Abstract
Radiolytic decomposition of high specific concentration radiopharmaceuticals is an undesired side-effect that can hamper development of novel PET tracers. This was particularly evident in a series of carbon-11 and fluorine-18 labeled mono- and dimethyl-substituted aryl amines, where rapid decomposition was observed in isolation and formulation steps. We tested a number of additives that inhibit radiolysis and can be safely added to the synthesis procedures (purification and isolation) and reformulation steps to provide suitable clinical formulations. Ethanol and sodium ascorbate are established anti-oxidant stabilizers that completely inhibit radiolytic decomposition and are amenable to human use. Herein, we also demonstrate for the first time that nitrones are non-toxic radical scavengers that are capable of inhibiting radiolysis.
Keywords: Tomography, Emission Computed, Radiolysis, Stability, Anti-oxidants
1. INTRODUCTION
Positron emission tomography (PET) imaging is a rapidly growing field of research in which molecules labeled with short-lived radionuclides such as carbon-11 or fluorine-18 are utilized to non-invasively examine biochemistry in living human subjects. As part of a broad program to deliver not only established PET radiopharmaceuticals for clinical care (2-deoxy-2-[18F]fluoro-D-glucose, [18F]FDG) and for clinical research (carbon-11 and fluorine-18 labeled radiotracers for brain, heart, pancreas and tumor imaging) we have a research program dedicated to the development and clinical implementation of new PET radiopharmaceuticals. In particular, we and others have significant interest in the development of new fluorine-18 labeled PET imaging agents for neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). The application of fluorine-18, with a convenient 110 minute half-life, will facilitate the more widespread distribution and use of such radiopharmaceuticals in clinical populations. During the development of radiochemical syntheses of such new fluorine-18 labeled radiopharmaceuticals, including the validation of methods to prepare suitably high amounts for potential distribution, we observed a distressing occurrence of decomposition of the final formulated products. As we suspected this might be due to radiolysis, and that it was particularly acute for the types of structures (N-methylanilines) common in our recently developed radiotracers, we undertook and report here a limited study of the factors involved in this decomposition process, examining what chemical structures seem particularly susceptible and what steps can be taken to effectively prevent decomposition. Radiolysis in radiopharmaceutical preparations is certainly not an unknown phenomenon, having been implicated originally in the decomposition of carbon-14 and hydrogen-3 labeled species [Bayly and Evans, 1966] and more recently of carbon-11 labeled species [Suzuki et al., 1990; Bogni et al., 2003; Fukumura et al., 2003, 2004, 2004]. These studies suggest that the degree of radiolysis of labeled compounds depends on the level of radioactivity, the level of specific activity, the structure of the radiopharmaceutical and the position of the radiolabel. These hypotheses are all supported by the results of the current study.
In contrast to the above studies, radiolytic decomposition has not been extensively studied for fluorine-18 labeled compounds, although many of the mechanisms are expected to be similar to those discussed by Fukumura [Fukumura et al., 2004] One example, a recent study of the stability of [18F]fluorodeoxyglucose (FDG), [Fawdry, 2007] reported a slow but steady decomposition of the radiopharmaceutical to release free [18F]fluoride ion. Similarly, MacGregor reported on the decomposition of [18F]-N-methylspiroperidol, in which it was shown that rate of radiolysis was proportional to specific activity [MacGregor et al., 1987]. With the more widespread availability of medical cyclotrons capable of producing high levels of [18F]fluoride ion coupled with the development and implementation of automated synthesis modules, high level preparations of fluorine-18 compounds are becoming increasingly common. Therefore, the problem of radiolytic decomposition of fluorine-18 labeled radiopharmaceuticals needs to be recognized and addressed as part of the overall radiopharmaceutical development program.
2. MATERIALS AND METHODS
2.1 General Considerations
Fluorine-18 ([18F]fluoride in H2[18O]O) and carbon-11 ([11C]CO2 in nitrogen gas) radionuclides were generated using a General Electric Medical Systems (GEMS) PETtrace cyclotron. Fluorine-18 labeled radiopharmaceuticals were prepared in a GEMS TRACERlab FX F-N synthesis module and carbon-11 tracers were synthesized using a GEMS TRACERlab FX C-Pro synthesis module or Bioscan Loop synthesis system. N,N-Dimethylaniline, N-methylaniline, 4-aminophenol and N-tert-butyl-α-phenylnitrone (PBN) were purchased from Sigma-Aldrich and used as received. Sodium ascorbate (Cenolate® Ascorbic Acid (as sodium ascorbate) Injection, USP, 500 mg/mL) was purchased from Hospira Inc. and used as received. Ethanol (sterile dehydrated alcohol injection, USP) was supplied by American Regent Inc. HPLC analysis of radiochemical purity was conducted using a Shimadzu LC-2010AHT Liquid Chromatograph fitted with UV and Bioscan γ-detectors. HPLC peaks corresponding to products were identified by co-injection with unlabeled reference standards. Gas chromatography (GC) analysis used to determine residual levels of organic solvents was performed on a Shimadzu GC-2010 Gas Chromatograph.
2.2 Typical Fluorine-18 Radiolabeling Procedure
[18F]Fluoride in [18O]H2O (37 – 74 GBq) was delivered to the TRACERlab FX F-N synthesis module and collected on a QMA-light Sep-Pak. The [18F]fluoride was then eluted from the QMA cartridge using aqueous potassium carbonate (3.5 mg in 0.5 mL) and transferred into the reactor vessel. Kryptofix-2.2.2 in acetonitrile (15 mg in 1 mL) was added and the water-acetonitrile azeotrope was evaporated (60 °C for 7 min under vacuum with argon stream followed by 120 °C for 5 min under vacuum). Precursors (1 – 1.5 mg) in anhydrous dimethylsulfoxide (DMSO, 1 mL) were added and the reaction was heated to 120°C for 10 min. Following labeling, the reactor was cooled to 50°C and the reaction mixture was diluted with HPLC solvent (3 mL). The crude mixture was passed through an alumina-light Sep-Pak and purified by semi-preparative HPLC. Those compounds purified using HPLC solvent systems suitable for injection (e.g., ethanol and water) were diluted with saline and transferred into a sterile 10 mL dose vial through a Millex 0.22 µm sterile filter.
For those compounds purified using solvent systems unsuitable for injection (e.g., acetonitrile-water), HPLC fractions containing desired products were collected, diluted with sterile water (50 mL), and the resulting solution was passed through a C18-light Sep-Pak. Radiopharmaceuticals remained bound to the Sep-Pak whilst residual HPLC solvent was washed away with further sterile water (10 mL). Products were then eluted with USP ethanol (0.5 mL) and diluted with 0.9% sterile saline (9.5 mL). The resulting isotonic solution was passed into a sterile 10 mL dose vial through a Millex 0.22 µm sterile filter.
2.3 Typical Carbon-11 Radiolabeling Procedure
[11C]CO2 (100 GBq) in a nitrogen stream was delivered to the TRACERlab FX C-Pro and trapped on molecular sieves. Heating (350 °C) under an atmosphere of hydrogen over a nickel catalyst reduced the [11C]CO2 to [11C]CH4 and subsequent reaction with iodine vapor at 720 °C provided [11C]CH3I. [11C]CH3I was then converted to [11C]CH3OTf by passing over silver triflate. Labeling reactions were carried out by bubbling methyl triflate through a solution of precursor either in the TRACERlab FX C-Pro reactor or in a Bioscan Autoloop methylation system. In both cases, purification by semi-preparative HPLC or Sep-Pak provided 11C-labeled radiopharmaceuticals as isotonic solutions suitable for injection that were released for QC analysis.
2.4 Modified Radiopharmaceutical Preparation Procedures Incorporating Anti-Oxidant Stabilizers
Radiopharmaceuticals subject to radiolytic decomposition were prepared following modified versions of the general procedures described above to allow for incorporation of anti-oxidant stabilizers into the manufacturing processes. HPLC solvent systems were prepared containing 0.5% w/v (5 g/L) sodium ascorbate (or ascorbic acid depending upon the pH of the buffer in question). HPLC fractions were simultaneously collected and diluted into aqueous sodium ascorbate (0.5% w/v, 0.25 g in 50 mL sterile water). The resulting solution was passed through a C18-light Sep-Pak that trapped the product and residual solvents were washed away with additional aqueous sodium ascorbate (50 mg in 10 mL sterile water). Products were eluted with ethanol (USP, 0.5 mL) and diluted with sodium ascorbate (USP, 500 mg/mL, 0.1 mL) in 0.9% saline (USP, 9.4 mL). This provided formulations (5% v/v ethanol in saline containing 0.5% w/v sodium ascorbate) suitable for injection that were transferred into sterile 10 mL dose vials through Millex 0.22 µm sterile filters and released for QC analysis.
2.5 Quality Control
All radiopharmaceuticals prepared for human use undergo extensive quality control according to the USP guidelines before they are released for use. Formulations are analyzed for chemical and radiochemical purity (HPLC); specific activity (HPLC); pyrogenicity (Charles Rivers); residual Kryptofix-2.2.2 (TLC); residual organic solvents (GC); formulation pH; radionuclidic half-life; and sterile filter integrity (bubble-point test). Every batch must pass all of these tests before release to the clinic is approved.
2.6. Determination of rates of radiolytic decomposition
The radiolytic decomposition of fluorine-18 and carbon-11 labeled products was monitored as a function of radiochemical purity through repeated injections of formulated products on the analytical HPLC. Decomposition of unlabeled standard compounds placed either next to or into a vial containing high concentrations of [18F]fluoride ion was monitored by HPLC coupled with UV detection of the mass peak.
3. RESULTS AND DISCUSSION
3.1 Potential PET Ligands for Imaging Alzheimer’s Disease Pathophysiology
We have been involved in two research projects investigating new tracers to image AD pathophysiology. The disease is characterized by the deposition of β-amyloid in plaques and formation of neurofibrillary tangles resulting from the abnormal aggregation of tau protein [La Ferla et al., 2007; Pallas and Camins, 2006]. The ability to determine levels of plaques and tangles early enough to allow effective diagnosis and treatment represents a major goal in AD therapy and radionuclide imaging techniques may play a key role in meeting this need [Nordberg, 2004; Cohen, 2007; Cai et al., 2007;].
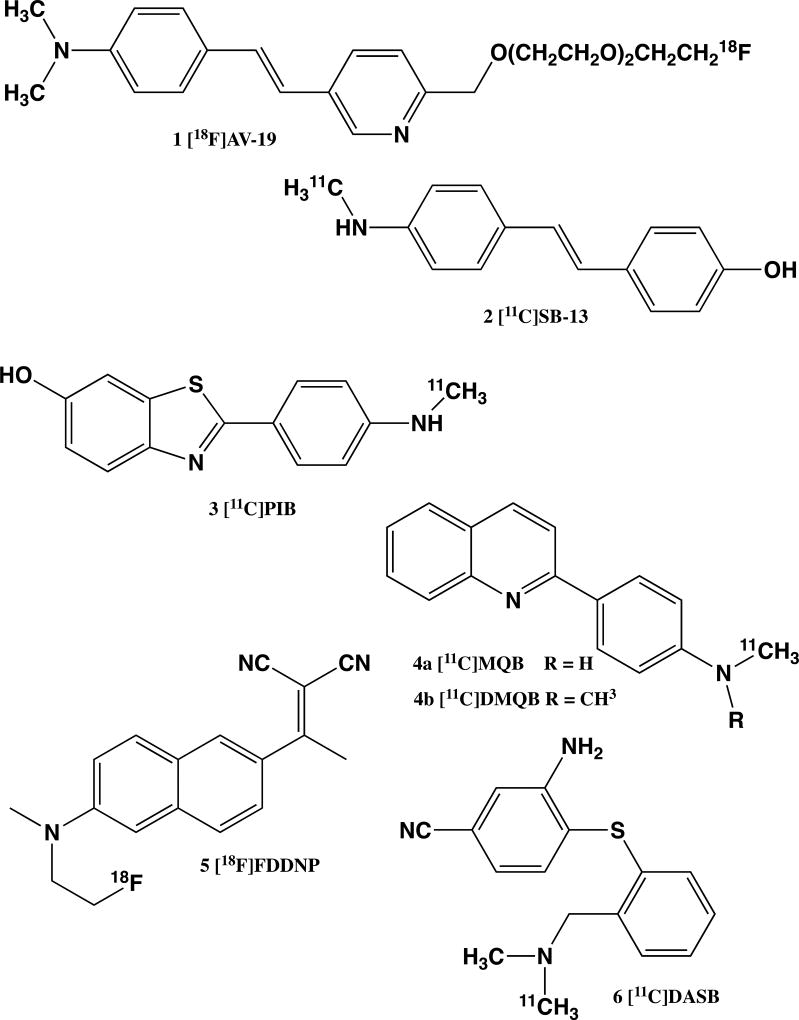
Currently, 2-(4-([11C]methylamino)phenyl)benzo[d]thiazol-6-ol ([11C]PIB, 3, Fig. 1) is the most commonly employed radioligand for imaging amyloid pathology [Mathis et al., 2007]. However, due to the short 20 min half-life of carbon-11, there is significant interest in developing fluorine-18 labeled amyloid imaging agents: the 110 min half-life of fluorine-18 is advantageous because it allows for multiple patients to be scanned from a single synthetic preparation and provides for possible distribution of radiopharmaceuticals to PET imaging centers that do not possess cyclotrons or the synthetic radiochemistry facilities. Numerous structures of potential fluorine-containing amyloid binding agents have now been reported, among them 2-(1-(6-((2-[18F]fluoroethyl)(methyl)amino)naphthalen-2-yl)ethylidene)malononitrile ([18F]FDDNP, 5) [Liu et al., 2007; Small et al., 2006], stilbenes related to SB-13 ((E)-4-(4-(dimethylamino)styryl)phenol 2) [Ono et al., 2003], and styrylpyridines (e.g., (E)-4-(2-(6-(2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N,N-dimethylbenzenamine [Zhang et al., 2007], ([18F]AV-19, 1, Fig. 1). Our interests were in the scale-up of the preparation of this last compound, [18F]AV-19, to allow initial clinical trials.
Figure 1.
Structures of representative N-methyl substituted anilines used as PET radiopharmaceuticals.
The second project targets neurofibrillary tangles, resulting from abnormal aggregation of tau protein, using substituted quinolines (e.g., N-[11C]methyl-4-(quinolin-2-yl)benzenamine (MQB, 4a) and N,N-[11C]dimethyl-4-(quinolin-2-yl)benzenamine (DMQB, 4b), Fig. 1) [Okamura et al., 2005; Kudo et al., 2004]. A common structural element in all of the radiopharmaceuticals illustrated in Figure 1, and seen in many drug candidates, is the presence of N-methylamine and N,N-dimethylamine substituents on the aryl rings. N-Methylamine groups are hydrogen bond donors that are able to coordinate to active sites and also allow for metabolism and subsequent elimination of drugs by, for example, cytochrome P450 mediated oxidative N-demethylation [Sheweita S A, 2000; Uehleke, 1973; Hlavica, 2002] or amine-oxidase/monoamine oxidase driven N-oxidation [Tipton et al., 2004; Edmondson et al., 2004]. However, despite the benefits of incorporating amines into potential tracer molecules, it quickly became apparent during lead development studies for both the amyloid and tau imaging projects that many potential tracers containing methylaniline components are prone to radiolytic decomposition.
3.2 Radiolytic Decomposition of Amino-substituted Molecules
[18F]AV-19 1 has been evaluated as an imaging agent for quantifying levels of amyloid plaques in the brains of AD patients [Skovronsky et al, 2008]. During initial development of a production method for [18F]AV-19 using a TRACERlab FX F-N, trial labeling studies were attempted by reacting a tosylated precursor in DMSO with 11 GBq of [18F]fluoride. A method was developed which routinely provided 1.5 GBq of [18F]AV-19 in >85% radiochemical purity at end-of-synthesis (Table 1, Entry 1, n = 3). However, the product as formulated in a standard 5% ethanol : saline formulation was unstable and found to decompose into 4 polar radioactive species (having lower retention times in the radiochemical HPLC traces) such that after 20 min radiochemical purity had decreased to 73%. This decomposition was attributed to radiolysis as the corresponding formulations of unlabeled reference standard are known to be chemically stable for extended times and at elevated temperatures. Unfortunately, and in agreement with previous reports [Fukumura et al., 2004, MacGregor et al., 1987], the rate of radiolytic decomposition greatly increased when the synthesis was scaled up to provide high specific concentration formulations suitable for imaging multiple patients from a single dose. Full-scale production using 66.6 GBq of [18F]fluoride gave the expected increase in yield of [18F]AV-19 (7.4 GBq) but there was a dramatic decrease in radiochemical purity (72% at EOS). Repeated analysis of the product by HPLC after end-of-synthesis revealed that rapid radiolytic decomposition was occurring to the extent that after 20 min, radiochemical purity had decreased to 37% and radiolabeled decomposition products were the major components of the formulation (Table 1, Entry 2). As per previous reports into radiolytic decomposition of radiopharmaceuticals [Suzuki et al., 1990; Bogni et al., 2003; Fukumura et al., 2003, 2004, 2004; Fawdry, 2007; MacGregor et al., 1987], this decomposition was thought to be due to transient reactive species such as hydroxyl radicals generated by radiolysis of water or hydrated electrons. In further experiments intended to elucidate the mechanism of decomposition, a vial containing a solution of [19F]AV-19 standard was placed in close proximity (1 cm away) to the TRACERlab FX F-N target vial containing 66.6 GBq of fluoride in an aqueous solution. Under these conditions [19F]AV-19 was found to be very stable and underwent none of the expected decomposition (Table 1, Entry 3). This confirmed that decomposition and/or the free-radicals suspected of causing radiolysis were not the result of the high concentration of gamma rays. Rather, for radiolysis to occur the radioactivity had to be in the same vial as the product and this was suggestive of decomposition possibly involving short-range positrons. Since the range of a fluorine-18 positron in water is 2.4 mm and they are unable to penetrate glass [Fowler and Wolf, 1982], decomposition does not occur when radioactivity is isolated in the target vial and separate from the solution of [19F]AV-19. As expected, when the [19F]AV-19 standard was exposed to 66.6 GBq of [18F]fluoride in solution, rapid decomposition occurred (Table 1, Entry 4).
Table 1.
Time-dependent stabilities of selected PET radioligands. All radiochemical products formulated in 8 ml of 5% ethanol in isotonic saline.
| Entry | Compound | Conditions | Puritya | ||
|---|---|---|---|---|---|
| 0 | 10 min | 20 min | |||
| 1 | [18F]AV-19 (1) | 1.48 GBq dose | 85% | 77% | 73% |
| 2 | [18F]AV-19 (1) | 7.4 GBq dose | 72% | 55% | 37% |
| 3 | [19F]AV-19 (1) | 1 cm from vial with 66.6 GBq of [18F]fluoride | 100% | 100% | 100% |
| 4 | [19F]AV-19 (1) | In vial with 66.6 Gbq [18F]fluoride | 100% | 73% | - |
| 5 | [11C]PIB (3) | 2.5 GBq dose; | 99% | 99% | 99% |
| 6 | [12C]PIB (3) | 1 cm from vial with 66.6 GBq of [18F]fluoride | 100% | 100% | 100% |
| 7 | [12C]PIB (3) | In vial via with 66.6 GBq [18F]fluoride | 100% | 100% | 100% |
| 8 | [11C]MQB (4a) | 1.73 GBq dose | 95% | 70% | - |
| 9 | [11C]DMQB (4b) | 1.85 GBq dose | 58% | 21% | 5% |
| 10 | [12C]DMQB (4b) | In vial with 11.1 Gbq [18F]fluoride | 60% | 22% | 0%b |
| 11 | [11C]DASB (6) | 1.85 GBq dose | 90% | - | - |
Radiochemical purity is reported for labeled compounds and UV purity for unlabeled standards
Purity recorded after 1 h to assess long term stability
It is noteworthy that in contrast to the above findings and despite similarities in structure, [11C]PIB (3) was very stable in high-specific activity formulations prepared from 37 GBq of methyl triflate using a GE TRACERlab FX C-Pro and Bioscan Autoloop (Table 1, Entry 5). Similarly, when placed into or next to a vial containing 66.6 GBq of [18F]fluoride, no decomposition occurred (Table 1, Entries 6 and 7). Presumably, the thiazole linkage provided formulated [11C]PIB with enhanced stability when compared to the alkene linkage found in AV-19.
Concomitantly with these studies, we have also investigated the possibility of radiolabeling quinolines (4) according to a literature procedure and using them to image tau pathology in AD. Labeling of these compounds was carried out with solutions of the corresponding aniline or N-methylaniline and carbon-11 methyl triflate (~37 GBq) in methylethylketone (MEK) to provide N-methyl- (MQB, 4a) and N,N-dimethyl-labeled (DMQB, 4b) tracers respectively. We were surprised during this work to observe similar radiolytic decomposition to that described above for the [18F]styrylpyridines. [11C]MQB (4a) was 95% radiochemically pure at EOS but this dropped off to 70% after 10 min (Table 1, Entry 8). Similar to that found earlier with the fluorine-18 labeled compounds, the rate of radiolysis was faster for the N,N-dimethylamine (DMQB, 4b) than for the N-methylamine (MQB, 4a). The dimethyl derivative [11C]DMQB (4b) was only 58% pure at EOS and by 20 min post-EOS the radiochemical purity had dropped to 5% (Table 1, Entry 9). Similar results were obtained when unlabeled standards of these compounds were exposed in solution to high levels of [18F]fluoride (Table 1, Entry 10). An additional drawback is that solutions of the corresponding unlabelled MQB and DMQB standards are known to be chemically unstable, however the process is noticeably slower (weeks to months) and is not responsible for the rapid decomposition of the labeled species.
3.3 Radiolysis of Simple Anilines
The results with the amyloid and tau radioligands raised the question of whether or not anilines in general are particularly prone to radiolytic decomposition. As one potential reaction of anilines is the formation of the N-oxide species, that might be expected to occur with most if not all anilines. Therefore three simple anilines (N,N-dimethylaniline, N-methylaniline and 4-aminophenol) were exposed to high levels of fluorine-18 to test this hypothesis. There was no evidence of decomposition any of the three were combined with 66.6 GBq of fluoride-18 in aqueous solution, using UV-HPLC analysis after 2 h of exposure. At present it is unclear what intrinsic feature of substituted anilines may make them more susceptible to radiolysis and further investigation is ongoing.
3.4 Inhibition of Radiolysis
All of the potential radiopharmaceuticals described in this note have been successfully radiolabeled with carbon-11 or fluorine-18. However, to progress any of them into clinical studies required inhibition of radiolytic decomposition so that stable formulations meeting all of the high radiochemical purity and specific activity requirements could be prepared. Therefore we investigated a number of means of inhibiting radiolytic decomposition.
3.41 Dilution of Formulated Products
The radiolytic decomposition of tracers was thought to be caused by hydroxyl radicals resulting from the reaction of high energy positrons with the aqueous medium. Since the range of a fluorine-18 positron in water is 2.4 mm, it followed that radiolytic decomposition should be slowed down simply by diluting the radiopharmaceutical formulations. Therefore, [18F]AV-19 (7.4 GBq) was prepared as described above and formulated in 0.5 mL ethanol and 9.5 mL saline. Five mL of this formulation was then removed and diluted with additional saline (5 mL) and the rate of radiolysis of the parent dose vs. the diluted dose were compared (Table 2). Radiolysis occurred in both cases, but decomposition was noticeably slower in the diluted formulation, despite the lower ethanol concentration. Whilst additional dilution might further slow radiolysis occurring during short-term storage of a fluorine-18 preparation, large injection volumes would begin to be required to obtain levels of injected activity necessary for successful PET imaging. Furthermore, the initial 60% radiochemical purity at formulation demonstrated the difficulties in preparation of this radiochemical without using some means to prevent the decomposition during the synthesis and formulation steps.
Table 2.
Test of dilution as a method for inhibiting radiolysis of a fluorine-18 labeled N,N-dimethylaniline.
| Compound | Formulation | Radiochemical Purity | ||||
|---|---|---|---|---|---|---|
| EOS | 10 min | 20 min | 3 h | |||
| 1 | [18F]AV-19 (1) | 3.7 GBq in 5% EtOH/Saline (5 mL) | 60% | 48% | 21% | 5% |
| 2 | [18F]AV-19 (1) | 3.7 GBq in 2.5% EtOH/Saline (10 mL) | 60% | 62% | 60% | 52% |
3.4.2 Addition of Anti-Oxidant Stabilizers
Since dilution was capable of slowing down radiolysis but not completely inhibiting it, incorporation of additional stabilizers into radiopharmaceutical formulations was considered (Table 3). Working on our hypothesis that radiolysis was attributable to reactive oxygen species, such as transient free-radicals, addition of anti-oxidant stabilizers was considered. Ethanol is noted for its anti-oxidant properties and ability to scavange hydroxyl radicals in aqueous solutions. Formulating [18F]AV-19 in a solution of 50% ethanol : 50% saline or 100% ethanol was found to completely inhibit radiolytic decomposition of the formulated product (Table 3, Entries 1 and 2), although it should be noted that the product was isolated in poor radiochemical purity after HPLC purification (a method for solving this problem is discussed later). These results using ethanol as inhibitor agree with Fukumura’s results with carbon-11 labelled species [Fukumura et al., 2003, 2004, 2004] and studies of the radiolytic decomposition of [18F]FDDNP reported in the literature [Liu et al., 2007; Small et al., 2006; Klok et al., 2007]. [18F]FDDNP contains a mono-methylaniline and is stable in neat ethanol but undergoes rapid radiolytic decomposition when the ethanol is diluted with water. High concentrations of ethanol do not represent a practical solution to the problem of radiolysis as 5–10% is the maximum amount of ethanol permitted in isotonic solutions formulated for injection. As formulating in 5% ethanol was not sufficient to prevent radiolysis in formulated products and also could not be employed to prevent radiolytic decomposition occurring during synthesis, alternative anti-oxidants were considered. A range of compounds are known to inhibit decomposition attributable to free radicals including ascorbic acid [Chen et al., 2005; Elmore, 2005; Liu et al., 2003; Werner et al., 1990], potassium iodide [Suzuki et al., 1990], nitrones [Reybier et al., 2006; Green et al., 2003] and thiourea [Halliwell and Gutteridge, 2005;]. Thiourea is highly toxic and unsuitable for clinical formulations and so we focused our efforts on ascorbic acid and nitrones as non-toxic anti-oxidants amenable for human use.
Table 3.
Applications of antioxidants (EtOH, PBN and sodium ascorbate) to inhibit radiolytic decomposition of PET radiopharmaceuticals containing N-methylaniline structures.
| Compound | Formulation | Purity | Long- term Stability |
|||
|---|---|---|---|---|---|---|
| EOS | 10 min | 20 min | ||||
| 1 | [18F]AV-19 (1) | 50 : 50 EtOH Saline | 60% | 62% | 64% | 66% (3 h) |
| 2 | [18F]AV-19 (1) | 100% EtOH | 66% | 66% | 66% | - |
| 3 | [18F]AV-19 (1) | 5% EtOH in Saline + 0.5% w/v PBN | 92% | 93% | 93% | 93% (1 h) |
| 4 | [18F]AV-19 (1) | 5% EtOH in Saline + 0.5% w/v Na-ascorbate | 96% | 96% | 96% | 100% (6 h) |
| 5 | [12C]DMQB (4b) | 0.3 Ci Fluoride added to 5% EtOH in Saline + 0.5% w/v Na-ascorbate | 100% | 100% | 100% | 100% (1h) |
| 6 | [11C]DASB (6) | 5% EtOH in Saline + 0.2% w/v Na-ascorbate | 99% | 99% | 99% | 99% (1 h) |
N-tert-Butyl-α-phenylnitrone (PBN) is a nitrone widely used as a means of trapping and detecting free-radicals in both chemistry and biology [Halliwell and Gutteridge, 2005;], particularly finding widespread application in electron spin resonance (ESR) spectroscopy. The ability of PBN to trap reactive oxygen species in vivo has also led to extensive investigation as a neuroprotective agent [Kotake, 1999; Kim et al., 2007]. We have also previously worked with PBN and radiolabeled analogs as a means of quantifying levels of free-radicals in vivo [Bormans and Kilbourn, 1995]. We were therefore interested in employing PBN to prevent radiolytic decomposition by trapping free radicals potentially formed in high specific concentration radiopharmaceutical formulations. [18F]AV-19 (1) was prepared as outlined above but the procedure was modified so that the semi-preparative HPLC buffer contained PBN (0.1% w/v) and the final product was formulated in 5% ethanol in saline containing 0.5% w/v PBN. This provided a high specific concentration formulation of [18F]AV-19 which had a radiochemical purity >93%. Incorporation of PBN inhibited radiolytic decomposition during synthesis and the high radiochemical purity of the formulation remained unchanged for 1 h after end-of-synthesis (Table 3, Entry 3). It thus appears that the inclusion of a nitrone effectively inhibits radiolytic decomposition; application to human studies is feasible as nitrones have been investigated in clinical studies as neuroprotective agents in cerebral ischemia [Green et al., 2003]. This is a preliminary proof-of-concept study and further investigations to determine the minimum effective level of PBN required to inhibit radiolytic decomposition are ongoing and will be reported in due course.
Ascorbic acid is also a well known anti-oxidant that a number of groups have employed to inhibit radiolytic decomposition [Fukumura et al., 2004; Chen et al., 2005; Elmore, 2005; Liu et al., 2003; Werner et al., 1990]. For example, Klok et al. used it to overcome stability problems associated with the preparation of [18F]FDDNP [Klok et al., 2007]. One disadvantage is that addition of ascorbic acid is known to make final formulations very acidic (pH ~ 2) and outside of physiological pH, making them unsuitable for human use without additional buffering. However, we have found that sodium ascorbate (SA) is a suitable alternative. Sodium ascorbate has the same excellent anti-oxidant properties as the free acid, but its addition to clinical formulations has negligible affect on pH. However, a drawback of adding sodium ascorbate to [18F]-labeled radiopharmaceutical formulations is that it complicates analysis of residual Kryptofix-2.2.2 in formulations during quality control. Kryptofix-2.2.2 is a crown-ether phase transfer reagent used to enhance nucleophilic substitution reactions using [18F]fluoride. Due to its toxicity, Kryptofix must be removed after synthesis and levels in clinical formulations must be quantified during quality control. Typically this is achieved using the classical iodoplatinate TLC spot-test [Mock et al., 1997]. However, if formulations containing sodium ascorbate are analyzed using this spot-test then bleaching of the iodoplatinate TLC strips occurs and renders the test subject to false negative results. To overcome this problem, we have recently developed an alternative TLC method that allows for the analysis of residual Kryptofix in the presence of sodium ascorbate [Scott and Kilbourn, 2007].
The full-scale synthesis of [18F]AV-19 (1) was repeated after incorporation of 0.5% w/v sodium ascorbate into the semi-preparative HPLC buffer (5 g/L), reformulation flask (250 mg/50 mL water) and final formulation (50 mg/10mL). The result was a high-specific concentration formulation of [18F]AV-19 (1) with excellent radiochemical purity (>95%,n = 20) that was suitable for injection (typical formulation pH = 6.5). As with PBN, sodium ascorbate also inhibited radiolysis during and after synthesis. The formulation was stable up to 6 h post end-of-synthesis and there was no decrease in radiochemical purity as analyzed by HPLC (Table 3, Entry 4). Similarly, carbon-11 labeled quinoline compounds (MQB, 4a and DMQB, 4b) were stable when formulated with sodium ascorbate in a similar fashion (Table 3, Entry 5).
The representative examples provided here show the worst-case scenario of radiolytic decomposition since without addition of anti-oxidant stabilizers, the tracers are unusable. However, given that sodium ascorbate is very efficient at inhibiting radiolysis, we re-visited formulation of other aniline containing radiopharmaceuticals that we routinely prepare and consider to be stable. [11C]DASB (3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)benzonitrile 6) is known to undergo very slow radiolysis and, not surprisingly given our previous findings, contains an aniline component. Slow radiolysis of a primary aniline is in keeping with the apparent trend that the rate of radiolysis increases with substitution at the aniline: ArNMe2 > ArNHMe > ArNH2. Seemingly, the more electron rich the aniline nitrogen, the more susceptible the compound is to radiolysis. Whilst formulation without ascorbate does not give an unusable product (radiochemical purity = 90%), addition of 0.4% w/v sodium ascorbate to the isolation and formulation steps increases radiochemical purity of the formulated product to 99% and the product is stable for at least three half-lives of the carbon-11 radionuclide with no evidence of any decomposition (Table 3, Entry 6).
4. CONCLUSIONS
In conclusion we have shown that a number of potential new PET ligands as well as some established compounds in human clinical trials are prone to radiolytic decomposition in the presence of high concentrations of radionuclide. Whilst these compounds are diverse in structure as shown in Figure 1, the common feature shared by all of them is an aniline functionality. The rate of radiolysis appears to be ArNMe2 > ArNHMe > ArNH2. To date the mechanism of radiolysis is not obvious, as relatively simple anilines and [11C]PIB appear stable, and further investigation of what ring substituents enhance radiolytic susceptibility is required. Nevertheless, this effect should be taken into account when designing novel PET ligands, particularly when aniline components are being considered. We speculate that high energy positrons result in the production of reactive oxygen species (for example, hydroxyl radicals or hydrogen peroxide) which have a detrimental effect upon formulated radiopharmaceuticals. As radiolytic decomposition can occur even during the isolation and formulation steps of a synthesis, promising radiotracers might be unnecessarily abandoned. This study demonstrates that radiolysis is a problem that can be easily overcome. Established none-toxic anti-oxidants and free-radical scavengers such as ethanol and sodium ascorbate completely inhibit radiolytic decomposition and provide solutions suitable for injection that are stable for at least several half-lives of the carbon-11 and fluorine-18 radionuclides (and consequently the lifetime of the radiopharmaceutical). We have also demonstrated for the first time that nitrones represent viable stabilizers for the inhibition of radiolysis. Of particular note in this study is that radiolysis can occur during the preparation and purification of a radiochemical, and an anti-oxidant stabilizer such as sodium ascorbate or nitrone can be utilized during these steps to provide a radiochemically pure and stable product for subsequent formulation.
Acknowledgments
Financial support of this work by the National Institute of Health (NIH Grant NS15655) is gratefully acknowledged.
References
- Bayly RJ, Evans EA. Stability and storage of compounds labeled with radioisotopes. J Labelled Compd. 1966;2:1–34. [Google Scholar]
- Bogni A, Bombardieri E, Iwata R, Cadini L, Pascali C. Stability of L-[S-methyl-11C]methionine molutions. J. Radioanal. Nucl. Chem. 2003;256:199–203. [Google Scholar]
- Bormans G, Kilbourn MR. Synthesis of N-tert-butyl-α-(4-[18F]fluorophenyl)-nitrone ([18F]FPBN) for in vivo detection of free radicals. J Labelled Compd. Radiopharm. 1995;36:103–110. [Google Scholar]
- Cai L, Innis RB, Pike VW. Radioligand development for PET imaging of β-amyloid (Ab)-current status. Curr. Med. Chem. 2007;14:19–52. doi: 10.2174/092986707779313471. [DOI] [PubMed] [Google Scholar]
- Chen J, Linder KE, Marinelli ER, Metcalfe E, Nunn A, Swenson RE, Tweedle M. Stable radiopharmaceutical compositions and methods for their preparations. PCT Int. Appl. WO2005009393. 2005:117. [Google Scholar]
- Cohen RM. The application of positron-emitting molecular imaging tracers in Alzheimer's disease. Mol. Imaging Biol. 2007;9:204–216. doi: 10.1007/s11307-007-0094-3. [DOI] [PubMed] [Google Scholar]
- Edmondson DE, Mattevi A, Binda C, Li M, Hubalek F. Structure and mechanism of monoamine oxidase. Curr. Med. Chem. 2004;11:1983–1993. doi: 10.2174/0929867043364784. [DOI] [PubMed] [Google Scholar]
- Elmore AR. Final report of the safety assessment of L-ascorbic Acid, calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate as used in cosmetics. Int. J. Toxicol. 2005;24(Suppl 2):51–111. doi: 10.1080/10915810590953851. [DOI] [PubMed] [Google Scholar]
- Fawdry RM. Radiolysis of 2-[18F]fluoro-2-deoxy-D-glucose (FDG) and the role of reductant stabilizers. Appl. Radiat. Isot. 2007;65:1193–1201. doi: 10.1016/j.apradiso.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Wolf AP. The synthesis of carbon-11, fluorine-18, and nitrogen-13 labeled radiotracers for biomedical applications. Technical Information Center, U.S. Department of Energy. 1982:124. [Google Scholar]
- Fukumura T, Akaike S, Yoshida Y, Suzuki K. Decomposition of an aqueous solution of [11C]Ro 15-4513: implication of hydrated electrons in the radiolysis of [11C]Ro 15-4513. Nucl. Med. Biol. 2003;30:389–395. doi: 10.1016/s0969-8051(03)00018-0. [DOI] [PubMed] [Google Scholar]
- Fukumura T, Nakao R, Yamaguchi M, Suzuki K. Stability of 11C-labeled PET radiopharmaceuticals. Appl. Rad. Isot. 2004;61:1279–1287. doi: 10.1016/j.apradiso.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Fukumura T, Yamaguchi M, Suzuki K. Radiolysis of [11C]iomazenil solution. Radiochim. Acta. 2004;92:119–123. [Google Scholar]
- Green AR, Ashwood T, Odergren T, Jackson DM. Nitrones as neuroprotective agents in cerebral ischemia, with particular reference to NXY-059. Pharmacology and Therapeutics. 2003;100:195–214. doi: 10.1016/j.pharmthera.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3. Oxford University Press; 2005. [Google Scholar]
- Hlavica P. N- oxidative transformation of free and N-substituted amine functions by cytochrome P450 as means of bioactivation and detoxication. Drug Metabolism Reviews. 2002;34:451–477. doi: 10.1081/dmr-120005646. [DOI] [PubMed] [Google Scholar]
- Kim S, de A Vilela GVM, Bouajila J, Dias AG, Cyrino FZGA, Bouskela E, Costa PRR, Nepveu F. α-Phenyl-N-tert-butyl nitrone (PBN) derivatives: Synthesis and protective action against microvascular damages induced by ischemia/reperfusion. Bioorg. Med. Chem. 2007;15:3572–3578. doi: 10.1016/j.bmc.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Klok RP, Klein PJ, van Berckel BNM, Tolboom N, Lammertsma AA, Windhorst AD. Synthesis of 2-(1,1-Dicyanopropen-2-yl)-6-(2-[18F]-fluoroethyl)-methylamino-naphthalene ([18F]FDDNP) Appl Rad Isotop. 2008;66:203–207. doi: 10.1016/j.apradiso.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Kotake Y. Pharmacologic properties of phenyl N-tert-butyl nitrone. Antiox. Redox. Signal. 1999;1:481–499. doi: 10.1089/ars.1999.1.4-481. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Suzuki M, Suemoto T, Okamura N, Shiomitsu T, Shimazu H. Quinoline derivative as diagnostic probe for disease with tau protein accumulation. PCT Int. Appl. WO 2004054978. 2004:47. [Google Scholar]
- La Ferla FM, Green KN. Oddo Salvatore, Intracellular amyloid-beta in Alzheimer's disease. Nature Reviews Neuroscience. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Liu S, Ellars CE, Edwards DS. Ascorbic acid: useful as a buffer agent and radiolytic stabilizer for metalloradiopharmaceuticals. Bioconjugate Chem. 2003;14:1052–1056. doi: 10.1021/bc034109i. [DOI] [PubMed] [Google Scholar]
- Liu J, Kepe V, Žabjek A, Petrič A, Padgett HC, Satyamurthy N, Barrio JR. High-yield, automated radiosynthesis of 2-(1-{6-[(2-[18F]Fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile ([18F]FDDNP) ready for animal or human administration. Mol. Imaging Biol. 2007;9:6–16. doi: 10.1007/s11307-006-0061-4. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Schlyer DJ, Fowler JS, Wolf AP, Shiue CY. Fluorine-18-N-methylspiroperidol:radiolytic decomposition as a consequence of high specific activity and high dose levels. J. Nucl. Med. 1987;28:60–67. [PubMed] [Google Scholar]
- Mathis CA, Lopresti BJ, Klunk WE. Impact of amyloid imaging on drug development in Alzheimer's disease. Nucl. Med. Biol. 2007;34:809–822. doi: 10.1016/j.nucmedbio.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock MB, Winkle W, Vavrek MT. A color spot test for the detection of Kryptofix 2.2.2 in [18F]FDG preparations. Nucl. Med. Biol. 1997;24:193–195. doi: 10.1016/s0969-8051(96)00212-0. [DOI] [PubMed] [Google Scholar]
- Nordberg A. PET imaging of amyloid in Alzheimer's disease. The Lancet Neurology. 2004;3:519–527. doi: 10.1016/S1474-4422(04)00853-1. [DOI] [PubMed] [Google Scholar]
- Okamura N, Suemoto T, Furumoto S, Suzuki M, Shimadzu H, Akatsu H, Yamamoto T, Fujiwara H, Nemoto M, Maruyama M, Arai H, Yanai K, Sawada T, Kudo Y. Quinoline and benzimidazole derivatives: Candidate probes for in vivo imaging of tau pathology in Alzheimer's disease. J. Neurosci. 2005;25:10857–10862. doi: 10.1523/JNEUROSCI.1738-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Wilson A, Nobrega J, Westaway D, Verhoeff P, Zhuang ZP, Kung MP, Kung HF. 11C-labeled stilbene derivatives as Ab-aggregate-specific PET imaging agents for Alzheimer’s disease. Nucl Med Biol. 2003;30:565–571. doi: 10.1016/s0969-8051(03)00049-0. [DOI] [PubMed] [Google Scholar]
- Pallas M, Camins A. Molecular and biochemical features in Alzheimer's disease. Curr. Pharm. Design. 2006;12:4389–408. doi: 10.2174/138161206778792967. [DOI] [PubMed] [Google Scholar]
- Reybier K, Boyer J, Farines V, Camus F, Souchard JP, Monje MC, Bernardes-Genisson V, Goldstein S, Nepveu F. Radical trapping properties of imidazolyl nitrones. Free Radical Research. 2006;40:11–20. doi: 10.1080/10715760500329598. [DOI] [PubMed] [Google Scholar]
- Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang SC, Satyamurthy N, Phelps ME, Barrio JR. PET of brain amyloid and tau in mild cognitive impairment. N. Engl. J. Med. 2006;355:2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- Scott PJH, Kilbourn MR. Determination of residual Kryptofix 2.2.2 levels in [18F]-labeled radiopharmaceuticals for human use. Appl. Rad. Isotop. 2007;65:1359–1362. doi: 10.1016/j.apradiso.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Skovronsky DM, Coleman R, Frey K, Garg P, Ichise M, Lowe V, Mintun M, Wong D, Kung HF. Results of multi-center trials comparing four 18F PET amyloid-imaging agents: Preclinical to clinical correlations. J Nuc Med. 49:34P. [Google Scholar]
- Sheweita SA. Drug- metabolizing enzymes: mechanisms and functions. Curr. Drug Metab. 2000;1:107–132. doi: 10.2174/1389200003339117. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Inoue O, Tamate K, Mikado F. Production of 3-N-[11C]methylspiperone with high specific activity and high radiochemical purity for PET studies: Suppression of its radiolysis. Appl. Radial. Isot. 1990;41:593–599. doi: 10.1016/0883-2889(90)90046-j. [DOI] [PubMed] [Google Scholar]
- Tipton KF, Boyce S, O'Sullivan J, Davey GP, Healy J. Monoamine oxidases : Certainties and uncertainties. Curr. Med. Chem. 2004;11:1965–1982. doi: 10.2174/0929867043364810. [DOI] [PubMed] [Google Scholar]
- Uehleke H. Role of cytochrome P-450 in the N - oxidation of individual amines. Drug Metab. Dispos. 1973;1:299–313. [PubMed] [Google Scholar]
- Werner IA, Altorfer H, Perlia X. The effectiveness of various scavengers on the g-irradiated, methanolic solution of medazepam. Int. J. Pharmaceutics. 1990;63:155–166. [Google Scholar]
- Zhang W, Kung MP, Oya S, Hou C, Kung HF. 18F-labeled styrylpyridines as PET agents for amyloid plaque imaging. Nucl. Med. Biol. 2007;34:89–97. doi: 10.1016/j.nucmedbio.2006.10.003. [DOI] [PubMed] [Google Scholar]