Abstract
Given that neurocognitive impairment is a frequent complication of HIV-1 infection in Spanish-speaking adults, the limited number of studies assessing HIV-associated neurocognitive disorders (HAND) in this population raises serious clinical concern. In addition to being appropriately translated, instruments need to be modified, normed, and validated accordingly. The purpose of the current study was to examine the diagnostic utility of the HIV Dementia Scale (HDS) and International HIV Dementia Scale (IHDS) to screen for HAND in Spanish-speaking adults living with HIV infection. Participants were classified as either HAND (N = 47) or No-HAND (N = 53) after completing a comprehensive neuropsychological evaluation. Receiver operating characteristic analyses found the HDS (AUC = .706) was more sensitive to detecting HAND than the IHDS (AUC = .600). Optimal cutoff scores were 9.5 for the HDS (PPV = 65.2%, NPV = 71.4%) and 9.0 for the IHDS (PPV = 59.4%, NPV = 59.1%). Canonical Correlation Analysis found the HDS converged with attention and executive functioning. Findings suggest that while the IHDS may not be an appropriate screening instrument with this population, the HDS retains sufficient statistical validity and clinical utility to screen for HAND in Spanish-speaking adults as a time-efficient and cost-effective measure in clinical settings with limited resources.
Keywords: AIDS, cognitive disorders, dementia, infectious disease, Latino/a, neuropsychology, Spanish
Hispanics are the largest racial and ethnocultural minority within the United States comprising approximately 16% of the population (U.S. Census Bureau, 2010). To date, it is estimated that there are 53 million Hispanics living in the United States, placing citizens of Hispanic origin as the largest racial or ethnic minority group in the nation (U.S. Census Bureau, 2013). The projected Hispanic population of the United States in 2050 is 132.8 million, which will constitute 30% of the nation's population amongst whom 35 million will speak Spanish at home (U.S. Census Bureau, 2010).
It is well documented that HIV invades the CNS during the acute stages of the infectious process (Davis et al., 1992), resulting in neuropathology through both direct and indirect mechanisms, though predominantly indirect (Goodkin, Lopez, Hardy, & Hardy, 2013). Neurocognitive disorders related to HIV are referred to as HIV-associated neurocognitive disorders (HAND; Antinori et al., 2007). Unfortunately, individuals who have compromised neuropsychological functioning as a result of HAND are at an increased risk of medication nonadherence to effective Antiretroviral Therapy (ART) and of worse self-care when compared to individuals without HAND (Becker, Thames, Woo, Castellon, & Hinkin, 2011; Hinkin et al., 2002; Price et al., 1999). As a consequence, while the incidence of the most severe form of HAND (i.e., HIV-associated dementia) have declined since the era prior to effective ART (Sacktor et al., 2001), the prevalence rates of milder forms of HAND have continued at a stable rate (Heaton et al., 2010; McArthur, 2004) and, in fact, appear to have increased.
Hispanics living in the United States are disproportionately tested for infection later during the course of the disease, are more likely to present with an opportunistic infection, and may have a significantly greater risk for developing HAND upon HIV diagnosis (Dennis, Napravnik, Sena, & Eron, 2011). Additionally, as individuals live longer with HIV infection, neurocognitive disorders increase concomitantly—due to a possible synergistic effect between HIV and aging (Ettenhofer et al., 2009; Goodkin et al., 2001; Hardy & Vance, 2009). Furthermore, individuals aging with HIV may experience possible long-term neurotoxic effects of ART (Brew, 2010). Detection of asymptomatic neurocognitive impairment (ANI) is essential because mild cognitive impairments may appear to predict the later occurrence of dementia (Grant et al., 2014). Therefore, it is of critical importance to continue refining methods for detecting HAND, especially among this underserved population.
Previous researchers have questioned the diagnostic validity of the limited measures available, as the assessment of ethnic minorities runs the potential risk of classifying healthy individuals as having impairment when in fact they do not (Pedraza & Mungas, 2008). This poor specificity is believed to stem from the paucity of measures that have been adequately translated, validated, and normed appropriately with large sample sizes for primary Spanish speakers. In addition, other researchers are in agreement and suggest that neuropsychological measures have relatively strong sensitivity across a variety of ethnic cohorts but lack good specificity (Heaton, Taylor, & Manly, 2003; Norman, Evans, Miller, & Heaton, 2000).
While neuropsychological (NP) test batteries are regarded as the gold standard by which to diagnose HAND, there are still a limited number of neuropsychologists available that can assess Spanish-speakers infected with HIV (Mindt et al., 2008, 2009; Mindt, Byrd, Saez, & Manly, 2010). As the Spanish-speaking population continues to increase in the United States, the demand to provide appropriate clinical services to Spanish-speakers also increases (Ardila, 2000; Artiola i Fortuny & Mullaney, 1997; López, Steiner, Hardy, IsHak, & Anderson, 2016; Pontón et al., 1996; Wilkie et al., 2004). Thus, there is an existing need for more research that validates cognitive screening tools specifically for Spanish-speaking individuals living with HIV infection to determine if a comprehensive neuropsychological assessment is further warranted in this disenfranchised group.
Two measures in particular have been developed to screen for HAND such as the HIV Dementia Scale (HDS; Power, Selnes, Grim, & McArthur, 1995) and International HIV Dementia Scale (IHDS; Sacktor et al., 2005). The HDS was specifically developed to screen for dementia in HIV seropositive individuals. The HDS has previously been documented to have a sensitivity of 80% and a specificity of 91% for detecting HIV-associated dementia (HAD; Power et al, 1995). It has also been used in clinical trials and resource limited medical settings globally, including Northern and Central America, sub-Saharan Africa, South Asia and Europe (Goodkin, Hardy, Singh, & Lopez, 2014; Kvalsund, Haworth, Murman, Velie, & Birbeck, 2009; Morgan et al., 2007). The IHDS is an adapted form of the HDS developed for use with non-English-speaking populations by replacing several tasks on the HDS assumed to be culturally dependent.
One recent meta-analytic study reviewing the HDS and IHDS demonstrated that the HDS has poor pooled sensitivity and the IHDS had moderate pooled sensitivity in detecting a range of cognitive impairment; the authors of this study noted that while the HDS and IHDS performed well to screen for HAD, they were less sensitive to milder HAND conditions (Zipursky et al., 2013). Another systematic review (Haddow, Floyd, Copas, & Gilson, 2013) examining the screening accuracy of the HDS and IHDS reported that both scales were low in predictive accuracy. Haddow et al. (2013) reported that the pooled diagnostic odds ratios (DOR) for the HDS was 7.52, while the pooled DOR for the IHDS was 3.49. Notably, Haddow et al. (2013) propose that the literature is limited by the lack of a “gold standard” in categorizing HAND. Other studies have documented strong psychometric properties in the abilities of the HDS and IHDS to screen for HAND. To date there is a dearth of studies that have examined the clinical utility of these measures in Spanish-speaking cohorts.
Previous work by Wojna et al. (2007) were the first to validate a Spanish translation of the HDS in a sample of Hispanic women. Since that time, few studies have examined the psychometric properties of the HDS and IHDS with Spanish-speaking individuals, particularly with individuals from Mexico and Central America now living in the United States. Levine et al. (2011) administered a Spanish translation of the HDS to a small sample of participants primarily from Mexico and Central America and found that while using the standard cutoff score of ≤10, the sensitivity and specificity for the HDS were 67% and 50% respectively. Yet, no studies to date have examined the diagnostic utility of the HDS and IHDS to screen for HAND with a larger sample of Spanish-speaking participants primarily from Mexico and Central America. Levine et al. (2011) recommended that further investigation of other cognitive screening measures, such as the IHDS, might be helpful in detecting mild forms of HAND among disenfranchised Spanish-speaking populations. The purpose of this study was to highlight the strengths and weaknesses of two screening measures commonly used to detect HAND, namely the HDS and IHDS, with a Spanish-speaking population, with the primary goal of expanding upon the limited literature base regarding the assessment of Spanish-speaking adults.
Method
Participants
Participants were recruited from AIDS Service Organizations, including UCLA, Harbor-UCLA Medical Center, AIDS Project Los Angeles, Cedars-Sinai Medical Center (CSMC) research advertisements in the community, and local physicians who provided their patients with the study's contact information. All participants successfully pre-screened were provided with a Spanish consent form approved by the CSMC IRB. Written informed consent was obtained from all participants after the procedures in the current study were fully explained. Study inclusion criteria were the following: (a) primary Spanish-speaking women and men; (b) ages ≥18 years of age; (c) willing to provide documentation of HIV serostatus; (d) Spanish as their primary language in terms of speaking, reading, and writing; and (e) HIV infection. The exclusion criteria in this study were: (a) systemic, acute opportunistic infection or tumor requiring chemotherapy; (b) CNS infections or tumors associated with HIV infection that would interfere with NP testing or completion of the study procedures; (c) severe HIV-associated dementia [as indicated by the Antinori et al., 2007 criteria]; (d) non-HIV-associated neurological disease [e.g., history of epilepsy; non-correctable visual or hearing impairments; prior cerebrovascular accident; Alzheimer's disease]; (e) history of a previous or current major psychiatric disorder [e.g., schizophrenia; bipolar affective disorder; major depressive disorder with melancholia]; (f) mental retardation, learning disorders, and pervasive developmental disorder; (g) current alcohol or substance dependence (per the standardized psychiatric interview SCID modified for the DSM-IV), as well as a history of alcohol or substance abuse within the past three months; (h) collagen vascular disease; (i) severe chronic obstructive pulmonary disease (i.e., resting hypercarbia, O2, or steroid dependency); (j) severe congestive heart failure (class IV); (k) unstable angina; (l) myocardial infarction (within prior 6 months); (m) use of systemic steroids—catabolic or anabolic; (n) hepatic failure; (o) renal failure; and (p) use of immunostimulant therapies or participation in trials of non-FDA-approved antiretroviral medications.
Procedures
Eligible participants were assessed using two Spanish-language batteries including a comprehensive neuropsychological testing battery (for a detailed list of the measures comprising the neuropsychological battery see Smith et al., 2014) and a psychosocial battery. Trained bilingual English and Spanish-speaking neuropsychologists and doctoral level clinical psychology students administered the comprehensive neuropsychological battery, which was an adapted version of the HIV/ University of Miami Annotated Neuropsychological test battery in Spanish (HUMANS; Wilkie et al., 2004). Testing was approximately 5 to 8 hours; the total duration included a lunch break and administration of the psychosocial battery and structured clinical interview. Participants were scheduled for a comprehensive neuropsychological assessment starting at approximately 9 AM. Participants signed an informed consent before any procedures were initiated. Participants were compensated $150 for completing the assessment and given a parking validation and meal voucher redeemable from the CSMC cafeteria for the day of assessment.
HAND classification
The assessment data were used to determine if participants met diagnostic criteria for HAND, according to the current recommended nosology (Antinori et al., 2007). HIV-associated asymptomatic neurocognitive impairment (ANI) and HIV-associated mild neurocognitive disorder (MND) require an individual's cognitive performance to be greater than one standard deviation but less than two standard deviations in at least two ability domains for age-education-appropriate norms on standardized neuropsychological testing and no evidence of another preexisting cause, with the only difference being that cognitive impairment does not interfere with daily function in the ANI group and caused mild impairment in daily functioning in the MND group. For a diagnosis of HIV-associated dementia (HAD), the individual's cognitive performance must have demonstrated marked impairment in at least two ability domains (with at least two standard deviations or greater below demographically corrected means), and marked interference with daily function in multiple areas (i.e., work, home life, social activities) without evidence of another pre-existing cause for the dementia (see Table 1 for a complete list of neuropsychological and functional measures used to derive HAND for the current analysis). In this study, if participants met criteria for ANI, MND, or HAD they were classified as HAND. If they did not meet diagnostic criteria for any HAND disorder, they were classified as No-HAND.
Table 1.
Raw Score Means (Standard Deviations) for the comprehensive neurocognitive battery measures used to determine HAND status for Neuropsychological Performance.
| Domain | No-HAND | HAND |
|---|---|---|
| Attention/Working Memory | ||
| Digit Span (forward) | 7.26 (1.97) | 6.30 (1.40) |
| Spatial Span (forward) | 8.08 (2.00) | 6.70 (1.49) |
| CPT-II Omissions | 7.00 (14.72) | 11.05 (9.35) |
| CPT-II Commissions | 10.33 (6.63) | 14.67 (7.98) |
| Digit Span (backward) | 5.06 (1.93) | 3.87 (1.31) |
| Spatial Span (backward) | 7.36 (1.91) | 5.32 (1.76) |
| Speed of Info Processing | ||
| Symbol Search | 23.34 (5.81) | 17.34 (8.44) |
| Digit Symbol (Coding) | 53.15 (14.50) | 41.21 (14.23) |
| CPT-II (Hit Rate) | 445.41 (92.82) | 456.93 (76.60) |
| Episodic Memory | ||
| RAVLT Long Delay | 9.53 (2.95) | 8.19 (2.20) |
| Logical Memory Delay | 18.38 (7.21) | 12.91 (5.81) |
| Visual Reproduction Delay | 29.08 (6.92) | 20.04 (8.99) |
| PMIT 3 (TP) | 16.13 (2.77) | 15.27 (3.31) |
| Abstraction/Executive | ||
| Color Trails 2 | 100.53 (33.76) | 142.74 (49.32) |
| WCST Perseverative Errors | 11.15 (5.39) | 16.30 (9.50) |
| WCST Categories | 2.29 (1.35) | 1.76 (1.27) |
| Stroop Interference | 37.47 (9.64) | 27.81 (8.75) |
| Language | ||
| Boston Naming Test | 52.81 (4.13) | 49.57 (6.50) |
| COWAT (PMR) | 37.79 (9.91) | 27.66 (9.14) |
| Category Fluency (Animals) | 18.21 (3.99) | 14.09 (4.31) |
| Visuospatial Skills | ||
| Block Design | 31.23 (10.76) | 21.74 (10.41) |
| Matrices | 9.83 (4.31) | 7.68 (4.24) |
| Motor Functioning | ||
| Grooved Pegboard (Non-dom) | 74.60 (11.18) | 92.22 (32.42) |
| Finger Tapping (Non-dom) | 45.41 (9.26) | 43.51 (11.51) |
| Gait | 8.12 (1.48) | 8.71 (1.85) |
| Functional Status | ||
| MOS-HIV (Cognitive) | 16.77 (4.91) | 16.38 (4.61) |
| Cognitive Difficulties Scale | 53.89 (28.96) | 54.43 (31.88) |
Note. Raw Score Means (Standard Deviations) for participants. CPT-II = Conner's Continuous Performance Test-II; RAVLT = Rey Auditory Verbal Learning Test; PMIT = Picture Memory Interference Test; WCST = Wisconsin Card Sorting Test; COWAT = Controlled Oral Word Association Test; MOS-HIV = Dutch Four Item Medical Outcomes Study-HIV.
Measures
Biological markers
Clinical laboratory measures were used to confirm HIV serostatus by HIV antibody testing in medical record reviews.
HIV dementia scale (HDS)
The HDS targets abilities that are often impaired in persons with HAD (i.e., attention, episodic memory and psychomotor speed). The HDS consists of four subtests including: antisaccadic eye movement error rate, the timed written alphabet, recall of four items at 5 minutes, and a cube copy time. HDS scores range from 0 to 16 with a raw cutoff score of ≤10 indicating increased risk of dementia.
International HIV dementia scale (IHDS)
The IHDS is an adapted form of the HDS developed for use with non-English-speaking populations by replacing the antisacaccadic eye movement error, timed alphabet writing, and cube copy items with more culture-free tests of motor and psychomotor speed. It is comprised of three items: Finger Tapping (motor speed), an alternating hand sequence item (psychomotor speed), and a four-word verbal memory item. The highest possible score on the IHDS is 12, with a cutoff of 10 or below indicative of risk for dementia (Sacktor et al., 2005).
Statistical analyses
Demographic and clinical characteristics were compared between the HAND and No-HAND participants using between-factors one-way ANOVAs (comparing group means) or Chi-square tests of independence (comparing frequencies) (see Table 2). These included sociodemographic and clinical background characteristics (e.g., age, educational level, pre-morbid IQ, current CD4 cell count, current ART treatment, percent with undetectable viral load, cognitive complaints, and depression).
Table 2.
Sociodemographic and medical characteristics of the participant groups and analysis of variance (ANOVA) p values for characteristic differences between No-Hand and Hand groups infected with HIV.
| Characteristic | No-HAND | HAND | p |
|---|---|---|---|
| N | 53 | 47 | |
| Age (years) | 44.72 (7.68) | 45.21 (7.66) | .75 |
| Education Level (years) | 9.74 (2.62) | 10.81 (3.99) | .11 |
| Pre-Morbid IQ (Vocabulary) | 35.66 (11.44) | 30.77 (12.70) | .05 |
| CD4 Cell Count | 475.45 (232.50) | 484.51 (218.08) | .85 |
| CDS | 53.89 (28.96) | 54.43 (31.88) | .93 |
| BDI | 12.32 (10.05) | 13.09 (9.62) | .70 |
| ND Viral Load (%) | 51.8 | 48.2 | .65 |
| Gender (%) | |||
| Male | 79.2 | 80.9 | |
| Female | 11.3 | 10.6 | |
| MTF | 9.4 | 8.5 |
Note. Means (Standard Deviations) and %. CDS = Cognitive Difficulty Scale; ND = Non-detectable (≤50); BDI = Beck Depression Inventory; MFT = Transgender Identity Male-to-Female.
Statistically significant at p < .05.
The HDS and IHDS total scores were compared between the HAND and No-HAND group using receiver operator characteristic (ROC) analyses (Swets, 1996). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were obtained for the measures. An optimal cutoff score for each measure was determined to be a score that had the maximum likelihood of detecting HAND while minimizing the likelihood of a false positive result, which was estimated by summing the sensitivity and specificity of each score and identifying the highest value. ROC analyses were used to identify whether the IHDS was comparable to the HDS in detecting HAND and to what degree both measures could correctly identify impairment. ROC curves were compared using the area under the curve (AUC), a measure of a test score's ability to distinguish two groups. As outlined by Hosmer and Lemeshow (2000), an AUC of .50 indicates a classification rate no better than chance while an AUC of 1.00 indicates perfect classification. Scores between .70 and .80 are considered to have adequate classification accuracy, while scores above .80 are regarded as good classification rates.
Finally, canonical correlation analysis (CCA) was run to examine the shared overlap in variance between items on the HDS/IHDS and neuropsychological domains. CCA is a multivariate technique that examines the relationships between two sets of variables. This is accomplished by identifying “variates,” or latent variables that explain covariance between the two groups. The two groups in this case are the screening items and the neuropsychological domains. This procedure identifies linear combinations within variable sets that maximizes their correlations, which, in turn, identifies the individual variables that contribute most to each linear combination (Tabachnick & Fidell, 2007). Neuropsychological domains were formed by taking within-group z-scores derived from the raw scores of individual sub-tests and averaging them into their respective domains. Domains in this study included memory (Rey Auditory Verbal Learning Test - Long Delay trial; Wechsler Memory Scale - Logical Memory II; Wechsler Memory Scale - Visual Reproduction II), attention (Wechsler Adult Intelligence Scale - Digit Span and Wechsler Memory Scale - Spatial Span), language (Boston Naming Test; Controlled Oral Word Association Test PMR and Animals), visuospatial (Wechsler Adult Intelligence Scale - Matrix Reasoning and Block Design subtests), executive functioning (Colors Trails 2; Wisconsin Card Sorting Test - Perseverative Errors; Stroop Interference trial), and motor speed (Grooved Pegboard and Finger Tapping Test, nondominant hand trials).
Results
Group differences
The current study included 100 Spanish-speaking HIV seropositive participants [(80 males, 11 females, nine transgender), mean age = 44.95 years, SD = 7.63, age range: 18–62 years; mean education = 10.24 years, SD = 3.36, education range: 1–20 years; mean Vocabulary subtest score = 33.36 SD = 12.23; mean current CD4 = 479.93, SD = 224.22; mean Beck Depression Inventory score = 12.68, SD = 9.81; mean Cognitive Difficulty Scale score = 54.14, SD = 30.22, and 65.1% had an undetectable HIV viral load. After Log10 transformation of plasma viral load, the total sample plasma viral load was (M = 2.14, SD = .94), and there were no statistically significant group differences between the HAND (M = 2.29, SD = 1.07) and No-HAND (M = 2.00, SD = .78) groups. All participants were living in the Los Angeles area at the time of assessment. The country of origin rates were as follows: Mexico 67%, Central America 25%, United States 2% and South America 6%. The demographic characteristics of the two different groups, No-HAND and HAND are provided (See Table 2). No differences were found between groups with respect to age, years of education, gender, pre-morbid IQ, current CD4 cell count, complaints of cognitive difficulties, depression, and nondetectable plasma viral load (%).
HDS and IHDS pearson's correlations with age and education
Analyses were conducted to determine the relationships between the HDS and IHDS with age and years of education. For the HDS, there was no significant correlation with age (r = −.14, p > .05) while years of education was significantly correlated with the HDS (r = .38, p < .01). For the IHDS, there was also no significant correlation with age (r = −.18, p > .05) while years of education was significantly correlated with the IHDS (r = .20, p < .05).
ROC analysis
The HDS and IHDS total scores were next subjected to ROC analyses. Figure 1 displays the two curves.
Figure 1.
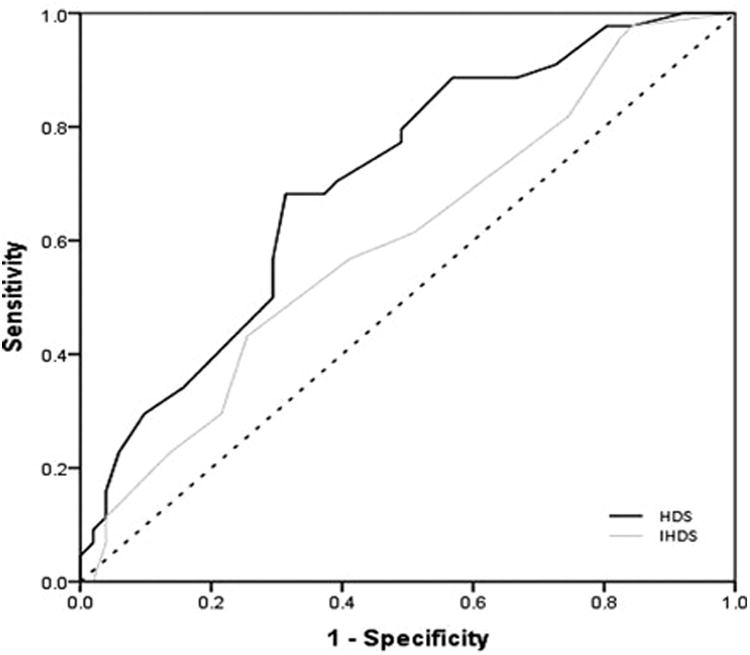
Receiver operating model characteristics for the HDS and IHDS in detecting HAND.
As seen in the figure, the HDS was the more accurate measure with an AUC of .706 (SE = .053, 95% CE = .602–.810) compared to the IHDS with an AUC of .600 (SE = .058, 95% CE = .487–.714). Furthermore, the HDS curve rejected the null hypothesis that the true AUC was .500 (p = .001) while the IHDS curve failed to reject the null (p = .092). See Tables 3 and 4 for the sensitivity, specificity, PPV, and NPV for the HDS and IHDS.
Table 3.
Classification statistics of the HDS in detecting HAND.
| Cutoff score | Sensitivity | Specificity | PPV | NPV | Overall accuracy |
|---|---|---|---|---|---|
| 3.0 | 0.045 | 1.000 | 1.000 | 0.548 | 1.045 |
| 3.5 | 0.068 | 0.980 | 0.750 | 0.549 | 1.049 |
| 4.0 | 0.091 | 0.980 | 0.800 | 0.556 | 1.071 |
| 4.5 | 0.114 | 0.961 | 0.714 | 0.557 | 1.074 |
| 5.0 | 0.136 | 0.961 | 0.750 | 0.563 | 1.097 |
| 5.5 | 0.159 | 0.961 | 0.778 | 0.570 | 1.120 |
| 6.0 | 0.227 | 0.941 | 0.769 | 0.585 | 1.168 |
| 6.5 | 0.295 | 0.902 | 0.722 | 0.597 | 1.197 |
| 7.0 | 0.341 | 0.843 | 0.652 | 0.597 | 1.184 |
| 7.5 | 0.386 | 0.804 | 0.630 | 0.603 | 1.190 |
| 8.0 | 0.409 | 0.784 | 0.621 | 0.606 | 1.193 |
| 8.5 | 0.500 | 0.706 | 0.595 | 0.621 | 1.206 |
| 9.0 | 0.568 | 0.706 | 0.625 | 0.655 | 1.274 |
| 9.5 | 0.682 | 0.686 | 0.652 | 0.714 | 1.368 |
| 10.0 | 0.682 | 0.667 | 0.638 | 0.708 | 1.348 |
| 10.5 | 0.682 | 0.627 | 0.612 | 0.696 | 1.309 |
| 11.0 | 0.705 | 0.608 | 0.608 | 0.705 | 1.312 |
| 11.5 | 0.773 | 0.510 | 0.576 | 0.722 | 1.283 |
| 12.0 | 0.795 | 0.510 | 0.583 | 0.743 | 1.305 |
| 12.5 | 0.864 | 0.451 | 0.576 | 0.793 | 1.315 |
| 13.0 | 0.886 | 0.431 | 0.574 | 0.815 | 1.318 |
| 13.5 | 0.886 | 0.333 | 0.534 | 0.773 | 1.220 |
| 14.0 | 0.909 | 0.275 | 0.519 | 0.778 | 1.184 |
| 14.5 | 0.977 | 0.196 | 0.512 | 0.909 | 1.173 |
| 15.0 | 0.977 | 0.157 | 0.500 | 0.889 | 1.134 |
| 15.5 | 1.000 | 0.078 | 0.484 | 1.000 | 1.078 |
| 16.0 | 1.000 | 0.059 | 0.478 | 1.000 | 1.059 |
Note. HDS = HIV Dementia Scale; PPV = Positive Predictive Value; NPV = Negative Predictive Value.
Table 4.
Classification statistics of the IHDS in detecting HAND.
| Cutoff score | Sensitivity | Specificity | PPV | NPV | Overall accuracy |
|---|---|---|---|---|---|
| 6.0 | 0.000 | 0.981 | 0.000 | 0.526 | 0.981 |
| 6.5 | 0.065 | 0.962 | 0.600 | 0.538 | 1.027 |
| 7.0 | 0.109 | 0.962 | 0.714 | 0.549 | 1.070 |
| 7.5 | 0.196 | 0.885 | 0.600 | 0.554 | 1.080 |
| 8.0 | 0.217 | 0.865 | 0.588 | 0.556 | 1.083 |
| 8.5 | 0.283 | 0.788 | 0.542 | 0.554 | 1.071 |
| 9.0 | 0.413 | 0.750 | 0.594 | 0.591 | 1.163 |
| 9.5 | 0.543 | 0.596 | 0.543 | 0.596 | 1.140 |
| 10.0 | 0.609 | 0.500 | 0.519 | 0.591 | 1.109 |
| 10.5 | 0.761 | 0.346 | 0.507 | 0.621 | 1.107 |
| 11.0 | 0.826 | 0.269 | 0.500 | 0.636 | 1.095 |
| 11.5 | 0.957 | 0.192 | 0.512 | 0.833 | 1.149 |
| 12.0 | 0.978 | 0.173 | 0.511 | 0.900 | 1.151 |
Note. IHDS = International HIV Dementia Scale; PPV = Positive Predictive Value; NPV = Negative Predictive Value.
As seen in Table 3, the optimal cutoff score for the HDS was 9.5 (Sensitivity = 68.2%, Specificity = 68.6%, PPV = 65.2%, NPV = 71.4%). In Table 4, the optimal cutoff score for the IHDS was 9.0, although classification rates were considerably lower than that of the HDS (Sensitivity = 41.3%, Specificity = 75.0%, PPV = 59.4%, NPV = 59.1%).
Canonical correlation analysis
Prior to conducting the CCA, data were screened to ensure that variables met assumptions of normality and linearity. For the HDS and the neuropsychological domains, the first of the four canonical correlations was statistically significant (Wilks λ = .571, p = .002), indicating that the two sets of variables formed one linear combination that was unlikely to have occurred by chance alone. Items within the HDS that significantly correlated with the first canonical variate included the attention score (adjusted R2 = .076, p = .042), the psy-chomotor score (adjusted R2 = .183, p < .001), and the construction score (adjusted R2 = .093, p = .023). The memory score was not statistically significant (adjusted R2; = .026, p = .214). Inspection of the second variate (Wilks λ = .706, p > .05) suggests that this might have accounted for much of the memory score's variance, although as mentioned the second, third, and fourth canonical dimensions did not approach statistical significance and cannot be not further interpreted (See Table 1 for the means and standard deviations between the HAND and No-HAND groups on the neuropsychological measures).
As seen in Table 5, the HDS psychomotor score had the greatest association with the variate among all the HDS variables. From the neuropsychological domains, the attention and executive functioning domains contributed the most variance.
Table 5.
Standardized canonical coefficients between the HDS and neuropsychological domains with the first canonical variate.
| Variable | Coefficient |
|---|---|
| HDS | |
| Attention | .254 |
| Psychomotor | .706 |
| Memory | .064 |
| Construction | .342 |
| Neurocognitive | |
| Attention | .512 |
| Memory | −.014 |
| Language | .298 |
| Visuospatial | −.038 |
| Executive | .450 |
| Motor | −.013 |
CCA was run next to compare the neuropsychological domains to the IHDS. The overall analysis was not significant (Wilks λ = .740, p = .067), indicating that there were no significant associations between the IHDS and neuropsychological domains. Although the IHDS was not statistically significant at detecting HAND, a trend emerged.
Discussion
Validated screening measures for HAND are essential for assessing Spanish-speaking individuals infected with HIV infection. In particular, the HDS and the IHDS are rapid screening measures that have been utilized widely for individuals infected with HIV in other populations. These screening measures may be of great benefit to use especially in resource-limited settings (e.g., see Goodkin et al., 2014), when comprehensive neuropsychological testing is not readily available for marginalized groups, such as primary Spanish speakers in the United States.
Overall, the results from this study demonstrate that the HDS is more sensitive than the IHDS in detecting HAND among Spanish-speaking populations. Findings demonstrated that the optimal cutoff score for the HDS was 9.5 (PPV = 65.2%, NPV = 71.4%), and that the optimal cutoff for the IHDS was 9.0 although classification rates were considerably lower than that of the HDS (PPV = 59.4%, NPV = 59.1%). Results from the CCA indicated that the HDS converged with traditional neuropsychological assessment measures such that the psychomotor, attention, and construction items correlated with attention and executive functioning—two domains that are commonly impacted in HAND. Therefore, our findings are congruent with previous research studies that have validated the HDS to screen for HAND with Spanish-speakers (Levine et al., 2011; Wojna et al., 2007) as the HDS does appear to be an effective screening tool for primary Spanish-speaking in the USA (at least from a predominantly Mexican culture). In addition, 47% of our sample demonstrated HAND. Rates of HAND among varying groups of Spanish-speakers are often difficult to assess (particularly with Spanish-speakers from Mexico and Central America), given the dearth of information in the literature for these specific cohorts as well as the small sample sizes of each recent study. Yet, the rates of HAND observed in the current study are comparable to the CHARTER Study cohort (English speaking), which to date remains one of the largest and most diverse samples of individuals living with HIV infection in the era of combination antiretroviral therapy (CART). Specifically, Heaton et al. (2010) reported that 52% of the total Charter Study sample had neuropsy-chological impairment, with 33% classified with ANI, 12% classified with MND, and 2% classified as having HAD. We encourage researchers to continue reporting on the prevalence rates of HAND among Spanish-speaking samples.
Our finding are congruent with one previous meta-analytic study (see Haddow et al., 2013) as our results showed that the IHDS was not as sensitive at detecting HAND, despite the fact that this measure was developed for the purpose of being more culturally sensitive. Furthermore, item convergence between the IHDS and neuropsychological domains was non-significant, indicating that the IHDS does not adequately tap into the neuropsychological constructs sensitive to HAND. One possible factor for this may stem from the reality that the IHDS was developed to assess HAD rather than more mild forms of HAND, like ANI or MND (Sacktor et al., 2005). However, it should also be noted that the IHDS did have an overall trend with the domains, suggesting that there may be a convergence between the variables, albeit a convergence that was weaker than that observed with the HDS.
Despite the fact that the HDS was sensitive in detecting HAND, it is important to discuss specific components of the scale to shed light upon areas of caution when utilizing it among Spanish-speaking individuals. For example, the IHDS was developed to reduce culturally biased items from the HDS (i.e., timed written alphabet and cube copy time, which are not easily performed by certain non-Western cultures), although these timed measures do tend to corroborate the notion of a subcortical syndrome in HIV infection. Our data on the written alphabet sub-test revealed that the number of letters provided by all of the participants ranged from 7 to 29, with an average of 24.2 (SD = 4.0) letters. To date, there is controversy and debate pertaining to how many letters exists in Spanish, because of the inclusion of additional letters in that language's alphabet (i.e., ch, ñ, ll and rr). Additionally, the letters “w” and “k” are not found in any original Spanish words. Therefore, individuals can vary in how many letters they provide on such a task. In fact, there was a significantly strong correlation between years of education and the number of letters produced (r = 0.354, p < .001). Similarly, the cube copy HDS item among all individuals was significantly correlated with education (r = 0.349, p < .001). While approximately 67% of individuals copied the cube correctly, a significant number of individuals rotated the direction of the cube (29%). Although the data suggests that the HDS is sensitive for detecting HAND among primary Spanish-speakers, it is recommended that future research explore the efficacy of utilizing both the HDS and the IHDS to aid in consideration of HAND status among this at-risk group.
We suspect that when sampling a population of primary Spanish-speakers in the United States, there may be significant variance in acculturation that is reflected in higher performance on the HDS than on the IHDS amongst more acculturated individuals – as opposed to what would be the case in, for example, a South African sample. In addition, we did not conduct a comprehensive assess of bilingualism proficiency. While all participants' language preference and language dominance was Spanish, we encourage future research studies to incorporate measuring bilingualism into the design and methodology as bilingualism can have a distinct influence on neurocognitive function (López et al., 2016; Mindt et al., 2008). Given that the IHDS was developed in response to the need to assess other racial, ethnic, and cultural groups outside of the United States, it follows that the IHDS would not have had as much of an advantage in sensitivity and specificity compared to the HDS in this study.
In addition to the HDS and IHDS, other research studies have identified a number of measures that have been used to screen for HAND in Spanish-speaking populations. Previous work by Muñoz-Moreno et al. (2013) developed a brief and feasible paper-based tool, The NEU Screen, to aid practicing clinicians in detecting HAND in Spanish-speaking adults, and demonstrated promising results to rapidly detect cognitive impairment in resource-limited settings. Furthermore, Levine et al. (2011) examined the clinical utility of both the Mini-Mental State Examination (MMSE) and the NEUROPSI to screen for HAND in a Spanish-speaking sample, and found the NEUROPSI demonstrated reasonable accuracy in detecting neurocognitive impairment, while the MMSE demonstrated very poor accuracy. Conversely, the dearth of available literature on screening instruments for HAND in Spanish-speaking populations remains a notable and prominent issue to date. Nevertheless, more recent research efforts with other language groups have made substantial progress. For example, Valcour, Paul, Chiao, Wendelken, and Miller (2011) have an excellent review of screening measures for HAND in aging HIV-positive cohorts. In addition, Overton et al. (2013) found that the Montreal Cognitive Assessment (MoCA) and the Alzheimer's Disease (AD-8) screening tools correlated with formal neuropsychological testing in an HIV-positive sample, but reported that the sensitivity and specificity of these instruments were moderate in predictive accuracy. Milanini et al. (2016) recently reported similar results in an Italian sample, as they found that while the MMSE had relatively poor predictive accuracy, the MoCA demonstrated moderate predictive accuracy to more mild forms of HAND in their sample. Taken together, our findings contribute to the literature by enhancing clinicians “tool kit” of validated neuropsychological measures that demonstrate efficient and robust psychometric properties to detect HAND in resource limited settings for Spanish-speakers living with HIV-infection. It follows, that future researchers are encouraged to continue refining and investigating the sensitivity and specificity of these screening instruments to ensure optimal care is provided to this at-risk and historically disenfranchised population.
This study has several strengths. We used a focused, comprehensive, and culturally sensitive neuropsychological battery of tests for Spanish-speakers to determine HAND classification, and applied validated, published norms for this population to diagnose HAND. We also accrued a sample representative of the HIV-seropositive Spanish-speaking population in the Los Angeles area. We selected a sample of patients who were screened to exclude relevant neurological and psychiatric disorders, which resulted in the elimination of any confounding conditions that could have been misrepresented as HAND. Most importantly, we validated the clinical utility of the HDS and the IHDS in screening for HAND and in aiding diagnostic classification, in addition to providing optimal cut-off scores.
Study limitations
One limitation was that participants were recruited from the Los Angeles area and may not fully represent a heterogeneous sample of Spanish speakers. Considering the majority of our participants were individuals of Mexican and Central American descent, a more inclusive sample of Spanish speakers could potentially have yielded different results. Findings even for the HDS were relatively weak in diagnosing HAND, with an AUC of .706 barely meeting the cutoff for adequate sensitivity. However, from a clinical perspective, this was deemed acceptable, as screening measures should not be used for diagnosis anyway but rather serve as a way to identify patients who may require a more rigorous evaluation. Furthermore, our study did not include HIV negative controls for comparison, which limits our ability to examine demographic associations in individuals without brain dysfunction. Additionally, this study did not include individuals with HAD. As one previous meta-analytic study (Zipursky et al., 2013) suggested that the HDS and IHDS were sensitive in screening for more severe forms of HAND, such as HIV-associated dementia, and were less sensitive to milder forms of HAND, our findings may be stem from these previously reported finding, as our study did not support inclusion of individuals with HAD. Recruiting individuals with HAD would have proven difficult, given our study required all individuals to complete a comprehensive neuropsychological assessment.
Additionally, we were unable to conduct separate statistical analyses on the specific HAND categories of ANI, MND, and HAD due to limitations of statistical power. In line with this, the range of the HDS and IHDS were somewhat limited, which may account for some of their non-significant correlations with demographic variables. Future studies are encouraged to over-recruit individuals with HAD in order to determine the clinical utility of the HDS and the IHDS to aid in clarifying the degree of impairment severity between Spanish-speakers with ANI, MND, and HAD. We recommend future research studies employ larger sample sizes to increase power and ultimately strengthen the generalizability of their research findings. In addition, the clinical utility of developing normative corrections for the HDS and IHDS in Spanish-speaking adults should be explored. Future researchers are encouraged to design and pursue research studies that are able to generate such normative samples with the aforementioned demographic corrections. Lastly, given the growing number of monolingual Spanish-speakers in the United States of America, it is becoming increasingly important for clinicians to draw upon measures with cost-efficient and time-effective properties that have also previously demonstrated robust psychometric properties. The findings in the current study have helped in this effort, as our findings suggest that the HDS has good predictive accuracy in the detection of more mild forms of HAND in a Spanish-speaking population.
Acknowledgments
Funding: This study was supported by a grant from the National Institute of Mental Health awarded to Dr. Enrique López (K23 MH087290).
Footnotes
Declaration of interest: None of the other authors have competing commercial or financial interests that would arise in relation to the findings and direct applications of this research.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A. Assessment of Spanish-speaking populations. Applied Neuropsychology. 2000;7(1):1–2. doi: 10.1207/S15324826AN0701_1. [DOI] [PubMed] [Google Scholar]
- Artiola i Fortuny L, Mullaney HA. Neuropsychology with Spanish speakers: Language use and proficiency issues for test development. Journal of Clinical and Experimental Neuropsychology. 1997;19(4):615–622. doi: 10.1080/01688639708403747. [DOI] [PubMed] [Google Scholar]
- Becker BW, Thames AD, Woo E, Castellon SA, Hinkin CH. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS and Behavior. 2011;15(8):1888–1894. doi: 10.1007/s10461-011-9924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ. Benefit or toxicity from neurologically targeted antiretroviral therapy? Clinical Infectious Diseases. 2010;50(6):930–932. doi: 10.1086/650744. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, et al. Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42(9):1736. doi: 10.1212/WNL.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Dennis AM, Napravnik S, Sena AC, Eron JJ. Late entry to HIV care among Latinos compared with non-Latinos in a southeastern US cohort. Clinical Infectious Diseases. 2011;53(5):480–487. doi: 10.1093/cid/cir434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenhofer ML, Hinkin CH, Castellon SA, Durvasula R, Ullman J, Lam M, et al. Foley J. Aging, neurocognition, and medication adherence in HIV infection. The American Journal of Geriatric Psychiatry. 2009;17(4):281–290. doi: 10.1097/JGP.0b013e31819431bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin K, Hardy DJ, Singh D, Lopez E. Diagnostic utility of the international HIV dementia scale for HIV-associated neurocognitive impairment and disorder in South Africa. The Journal of Neuropsychiatry and Clinical Neurosciences. 2014;26(4):352–358. doi: 10.1176/appi.neuropsych.13080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin K, Lopez E, Hardy DJ, Hardy WD. Neurocognitive decline in HIV infection. Psychiatric Annals. 2013;43(5):204–211. doi: 10.3928/00485713-20130503-04. [DOI] [Google Scholar]
- Goodkin K, Wilkie FL, Concha M, Hinkin CH, Symes S, Baldewicz TT, et al. Eisdorfer C. Aging and neuro-AIDS conditions and the changing spectrum of HIV-1-associated morbidity and mortality. Journal of Clinical Epidemiology. 2001;54(12, Supplement 1):S35–S43. doi: 10.1016/S0895-4356(01)00445-0. [DOI] [PubMed] [Google Scholar]
- Grant I, Franklin DR, Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Forthe Charter Group. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82(23):2055–2062. doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow LJ, Floyd S, Copas A, Gilson RJC. A systematic review of the screening accuracy of the HIV dementia scale and international HIV dementia scale. PLoS ONE. 2013;8(4):e61826. doi: 10.1371/journal.pone.0061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D, Vance D. The neuropsychology of HIV/AIDS in older adults. Neuropsychological Review. 2009;19(2):263–272. doi: 10.1007/s11065-009-9087-0. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: Charter study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Taylor MJ, Manly J. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. In: Tulsky DS, Saklofske DH, Chelune GJ, Heaton RK, Ivnik RJ, Bornstein R, et al.Ledbetter MF, editors. Clinical interpretation of the WAIS-III and WMS-III. San Diego, CA: Academic Press; 2003. pp. 181–210. [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Stefaniak M. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59(12):1944–1950. doi: 10.1212/01.WNL.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd. Wiley-Interscience; Publication: 2000. Introduction to the logistic regression model; pp. 1–30. [Google Scholar]
- Kvalsund MP, Haworth A, Murman DL, Velie E, Birbeck GL. Closing gaps in antiretroviral therapy access: Human immunodeficiency virus–associated dementia screening instruments for non-physician healthcare workers. The American Journal of Tropical Medicine and Hygiene. 2009;80(6):1054–1059. [PubMed] [Google Scholar]
- Levine A, Palomo M, Lopez E, Singer EJ, Valdes-Sueiras M, Hinkin CH, et al. Donovan S. A comparison of screening batteries in the detection of neurocognitive impairment in HIV-infected Spanish speakers. Neurobehavioral HIV Medicine. 2011;3:79–86. doi: 10.2147/nbhiv.s22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López E, Steiner AJ, Hardy DJ, IsHak WW, Anderson B. Discrepancies between bilinguals' performance on the Spanish and English versions of the WAIS digit span task: Cross-cultural implications. Applied Neuropsychology: Adult. 2016;23(5):343–352. doi: 10.1080/23279095.2015.1074577. [DOI] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: An evolving disease. Journal of Neuroimmunology. 2004;157(1–2):3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Milanini B, Ciccarelli N, Fabbiani M, Baldonero E, Limiti S, Gagliardini R, et al. Di Giambenedetto S. Neuropsychological screening tools in Italian HIV+ patients: A comparison of Montreal Cognitive Assessment (MoCA) and Mini Mental State Examination (MMSE) The Clinical Neuropsychologist. 2016:1–12. doi: 10.1080/13854046.2016.1183048. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Mindt MR, Arentoft A, Germano KK, D'Aquila E, Scheiner D, Pizzirusso M, et al. Gollan TH. Neuropsychological, cognitive, and theoretical considerations for evaluation of bilingual individuals. Neuropsychological Review. 2008;18(3):255–268. doi: 10.1007/s11065-008-9069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindt MR, Byrd D, Ryan EL, Robbins R, Monzones J, Arentoft A, et al. Morgello S. “Characterization and sociocultural predictors of neuropsychological test performance in HIV+Hispanic individuals”: Correction. Cultural Diversity and Ethnic Minority Psychology. 2009;15(4):433. doi: 10.1037/a0017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindt MR, Byrd D, Saez P, Manly J. Increasing culturally competent neuropsychological services for ethnic minority populations: A call to action. The Clinical Neuropsychologist. 2010;24(3):429–453. doi: 10.1080/13854040903058960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Scott JC, Childers M, Beck JM, Ellis RJ, et al. HIV Neurobehavioral Research Center (HNRC) Group. Predictive validity of demographically adjusted normative standards for the HIV Dementia Scale. Journal of Clinical and Experimental Neuropsychology. 2007;30(1):83–90. doi: 10.1080/13803390701233865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Moreno JA, Prats A, Pérez-Álvarez N, Fumaz CR, Garolera M, Doval E, et al. for the NEU Study Group. A brief and feasible paper-based method to screen for neurocognitive impairment in HIV-infected patients: The NEU screen. Journal of Acquired Immune Deficiency Syndromes. 2013;63(5):585–592. doi: 10.1097/QAI.0b013e31829e1408. [DOI] [PubMed] [Google Scholar]
- Norman MA, Evans JD, Miller SW, Heaton RK. Demographically corrected norms for the California Verbal Learning Test. Journal of Clinical and Experimental Neuropsychology. 2000;22(1):80–94. doi: 10.1076/1380-3395(200002)22:1;1-8;FT080. [DOI] [PubMed] [Google Scholar]
- Overton ET, Azad TD, Parker N, Shaw D, Frain J, Spitz T, et al. Ances BM. The Alzheimer's disease-8 and Montreal cognitive assessment as screening tools for neurocognitive impairment in HIV-infected persons. Journal of Neurovirology. 2013;19(1):109–116. doi: 10.1007/s13365-012-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza O, Mungas D. Measurement in cross-cultural neuropsychology. Neuropsychological Review. 2008;18(3):184–193. doi: 10.1007/s11065-008-9067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontón MO, Satz P, Herrera L, Ortiz F, Urrutia CP, Young R, et al. Namerow N. Normative data stratified by age and education for the neuropsychological screening battery for Hispanics (NeSBHIS): Initial report. Journal of the International Neuropsychological Society. 1996;2(2):96–104. doi: 10.1017/S1355617700000941. [DOI] [PubMed] [Google Scholar]
- Power C, Selnes OA, Grim JA, McArthur JC. HIV dementia scale: A rapid screening test. Journal of Acquired Immune Deficiency Syndromes. 1995;8(3):273–278. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- Price RW, Yiannoutsos CT, Clifford DB, Zaborski L, Tselis A, Sidtis JJ, et al. for the AIDS Clinical Trial Group, and Neurological AIDS Research Consortium Study Team. Neurological outcomes in late HIV infection: adverse impact of neurological impairment on survival and protective effect of antiviral therapy. AIDS. 1999;13(13):1677–1685. doi: 10.1097/00002030-199909100-00011. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, et al. MPH, and the Multicenter AIDS Cohort Study. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56(2):257–260. doi: 10.1212/WNL.56.2.257. [DOI] [PubMed] [Google Scholar]
- Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, et al. Katabira E. The International HIV Dementia Scale: A new rapid screening test for HIV dementia. AIDS. 2005;19(13):1367–1374. [PubMed] [Google Scholar]
- Smith K, Steiner A, IsHak W, Acosta J, Erich B, D'Elia L, et al. López E. Utilizing the color figure mazes test to assess executive functioning while screening for HIV-associated neurocognitive disorders in HIV-1 seropositive Spanish-speaking adults. Journal of AIDS and Clinical Research. 2014;5(10):1–8. doi: 10.4172/2155-6113.1000357. [DOI] [Google Scholar]
- Swets JA. Signal detection theory and ROC analysis in psychology and diagnostics: Collected papers. Psychology Press; 1996. [Google Scholar]
- Tabachnick B, Fidell L. Multivariate analysis of variance and covariance. Using Multivariate Statistics. 2007;3:402–407. [Google Scholar]
- U.S. Census Bureau. The Hispanic population: 2010. Washington, DC: U.S. Department of Commerce; 2010. Retrieved from http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. [Google Scholar]
- U.S. Census Bureau. Hispanic heritage month 2012: Sept. 15–Oct. 15. 2013 Retrieved from http://www.census.gov/newsroom/releases/pdf/cb13ff-19_hispanicheritage.pdf.
- Valcour V, Paul R, Chiao S, Wendelken LA, Miller B. Screening for cognitive impairment in human immunodeficiency virus. Clinical Infectious Diseases. 2011;53(8):836–842. doi: 10.1093/cid/cir524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie FL, Goodkin K, Ardila A, Concha M, Lee D, Lecusay R, et al. O'Mellan S. Humans: An English and Spanish neuropsychological test battery for assessing HIV-1-infected individuals–initial report. Applied Neuropsychology. 2004;11(3):121–133. doi: 10.1207/s15324826an1103_1. [DOI] [PubMed] [Google Scholar]
- Wojna V, Skolasky RL, McArthur JC, Maldonado E, Hechavarria R, Mayo R, et al. Nath A. Spanish validation of the HIV dementia scale in women. AIDS Patient Care STDS. 2007;21(12):930–941. doi: 10.1089/apc.2006.0180. [DOI] [PubMed] [Google Scholar]
- Zipursky AR, Gogolishvili D, Rueda S, Brunetta J, Carvalhal A, McCombe JA, et al. Rourke SB. Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS: A systematic review of the literature. AIDS. 2013;27(15):2385–2401. doi: 10.1097/QAD.0b013e328363bf56. [DOI] [PMC free article] [PubMed] [Google Scholar]


