Abstract
Aerobic exercise helps to maintain cardiovascular health in part by mitigating age-induced arterial stiffening. However, the long-term effects of exercise regimens on aortic stiffness remain unknown, especially in the intimal extracellular matrix layer known as the subendothelial matrix. To examine how the stiffness of the subendothelial matrix changes following exercise cessation, mice were exposed to an 8 week swimming regimen followed by an 8 week sedentary rest period. Whole vessel and subendothelial matrix stiffness were measured after both the exercise and rest periods. After swimming, whole vessel and subendothelial matrix stiffness decreased, and after 8 weeks of rest, these values returned to baseline. Within the same time frame, the collagen content in the intima layer and the presence of advanced glycation end products (AGEs) in the whole vessel were also affected by the exercise and the rest periods. Overall, our data indicate that consistent exercise is necessary for maintaining compliance in the subendothelial matrix.
Introduction
Increased stiffness within the vasculature is independently associated with cardiovascular disease risk, and is often measured using pulse wave velocity (PWV), a standard clinical measurement of bulk arterial stiffness [1]. Well-known risk factors for cardiovascular disease include increased age and lack of exercise [2,3], which are also associated with increased artery stiffness [1,4,5]. Changes in subendothelial matrix stiffness are associated with endothelial cell contraction, endothelial cell–cell junction disruption, and increased vessel permeability [6,7]. Previously, we showed that increased vascular stiffness in aged mice can be decreased through exercise on both the macro and microscales [5]. However, the lasting effects of exercise on microscale intimal stiffness after exercise cessation are unknown. Here, mice underwent exercise and rest periods, and changes in PWV, subendothelial matrix stiffness and cardiac ejection fraction were measured. To investigate the underlying causes for vascular stiffness changes, we examined collagen content in the intima and advanced glycation end product (AGE) accumulation within the whole artery, as both collagen [4,8] and AGEs [9,10] are associated with arterial stiffening.
Methods
Animal Model.
All animal experiments were carried out under approved Cornell University IACUC protocols. C57Bl/6 male mice were acquired from the National Institute on Aging at 18 months of age. Mice were weighed multiple times a week as a health assessment.
Swimming Protocol.
Mice were swum in groups of 2–5 mice per tank as previously described [5]. Mice swam five days a week for 10 min per day in week 1, 30 min per day in weeks 2–3, and 45 min per day for weeks 4–8.
Pulse Wave Velocity.
Each mouse was anesthetized using 0.5–2% isoflurane, hair was removed, and an MS550D transducer was used to acquire Doppler images on a Vevo2100 (VisualSonics, Toronto, ON, Canada) ultrasound, as previously described [5]. The arrival times at each location were measured from the peak of the R-wave on the electrocardiogram to the velocity upstroke, and the difference between arrival times was used as the transit time. Distance between the points was measured over the animal's body, and PWV was calculated as distance over time.
Ejection Fraction.
Following PWV measurement, left ventricle (LV) long-axis images were acquired at systole and diastole on the Vevo2100 ultrasound. The electrocardiogram-gated kilohertz visualization imaging method was used to create video of LV expansion and contraction, and ejection fraction was determined using manual outlines of the chamber and the ultrasound software. This method was limited to one researcher performing the LV traces.
Thoracic Aorta Collection.
Mice were anesthetized using 2–3.5% isoflurane, the thoracic cavity was opened, and the heart was perfused with phosphate buffered saline (PBS). The thoracic aorta was excised, excess fat was removed, and the tissue was cut longitudinally and stored at 4 °C in PBS until use, as previously described [5].
Atomic Force Microscopy.
Aorta tissue was used within 12 h postmortem. Endothelial cells were scraped from the intima using a cotton swab as previously described [11], and the tissue was glued to a Petri dish (Loctite Super Glue, Westlake, OH). The tissue was submerged in a drop of room temperature PBS and acclimated to room temperature before measurements were recorded. Indentations were made on an Asylum (Santa Barbara, CA) atomic force microscope (MFP-3D) using a 0.12 N/m cantilever, as specified by the manufacturer (Novascan, Boone, IA) and calibrated to 0.109 ± 0.019 N/m before use. The cantilever was affixed with a 10 μm diameter polystyrene bead, and the Hertz model (with an assumed Poisson's ratio of 0.5) was used to fit the force indentation curves to determine elastic modulus as previously described [5,6,11].
Structured Illumination Microscope Setup.
A structured illumination microscope built on an inverted Axiovert microscope (Zeiss, Thornwood, NY) was used to measure the picrosirius red (PSR) polarization signal of the intima layer. Briefly, a linear polarizer was rotated above the condenser by a linear motor actuator (Thorlabs, Newton, NJ). A 20 lines/mm Ronchi grid (Edmunds Optics, Barrington, NJ) was positioned in the illumination plane above the linear polarizer and moved 2π/3 for each image acquisition. A polarizer analyzer was positioned in the imaging plane with its optical axis turned 90 deg relative to the illumination polarizer (cross polarizer configuration).
Collagen Content.
Aorta sections were fixed in 3.7% formaldehyde for 15 min and stored in PBS at 4 °C until use. Arteries were stained with a PSR kit (Polysciences, Inc. (Warrington, PA) 24901) by soaking the tissue in solutions provided by the kit for 1 h with solution A, 3 h with solution B and 1 h with solution C, and then soaked overnight at 4 °C in PBS tissue was also stained with primary 1:100 (Santa Cruz Biotechnology (Dallas, TX) sc-6458) and secondary 1:200 (Alexaflour-488, A11055, ThermoFisher Scientific, Waltham, MA) both overnight. To determine collagen content, the intima layer was identified using fluorescence imaging of the vascular endothelial cadherin layer. Samples where the intima layer could not be identified were removed from analysis. White light was then used to illuminate the sample and a sequence of three images under structured illumination microscope was taken. The images were processed by a matlab code to reconstruct a thin optical section of the PSR polarization signal [12], which correlates with collagen content [13,14]. The resulting images are composed of three color channels (red, green, and blue), and their intensity values were measured (imagej). The composite intensity value at each artery location was calculated using grayscale correction factors: 0.2989*red, 0.587*green, and 0.114*blue. Images taken on different days were normalized to a control artery and were range corrected. Data were log transformed prior to group comparisons.
N(ε)-Carboxymethyl-Lysine Content.
Aorta pieces were flash frozen in liquid nitrogen and stored at −80 °C. The night before the enzyme-linked immunosorbent assay, the samples were ground and dissolved in 300 μL of radioimmunoprecipitation assay buffer containing 1:1000 protease inhibitor. A bicinchoninic acid assay (Pierce, Thermo Scientific, Waltham, MA) measured protein concentration and N(ε)-carboxymethyl-lysine (CML) content was measured using a competitive enzyme-linked immunosorbent assay (Cell Biolabs, Inc., San Diego, CA, STA-816) according to manufacturer's instructions. A minimum of 0.09 mg of protein for each sample was required to run the assay, and artery sections which were too small were removed from analysis. CML concentration was normalized against protein concentration to determine weight percent (ng CML per mg protein for each sample).
Statistics.
Normality was assessed using the Shapiro-Wilks test. Groups were compared according to each time point using a Student's t-test, where a p-value less than 0.05 was determined to be significant. Linear correlation was assessed with a Pearson correlation p-value less than 0.05.
Results
Exercise and Rest Regimen.
To examine the effects of exercise and subsequent exercise cessation on arterial stiffness, mice underwent an 8 week exercise regimen followed by an 8 week rest period (Fig. 1(a)). Before, during, and following these periods, PWV values were recorded. At time of sacrifice, the thoracic aorta was removed and used for subendothelial matrix stiffness measurements, whole vessel AGE measurements, and intimal collagen analysis. Throughout the study, the mice were weighed multiple times a week. Even though the mice were advanced in aged, mouse body weight remained constant over the study (Fig. 1(b)), a marker of good health.
Fig. 1.
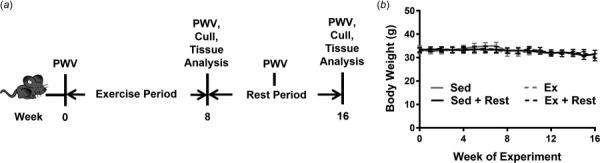
Mice undergo an exercise regimen and a rest period. (a) Aged C57Bl/6 mice swim for 8 weeks during the exercise period, and then rest for 8 weeks. PWV is measured throughout the study, and at week 16 the mice are culled and their aortas are removed for further analysis. (b) Mouse body weight remains steady over the study in all groups; n = 4–7 mice per group, error bars are standard error of the mean (SEM).
Pulse Wave Velocity.
Pulse wave velocity of the exercised mice significantly decreased compared to the sedentary cohort following 8weeks of exercise. After 4 weeks of postexercise rest, PWV increased to the level of the sedentary counterparts and remained elevated for the remainder of the 8 week rest period (Fig. 2(a)). These data indicate that PWV is affected by exercise and rest periods.
Fig. 2.
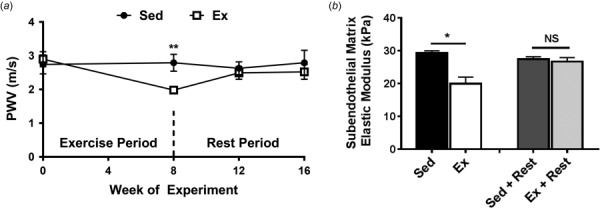
Pulse wave velocity and subendothelial matrix stiffness are affected by exercise and rest periods. (a) PWV decreases after 8 weeks of exercise and increases to baseline following 4 weeks of rest; **p < 0.005 (Student's t-test), n = 7–12 mice per data point. (b) Subendothelial matrix stiffness decreases following an exercise regimen and returns to baseline following a rest period; *p < 0.05 (student's t-test), n = 4–7 mice per group, NS = not statistically significant. Error bars are SEM in the whole figure.
Subendothelial Matrix Stiffness.
Ex vivo murine aortas were indented with atomic force microscopy (AFM) to assess the subendothelial matrix elastic moduli, which decreased after exercise and restiffened following the rest period (Fig. 2(b)). Together, the combined PWV and AFM measurements demonstrate that both the subendothelial matrix stiffness and PWV changes due to exercise have transient effects.
Left Ventricular Ejection Fraction.
To determine left ventricular ejection fraction (LVEF), high-resolution ultrasound electrocardiogram-gated kilohertz visualization images were acquired and the left ventricle cross-sectional areas were measured at systole and diastole (Fig. 3(a)). Exercise increased LVEF, and following the 8 week rest period, the heart returned to lower baseline function (Fig. 3(b)). Following the rest period both macroscale and microscale stiffness of the aorta show a negative linear correlation with LVEF (Figs. 3(c) and 3(d)).
Fig. 3.
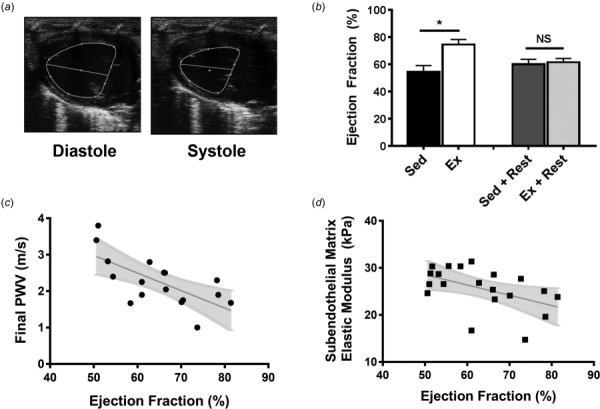
Left ventricular ejection fraction is inversely correlated with arterial stiffness. (a) Representative ultrasound images of the long-axis view of the left ventricle, used to calculate ejection fraction. (b) Ejection fraction increases with exercise and returns to baseline following a rest period; *p < 0.05 (student's t-test), n = 5–7 mice per group, error bars are SEM. (c) There is a significant inverse correlation between PWV and ejection fraction; p < 0.005 (Pearson correlation). (d) There is a significant inverse correlation between the subendothelial matrix elastic modulus and ejection fraction; p < 0.05 (Pearson correlation).
Intima Collagen Content.
Increased collagen is a major cause of increased arterial stiffness associated with advanced age [4,8,15]. While previous studies focus on changes in total vessel or medial collagen [4,8,15,16], here, we focus on the collagen in the subendothelial matrix. Intimal collagen content was assessed based on the PSR polarization signal assessed using structured illumination microscopy (Fig. 4(a)), which measures collagen signal from a defined tissue layer based on the birefringent nature of collagen structure [17]. Representative images of the polarized signal from sedentary and exercise groups demonstrate different levels of intima collagen in tissue sections (Fig. 4(b)). Collagen content within the intima decreases following exercise and begins to increase following rest (Fig. 4(c)).
Fig. 4.
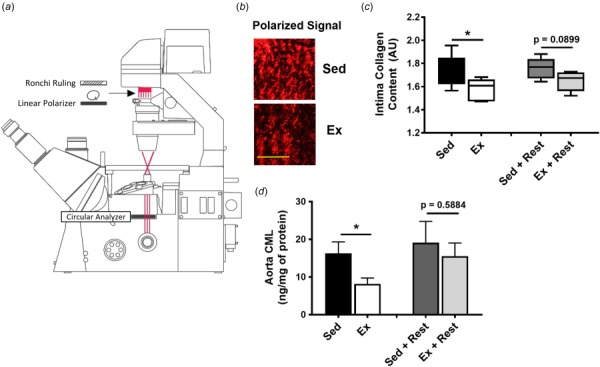
Intimal collagen and aortic CML content are affected by exercise and rest. (a) Schematic of the structured illumination microscope used to measure intimal collagen content. (b) Representative images of polarized signal, representing intimal collagen content, from sedentary and exercise mice; scale bar is 100 μm. (c) Intimal collagen content decreases with exercise and begins to increase following a rest period; n = 5–8 mice per group, error bars are minimum and maximum values. (d) Aortic content of CML, an advanced glycation product, decreases with exercise and increases following a rest period in the whole vessel; *p < 0.05 (student's t-test), n = 5–9 mice per group, error bars are SEM. NS = not statistically significant.
Aortic Advanced Glycation End Product Content.
Advanced glycation end products act through a variety of mechanisms [18], and have previously been associated with vascular stiffening [10]. Here, we measured the aortic content of CML, a dominant protein modification found in vivo [19] which increases with cardiovascular risk factors such as poor diet, sedentary lifestyle, and increased age [10,20]. CML AGE content in the vessel decreases following exercise and returns to baseline after the rest period (Fig. 4(d)).
Discussion
While prior studies have demonstrated that exercise modulates macroscale arterial compliance and stiffness [4,21,22], our study examines the impact of exercise and rest on both macro and microscale arterial stiffness. Here, we found that PWV and subendothelial matrix stiffening deceased after exercise, but these effects do not persist; macro and microscale stiffness return to baseline following a rest period. We also find that cardiac function, as assessed by LVEF, is inversely correlated with PWV and subendothelial matrix stiffness. Overall, our study demonstrates that continued exercise is important to maintaining cardiovascular health, especially with respect to the intimal layer.
We also examined possible causes for the observed changes in the arterial stiffness changes. While it is known that age-induced whole vessel [23] or diet-induced medial [16] collagen content is decreased following exercise, here we examined the role of collagen in the intimal layer alone. Different collagen types are present in the intima layer, such as type IV collagen and collagen I and III fibers [24–26]. Here, we used PSR staining, which enhances the collagen I and III signal. Our data demonstrate that intima collagen content decreases with exercise, and begins to return to baseline following exercise cessation. However, the extent to which each type of collagen changes remains unknown and is an interesting research question for future studies. In the whole artery, aortic CML has been shown to increase with age and decrease with exercise [10]. CML mainly influences arterial stiffness through its interaction as a ligand with the receptor for AGEs [20,27]. Here, we find that CML content in the aorta decreases with exercise, and begins to return to baseline after exercise cessation. Since collagen and CML content follow similar trends to the macro and microscale stiffness measurements in this study, we propose that these factors may play a role in modulating arterial stiffness following exercise and exercise cessation. These findings underscore the importance of future studies to analyze the roles of extracellular matrix proteins and their effectors in the intima layer to further understand the mechanisms of subendothelial matrix stiffening.
Acknowledgment
Imaging data was acquired in the Cornell BRC-Imaging Facility using the shared, NIH-funded VisualSonics high-resolution ultrasound. For AFM data collection, this work made use of the Cornell Center for Materials Research Shared Facilities which are supported through the NSF MRSEC program.
Contributor Information
Julie C. Kohn, Nancy E. and Peter C. Meinig School , of Biomedical Engineering, , Cornell University, , Weill Hall, , Ithaca, NY 14853 , e-mail: jck256@cornell.edu
François Bordeleau, Department of Biomedical Engineering, , Vanderbilt University, , Engineering and Science Building, , Nashville, TN 351631 , e-mail: francois.bordeleau@vanderbilt.edu.
Joseph Miller, Nancy E. and Peter C. Meinig School , of Biomedical Engineering, , Cornell University, , Weill Hall, , Ithaca, NY 14853 , e-mail: jpm386@cornell.edu.
Hannah C. Watkins, Nancy E. and Peter C. Meinig School , of Biomedical Engineering, , Cornell University, , Weill Hall, , Ithaca, NY 14853 , e-mail: hcw47@cornell.edu
Shweta Modi, Nancy E. and Peter C. Meinig School , of Biomedical Engineering, , Cornell University, , Weill Hall, , Ithaca, NY 14853 , e-mail: sm2258@cornell.edu.
Jenny Ma, Nancy E. and Peter C. Meinig School , of Biomedical Engineering, , Cornell University, , Weill Hall, , Ithaca, NY 14853 , e-mail: jm2386@cornell.edu.
Julian Azar, Nancy E. and Peter C. Meinig School , of Biomedical Engineering, , Cornell University, , Weill Hall, , Ithaca, NY 14853 , e-mail: ja522@cornell.edu.
David Putnam, Nancy E. and Peter C. Meinig School , of Biomedical Engineering, , Cornell University, , Weill Hall, , Ithaca, NY 14853;; Robert Frederick Smith School of Chemical , and Biomolecular Engineering, , Cornell University, , Weill Hall, , Ithaca, NY 14853 , e-mail: dap43@cornell.edu
Cynthia A. Reinhart-King, Nancy E. and Peter C. Meinig School , of Biomedical Engineering, , Cornell University, , Weill Hall, , Ithaca, NY 14853;; Cornelius Vanderbilt Professor of Engineering, , Department of Biomedical Engineering, , Vanderbilt University, , Mailbox PMB 351631, , 440 Engineering and Science Building, , Nashville, TN 351631 , e-mails: cak57@cornell.edu; , cynthia.reinhart-king@vanderbilt.edu
Funding Data
National Institutes of Health (Grant No. S10OD016191).
Cornell University National Science Foundation (Grant Nos. 1435755, 2013165170, and DMR-1719875).
Nomenclature
- AFM =
atomic force microscopy
- AGE =
advanced glycation end product
- CML =
N(ε)-carboxymethyl-lysine
- LVEF =
left ventricular ejection fraction
- PBS =
phosphate buffered saline
- PWV =
pulse wave velocity
- SEM =
standard error of the mean
- π =
Pi
References
- [1]. Mitchell, G. F. , Shih-jen, H. , Vasan, R. S. , Larson, G. , Pencina, M. J. , Hamburg, N. M. , Vita, J. A. , Levy, D. , and Benjamin, E. J. , 2010, “ Arterial Stiffness and Cardiovascular Events: The Framingham Heart Study,” Circulation, 121(4), pp. 505–511. 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Fletcher, G. F. , Balady, G. , Blair, S. N. , Blumenthal, J. , Caspersen, C. , Chaitman, B. , Epstein, S. , Froelicher, E. S. S. , Froelicher, V. F. , Pina, I. L. , and Pollock, M. , 1996, “ Statement on Exercise: Benefits and Recommendations for Physical Activity Programs for All Americans,” Circulation, 94(4), pp. 857–862. 10.1161/01.CIR.94.4.857 [DOI] [PubMed] [Google Scholar]
- [3]. Grundy, S. M. , Pasternak, R. , Greenland, P. , Smith, S. , and Fuster, V. , 1999, “ Assessment of Cardiovascular Risk by Use of Multiple-Risk-Factor Assessment Equations,” Circulation, 100, pp. 1481–1492. 10.1161/01.CIR.100.13.1481 [DOI] [PubMed] [Google Scholar]
- [4]. Gu, Q. , Wang, B. , Zhang, X. F. , Ma, Y. P. , Liu, J. D. , and Wang, X. Z. , 2014, “ Chronic Aerobic Exercise Training Attenuates Aortic Stiffening and Endothelial Dysfunction Through Preserving Aortic Mitochondrial Function in Aged Rats,” Exp. Gerontol., 56, pp. 37–44. 10.1016/j.exger.2014.02.014 [DOI] [PubMed] [Google Scholar]
- [5]. Kohn, J. C. , Chen, A. , Cheng, S. , Kowal, D. R. , King, M. R. , and Reinhart-King, C. A. , 2016, “ Mechanical Heterogeneities in the Subendothelial Matrix Develop With Age and Decrease With Exercise,” J. Biomech., 49(9), pp. 1447–1453. 10.1016/j.jbiomech.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Huynh, J. , Nishimura, N. , Rana, K. , Peloquin, J. M. , Califano, J. P. , Montague, C. R. , King, M. R. , Schaffer, C. B. , and Reinhart-King, C. A. , 2011, “ Age-Related Intimal Stiffening Enhances Endothelial Permeability and Leukocyte Transmigration,” Sci. Transl. Med., 3(112), p. 112ra122. 10.1126/scitranslmed.3002761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Krishnan, R. , Klumpers, D. D. , Park, C. Y. , Rajendran, K. , Trepat, X. , van Bezu, J. , van Hinsbergh, V. W. M. , Carman, C. V. , Brain, J. D. , Fredberg, J. J. , Butler, J. P. , and van Nieuw Amerongen, G. P. , 2011, “ Substrate Stiffening Promotes Endothelial Monolayer Disruption Through Enhanced Physical Forces,” Am. J. Physiol. Cell. Physiol., 300(1), pp. C146–C154. 10.1152/ajpcell.00195.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Fleenor, B. S. , and Berrones, A. J. , 2015, “ Structural Changes Contributing to Arterial Stiffness: Compositional Changes,” Arterial Stiffness: Implications and Interventions, Springer International Publishing, New York, pp. 19–20. [Google Scholar]
- [9]. Fleenor, B. S. , Sindler, A. L. , Eng, J. S. , Nair, D. P. , Dodson, R. B. , and Seals, D. R. , 2012, “ Sodium Nitrite De-Stiffening of Large Elastic Arteries With Aging: Role of Normalization of Advanced Glycation End-Products,” Exp. Gerontol., 47(8), pp. 588–594. 10.1016/j.exger.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Gu, Q. , Wang, B. , Zhang, X.-F. , Ma, Y.-P. , Liu, J.-D. , and Wang, X.-Z. , 2014, “ Contribution of Receptor for Advanced Glycation End Products to Vasculature-Protecting Effects of Exercise Training in Aged Rats,” Eur. J. Pharmacol., 741, pp. 186–194. 10.1016/j.ejphar.2014.08.017 [DOI] [PubMed] [Google Scholar]
- [11]. Peloquin, J. , Huynh, J. , Williams, R. M. , and Reinhart-King, C. A. , 2011, “ Indentation Measurements of the Subendothelial Matrix in Bovine Carotid Arteries,” J. Biomech., 44(5), pp. 815–821. 10.1016/j.jbiomech.2010.12.018 [DOI] [PubMed] [Google Scholar]
- [12]. Neil, M. A. , Juskaitis, R. , and Wilson, T. , 1997, “ Method of Obtaining Optical Sectioning by Using Structured Light in a Conventional Microscope,” Opt. Lett., 22(24), pp. 1905–1907. 10.1364/OL.22.001905 [DOI] [PubMed] [Google Scholar]
- [13]. Jalil, J. E. , Doering, C. W. , Janicki, J. S. , Pick, R. , Clark, W. A. , Abrahams, C. , and Weber, K. T. , 1988, “ Structural vs. Contractile Protein Remodeling and Myocardial Stiffness in Hypertrophied Rat Left Ventricle,” J. Mol. Cell. Cardiol., 20(12), pp. 1179–1187. 10.1016/0022-2828(88)90597-4 [DOI] [PubMed] [Google Scholar]
- [14]. Rich, L. , and Whittaker, P. , 2005, “ Collagen and Picrosirius Red Staining: A Polarized Light Assessment of Fibrillar Hue and Spatial Distribution,” Braz. J. Morphol. Sci., 22(2), pp. 97–104.https://pdfs.semanticscholar.org/8128/2ace6a9024d0bd96b46dcc47aabc6e669a30.pdf [Google Scholar]
- [15]. Kohn, J. C. , Lampi, M. C. , and Reinhart-King, C. A. , 2015, “ Age-Related Vascular Stiffening: Causes and Consequences,” Front. Genet., 6, p. 112. 10.3389/fgene.2015.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Padilla, J. , Ramirez-perez, F. I. , Habibi, J. , Bostick, B. , Aroor, A. R. , Hayden, M. R. , Jia, G. , Garro, M. , Demarco, V. G. , Manrique, C. , Booth, F. W. , Martinez-lemus, L. A. , and Sowers, J. R. , 2016, “ Regular Exercise Reduces Endothelial Cortical Stiffness in Western Diet—Fed Female Mice,” Hypertension, 68(5), pp. 1236–1244. 10.1161/HYPERTENSIONAHA.116.07954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Wolman, M. , and Kasten, F. H. , 1986, “ Polarized Light Microscopy in the Study of the Molecular Structure of Collagen and Reticulin,” Histochemistry, 85(1), pp. 41–49. 10.1007/BF00508652 [DOI] [PubMed] [Google Scholar]
- [18]. Goldin, A. , Beckman, J. A. , Schmidt, A. M. , and Creager, M. A. , 2006, “ Advanced Glycation End Products: Sparking the Development of Diabetic Vascular Injury,” Circulation, 114(6), pp. 597–605. 10.1161/CIRCULATIONAHA.106.621854 [DOI] [PubMed] [Google Scholar]
- [19]. Dyer, D. G. , Dunn, J. A. , Thorpe, S. R. , Bailie, K. E. , Lyons, T. J. , McCance, D. R. , and Baynes, J. W. , 1993, “ Accumulation of Maillard Reaction Products in Skin Collagen in Diabetes and Aging,” J. Clin. Invest., 91(6), pp. 2463–2469. 10.1172/JCI116481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Grossin, N. , Auger, F. , Niquet-Leridon, C. , Durieux, N. , Montaigne, D. , Schmidt, A. M. , Susen, S. , Jacolot, P. , Beuscart, J.-B. , Tessier, F. J. , and Boulanger, E. , 2015, “ Dietary CML-Enriched Protein Induces Functional Arterial Aging in a RAGE-Dependent Manner in Mice,” Mol. Nutr. Food Res., 59(5), pp. 927–938. 10.1002/mnfr.201400643 [DOI] [PubMed] [Google Scholar]
- [21]. Santos-Parker, J. R. , LaRocca, T. J. , and Seals, D. R. , 2014, “ Aerobic Exercise and Other Healthy Lifestyle Factors That Influence Vascular Aging,” Adv. Physiol. Educ., 38(4), pp. 296–307. 10.1152/advan.00088.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Tanaka, H. , Dinenno, F. A. , Monahan, K. D. , Clevenger, C. M. , DeSouza, C. A. , and Seals, D. R. , 2000, “ Aging, Habitual Exercise, and Dynamic Arterial Compliance,” Circulation, 102(11), pp. 1270–1275. 10.1161/01.CIR.102.11.1270 [DOI] [PubMed] [Google Scholar]
- [23]. Fleenor, B. S. , Marshall, K. D. , Durrant, J. R. , Lesniewski, L. A. , and Seals, D. R. , 2010, “ Arterial Stiffening With Ageing is Associated With Transforming Growth Factor-β1-Related Changes in Adventitial Collagen: Reversal by Aerobic Exercise,” J. Physiol., 588(20), pp. 3971–3982. 10.1113/jphysiol.2010.194753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Murata, K. , Motayama, T. , and Kotake, C. , 1986, “ Collagen Types in Various Layers of the Human Aorta and Their Changes With the Atherosclerotic Process,” Atherosclerosis, 60(3), pp. 251–262. 10.1016/0021-9150(86)90172-3 [DOI] [PubMed] [Google Scholar]
- [25]. Palotie, A. , Tryggvason, K. , Peltonen, L. , and Seppa, H. , 1983, “ Components of Subendothelial Aorta Basement Membrane: Immunohistochemical Localization and Role in Cell Attachment,” Lab. Invest., 49(3), pp. 362–370.https://www.ncbi.nlm.nih.gov/pubmed/6224965 [PubMed] [Google Scholar]
- [26]. VanderBurgh, J. A. , and Reinhart-King, C. A. , 2018, “ The Role of Age-Related Intimal Remodeling and Stiffening in Atherosclerosis,” Advances in Pharmacology, Vol. 81, pp. 365–391. [DOI] [PubMed] [Google Scholar]
- [27]. Kislinger, T. , Fu, C. , Huber, B. , Qu, W. , Taguchi, A. , Yan, S. D. , Hofmann, M. , Yan, S. F. , Pischetsrieder, M. , Stern, D. , and Schmidt, A. M. , 1999, “ N(Epsilon)-Carboxymethyl Lysine Adducts of Proteins are Ligands for Receptor for Advanced Glycation End Products That Activate Cell Signaling Pathways and Modulate Gene Expression,” J. Biol. Chem., 274(44), pp. 31740–31749. 10.1074/jbc.274.44.31740 [DOI] [PubMed] [Google Scholar]


