Abstract
Background
Bedroom allergen exposures contribute to allergic disease morbidity because people spend considerable time in bedrooms, having close contact with allergen reservoirs.
Objective
We investigated participant and housing characteristics, including sociodemographic, regional and climatic factors, associated with bedroom allergen exposures in a nationally representative sample of the US population.
Methods
Data were obtained from National Health and Nutrition Examination Survey (NHANES) 2005–2006. Information on participant and housing characteristics was collected by questionnaire and environmental assessments. Concentrations of 8 indoor allergens (Alt a 1, Bla g 1, Can f 1, Fel d 1, Der f 1, Der p 1, Mus m 1, and Rat n 1) in dust vacuumed from nearly 7,000 bedrooms were measured by immunoassays. Exposure levels were classified as elevated based on percentile (75th/90th) cut-offs. We estimated the burden of exposure to multiple allergens and used multivariable logistic regression to identify independent predictors for each allergen and household allergen burden.
Results
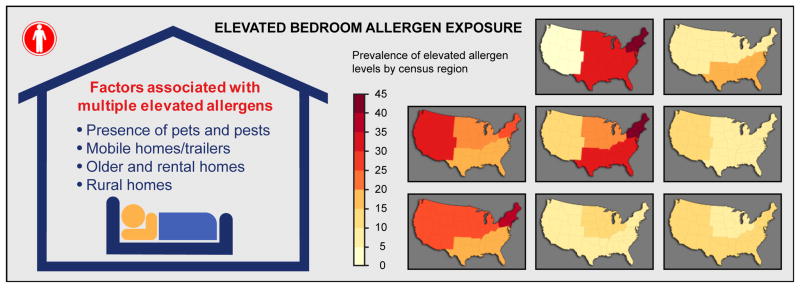
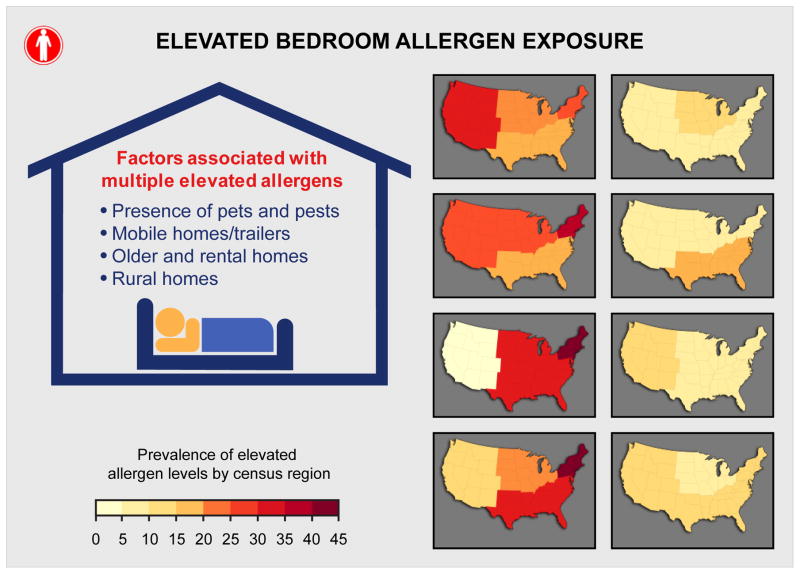
Almost all participants (>99%) had at least one and 74.2% had 3–6 allergens detected. Over 2/3 of participants (72.9%) had at least one allergen and 18.2% had ≥3 allergens exceeding elevated levels. Although exposure variability showed significant racial/ethnic and regional differences, high exposure burden to multiple allergens was most consistently associated with the presence of pets and pests, living in mobile homes/trailers, older and rental homes, and in non-metropolitan areas.
Conclusions
Exposure to multiple allergens is common. Despite highly variable exposures, bedroom allergen burden is strongly associated with the presence of pets and pests.
Clinical implications
In the general US population, exposure to indoor allergens varies by sociodemographic and housing characteristics; however, exposure patterns show sociodemographic differences when compared to sensitization patterns.
Keywords: allergen, indoor, exposure, home, allergy
INTRODUCTION
Indoor allergen exposures are important risk factors of allergic respiratory disease as people spend a large portion of their time indoors, especially at home.1–3 Although the relationship between allergen exposures and the development of allergic sensitization and disease is complex and not fully understood,1 indoor allergens can trigger and exacerbate asthma and allergy symptoms in sensitized individuals.1, 2, 4–8 Despite extensive research, most indoor allergen studies have focused on selected high-risk populations (e.g., asthmatics, children, and inner-city populations), single allergens, or both.2, 9–11 Recently released data from National Health and Nutrition Examination Survey (NHANES) provide the largest resource to date for assessment of indoor allergen exposures in a nationally representative sample of the United States (US) population,12 following the National Survey of Lead and Allergens in Housing (NSLAH), which was conducted on a much smaller scale in 1998–1999.13 NHANES 2005–2006 assessed residential indoor allergen levels in bedroom dust collected from nearly 7,000 US households.
Bedrooms are often considered an important site for allergen exposures, not only because of the time spent in bed but also due to the close proximity of allergen reservoirs (e.g., bedding) to a person’s breathing zone and associated reservoir disturbances.14 Recent studies demonstrate that a significant fraction of airborne particles, which are resuspended by human movements in bed, can be inhaled during sleep.15, 16 Although residential exposures may vary spatially and temporally, several studies link bedroom allergen exposures with allergic sensitization and disease morbidity,17 especially among those who are exposed to elevated levels of the allergen(s) to which they are sensitized.4–7
To advance our knowledge of the prevalence and determinants of indoor allergen exposures, we investigated the importance of different sociodemographic and housing factors in residential allergen exposures. This manuscript provides the most comprehensive report on bedroom allergen exposures in US households, estimating the exposure burden to individual and multiple allergens, as well as identifying independent predictors of these exposures. No study to date has provided such detailed information on how allergen levels vary by regional and climatic factors, as well as by level of urbanization. Since NHANES 2005–2006 is the first large population-based study to enable comparisons between exposure and sensitization patterns in the general US population, we also discuss sociodemographic similarities and differences between allergen exposures and the previously reported sensitization patterns.18
METHODS
Study data and design
Data were collected as part of NHANES 2005–2006, which employed a complex, multistage probability sampling design to select a sample of the civilian, non-institutionalized US population. The survey oversampled adolescents (aged 12–19 years), elderly people (aged 60 and older), African Americans, Mexican Americans, and low-income individuals, to ensure adequate samples for subgroup analyses. Information on participant and housing characteristics was obtained by questionnaires,19–21 as well as environmental assessments (e.g., room temperature and relative humidity were assessed with a digital hygro-thermometer).22 NHANES 2005–2006 included a component that assessed indoor allergen levels in reservoir dust samples collected from participants’ bedrooms. A detailed description of the study procedures is available in the NHANES Allergen Dust Collection Procedures Manual.22 Due to delays in the laboratory analysis phase of this component, dust data were not available until 2014. Our data analysis is limited to participants with dust data available (N=6,963). Table E1 in the Online Repository shows selected population characteristics of the NHANES 2005–2006 participants. To protect participant confidentiality, all data analysis using restricted, not publicly available variables (census region, level of urbanization, climate region, and presence of children in the household) was conducted at the National Center for Health Statistics (NCHS) Atlanta Research Data Center (RDC). All restricted variables were provided and/or created by the NCHS. The climate regions were determined based on the guidelines of the US Department of Energy Building America Program, which use heating degree-days, average temperatures, and precipitation to differentiate each region.23 To avoid data suppression due to small cell sizes, eight US climate regions were aggregated into four categories: subarctic/very cold/cold; mixed-humid/marine; hot-humid; and mixed-dry/hot-dry. The survey protocol was approved by the NCHS Ethics Review Board,24 and written informed consent was obtained from all participants. Information on the NHANES survey design and implementation can be found at http://www.cdc.gov/nchs/nhanes/survey_methods.htm.25
Exposure assessment
Participants aged 1 year and older were eligible for dust allergen testing. Dust samples were collected from each participant’s bed and bedroom floor using a Sanitare™ Model 3683 vacuum cleaner and a Mitest™ Dust Collector (Indoor Biotechnologies, Inc., Charlottesville, VA). For a combined sample, each sampling location (1 yd2) was vacuumed for 2 minutes, and allergen levels were assessed with immunoassays. Concentrations of dog (Can f 1), dust mite (Der f 1 and Der p 1), cat (Fel d 1), Alternaria alternata (Alt a 1), mouse (Mus m1), and rat (Rat n 1) allergens were assessed with the MARIA® Multiplex Array for Indoor Allergens (Charlottesville, VA), whereas concentrations of cockroach allergen (Bla g 1) were measured with ELISA.12 For allergens assessed with MARIA®, the lower limit of detection (LLOD) varied from 0.002 μg/g to 0.013 μg/g. The lowest LLOD for Bla g1 was 0.002 U/g. To maximize the number of samples in the analyses, fill values equal to the allergen-specific LLOD divided by the square root of 2 were used for samples below LLOD.12 Details of the laboratory methods and quality control procedures are published elsewhere.26, 27
Statistical analysis
To estimate exposure to elevated allergen levels and the burden to multiple allergens, allergen concentrations were dichotomized to high and low-to-medium levels. Since clinically relevant thresholds have not been established for all measured allergens, we used percentile cut-offs to ensure a consistent analytical approach across all allergens. For allergens that had relatively non-skewed distributions, allergen exposures were classified as elevated if the allergen concentration exceeded the 75th percentile (Can f 1: 8.472 μg/g; Fel d 1: 6.369 μg/g; Der f 1: 0.338 μg/g; Der p 1: 0.219 μg/g). For allergens with skewed distributions, a cut-off of the 90th percentile was used to differentiate exposure levels (Alt a 1: 0.009 μg/g; Bla g 1: 1.778 U/g; Mus m 1: 0.238 μg/g; Rat n 1: 0.013 μg/g). To evaluate the overall allergen burden, we created four separate outcomes that reflected different exposure levels. Exposure burden was classified high when 1) ≥ 3 allergens exceeded elevated levels (Multiple Elevated Exposures), or 2) ≥ 7 allergens were above detection limits and at least one allergen exceeded elevated levels (Multiple Detectable Exposures). Exposure burden was considered low to medium when none of the allergens exceeded elevated levels (No Elevated Exposures), and low when ≤ 2 allergens were detected and none of the allergens exceeded elevated levels (Low Exposure).
We performed logistic regression analyses to identify independent predictors for individual allergen exposures and for the four allergen burden related outcomes. First, all potential participant- and housing-related variables were evaluated using bivariate analyses. Of these variables [age; gender; race/ethnicity; poverty index ratio (PIR)21; census and climate region; level of urbanization; housing type; year home constructed; tenure; length of residency; number of household members; presence of pets (cats/dogs), cockroaches, mildew/musty smell, children, smokers or mattress cover; reported pet avoidance; type of floor covering; room temperature and relative humidity], we selected those with p-values less than 0.25 for inclusion in the regression analysis. We chose a data-driven modeling approach and used backward elimination for model selection; starting from the full model, variables with the highest p-value (Wald F) were dropped until all remaining predictors in each model had p-values less than or equal to 0.05. The climate and census region variables were interrelated, and thus were modeled separately. Because climate region was not an independent predictor for elevated allergen levels, except for Der f 1, complete modeling results are presented only for census region.
All descriptive statistics and predictive modeling were performed using SAS software (version 9.3; SAS Institute, Cary, NC). Cluster analysis was conducted with the R system for statistical computing (version 2.10.1). To obtain unbiased national prevalence and variance estimates, the sampling weights and design variables were applied to all analyses.25 Separate dust allergen examination subsample weights were used to account for selective non-response and any missing laboratory data.12
RESULTS
Distributions of allergen levels
Of the NHANES 2005–2006 participants with available dust data, 98.1% (N=6832) had data for each of the eight allergens. Figure 1 shows percentages of participants with detectable levels of allergens. Cat (Fel d 1, 93.0%), dog (Can f 1, 86.7%), and mouse (Mus m 1, 80.9%) were the most commonly detected allergens, followed by dust mite allergens (Der f 1, 58.2%; Der p 1, 48.9%). More detailed information on the distributional characteristics and clustering patterns is presented in the Online Repository (Table E2; Figure E1; Table E3).
Figure 1.
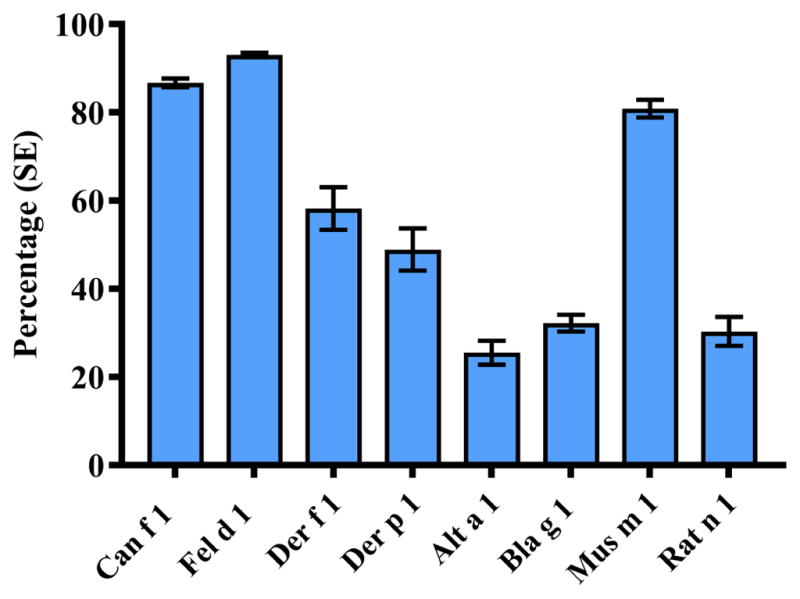
Prevalence (±SE) of exposure to detectable bedroom allergen levels in the US population.
Overall burden to multiple allergens
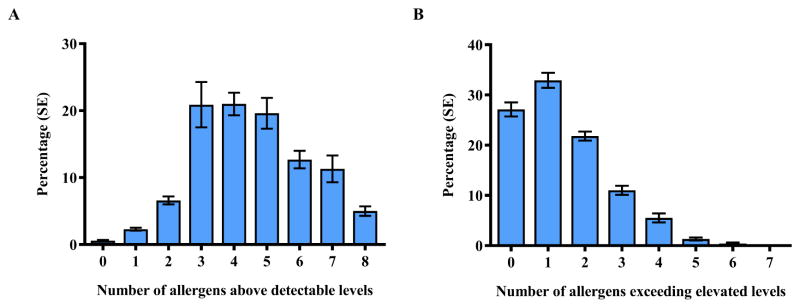
Exposure to multiple allergens was common. Detectable levels of allergens were present in almost all bedrooms (>99%), and over 90% of participants had at least 3 allergens detected (Figure 2A). Of the participants, 15.8% had 7 or more allergens detected and at least one allergen exceeding elevated levels. The majority of the participants were exposed to elevated allergen levels; more than 2/3 of participants (72.9%) had at least one allergen, and 18.2% had 3 or more allergens exceeding elevated levels (Figure 2B). Only 6.4% of the participants had a low allergen burden (≤ 2 allergens detected, no allergens exceeded elevated levels) in their bedrooms.
Figure 2.
Exposure to multiple allergens in bedrooms. Percentages (±SE) of NHANES 2005–2006 participants with detectable (A) and elevated (B) levels of allergens by numbers of allergens exceeding allergen-specific thresholds (LOD, 75th/90th percentile). Can f 1, Fel d 1, Der f 1, and Der p 1 levels were elevated if allergen concentration >75th percentile; Alt a 1, Bla g 1, Mus m 1 and Rat n 1 levels were elevated if allergen concentration > 90th percentile.
Individual allergen levels in relation to participant and housing characteristics
The bar graphs in Figure E2 (Online Repository) illustrate the prevalence of elevated allergen levels across categories of the variables of interest. Of the participant characteristics, race/ethnicity and family PIR were most consistently associated with elevated allergen levels. Non-Hispanic blacks had the lowest prevalence of elevated dog, cat, Alternaria allergens, whereas elevated cockroach allergen levels were least prevalent among non-Hispanic whites. Elevated pet allergen levels were detected most frequently in more affluent households, while elevated Der f 1 levels were most common in middle-income households and elevated levels of Der p 1, cockroach and mouse allergens were most prevalent in low-income homes. Allergen levels were also associated with participant’s age and the presence of children in the household. For example, elevated Der p 1 and mouse allergen levels were more common among children than adults, whereas elevated Der f 1 levels were least prevalent in households with young children. Females were more likely to have elevated levels of cat and Alternaria allergens in their bedrooms.
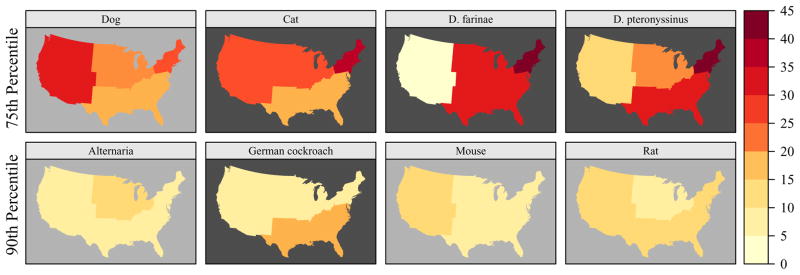
Allergen levels varied by region and climatic factors (Figure 3, Figure E3). Elevated cockroach allergen levels were most prevalent in hot-humid and mixed-humid/marine climates, particularly in the South. Dust mite allergen levels were strongly influenced by region and climate. While elevated dust mite allergen levels were most prevalent in the Northeastern region, climatic factors influenced allergen levels. Der p 1 levels were elevated most frequently in hot-humid areas, whereas elevated Der f 1 levels were most common in mixed-hot/marine climates. Pet and dust mite allergen levels differed significantly by level of urbanization (Figure E2). Elevated dog allergen levels were less frequently found in non-metropolitan homes, while the prevalence of elevated cat allergen levels was least common in large, central metropolitan areas. The prevalence of elevated dust mite allergen levels decreased with increasing level of urbanization.
Figure 3.
Prevalence of elevated allergen levels by census region. Can f 1, Fel d 1, Der f 1, and Der p 1 levels were elevated if allergen concentration >75th percentile; Alt a 1, Bla g 1, Mus m 1 and Rat n 1 levels were elevated if allergen concentration > 90th percentile. The dark background color in the map indicates statistically significant differences (P < 0.05) across US census regions (Northeast, Midwest, South, West).
Multi-family homes were less likely to have elevated allergen levels, except for mouse and cockroach allergens (Figure E2). For most allergens, the prevalence of elevated levels was highest in mobile homes. Elevated dust mite allergen levels were prevalent in older homes and the prevalence of elevated allergen levels tended to increase with increasing years of residency. Elevated pet allergen levels were more common in resident-owned homes and less crowded households, whereas elevated Der p 1, cockroach and mouse allergen levels were more prevalent in rental homes. The presence of pets and pests was strongly associated with elevated levels of Can f 1, Fel d 1 and Bla g 1. The presence of cockroaches, however, was also associated with elevated Der p 1 and rodent allergen levels. Elevated cat, rodent and dust mite allergen levels were less prevalent in households that reported pet avoidance measures due to participant’s allergies or asthma. Reported mildew and/or musty smell in the home was associated with elevated Der p 1 levels, whereas increased Der f 1 levels were strongly associated with the presence of carpeting. Only dust mite allergen levels were influenced by room temperature and relative humidity. Having an impermeable mattress cover was associated with a lower prevalence of elevated allergen levels, particularly for mouse allergen. The presence of smoker(s), which is often associated with lower socioeconomic status,9, 28 was considered as a potential predictor of elevated Der p 1, Alt a 1 and Bla g 1 levels, but it did not remain as an independent predictor for any allergens.
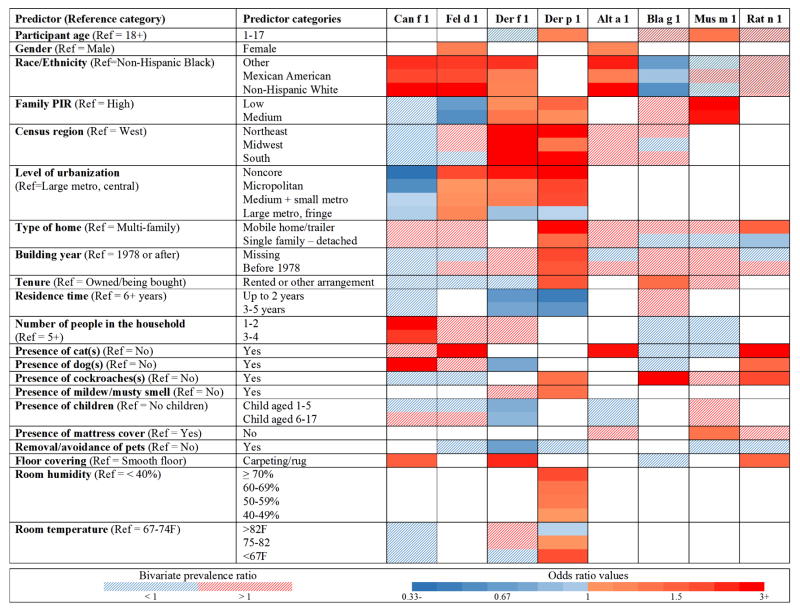
Independent predictors of individual allergen exposures
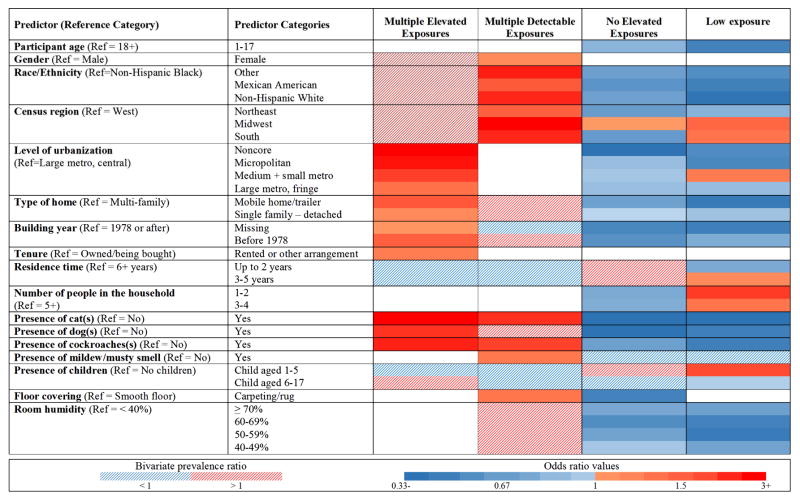
Figure 4 summarizes results from the predictor modeling for each allergen. In the heat map, the direction and strength of the associations are indicated by color, while numeric odds ratios for the predictors are presented in Table E4 (Online Repository). Race/ethnicity, family PIR, and level of urbanization were the most consistent predictors of individual allergen levels, whereas the strongest associations were observed between elevated pet allergen levels and the presence of cat(s)/dog(s). Participant’s age and gender remained independent predictors for elevated dust mite, mouse, cat and Alternaria levels. Region and climatic factors predicted only elevated dust mite levels. Odds of having elevated Der f 1 and Der p 1 levels were highest in the Northeast and South. Climate region was an independent predictor for elevated Der f 1 levels; compared to mixed-dry/hot-dry climates, living in mixed-humid/marine or hot-humid climates increased the odds of elevated Der f 1 levels 3–4 fold (data not shown). Overall, housing characteristics were most frequently associated with elevated Der p 1 levels, but the presence of carpeting increased the odds of having elevated levels for several allergens. The presence of pets and cockroaches increased Can f 1, Fel d 1 and Bla g 1 levels, and was associated with elevated levels of other allergens. Interestingly, reported pet avoidance was associated with lower odds of having elevated Der f 1 levels, and did not predict lower pet allergen levels in the bedroom.
Figure 4.
Independent predictors of elevated allergen levels. The odds ratios from multivariable regression models are presented in the online repository (Table E4). For the independent predictors, the solid red/blue color indicates the direction of the associations (red increasing; blue decreasing) when each of the participant/housing characteristic categories were compared to the reference category adjusting for the other independent predictor variables in the model. The dashed areas indicate participant/housing characteristics included in initial prediction models based on bivariate analysis (P < 0.25; Figure E2). The metropolitan counties are subdivided based on the population of their metropolitan statistical area (MSA): large (central, fringe), for MSA population of 1 million or more; medium, for MSA population of 250,000–999,999; and small, for MSA population below 250,000. Non-metropolitan counties not defined as micropolitan are considered to be noncore and are thought of as the most rural areas.
Allergen burden in relation to participant and housing characteristics
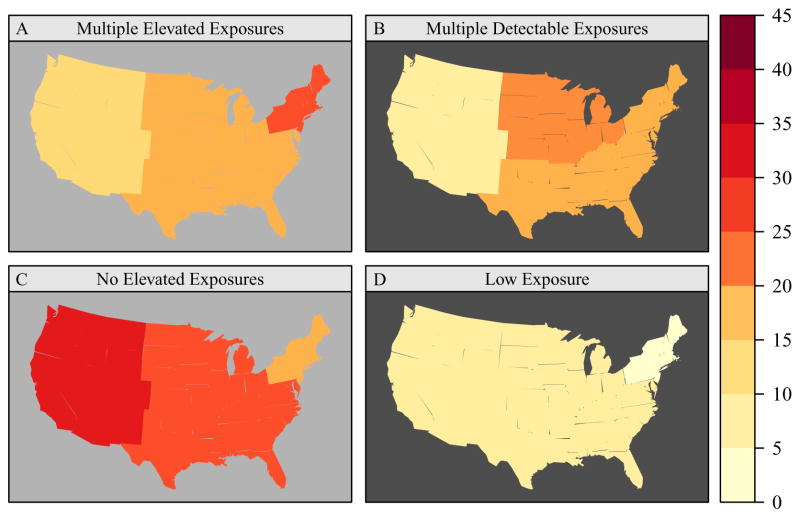
The bar graphs in Figure E4 in the Online Repository illustrate allergen burden across categories of the studied characteristics. Allergen burden varied significantly by race/ethnicity. Non-Hispanic blacks were more likely to have low (≤ 2 detectable allergens; no elevated exposures) or low-to-medium (no elevated exposures) allergen burden than other racial/ethnic groups. Although children were less likely to have low allergen burden than adults, low allergen burden was more prevalent in homes with children aged 1 to 5 years than in those with older children. Having multiple detectable allergens with at least one exceeding the elevation threshold was more common among females than males. While allergen burden was not strongly influenced by socioeconomic status (i.e., PIR), regional differences were apparent (Figure 5). Low-to-medium exposure burden was most prevalent in the West, whereas the highest prevalence of multiple elevated exposures was found in the Northeast. However, the number of detectable allergens was highest in the Midwest. Allergen burden did not differ significantly across the four climate regions (Figure E5). Compared to metropolitan areas, non-metropolitan areas were more likely to have several allergens at elevated levels.
Figure 5.
Prevalence of allergen burden by census region. Allergen Burden Outcomes: Multiple Elevated Exposures (≥3 allergens at elevated levels); Multiple Detectable Exposures (≥7 allergens >LOD, ≥1 allergens at elevated levels); No Elevated Exposures (0 allergens at elevated levels); Low Exposure (≤2 allergens > LOD, 0 allergens at elevated levels). Can f 1, Fel d 1, Der f 1, and Der p 1 levels were elevated if allergen concentration >75 percentile; Alt a 1, Bla g 1, Mus m 1 and Rat n 1 levels were elevated if allergen concentration > 90 percentile. The dark background color in the map indicates statistically significant differences (P < 0.05) across census regions (Northeast, Midwest, South, West).
Of the housing characteristics, type and age of housing were consistently associated with allergen burden (Figure E4). Allergen burden was highest in mobile homes, and lowest in multi-family housing. The prevalence of high allergen burden increased with increasing age of the home. The presence pets and pests contributed strongly to high allergen burden. While the presence of carpeting and mildew/musty smell was associated with a greater likelihood of having higher allergen burden, low allergen burden was more prevalent in homes with fewer occupants and low humidity. Although allergen burden tended to be higher in homes of smokers and lower among those who reported avoiding pets, neither of the variables was an independent predictor of allergen burden.
Independent predictors of allergen burden
Figure 6 summarizes modeling results, showing the direction and strength of the associations by color, while odds ratios for the predictors are presented in the Online Repository (Table E5).
Figure 6.
Independent predictors of allergen burden. Allergen Burden Outcomes: Multiple Elevated Exposures (≥3 allergens at elevated levels); Multiple Detectable Exposures (≥7 allergens LOD, ≥1 allergens at elevated levels); No Elevated Exposures (0 allergens at elevated levels); Low Exposure (≤2 allergens > LOD, 0 allergens at elevated levels). The odds ratios from multivariable regression models are presented in the online repository (Table E5). For the independent predictors, the solid red/blue color indicates the direction of the associations (red increasing; blue decreasing) when each of the participant/housing characteristic categories were compared to the reference category adjusting for the other independent predictor variables in the model. The dashed areas indicate participant/housing characteristics included in initial prediction models based on bivariate analysis (P < 0.25; Figure E4).
Race/ethnicity and presence of pets and pests predicted allergen burden most consistently. Although low to medium allergen burden was more prevalent among adults than children, the presence of young children aged 1 to 5 years was associated with low allergen burden. Females tended to be exposed to greater number of allergens than males. Allergen burden was strongly affected by census region and level of urbanization. For example, the odds of having high allergen burden significantly increased with decreasing level of urbanization. High allergen burden was prevalent in mobile homes/trailers, as well as older and rental homes. Reported mildew and/or musty smell and the presence of carpeting increased the odds of exposure to multiple allergens. Low allergen burden was most prevalent in homes with few occupants. Increased humidity levels were associated with reduced odds of having low to medium allergen burden.
DISCUSSION
The role and characteristics of residential indoor allergen exposures have been investigated in numerous studies, but only two, NHANES 2005–2006 and NSLAH, have examined exposure characteristics and variability in nationally representative samples of the US population. While NSLAH advanced our understanding of prevalence and determinants of indoor allergen exposures,29–34 NHANES 2005–2006 provided a much larger cohort to support enhanced power and precision associated with subgroup analyses on a national scale. NHANES data demonstrated that individual allergen levels and allergen burden varied significantly by participant and housing characteristics; however, climatic conditions and socioeconomic status were associated only with individual allergen levels, not with allergen burden. High allergen burden was most consistently associated with the presence of pets and pests, living in mobile homes/trailers, older and rental homes, and in non-metropolitan areas. Compared to the previously published sensitization patterns in this population,18 allergen exposures show distinct differences by gender, race/ethnicity, and level of urbanization, as highlighted in the discussion.
Agreeing with NSLAH,33 exposure to multiple allergens was common. Allergens were detected ubiquitously, practically in every home, and in most homes, allergens were found at elevated levels. However, due to methodological differences (e.g., use of different types of antibodies and standards), it is difficult to make direct quantitative comparisons between the surveys. Allergens were assessed with individual ELISAs in NSLAH, while a more sensitive multiplex assay, MARIA®,35 was used to quantify most allergens in NHANES 2005–2006. In NSLAH, Alternaria alternata and mouse allergens were also measured with polyclonal antibodies, which have higher potential for cross-reactivity. Indeed, polyclonal antibodies against Alternaria alternata have been found to cross-react with other fungi.36
Clustering patterns of the allergens were largely similar in both surveys.33 Rat allergen, which was not measured in NSLAH, showed very strong clustering with other allergens in high levels, reflecting high overall allergen burden in the home. In contrast, clustering of allergens showed less distinct patterns than allergen-specific IgEs (sIgE) in NHANES 2005–2006.18 While interesting, this is not completely unexpected, given that additional factors, including host susceptibility, other environmental exposures, and biological cross-reactivity influence clustering of sIgEs.
Participant’s race/ethnicity was strongly associated with individual allergen levels and allergen burden, supporting NSLAH findings.33 Although racial differences in Alternaria and pet allergen exposures might explain why non-Hispanic blacks were more likely to have lower allergen burden than other groups, the finding is contradictory with the observed racial disparities in sensitization. Several studies have demonstrated that allergic sensitization and disease rates are higher in non-Hispanic blacks than other groups.18, 37–39 In NHANES 2005–2006, racial/ethnic exposure patterns differed from patterns observed in allergic sensitization,18 except for cockroach. Among non-Hispanic blacks, both exposure and sensitization to cockroach allergen were more prevalent than in other groups.
Children were less likely than adults to have low allergen burden in their homes. However, the odds of having low allergen burden were 2-fold higher among homes with children aged 1 to 5 years compared to those without children. Because NHANES did not collect information on cleaning frequency, it remains unclear whether this relates to more frequent cleaning activities in homes with young children. Yet, the presence of young children, as well as reported pet avoidance measures, conferred lower odds of having elevated Der f 1 levels. Reported pet avoidance, on the other hand, did not lower the odds of having elevated pet allergen levels or high allergen burden in the bedroom. While exposure to pets, especially in early life, can have a protective effect on the development of allergic sensitization and asthma,40–42 lowering exposure is advisable for those who are sensitized to pet allergens.5, 40 In fact, national data suggest that exposure to elevated dog and cat allergen levels in the bedroom is associated with excess asthma attacks and emergency care visits among pet-sensitive asthmatics in the US.43
Although positive sIgE tests and increased sIgE levels are reported more commonly among males than females,18 the odds of being exposed to multiple allergens were higher for females than males. In particular, Alternaria and cat allergen levels were elevated among females. The results may reflect behavioral differences related to pet ownership. Alternaria spore concentrations are often found higher outdoors than indoors,44 which may explain why Alt a 1 levels were detected at much lower levels than Fel d 1. Previous studies support that presence of pets is associated with higher residential fungal levels.34, 45, 46 While pets can transport outdoor allergens on their fur, potential indoor sources cannot be ruled out.
Socioeconomic status (SES) was associated with individual allergen levels, agreeing with published data.29, 31, 34, 47 Exposure and sensitization patterns showed similarities, most consistently for pet and cockroach allergens. However, PIR, an indicator of SES, was not an independent predictor of allergen burden, suggesting that residential allergen burden may not vary significantly across different socioeconomic groups. Although allergen burden in the home can differ, depending on which allergens are measured, another study has reported that the risk of having at least one allergen at elevated levels may not vary between high- and low-poverty area homes.47
Regional variation was apparent for allergen exposures and burden. Dust mite allergen levels, which are not necessarily highly correlated and tend to differ by housing conditions and characteristics (e.g., humidity levels, age and type of house),10, 30, 48 also exhibited substantial regional differences. Consistent with NSLAH, the prevalence of elevated dust mite allergens was significantly lower in the West compared to northeastern and southern regions in the US.30 In NHANES 2005–2006, regional exposure and sensitization patterns showed similarities for dust mite and cockroach allergens.18 For dust mites, elevated exposure levels, as well as prevalence of sensitization and increased sIgE levels were highest in the Northeast and South.18 Exposure and sensitization to cockroach allergen tended to be highest in the South,18 although census region was not an independent predictor for elevated cockroach allergen levels. The NHANES 2005–2006 data suggested that allergen burden might vary both across and within census regions. For example, participants in the South were less likely to have low-to-medium exposures and more likely to have multiple detectable exposures than those living in the West. On the other hand, while multiple detectable exposures were more prevalent in the Midwest than in the western US, participants in the Midwest had higher odds of having low allergen burden than those living in the West.
Although climatic conditions are often associated with housing characteristics (e.g., building codes and types differ by climate zones/regions), only individual allergen levels, but not allergen burden, showed variation by climatic conditions. Elevated dust mite allergen levels showed species-specific differences, which most likely relate to biologic and ecologic differences (e.g., relative humidity and temperature requirements for reproduction and survival) between the species;49 elevated Der p 1 levels were most prevalent in hot humid regions, whereas elevated Der f 1 levels were most frequently detected in mixed-humid/marine climates. Nonetheless, climate region remained as an independent predictor only for elevated Der f 1 levels.
Allergen burden and levels of pet and dust mite allergens varied significantly across levels of urbanization in NHANES 2005–2006, while geographic variation was less notable in NSLAH,33 reflecting perhaps a smaller sample size (831 households) and/or differences in urban-rural and exposure classification schemes. Allergen exposures exhibited opposite patterns compared to allergic sensitization, which was found more common in urban areas.18 Living in the most rural areas increased the odds of having high allergen burden (≥ 3 allergens exceeding elevated levels) by more than 3-fold compared to living in a highly urbanized area. While the odds of having elevated cat and dust mite levels were significantly increased in rural homes, increased dog allergen levels were much less frequently detected in rural homes than in more urbanized areas, suggesting that dogs might be kept outdoors rather than indoors in less urbanized areas. Level of urbanization may influence environmental exposures, not only contributing to diversity and levels of microbial, pollen and/or air pollution exposures,50–52 but also affecting characteristics of indoor allergen exposures.
Housing characteristics, which are closely associated with sociodemographic and environmental factors, influenced individual allergen levels and allergen burdens notably. Of the allergens, Der p 1 levels were most frequently associated with housing-related factors, especially with those reflecting lower SES and increased humidity/excess moisture in the home. Compared to NSLAH, NHANES 2005–2006 provided less detailed information on housing characteristics, since it was not designed to survey housing conditions. However, both studies showed that elevated allergen exposures in the home were consistently associated with housing type and the presence of pets and pests.
The national representativeness is one of the greatest strengths of NHANES 2005–2006; however, the cross-sectional design is a limitation. Although using concentrations in reservoir dust as surrogates of exposures may not be an optimal method for exposure assessment, it is an accepted and widely used method in large epidemiological studies.10, 53, 54 Indeed, combined dust samples collected from participant’s bed and bedroom floor may be less influenced by temporal and spatial variability, more reproducible, and represent long-term exposure better than short-term air sampling. Even though repeated measurements might have improved the accuracy of exposure estimates, the within-home variance of exposures tends to be smaller than the between-home variance.55 We acknowledge that exposures could be assessed more accurately with personal monitors, as an individual’s exposure to allergens varies in intensity, content, and particle size over time.56 While new, improved exposure assessment techniques may provide novel insight into the role of allergens and other environmental exposures in asthma and allergies,57 studies, especially large-scale ones, showing significant advantages for personal sampling over reservoir dust analysis are still scant.58 Assessment of allergen burden is complicated, not only because it is unknown whether each allergen imposes an equivalent risk but also due to residential exposure variability.47 Our outcomes, which were based on measurements of eight common indoor allergens, may have limited ability to reflect total allergen burden in the home. Due to the NHANES survey design, seasonal and regional variables were interrelated,59 which made the assessment of potential seasonal effects difficult. Thus, caution is warranted when interpreting regional differences (e.g., the survey did not account for seasonal variability in indoor temperature and humidity levels). Furthermore, the methodological differences between NHANES 2005–2006 and NSLAH precluded the assessment of temporal trends in allergen exposures. However, despite all limitations, NHANES 2005–2006 not only extends the knowledge on geographic and climatic variation of indoor allergens, but also provides information on variability of residential allergen burden and complements the previously published sensitization findings,18 enabling comparisons between exposure and sensitization patterns in the general US population.
This comprehensive report on indoor allergen exposures highlights factors that influence allergen levels and burden in US homes. While exposure variability showed significant racial/ethnic and regional differences, high exposure burden to multiple allergens was consistently found in homes with pets and pests, mobile homes and/or trailers, older and rental homes, and in non-metropolitan areas. Although removing pets and pests from home may not always be easy, the national data suggest that the presence of pets and pests can make a significant difference in residential allergen burden. Low allergen burden, which was rather uncommon, was associated with several additional sociodemographic and housing-related factors. In comparison to the previously published data on allergen-specific sensitization in NHANES 2005–2006,18 exposure patterns showed notable differences by sociodemographic factors (e.g., gender, race/ethnicity, and level of urbanization). Further studies, preferably of longitudinal design, are needed to elucidate how genetic, socioeconomic, cultural, environmental, or other factors contribute to the complex relationships between allergen exposures and development of allergic diseases such as asthma.
Supplementary Material
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Grant number: Z01-ES-025041).
We acknowl edge the analyst Mr. Ajay Yesupriya at the National Center for Health Statistics (NCHS) Atlanta Research Data Center (RDC) for his invaluable assistance with conduct of the data analysis. We also thank Ms. Caroll Co for her assistance with geographic data visualization.
The findings and conclusions in this paper are those of the author(s) and do not necessarily represent the views of the Research Data Center, the National Center for Health Statis tics, or the Centers for Disease Control and Prevention.
Abbreviations used
- Alt a 1
Alternaria alternata 1 (Alternaria allergen)
- Bla g 1
Blattella germanica 1 (Cockroach allergen)
- Can f 1
Canis familiaris 1 (Dog allergen)
- CI
Confidence interval
- Der f 1
Dermatophagoides farinae 1 (Dust mite allergen)
- Der p 1
Dermatophagoides pteronyssinus 1 (Dust mite allergen)
- ELISA
Enzyme-linked immunosorbent assay
- Fel d
1 Felis domesticus 1 (Cat allergen)
- IgE
Immunoglobulin E
- LLOD
Lower limit of detection
- Mus m 1
Mus musculus 1 (Mouse allergen)
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- NIEHS
National Institute of Environmental Health Sciences
- NSLAH
National Survey of Lead and Allergens in Housing
- OR
Odds ratio
- PIR
Poverty index ratio
- Rat n 1
Rattus norvegicus 1 (Rat allergen)
- sIgE
Serum specific immunoglobulin E
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Custovic A. To what extent is allergen exposure a risk factor for the development of allergic disease? Clin Exp Allergy. 2015;45:54–62. doi: 10.1111/cea.12450. [DOI] [PubMed] [Google Scholar]
- 2.Kanchongkittiphon W, Mendell MJ, Gaffin JM, Wang G, Phipatanakul W. Indoor environmental exposures and exacerbation of asthma: an update to the 2000 review by the Institute of Medicine. Environ Health Perspect. 2015;123:6–20. doi: 10.1289/ehp.1307922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erwin EA, Platts-Mills TA. Allergens. Immunol Allergy Clin North Am. 2005;25:1–14. doi: 10.1016/j.iac.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Phipatanakul W, Matsui E, Portnoy J, Williams PB, Barnes C, Kennedy K, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375–87. doi: 10.1016/j.anai.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portnoy J, Kennedy K, Sublett J, Phipatanakul W, Matsui E, Barnes C, et al. Environmental assessment and exposure control: a practice parameter--furry animals. Ann Allergy Asthma Immunol. 2012;108:223e1–15. doi: 10.1016/j.anai.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portnoy J, Miller JD, Williams PB, Chew GL, Miller JD, Zaitoun F, et al. Environmental assessment and exposure control of dust mites: a practice parameter. Ann Allergy Asthma Immunol. 2013;111:465–507. doi: 10.1016/j.anai.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portnoy J, Chew GL, Phipatanakul W, Williams PB, Grimes C, Kennedy K, et al. Environmental assessment and exposure reduction of cockroaches: a practice parameter. J Allergy Clin Immunol. 2013;132:802–8. e1–25. doi: 10.1016/j.jaci.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsui EC, Abramson SL, Sandel MT Section On A, Immunology, Council On Environmental H. Indoor Environmental Control Practices and Asthma Management. Pediatrics. 2016:138. doi: 10.1542/peds.2016-2589. [DOI] [PubMed] [Google Scholar]
- 9.Crain EF, Walter M, O’Connor GT, Mitchell H, Gruchalla RS, Kattan M, et al. Home and allergic characteristics of children with asthma in seven U.S urban communities and design of an environmental intervention: the Inner-City Asthma Study. Environ Health Perspect. 2002;110:939–45. doi: 10.1289/ehp.02110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson J, Dixon SL, Breysse P, Jacobs D, Adamkiewicz G, Chew GL, et al. Housing and allergens: a pooled analysis of nine US studies. Environ Res. 2010;110:189–98. doi: 10.1016/j.envres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld L, Chew GL, Rudd R, Emmons K, Acosta L, Perzanowski M, et al. Are building-level characteristics associated with indoor allergens in the household? J Urban Health. 2011;88:14–29. doi: 10.1007/s11524-010-9527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Health and Nutrition Examination Survey 2005 – 2006, Data Documentation, Codebook, and Frequencies: Allergens - Household Dust (ALDUST_D) Hyattsville, MD: National Center for Health Statistics; 2014. [Cited 2017 May 31.] Available from http://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/ALDUST_D.htm. [Google Scholar]
- 13.Vojta PJ, Friedman W, Marker DA, Clickner R, Rogers JW, Viet SM, et al. First National Survey of Lead and Allergens in Housing: survey design and methods for the allergen and endotoxin components. Environ Health Perspect. 2002;110:527–32. doi: 10.1289/ehp.02110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Meara T, Tovey E. Monitoring personal allergen exposure. Clin Rev Allergy Immunol. 2000;18:341–95. doi: 10.1385/CRIAI:18:3:341. [DOI] [PubMed] [Google Scholar]
- 15.Boor BE, Spilak MP, Corsi RL, Novoselac A. Characterizing particle resuspension from mattresses: chamber study. Indoor Air. 2015;25:441–56. doi: 10.1111/ina.12148. [DOI] [PubMed] [Google Scholar]
- 16.Spilak MP, Boor BE, Novoselac A, Corsi RL. Impact of bedding arrangements, pillows, and blankets on particle resuspension in the sleep microenvironment. Build Environ. 2014;81:60–8. [Google Scholar]
- 17.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102:563–70. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 18.Salo PM, Arbes SJ, Jr, Jaramillo R, Calatroni A, Weir CH, Sever ML, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J Allergy Clin Immunol. 2014;134:350–9. doi: 10.1016/j.jaci.2013.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Health and Nutrition Examination Survey 2005 – 2006, Data Documentation, Codebook, and Frequencies: Allergy (AGQ_D) Hyattsville, MD: National Center for Health Statistics; 2008. [Cited 2017 May 31.] Available from https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/AGQ_D.htm. [Google Scholar]
- 20.National Health and Nutrition Examination Survey 2005 – 2006, Data Documentation, Codebook, and Frequencies: Housing Caracteristics (HOQ_D) National Center for Health Statistics; 2008. [Cited 2017 May 31.] Available from https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/HOQ_D.htm. [Google Scholar]
- 21.National Health and Nutrition Examination Survey 2005 – 2006, Data Documentation, Codebook, and Frequencies: Demographic variables & Sample Weights (DEMO_D) Hyattsville, MD: National Center for Health Statistics; 2009. [Cited 2017 May 31.] Available from https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/DEMO_D.htm. [Google Scholar]
- 22.Allergen Dust Collection Procedures Manual. Hyattsville, MD: National Center for Health Statistics; 2006. [Cited 2017 May 31.] Available from https://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/allergen_manual_06.pdf. [Google Scholar]
- 23.Baechler MC, Williamson JL, Gilbride TL, Cole PC, Hefty MG, Love PM. Building America Best Practices Series Volume 7.1. U.S. Department of Energy; 2010. Guide to Determining Climate Regions by County. [Google Scholar]
- 24.Public data general release file documentation. Hyattsville, MD: National Center for Health Statistics; 2005. [Cited 2017 May 31.] Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/general_data_release_doc_05_06.pdf. [Google Scholar]
- 25.Survey Methods and Analytic Guidelines. Hyattsville, MD: National Center for Health Statistics; 2014. [Cited 2017 May 31.] Available from https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx. [Google Scholar]
- 26.Laboratory Procedure Manual: Indoor Allergens, Dust, Multiplex Array for Indoor Allergens (MARIA) Hyattsville, MD: National Center for Health Statistics; 2014. [Cited 2017 May 31.] Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/aldust_d_met_-indoor_allergens_maria.pdf. [Google Scholar]
- 27.Laboratory Procedure Manual: Cockroach (Bla g 1), Dust, Enzyme-linked immunosorbent assay. Hyattsville, MD: National Center for Health Statistics; 2014. [Cited 2017 May 31.] Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/aldust_d_met_cockroach_bla-g-1.pdf. [Google Scholar]
- 28.Rauh VA, Landrigan PJ, Claudio L. Housing and health: intersection of poverty and environmental exposures. Ann N Y Acad Sci. 2008;1136:276–88. doi: 10.1196/annals.1425.032. [DOI] [PubMed] [Google Scholar]
- 29.Cohn RD, Arbes SJ, Jr, Jaramillo R, Reid LH, Zeldin DC. National prevalence and exposure risk for cockroach allergen in U.S. households. Environ Health Perspect. 2006;114:522–6. doi: 10.1289/ehp.8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arbes SJ, Jr, Cohn RD, Yin M, Muilenberg ML, Burge HA, Friedman W, et al. House dust mite allergen in US beds: results from the First National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2003;111:408–14. doi: 10.1067/mai.2003.16. [DOI] [PubMed] [Google Scholar]
- 31.Arbes SJ, Jr, Cohn RD, Yin M, Muilenberg ML, Friedman W, Zeldin DC. Dog allergen (Can f 1) and cat allergen (Fel d 1) in US homes: results from the National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2004;114:111–7. doi: 10.1016/j.jaci.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 32.Cohn RD, Arbes SJ, Jr, Yin M, Jaramillo R, Zeldin DC. National prevalence and exposure risk for mouse allergen in US households. J Allergy Clin Immunol. 2004;113:1167–71. doi: 10.1016/j.jaci.2003.12.592. [DOI] [PubMed] [Google Scholar]
- 33.Salo PM, Arbes SJ, Jr, Crockett PW, Thorne PS, Cohn RD, Zeldin DC. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J Allergy Clin Immunol. 2008;121:678–84. e2. doi: 10.1016/j.jaci.2007.12.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salo PM, Yin M, Arbes SJ, Jr, Cohn RD, Sever M, Muilenberg M, et al. Dustborne Alternaria alternata antigens in US homes: results from the National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2005;116:623–9. doi: 10.1016/j.jaci.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King EM, Filep S, Smith B, Platts-Mills T, Hamilton RG, Schmechel D, et al. A multi-center ring trial of allergen analysis using fluorescent multiplex array technology. J Immunol Methods. 2013;387:89–95. doi: 10.1016/j.jim.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmechel D, Green BJ, Blachere FM, Janotka E, Beezhold DH. Analytical bias of cross-reactive polyclonal antibodies for environmental immunoassays of Alternaria alternata. J Allergy Clin Immunol. 2008;121:763–8. doi: 10.1016/j.jaci.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 37.Wegienka G, Havstad S, Joseph CL, Zoratti E, Ownby D, Woodcroft K, et al. Racial disparities in allergic outcomes in African Americans emerge as early as age 2 years. Clin Exp Allergy. 2012;42:909–17. doi: 10.1111/j.1365-2222.2011.03946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wegienka G, Johnson CC, Zoratti E, Havstad S. Racial differences in allergic sensitization: recent findings and future directions. Curr Allergy Asthma Rep. 2013;13:255–61. doi: 10.1007/s11882-013-0343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhan N, Kawachi I, Glymour MM, Subramanian SV. Time Trends in Racial and Ethnic Disparities in Asthma Prevalence in the United States From the Behavioral Risk Factor Surveillance System (BRFSS) Study (1999–2011) Am J Public Health. 2015;105:1269–75. doi: 10.2105/AJPH.2014.302172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konradsen JR, Fujisawa T, van Hage M, Hedlin G, Hilger C, Kleine-Tebbe J, et al. Allergy to furry animals: New insights, diagnostic approaches, and challenges. J Allergy Clin Immunol. 2015;135:616–25. doi: 10.1016/j.jaci.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 41.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–72. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 42.Kerkhof M, Wijga AH, Brunekreef B, Smit HA, de Jongste JC, Aalberse RC, et al. Effects of pets on asthma development up to 8 years of age: the PIAMA study. Allergy. 2009;64:1202–8. doi: 10.1111/j.1398-9995.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- 43.Gergen PJ, Mitchell HE, Calatroni A, Sever ML, Cohn RD, Salo PM, et al. Sensitization and Exposure to Pets: The Effect on Asthma Morbidity in the US Population. J Allergy Clin Immunol Pract. 2017 doi: 10.1016/j.jaip.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–34. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 45.Dharmage S, Bailey M, Raven J, Mitakakis T, Thien F, Forbes A, et al. Prevalence and residential determinants of fungi within homes in Melbourne, Australia. Clin Exp Allergy. 1999;29:1481–9. doi: 10.1046/j.1365-2222.1999.00640.x. [DOI] [PubMed] [Google Scholar]
- 46.Kettleson EM, Adhikari A, Vesper S, Coombs K, Indugula R, Reponen T. Key determinants of the fungal and bacterial microbiomes in homes. Environ Res. 2015;138:130–5. doi: 10.1016/j.envres.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitch BT, Chew G, Burge HA, Muilenberg ML, Weiss ST, Platts-Mills TA, et al. Socioeconomic predictors of high allergen levels in homes in the greater Boston area. Environ Health Perspect. 2000;108:301–7. doi: 10.1289/ehp.00108301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Strien RT, Gehring U, Belanger K, Triche E, Gent J, Bracken MB, et al. The influence of air conditioning, humidity, temperature and other household characteristics on mite allergen concentrations in the northeastern United States. Allergy. 2004;59:645–52. doi: 10.1111/j.1398-9995.2004.00470.x. [DOI] [PubMed] [Google Scholar]
- 49.Arlian LG, Morgan MS, Neal JS. Dust mite allergens: ecology and distribution. Curr Allergy Asthma Rep. 2002;2:401–11. doi: 10.1007/s11882-002-0074-2. [DOI] [PubMed] [Google Scholar]
- 50.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10:861–8. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 51.Schroder PC, Li J, Wong GW, Schaub B. The rural-urban enigma of allergy: what can we learn from studies around the world? Pediatr Allergy Immunol. 2015;26:95–102. doi: 10.1111/pai.12341. [DOI] [PubMed] [Google Scholar]
- 52.Parker JD, Akinbami LJ, Woodruff TJ. Air pollution and childhood respiratory allergies in the United States. Environ Health Perspect. 2009;117:140–7. doi: 10.1289/ehp.11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton RG. Assessment of indoor allergen exposure. Curr Allergy Asthma Rep. 2005;5:394–401. doi: 10.1007/s11882-005-0013-0. [DOI] [PubMed] [Google Scholar]
- 54.Takaro TK, Scott JA, Allen RW, Anand SS, Becker AB, Befus AD, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort study: assessment of environmental exposures. J Expo Sci Environ Epidemiol. 2015;25:580–92. doi: 10.1038/jes.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antens CJ, Oldenwening M, Wolse A, Gehring U, Smit HA, Aalberse RC, et al. Repeated measurements of mite and pet allergen levels in house dust over a time period of 8 years. Clin Exp Allergy. 2006;36:1525–31. doi: 10.1111/j.1365-2222.2006.02603.x. [DOI] [PubMed] [Google Scholar]
- 56.Tovey E, Ferro A. Time for new methods for avoidance of house dust mite and other allergens. Curr Allergy Asthma Rep. 2012;12:465–77. doi: 10.1007/s11882-012-0285-0. [DOI] [PubMed] [Google Scholar]
- 57.Gold DR, Adamkiewicz G, Arshad SH, Celedon JC, Chapman MD, Chew GL, et al. NIAID, NIEHS, NHLBI, MCAN Workshop Report: The Indoor Environment and Childhood Asthma: Implications for Home Environmental Intervention in Asthma Prevention and Management. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gore RB, Curbishley L, Truman N, Hadley E, Woodcock A, Langley SJ, et al. Intranasal air sampling in homes: relationships among reservoir allergen concentrations and asthma severity. J Allergy Clin Immunol. 2006;117:649–55. doi: 10.1016/j.jaci.2005.12.1351. [DOI] [PubMed] [Google Scholar]
- 59.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2:1–24. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.