierold C, Nehring JA, DeLuca HF. Nuclear receptor 4A2 and C/EBPbeta regulate the parathyroid hormone-mediated transcriptional regulation of the 25-hydroxyvitamin D3-1alpha-hydroxylase. Arch. Biochem Biophys. 2007;460:233–239. doi: 10.1016/j.abb.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 51.Rost CR, Bikle DD, Kaplan RA. In vitro stimulation of 25-hydroxycholecalciferol 1 alpha-hydroxylation by parathyroid hormone in chick kidney slices: evidence for a role for adenosine 3’,5’-monophosphate. Endocrinology. 1981;108:1002–1006. doi: 10.1210/endo-108-3-1002. [DOI] [PubMed] [Google Scholar]
- 52.Zierold C, Reinholz GG, Mings JA, Prahl JM, DeLuca HF. Regulation of the procine 1,25-dihydroxyvitamin D3-24-hydroxylase (CYP24) by 1,25-dihydroxyvitamin D3 and parathyroid hormone in AOK-B50 cells. Arch. Biochem. Biophys. 2000;381:323–327. doi: 10.1006/abbi.2000.1964. [DOI] [PubMed] [Google Scholar]
- 53.Zierold C, Mings JA, DeLuca HF. Parathyroid hormone regulates 25-hydroxyvitamin D(3)-24-hydroxylase mRNA by altering its stability. Proc. Natl. Acad. Sci. USA. 2001;98:13572–13576. doi: 10.1073/pnas.241516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am. J. Physiol. Ren. Physiol. 2010;298:F485–F499. doi: 10.1152/ajprenal.00608.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perwad F, et al. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 56.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am. J. Physiol. Ren. Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 57.Shimada T, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Ren. Physiol. 2005;289:F1088–F1095. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 58.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 59.Krishnan AV, Feldman D. Molecular pathways mediating the anti-inflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr. Relat. Cancer. 2010;17:R19–R38. doi: 10.1677/ERC-09-0139. [DOI] [PubMed] [Google Scholar]
- 60.Moreno J, et al. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65:7917–7925. doi: 10.1158/0008-5472.CAN-05-1435. [DOI] [PubMed] [Google Scholar]
- 61.Yuan L, Jiang R, Yang Y, Ding S, Deng H. 1,25-Dihydroxyvitamin D3 inhibits growth of the breast cancer cell line MCF-7 and downregulates cytochrome P4501B1 through the COX-2/PGE2 pathway. Oncol. Rep. 2012;28:2131–2137. doi: 10.3892/or.2012.2031. [DOI] [PubMed] [Google Scholar]
- 62.Thill M, et al. Expression of prostaglandin- and vitamin D-metabolising enzymes in benign and malignant breast cells. Anticancer Res. 2012;32:367–372. [PubMed] [Google Scholar]
- 63.Cordes T, et al. Correlation of prostaglandin metabolizing enzymes and serum PGE2 levels with vitamin D receptor and serum 25(OH)2D3 levels in breast and ovarian cancer. Anticancer Res. 2012;32:351–357. [PubMed] [Google Scholar]
- 64.Nonn L, Peng L, Feldman D, Peehl DM. Inhibition of p38 by vitamin D reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: implications for prostate cancer prevention by vitamin D. Cancer Res. 2006;66:4516–4524. doi: 10.1158/0008-5472.CAN-05-3796. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q, et al. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J. Biol. Chem. 2014;289:11681–11694. doi: 10.1074/jbc.M113.517581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, et al. Vitamin D receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein. J. Biol. Chem. 2013;288:19450–19458. doi: 10.1074/jbc.M113.467670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun J, et al. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am. J. Physiol. Endocrinol. Metab. 2006;291:E315–E322. doi: 10.1152/ajpendo.00590.2005. [DOI] [PubMed] [Google Scholar]
- 69.Bessler H, Djaldetti M. 1alpha,25-Dihydroxyvitamin D3 modulates the interaction between immune and colon cancer cells. Biomed. Pharmacother. 2012;66:428–432. doi: 10.1016/j.biopha.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Uberti, F., Morsanuto, V. & Molinari, C. Vitamin D in oxidative stress and diseases (ed Gowder S) A critical evaluation of vitamin D - basic overview. Ch. 02 (InTech, Rijeka, Croatia, 2017). 10.1016/j.jsbmb.2017.06.013.
- 71.Nair-Shalliker V, Armstrong BK, Fenech M. Does vitamin D protect against DNA damage? Mutat. Res. 2012;733:50–57. doi: 10.1016/j.mrfmmm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Kallay E, et al. Vitamin D receptor activity and prevention of colonic hyperproliferation and oxidative stress. Food Chem. Toxicol. 2002;40:1191–1196. doi: 10.1016/s0278-6915(02)00030-3. [DOI] [PubMed] [Google Scholar]
- 73.Banakar MC, et al. 1alpha, 25-dihydroxyvitamin D3 prevents DNA damage and restores antioxidant enzymes in rat hepatocarcinogenesis induced by diethylnitrosamine and promoted by phenobarbital. World J. Gastroenterol. 2004;10:1268–1275. doi: 10.3748/wjg.v10.i9.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fedirko V, et al. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: a randomized clinical trial. Cancer Epidemiol. Biomark. Prev. 2010;19:280–291. doi: 10.1158/1055-9965.EPI-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peehl DM, et al. Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J. Steroid Biochem. Mol. Biol. 2004;92:131–141. doi: 10.1016/j.jsbmb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 76.Lambert JR, et al. Prostate derived factor in human prostate cancer cells: gene induction by vitamin D via a p53-dependent mechanism and inhibition of prostate cancer cell growth. J. Cell. Physiol. 2006;208:566–574. doi: 10.1002/jcp.20692. [DOI] [PubMed] [Google Scholar]
- 77.Swami S, Raghavachari N, Muller UR, Bao YP, Feldman D. Vitamin D growth inhibition of breast cancer cells: gene expression patterns assessed by cDNA microarray. Breast Cancer Res. Treat. 2003;80:49–62. doi: 10.1023/A:1024487118457. [DOI] [PubMed] [Google Scholar]
- 78.Bao BY, Ting HJ, Hsu JW, Lee YF. Protective role of 1 alpha, 25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. Int J. Cancer. 2008;122:2699–2706. doi: 10.1002/ijc.23460. [DOI] [PubMed] [Google Scholar]
- 79.Zhang X, et al. Suppression of death receptor-mediated apoptosis by 1,25-dihydroxyvitamin D3 revealed by microarray analysis. J. Biol. Chem. 2005;280:35458–35468. doi: 10.1074/jbc.M506648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manna P, Jain S. Vitamin D (VD) prevents oxidative stress via regulating NOX4/Nrf2/Trx signaling cascade and upregulates SIRT1-mediated AMPK/IRS1/GLUT4 pathway and glucose uptake in high glucose treated 3T3L1 adipocytes. FASEB J. 2015;29(1 Supplement):253. [Google Scholar]
- 81.Nakai K, et al. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am. J. Hypertens. 2014;27:586–595. doi: 10.1093/ajh/hpt160. [DOI] [PubMed] [Google Scholar]
- 82.Teixeira TM, et al. Activation of Nrf2-antioxidant signaling by 1,25-dihydroxycholecalciferol prevents leptin-induced oxidative stress and inflammation in human endothelial cells. J. Nutr. 2017;147:506–513. doi: 10.3945/jn.116.239475. [DOI] [PubMed] [Google Scholar]
- 83.Berridge MJ. Vitamin D cell signalling in health and disease. Biochem Biophys. Res. Commun. 2015;460:53–71. doi: 10.1016/j.bbrc.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 84.Ting HJ, et al. A positive feedback signaling loop between ATM and the vitamin D receptor is critical for cancer chemoprevention by vitamin D. Cancer Res. 2012;72:958–968. doi: 10.1158/0008-5472.CAN-11-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang F, Li P, Fornace AJ, Jr., Nicosia SV, Bai W. G2/M arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through the induction of GADD45 via an exonic enhancer. J. Biol. Chem. 2003;278:48030–48040. doi: 10.1074/jbc.M308430200. [DOI] [PubMed] [Google Scholar]
- 86.Akhter J, Chen X, Bowrey P, Bolton EJ, Morris DL. Vitamin D3 analog, EB1089, inhibits growth of subcutaneous xenografts of the human colon cancer cell line, LoVo, in a nude mouse model. Dis. Colon Rectum. 1997;40:317–321. doi: 10.1007/BF02050422. [DOI] [PubMed] [Google Scholar]
- 87.Gonzalo S. Novel roles of 1alpha,25(OH)2D3 on DNA repair provide new strategies for breast cancer treatment. J. Steroid Biochem. Mol. Biol. 2014;144(Pt A):59–64. doi: 10.1016/j.jsbmb.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008;66:S116–S124. doi: 10.1111/j.1753-4887.2008.00094.x. [DOI] [PubMed] [Google Scholar]
- 89.Larriba M, et al. Vitamin D is a multilevel repressor of Wnt/b-catenin signaling in cancer cells. Cancers. 2013;5:1242. doi: 10.3390/cancers5041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pendás-Franco N, et al. DICKKOPF-4 is induced by TCF/β-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1α,25-dihydroxyvitamin D3. Oncogene. 2008;27:4467. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 91.An BS, et al. Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol. Cell Biol. 2010;30:4890–4900. doi: 10.1128/MCB.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kasiappan R, et al. 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J. Biol. Chem. 2012;287:41297–41309. doi: 10.1074/jbc.M112.407189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen A, Davis BH, Sitrin MD, Brasitus TA, Bissonnette M. Transforming growth factor-beta 1 signaling contributes to Caco-2 cell growth inhibition induced by 1,25(OH)(2)D(3) Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G864–G874. doi: 10.1152/ajpgi.00524.2001. [DOI] [PubMed] [Google Scholar]
- 94.Yang L, Yang J, Venkateswarlu S, Ko T, Brattain MG. Autocrine TGFbeta signaling mediates vitamin D3 analog-induced growth inhibition in breast cells. J. Cell Physiol. 2001;188:383–393. doi: 10.1002/jcp.1125. [DOI] [PubMed] [Google Scholar]
- 95.Díaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res. 2000;60:2304–2312. [PubMed] [Google Scholar]
- 96.Pálmer HG, et al. Genetic signatures of differentiation induced by 1α,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63:7799–7806. [PubMed] [Google Scholar]
- 97.Pan L, et al. Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A /sodium butyrate-induced and 5-aza-2′-deoxycytidine-induced PTEN upregulation. FEBS J. 2010;277:989–999. doi: 10.1111/j.1742-4658.2009.07542.x. [DOI] [PubMed] [Google Scholar]
- 98.Sergeev IN. Vitamin D and cellular Ca2+ signaling in breast cancer. Anticancer Res. 2012;32:299–302. [PubMed] [Google Scholar]
- 99.Wilson EN, et al. A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Horm. Cancer. 2011;2:272–285. doi: 10.1007/s12672-011-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma K, et al. A novel cytostatic form of autophagy in sensitization of non-small cell lung cancer cells to radiation by vitamin D and the vitamin D analog, EB 1089. Autophagy. 2014;10:2346–2361. doi: 10.4161/15548627.2014.993283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 2005;12:1297–1309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- 102.Tavera-Mendoza L, et al. Convergence of vitamin D and retinoic acid signalling at a common hormone response element. EMBO Rep. 2006;7:180–185. doi: 10.1038/sj.embor.7400594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lisse TS, et al. Gene targeting by the vitamin D response element binding protein reveals a role for vitamin D in osteoblast mTOR signaling. FASEB J. 2011;25:937–947. doi: 10.1096/fj.10-172577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lisse TS, Hewison M. Vitamin D: a new player in the world of mTOR signaling. Cell Cycle. 2011;10:1888–1889. doi: 10.4161/cc.10.12.15620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bikle DD. Clinical counterpoint: vitamin D: new actions, new analogs, new therapeutic potential. Endocr. Rev. 1992;13:765–784. doi: 10.1210/edrv-13-4-765. [DOI] [PubMed] [Google Scholar]
- 106.Bikle DD. Extra renal synthesis of 1,25-dihydroxyvitamin D and its health implications. Clin. Rev. Bone Miner. Metab. 2009;7:114–125. [Google Scholar]
- 107.Al-Azhri J, et al. Tumor expression of vitamin D receptor and breast cancer histopathological characteristics and prognosis. Clin. Cancer Res. 2017;23:97–103. doi: 10.1158/1078-0432.CCR-16-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum. Pathol. 2011;42:618–631. doi: 10.1016/j.humpath.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y, et al. VDR status arbitrates the prometastatic effects of tumor-associated macrophages. Mol. Cancer Res. 2014;12:1181–1191. doi: 10.1158/1541-7786.MCR-14-0036. [DOI] [PubMed] [Google Scholar]
- 110.Lopes N, et al. Alterations in vitamin D signalling and metabolic pathways in breast cancer progression: a study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer. 2010;10:483. doi: 10.1186/1471-2407-10-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thill M, et al. Expression of vitamin D receptor (VDR), cyclooxygenase-2 (COX-2) and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in benign and malignant ovarian tissue and 25-hydroxycholecalciferol (25(OH2)D3) and prostaglandin E2 (PGE2) serum level in ovarian cancer patients. J. Steroid Biochem. Mol. Biol. 2010;121:387–390. doi: 10.1016/j.jsbmb.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 112.Hendrickson WK, et al. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J. Clin. Oncol. 2011;29:2378–2385. doi: 10.1200/JCO.2010.30.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srinivasan M, Parwani AV, Hershberger PA, Lenzner DE, Weissfeld JL. Nuclear vitamin D receptor expression is associated with improved survival in non-small cell lung cancer. J. Steroid Biochem. Mol. Biol. 2011;123:30–36. doi: 10.1016/j.jsbmb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jozwicki W, Brozyna AA, Siekiera J, Slominski AT. Expression of vitamin D receptor (VDR) positively correlates with survival of urothelial bladder cancer patients. Int J. Mol. Sci. 2015;16:24369–24386. doi: 10.3390/ijms161024369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Palmer HG, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat. Med. 2004;10:917–919. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 116.Pena C, et al. E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: clinicopathological correlations. Hum. Mol. Genet. 2005;14:3361–3370. doi: 10.1093/hmg/ddi366. [DOI] [PubMed] [Google Scholar]
- 117.Mittal MK, Myers JN, Misra S, Bailey CK, Chaudhuri G. In vivo binding to and functional repression of the VDR gene promoter by SLUG in human breast cells. Biochem. Biophys. Res. Commun. 2008;372:30–34. doi: 10.1016/j.bbrc.2008.04.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maruyama R, et al. Comparative genome analysis identifies the vitamin D receptor gene as a direct target of p53-mediated transcriptional activation. Cancer Res. 2006;66:4574–4583. doi: 10.1158/0008-5472.CAN-05-2562. [DOI] [PubMed] [Google Scholar]
- 119.Stambolsky P, et al. Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell. 2010;17:273–p285. doi: 10.1016/j.ccr.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.DeSmet ML, Fleet JC. Constitutively active RAS signaling reduces 1,25 dihydroxyvitamin D-mediated gene transcription in intestinal epithelial cells by reducing vitamin D receptor expression. J. Steroid Biochem. Mol. Biol. 2017;173:194–201. doi: 10.1016/j.jsbmb.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Z, et al. Constitutive activation of the mitogen-activated protein kinase pathway impairs vitamin D signaling in human prostate epithelial cells. J. Cell. Physiol. 2010;224:433–442. doi: 10.1002/jcp.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Solomon C, White JH, Kremer R. Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3-dependent signal transduction by phosphorylating human retinoid X receptor alpha. J. Clin. Invest. 1999;103:1729–1735. doi: 10.1172/JCI6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marik R, et al. DNA methylation-related vitamin D receptor insensitivity in breast cancer. Cancer Biol. Ther. 2010;10:44–53. doi: 10.4161/cbt.10.1.11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liel Y, Shany S, Smirnoff P, Schwartz B. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology. 1999;140:280–285. doi: 10.1210/endo.140.1.6408. [DOI] [PubMed] [Google Scholar]
- 125.Mohri T, Nakajima M, Takagi S, Komagata S, Yokoi T. MicroRNA regulates human vitamin D receptor. Int. J. Cancer. 2009;125:1328–1333. doi: 10.1002/ijc.24459. [DOI] [PubMed] [Google Scholar]
- 126.Essa S, et al. VDR microRNA expression and epigenetic silencing of vitamin D signaling in melanoma cells. J. Steroid Biochem. Mol. Biol. 2010;121:110–113. doi: 10.1016/j.jsbmb.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 127.Hsu JW, et al. Suppression of prostate cancer cell rolling and adhesion to endothelium by 1alpha,25-dihydroxyvitamin D3. Am. J. Pathol. 2011;178:872–880. doi: 10.1016/j.ajpath.2010.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Segersten U, et al. 25-Hydroxyvitamin D(3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. J. Clin. Endocrinol. Metab. 2002;87:2967–2972. doi: 10.1210/jcem.87.6.8604. [DOI] [PubMed] [Google Scholar]
- 129.Matusiak D, Benya RV. CYP27A1 and CYP24 expression as a function of malignant transformation in the colon. J. Histochem. Cytochem. 2007;55:1257–1264. doi: 10.1369/jhc.7A7286.2007. [DOI] [PubMed] [Google Scholar]
- 130.Chen, T. C., Holick, M. F., Lokeshwar, B. L., Burnstein, K. L. & Schwartz, G. G. Evaluation of vitamin D analogs as therapeutic agents for prostate cancer. (eds Reichrath, J., Tilgen, W., Friedrich, M.) Vitamin D analogs in cancer prevention and therapy pp. 273-288 (Springer, Berlin, Heidelberg, Germany, 2003) 10.1007/978-3-642-55580-0_20. [DOI] [PubMed]
- 131.Mawer EB, et al. Constitutive synthesis of 1,25-dihydroxyvitamin D3 by a human small cell lung cancer cell line. J. Clin. Endocrinol. Metab. 1994;79:554–560. doi: 10.1210/jcem.79.2.8045976. [DOI] [PubMed] [Google Scholar]
- 132.Hansdottir S, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hummel DM, Fetahu IS, Groschel C, Manhardt T, Kallay E. Role of proinflammatory cytokines on expression of vitamin D metabolism and target genes in colon cancer cells. J. Steroid Biochem. Mol. Biol. 2014;144(Pt A):91–95. doi: 10.1016/j.jsbmb.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Clinckspoor I, et al. Altered expression of key players in vitamin D metabolism and signaling in malignant and benign thyroid tumors. J. Histochem. Cytochem. 2012;60:502–511. doi: 10.1369/0022155412447296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lopes N, et al. 1Alpha,25-dihydroxyvitamin D3 induces de novo E-cadherin expression in triple-negative breast cancer cells by CDH1-promoter demethylation. Anticancer Res. 2012;32:249–257. [PubMed] [Google Scholar]
- 136.Blomberg Jensen M, et al. Expression of the vitamin D receptor, 25-hydroxylases, 1alpha-hydroxylase and 24-hydroxylase in the human kidney and renal clear cell cancer. J. Steroid Biochem. Mol. Biol. 2010;121:376–382. doi: 10.1016/j.jsbmb.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 137.Urbschat A, et al. Vitamin D hydroxylases CYP2R1, CYP27B1 and CYP24A1 in renal cell carcinoma. Eur. J. Clin. Invest. 2013;43:1282–1290. doi: 10.1111/eci.12176. [DOI] [PubMed] [Google Scholar]
- 138.Yokomura K, et al. Increased expression of the 25-hydroxyvitamin D(3)-1alpha-hydroxylase gene in alveolar macrophages of patients with lung cancer. J. Clin. Endocrinol. Metab. 2003;88:5704–5709. doi: 10.1210/jc.2003-030537. [DOI] [PubMed] [Google Scholar]
- 139.Szeles L, et al. 1,25-Dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J. Immunol. 2009;182:2074–2083. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 140.Zehnder D, et al. Synthesis of 1,25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J. Am. Soc. Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 141.Albertson DG, et al. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat. Genet. 2000;25:144–146. doi: 10.1038/75985. [DOI] [PubMed] [Google Scholar]
- 142.Horvath HC, et al. The candidate oncogene CYP24A1: a potential biomarker for colorectal tumorigenesis. J. Histochem. Cytochem. 2010;58:277–285. doi: 10.1369/jhc.2009.954339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cross HS, Bises G, Lechner D, Manhardt T, Kallay E. The vitamin D endocrine system of the gut–its possible role in colorectal cancer prevention. J. Steroid Biochem. Mol. Biol. 2005;97:121–128. doi: 10.1016/j.jsbmb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 144.Brozek W, Manhardt T, Kallay E, Peterlik M, Cross HS. Cancers (Basel) 2012. Relative expression of vitamin D hydroxylases, CYP27B1 and CYP24A1, and of cyclooxygenase-2 and heterogeneity of human colorectal cancer in relation to age, gender, tumor location, and malignancy: results from factor and cluster analysis; pp. 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen G, et al. CYP24A1 is an independent prognostic marker of survival in patients with lung adenocarcinoma. Clin. Cancer Res. 2011;17:817–826. doi: 10.1158/1078-0432.CCR-10-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tannour-Louet M, et al. Increased expression of CYP24A1 correlates with advanced stages of prostate cancer and can cause resistance to vitamin D3-based therapies. FASEB J. 2014;28:364–372. doi: 10.1096/fj.13-236109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother. Pharmacol. 2006;57:234–240. doi: 10.1007/s00280-005-0059-7. [DOI] [PubMed] [Google Scholar]
- 148.Mitschele T, et al. Analysis of the vitamin D system in basal cell carcinomas (BCCs) Lab. Invest. 2004;84:693–702. doi: 10.1038/labinvest.3700096. [DOI] [PubMed] [Google Scholar]
- 149.Diesing D, et al. Analysis of 25-hydroxyvitamin D3–1alpha-hydroxylase in normal and malignant breast tissue. J. Clin. Oncol. 2005;23:806–806. [PubMed] [Google Scholar]
- 150.Sun H, et al. CYP24A1 is a potential biomarker for the progression and prognosis of human colorectal cancer. Hum. Pathol. 2016;50:101–108. doi: 10.1016/j.humpath.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 151.Mimori K, et al. Clinical significance of the overexpression of the candidate oncogene CYP24 in esophageal cancer. Ann. Oncol. 2004;15:236–241. doi: 10.1093/annonc/mdh056. [DOI] [PubMed] [Google Scholar]
- 152.Shiratsuchi H, et al. Oncogenic potential of CYP24A1 in lung adenocarcinoma. J. Thorac. Oncol. 2017;12:269–280. doi: 10.1016/j.jtho.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 153.Osanai M, Lee GH. CYP24A1-induced vitamin D insufficiency promotes breast cancer growth. Oncol. Rep. 2016;36:2755–2762. doi: 10.3892/or.2016.5072. [DOI] [PubMed] [Google Scholar]
- 154.Muindi JR, et al. CYP24A1 inhibition enhances the antitumor activity of calcitriol. Endocrinology. 2010;151:4301–4312. doi: 10.1210/en.2009-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luo W, et al. Epigenetic regulation of vitamin D 24-hydroxylase/CYP24A1 in human prostate cancer. Cancer Res. 2010;70:5953–5962. doi: 10.1158/0008-5472.CAN-10-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brozyna AA, et al. CYP24A1 expression inversely correlates with melanoma progression: clinic-pathological studies. Int J. Mol. Sci. 2014;15:19000–19017. doi: 10.3390/ijms151019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hobaus J, et al. Increased copy-number and not DNA hypomethylation causes overexpression of the candidate proto-oncogene CYP24A1 in colorectal cancer. Int J. Cancer. 2013;133:1380–1388. doi: 10.1002/ijc.28143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meijer GA, et al. Progression from colorectal adenoma to carcinoma is associated with non- random chromosomal gains as detected by comparative genomic hybridisation. J. Clin. Pathol. 1998;51:901–909. doi: 10.1136/jcp.51.12.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Komagata S, et al. Human CYP24 catalyzing the inactivation of calcitriol is post-transcriptionally regulated by miR-125b. Mol. Pharmacol. 2009;76:702–709. doi: 10.1124/mol.109.056986. [DOI] [PubMed] [Google Scholar]
- 160.Borkowski R, et al. Genetic mutation of p53 and suppression of the miR-17 approximately 92 cluster are synthetic lethal in non-small cell lung cancer due to upregulation of vitamin D Signaling. Cancer Res. 2015;75:666–675. doi: 10.1158/0008-5472.CAN-14-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Luo W, et al. Inhibition of protein kinase CK2 reduces Cyp24a1 expression and enhances 1,25-dihydroxyvitamin D(3) antitumor activity in human prostate cancer cells. Cancer Res. 2013;73:2289–2297. doi: 10.1158/0008-5472.CAN-12-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Trembley JH, et al. Emergence of protein kinase CK2 as a key target in cancer therapy. Biofactors. 2010;36:187–195. doi: 10.1002/biof.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ramnath N, et al. Epigenetic regulation of vitamin D metabolism in human lung adenocarcinoma. J. Thorac. Oncol. 2014;9:473–482. doi: 10.1097/JTO.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hobaus J, Fetahu I, Khorchide M, Manhardt T, Kallay E. Epigenetic regulation of the 1,25-dihydroxyvitamin D3 24-hydroxylase (CYP24A1) in colon cancer cells. J. Steroid Biochem. Mol. Biol. 2013;136:296–299. doi: 10.1016/j.jsbmb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tieu EW, et al. Rat CYP24A1 acts on 20-hydroxyvitamin D(3) producing hydroxylated products with increased biological activity. Biochem. Pharmacol. 2012;84:1696–1704. doi: 10.1016/j.bcp.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brozyna AA, Jozwicki W, Skobowiat C, Jetten A, Slominski AT. RORalpha and RORgamma expression inversely correlates with human melanoma progression. Oncotarget. 2016;7:63261–63282. doi: 10.18632/oncotarget.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fan Z, Wang Z, Chen W, Cao Z, Li Y. Association between the CYP11 family and six cancer types. Oncol. Lett. 2016;12:35–40. doi: 10.3892/ol.2016.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jernberg E, et al. Characterization of prostate cancer bone metastases according to expression levels of steroidogenic enzymes and androgen receptor splice variants. PLoS ONE. 2013;8:e77407. doi: 10.1371/journal.pone.0077407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fu RD, Qiu CH, Chen HA, Zhang ZG, Lu MQ. Retinoic acid receptor-related receptor alpha (RORalpha) is a prognostic marker for hepatocellular carcinoma. Tumour Biol. 2014;35:7603–7610. doi: 10.1007/s13277-014-2007-9. [DOI] [PubMed] [Google Scholar]
- 170.Oh TG, et al. PRMT2 and RORgamma expression are associated with breast cancer survival outcomes. Mol. Endocrinol. 2014;28:1166–1185. doi: 10.1210/me.2013-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lekva T, et al. Attenuated RORC expression in the presence of EMT progression in somatotroph adenomas following treatment with somatostatin analogs is associated with poor clinical recovery. PLoS ONE. 2013;8:e66927. doi: 10.1371/journal.pone.0066927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Oh TG, et al. The nuclear receptor, RORgamma, regulates pathways necessary for breast cancer metastasis. EBioMedicine. 2016;6:59–72. doi: 10.1016/j.ebiom.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Trump DL, Deeb KK, Johnson CS. Vitamin D: considerations in the continued development as an agent for cancer prevention and therapy. Cancer J. 2010;16:1–9. doi: 10.1097/PPO.0b013e3181c51ee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen TC, Kittaka A. Novel vitamin d analogs for prostate cancer therapy. ISRN Urol. 2011;2011:301490. doi: 10.5402/2011/301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Byers SW, Rowlands T, Beildeck M, Bong YS. Mechanism of action of vitamin D and the vitamin D receptor in colorectal cancer prevention and treatment. Rev. Endocr. Metab. Disord. 2012;13:31–38. doi: 10.1007/s11154-011-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Luo W, Hershberger PA, Trump DL, Johnson CS. 24-Hydroxylase in cancer: impact on vitamin D-based anticancer therapeutics. J. Steroid Biochem. Mol. Biol. 2013;136:252–257. doi: 10.1016/j.jsbmb.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nagarajan A, Malvi P, Wajapeyee N. Oncogene-directed alterations in cancer cell metabolism. Trends Cancer. 2016;2:365–377. doi: 10.1016/j.trecan.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]