Abstract
Mitochondrial oxidative phosphorylation (OXPHOS) is responsible for producing most of the adenosine triphosphate required by eukaryotic cells. Lymphocytes make up the majority of the peripheral blood mononuclear cells. Peripheral blood mononuclear cells are readily obtainable, providing an ideal sample to monitor systemic changes and understand molecular signaling mechanisms in disease processes. Mitochondrial energy metabolism of lymphocyte has been used to screen for OXPHOS disorders. While there are increasing studies of lymphocyte OXPHOS, few studies examined activity of electron transport chain of lymphocyte mitochondria. We present an optimal protocol to harvest fresh peripheral blood mononuclear cells from human whole blood, determine integrated mitochondrial function, and analyze electron transport chain complex activity. Analyzing integrated mitochondrial function using OXPHOS provides data to uncover defects in the transport of substrates into the mitochondria, generation of reducing equivalents, the electron transport chain, and coupling to the production of adenosine triphosphate. The optimal conditions to harvest peripheral blood mononuclear cells were using blood anticoagulated with ethylenediaminetetraacetic acid, processed utilizing Lymphoprep™, and washed in phosphate buffered saline, all at room temperature. Using isolated peripheral blood mononuclear cells, integrated mitochondrial function and the activities of electron transport chain were determined.
Keywords: Integrated mitochondrial function, oxidative phosphorylation, electron transport chain complex activity, human lymphocyte, peripheral blood mononuclear cells, bioenergetics
1. Introduction
Mitochondrial oxidative phosphorylation (OXPHOS) is a pivotal system, defined as the oxidation of fuel molecules by oxygen and the concomitant transduction of this energy into adenosine triphosphate (ATP) [1]. Integrated mitochondrial function is measured as OXPHOS and involves transport of substrates into the mitochondria, the generation of reducing equivalents (NADH and FADH2) by specific dehydrogenases, entry into the electron transport chain (ETC), and coupling to the production of ATP [2, 3]. The OXPHOS system is composed of five enzyme complexes situated in the inner mitochondrial membrane, including complex I (NADH: ubiquinone oxidoreductase), complex II (succinate: ubiquinone oxidoreductase), complex III (the bc1 complex or ubiquinone: cytochrome c oxidoreductase), complex IV (cytochrome c oxidase or reduced cytochrome c: oxygen oxidoreductase), and complex V (ATP synthase). Additionally, OXPHOS involves two electron transport carriers, ubiquinone (coenzyme Q10) and cytochrome c [4].
Deficiencies in energy metabolism have been implicated in a variety of human disease states.[5] Mitochondria are involved in many pathological disorders, including neurodegenerative disease, type 2 diabetes, inflammation, cardiovascular disease, and cancer [6, 7]. In addition to the mitochondrial theory of aging, age-associated changes in mitochondrial respiratory chain function and mitochondrial ATP production have been studied and linked to the aging process [8]. Diagnosis of mitochondrial disease or OXPHOS deficiency relies on clinical symptoms with biochemical and molecular analysis. It is important to consider the transport and oxidation of respiratory substrates upstream of entry of the reducing equivalents into the electron transport system. Integrated mitochondrial function using OXPHOS can help define sites of impairment in mitochondrial pathologies previously described [3]. Assessing mitochondrial bioenergetics function involves measuring OXPHOS by polarographic methods and enzymatic measurement of ETC complexes is mainly performed in tissue homogenates, insolated mitochondria, muscle biopsies, and cultured fibroblasts [9]. These methods require either an invasive skin or skeletal muscle biopsy.
Systemic and local changes following injury and disease are most immediately reflected as changes in molecular and cellular constituents of blood [10]. Peripheral blood cells have been used as a source to assess bioenergetics function in translational research [11]. Lymphocytes are readily obtainable and less invasive [12, 13]. Lymphocytes make up the majority of the peripheral blood mononuclear cells. Lymphocyte energy metabolism has been used to measure defects in mitochondrial OXPHOS and to screen for OXPHOS disorders [9, 14, 15]. Altered mitochondrial OXPHOS in lymphocytes and peripheral blood mononuclear cells has been detected in patients with systematic lupus erythematosus [16], depression [17], inflammation of overweight elderly [18], septic shock [19–21], Alzheimer disease [13], Huntington’s disease [22, 23], and medications and age [24].
As noted above there are multiple studies of peripheral blood mononuclear cells OXPHOS, but few studies examined lymphocyte ETC activity [24–27]. In this paper, we present an optimal protocol to harvest human peripheral blood mononuclear cells and describe methods to determine integrated mitochondrial function in mononuclear cells as OXPHOS, coupled with the analysis of ETC enzyme activity. Integrated mitochondrial function can be assessed by measuring oxygen consumption under a variety of conditions with various substrates; for example, a high-resolution respirometry approach can assess OXPHOS efficiency in intact and permeabilized living cells [28]. Analysis of peripheral blood mononuclear cells mitochondrial OXPHOS and ETC complex activity can help to identify systemic mitochondrial dysfunction and specific complex defects.
2. Materials and Methods
2.1 Patient Section
The study sample was drawn from a population comprised patients with localized prostate cancer before any form of treatment. The study protocol (061409C) was reviewed and approved by the Institutional Review Board of the Case Comprehensive Cancer Center and the University Hospitals Cleveland Medical Center. We excluded potential subjects from the study if they had progressive disease causing significant fatigue; documented major psychiatric illness within 5 years; had uncorrected hypothyroidism or untreated anemia; took sedatives, steroids or nonsteroidal anti-inflammatory agents; or had a second malignancy or mitochondrial disease. Written informed consent form was obtained prior to inclusion. Forty-eight males with localized prostate cancer (age 66.7±8.1 years) were enrolled in the study between December 2015 and December 2017. The majority of participants were married (71%), Caucasian (69%), and employed (60%). More than half of patients has a clinical stage T1 (b–c) with a Gleason score of 6 or 7. All participants scored 90 on the Karnofsky Performance Scale indicating these participants were able to carry out normal activities with only minor signs or symptoms of disease.
2.2 Mononuclear Cells Preparation
To determine optimal conditions to harvest fresh human mononuclear cells, we have used different approaches to isolate lymphocytes from a healthy volunteer.
Approach 1 (ACD/Ficoll/PBS)
a total of 20 ml blood was collected using vacutainers (BD Biosciences) containing acid-citrate-dextrose (ACD), stored at room temperature, and processed within 24 hours. Blood was diluted with an equal amount of phosphate-buffered saline (PBS), layered over a Ficoll-Paque (Sigma-Aldrich) density gradient, and centrifuged at 800 ×g (1854 rpm) for 30 minutes in room temperature. Following centrifugation, the mononuclear cells were removed from the interface between Ficoll-Paque and plasma using a pipettor. Mononuclear cells were collected by centrifugation at 800 ×g for 10 minutes and then washed twice with PBS. To lyse red blood cells, the mononuclear cells were resuspended in hypotonic sodium chloride (0.2% NaCl) for 1 minute following by hypertonic sodium chloride (1.6% NaCl) for another 1 minute before the last wash with PBS and centrifugation.
Approach 2A (EDTA/Ficoll/PBS)
Vacutainers containing ethylenediaminetetraacetic acid (EDTA) were used to collect blood (20 ml). The same amount of PBS was mixed with the blood, layered on Ficoll-Paque, and the same procedures as approach 1 were followed.
Approach 2B (EDTA/Ficoll/DPBS)
EDTA tubes were used to collect 20 ml of blood. Twenty ml of Dulbecco’s phosphate-buffered saline (DPBS) was used to dilute the blood and layered on Ficoll-Paque, following 2 washers with DPBS and centrifuged (800 ×g, 10 minutes at room temperature). Lysis RBC with sodium chloride and the same procedures as approach 1 was followed to obtain the mononuclear cell pallet.
Approach 2C (EDTA/Ficoll/HBSS)
Twenty ml of Hank’s balanced salt solution (HBSS) was used to mix/dilute 20 ml EDTA tubes collected blood, layered over Ficoll-Paque, and the same procedures as approach 1 was followed (collecting buffy coat, washing twice, and RBC lysis).
Approach 3 (EDTA/Lymphoprep ™/PBS)
EDTA tubes were used to collect 20 ml blood, diluted with 20 ml PBS, layered over Lymphoprep ™ (Accurate Chemical & Scientifix Corp. Cat # 1001967), and centrifuged (800 ×g, 30 minutes, room temperature). Following centrifugation, the mononuclear cells (buffy coat/cloudy layer) were removed from the interface between Lymphoprep ™ and plasma using a pipettor, and centrifuged at 800 ×g for 10 minutes. The same procedure as approach 1 was followed to lyse RBC, wash twice with PBS, and centrifugation to obtain the mononuclear cell pellet. To determine how long blood can be stored before harvesting mononuclear cells, we kept aliquots at room temperature and processed the sample at 0-hour, 24-hour, 48-hour, and 72-hour after the blood was collected. After comparing different approaches of mononuclear cells isolation, the 3rd approach (EDTA/Lymphoprep ™/PBS) was used to collect and process blood from all research participants.
2.3 Measurement of Oxidative Phosphorylation with High-Resolution Respirometry
To prepare for OXPHOS, the mononuclear cell pellet was suspended with pre-warmed (37°C) mitochondria respiration medium [29] (MiR05, 110mM sucrose, 60mM potassium lactobionate, 0.5mM EGTA, 3mM MgCl2 • 6 H2O, 20mM taurine, 10mM KH2PO4, 20 mM HEPES, and 2mg/ml BSA, pH=7.1). The number of mononuclear cells was counted using a hemocytometer and adjusted to a final concentration of 1.5–2 million cells/ml with MiR05. Mononuclear cell mitochondrial OXPHOS was determined employing a high-resolution respirometer, Oxygraph-2 K using DatLab 4 software (Oroboros instruments, Innsbruck, Austria).
After air calibration of the O2k system, the cell suspension of intact mononuclear cells in MiR05 was added to two chambers of the OROBOROS O2k system. Two different substrate-uncoupler-inhibitor protocols [29, 30] were used to determine flux through complex I, II, III, and IV, as well as fatty acid oxidation. At the end, the contents of each chamber were collected and frozen for later citrate synthase measurement. The protocol was previously published [30, 31], and the final concentration of the substrates and inhibitors for mononuclear cell mitochondrial OXPHOS was tailored for human mononuclear cells, including 2mM malate, 2.5mM pyruvate, 2.5mM adenosine diphosphate (ADP), 10mM glutamate, 10mM succinate, 10µM palmitoylcarnitine, 0.5mM duroquinol, 1mM tetramethyl-p-phenylenediamine (TMPD), 2mM ascorbate, 1.25µM carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP), 150 nM rotenone, 125 nM antimycin A, and 200 mM sodium azide. One ml of the incubation mixture with cells in each experiment was saved and citrate synthase determined as a marker of the number of mitochondria per chamber.
2.4 Measurement of Electron Transport Complex Activity (ETC)
For the ETC assay, the lymphocyte pellet was resuspended at 4°C in 750µl of MSM/2mM EDTA buffer (220 mM mannitol, 70 mM sucrose, 5 mM MOPS, pH 7.4, and 0.1 M ethylenediaminetetraacetic acid, disodium salt) with 7.5 µl of proteinase inhibitor (Sigma-Aldrich, Cat # P8340) and kept on ice. Protein concentration was determined by the Lowry assay using bovine serum album as a standard [32] and the sample prepared using 5% cholate and MSM/2mM EDTA at 1mg/ml of protein for assays except for cytochrome c oxidase [33]. A second sample for cytochrome c oxidase was prepared in MSM/2mM EDTA at 1mg/ml of protein without cholate [34, 35].
For ETC enzyme activity measurement, the methods used in the Hoppel lab were published previously [3, 36, 37]. Enzymatic activities of complexes I, II, III, and IV in fresh isolated lymphocytes were determined using spectrophotometry. Rotenone-sensitive NADH cytochrome c reductase was used to measure the linked activity of complex I and III and antimycin A-sensitive succinate cytochrome c reductase was used to measure the linked activity of complexes II and III. Complex II activity was measured as the thenoyltrifluoroacetone (TTFA)-sensitive succinate-2,6 Dichlorophenol-indophenol (DCPIP) reductase and total complex II determined in the same way but with Coenzyme Q1 added [37]. Succinate dehydrogenase (SDH) was used to measure the first 2 subunits (A and B) of complex II and served as a membrane-bonded mitochondrial marker enzyme. In addition to SDH, we measured citrate synthase activity as the other mitochondrial marker, as well as the activity of lactate dehydrogenase (LDH) as cytoplasmic marker. Antimycin A-sensitive decylubiquinol cytochrome c reductase (complex III) was utilized to determine the reduction of cytochrome c coupled to the oxidation of decylubiquinol to decylubiquinone. Complex III was inhibited by antimycin A and the assay was measured as an antimycin A-sensitive component. Cytochrome c oxidase (complex IV) was measured as the first order rate constant.
2.5 Data Analysis
Descriptive statistics were calculated for the mean, standard deviation, standard errors, and range for mitochondrial OXPHOS and enzymatic activities of respiratory complexes. Pair-t tests were used to compare differences between intact cell respiration with and without adding substrates, as well as the difference between OXPHOS and maximal oxidative capacity. The OXPHOS data were normalized to cell number. Data are expressed as means ± S.E. of the mean of 48 for OXPHOS, and of 46 for ETC because the technician was on sick leave and not be able to perform 2 ETC assays.
3. Results
3.1 Mononuclear Cell Harvest
Comparisons were made between the different approaches for lymphocytes isolation (Table 1). Approach 1 (ACD/Ficoll/PBS) showed less yield (3.4 × 106 cells/ml), lower intact cell respiration (3.3 pmol/sec/million cells), and lower OXPHOX (8.6 pmol/sec/million cells) of lymphocyte compared to Approach 2A (EDTA/Ficoll/PBS) with a yield (4.1 × 106 cells/ml) and higher intact cell respiration (6.6 pmol/sec/million cells) with OXPHOX (19.7 pmol/sec/million cells). The different washing buffers, Approach 2B (EDTA/Ficoll/DPBS) and Approach 2C (EDTA/Ficoll/HBSS) both were less than Approach 2A (EDTA/Ficoll/PBS). Mononuclear cell viability was compared with different density-gradient mediums, Approach 2 A (EDTA/Ficoll/PBS) and Approach 3 (EDTA/Lymphoprep™/PBS); Approach 3 provided greater yield (4.6 × 106 cells/ml), higher viability (95%), and higher intact cell respiration (6.6 pmol/sec/million cells) as well as OXPHOS (23.7 pmol/sec/million cells) compared to Approach 2A.
Table 1.
Comparison Table (n=5)
| Approach 1 | Approach 2 | Approach 3 | |||
|---|---|---|---|---|---|
| (ACD/Ficoll/ PBS) |
(EDTA/Ficoll/ PBS) |
(EDTA/Ficoll/ DPBS) |
(EDTA/Ficoll/ HBSS) |
(EDTA/Lymphoprep™/ PBS) |
|
| Sample size | 5 | 5 | 5 | 5 | 5 |
| Yield (106 cells/ml whole blood) | 3.4± 0.2 | 4.1± 0.4 | 3.0± 0.4 | 2.9± 0.2 | 4.6± 0.3 |
| Viability | ND | 90% | ND | ND | 95% |
| Intact cell respiration (pmol/sec/milli on cells) | 3.3± 0.2 | 6.6± 0.5 | 4.4± 0.6 | 4.7± 0.5 | 7.7 ± 0.6 |
| OXPHOS (pmol/sec/milli on cells) | 8.6± 0.8 | 19.7± 0.9 | 8.4± 0.6 | 21.7± 1.1 | 23.7± 1.6 |
ACD: acid-citrate-dextrose; EDTA: ethylenediaminetetraacetic acid; PBS: phosphate buffered saline; DPBS: Dulbecco’s phosphate buffered saline; HBSS: Hank’s balanced salt solution; ND: Not done; OXPHOS: oxidative phosphorylation.
Whole blood has collected using vacutainers containing EDTA or ACD from single research participant. Lymphocytes were harvested with 3 different washing buffers (DPBS, PBS, and HBSS) and 2 density-gradient mediums (Ficoll-Paque and Lymphoprep™). The number of lymphocytes was counted using a hemocytometer. Viability was measured using trypan blue. Intact cell respiration and OXPHOS were measured using the Oxygraph-2K. Intact cell respiration was measured without any substrates addition. OXPHOS was determined in permeabilized cells with additions of malate (2mM), pyruvate (2.5mM), adenosine diphosphate (2.5mM), glutamate (10mM), and succinate (10mM). Data are presented as mean ± standard error (n=5).
To evaluate how long blood can be stored, Table 2 contains data from two experiments concerning the yield of mononuclear cells and the bienergetic function of cells at 0-hour, 24-hour, 48-hour, and 72-hour after blood was collected. Processed blood at 0-hour provided the greatest yield (4.9–5.3 × 106 cells/ml) and highest intact cell respiration (8.1–8.2 pmol/sec/million cells) and OXPHOS (20.8–23.8 pmol/sec/million cells). Decreases of 13–18% yield (4.0–4.6 × 106 cells/ml), 30–37% of intact cell respiration (5.1–5.6 pmol/sec/million cells) and OXPHOS (14.9–16.8 pmol/sec/million cells) of mononuclear cells in the blood processed at 24-hour compared to processing blood at 0-hour in both experiments. There was a significant reduction in bioenergetic function at 48- and 72-hour of processing blood compared to blood processed at 24-hour in experiment 2, whereas in experiment 1 no significant decrease was observed. Since we needed to accept blood collected from off-site, we selected the 24-hour time point as our method to process the blood. Intact cell respiration was not detectable when mononuclear cells were processed using cold buffers (data not shown). We also determined that the optimal amount of peripheral blood to perform OXPHOS was 20 ml and 20 ml for ETC activity.
Table 2.
Comparison of Mononuclear Cell Yield, Intact Cell Respiration and OXPHOS at 0-hour, 24-hours, 48-hours, and 72-hours after Blood Sample Collected (n=2)
| Time to process sample | 0-hour | 24-hours | 48-hours | 72-hours | ||||
|---|---|---|---|---|---|---|---|---|
| Experiment | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Yield (106 cells/ml whole blood) | 5.3 | 4.9 | 4.6 | 4.0 | 4.2 | 3.4 | 3.5 | 2.7 |
| Intact cell respiration (pmol/sec/million cells) | 8.1 | 8.2 | 5.6 | 5.1 | 5.2 | 3.8 | 4.2 | 3.8 |
| OXPHOS (pmol/sec/million cells) | 23.8 | 20.8 | 16.8 | 14.9 | 16.2 | 8.6 | 14.6 | 7.1 |
OXPHOS: oxidative phosphorylation.
3.2 Protocols Development of Human Mitochondrial Mononuclear Cell OXPHOS
Mitochondrial OXPHOS was measured using a modified approach with two protocols [29–31] from freshly harvested human mononuclear cells. The modified approach include 20 µg/ml of digitonin to permeabilize the plasma membrane, 1.25 µM of FCCP to reach maximal oxidation capacity, 150 nM of rotenone for complex I inhibition, and 1mM of TMPD for complex IV substrate. Figs 1A and 1B contain examples of protocol 1 and protocol 2 as representative sample. We measured mitochondrial OXPHOS starting with intact cellular respiration after air calibration of the O2k system. Raw data for mitochondrial OXPHOS are shown in Figs. 2A, 2B, and 2C.
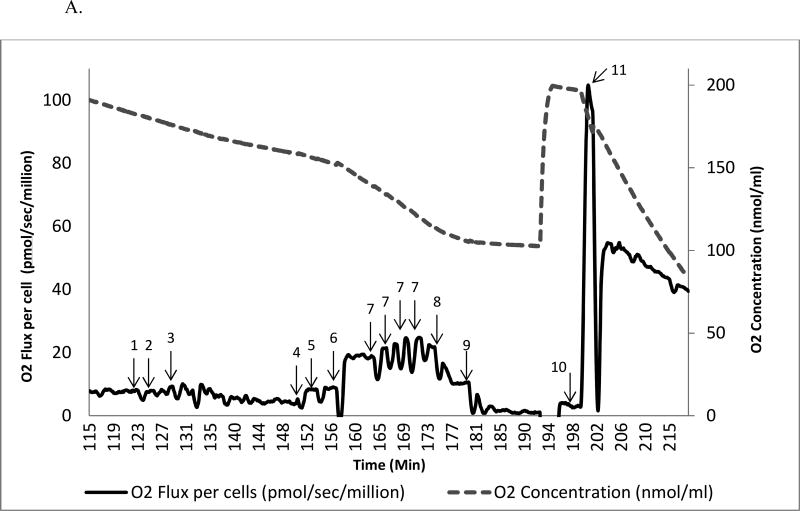
Figure 1.
A. Substrate-inhibitor tracing protocol 1 in human mononuclear cell
The oxygen consumption rate is plotted on the left y axis and the oxygen concentration curve is plotted on the right y axis. A total of 11 additions of reagents and inhibitors are tagged with a numeric symbol. 1=2 mM malate; 2=2.5 mM pyruvate; 3=20 µg/ml digitonin; 4=2.5 mM ADP; 5=10 mM glutamate; 6=10 mM succinate; 7= 0.25 µM increment of FCCP; 8=150 nM rotenone; 9=125 nM antimycin A; 10=1 mM TMPD + 2 mM ascorbate; 11=200 mM sodium azide.
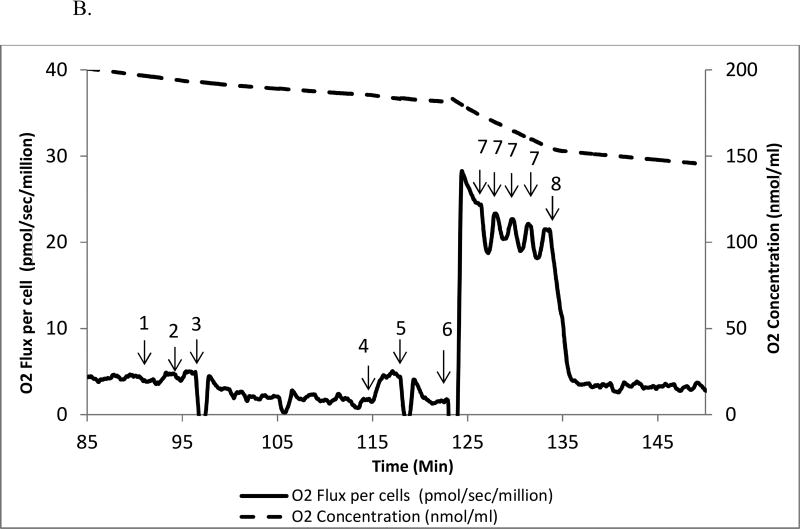
B. Substrate-inhibitor tracing protocol 2 in human mononuclear cell
The oxygen consumption rate is plotted on the left y axis and the oxygen concentration curve is plotted on the right y axis. Eight additions of reagents and inhibitors are tagged with a numeric symbol. 1=2 mM malate; 2=10 µM palmitoylcarnitine; 3=20µg/ml digitonin; 4=2.5 mM ADP; 5=150 nM rotenone; 6=0.5 mM duroquinol; 7=0.25µM increment of FCCP; 8=125 nM antimycin A.
Figure 2.
A. Oxidative phosphorylation: complexes I and II
B. Oxidative phosphorylation: complex IV
C. Oxidative phosphorylation: fatty acid oxidation and complex III
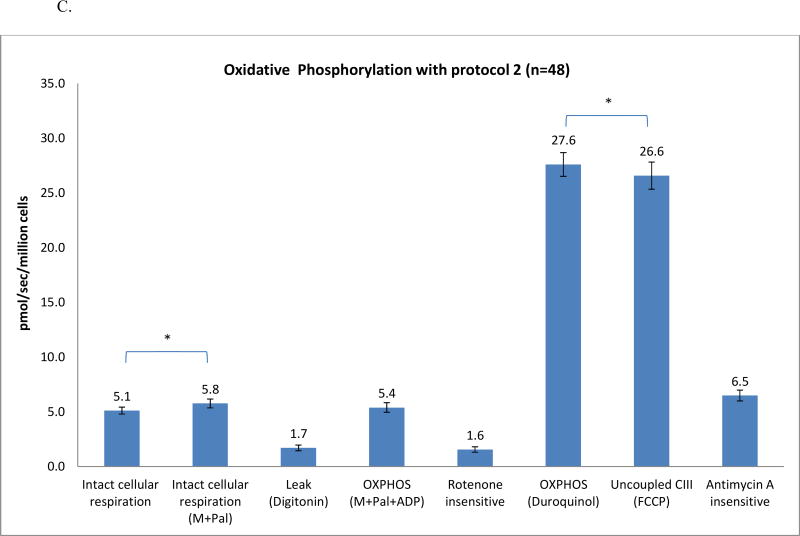
Mitochondrial OXPHOS flux of human mononuclear cells. Data are presented as mean ± standard error from 48 patients with prostate cancer without treatments. M: malate; P: pyruvate; ADP: adenosine diphosphate; G: glutamate; S: succinate; FCCP: carbonylcyanide-p-trifluoromethoxyphenylhydrazone; Rot: rotenone; AA: antimycin A; TMPD: tetramethyl-p-phenylenediamine; Pal: palmitoylcarnitine. OXPHOS complex I, complex II (Fig. 1A), and complex IV (Fig. 1B) determined with substrates uncoupler inhibitors titrations protocol 1; protocol 2 was used to determine fatty acid oxidation and complex III (Fig. 1C).
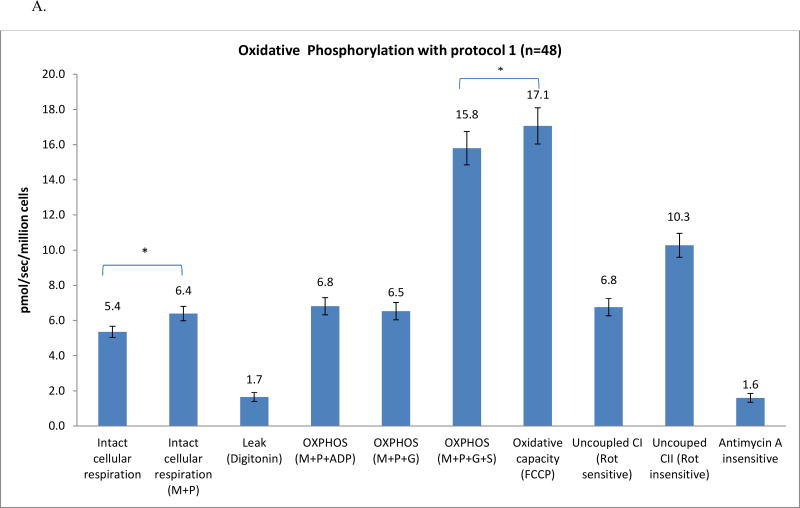
In protocol 1, the intact cellular respiration rate significantly increased from 5.4 pmol/sec/million cells to 6.4±0.4 pmol/sec/million cells (Fig. 2A) by adding exogenous substrates (malate and pyruvate). To access mitochondria, digitonin at a final concentration of 20 µg/ml was used to permeabilize the plasma membrane while maintaining the integrity of the mitochondrial outer membrane of isolated mononuclear cells [30, 38]. The oxygen consumption rate gradually decreased over 20–30 minutes to 1.7±0.3 pmol/sec/million cells (Fig. 1). The digitonin-permeabilized rate reflects the oxidation of the substrates (malate+pyruvate) in the absence of a significant amount of ADP [30], referred to as the leak rate by Graiger [29]. When the rate reaches this nadir, ADP is added to obtain the ADP-stimulated OXPHOS (6.8±0.5 pmol/sec/million cells), a 3-fold increase over the leak rate. Glutamate is then added, and the rate (6.5± 0.5 pmol/sec/million cells) either does not change or increases slightly. Succinate, the substrate for complex II, was added and the rate was higher (15.8±0.9 pmol/sec/million cells), reflecting the convergence of complex I and II on coenzyme Q (CoQ) [39].
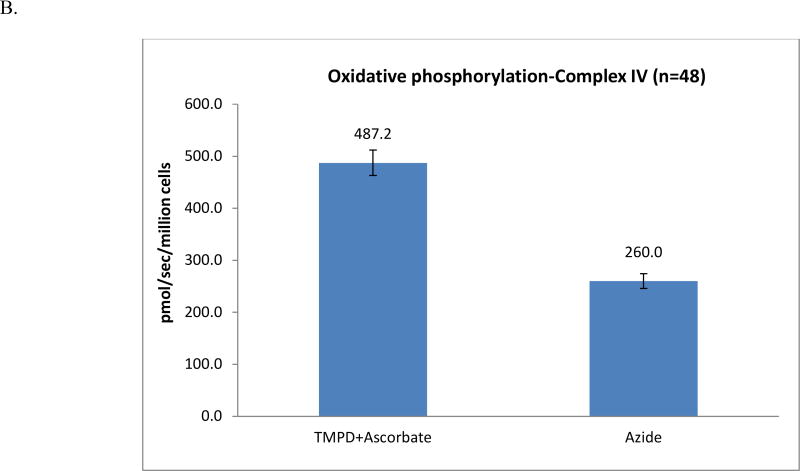
To assess maximal oxidative capacity, the uncoupler (FCCP) was added. A small but significant increase in oxidation rate (17.1±1.0 pmol/sec/million cells) was obtained (7.6% increase, p<0.05). When the maximal oxidation rate was reached, rotenone was added to inhibit complex I. Rotenone-sensitive uncoupled complex I was calculated (10.3 pmol/sec/million cells) after the rotenone-insensitive rate was obtained (6.8±0.5 pmol/sec/million cells). Antimycin A is then injected to inhibit complex III; the antimycin A-insensitive rate (1.6±0.2 pmol/sec/million cells) was the residual oxygen consumption rate. To evaluate uncoupled complex IV, we add TMPD and ascorbate to reduce endogenous cytochrome c; the rate was measured and is displayed in Fig. 2B. To calculate the rate of uncoupled complex IV (227.2 pmol/sec/million cells), we injected sodium azide to inhibit complex IV.
In protocol 2, we measured OXPHOS of fatty acid and complex III oxidation. The rate remained unchanged after malate was added. We then injected palmitoylcarnitine, the substrate of fatty acid, the rate was measured as 5.8±0.4 pmol/sec/million cells, a small, but significant increase compared to intact cell (5.1±0.3 pmol/sec/million cells). To access mitochondria, digitonin (20µg/ml) was added to the chamber and the rate was decreased to 1.7±0.3 pmol/sec/million cells. Then ADP was added to measure the ADP-stimulated oxidation rate of fatty acid (5.4±0.4 pmol/sec/million cells). Rotenone was added to inhibit complex I and to measure the rotenone-insensitive rate (1.6±0.2 pmol/sec/million cells).
To measure the OXPHOS rate of complex III, we added a reduced analog of coenzyme Q, duroquinol, a substrate for complex III, leading to a rate of 27.6±1.1 pmol/sec/million cells. To obtain the maximum oxidative capacity of complex III, an uncoupler, FCCP, was added. The rate of oxidation (26.6±1.2 pmol/sec/million cells) decreased 4% but this was significant compared to the OXPHOS coupled complex III rate (p<0.05). Lastly, antimycin A was added to inhibit complex III; the antimycin A-insensitive rate was 6.5±0.5 pmol/sec/million cells. Raw data are shown in Fig. 2 C.
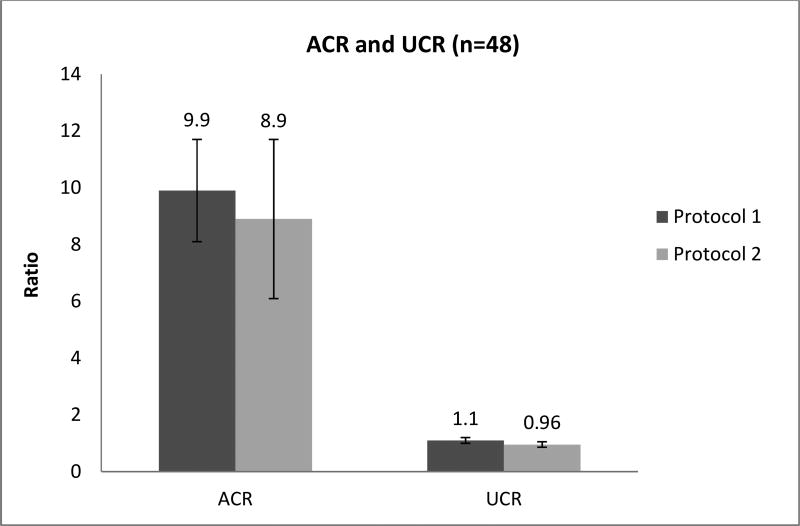
To estimate the coupling of mitochondrial oxidation to the production of ATP (OXPHOS), the acceptor control ratio (ACR) was calculated as the ADP-stimulated OXPHOS of malate+pyruvate+ADP oxidation divided by the leak rate (digtonin-permeabilized respiration). The uncoupled control ratio (UCR) was defined as the maximum oxidative capacity (evoked by FCCP) divided by the ADP-stimulated OXPHOS linked complex I through V of malate+pyruvate+glutamate+succinate. The ratios are presented in Fig. 5; the ACR is high whereas the UCR shows that oxidative capacity is basically equal to OXPHOS capacity.
Figure 5.
Acceptor and uncoupled control ratio
ACR: acceptor control ratio, UCR: uncoupled control ratio. Data are presented as mean ± standard error from 48 patients with prostate cancer without treatment.
3.3 Electron Transport Chain (ETC) Enzyme Activity
The procedures used in the Hoppel Laboratory for ETC enzymatic activity measurement [34, 36, 37] were tailored to fresh human lymphocytes. The mitochondrial ETC activity assays consisted of four measurements, the activity of citrate synthase as a marker enzyme for mitochondrial content, and lactate dehydrogenase activity as a cytoplasmic marker.
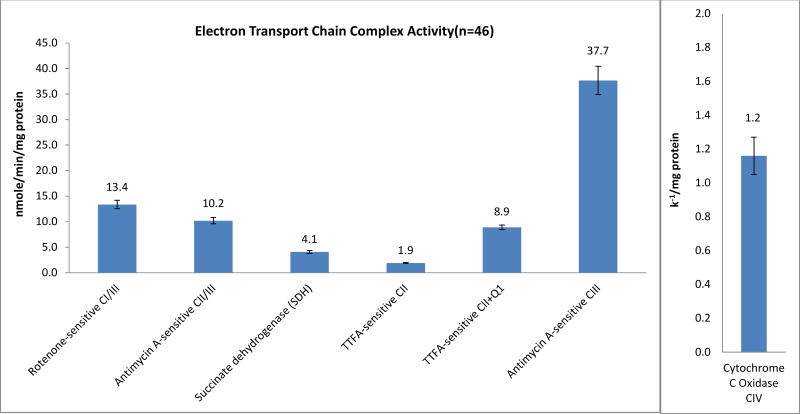
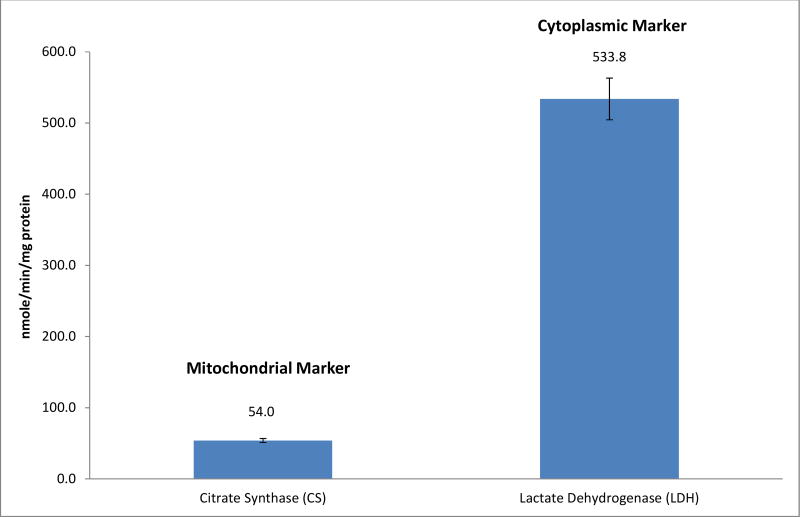
Raw data are showed in Fig. 3. Rotenone-sensitive linked activity of complexes I and III was 13.6±0.8 nmole/min/mg protein, antimycin A-sensitive linked activity of complexes II and III was 10.2±0.6 nmole/min/mg protein. SDH was the assay that measured the first 2 subunits of complex II and the rate was 4.1±0.3 nmole/min/mg protein. Complex II, TTFA-sensitive complex II with CoQ1 was 9.2±0.5 nmole/min/mg protein and without CoQ1 was 1.9±0.1 nmole/min/mg protein. The rate of complex III, antimycin A-sensitive was 37.7±3.0 nmole/min/mg protein, and the complex IV, cytochrome c oxidase was 1.2±0.1 k−1/mg protein. In addition, the content of the mitochondrial marker, citrate synthase, was 54±2.9 nmole/min/mg protein, and the marker of cytoplasm, lactate dehydrogenase was 533.8±30.1 nmole/min/mg protein. (Fig. 4)
Figure 3.
Activity of electron transport chain complexes
Mitochondrial ETC activity of human mononuclear cells. Data are presented as mean ± standard error from 46 patients with prostate cancer without treatments.
Figure 4.
Markers of mitochondria and cytoplasm (n=46)
Mitochondrial and cytoplasmic markers of human mononuclear cells. Data are presented as mean ± standard error from 46 patients with prostate cancer without treatment.
In a preliminary experiment, measurement of ETC activity from frozen lymphocytes or from those stored at 4 degrees after harvest was performed. The activities of citrate synthase, lactate dehydrogenase, succinate dehydrogenase, and complex IV were not different from those of fresh lymphocytes, but the linked activity of complexes I and III, II and III, and complex III activity were markedly decreased (data are not shown).
4. Discussion
A protocol was developed to identify the optimal conditions to collect blood sample and harvest fresh human mononuclear cells. Two substrate-uncoupler-inhibitor protocols to determine integrated mitochondrial function and ETC activity measurements were tailored to analyze these activities of human mononuclear cells. The capacity of OXPHOS-complex I, II, and complex IV was determined by protocol 1, and protocol 2 was used to measure fatty acid oxidation and complex III. As described, protocol 2 was a new approach [30], first applied to human lymphocyte mitochondria, that measures long-chain fatty acid oxidation using the substrate, palmitoylcarnitine; specifically, coupled and uncoupled oxidation of complex III were determined with duroquinol. Additionally, human lymphocyte ETC enzyme activity was investigated.
To determine optimal conditions of collecting blood and harvesting mononuclear cells, we compared different protocols [16, 27, 40, 41]. Vacutainers containing ACD and EDTA have been used to collect human blood in studying peripheral blood monocytes [11, 41], with different phosphate buffers (DPBS, PBS, HBSS) and density gradients (Ficoll-Paque and Lymphoprep™) [11, 27, 42–44]. All Whereas all published reports harvesting lymphocytes are done at room temperature, the resultant mononuclear cells pellet is kept at either room temperature, 4°C,or −70 °C before study [9, 27, 42, 45]. Similar to previous report [43], Lymphoprep™ provides greater yield and higher viability of lymphocytes than Ficoll-Paque; however, there was no statistical significance. Blood processed immediately (0-hour) after blood was collected provides the greatest yield and bioenergetic function of mononuclear cells. The data were similar on mononuclear cell yield, intact cell respiration and OXPHOS at 0-hour and 24 hours in both experiments. There was no significant decrease of mononuclear cell yield and bioenergetic function at 48-hours and 72 hours compared to 24-hours in experiment 1, but 35%–50% decrease was observed in experiment 2 (Table 2). However, blood was collected from distant clinical settings and transported to the mitochondrial research laboratory, to be consistent to generate date we had decided to process the blood within 24-hour after blood was collected.
4.1 OXPHOS
Intact cell respiration, representing endogenous cell respiration, in both protocols as showed in Figures 2A and 2B, are identical and are not significantly difference by t test (p=0.6). In both protocols, intact cell respiration did not change with the addition of malate because there is no transporter of malate in plasma membranes [46]. The rate was small and significantly slow increased after the addition of pyruvate (protocol 1) and palmitoylcarnitine (protocol 2), because there are monocarboxylate transporters for pyruvate and carnitine transporter for palmitoylcarnitine located in plasma membrane [47]. Digitonin-permeabilized cell respiration rate was the same in both protocols; it was 70% rate lower than in intact cells. Digitonin is a detergent used to disrupt the plasma membrane without interference with mitochondrial integrity [48].
We found that the antimycin-A-insensitive rate (1.6 pmol/sec/million cells) in protocol 1 was the same as the rotenone-insensitive rate in protocol 2, indicating a non-mitochondrial respiration rate [29]. In Table 3, data are corrected for non-mitochondrial respiration (antimycin A respiration for protocol 1, rotenone and antimycin A respiration for protocol 2); our data using mononuclear cells for intact cellular respiration, OXPHOS (complex I), OXPHOS (complex I+complex II) and uncoupled complex I are similar to the data reported for controls in peripheral blood monocytes by the Elmer group [22, 49]. Additionally, our data for OXPHOS (complex I+complex II) is similar to that derived from fresh isolated lymphocytes [50].
Table 3.
Integrated Mitochondrial Function in Human Peripheral Blood Mononuclear Cells (n=48)
| Prorocol 1 | (pmol/sec/million cells) mean ± standard error |
|---|---|
| Intact Cell (Malate+Pyruvate) | 4.8 ± 0.2 |
| Permeabilized Cell (Digitonin) | 0.1 ± 0.1 |
| OXPHOS (CI) | 5.0 ± 0.4 |
| OXPHOS (CI+CII) | 14.2 ± 0.9 |
| Maximal oxidative capacity (Uncoupler) | 15.5 ± 0.9 |
| Uncoupled CI | 6.8 ± 0.5 |
| Uncoupled CII | 8.7 ± 0.6 |
| Uncoupled CIV | 227.3 ± 12.9 |
|
| |
| Protocol 2 | |
|
| |
| Intact Cell (Malate+ Palmitoylcarnitine) | 4.2 ± 0.2 |
| Permeabilized Cell (Digitonin) | 0.2 ± 0.1 |
| OXPHOS (Fatty acid oxidation) | 3.8 ± 0.3 |
| OXPHOS (Coupled CIII; Duroquinol) | 21.1 ± 1.1 |
| Uncoupled Complex III (Uncoupler) | 20.1 ± 1.2 |
OXPHOS=oxidative phosphorylation; CI=complex I; CII=complex II; CIII=complex III; CIV=complex IV. Data are subtracted from non-mitochondrial respiration (antimycin A respiration for protocol 1, rotenone and antimycin A respiration for protocol 2).
The rate of OXPHOS complex I+complex II was 3-fold higher than OXPHOS complex I alone. After the addition of uncoupler, the maximum oxidative capacity was small and slightly increased, although significantly (8%, p=0.02), indicating a small reserve capacity of lymphocyte mitochondria. As described [30], protocol 2 was a novel development to measure OXPHOS studying at fatty acid oxidation using palmitoylcarnitine, a long-chain fatty acid substrate and complex III, with duroquinol, a reduced CoQ analog, a substrate for complex III. The OXPHOS fatty acid oxidation-rotenone sensitive rate was 3.5-fold higher than the leak rate. In addition to investigation of lymphocyte mitochondrial OXPHOS fatty acid oxidation, the uncoupled oxidation using duriquinol as substrate was slightly decreased compared to OXPHOS (4%, p< 0.05) but it was statistically significant as determined by a paired t-test.
As shown in Fig. 5, the ACR of 9.9 (in protocol 1) and of 8.9 (in protocol 2) indicates that oxidative phosphorylation is highly dependent on the presence of ADP. The UCR reflects the respiratory capacity to the rate of oxidative phosphorylation [29, 30]. There was a little difference in the maximum oxidative capacity because the UCR was close to 1 (1.1 in protocol 1 and 0.96 in protocol 2).
4.2 ETC enzyme activity
We presented a comprehensive approach to evaluating the lymphocyte ETC including the rotenone-sensitive linked activity of complex I and III, the antimycin A-sensitive linked activity of complex II and III, complex II activity as the TTFA-sensitive with CoQ1 or without CoQ1 and SDH, the first 2 subunits of complex II, the antimycin A-sensitive of complex III activity, and cytochrome c oxidase, the complex IV activity. In addition to SDH, the other mitochondrial marker measured was citrate synthase; the cytoplasmic enzyme used was lactate dehydrogenase.
In Table 4, our data are compared to studies on lymphocyte and mononuclear cell ETC activity [25–27, 50]. Some studies reported activity of succinate cytochrome c reductase without indicating antimycin a-sensitive [26, 27, 50]; however, our findings at total activity and the antimycin A-sensitive linked activity of complex II and III are lower than report from adult controls by Negari [27] and from studies in patients at respiratory chain risk by Rustin [26]. Citrate synthase activity is the most commonly used as a mitochondrial enzyme marker [25–27, 50, 51]; additionally, our data at citrate synthase activity is similar to Chretien’s study [50]. Comparing to the previous report of human lymphocyte and mononuclear cell ETC activity, our data provide inclusive analysis of ETC from mononuclear cell mitochondria because others did selective analysis. (Table 4)
Table 4.
Comparison of ETC Enzymatic Assay in Mononuclear Cells and Lymphocytes (nmole/min/mg)
| References | Our data (N=46) | ||||
|---|---|---|---|---|---|
| Measures | Berger I. | Negari S.B. | Drouet M. | Rustin P. | |
| CS | NP | 75 | ND | 47–114 | 54±2.8 |
| SDH | ND | 15 | ND | ND | 4.1± 0.3 |
| I/III | ND | ND | ND | ND | 13.4±0.8 |
| II/III (Without antimycin A-sensitive) | ND | 20 | 24.3–32.9 | 23–46 | 11.2±0.7 |
| II/III (With antimycin A-sensitive) | 10.2±0.6 | ||||
| II−Q1 | ND | ND | ND | ND | 1.9±0.1 |
| II+Q1 | ND | ND | ND | ND | 9.2±0.5 |
| III | ND | ND | 44.2–51.5 | ND | 37.7±3 |
| IV | 0.75±0.12* | 80 | 49.4–50.1 | 69–146 | 1.2±0.1** |
| LDH | ND | ND | ND | 706–1656 | 533.8±30 |
ETC= electron transport chain; CS=citrate synthase; NP=Not provide; ND=Not determined; SDH=succinate dehydrogenase; I/III= rotenone-sensitive linked activity of complex I and III; II/III=antimycin Asensitive linked activity of complex II and III; II−Q1 and II+Q1=complex II activity as the thenoyltrifluoroacetone-sensitive without CoQ1 or with CoQ1; III= antimycin A-sensitive, complex III activity; IV= cytochrome c oxidase, complex IV activity ; LDH= lactate dehydrogenase;
= ratio of complex IV/CS;
= k−1/mg. A total of 46 samples were used for ETC analysis because the technician was on sick leave and not be able to perform 2 assays.
5. Conclusions
We compared different protocols, performing preliminary studies with different conditions of density gradients and saline buffers, and provide optimal conditions to harvest human mononuclear cells from fresh peripheral whole blood. Integrated mitochondrial function as measured from freshly mononuclear cell mitochondrial OXPHOS was determined and the activity of mononuclear cell ETC was analyzed. To our knowledge, this is the first comprehensively report on mononuclear cell mitochondrial OXPHOS and ETC enzyme activity. Molecular and biochemical analysis of mitochondrial OXPHOS and ETC complex activity in peripheral blood mononuclear cells can assist in identifying systemic mitochondrial dysfunction and specific complex defects.
Acknowledgments
We appreciate the analysis of ETC by Hiral Patel and Maria Stoll; Paul Minkler provided figures support.
Funding
This work was supported by the National Institute of Nursing Research, National Institutes of Health (K01 NR015246, 2015–2018); the Oncology Nursing Society Foundation, Pittsburg, PA (RES125833, 2014–2015); and the Center for Mitochondrial Disease, Case Western Reserve University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There are no conflicts of interest to declare.
References
- 1.van den Heuvel L, Smeitink J. The oxidative phosphorylation (OXPHOS) system: nuclear genes and human genetic disease. BioEssays. 2001;23(6):518–525. doi: 10.1002/bies.1071. [DOI] [PubMed] [Google Scholar]
- 2.Davis RE, Williams M. Mitochondrial function and dysfunction: an update. J Pharmacol Exp Ther. 2012;342(3):598–607. doi: 10.1124/jpet.112.192104. [DOI] [PubMed] [Google Scholar]
- 3.Puchowicz MA, et al. Oxidative phosphorylation analysis: assessing the integrated functional activity of human skeletal muscle mitochondria--case studies. Mitochondrion. 2004;4(5–6):377–85. doi: 10.1016/j.mito.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Chandra D, Singh KK. Genetic insights into OXPHOS defect and its role in cancer. Biochimica et biophysica acta. 2011;1807(6):620–625. doi: 10.1016/j.bbabio.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace DC, Fan W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion. 2010;10(1):12–31. doi: 10.1016/j.mito.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace DC, Lott MT, Procaccio V. Chapter 11 - Mitochondrial Medicine: The Mitochondrial Biology and Genetics of Metabolic and Degenerative Diseases, Cancer, and Aging. In: David LR, et al., editors. Emery and Rimoin's Principles and Practice of Medical Genetics. Sixth. Academic Press; Oxford: 2013. pp. 1–153. [Google Scholar]
- 7.Wallace DC. A mitochondrial bioenergetic etiology of disease. J Clin Invest. 2013;123(4):1405–12. doi: 10.1172/JCI61398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutter E, et al. High-resolution respirometry--a modern tool in aging research. Exp Gerontol. 2006;41(1):103–9. doi: 10.1016/j.exger.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Marriage BJ, et al. The use of lymphocytes to screen for oxidative phosphorylation disorders. Analytical Biochemistry. 2003;313(1):137–144. doi: 10.1016/s0003-2697(02)00539-0. [DOI] [PubMed] [Google Scholar]
- 10.Parichehreh V, et al. Exploiting osmosis for blood cell sorting. Biomedical Microdevices. 2011;13(3):453–462. doi: 10.1007/s10544-011-9513-y. [DOI] [PubMed] [Google Scholar]
- 11.Chacko BK, et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest. 2013 doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Amero KK, Bosley TM. Detection of Mitochondrial Respiratory Dysfunction in Circulating Lymphocytes Using Resazurin. Archives of Pathology & Laboratory Medicine. 2005;129(10):1295–8. doi: 10.5858/2005-129-1295-DOMRDI. [DOI] [PubMed] [Google Scholar]
- 13.Leuner K, et al. Peripheral Mitochondrial Dysfunction in Alzheimer’s Disease: Focus on Lymphocyte. Molecular Neurobiology. 2012;46(1):194–204. doi: 10.1007/s12035-012-8300-y. [DOI] [PubMed] [Google Scholar]
- 14.Cortez E, et al. Lymphocytes Mitochondrial Physiology as Biomarker of Energy Metabolism during Fasted and Fed Conditions. The Scientific World Journal. 2012;2012:629326. doi: 10.1100/2012/629326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pecina P, et al. Noninvasive diagnostics of mitochondrial disorders in isolated lymphocytes with high resolution respirometry. BBA Clinical. 2014;2:62–71. doi: 10.1016/j.bbacli.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perl A, Hanczko R, Doherty E. Assessment of Mitochondrial Dysfunction in Lymphocytes of Patients with Systemic Lupus Erythematosus. In: Perl A, editor. Autoimmunity: Methods and Protocols. Humana Press; Totowa, NJ: 2012. pp. 61–89. [DOI] [PubMed] [Google Scholar]
- 17.Karabatsiakis A, et al. Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Translational Psychiatry. 2014;4(6):e397. doi: 10.1038/tp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyrrell DJ, et al. Blood-cell bioenergetics are associated with physical function and inflammation in overweight/obese older adults. Experimental Gerontology. 2015;70(Supplement C):84–91. doi: 10.1016/j.exger.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maestraggi Q, et al. Skeletal Muscle and Lymphocyte Mitochondrial Dysfunctions in Septic Shock Trigger ICU-Acquired Weakness and Sepsis-Induced Immunoparalysis. BioMed Research International. 2017;2017:7897325. doi: 10.1155/2017/7897325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Japiassu AMMDP, et al. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5'-triphosphate synthase activity *. Critical Care Medicine. 2011;39(5):1056–1063. doi: 10.1097/CCM.0b013e31820eda5c. [DOI] [PubMed] [Google Scholar]
- 21.Sjövall F, et al. Patients with sepsis exhibit increased mitochondrial respiratory capacity in peripheral blood immune cells. Critical Care. 2013;17(4):R152–R152. doi: 10.1186/cc12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehinger JK, et al. Mitochondrial Respiratory Function in Peripheral Blood Cells from Huntington's Disease Patients. Movement Disorders Clinical Practice. 2016;3(5):472–482. doi: 10.1002/mdc3.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida S, et al. Evidence of apoptosis and mitochondrial abnormalities in peripheral blood cells of Huntington’s disease patients. Biochemical and Biophysical Research Communications. 2008;374(4):599–603. doi: 10.1016/j.bbrc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Drouet M, et al. Age-associated changes in mitochondrial parameters on peripheral human lymphocytes. Experimental Gerontology. 1999;34(7):843–852. doi: 10.1016/s0531-5565(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 25.Berger I, et al. The Effect of Antiepileptic Drugs on Mitochondrial Activity: A Pilot Study. Journal of Child Neurology. 2010;25(5):541–545. doi: 10.1177/0883073809352888. [DOI] [PubMed] [Google Scholar]
- 26.Rustin P, et al. Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta. 1994;228(1):35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 27.Negari SB, et al. Mitochondrial OXPHOS function is unaffected by chronic azithromycin treatment. J Cyst Fibros. 2013;12(6):682–7. doi: 10.1016/j.jcf.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Stary CM. A high-resolution method for assessing cellular oxidative phosphorylation efficiency: bringing mitochondrial bioenergetics into focus. Focus on "Direct real-time quantification of mitochondrial oxidative phosphorylation efficiency in permeabilized skeletal muscle myofibers". Am J Physiol Cell Physiol. 2016;311(2):C237–8. doi: 10.1152/ajpcell.00203.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol. 2012;810:25–58. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- 30.Ye F, Hoppel CL. Measuring oxidative phosphorylation in human skin fibroblasts. Anal Biochem. 2013;437(1):52–8. doi: 10.1016/j.ab.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Hsiao C-P, Daly B, Hoppel C. Association between Mitochondrial Bioenergetics and Radiation-Related Fatigue: A Possible Mechanism and Novel Target. Archives in Cancer Research. 2015;3(2):14. [Google Scholar]
- 32.Lowry OH, et al. PROTEIN MEASUREMENT WITH THE FOLIN PHENOL REAGENT. Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 33.Hoppel C, Cooper C. An improved procedure for preparation of inner membrane vesicles from rat liver mitochondria by treatment with digitonin. Arch Biochem Biophys. 1969;135(1):173–83. doi: 10.1016/0003-9861(69)90528-1. [DOI] [PubMed] [Google Scholar]
- 34.Krahenbuhl S, et al. Development and evaluation of a spectrophotometric assay for complex III in isolated mitochondria, tissues and fibroblasts from rats and humans. Clin Chim Acta. 1994;230(2):177–87. doi: 10.1016/0009-8981(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 35.Slater EC. The measurement of the cytochrome oxidase activity of enzyme preparations. Biochemical Journal. 1949;44(3):305. doi: 10.1042/bj0440305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoppel CL, et al. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. Journal of Clinical Investigation. 1987;80(1):71–77. doi: 10.1172/JCI113066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krahenbuhl S, et al. Decreased activities of ubiquinol:ferricytochrome c oxidoreductase (complex III) and ferrocytochrome c: oxygen oxidoreductase (complex IV) in liver mitochondria from rats with hydroxycobalamin[c-lactam]-induced methylmalonic aciduria. Journal of Biological Chemistry. 1991;266(31):20998–1003. [PubMed] [Google Scholar]
- 38.Tsai H-H, et al. Exercise Training Alleviates Hypoxia-induced Mitochondrial Dysfunction in the Lymphocytes of Sedentary Males. Scientific Reports. 2016;6:35170. doi: 10.1038/srep35170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gnaiger E. MitoPathways to Complexes I+II. In: Gnaiger E, editor. Mitochondrial Pathways and Respiratory Control: An Introduction to OXPHOS Analysis. OROBOROS: Innsbruck, Austria; 2012. pp. 29–44. [Google Scholar]
- 40.Nauseef W. Isolation of Human Neutrophils from Venous Blood. In: Quinn MT, DeLeo FR, editors. Neutrophil Methods and Protocols. Humana Press; 2014. pp. 13–18. [DOI] [PubMed] [Google Scholar]
- 41.Sumbalova Z, et al. Mitochondrial Physiology Network. OROBOROS: Austria; 2016. Isolation of blood cells for HRR; p. 14. [Google Scholar]
- 42.Pecina P, et al. Noninvasive diagnostics of mitochondrial disorders in isolated lymphocytes with high resolution respirometry. BBA Clin. 2014;2:62–71. doi: 10.1016/j.bbacli.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeo C, et al. Ficoll-Paque versus Lymphoprep: a comparative study of two density gradient media for therapeutic bone marrow mononuclear cell preparations. Regen Med. 2009;4(5):689–96. doi: 10.2217/rme.09.44. [DOI] [PubMed] [Google Scholar]
- 44.Leuner K, et al. Peripheral mitochondrial dysfunction in Alzheimer's disease: focus on lymphocytes. Mol Neurobiol. 2012;46(1):194–204. doi: 10.1007/s12035-012-8300-y. [DOI] [PubMed] [Google Scholar]
- 45.Perl A, Hanczko R, Doherty E. Assessment of mitochondrial dysfunction in lymphocytes of patients with systemic lupus erythematosus. Methods Mol Biol. 2012;900:61–89. doi: 10.1007/978-1-60761-720-4_4. [DOI] [PubMed] [Google Scholar]
- 46.Pajor AM, et al. Molecular properties of the SLC13 family of dicarboxylate and. Pflügers Archiv - European Journal of Physiology. 2006;451(5):597–605. doi: 10.1007/s00424-005-1487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. American Journal of Physiology - Cell Physiology. 1993;264(4):C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- 48.Vercesi AE, et al. Digitonin permeabilization does not affect mitochondrial function and allows the determination of the mitochondrial membrane potential of Trypanosoma cruzi in situ. Journal of Biological Chemistry. 1991;266(22):14431–14434. [PubMed] [Google Scholar]
- 49.Sjövall F, et al. Patients with sepsis exhibit increased mitochondrial respiratory capacity in peripheral blood immune cells. Critical Care. 2013;17(4):R152. doi: 10.1186/cc12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chretien D, et al. Reference charts for respiratory chain activities in human tissues. Clin Chim Acta. 1994;228(1):53–70. doi: 10.1016/0009-8981(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 51.Drouet M, et al. Age-associated changes in mitochondrial parameters on peripheral human lymphocytes. Exp Gerontol. 1999;34(7):843–52. doi: 10.1016/s0531-5565(99)00058-3. [DOI] [PubMed] [Google Scholar]