Abstract
Pulmonary vascular remodeling due to excessive proliferation and resistance to apoptosis of pulmonary artery smooth muscle cells (PASMCs) is the hallmark feature of pulmonary arterial hypertension (PAH). Recent evidence suggests that miR-125a-5p plays a role in a rat model of monocrotaline-induced PAH (MCT-PAH); however, the underlying mechanism is currently unknown. Here, we examined the expression profile of miR-125a-5p in MCT-PAH rats and investigated the putative therapeutic effect of miR-125a-5p using the miR-125a-5p agomir. In addition, the miR-125a-5p agomir or antagomir was transfected into rat PASMCs, and proliferation and apoptosis were measured. Activity of the miR-125a-5p target STAT3 was measured using a luciferase reporter assay, and the expression of downstream molecules was measured using RT–qPCR and/or western blot analysis. Importantly, inducing miR-125a-5p expression in vivo slowed the progression of MCT-PAH by reducing systolic pulmonary arterial pressure, the Fulton index, and pulmonary vascular remodeling. Moreover, overexpressing miR-125a-5p inhibited the proliferation and promoted the apoptosis of PASMCs. In addition, stimulating PASMCs with TGF-β1 or IL-6 upregulated miR-125a-5p expression, whereas overexpressing miR-125a-5p reduced TGF-β1 and IL-6 production, as well as the expression of their downstream targets STAT3 and Smad2/3; in contrast, downregulating miR-125a-5p increased TGF-β1 and IL-6 production. Finally, a dual-luciferase reporter assay revealed that miR-125a-5p targets the 3′-UTR of STAT3, suppressing the downstream molecules PCNA, Bcl-2, and Survivin. Taken together, these findings suggest that miR-125a-5p ameliorates MCT-PAH in rats, has a negative feedback regulation with TGF-β1 and IL-6, and regulates the proliferation and apoptosis of PASMCs by directly targeting STAT3.
Pulmonary hypertension: Alleviating the pressure
A study in rats suggests that the small RNA molecule miR-125a-5p is a promising therapeutic target for treating pulmonary arterial hypertension (PAH). This type of high blood pressure is due to the narrowing of arteries that carry blood from the heart to the lungs and at present has no cure. Jieyan Shen at Shanghai Jiao Tong University, China, and colleagues found that PAH lowers the levels of miR-125a-5p in rat pulmonary arteries and that administration of miR-125a-5p as an early preventative treatment reduced disease progression. miR-125a-5p slowed the proliferation of pulmonary artery smooth muscle cells and triggered cell death by directly interacting with a gene expression regulator and reducing the production of certain pro-inflammatory signaling molecules. Targeting miR-125a-5p’s mechanism of action could represent a new treatment approach for this chronic, life-changing disease.
Introduction
Pulmonary arterial hypertension (PAH) is a devastating disease characterized by pulmonary vascular remodeling with medial hypertrophy, intimal proliferative and adventitial thickening, and perivascular inflammatory infiltrates1. The hallmark feature of PAH—pulmonary vascular remodeling—is associated with the excessive proliferation and resistance to apoptosis of pulmonary artery smooth muscle cells (PASMCs). Advances in the treatment of PAH have led to improved outcome and prognosis of PAH patients, with 5-year and 10-year survival rates of 52–75% and 45–66%, respectively;2 however, these patients still have functional limitations and reduced long-term survival, possibly due to irreversible pulmonary vascular remodeling. Thus, novel therapies are needed in order to reverse or reduce pulmonary vascular remodeling in PAH.
MicroRNAs (miRNAs) are endogenous small noncoding RNAs (∼22 nt) that negatively regulate gene expression by binding to the 3′ untranslated region (3′-UTR) of their target coding mRNA to inhibit expression by blocking translation and/or accelerating mRNA degradation3. A growing number of studies have reported that specific miRNAs can improve the outcome of PAH, suggesting that miRNAs may play a role in the pathogenesis of PAH4, 5. Recent studies have shown that the miR-125a-5p plays a key role in several regulatory processes, including the proliferation, migration, and apoptosis of various cell types, thereby affecting the development of certain diseases. However, the precise role of miR-125a-5p in PAH is poorly understood. Here, we investigated the role of miR-125a-5p in the development of monocrotaline-induced PAH (MCT-PAH) in rats, and we examined its underlying mechanism.
Materials and methods
Ethics
All experiments were performed in accordance with the Shanghai JiaoTong University Ethics Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and the tenets outlined in the Declaration of Helsinki.
Experimental animal model and treatment
Monocrotaline (MCT; Sigma-Aldrich, St. Louis, MO) was dissolved in phosphate-buffered saline. The pH was adjusted to 7.4 with 0.1 N HCl, and the volume was increased to achieve a final concentration of 20 mg/ml. The MCT solution was sterilized by passing through a 0.45-μm syringe filter, aliquoted, and stored at 20 °C. On day 1, rats were given an intraperitoneal injection of 50 mg/kg MCT to induce PAH; control rats received normal saline (0.9% NaCl)6, 7. To investigate the effect of increasing miR-125a-5p expression in vivo, MCT-PAH rats received the miR-125a-5p agomir (1 nM or 2 nM; RiboBio, Guangzhou, China) via a nebulizer. One set of rats received the miR-125a-5p agomir at week 2 (relative to the MCT injection) and were sacrificed at week 3 (Early Therapy); a second set of rats received the miR-125a-5p agomir at week 3 and were sacrificed at week 4 (Late Rescue Therapy).
Hemodynamic measurements
At week 3 or week 4, rats were anesthetized with pentobarbital (40 mg/kg), and the right heart was catheterized. A specially designed J-shaped polyethylene catheter (0.9 mm) filled with heparinized saline was introduced into the right external jugular vein, and then advanced through the right atrium and right ventricle (RV). Proper positioning of the catheter was confirmed by the shape of the pressure trace curve. Once the shape of the pressure curve was stable, the following hemodynamic parameters were measured using an ML110 pressure transducer and recorded using a PowerLab data acquisition system (both from ADInstruments, New South Wales, Australia): mean right ventricular pressure (mRVP) and systolic pulmonary arterial pressure (SPAP).
Tissue preparation and histology
After the hemodynamic measurements were obtained, the rats were immediately sacrificed. The lung tissue was infused with 0.9% normal saline via the pulmonary artery (PA). The PA was then isolated from the lungs and stored at −80 °C for molecular experiments. The lower lobe of the right lung was fixed in 4% paraformaldehyde at 4 °C for 24 h, then embedded in paraffin. To quantify remodeling of the RV, the free wall of the RV and the left ventricle plus septum (LV + S) were dissected and weighed, and the Fulton index was calculated using the formula [RV/(LV + S)]. In addition, paraffin-embedded lung tissue samples were sectioned at 5-µm thickness, and the sections were stained with hematoxylin-and-eosin (H&E); pulmonary vascular remodeling was then measured by calculating the percentage of pulmonary artery wall area relative to the total artery area (10 arteries were measured per animal).
Culture, treatment, and stimulation of PASMCs
Pulmonary artery smooth muscle cells (PASMCs) were isolated from male control and MCT-PAH rats and cultured for 3–5 passages prior to use in all experiments. PASMCs were grown in high-glucose DMEM (Shanghai Basalmedia Technologies Co., Ltd., Shanghai, China) with 10% FBS (Sigma-Aldrich) and 0.5% antibiotic/antimitotic (Shanghai Basalmedia Technologies, Shanghai, China). PASMCs were positively identified using immunofluorescence with the anti-α-SMA antibody (Abcam, Cambridge, UK) (Supplemental Figure S1). The role of miR-125a-5p was assessed using the miR-125a-5p agomir (50 nM) or the miR-125a-5p antagomir (100 nM) (RiboBio, Guangzhou, China), with high transfection efficiency (Supplemental Figure S2). For each experiment, the respective negative controls for the miR-125a-5p agomir or antagomir (RiboBio, Guangzhou, China) were used. To stimulate PASMCs, the cells were incubated in medium alone (as a control) or in medium containing 10 ng/ml TGF-β1 or IL-6 (PeproTech, Rocky Hill, NJ).
Proliferation and apoptosis measurements
To study the in vitro effect of miR-125a-5p on PASMC proliferation and apoptosis, unstimulated PASMCs were transfected with either the miR-125a-5p agomir or the miR-125a-5p antagomir (or the respective negative control). PASMC proliferation was measured using the CCK8 kit (Dojindo Molecular Technologies, Inc., Rockville, MD) in accordance with the manufacturer’s instructions. Apoptosis of PASMCs was quantified using the Annexin V-FITC Apoptosis kit (Miltenyi Biotec, Inc., Bergisch Gladbach, Germany) in accordance with the manufacturer’s instructions followed by flow cytometry.
Enzyme-linked immunosorbent assay
The culture medium from PASMCs was collected 48 h after transfection with the miR-125a agomir or antagomir (or the respective negative control) and centrifuged at 10,000×g for 10 min at 4 °C. The supernatant was placed immediately at −80 °C in aliquots and thawed only once before measurement. The protein levels of TGF-β1 and IL-6 were measured using an enzyme-linked immunosorbent assay (ELISA) as described previously8.
RT–qPCR and western blot analysis
As described previously7, total RNA was extracted, and target mRNA was quantified using real-time quantitative reverse-transcription polymerase chain reaction (RT–qPCR) with the primer sequences (designed using NCBI’s Primer-BLAST program) listed in Supplemental Table S1; β-actin was used as a reference gene. miR-125a-5p was measured using the TaqMan MicroRNA Assay (MIMAT0000829, Applied Biosystems, Waltham, MA) in accordance with the manufacturer’s instruction; U6 was used as a reference gene. For western blot analysis to measure proteins, the following primary antibodies were used (all from Cell Signaling Technology, Inc., Danvers, MA): STAT3 (signal transducer and activator of transcription 3), p-STAT3, Smad2/3, PCNA (proliferating cell nuclear antigen), Bcl-2 (B-cell lymphoma 2), Survivin, and β-actin.
Verification that miR-125a-5p targets STAT3
The TargetScan, miRanda, and miRDB algorithms were used to predict possible targets of miR-125a-5p, followed by verification using a 3′-UTR reporter assay. From a list of 35 predicted targets, STAT3 was selected for verification, as STAT3 plays a key role in the pathogenesis of PAH9. The 3′-UTR of the STAT3 gene was amplified using PCR from rat genomic DNA, and the amplification product was inserted into a modified pmirGLO dual-luciferase vector (Promega, Madison, WI) at EcoRI and XhoI restriction sites, resulting in the pFLuc-3′-UTR-STAT3 construct. The following primer pairs were used for PCR amplification: 5′-CAGAATTCCACACTGAAACAGCATAGCCTTT-C-3′ and 5′-CACCTCGAGACCAGGACAGAATAAAAGCCACTG-3′. To generate a mutated 3′-UTR reporter construct, seven nucleotides in the seeding sequence of the predicted miR-125a-5p recognition site were mutated using overlap PCR (see Fig. 1a). The 3′-UTR reporter assay was performed in HEK-293T cells seeded on 24-well plates. When the cells reached 80–90% confluence, they were transfected with 50 ng of the pFLuc-3′-UTR-STAT3 construct and 50 pmol of the miR-125a-5p agomir or antagomir using the K2 transfection system (Biontex Laboratories GmbH, Martinsried Munich, Germany). Two days after transfection, the cells were harvested and measured using the dual-luciferase assay.
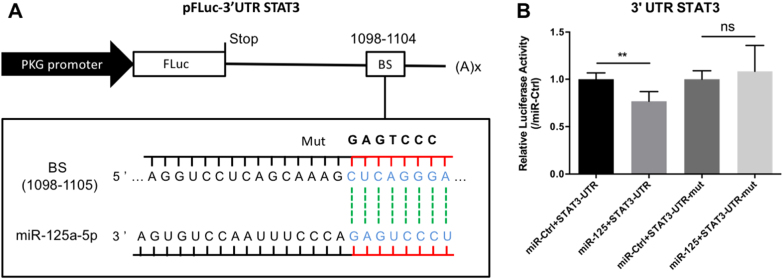
Fig. 1. STAT3 is a direct regulatory target of miR-125a-5p in HEK-293T cells.
a Schematic depiction of the pFLuc-3′-UTR-STAT3 luciferase reporter construct containing the 3′-UTR of the rat STAT3 gene; the predicted miR-125a-5p‒binding site (BS) is indicated. Shown below is the wild-type miRNA binding site (CUCAGGGA), along with the corresponding complementary sequence in the miR-125a-5p miRNA; the sequence of the mutated binding site (GAGTCCC) is also shown. b HEK-293T cells were transfected with the indicated constructs; two days after transfection, luciferase activity was measured and is expressed relative to cells transfected with the respective control miRNA. **p < 0.01; NS not significant (p > 0.05)
Statistics
All summary data are presented as fold change or mean value ± SD. The unpaired Student’s t-test was used to compare two groups; to compare > 2 groups, a one-way ANOVA followed by Tukey’s multiple comparison test or a two-way ANOVA followed by either Bonferroni’s or Tukey’s multiple comparisons test as used. Differences with a two-sided p-value <0.05 were considered statistically significant.
Results
MiR-125a-5p is downregulated in a rat model of monocrotaline-induced PAH
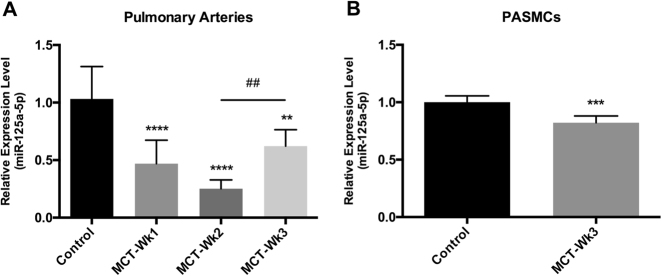
We first measured miR-125a-5p in a wide range of tissues and found that miR-125a-5p is highly expressed in several tissues, including the pulmonary artery (PA) (Supplemental Figure S3). Next, to examine the change in the expression of miR-125a-5p in the PA of rats with monocrotaline-induced PAH (MCT-PAH), we injected rats with MCT or saline (as a control group), isolated the PA 1, 2, or 3 weeks after injection, and then measured the expression level of miR-125a-5p using RT–qPCR. Our analysis revealed that miR-125a-5p was significantly downregulated in the PA at all three time points compared with control rats (Fig. 2a). The expression of miR-125a-5p was lowest in week 2, and then increased significantly in week 3 (but remained significantly lower than the control group).
Fig. 2. The expression profile of miR-125a-5p in the pulmonary artery (PA) of MCT-PAH rats and in rat PASMCs.
a Compared to control rats (n = 6), miR-125a-5p was significantly downregulated in the PA of MCT-PAH rats at week 1 (n = 6), week 2 (n = 8), and week 3 (n = 8). b miR-125a-5p was significantly downregulated in MCT-PASMCs compared with control PASMCs. The expression level of miR-125a-5p was normalized to U6. **p < 0.01, ***p < 0.001, and ****p < 0.0001 vs. control; ##p < 0.01
As discussed above, excessive proliferation and resistance to apoptosis of pulmonary artery smooth muscle cells (PASMCs) is the hallmark feature of PAH. We therefore hypothesized that miR-125a-5p may play a pathological role in this process. To test this hypothesis, we measured the expression of miR-125a-5p in PASMCs obtained from control rats and MCT-PAH rats 3 weeks after MCT injection. Our RT–qPCR data showed that the expression of miR-125a-5p was significantly downregulated in MCT-PASMCs compared with control PASMCs (Fig. 2b).
MiR-125a-5p therapy partly reduces the development of MCT-PAH
To examine the effect of miR-125a-5p on the development of MCT-PAH, we increased the expression of miR-125a-5p by treating rats with the miR-125a-5p agomir. Two different doses of the miR-125a-5p agomir (1 or 2 nM) were applied using a nebulizer, and the effects were measured at week 3 (Fig. 3a) and week 4 (Fig. 3b) to test the difference between early preventive therapy and late rescue therapy.
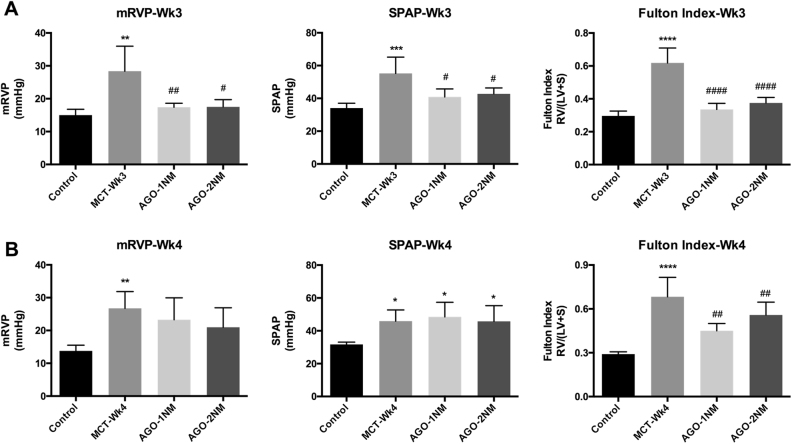
Fig. 3. miR-125a-5p slows the progression of MCT-PAH.
The miR-125a-5p agomir (1 nM or 2 nM) was administered by nebulizer, and mRVP, SPAP, and the Fulton index were measured 3 weeks (a) or 4 weeks (b) after MCT injection (n = 5–6 rats per group). AGO, miR-125a-5p agomir; mRVP, mean right ventricular pressure; SPAP, systolic pulmonary arterial pressure. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 vs. control; #p < 0.05, ##p < 0.01, and ####p < 0.0001 vs. MCT-WK3
At week 3, both doses of the miR-125a-5p agomir significantly reduced systolic pulmonary arterial pressure (SPAP), mean right ventricular pressure (mRVP), and the Fulton index (Fig. 3a); however, we found no significant difference between the two doses. At week 4, both doses of the miR-125a-5p agomir significantly reduced the Fulton index, but neither dose had any effect on SPAP or mRVP (Fig. 3b).
Taken together, these results indicate that miR-125a-5p agomir therapy partly reduces the progression of MCT-PAH.
MiR-125a-5p reduces pulmonary vascular remodeling
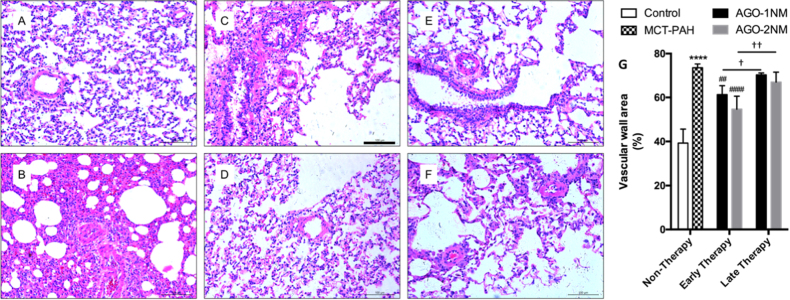
Next, we examined whether miR-125a-5p affects the hallmark histological feature of PAH, pulmonary vascular remodeling, by performing a histological analysis. Rats were sacrificed, and paraffin embed sections were prepared from the lower lobe of the right lung and stained with hematoxylin-and-eosin (H&E). We found that for the early therapy (sacrificed at week 3), both doses of the miR-125a-5p agomir (i.e., 1 and 2 nM) significantly reduced pulmonary vascular remodeling by reducing the percentage of pulmonary artery wall area in MCT-PAH rats (Fig. 4c, d, g). At week 4, however, neither dose of the miR-125a-5p agomir had a significant effect on pulmonary vascular remodeling (Fig. 4e, f, g).
Fig. 4. miR-125a-5p reduces pulmonary vascular remodeling in vivo.
Early miR-125a-5p agomir treatment significantly reduced pulmonary vascular remodeling. The images in A-F show representative H&E-stained sections of right lung tissue sections in the indicated groups; the scale bars represent 100 μm. a Control, b MCT-PAH, c the early therapy group with 1 nM miR-125a-5p agomir, d the early therapy group with 2 nM miR-125a-5p agomir, e the late therapy group with 1 nM miR-125a-5p agomir, and f the late therapy group with 2 nM miR-125a-5p agomir. g Summary of vascular wall area in the indicated groups (n = 5–6 rats per group). ****p < 0.0001 vs. control; ##p < 0.01 and ####p < 0.0001 vs. MCT-PAH; †p < 0.05 and ††p < 0.01
MiR-125a-5p inhibits proliferation and promotes apoptosis of PASMCs
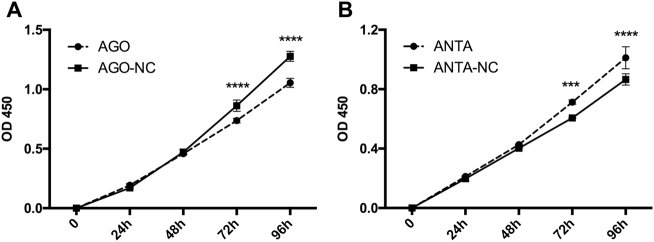
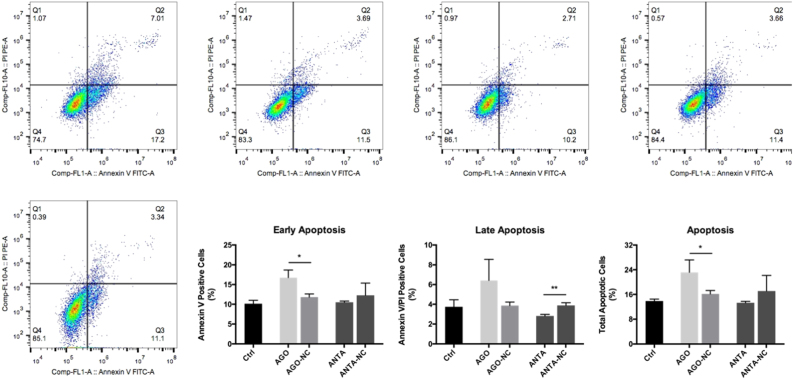
Based on the above results, we hypothesized that miR-125a-5p can affect the proliferation and apoptosis of PASMCs. To test this hypothesis, we transfected cultured rat PASMCs with either the miR-125a-5p agomir (50 nM) to induce overexpression of miR-125a-5p or the miR-125a-5p antagomir (100 nM) to inhibit miR-125a-5p expression (Figs. 5 and 6). We found that the miR-125a-5p agomir inhibited the proliferation of PASMCs (Fig. 5a) and promoted apoptosis of PASMCs selectively in the early stage (Fig. 6). In contrast, the miR-125a-5p antagomir increased the proliferation of PASMCs (Fig. 5b) and inhibited late apoptosis (Fig. 6).
Fig. 5. miR-125a-5p inhibits the proliferation of PASMCs.
a Overexpressing miR-125a-5p with the agomir (50 nM) significantly inhibited the proliferation of rat PASMCs (interaction: p < 0.0001). b Downregulating miR-125a-5p expression with the antagomir (100 nM) increased the proliferation of PASMCs (interaction: p < 0.001). AGO-NC and ANTA-NC refer to negative controls for the miR-125a-5p agomir and antagomir, respectively. ***p < 0.001 and ****p < 0.0001 vs. the respective negative control
Fig. 6. miR-125a-5p promotes apoptosis in PASMCs.
Overexpressing miR-125a-5p with the agomir promoted the early stage—but not the late stage—of apoptosis in PASMCs. Downregulating miR-125a-5p with the antagomir inhibited the late stage of apoptosis in PASMCs. Q2: cells that stained positive for both Annexin V and propidium iodide (i.e., late apoptosis); Q3: cells that stained positive for Annexin V but not propidium iodide (i.e., early apoptosis). AGO-NC and ANTA-NC refer to negative controls for the miR-125a-5p agomir and antagomir, respectively. *p < 0.05 and **p < 0.01
Reciprocal regulation between TGF-β1/IL-6 signaling and miR-125a-5p expression in PASMCs
Previous studies10 showed that the pro-inflammatory cytokines TGF-β1 and IL-6 play an important role in the proliferation and apoptosis of PASMCs. Moreover, the expression of TGF-β1 and IL-6 in the lung tissue of MCT-PAH rats increases progressively as PAH progresses.
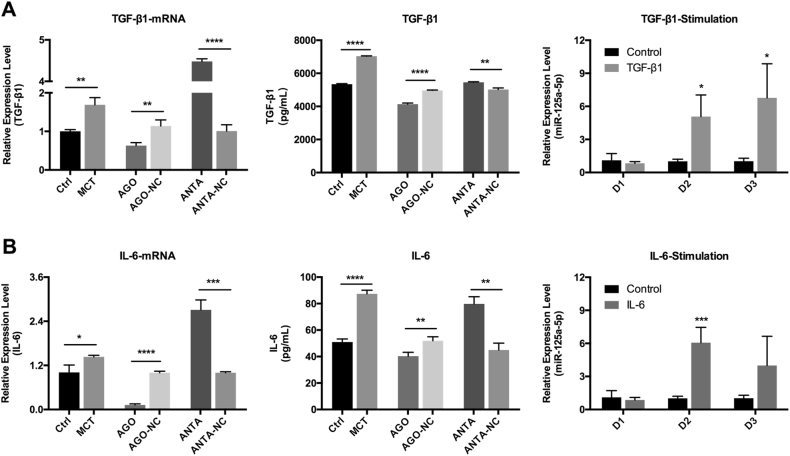
To study whether TGF-β1 and/or IL-6 affects the expression of miR-125a-5p, we stimulated cultured PASMCs with TGF-β1 or IL-6, and then measured the expression levels of miR-125a-5p using RT–qPCR. After stimulation with TGF-β1, miR-125a-5p expression was significantly increased on both day 2 and day 3 (Fig. 7a, right). Moreover, after stimulation with IL-6, miR-125a-5p expression was also significantly increased, although only on day 2 (Fig. 7b, right).
Fig. 7. Reciprocal negative regulation between TGF-β1/IL-6 and miR-125a-5p in PASMCs.
a Overexpressing miR-125a-5p inhibited the expression of TGF-β1, whereas downregulating miR-125a-5p upregulated the expression of TGF-β1. Stimulating cells with TGF-β1 upregulated the expression of miR-125a-5p beginning on day 2. b Overexpressing miR-125a-5p inhibited the expression of IL-6, whereas downregulating miR-125a-5p upregulated the expression of IL-6. Stimulating cells with IL-6 upregulated the expression of miR-125a-5p measured on day 2. AGO-NC and ANTA-NC refer to negative controls for the miR-125a-5p agomir and antagomir, respectively. D, Day. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
Next, to test whether this regulation is reciprocal (i.e., whether miR-125a-5p expression affects the expression of TGF-β1 and IL-6), we incubated PASMCs with either the miR-125a-5p agomir (50 nM) or the miR-125a-5p antagomir (100 nM), and then measured the mRNA and protein levels of TGF-β1 and IL-6 using RT–qPCR and ELISA, respectively. We found that increasing miR-125a-5p expression inhibited the expression of both TGF-β1 and IL-6, whereas downregulating miR-125a-5p expression increased the expression of both TGF-β1 and IL-6 (Fig. 7).
Taken together, these results suggest the presence of a reciprocal negative feedback system between miR-125a-5p and TGF-β1/IL-6 in PASMCs.
MiR-125a-5p inhibits key signaling molecules downstream of TGF-β1 and IL-6
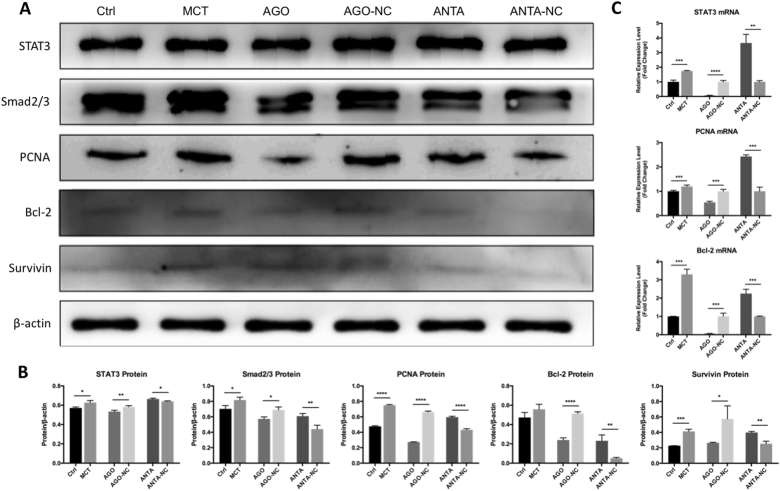
Previous studies found that the TGF-β1/Smad2/3 and IL-6/STAT3 signaling pathways play an essential role in the development of PAH9, 11, 12. We therefore examined the effect of modulating miR-125a-5p expression on Smad2/3 and STAT3, two key signaling molecules downstream of TGF-β1/IL-6 signaling. Our results show that upregulating miR-125a-5p decreased the expression of both STAT3 and Smad2/3; in contrast, downregulating miR-125a-5p increased the expression of STAT3 and Smad2/3 (Fig. 8).
Fig 8. miR-125a-5p regulates downstream molecules of TGF-β1 and IL-6 signaling pathway.
a Western blot analysis of STAT3, Smad2/3, PCNA, Survivin, and Bcl-2 in PASMCs treated as indicated. b Summary of the data shown in a. c RT–qPCR results showing the mRNA levels of STAT3, PCNA, and Bcl-2 in PASMCs treated as indicated, expressed relative to the respective control groups. AGO-NC and ANTA-NC refer to negative controls for the miR-125a-5p agomir and antagomir, respectively. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
MiR-125a-5p directly suppresses STAT3 expression via the STAT3 3′-UTR
Bioinformatics analysis predicted that the 3′-UTR of the STAT3 gene contains a conserved putative binding site for miR-125a-5p (5′-CUCAGGGA-3′). Therefore, to determine whether miR-125a-5p directly regulates STAT3 expression via its 3′-UTR, we cloned the STAT3 3’-UTR fragment containing either the putative miR-125a-5p‒binding site or the same sequence with a mutated miR-125a-5p‒binding site; these sequences were inserted downstream of a Renilla luciferase reporter gene in the pmirGLO vector (Fig. 1a). We then co-transfected HEK-293T cells with either the wild-type or mutant luciferase reporter vector together with the miR-125a-5p agomir and measured luciferase activity.
In cells expressing the STAT3 3′-UTR fragment containing the putative miR-125a-5p-binding site, the miR-125a-5p agomir reduced luciferase activity by 24%, whereas an unrelated (control) miRNA agomir had no effect (Fig. 1b). In contrast, cells expressing the reporter construct containing the mutated STAT3 3′-UTR were unaffected by the miR-125a-5p agomir (Fig. 1b). These results provide a mechanistic explanation for our finding that miR-125a-5p inhibits the expression of STAT3 in cultured PASMCs (Fig. 8).
MiR-125a-5p suppresses proliferation and apoptosis markers downstream of STAT3 signaling
Finally, we examined the effect of miR-125a-5p on proliferation and apoptosis markers downstream of STAT3 using RT–qPCR and western blot analysis to measure the expression levels of PCNA (proliferating cell nuclear antigen), Survivin, and Bcl-29. We found that the miR-125a-5p agomir led to reduced expression of all three markers at both the mRNA and protein levels, whereas the miR-125a-5p antagomir led to increased expression of these three markers, suggesting that miR-125a-5p regulates signaling molecules downstream of STAT3.
Discussion
In the current study, we examined the therapeutic potential of increasing miR-125a-5p expression in the development of pulmonary arterial hypertension using an established rat model of monocrotaline-induced PAH (MCT-PAH). Our results provide evidence that miR-125a-5p has both anti-proliferative and pro-apoptotic effects on pulmonary artery smooth muscle cells (PASMCs) via the TGF-β1/Smad2/3 and IL-6/STAT3 signaling pathways, consistent with their known pathophysiological role in PAH10, 13. Therefore, in addition to demonstrating the important role of miR-125a-5p in PAH, our findings suggest that this miRNA may be a new therapeutic target for patients with PAH.
We found that miR-125a-5p is downregulated in the pulmonary artery (PA) of MCT-PAH rats compared with control rats. Interestingly, however, the time course of this downregulation was dynamic, reaching maximum downregulation two weeks after MCT-PAH was induced; by three weeks, miR-125a-5p expression had recovered significantly, although expression had not yet returned to control levels. Previous reports emphasized the importance of miR-125a in hypoxia-induced pulmonary hypertension (PH), but with conflicting results. Huber et al. found that miR-125a expression was increased in the lung tissue of PH mice14, whereas Ma et al.15 found decreased expression in the PA of PH mice. Thus, further research is clearly needed in order to clarify the role of miR-125a in hypoxia-induced PH. With respect to our study, our expression profile regarding miR-125a-5p in our MCT-PAH model is consistent with previous findings. For example, Caruso et al.16 found a dynamic miRNA expression profile in the lung tissues of MCT-PAH rats. In their control group, the absolute expression level of miR-125a-5p was ~2 ng/nl; in the MCT-PAH group, the level of miR-125a-5p was ~1.6, 0.8, and 1.2 ng/nl on days 2, 7, and 21, respectively, consistent with peak downregulation occurring after 1 week. Moreover, Gareri et al.17 found that miR-125a-5p is robustly expressed in aortic vascular smooth muscle cells, but is downregulated following vascular injury, which is similar with our findings. These results suggest that miR-125a-5p plays an important role in MCT-PAH. To demonstrate clinical relevance, we found that inducing miR-125a-5p expression using an agomir delivered via a nebulizer slowed the progression of MCT-PAH by improving hemodynamics and the Fulton index; moreover, these beneficial effects were stronger in early preventive therapy at week 2 than in late rescue therapy at week 3, suggesting that early preventive therapy is more efficacious. In addition, miR-125a-5p therapy reduced pulmonary vascular remodeling, and again the early time point had more improvement than the later time point. Thus, early intervention is important in treating PAH.
Our histological finding that miR-125a-5p reduces pulmonary vascular remodeling prompted us to focus on the role of miR-125a-5p on the proliferation and resistance to apoptosis of PASMCs; however, we cannot exclude the possibility that miR-125a-5p plays a role in pulmonary artery endothelial cells (PAECs), which have also been implicated in the pathogenesis of PAH18. In addition, Huber et al. found that miR-125a plays a role in PAECs in their hypoxia-induced mouse model; however, they did not make a distinction between miR-125a-5p and miR-125a-3p14. In our study, we found that overexpressing miR-125a-5p inhibits proliferation and promotes apoptosis of PASMCs, whereas downregulating miR-125a-5p increases proliferation and inhibits late apoptosis. Based on our findings, as well as the recent report by Gareri et al.17 showing that overexpressing miR-125a-5p is sufficient to reduce VSMCs proliferation, we propose that miR-125a-5p reduces pulmonary vascular remodeling in PAH by inhibiting proliferation and promoting apoptosis in PASMCs. However, the precise mechanism by which miR-125a-5p regulates these cellular processes in PASMCs remains unclear and warrants further study.
Early and persistent inflammation contributes to pulmonary vascular remodeling in PH13. Previous studies found that the pro-inflammatory cytokines TGF-β1 and IL-6 play an essential role in pulmonary vascular remodeling. In addition, several groups reported that TGF-β1 signaling pathway plays a key pathogenic role in PAH11, 12, 19, 20. For example, activation of TGF-β1 can induce excessive proliferation and reduced apoptosis of PASMCs via Smad2/3, thus driving pulmonary vascular remodeling11. Similarly, activation of IL-6 can up-regulate STAT3 (signal transducer and activator of transcription 3), ultimately inducing excessive proliferation and apoptosis resistance in PASMCs9. Recently, several studies found that TGF-β1 can also regulate cell proliferation and apoptosis via STAT321, 22. Thus, activation of the TGF-β1/IL-6/STAT3 signaling pathway appears to play an essential role in the pathogenesis of PAH. Consistent with this notion, we found that miR-125a-5p modulates the proliferation and apoptosis of PASMCs via the TGF-β1/Smad2/3 and TGF-β1/IL-6/STAT3 signaling pathways.
Interestingly, our results provide the first evidence of reciprocal negative feedback regulation between miR-125a-5p and TGF-β1/IL-6 in PASMCs. Specifically, upregulating and downregulating miR-125a-5p reduced and increased, respectively, the expression of both TGF-β1 and IL-6; in contrast, treating PASMCs with either TGF-β1 or IL-6 led to increased expression of miR-125a-5p. Because miR-125a-5p expression is decreased early in MCT-PAH, whereas TGF-β1 and IL-6 levels are increased late in MCT-PAH, we speculate that downregulation of miR-125a-5p may be an initial triggering factor in the pathogenesis, especially inflammation response of MCT-PAH. Thus, following MCT injection in rats, the early decrease in miR-125a-5p expression may serve as a signal to recruit inflammatory cells such as macrophages to the pulmonary artery, thus producing more and more TGF-β1 and IL-6 to PASMCs, including self-secreting, in order to rescue miR-125a-5p expression and restore cellular homeostasis. In this respect, it is important to note that Chen et al. found that inhibiting miR-125a-5p expression in macrophages significantly increased the levels of secreted TGF-β by nearly three-fold23. These results may explain why miR-125a-5p expression began to recover within three weeks following MCT injection. Although a clear causal relationship between miR-125a-5p and TGF-β1/IL-6 has not been established, these results provide further evidence that the downregulation of miR-125a-5p is an initial pathogenic factor in MCT-PAH.
Consistent with signaling pathways downstream of TGF-β1 and IL-6, we found that the expression of both Smad2/3 and STAT3 is regulated by miR-125a-5p expression. Interestingly, some miRNAs—for example, miR-17/9224 and miR-20425 have been linked to the STAT3 signaling pathway in PAH9; however, these miRNAs act downstream of STAT3. In our study, we found that miR-125a-5p can directly suppress the expression of STAT3 by targeting its 3′-UTR, thereby reducing the expression of downstream markers of cell proliferation and apoptosis, including PCNA, Survivin, and BcL-2, which are also downstream markers of Smad2/3.
In conclusion, our findings suggest that the microRNA miR-125a-5p plays an important role in the development of MCT-PAH. These data may have clinical relevance, suggesting that targeting miR-125a-5p may provide a novel approach to treating PAH; consistent with this notion, we found that treating rats with the miR-125a-5p agomir can slow the progression of MCT-PAH and reduces pulmonary vascular remodeling. Finally, we provide the first evidence that miR-125a-5p has reciprocal negative feedback regulation with TGF-β1/IL-6, and regulates the proliferation and apoptosis of PASMCs by directly targeting the STAT3 3′-UTR.
Electronic supplementary material
Acknowledgements
This work was supported by National Natural Science Foundation of China (CN) (81570040).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Zongye Cai and Jian Li.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s12276-018-0068-3.
References
- 1.Galie N, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, et al. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2015;2016:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Chelladurai P, Seeger W, Pullamsetti SS. Epigenetic mechanisms in pulmonary arterial hypertension: the need for global perspectives. Eur. Respir. Rev. 2016;25:135–140. doi: 10.1183/16000617.0036-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courboulin A, Ranchoux B, Cohen-Kaminsky S, Perros F, Bonnet S. MicroRNA networks in pulmonary arterial hypertension: share mechanisms with cancer? Curr. Opin. Oncol. 2016;28:72–82. doi: 10.1097/CCO.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 6.Shen L, Shen J, Pu J, He B. Aspirin attenuates pulmonary arterial hypertension in rats by reducing plasma 5-hydroxytryptamine levels. Cell Biochem. Biophys. 2011;61:23–31. doi: 10.1007/s12013-011-9156-x. [DOI] [PubMed] [Google Scholar]
- 7.Sun LY, et al. 5-Aminosalicylic Acid Attenuates Monocrotaline-Induced Pulmonary Arterial Hypertension in Rats by Increasing the Expression of Nur77. Inflammation. 2017;40:806–817. doi: 10.1007/s10753-017-0525-5. [DOI] [PubMed] [Google Scholar]
- 8.Shen JY, Cai ZY, Sun LY, Yang CD, He B. The Application of Intravascular Ultrasound to Evaluate Pulmonary Vascular Properties and Mortality in Patients with Pulmonary Arterial Hypertension. J. Am. Soc. Echocardiogr. 2016;29:103–111. doi: 10.1016/j.echo.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Paulin R, Meloche J, Bonnet S. STAT3 signaling in pulmonary arterial hypertension. JAKSTAT. 2012;1:223–233. doi: 10.4161/jkst.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Upton PD, Davies RJ, Tajsic T, Morrell NW. Transforming growth factor-beta(1) represses bone morphogenetic protein-mediated Smad signaling in pulmonary artery smooth muscle cells via Smad3. Am. J. Respir. Cell Mol. Biol. 2013;49:1135–1145. doi: 10.1165/rcmb.2012-0470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long L, et al. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation. 2009;119:566–576. doi: 10.1161/CIRCULATIONAHA.108.821504. [DOI] [PubMed] [Google Scholar]
- 13.Tuder RM, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J. Am. Coll. Cardiol. 2013;62:D4–D12. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber LC, et al. Featured Article: microRNA-125a in pulmonary hypertension: Regulator of a proliferative phenotype of endothelial cells. Exp. Biol. Med. (Maywood) 2015;240:1580–1589. doi: 10.1177/1535370215579018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma C, et al. MiR-125a regulates mitochondrial homeostasis through targeting mitofusin 1 to control hypoxic pulmonary vascular remodeling. J. Mol. Med. 2017;95:977–993. doi: 10.1007/s00109-017-1541-5. [DOI] [PubMed] [Google Scholar]
- 16.Caruso P, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler. Thromb. Vasc. Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 17.Gareri C, et al. miR-125a-5p modulates phenotypic switch of vascular smooth muscle cells by targeting ETS-1. J. Mol. Biol. 2017;429:1817–1828. doi: 10.1016/j.jmb.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Jurasz P, Courtman D, Babaie S, Stewart DJ. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol. Ther. 2010;126:1–8. doi: 10.1016/j.pharmthera.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Thomas M, et al. Activin-like kinase 5 (ALK5) mediates abnormal proliferation of vascular smooth muscle cells from patients with familial pulmonary arterial hypertension and is involved in the progression of experimental pulmonary arterial hypertension induced by monocrotaline. Am. J. Pathol. 2009;174:380–389. doi: 10.2353/ajpath.2009.080565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies RJ, et al. BMP type II receptor deficiency confers resistance to growth inhibition by TGF-beta in pulmonary artery smooth muscle cells: role of proinflammatory cytokines. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;302:L604–L615. doi: 10.1152/ajplung.00309.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitt N, et al. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat. Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Davies KJ, Forman HJ. TGFbeta1 rapidly activates Src through a non-canonical redox signaling mechanism. Arch. Biochem. Biophys. 2015;568:1–7. doi: 10.1016/j.abb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen T, et al. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc. Res. 2009;83:131–139. doi: 10.1093/cvr/cvp121. [DOI] [PubMed] [Google Scholar]
- 24.Brock M, et al. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ. Res. 2009;104:1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 25.Courboulin A, et al. Role for miR-204 in human pulmonary arterial hypertension. J. Exp. Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.