Abstract
Aims
To estimate the prevalence of and risk factors for cardiovascular autonomic neuropathy (CAN) in adolescents and young adults with type 1 and type 2 diabetes enrolled in the SEARCH for Diabetes in Youth Study.
Methods
The study included 1646 subjects with type 1 diabetes (age 18 ± 4 years, diabetes duration 8 ± 2 years, HbA1c 9.1 ± 1.9%, 76% Non-Hispanic Whites) and 252 with type 2 diabetes (age 22 ± 4 years, diabetes duration 8 ± 2 years, HbA1c 9.2 ± 3.0%, 45% Non-Hispanic Blacks). Cross-sectional and longitudinal risk factors were assessed at baseline and follow-up visits. Area under the curve (AUC) was used to assess the longitudinal glycemic exposure and cardiovascular risk factors. CAN was assessed by time and frequency domain indices of heart rate variability (HRV). CAN was defined as the presence of ≥3 of 5 abnormal HRV indices.
Results
The prevalence of CAN was 12% in adolescents and young adults with type 1 diabetes and 17% in those with type 2 diabetes. Poor long-term glycemic control (AUC HbA1c), high blood pressure, and elevated triglyceride levels were correlates of CAN in subjects with type 1 diabetes. In those with type 2 diabetes, CAN was associated with elevated triglycerides and increased urinary albumin excretion.
Conclusions
The prevalence of CAN in this multiethnic cohort of adolescents and young adults with type 1 and type 2 diabetes are comparable to those reported in adults with diabetes. Suboptimal glycemic control and elevated triglycerides were the modifiable risk factors associated with CAN.
INTRODUCTION
Cardiovascular autonomic neuropathy (CAN) is a serious complication of diabetes affecting the autonomic nerves innervating the heart and blood vessels, with subsequent sympathovagal imbalance and impact on heart rate regulation and cardiac performance (1–3). Although asymptomatic in earlier stages, CAN has been shown to be an independent predictor of cardiovascular disease (CVD) mortality risk (4,5), silent myocardial ischemia (6) and/or major CVD events (7), cardiac remodeling and left ventricular dysfunction (8), and progression of diabetic nephropathy and chronic kidney disease (9,10). Yet, CAN is one of the least recognized complications of diabetes, especially in youth.
Reduction in the heart rate variability (HRV) parameters is the earliest manifestations of CAN. Evaluation of HRV with time and frequency domain indices are non-invasive methods to assess the presence and severity of CAN (2). Several small cross-sectional studies in the various pediatric population, using diverse definitions, have reported CAN prevalence estimates between 18% and 75% (11–15). In one of the largest pediatric epidemiological studies assessing the burden of diabetes-related complications in an Australian cohort of adolescents with diabetes, Eppens and colleagues found strikingly high prevalence of autonomic neuropathy (using pupillometry) in adolescents with type 1 and type 2 diabetes (61% and 57%, respectively) (15).
Apart from the SEARCH study, there have been no systematic efforts to assess the burden of neuropathy, including CAN, in adolescents and young adults with diabetes in the United States. We previously reported that youth with type 1 diabetes have reduced HRV as compared to age-matched healthy controls enrolled in the SEARCH Cardiovascular Disease (CVD) study (16). Although, we have recently reported the age-adjusted prevalence of several diabetic complications (including CAN) in the SEARCH participants as part of the SEARCH Cohort Study (17), the specific objective of this study was to examine the prevalence of CAN more closely by age group, diabetes duration, gender and race/ethnicity. Moreover, we were specifically interested in examining the cross-sectional and longitudinal risk factors (since diagnosis of diabetes to present time) in adolescents and young adults with and without CAN separately in those with type 1 and type 2 diabetes to better understand the underlying risk factors and the pathological processes that drive CAN in this young cohort.
The overall objectives of the current study were: 1) to estimate the prevalence of CAN in a large, ethnically diverse cohort of adolescents and young adults with type 1 and type 2 diabetes enrolled in the SEARCH for Diabetes in Youth Study by age, gender, diabetes duration and race/ethnicity; and 2) to identify the cross-sectional and longitudinal associations of CAN with anthropometric and metabolic parameters.
METHODS
SEARCH for Diabetes in Youth Study
SEARCH for Diabetes in Youth is a prospective cohort study following children and adolescents of diverse racial and ethnic backgrounds diagnosed with diabetes at less than 20 years of age in the United States of America (USA) (17). SEARCH participants are incident cases of diabetes identified at four geographically defined populations in Ohio, Washington, South Carolina, and Colorado, from health plan enrollees in California, and from Indian Health Service beneficiaries from American Indian populations in Arizona and New Mexico.
Study Population
Adolescents and young adults with diabetes diagnosed at < 20 years of age were identified from a population-derived incident registry network at five USA sites by the SEARCH for Diabetes in Youth Registry Study (17). Cases with newly diagnosed type 1 or type 2 diabetes in 2002-2006 or 2008, who completed a SEARCH baseline examination (on average 9.3 ± 6.4 months from diagnosis) and had at least 5 years of diabetes duration between 2011 and 2015, were recruited into the SEARCH Cohort Study (2011-2015) (on average at 7.9 ± 1.9 years from diagnosis) (Appendix Figure 1). Although the parent SEARCH Cohort Study enrolled 2777 individuals, we excluded children < 10 years of age (n=134) at the cohort visit, those with no diabetes antibody measures for the etiological definition of diabetes (n=440), and those with incomplete neuropathy assessment (n=305), which reduced the analytic sample size to 1898 individuals (Appendix Figure 2).
Prior to protocol implementation, local institutional review board approval was obtained for each center. Written informed consent was obtained from participants age 18 and older, while assent with parental written informed consent was obtained for participants younger than 18 years.
Baseline and Cohort Visits
The SEARCH baseline and cohort visits included a participant survey; measurement of height, weight, waist circumference, blood pressure; and blood and urine collection. Race and ethnicity were self-reported and categorized as Non-Hispanic White (NHW), Non-Hispanic Black (NHB), Hispanic, Asian or Pacific Islander, and Other. Current cigarette smoking was defined as having smoked cigarettes on ≥ 1 of the 30 days preceding the visit. Individuals who had tried smoking or smoked regularly (at least one cigarette every day for 30 days) but were not current smokers were considered former smokers. Subjects who had never smoked a whole cigarette were considered nonsmokers.
Waist circumference was measured using the natural waist location (17) and divided by height in centimeters to calculate the waist-to-height ratio (WHR). Body mass index (BMI) was defined as weight (kilograms) divided by height (meters)2. For participants < 20 years of age, the Centers for Disease Control and Prevention (CDC)-derived BMI z20 scores were used; for those ≥ 20 years the observed mean and standard deviation were used to standardize their BMI z20 values.
Resting systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured three times using an aneroid sphygmomanometer while the participants were seated for at least 5 minutes, and the average of the three measurements was taken.
A blood draw occurred after an 8-hour overnight fast, and medications, including short-acting insulin, were withheld the morning of the visit. Blood samples were obtained under conditions of metabolic stability, as defined by no episodes of diabetic ketoacidosis in the prior month. Specimens were processed locally at the sites and shipped within 24 h to the central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington), where they were analyzed for measurement of high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides, and glycated hemoglobin (HbA1c) as previously described (17). Urinary albumin and creatinine levels were assessed on a random spot urine sample to evaluate renal function using the albumin-to-creatinine ratio (ACR). The accuracy of HbA1c data was monitored by participation in the National Glycohemoglobin Standardization Program, and the accuracy and consistency of lipid data were monitored regularly by comparing results obtained by enzymatic methods with those obtained by CDC reference methods (CDC Reference Laboratory) (18).
In addition to a SEARCH baseline and a cohort visit, 57% of participants (n=1082) had one or more intermediate visits (at 1, 2 or 5 years after the baseline visit) at which the same cardio-metabolic risk factors were measured, including HbA1c, lipids, waist circumference, WHR, and BMI. The assay of biological samples has remained consistent over time.
Diabetes Type
Diabetes type was defined using an etiological classification developed by SEARCH (19) based on diabetes autoantibodies [Glutamate decarboxylase-2 (GAD-65), insulinoma-associated-2 antibodies IA-2A), and Zinc-T8 autoantibody] and estimated insulin sensitivity score (validated equation including waist circumference, HbA1c, and triglyceride levels) at the baseline visit (19). Type 1 diabetes was defined as at least one positive antibody, regardless of insulin sensitivity, or no positive antibodies and insulin sensitivity (score > 8.15). Type 2 diabetes was defined as negative antibodies and insulin resistance (score < 8.15) (19).
Assessment of CAN
CAN was assessed by HRV testing using the SphygmoCor (Atcor, PA). SEARCH staff from each center were centrally trained and certified to perform the HRV test. The HRV tests were performed under standardized conditions that included overnight fasting, avoidance of caffeine and tobacco products for 8 hours before the test, and withholding prescriptions and over-the-counter medicines (except for basal insulin) until testing was completed. Participants underwent a 5-minute continuous electrocardiogram recording while supine after a 10-minute rest. All traces were reviewed and analyzed to ensure R-waves were adequately identified from artifacts and ectopic beats. The term “NN interval” is used instead of RR interval of the ECG to emphasize that the processed beats are normal sinus rhythm (i.e., every QRS complex preceded a P-wave). We analyzed the following time- and frequency-domain HRV parameters from the SphgmoCor device: standard deviation of NN interval (SDNN), root mean square difference of the successive NN interval (RMSSD), high frequency (HF) power, low frequency (LF) power and LF: HF ratio. SDNN is a measure of overall HRV, while the RMSSD and HF power represent the parasympathetic component of the autonomic system and LF power the sympathetic component. HRV test was considered abnormal if the values were below the 5th percentile observed in 206 age- and sex-matched healthy controls (age 10-28 years, 54% females) from the SEARCH CVD study (16). CAN, as our primary outcome measure, was defined as the presence of ≥ 3 abnormal HRV indices.
Statistical Analysis
Cross-sectional Data
Anthropometric, demographic, and metabolic data collected at the cohort visit as described above were used to compare the characteristics distinguishing adolescents and young adults with and without CAN stratified by diabetes type.
Students t-test and Wilcoxon two-sample tests were used to compare the distribution of normally and non-normally (triglycerides, SDNN, RMSSD) distributed continuous variables, respectively, and the χ2 test was used for categorical variables separately for type 1 and type 2 diabetes participants. Fisher’s exact test was used whenever a cell count for a particular test was less than 5. The data is presented as mean ± standard deviation for normally distributed variables and as median (inter quartile range) for non-normally distributed variables such as triglycerides and log transformation was done for others such as ACR.
The prevalence of CAN was estimated overall and based on the age at diagnosis (≥ 10 years and < 10 years) and duration of diabetes (5 years, 6-10 years, and > 10 years) separately for persons with type 1 or type 2 diabetes.
Longitudinal Data
In addition to the data collected at baseline and at the cohort visit, the area under the curve (AUC) was computed to summarize the longitudinal trajectory of HbA1c and other continuous variables, such as lipids, blood pressure, and BMI collected over time (at the baseline, 1, 2, or 5-year follow-up and cohort visits), adjusting for the time interval between the first and last measurement.
To assess the association of long-term glycemic control with CAN, logistic regression models treating the presence of CAN as the outcome were fitted separately for participants with type 1 or type 2 diabetes. These models were adjusted for potential confounders (collected at current SEARCH 3 cohort visit) such as age and sex (model 2), BMI (model 3), blood pressure (model 4), triglycerides (model 5), and ACR (model 6). A fully adjusted model that included all of these variables as covariates was also fitted (model 7). Models were stratified by diabetes type to limit confounding effects of age and adiposity. Diagnostic tests were performed to ensure that modeling assumptions were satisfied. The data were analyzed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Characteristics of adolescents and young adults with type 1 (n=1646) and type 2 diabetes (n = 252) stratified by their CAN status are shown in Table 1. The prevalence of CAN was 12% in adolescents and young adults with type 1 diabetes, and 17% among those with type 2 diabetes (Table 1). Subjects with type 1 diabetes and CAN were older (mean age 19 ± 4 vs. 18 ± 4 years), more likely to have developed diabetes at age 10 or older, and had a larger waist circumference (81 ± 12 vs. 78 ± 12 cm), higher blood pressure (SBP 110 ± 11 vs. 106 ± 11 and DBP 72 ± 9 vs. 69 ± 9 mm Hg), poorer glycemic control (HbA1c 9.6 ± 2.1 vs. 9.1 ± 1.8 %), and elevated levels of triglycerides [median (IQR) 82(61,120) vs. 74(55,104) mg/dl] than those without CAN (all P < 0.05) (Table 1). Males with type 1 diabetes had a higher prevalence of CAN as compared to females (15% vs. 10%, P = 0.001). Subjects with type 2 diabetes and CAN had higher DBP (80 ± 12 vs. 75 ± 10 mm Hg) and elevated triglyceride levels [median (IQR) 151(102,254) vs. 110(78,183) mg/dl) and ACR (3.2 ± 1.5 vs. 2.7 ± 1.7 mg/g) compared to those without CAN (all P < 0.05) (Table 1). Hispanic and NHW subjects with type 2 diabetes had a higher prevalence of CAN (29% and 27%, respectively) as compared to NHB (7%) and other minority groups (12%) (P = 0.001).
Table 1.
Clinical characteristics of adolescents and young adults with type 1 and type 2 diabetes stratified by cardiovascular autonomic neuropathy status at the current SEARCH Cohort research visit(2011-2015).
| Type 1 diabetes = 1646 | Type 2 diabetes = 252 | |||||
|---|---|---|---|---|---|---|
| Variable | No CAN | CAN | P-value | No CAN | CAN | P-value |
| N (%) | 1443(88%) | 203(12%) | 209(83%) | 43(17%) | ||
| Age, years | 18 ± 4 | 19 ± 4 | <0.0001 | 22 ± 4 | 22 ± 3 | 0.99 |
| Age at diagnosis, years | 10 ± 4 | 11 ± 3 | <0.0001 | 14.1 ± 3 | 14 ± 3 | 0.82 |
| Age at diagnosis ≥ 10 | 723(84%) | 137(16%) | <0.0001 | 200(83%) | 40(17%) | 0.45 |
| Age at diagnosis < 10 | 720(92%) | 66(8%) | 9(75%) | 3(25%) | ||
| Diabetes duration, years | 7.8 ± 1.8 | 7.9 ± 1.9 | 0.44 | 7.9 ± 1.9 | 7.8 ± 2.0 | 0.82 |
| Diabetes duration, years | ||||||
| 5-10 years | 1233(88%) | 167(12%) | 0.24 | 174(82%) | 38(18%) | 0.49 |
| ≥10 years | 210(85%) | 36(15%) | 35(87%) | 5(13%) | ||
| Sex | ||||||
| Female | 746(90%) | 79(10%) | 0.001 | 141(83%) | 28(17%) | 0.77 |
| Male | 697(85%) | 124(15%) | 68(82%) | 15(18%) | ||
| Race/ethnicity | ||||||
| Non-Hispanic White | 1089(87%) | 158(13%) | 0.63 | 48(73%) | 18(27%) | 0.001 |
| Asian/Pacific Islander | 24(92%) | 2(8%) | 3(100%) | 0(0%) | ||
| Non-Hispanic Black | 143(91%) | 14(9%) | 105(93%) | 8(7%) | ||
| Hispanic | 175(86%) | 28(14%) | 37(71%) | 15(29%) | ||
| Others | 12(87%) | 1(13%) | 16(88%) | 2(12%) | ||
| Smoking | ||||||
| Never | 967(89%) | 122(11%) | 0.15 | 80(84%) | 15(16%) | 0.76 |
| Former | 259(85%) | 46(15%) | 61(80%) | 15(20%) | ||
| Current | 188(86%) | 30(14%) | 63(84%) | 12(16%) | ||
| Oral diabetes medication | ||||||
| Yes | 68(87.2%) | 10(12.8%) | 0.40 | 109(82.6%) | 23(17.4%) | 0.90 |
| No | 1372(87.7%) | 192(12.3%) | 99(83.2%) | 20(16.8%) | ||
| Insulin dose | 56.84 ± 29.09 | 64.22 ± 30.97 | 0.0007 | 66.19 ± 38.48 | 76.35 ± 36.26 | 0.20 |
| Insulin dose, per kg | 0.85 ± 0.40 | 0.90 ± 0.40 | 0.06 | 0.73 ± 0.39 | 0.86 ± 0.36 | 0.19 |
| Waist circumference, cms | 78 ± 12 | 81 ± 12 | 0.00011 | 104 ± 19 | 111 ± 20 | 0.06 |
| Waist-to-height ratio | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.48 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.33 |
| BMI, kg/m2 | 24 ± 5 | 25 ± 5 | 0.12 | 35 ± 9 | 37 ± 9 | 0.38 |
| SBP, mm Hg | 106 ± 11 | 110 ± 11 | <0.0001 | 118 ± 13 | 121 ± 17 | 0.36 |
| DBP, mm Hg | 69 ± 9 | 72 ± 9 | <0.0001 | 75 ± 10 | 80 ± 12 | 0.01 |
| HbA1c, % | 9.1 ± 1.8 | 9.6 ± 2.1 | 0.001 | 9.2 ± 3 | 9.6 ± 2.8 | 0.42 |
| HbA1c, mmol/mol | 75 ± 7 | 80 ± 8 | 0.001 | 77 ± 9 | 80 ± 9 | 0.42 |
| HDL-cholesterol mg/dl | 55 ± 14 | 54 ± 13 | 0.15 | 42 ± 12 | 39 ± 10 | 0.16 |
| LDL-cholesterol mg/dl | 96 ± 28 | 99 ± 32 | 0.50 | 106 ± 38 | 104 ± 37 | 0.88 |
| Triglycerides mg/dL | 74 (55,104) | 82 (61,120) | 0.005 | 110 (78,183) | 151 (102,254) | 0.002 |
| CRP mg/dL | 0.2 ± 0.5 | 0.4 ± 1.5 | 0.74 | 0.6 ± 0.8 | 0.7 ± 0.7 | 0.16 |
| Log ACR, μg/mg | 2.0 ± 0.93 | 2.1 ± 0.95 | 0.33 | 2.7 ± 1.7 | 3.2 ± 1.5 | 0.014 |
All data are presented as mean ± SD or n (%) or median (IQR). CAN: cardiovascular autonomic neuropathy, BMI: body mass index, SPB and DBP: systolic and diastolic blood pressure, HDL: high density lipoprotein, LDL: low density lipoprotein, CRP: C-reactive protein, ACR: albumin:creatinine ratio
The association of CAN with the AUC of cardio-metabolic risk factors is depicted in Table 2. Long-term poor glycemic control, summarized as AUC for HbA1c, triglyceride levels (AUC triglycerides), and blood pressure (AUC SBP and AUC DBP), were significantly higher among type 1 diabetes subjects with CAN as compared to those without CAN. Only higher triglycerides over time were significantly associated with CAN among subjects with type 2 diabetes (Table 2).
Table 2.
Cardio-metabolic risk factor burden over time by cardiovascular autonomic neuropathy status in adolescents and young adults with type 1 and type 2 diabetes.
| Type 1 diabetes = 1646 | Type 2 diabetes = 252 | |||||
|---|---|---|---|---|---|---|
| Variable | No CAN | CAN | P-value | No CAN | CAN | P-value |
| AUC BMI-z | 20.6 ± 0.9 | 20.6 ± 0.9 | 0.75 | 21.9 ± 0.7 | 22 ± 0.6 | 0.50 |
| AUC DBP | 65.9 ± 7.2 | 68 ± 7.6 | <0.001 | 73.1 ± 6.7 | 75.6 ± 9.7 | 0.18 |
| AUC SBP | 103.5 ± 9 | 107.5 ± 9 | <0.001 | 116.6 ± 9.3 | 119.7 ± 11.8 | 0.22 |
| AUC HbA1c | 8.5 ± 1.3 | 8.8 ± 1.5 | 0.005 | 8.4 ± 2.4 | 8.6 ± 2.3 | 0.65 |
| AUC LDL-cholesterol | 93.5 ± 22.3 | 95.1 ± 24.2 | 0.53 | 104.1 ± 30.7 | 99.6 ± 26.6 | 0.54 |
| AUC Triglycerides | 79.2 ± 43.8 | 92 ± 59.6 | 0.001 | 149.6 ± 121.7 | 225.3 ± 221.8 | 0.005 |
| AUC HDL-cholesterol | 56.1 ± 11.8 | 54.7 ± 10.6 | 0.10 | 42.4 ± 10.9 | 40.1 ± 10.4 | 0.13 |
All data are presented as mean ± SD. CAN: cardiovascular autonomic neuropathy, BMI: body mass index, SPB and DBP: systolic and diastolic blood pressure, HDL: high density lipoprotein, LDL: low density lipoprotein, AUC: area under the curve derived from data collected at baseline, 1,2, 5 year follow-up and the current cohort visits
Table 3 summarizes the results from the multiple logistic regression analyses for the association between longitudinal glycemic control (AUC HbA1c as the independent variable) and CAN (dependent variable) adjusted sequentially for covariates. Long-term poor glycemic control (AUC HbA1c) was significantly associated with CAN independent of age, sex, blood pressure, BMI, triglyceride levels, and ACR in subjects with type 1 diabetes, but not in those with type 2 diabetes (Table 3).
Table 3.
Multivariable regression models for association between long term glycemic control and cardiovascular autonomic neuropathy collected at the current SEARCH Cohort visit(2011-2015)
| Dependent Variable: CAN Independent Variable: AUC A1c adjusted for time between measures |
Type 1 diabetes | Type 2 diabetes | ||
|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Model 1 = AUC A1c | 1.28 (1.11;1.48) | 0.0006 | 1.07 (0.77;1.48) | 0.69 |
| Model 2 = Model 1+ age, sex | 1.3 (1.13;1.5) | 0.0002 | 1.07 (0.77;1.49) | 0.68 |
| Model 3 = Model 1+ BMI | 1.29 (1.11;1.49) | 0.0006 | 1.11 (0.79;1.56) | 0.54 |
| Model 4 = Model 1 + SBP, DBP | 1.26 (1.09;1.47) | 0.002 | 1 (0.71;1.41) | 1.00 |
| Model 5 = Model 1 + Trig | 1.22 (1.05;1.43) | 0.010 | 0.93 (0.65;1.33) | 0.69 |
| Model 6 = Model 1 + ACR | 1.26 (1.07;1.47) | 0.005 | 1.27 (0.86;1.88) | 0.22 |
| Model 7 = Model 1 + age, sex, BMI, SBP, DBP, Trig, ACR | 1.24 (1.04;1.46) | 0.01 | 1.08 (0.69;1.7) | 0.72 |
CAN: cardiovascular autonomic neuropathy, OR: odds ratio, CI: confidence interval, BMI: body mass index, SPB and DBP: systolic and diastolic blood pressure, trig: triglycerides, ACR: albumin: creatinine ratio, AUC: area under the curve
Since nearly 32% (n= 879) of the 2777 SEARCH participants were excluded from the analysis data set due to various reasons (age < 10 years, missing etiological definition of diabetes type, incomplete CAN assessment), we examined whether there were any significant differences in the anthropometric, demographic, and metabolic characteristics of the participants who were excluded versus included in this data set that could potentially affect the prevalence estimates. Individuals excluded (n=879) from the analysis were more likely to have a longer duration of diabetes (9 vs. 8 years, P < 0.001) and were younger at the time of diabetes diagnosis (9 vs. 11 years, P < 0.001), as compared to those included in the analytic sample (n=1898) (Appendix Table 1).
DISCUSSION
This study evaluating a large, multiethnic cohort of adolescents and young adults in the USA found high prevalence of CAN in subjects with type 1 and type 2 diabetes. Poor glycemic control and higher triglyceride levels over time were consistently associated with CAN. This is the first population-derived study in the USA that carefully characterized differences in the cross-sectional and longitudinal risk factors for CAN in a large, racially/ethnically diverse cohort of adolescents and young adults with type 1 diabetes and type 2 diabetes.
Placing our findings in perspective, in a meta-analysis including 3943 participants from 19 studies, the prevalence of subclinical CAN defined by either cardiovascular reflex tests or baroreflex sensitivity in young people (age < 24 years) with type 1 diabetes varied between 16 and 75% depending on the outcomes reported (20). For instance, in this meta-analysis pooled prevalence of CAN defined by measures of HRV was 21%, and ranged from 4-11% if CAN was defined by a single cardiovascular reflex test such as deep breathing or Valsalva (20). In contrast, a relatively small study that assessed HRV (LF and HF power) and included only 20 pediatric patients with type 1 diabetes (mean diabetes duration 7 years, mean HbA1c 8.2%) reported prevalence rates for CAN as high as 75% (11). The prevalence estimates for CAN we found in the SEARCH cohort are lower compared with the report by Eppens and colleagues in an Australian cohort that included 1433 youth with type 1 and 68 type 2 diabetes who found that 61% of the youth with type 1 diabetes and 57% of those with type 2 diabetes had evidence of autonomic neuropathy (15). However, in that cohort autonomic neuropathy was defined as an abnormal pupillometry test (assessed by measuring the pupil size before and 3 seconds after a light stimulus was delivered using an infrared pupillometer) (15). The difference in the method of assessment (pupillometry vs. HRV testing) likely explains the higher prevalence of autonomic neuropathy in the Australian cohort, in spite of that cohort being younger, with shorter diabetes duration and better glycemic control compared with the SEARCH cohort (15). Although pupillometry was considered in the past a simple non-invasive test to assess parasympathetic autonomic function, it has not been widely used in the research setting due to the lack of standardization in the techniques employed, and the lack of validation studies. Thus, differences in outcome definitions, type of autonomic dysfunction evaluated, and the methods of assessments are likely the reasons accounting for the high variability in the reported prevalence reported in the few pediatric populations studied.
Poor glycemic control, longer duration of diabetes, increasing age, microalbuminuria, DBP, and dyslipidemia (lower HDL, increased triglycerides) are some of the established risk factors for CAN in adults with diabetes (1, 21–25). This study found that poor glycemic control and hypertriglyceridemia over time were the strongest risk factors associated with CAN in adolescents and young adults with type 1 diabetes. There is ample biologic plausibility and evidence for the causal role of hyperglycemia in the development and progression of chronic complications, including CAN (1, 26–29). Hyperglycemia induces abnormal signaling of the autonomic neurons via accumulation of advanced glycation end products and microangiopathy, causing ischemic atrophy of autonomic nerve fibers innervating cardiac and vascular tissues (30). In this study, unfortunately, glucose control was quite poor in adolescents and young adults with type 1 or type 2 diabetes, with a mean HbA1c of ~ 9%, far exceeding the target HbA1c (<7.5%) recommended by the American Diabetes Association (31). These data further confirm that there is an urgent need for efforts focused at improving glycemic control among adolescents and young adults with diabetes to mitigate the elevated risk of the adverse outcomes associated with CAN and its downstream consequences, including increased CVD risk, in addition to numerous other adverse effects.
Dyslipidemia has also been implicated in the pathogenesis of diabetic neuropathy in a non-glucocentric paradigm involving linked metabolic and inflammatory insults that trigger neurodegeneration (32-36). However, there is a close link between glucose and lipid metabolism, as hypertriglyceridemia and reduced HDL commonly occur in poorly controlled type 1 and type 2 diabetes (33). Hyperglycemia and dyslipidemia are also known as pro-inflammatory triggers to the neurodegenerative processes (23). In a prior analysis of youth participating in the SEARCH CVD study, we found an atherogenic lipid profile in youth with type 1 diabetes and reduced HRV as compared to age-matched healthy controls (16).
The findings from this study have important clinical implications. We observed that youth and young adults with type 1 and type 2 diabetes have evidence of CAN, as documented by changes in HRV at a mean diabetes duration of 8 years. Emerging epidemiological evidence also suggests that CAN is associated with increased arterial stiffness in adolescents (37) and adults (38) with type 1 diabetes, and thus could have an additive effect on the risk of future cardiovascular events, which occur earlier and with poorer prognosis in individuals with diabetes compared with the general population (39,40,4).
Considering that SEARCH participants are a representative cohort of USA youth with diabetes and have suboptimal glycemic control (mean HbA1c well above the American Diabetes Association recommended target of < 7.5%) (19), these data provide evidence that similar screening for CAN, as is recommended for adults, may be beneficial in adolescents and young adults as in adults with diabetes (1), especially in those individuals who have additional risk factors associated with CAN. Recent recommendations from the American Heart Association call for a multifactorial approach to mitigate the increased CVD risk in youth with diabetes (41). Thus, targeting poor glycemic control and dyslipidemia in adolescents and young adults with diabetes as early as possible, which is also in line with current standard of care in diabetes (31), could help mitigate the increased CVD risk in this young population (28,36).
The large sample size, multiethnic composition of the cohort, use of a non-invasive, simple, validated instrument to assess CAN, and evaluation of the longitudinal and cross sectional risk factors are among the strengths of our study. The limited power to examine the association between long-term glycemic control and CAN among persons with type 2 diabetes (despite similar levels of HbA1c to those with type 1 diabetes) may have been due to a comparatively small sample size (although the association was in the same direction as that of the type 1 diabetes group) and is one of the limitations of our study. The lack of longitudinal measures of CAN is also a limitation of our study, although a subset of this cohort (2002-2012 incidence cases ≥ 10 years of age with least 5 years of duration of diabetes) will be re-evaluated for CAN as part of the next phase of SEARCH (2016-2020). Finally, although the SEARCH Cohort Study is drawn from population-based registries of youth with diabetes, those excluded from the analytic sample were more likely to be older at time of diagnosis and had a longer duration of diabetes. Each of these variables is associated with increased prevalence of CAN and may influence our estimates of CAN prevalence in youth and young adults with diabetes.
Overall, the current data support the contention that good glycemic control and better approaches to manage dyslipidemia, which have been the accepted standard of care for diabetes, need to be the mainstay, as they may also prevent the development and worsening of CAN in this young population. Given the independent risk of CAN for cardiovascular events and death, health care providers should motivate pediatric patients to reach and maintain optimal glucose control and better management of other risk factors including triglycerides to ameliorate the risk of premature cardiovascular events
Acknowledgments
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their health care providers, whose participation made this study possible.
FUNDING
Grant Support: SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases.
Site Contract Numbers: Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Children’s Hospital Medical Center (Cincinnati) (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708), University of Washington School of Medicine (U58/CCU019235-4, U01 DP000244, and U18DP002710-01), Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200-2010-35171).
The authors wish to acknowledge the involvement of the South Carolina Clinical & Translational Research Institute at the Medical University of South Carolina, NIH/National Center for Advancing Translational Sciences (NCATS) grant number UL1 TR000062; the Seattle Children’s Hospital and the University of Washington, NIH/NCATS grant number UL1 TR00423; the University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant Number UL1 TR000154; the Barbara Davis Center at the University of Colorado at Denver (DERC NIH grant number P30 DK57516); the University of Cincinnati, NIH/NCATS grant number UL1 TR000077; and the Children with Medical Handicaps program managed by the Ohio Department of Health.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
APPENDIX
Appendix Figure 1.
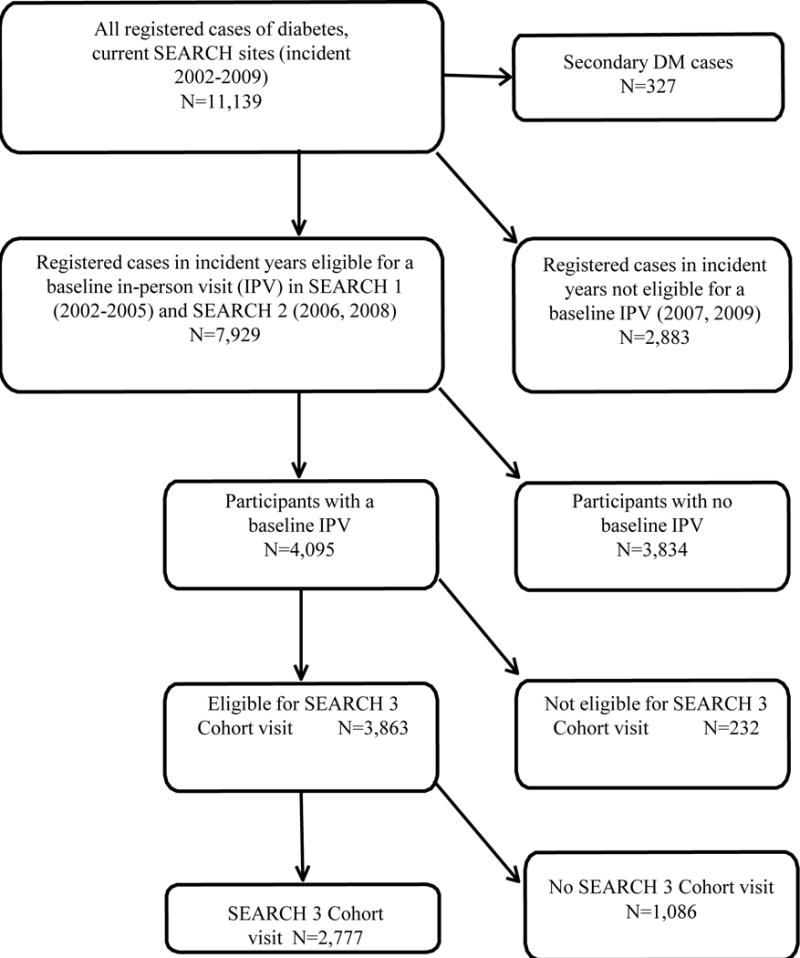
SEARCH 3 consort diagram.
Appendix Figure 2.
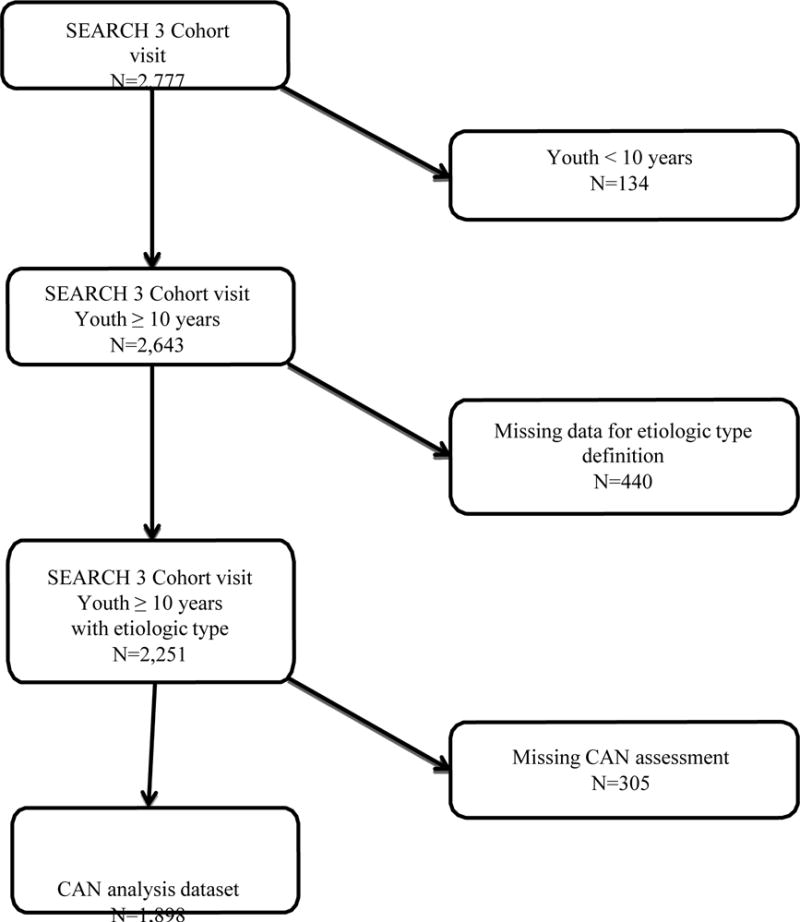
Exclusion and inclusion criteria
Appendix Table 1.
Characteristics of the participants excluded and included in the cardiovascular autonomic neuropathy analysis dataset at the current SEARCH Cohort visit(2011–2015).
| Variable | Excluded (N=879) | Included (N=1898) | P-value |
|---|---|---|---|
| Age at cohort visit (years) | 18 ± 5 | 19 ± 4 | <0.001 |
| Age at diagnosis (years) | 9 ± 5 | 11 ± 4 | <0.001 |
| Sex (Female) | 53% | 52% | 0.74 |
| Diabetes duration (months) | 104 ± 24 | 95 ± 23 | <0.001 |
| Race/ethnicity | 0.080 | ||
| Non-Hispanic White | 63.8% | 69.3% | |
| Non-Hispanic Black | 18.7% | 14.1% | |
| Hispanic | 13.7% | 13.4% | |
| Asian/Pacific Islander | 1.9% | 1.5% | |
| Native Americans | 1.5% | 1.3% | |
| Other | 0.4% | 0.3% | |
| BMI z-score | 0.8 ± 1.2 | 0.7 ± 1.1 | 0.29 |
| SBP (mm Hg) | 102 ± 14 | 102 ± 13 | 0.62 |
| DBP (mm Hg) | 64 ± 11 | 64 ± 10 | 0.86 |
| HbA1c (%) | 9.2 ± 2.1 | 9.2 ± 2.1 | 0.90 |
| Triglycerides (mg/dL) | 102 ± 100 | 105 ± 107 | 0.43 |
| LDL-cholesterol (mg/dL) | 97 ± 30 | 98 ± 30 | 0.49 |
| HDL-cholesterol (mg/dL) | 55 ± 15 | 53 ± 14 | 0.06 |
All data are presented as mean ± SD or n (%). BMI: body mass index, SPB and DBP: systolic and diastolic blood pressure, HDL: high density lipoprotein, LDL: low density lipoprotein
Footnotes
DUALITY OF INTEREST
All authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
MJ, JD, RPB and DD designed the study and analysis plan. MJ wrote the manuscript. JD performed the analysis and provided critical input to the manuscript. RPB reviewed data, provided input in study design, and critically reviewed the manuscript for intellectual content. EMU, DD, RAB, DJP, GI, CP, LMD, ADL, SM, BL, and ELF critically reviewed the manuscript and provided input. JD is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, et al. Toronto Consensus Panel on Diabetic Neuropathy Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27(7):639–53. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 3.Pop-Busui R. What do we know and we do not know about cardiovascular autonomic neuropathy in diabetes. J Cardiovasc Transl Res. 2011;5(4):463–78. doi: 10.1007/s12265-012-9367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(7):1578–84. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26(6):1895–901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 6.Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, et al. DIAD Investigators Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301(15):1547–55. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pop-Busui R, Braffett BH, Zinman B, Martin C, White NH, Herman WH, et al. DCCT/EDIC Research Group Cardiovascular Autonomic Neuropathy and Cardiovascular Outcomes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes Care. 2017;40(1):94–100. doi: 10.2337/dc16-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pop-Busui R, Cleary PA, Braffett BH, Martin CL, Herman WH, Low PA, et al. DCCT/EDIC Research Group Association between cardiovascular autonomic neuropathy and left ventricular dysfunction: DCCT/EDIC study (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications) J Am Coll Cardiol. 2013;61(4):447–54. doi: 10.1016/j.jacc.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlov S, Cherney DZ, Pop-Busui R, Lovblom LE, Ficociello LH, Smiles AM, et al. Cardiac autonomic neuropathy and early progressive renal decline in patients with nonmacroalbuminuric type 1 diabetes. Clin J Am Soc Nephrol. 2015;10(7):1136–44. doi: 10.2215/CJN.11441114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheelock KM, Jaiswal M, Martin CL, Fufaa GD, Weil EJ, Lemley KV, et al. Cardiovascular autonomic neuropathy associates with nephropathy lesions in American Indians with type 2 diabetes. J Diabetes Complications. 2016;30(5):873–9. doi: 10.1016/j.jdiacomp.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boysen A, Lewin MAG, Hecker W, Leichter HE, Uhlemann F. Autonomic function testing in children and adolescents with diabetes mellitus. Pediatr Diabetes. 2007;8:261–264. doi: 10.1111/j.1399-5448.2007.00254.x. [DOI] [PubMed] [Google Scholar]
- 12.Dalla Pozza R, Bechtold S, Bonfig W, et al. Impaired short-term blood pressure regulation and autonomic dysbalance in children with type 1 diabetes mellitus. Diabetologia. 2007;50:2417–2423. doi: 10.1007/s00125-007-0823-9. [DOI] [PubMed] [Google Scholar]
- 13.Mohsin F, Craig ME, Cusumano J, et al. Discordant trends in microvascular complications in adolescents with type 1 diabetes from 1990 to 2002. Diabetes Care. 2005;28:1974–1980. doi: 10.2337/diacare.28.8.1974. [DOI] [PubMed] [Google Scholar]
- 14.Riihimaa PH, Suominen K, Knip M, Tapanainen P, Tolonen U. Cardiovascular autonomic reactivity is decreased in adolescents with type 1 diabetes. Diabetic Medicine. 2002;19:932–38. doi: 10.1046/j.1464-5491.2002.00816.x. [DOI] [PubMed] [Google Scholar]
- 15.Eppens MC, Craig ME, Cusumano J, Hing S, Chan AK, Howard NJ, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29(6):1300–6. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 16.Jaiswal M, Urbina EM, Wadwa RP, Talton JW, D’Agostino RB, Jr, Hamman RF, et al. Reduced heart rate variability among youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36(1):157–62. doi: 10.2337/dc12-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabelea D, Stafford JM, Mayer-Davis EJ, D’Agostino R, Jr, Dolan L, Imperatore G, et al. SEARCH for Diabetes in Youth Research Group Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA. 2017;317(8):825–835. doi: 10.1001/jama.2017.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers GL, Kimberly MM, Waymack PW, Smith SJ, Cooper GR, Sampson EJ. A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements. Clin Chem. 2000;46:1762–72. [PubMed] [Google Scholar]
- 19.Dabelea D, Pihoker C, Talton JW, D’Agostino RB, Jr, Fujimoto W, Klingensmith GJ, et al. SEARCH for Diabetes in Youth Study Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34(7):1628–33. doi: 10.2337/dc10-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang M, Donaghue KC, Cho YH, Craig ME. Autonomic neuropathy in young people with type 1 diabetes: a systematic review. Pediatric Diabetes. 2013;14:239–248. doi: 10.1111/pedi.12039. [DOI] [PubMed] [Google Scholar]
- 21.Pop-Busui R, Low PA, Waberski BH, et al. DCCT/EDIC Research Group Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC) Circulation. 2009;119:2886–2893. doi: 10.1161/CIRCULATIONAHA.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kempler P, Tesfaye S, Chaturvedi N, et al. The EURODIAB IDDM Complications Study Group Autonomic neuropathy is associated with increased cardiovascular risk factors: the EURODIAB IDDM Complications Study. Diabet Med. 2002;19:900–09. doi: 10.1046/j.1464-5491.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- 23.Gottsäter A, Ahmed M, Fernlund P, Sundkvist G. Autonomic neuropathy in type 2 diabetic patients is associated with hyperinsulinaemia and hypertriglyceridaemia. Diabet Med. 1999;16:49–54. doi: 10.1046/j.1464-5491.1999.00001.x. [DOI] [PubMed] [Google Scholar]
- 24.Witte DR, Tesfaye S, Chaturvedi N, Eaton S, Kempler P, Fuller JH. Risk factors for cardiac autonomic neuropathy in type 1 diabetes mellitus. Diabetologia. 2005;48:164–171. doi: 10.1007/s00125-004-1617-y. [DOI] [PubMed] [Google Scholar]
- 25.Larsen JR, Sjøholm H, Berg TJ, Sandvik L, Brekke M, Hanssen KF, Dahl-Jørgensen K. Eighteen years of fair glycemic control preserves cardiac autonomic function in type 1 diabetes. Diabetes Care. 2004;27:963–966. doi: 10.2337/diacare.27.4.963. [DOI] [PubMed] [Google Scholar]
- 26.The Diabetes Control and Complications Trial Research Group. The effect of intensive therapy of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 27.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, et al. Diabetes Control and Complications Trial Epidemiology of Diabetes Interventions and Complications Research Group.ions study (DCCT/EDIC) Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348(23):2294–303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med J. 2003;348(5):383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 29.Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14(9):528. doi: 10.1007/s11892-014-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad A, Bekker P, Tsimikas S. Advanced glycation end products and diabetic cardiovascular disease. Cardiol Rev. 2012;20:177–183. doi: 10.1097/CRD.0b013e318244e57c. [DOI] [PubMed] [Google Scholar]
- 31.Standards of Medical Care in Diabetes—2016. 2016;39(Suppl. 1):S86–S9. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 32.Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: A new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14:257–67. doi: 10.1111/j.1529-8027.2009.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58(7):1634–40. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hur J, Sullivan KA, Pande M, Hong Y, Sima AA, Jagadish HV, et al. The identification of gene expression profiles associated with progression of human diabetic neuropathy. Brain. 2011;134(11):3222–35. doi: 10.1093/brain/awr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ginsberg HN. Diabetic dyslipidemia: basic mechanisms underlying the common hypertriglyceridemia and low HDL cholesterol levels. Diabetes. 1996;45(3):S27–S30. doi: 10.2337/diab.45.3.s27. [DOI] [PubMed] [Google Scholar]
- 36.Fried LF, Forrest KY, Ellis D, et al. Lipid modulation in insulin-dependent mellitus: effect on microvascular outcomes. J Diabetes Complications. 2001;15(3):113–9. doi: 10.1016/s1056-8727(01)00140-4. [DOI] [PubMed] [Google Scholar]
- 37.Jaiswal M, Urbina EM, Wadwa RP, Talton JW, D’Agostino RB, Jr, Hamman RF, et al. Reduced heart rate variability is associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36(8):2351–8. doi: 10.2337/dc12-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ. Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care. 2010;33(3):652–7. doi: 10.2337/dc09-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao D, Carnethon M, Evans G, Cascio W, Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in diabetics: the ARIC study. Am J Epidemiol. 2000;151:S35. doi: 10.2337/diabetes.51.12.3524. [DOI] [PubMed] [Google Scholar]
- 40.Valensi P, Sachs RN, Harfouche B, Lormeau B, Paries J, Cosson E, et al. Predictive value of cardiac autonomic neuropathy in diabetic patients with or without silent myocardial ischemia. Diabetes Care. 2001;24:339–343. doi: 10.2337/diacare.24.2.339. [DOI] [PubMed] [Google Scholar]
- 41.Maahs DM, Daniels SR, de Ferranti SD, Dichek HL, Flynn J, Goldstein BI, et al. American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council for High Blood Pressure Research, and Council on Lifestyle and Cardiometabolic Health Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014;130(17):1532–58. doi: 10.1161/CIR.0000000000000094. [DOI] [PubMed] [Google Scholar]


