Abstract
Background
Mechanisms for the development of food allergy in neonates are unknown but are clearly linked in patient populations to a genetic predisposition towards skin barrier defects. Whether skin barrier defects functionally contribute to development of food allergy is unknown.
Objective
The purpose of the study was to determine whether skin barrier mutations, that are primarily heterozygous in patient populations, contribute to the development of food allergy.
Methods
Mice heterozygous for the Flgft and Tmem79ma mutations were skin sensitized with environmental allergens and food allergens. After sensitization, mice received oral challenge with food allergen and then inflammation, inflammatory mediators, and anaphylaxis were measured.
Results
We define development of inflammation, inflammatory mediators, and food allergen-induced anaphylaxis in neonatal mice with skin barrier mutations following brief concurrent cutaneous exposure to food and environmental allergens. Moreover, neonates of allergic mothers have elevated responses to suboptimal sensitization with food allergens. Importantly, the responses to food allergens by these neonatal mice were dependent on genetic defects in skin barrier function and on exposure to environmental allergens. Blockade of ST2 during skin sensitization inhibited development of anaphylaxis, antigen-specific IgE and inflammatory mediators. The neonatal anaphylactic responses and antigen-specific IgE were also inhibited by oral pre-exposure to food allergen but, interestingly, this was blunted by concurrent pre-exposure of the skin to environmental allergen.
Conclusion
These studies uncover mechanisms for food allergy sensitization and anaphylaxis in neonatal mice that are consistent with features of human early life exposures and genetics in clinical food allergy and demonstrate that changes in barrier function drive development of anaphylaxis to food allergen.
Keywords: sensitization, anaphylaxis, food allergy, peanut, chicken egg ovalbumin, filaggrin, mattrin, alternaria, house dust mite, skin, ST2
Introduction
Acute allergic responses range from mild itching in mouth, hives, abdominal discomfort, to severe life-threatening anaphylaxis. There is no cure. Therapy includes strict avoidance of the food allergens and management of reactions with use of epinephrine for accidental consumption, though recent efforts in tolerance induction have shown some promise. Food allergy prevalence reports indicate 6% in young children have food allergies and up to an 18% increase in prevalence from 1997 and 20071–3.
Food allergies often develop early in life. Offspring of allergic mothers or fathers have increased incidence of food allergies4. The risk for allergic disease in humans is associated with in utero and early exposures to environmental factors5. In animal studies, we and others have reported that offspring from allergic mothers are predisposed to allergic lung responses to suboptimal allergen doses6–15. It is not known whether offspring of allergic mother mice have increased responsiveness to sensitization to food allergens and food-induced anaphylaxis.
In adult mouse models of food allergy, tolerance to food antigens is broken by oral administration of food antigens with cholera toxin (CT)16–19 or staphylococcal enterotoxin B (SEB)20. However, cholera is not prevalent in individuals with food allergies. Moreover, cholera outbreaks have not occurred in the countries with prevalent food allergies21–25. For SEB, the induction of food allergy by oral co-administration of food antigen and SEB is reported by some20 but not others26 to break tolerance to food antigens in wild type mice. The CT and SEB models have been used to examine approaches for inhibition of established food allergy. However, mechanisms for development of food allergy from environmental exposure are not known.
Early in life, food allergy is often associated with atopic dermatitis. Atopic dermatitis results in skin barrier dysfunction and itchy, red, swollen, and cracked skin. Early-onset of atopic dermatitis is associated with increased risk of allergic sensitization to food allergens by age 2 and asthma by age 727, 28. Moreover, IgE-mediated food allergy is observed in up to 35% in children affected with atopic dermatitis29. This suggests that interventions early in life may influence the association of atopic dermatitis with food allergies30.
Atopic dermatitis in humans is associated with mutations in the skin barrier genes SPINK5, a serine peptidase inhibitor, CDSN, a structural protein of corneodesmosomes in the stratum corneum, Flg, filaggrin, a filament-associated protein that crosslinks keratin fibers in keratinocytes31 and Matt, mattrin a transmembrane protein involved in stratum corneum barrier function32. Whether atopic patients without these skin barrier mutations have other unidentified mutations is not known. Filaggrin expression in keratinocytes can also be decreased during inflammation with IL-4 and IL-13 expression33. Several Flg mutations are a risk factor for development of allergies34, 35, including peanut allergy36, 37. Flg and Matt genes affect skin barrier function. Mutations in both of these genes predispose humans and mice to atopic dermatitis32. Flaky tail mice, with mutations in Flg (Flgft) and Matt (Tmem79ma)32, are used in studies of the generation of localized sites of atopic dermatitis, skin allergic responses and lung asthmatic responses34, 38–40. A report indicates that transcriptomes for inflammation vary between unchallenged flaky tail mice and Atopic Dermatitis patients41, but it is important to appreciate the differences in skin exposures and thus stimulation for humans versus mice in barrier facilities. Although it is speculated that decreased barrier function increases exposure to allergens, it is not known how skin barrier mutations impact sensitization to food allergens.
Atopic dermatitis and allergies are associated with fungal and house dust mite exposure42. House dust mites, the fungal allergen Alternaria alternata and food antigens such as peanuts are present in house dust43–47. House dust mite is a ubiquitous indoor allergen and the fungal allergen Alternaria alternata is a ubiquitous indoor and outdoor allergen to which neonates are exposed. Healthy and atopic dermatitis skin possess the fungi Alternaria and Malassezia48. Fungal extracts exhibit broad cross-reactivity among fungi42. Compared to healthy individuals, patients with atopic dermatitis show a higher frequency of IgE reactivity to the skin yeast Malassezia49–51 and the allergen house dust mite51. Atopic dermatitis patients can also have Alternaria-reactive IgE52. The mechanisms for these associations with atopic dermatitis are unclear but may be attributed to a combination of dysfunctional skin barrier, genetics, and environmental exposures early in life49.
In humans, expression of skin barrier mutations is predominantly heterozygous53. Thus, we used FT mice as a representative of skin barrier defects. Heterozygous FT+/− were used where the “−“ indicates the presence of the mutation. In our studies, neonatal FT+/− mice developed allergic skin responses with increased Th2 cytokines, chemokines, and antigen-specific IgE after skin sensitization with peanut or chicken egg ovalbumin and co-exposure to Alternaria alternata or house dust mite. These sensitized neonates had anaphylaxis in response to oral gavage with the food antigen. The responses required the heterozygous expression of the skin barrier mutations and required the co-stimulation with Alternaria alternata or house dust mite extract. Blockade of ST2 during skin sensitization inhibited anaphylaxis and inflammatory mediators. Interestingly, the FT+/− offspring of allergic wild type mothers had elevated responses to suboptimal skin challenges as compared to FT+/− offspring of non-allergic wild type mothers. In addition, skin pre-exposure to Alt reduced tolerance induced by oral pre-exposure to food allergen. These mechanistic studies reflect early life exposures to allergens and genetic mutations that are consistent with the clinical manifestations of the development of food allergies.
Materials and Methods
Mice
C57BL/6J mice were from Jackson Laboratories, Bar Harbor, Maine. Flaky tail mice (FT−/− mice) with homozygous mutations in Flgft/ft/Tmem79ma/ma and housed under specific pathogen-free barrier conditions. The Flaky tail mice on the C57BL/6 background have been previously described32, 34, 38–40. The mouse bedding was ¼ inch Coarse Aspen Sani Chips (catalog #7115, Harlan Lab) and the diet was Teklad Irradiated LM-485 mouse diet (catalog #7912, Teklad). All mice were randomly selected for the studies. The studies are approved by the Northwestern University Institutional Review Committee for animals.
Allergens
Alternaria alternata extract (Alt, catalog #XPM1D3A2.5) and house dust mite (HDM) extract (Dermatophagoides pteronyssius extract, catalog #XPB82D3A2.5) were from Greer Labs. These are standard extracts from GREER used for patient immunotherapy. Chicken egg ovalbumin fraction V (OVA, catalog # 9006-59-1) was from Sigma. To generate peanut extract (PNE), peanuts (Planters Lightly Salted Dry Roasted Peanuts) were ground and then 25g were homogenized in 250ml of 20 mM Tris buffered (pH 7.2) saline (TBS)54. This was stirred for 2 hours at room temperature and centrifuged at 3000 g for 30 min. The aqueous middle layer was collected and centrifuged at 1600 g for 45 min to remove residual particles and fat. The aqueous layer was collected. Protein concentrations were determined by Pierce BCA Protein Assay Kit (Thermo Fisher Sci). Aliquots were stored at −20°C. SDS-PAGE after co-incubation of the allergens in vitro was performed as described in the Supplemental Methods.
Allergen sensitization
A. Generation of allergic mothers
Female mice at 4-6 weeks old were sensitized by intraperitoneal injection (200 μl) of OVA grade V (5 μg)/alum (1mg) or saline/alum (1mg) on days 0 and 76–8, 14, 15, 55 and then received nebulized saline or 3% (w/v) OVA in saline for 20 min three times a week on weeks 4, 8, 12, and 16 weeks. At the last challenge on week 16, allergic or nonallergic female mice were mated with 3 to 4 month old wild type or homozygous flaky tail (FT−/−) non-allergic males.
B. Treatment of offspring
FT+/− offspring of the mating of wild type (WT) C57BL/6 females with FT−/− males were confirmed by genotyping (Supplemental Methods). At 3 days old, pups were gently taped five times on the back with 3M surgical hypoallergenic paper tape and wiped once with 4% SDS in sterile deionized water56 on a sterile gauze. After 3 minutes, Alt extract or HDM extract (10 μg protein in 5 μl sterile saline) was applied to the pup skin and then, immediately PNE or OVA (100 μg protein in 10 μl sterile saline) was applied to the same area on the back. The pups were placed in a cage without the mother for 40 minutes57. Then to prevent antigen consumption during grooming by the mother, the pups were washed gently with water on a paper towel and wiped dry to remove free antigen from the surface57 before placing the pups back with the mother. The antigen applications were repeated 1 to 2 times per week as indicated in the figures, but on pups that are older than 5 days, the fur was shaved before tape stripping. The taping did not overtly disrupt the skin but gently removed dried skin after birth or hair after shaving (Supplement Fig 1).
C. Tolerance induction
In studies with oral tolerance, pups received 100 μl of 10 mg/ml PNE by gavage on 4 days before skin sensitization as indicated in the Figure.
D. Blockade of ST2
For the pups treated with anti-ST2 (catalog # MAB10041, R&D Systems, Inc) or isotype control (catalog #400544, Biolegend), the pups received an intraperitoneal injection of 8 μg antibody in saline per g pup at 1 hr before skin sensitization #1, 12 μg antibody/g pup at 1 hr before skin sensitization #2 and #3, and then 15 μg antibody/g pup at 1 hr before skin sensitization #4.
Oral Antigen Challenge, Anaphylaxis and Tissue Analysis
At 48 hours after the last allergen skin sensitization indicated in the figure timelines, baseline rectal temperatures of the pups were taken using a BAT-12 Microprobe Thermometer with a RET-4 thermocouple sensor type T rectal probe for neonatal mice (Physitemp Instruments Inc). Then, the pups received PNE or OVA by gavage (100 μl of 10 mg protein/ml sterile saline) using a 24 gauge gavage needle (Pet Surgical, Agoura Hills, CA). Rectal temperatures were taken every 15-20 minutes for up to 105 minutes. The pups were placed back with the mothers until pups were euthanized and tissues collected. Tissue sections were stained for eosinophils and mast cells or examined by qRT-PCR for inflammatory mediators (Supplemental methods). Eosinophils and mast cells in tissue sections were counted by light microscopy in 10 high powered fields in a blinded fashion. Serum anti-peanut antibodies (IgE, IgG2b, IgG1, and IgA), serum anti-OVA antibodies, and Mcpt-1 were determined by ELISA (Supplemental Methods). Endotoxin was measured using a chromogenic quantitation kit (Supplemental Methods).
Statistics
Data were analyzed by a one way ANOVA followed by Tukey’s multiple comparisons test or T-test (SigmaStat, Jandel Scientific, San Ramon, CA). Presented are the means ± the standard errors. The statistical analysis for the change in temperature was done using the maximum change in temperature. The data in the figures include both genders for the offspring because there were no differences in outcomes by gender (Supplement Figure 2).
Results
Skin sensitization of neonates with skin barrier gene mutations induced responsiveness to oral food allergen-induced anaphylaxis
Because humans are predominantly heterozygous for skin barrier mutations, skin of neonatal mice with heterozygous mutations were exposed to environmental allergens and food allergens. Flaky Tail (FT) mice were used as a model representative of skin barrier mutations. Briefly, wild type female mice were bred with male FT−/− mice that are homozygous for the filaggrin flaky tail (ft) mutation and Tmem79 ma mutation, yielding offspring heterozygous FT+/− pups for both the Flgft and Tmem79ma mutations (Fig 1A). Pups were sensitized, on postnatal day (PND) 3 and then every 2-3 days (Fig 1A). To remove dry shedding skin after birth or fur after shaving, the pup skin received 5 gentle tape applications with 3M Micropore paper hypoallergenic surgical paper tape, a gentle, non-irritating general purpose paper tape that is gentle to the skin, resulting in very little trauma to the neonate skin (Supplement Figure 1); this is in contrast to the skin trauma induced by more adhesive tapes such as 3M Tegaderm adhesive film58. For applications after PND9, the pups were shaved to remove fur which can induce small nicks (Supplement Figure 1), analogous to infant scratching with atopic dermatitis. This skin site was then wiped once with 4% SDS in water which resembles cleansing wipe use on infants. Then, the pups received cutaneous application of Alternaria alternata extract (ALT), house dust mite extract (HDM), ALT with HDM or saline Figure 1A and B and Supplement Figure 2A and B. Immediately after this, a food allergen, peanut extract (PNE) (Fig 1B) or OVA (Supplement Fig 2B), was applied to the skin. Two days after the last skin application, the pups received oral gavage with 100 μg PNE (Fig. 1B) or OVA (Supplement Fig. 2B) and rectal temperature was monitored. All offspring were FT+/− as determined by genotyping (data not shown). Anaphylaxis occurred only in the pups that received skin application of HDM or ALT with food allergens PNE (Fig. 1B) or OVA (Supplement Fig. 2B). Pups with PNE-only or saline on the skin did not have anaphylaxis with oral challenge so for subsequent experiments, the PNE-only skin-treated groups served as the negative control group. For pups in Fig. 1B, there were no differences in anaphylaxis by gender (Supplement Fig. 3A) and no differences for gender or treatment groups in initial body temperature immediately prior to oral challenge (Supplement Fig. 3B). Thus, ALT or HDM were required for skin sensitization with PNE or OVA in the FT+/− pups. Serum contained anti-PNE specific IgE and IgG2b, but not serum anti-PNE specific IgG1 or monomeric IgA, in pups with co-application of HDM or ALT with PNE (Fig 1C). There were no anti-PNE specific antibodies in pups with PNE only skin sensitization (Fig. 1C). Serum of OVA sensitized pups contained anti-OVA-specific IgE with co-application of HDM or ALT with OVA, but not OVA alone (Supplement Fig 2C).
Figure 1.
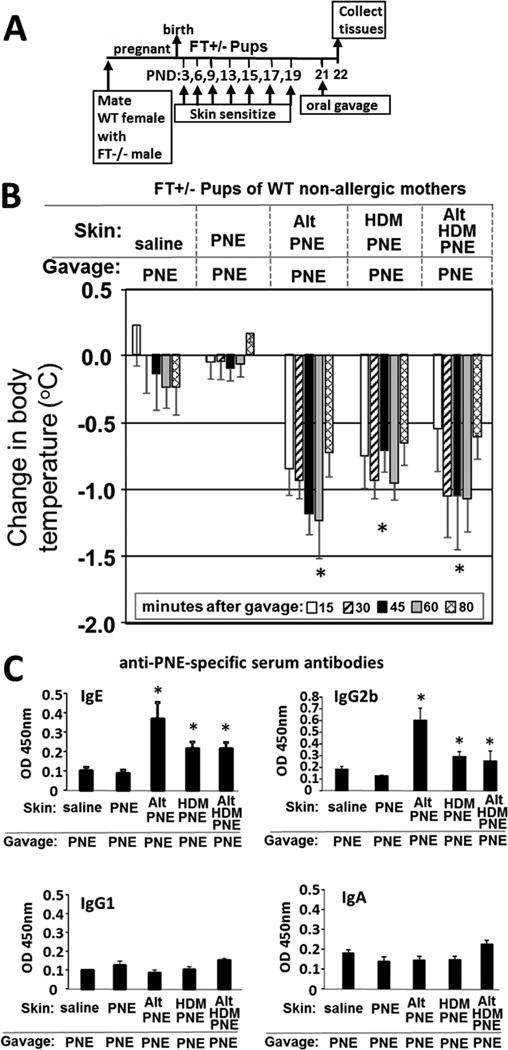
Skin sensitization of neonates with skin barrier gene mutations induced responsiveness to oral food allergen-induced anaphylaxis. (A) Timeline for mating and for FT+/− pup treatments with food and environmental allergens. (B) Food allergen-induced temperature (oC) changes on day 21. (C) Anti-peanut specific serum IgE, IgG1, IgG2b, and IgA as determined by ELISA. N=8-10/group. The data in all figures include both genders for pups because there were no differences in outcomes by gender (Supplement Data Figure 2). *, p<0.05 as compared to the saline skin-sensitized group and the PNE-only skin-sensitized group. Alt, Alternaria alternata extract; FT−/−, flaky tail mice homozygous for filaggrin and mattrin mutations; FT+/−, flaky tail mice heterozygous for filaggrin and for mattrin mutations; HDM, house dust mite extract; OVA, chicken egg ovalbumin; PND, postnatal day; PNE, peanut extract; WT, wild type.
The pup skin was not exposed to appreciable amounts of endotoxin in PNE, Alt, HDM or OVA but there wasb considerable endotoxin in fecal pellets in the mouse cage (Supplement Fig. 5). To determine whether ALT or HDM, which may contain proteases, altered the molecular weights of the major proteins in the PNE, ALT or HDM were incubated with PNE (Supplement Fig. 4). The amounts of ALT, HDM, PNE, Alt/PNE or HDM/PNE, that had been applied to the pup skin for 40 minutes (Figure 1B), were placed in a tube for 20 or 40 minutes at room temperature and then examined by SDS-PAGE and staining with Simply Blue protein dye (Supplement Fig. 4). There was no change in the size of the major protein bands (Supplement Fig. 4).
Skin barrier gene mutations were required for oral food allergen-induced anaphylaxis and generation of anti-peanut specific IgE in FT+/− offspring of allergic and non-allergic mothers
To determine whether the mutations in the FT+/− pups were required for the skin sensitization to food allergens, non-allergic wild type female mice or OVA-allergic wild type female mice (Fig 2A) were mated with wild type or FT−/− male mice. The OVA-allergic wild type females were allergic as determined by expression of serum OVA-specific IgE on gestational day 18 (Supplement Fig. 6). The FT+/− pups were skin sensitized with allergen and then received one oral gavage with PNE as in Fig 2A. Anaphylaxis responses of FT+/− pups of non-allergic mothers occurred in the ALT/PNE sensitized pups (Fig. 2B group 2), but not in the pups sensitized with PNE alone, and not in ALT/PNE-sensitized wild type pups (Fig. 2B Groups 1,3). For pups of allergic mothers, anaphylaxis responses occurred in the ALT/PNE sensitized or HDM/PNE heterozygous FT+/− pups (Fig. 2B Groups 5,6) but not in the pups sensitized with PNE alone (Fig. 2B Group 1) and not in wild type pups with skin treatments with saline, PNE, ALT/PNE or HDM/PNE (Fig 2B Groups 4,7,8,9,10). The Group 2 pups of non-allergic mothers and Group 5 pups of allergic mothers are not significantly different. These data demonstrate that the skin barrier mutations were required for development of the food allergen responses.
Figure 2.
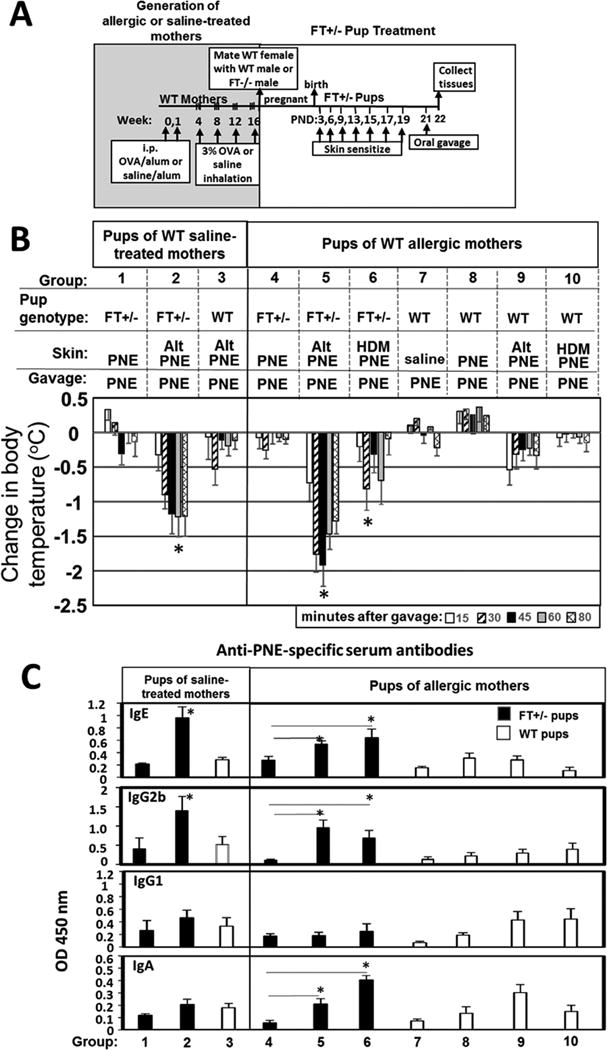
Skin barrier gene mutations are required for oral food allergen-induced anaphylaxis and generation of anti-peanut specific IgE in FT+/− offspring of allergic and non-allergic mothers. (A) Timeline for mating of females with FT−/− males or WT males and for pup treatments with food and environmental allergens. (B) Food allergen-induced temperature changes on day 21. (C) Anti-peanut specific antibodies were measured by ELISA. Serum was from pups in Figure 2B. Anti-peanut specific serum IgE was measured using a 1/10 serum dilution and a 12 minute TMB color development. Anti-peanut specific serum IgG2b was measured using a 1/1000 serum dilution and a 12 minute TMB color development. Anti-peanut specific serum IgG1 was measured using a 1/20,000 serum dilution and a 6 minute TMB color development. Anti-peanut specific monomeric serum IgA was measured using a 1/10 serum dilution and a 30 minute TMB substrate color development. N=8-10/group. *, p<0.05 as compared to the corresponding PNE only skin-sensitized groups, ie as compared group 1 for FT+/− pups of saline treated mothers, as compared to group 4 of FT+/− pups of allergic mothers. For WT pups, there was no difference as compared to group 8. Alt, Alternaria alternata extract; FT−/−, flaky tail mice homozygous for filaggrin and mattrin mutations; FT+/−, flaky tail mice heterozygous for filaggrin and for mattrin mutations; HDM, house dust mite extract; PND, postnatal day; PNE, peanut extract; WT, wild type.
Serum anti-PNE specific IgE was generated in FT+/− pups of non-allergic and allergic mothers that were sensitized with ALT/PNE but not in the pups sensitized with PNE alone, nor in the ALT/PNE-treated wild type pups (Fig 2C). Interestingly, the FT+/− pups of allergic mothers also had elevated relative levels of serum anti-peanut IgG2b (Fig. 2C). There were increased relative levels of serum anti-PNE specific monomeric IgA in pups of allergic mothers (Fig. 2C Group 5,6) but not pups of non-allergic mothers (Fig. 1C and 2C Group 2). The role of serum monomeric IgA in allergy is incompletely understood59.
Allergic mothers transmitted to FT+/− pups an elevated responsiveness to oral food allergen anaphylaxis after suboptimal pup skin sensitization
Food allergy in pups of allergic mothers was examined because, pups of allergic mothers have elevated allergic lung responses to a suboptimal number of sensitizations with allergen, even if the allergen of the pup differs from the allergen of the mother7, 8, 14, 15, 60, 61. This is also evident in humans where family history of allergic disease is one of the strongest risk factors for development of atopic disease in children4, 62–66. It is not known whether offspring of allergic mothers respond to suboptimal food allergen sensitization. It was determined whether 3 to 6 skin sensitizations were suboptimal in FT+/− pups of non-allergic mothers but sufficient for anaphylaxis in FT+/− pups of allergic mothers (Fig 3A). The oral challenge was designed to be at least 13 days after the first skin sensitization to ensure time for activation of acquired immune responses including T cell responses and plasma cell generation. After 3 skin sensitizations, anaphylaxis did not occur in pups of non-allergic mothers, but anaphylaxis did occur in pups of allergic mothers (Figure 3B upper panel). At 4, 5 or 6 skin sensitizations, the pups of allergic and non-allergic mothers generated anaphylactic responses (Fig 3B upper panel). The younger pups had lower basal body temperatures (Supplement Fig 7). The temperature drops were greatest in younger pups (Fig. 3B), consistent with the reduced ability of newborns and young infants to control temperature67–70. The FT+/−-pups sensitized 3 to 6 times with only PNE did not exhibit anaphylaxis to PNE (Fig 3B lower panel). Serum anti-peanut IgE was present in FT+/− pups of allergic mothers after 3 skin sensitizations (Fig 3C middle panel), whereas serum anti-peanut IgE was only detected after 4 skin sensitizations in pups of non-allergic mothers (Fig 3C right panel). Furthermore, Alt/PNE skin-treated pups had increased mast cell protease 1 in the serum as compared to PNE skin-treated pups (Fig 3D).
Figure 3.
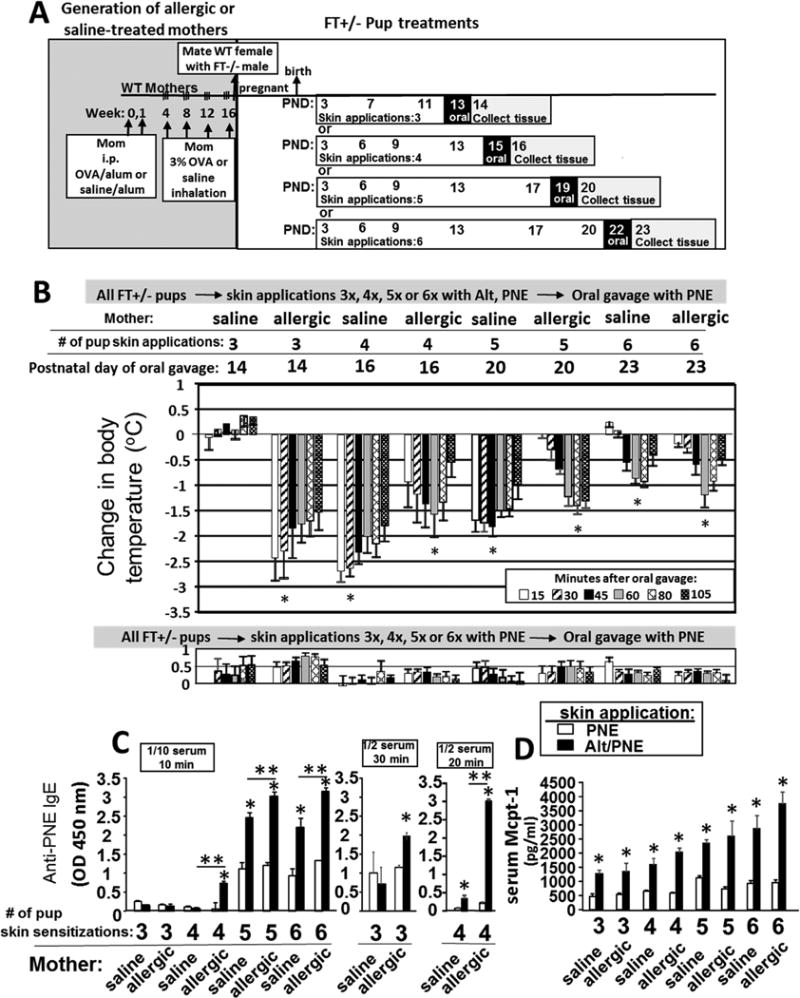
Allergic mothers transmitted to FT+/− pups an elevated responsiveness to oral food allergen anaphylaxis after suboptimal pup skin sensitization. (A) Timeline for induction of OVA-induced lung inflammation in females, mating of females with FT−/− (FLGft/ft/Tmem79ma/ma) males or WT males, and for pup treatments with food and environmental allergens. (B) Food allergen-induced temperature changes after oral gavage on days 13, 15, 19 or 22. (C) Serum was from pups in (B). In the panel on the left, anti-peanut specific serum IgE was measured using a 1/10 serum dilution and a 12 minute TMB substrate color development. In the two panels on the right, anti-peanut specific serum IgE was measured using a 1/2 serum dilution and a 20-30 minute TMB color development. (D) Serum Mcpt-1 was measured by ELISA. N=8-10/group. *, p<0.05 as compared to the corresponding PNE only skin-treated group. **, p<0.05 as compared to the indicated groups of Alt/PNE-treated pups from non-allergic saline-treated mothers.
Cells and mediators of allergic responses were examined in the pups from Figure 3. In the pups with 6 Alt/PNE skin sensitizations, there was an increase in total number of skin mast cells in pups of allergic mothers but not pups of non-allergic mothers (Fig 4A,B). Although there was no increase in mast cell numbers in pups of non-allergic mothers (Fig 4A,B), these mast cells degranulated (Fig 4C) and the pups underwent anaphylaxis (Fig 3B). The number of degranulated skin mast cells, a measure of mast cell activation, was increased in Alt/PNE-sensitized pups of allergic and non-allergic mothers as compared to pups with PNE-only treated skin (Fig 4C). There were more degranulated skin mast cells in Alt/PNE-sensitized pups of allergic as compared to Alt/PNE-sensitized pups of non-allergic mothers (Fig 4C). Skin and blood eosinophils were also increased in pups sensitized with Alt/PNE as compared to pups with PNE-only treated skin (Fig 4D–F). Skin and blood of Alt/PNE sensitized pups of allergic mothers had significantly more eosinophils than those of non-allergic mothers (Fig 4D–F). Since allergic inflammation is initiated in epithelial tissues by production of cytokines and chemokines, it was determined whether skin sensitization induced skin expression of CCL11, thymic stromal lymphopoietin (TSLP), and IL-33. CCL11, TSLP and IL-33 were increased in skin of pups with skin applications of Alt/PNE for pups as compared to pups with PNE-only treated skin (Fig 4G). Moreover, at 3 skin applications of Alt/PNE, the pup skin of allergic mothers had higher amounts of CCL11 and TSLP than the skin of pups from non-allergic mothers (Fig 4G). At 6 skin applications, there was no difference in CCL11 and TSLP for Alt/PNE treated pups of allergic and non-allergic mothers, suggesting that maximal induction may have occurred. In pups with 6 skin applications, TSLP was also elevated in the jejunum at 6 hrs after only a single oral gavage with PNE (Fig 4G). Eosinophils and degranulated mast cells were similarly increased in the intestines of the Alt/PNE sensitized pups of allergic and non-allergic mothers as compared to pups with PNE-only skin-treated pups (Fig. 5).
Figure 4.
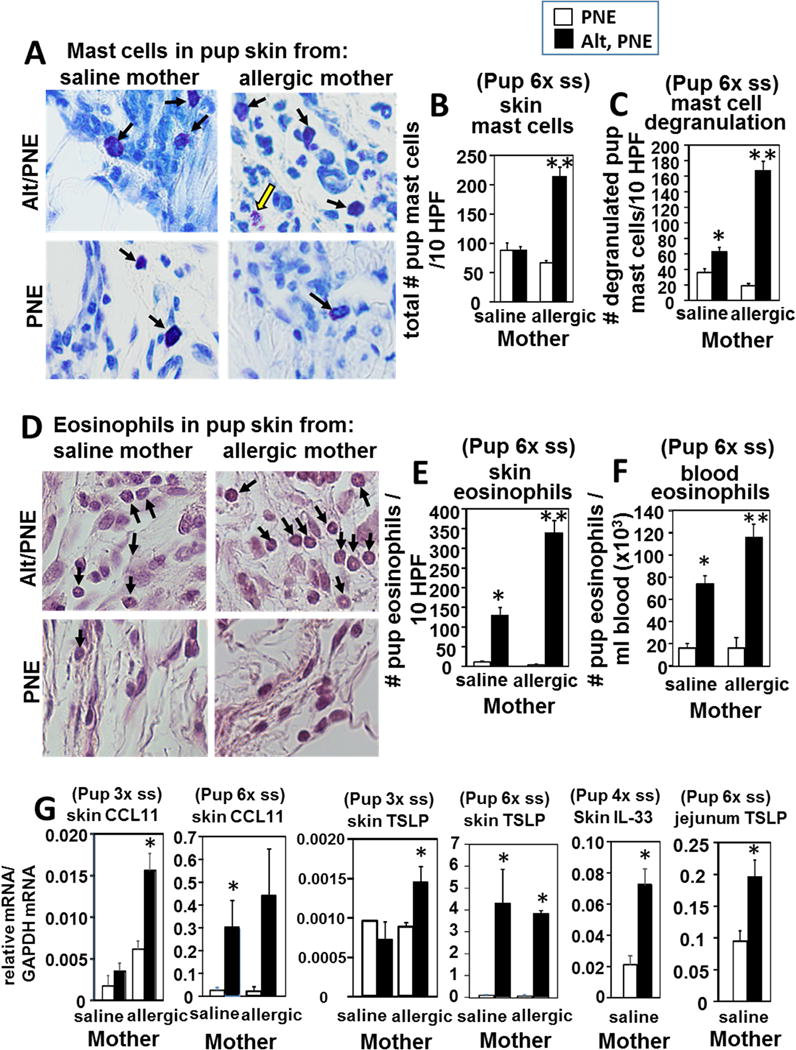
Skin Inflammation and mediators of allergic responses are elevated in Alt/PNE skin sensitized FT+/− pups. Pup skin tissues from the site of skin sensitizations and pup blood from the pups in Figure 4B. (A) Shown are representative micrographs of toluidine-stained pup skin tissue sections from the site of 6 skin sensitizations and skin collected on PND22. Black arrows indicate mast cells. Yellow arrow indicates a degranulated mast cell. (B) Pups had 6 skin sensitizations (6×ss) and skin was collected on PND22. Presented is the number of skin mast cells in 10 high powered fields at 40× (HPF) per pup tissue section from 8-10 pups. (C) Pups had 6 skin sensitizations and skin was collected on PND22. Presented is the number of degranulated skin mast cells in 10 high powered fields at 40× (HPF) per pup tissue section from 8-10 pups. (D) Shown are micrographs of eosin-stained skin tissue section with a light hematoxylin counter stain from pups with 6 skin sensitizations and skin collected on PND22. Black arrows indicate eosinophils. (E) Number of eosinophils in 10 high powered fields at 40× (HPF) from pups with 6 six sensitizations and skin collected on PND22. (F) Number of blood eosinophils as determined by discomb staining and hemacytometer counting from pups with 6 skin sensitizations and blood collected on PND22. (G) RT-qPCR of pup skin TSLP and CCL11 for pup skin after 3 skin sensitization (3× ss) with skin collected PND12 or 6 skin sensitizations (6×ss) with skin collected on PND22. RT-qPCR of IL-33 after 4 skin sensitizations (4× ss) with skin collected PND16. RT-qPCR of TSLP for jejunum from pups with 6 skin sensitizations (6× ss) and intestine collection on PND22. N=8-10/group. *, p<0.05 as compared to the corresponding PNE only skin-treated group. **, p<0.05 as compared to the Alt/PNE-treated pups from non-allergic saline-treated mothers and compared to corresponding PNE skin-treated group from allergic mothers.
Figure 5.
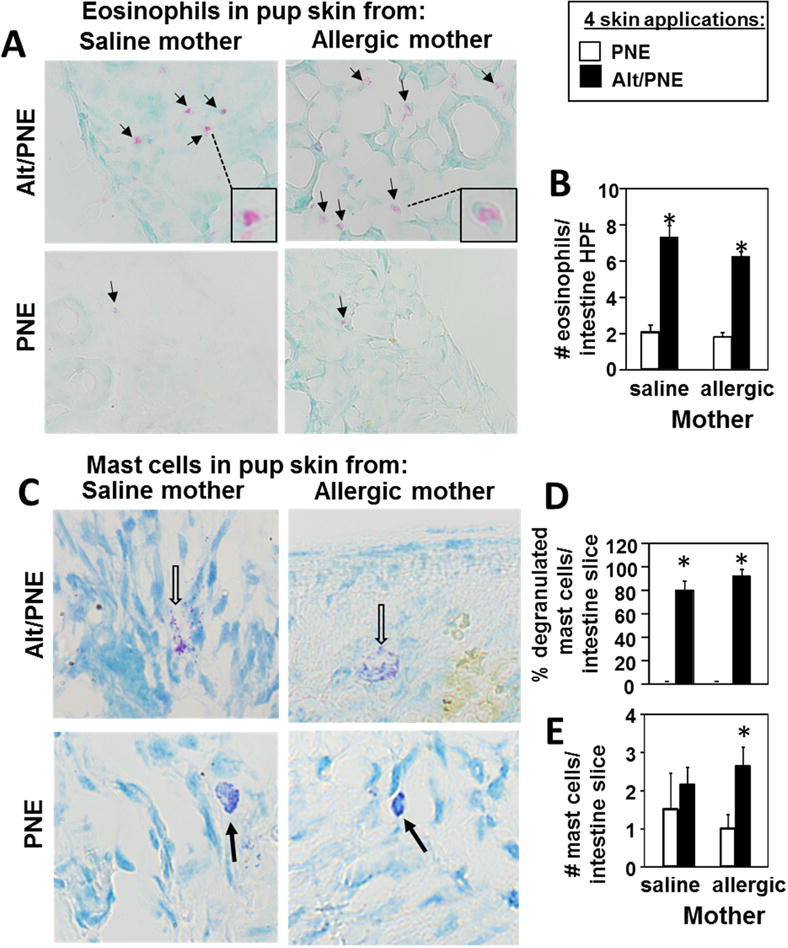
Intestinal Inflammation is elevated in Alt/PNE skin-sensitized FT+/− pups. Intestines were collected from pups from Figure 4B with 4 skin sensitizations. (A) Shown are micrographs of eosin-stained intestine tissue section with a light methyl-green counter stain. Black arrows indicate eosinophils. Box insert contains enlarged image of eosinophil indicated by dotted line. (B) Number of eosinophils per high powered 64× field (HPF) of pup intestine tissue section. (C) Shown are representative micrographs of toluidine-stained pup intestine tissue sections. Open arrows indicate degranulated purple mast cells. Closed black arrows indicate intact dark purple mast cells. (D) Percent degranulated mast cells per intestine tissue slice. (E) Number of cells per pup intestine tissue slice. N=8-10/group. *, p<0.05 as compared to the corresponding PNE only skin-treated group.
Anti-ST2 administration at time of skin sensitization blocked oral antigen-induced anaphylaxis
IL-33 has a key role in initiation of allergic responses in skin and lung71, 72, regulates mast cells73, 74 and reduces anaphylaxis in a food model with 49 days of sensitization75. In Figures 3–6, IL-33 was increased and mast cells degranulated in the skin and intestine. Therefore, we determined whether blocking the IL-33 receptor, ST2, blocks anaphylaxis. FT+/− pups were treated with blocking anti-ST2 antibodies or IgG2b isotype control antibodies 1 hr before each skin sensitization as in Figure 6A,B. Anti-ST2, but not the isotype control antibody, blocked oral-PNE-induced anaphylaxis (Fig. 6B), blocked the increase in anti-PNE-specific IgE, IgG2b and IgG1 (Fig. 6C) and blocked the increase in serum Mcpt-1 (Fig. 6D).
Figure 6.
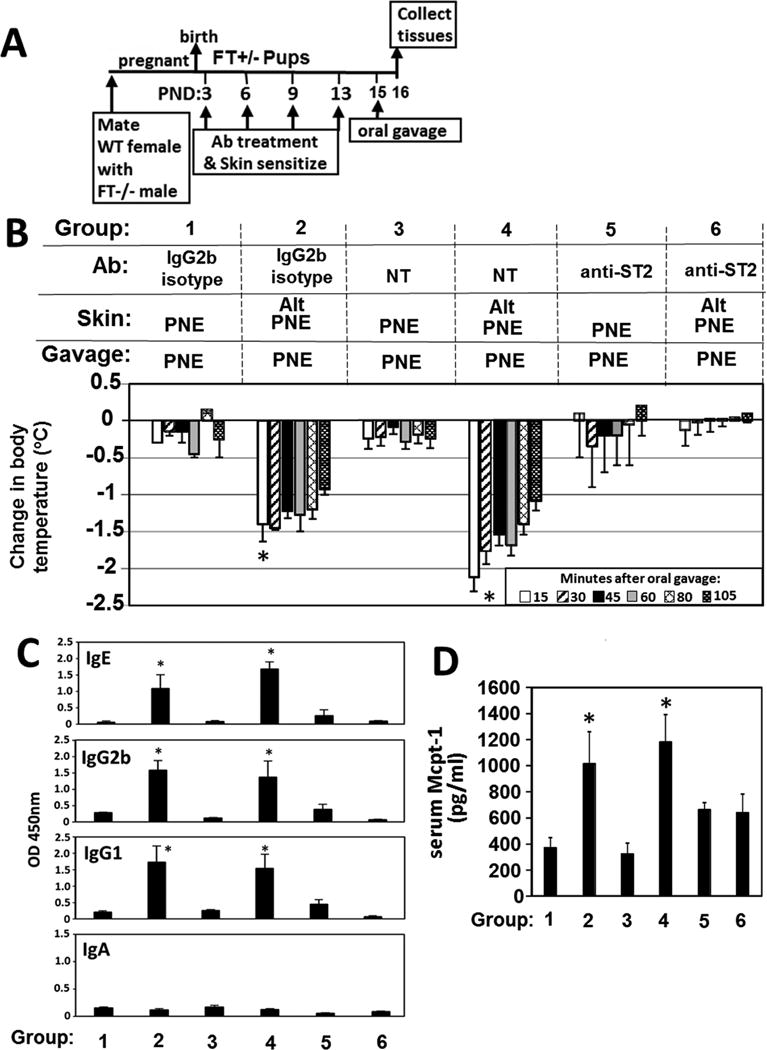
Anti-ST2 blocked development of oral food allergen-induced anaphylaxis. (A) Timeline for mating of females with FT−/− males or WT males, for antibody (Ab) treatment one hour before pup skin sensitization on PND3-13 with food and environmental allergens. FT+/− pups received an intraperitoneal injection of 8 8 μg anti-ST2 or IgG2b isotype control at 1 hr before skin sensitization #1, 12 μg antibody/g pup at 1 hr before skin sensitization #2 and #3, and then 15 μg antibody/g pup at 1 hr before skin sensitization #4. (B) Food allergen-induced temperature changes after oral gavage on postnatal day 15. (C) Anti-peanut specific IgE, IgG2b, IgG1 and monomeric IgA in serum from pups in panel B. (D) Serum Mcpt-1 was measured by ELISA. *, p<0.05 as compared to groups, 1, 3, 5 and 6.
Oral pre-exposure to PNE induced tolerance but this was less effective when the FT+/− pup skin was exposed to the ubiquitous environmental allergen Alt
Recent clinical data from the “Learning Early about Allergy to Peanut” (LEAP) study suggests that early life oral introduction of peanut reduces the frequency of development of peanut allergy76, 77. Whether early introduction of oral peanut prevents food allergen sensitization by cutaneous skin exposure is not known. We determined whether daily oral gavage on PND 3, 4, 5, and 6 with PNE in Tris-buffered saline (TBS) reduces FT+/− pup skin sensitization as compared to administration of the solvent control TBS (Fig 7A). After oral administrations, all pups received 5 skin applications of Alt/PNE on PND 6, 8, 10, 14 and 18 (Fig 7A). Pre-exposure to PNE by oral gavage blocked the development of anaphylaxis in FT+/− pups of non-allergic mothers (Figure 7B) and allergic mothers (Figure 8B). Wild type pups did not develop anaphylaxis (Fig 7B). Remarkably, for FT+/− pups of allergic mothers or nonallergic mothers (Fig 8B), skin exposures to Alt concurrent with each oral pre-exposure to PNE reduced the protective effect and had anaphylaxis (Fig 8B Group 4 and 7 and Supplement Fig. 8).
Figure 7.
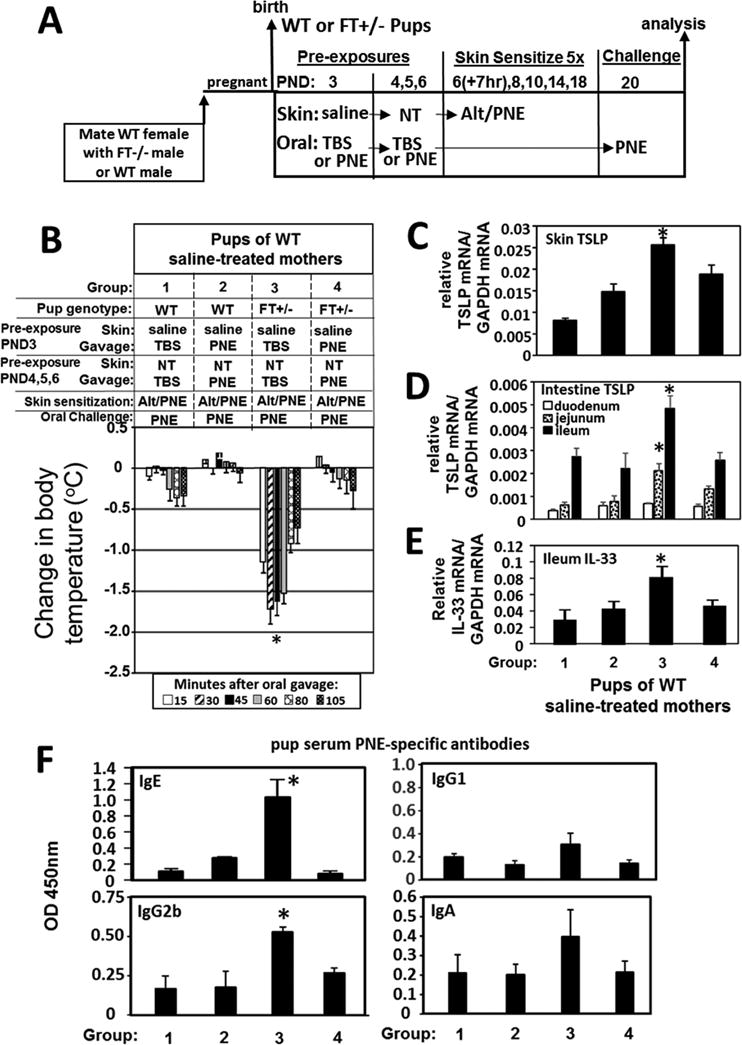
Oral pre-exposure to PNE induced tolerance in FT+/− pups. (A) Timeline for induction of mating of females with FT−/− males or WT males, for pup pre-exposures on PND 3-6, and for pup skin treatments on PND6-18 with food and environmental allergens. Intestines were flushed with saline before RT-qPCR analysis (B) Food allergen-induced temperature changes after oral gavage on days 20. *, p<0.05 as compared to all other groups. For pups from panel B, shown is the RT-qPCR of (C) pup skin TSLP, (D) pup intestine TSLP, and (E) pup ileum IL-33. (F) Anti-peanut specific serum IgE, IgG2b, IgG1 and IgA from pups in panel 2 as determined by ELISA. TBS, Tris-buffered saline. *, p<0.05 as compared to groups 1, 2, and 4.
Figure 8.
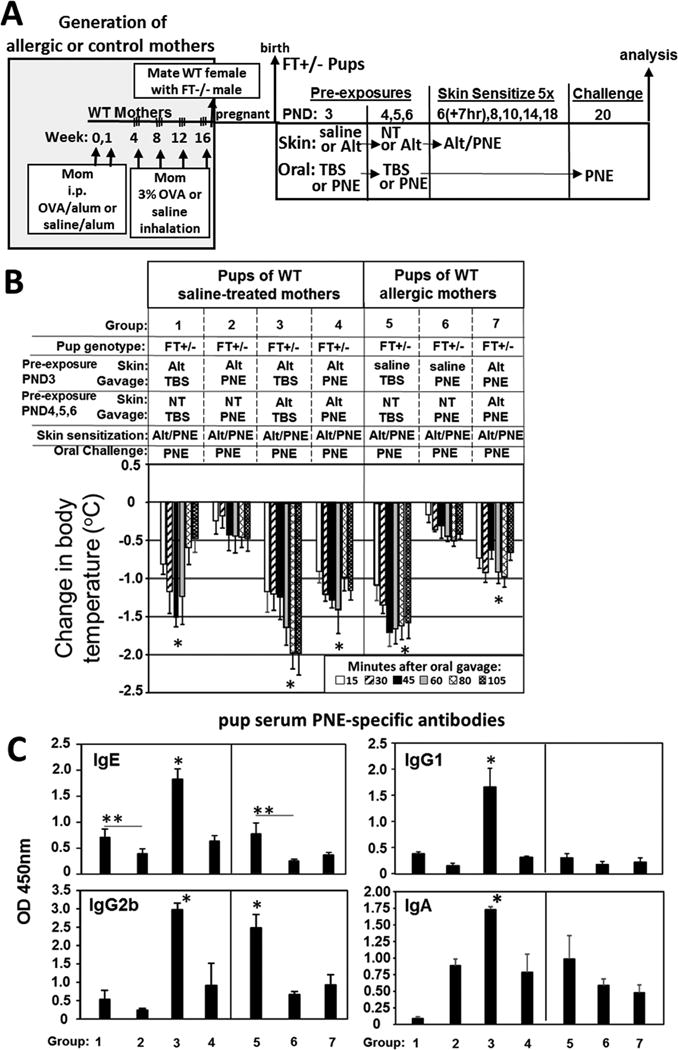
Oral pre-exposure to PNE to induce tolerance in FT+/− pups was less effective when their skin was pre-exposed to the ubiquitous environmental allergen Alt during oral pre-exposure to PNE. (A) Timeline for induction of OVA-induced lung inflammation in females, mating of females with FT−/− males, for pup pre-exposures on PND 3-6, for pup skin sensitization on PND6-18 with food and environmental allergens and for oral challenge on PND 20. (B) Food allergen-induced temperature changes after oral gavage on day 20. *, p<0.05 as compared to the group without the asterisk. (C) Anti-peanut specific serum IgE, IgG2b, IgG1 and monomeric IgA from pups in panel B as determined by ELISA. *, p<0.05 as compared to all groups without one asterisk. **, p<0.05 as compared to indicated group. N=8-10 pups/group.
Mediators of initiation of allergic responses were assessed at 6 hours after the PND20 oral PNE gavage for pups in Fig 7B. Alt/PNE-sensitized FT+/− pup intestines had increased TSLP and IL-33 (Fig 7C–E Group 3). This increase in TSLP was blocked by PND3-6 pre-exposure with oral PNE (Fig 7C–E Group 4). Pups that had 4 pre-exposures to Alt only before skin sensitization had a significant increase in antibodies (Fig 8C Group 3) as compared to skin sensitization without the Alt pre-exposures (Fig 8C Group 1 or 5). Interestingly, the Alt-PNE-induced anti-PNE-specific IgE, and IgG2b antibodies (Fig 7F Group 3) were blocked by pre-exposure oral PNE (Fig 7F Group 4). However, the reduction in anti-PNE-specific IgE (Fig 8C Group 1 compared to Group 2 and Fig 8C Group 5 compared to Group 6) was less effective when there was skin pre-exposure to Alt on PND3-6 at the time of pre-exposure with oral PNE (Fig 8C Group 1 compared to Group 4 and Fig 8C Group 5 compared to group 7).
Discussion
We demonstrated that neonatal mice heterozygous for skin barrier mutations develop food allergy by skin sensitization with food allergens and co-exposure to Alt or HDM. The skin barrier mutations and Alt or HDM were required to drive the development of food allergen sensitization, food-induced anaphylaxis, and inflammation and cytokines in the skin and intestine. Blocking ST2 during skin sensitization blocked anaphylaxis by FT+/− pups. The FT+/− neonates of allergic WT mothers had anaphylaxis and elevated serum allergen-specific IgE to suboptimal skin challenges as compared to FT+/− neonates of non-allergic WT mothers. Skin pre-exposure to the ubiquitous environmental allergen Alt reduced oral-PNE-induced tolerance in the presence of skin barrier mutations. These data demonstrate mechanisms for potent and rapid development of food allergies in offspring heterozygous for skin barrier mutations.
Animal studies of food allergy have included food exposure with bacterial toxins or prolonged antigen exposure. For instance, in small and large animals, food allergy models include sensitization of adult mice by oral administration of food antigens with cholera toxin or intraperitoneal injection of food antigens with alum78. In studies with prolonged allergen exposure, OVA sensitization of adult mice is induced by a 49 day protocol with tape stripping and OVA skin patches held by semi-permeable Tegaderm tape; this tape prevents the passage of liquids and microbes while allowing moisture vapor and gas exchange79. Since mice are weaned at 21 days, the 49 days precludes the ability to analyze development of food allergy in neonates, the time when food allergy often develops in humans. In contrast, our studies demonstrate neonate anaphylaxis with up to a 3°C temperature drop after rapid sensitization by 3 to 4 skin exposures to allergens. In another study, adult wild type mice were skin sensitized to OVA or peanut by two weeks of daily tape stripping and concurrent application of an adjuvant Calcipotriol (MC 903) but in these studies, gavage with PNE decreased body temperature by only about 0.5°C58. It is reported that skin exposure to OVA in PBS exacerbates spontaneous skin inflammation in homozygous flaky tail mice but not in heterozygous FT+/− mice80. Consistent with this, in our studies with heterozygous FT+/− mice, PNE alone did not induce skin inflammation. However, we demonstrated that, in heterozygous FT+/− neonatal mice, Alternaria alternata drove a robust food allergen-induced allergic inflammation and anaphylaxis. Thus, exposure to food allergens along with components of house dust may potently sensitize individuals with skin barrier mutations early in life.
Initiation of allergic responses at epithelial tissues in skin or lungs of adult mice is regulated by production of innate cytokines such as IL-33, and TSLP18, 81, 82. An increase in expression of CCL11 and IL33 is also associated with human atopic dermatitis33. In our report, sensitization by skin application of Alt/PNE on FT+/− neonates increased skin TSLP, CCL11, IL-33 and eosinophils and increased intestine TSLP, demonstrating that there is induction of signals for initiation of allergic responses. In a 49 day skin sensitization protocol, blockade of the IL33 receptor, ST2, just before oral challenge, significantly reduced the severity of oral anaphylaxis75. We demonstrate that blockade of ST2 before each skin sensitization completely blocked oral antigen-induced anaphylaxis. Thus, ST2 plays a key role in the development of anaphylaxis.
Food-specific IgE is low in food allergic children and correlates poorly with food allergy4. IgG1 can also mediate peanut antigen-induced anaphylaxis in the absence of IgE in animal models83. In adult mice, IgE is necessary but not sufficient for oral food anaphylaxis in a model with OVA sensitization in a protocol with 49 days of tape stripping and OVA skin patches79. In our studies with only 3 skin sensitizations with PNE/Alt in neonatal FT+/− mice, there was anaphylaxis to oral antigen challenge despite very low anti-PNE IgE levels. This is consistent with reports in humans that food antigen-specific antibodies are not always associated with food responses and may be below detection in the serum. At low antigen-specific serum antibody concentrations with allergen responses, the antibodies are likely in tissues bound to Fc receptors on inflammatory cells. Our ongoing studies are determining functions of the antibodies and Fc receptors.
Allergic lung responses to low dose allergen have been reported to be elevated in offspring of allergic mothers and these offspring responses are not specific to the allergen that the mother had been exposed6–12, 14, 15, 55, 60, 61, 84. We demonstrated that the allergen to which the mother responds (OVA) can be different than the food allergen (PNE) of the offspring and we demonstrate that offspring of the allergic mothers respond to a protocol that is suboptimal for offspring of nonallergic mothers. Our ongoing studies are determining maternal factors of allergic mothers that contribute to food allergy in FT+/− neonates.
Tolerance to food allergens hasg been studied in humans and mice. The clinical “LEAP” studies suggest that early life oral introduction of peanut at 4 to 11 months old reduces the frequency for development of peanut allergy in children76, 77. Tolerance to food allergens has been studied in adult but not neonatal mice. Briefly, low dose pre-exposure of allergic mother mice to peanut during pregnancy and lactation protects 5 week old adult offspring from sensitization with oral peanut plus cholera toxin, but protection in the Th2 environment of neonates was not studied85. Also, aerosolized OVA exposure of non-allergic mother mice during pregnancy protected 1 to 4 month old adult offspring from intraperitoneal sensitization with OVA86. In contrast, in another report, maternal exposure to peanut during mouse pregnancy and lactation did not protect development of anti-peanut antibodies in adult 5 week old offspring that received peanut with alum adjuvant twice at 2 week intervals; however, when the 5 week old adult offspring were fed peanut for 5 days before sensitization with peanut/alum, antibody generation was reduced87. In another study with adult mice, pre-exposure for 6 days with OVA in the drinking water blocks development of atopic dermatitis induced by in a 49 day sensitization protocol with OVA-loaded skin patches that were held on the skin with semi-permeable Tegaderm tape88. We demonstrated that tolerance can be induced in neonatal mice. In neonatal FT+/− mice, anaphylaxis to food allergen was prevented by pre-exposure with oral PNE before skin sensitizations with PNE/Alt. Of potential concern for individuals with skin barrier mutations, in the neonatal FT+/− mice, skin application with the ubiquitous allergen Alt at the same time of pre-exposure with oral PNE reduced the induction of tolerance to PNE. Future studies will address mechanisms of Alt inhibition of oral tolerance.
In humans, child peanut allergy associates with exposure to peanut in household dust, exposure of peanut oils to inflamed skin in children, and household peanut consumption associate with child peanut allergy89–91. House dust also contains Alt and HDM. Thus in the environment of infants and children, there is often ubiquitous exposure to HDM, Alt and food allergens. Specifically, household dust contains peanut allergens at a level of 5 to 2200 μg peanut/ g household dust45, Alt at 0.1 to 100 μg/ g household dust46, 47, and HDM antigens at about 12 μg DerP1DerP2/ g dust43, 44. In our cause and effect studies, the neonatal FT+/− mice received a 40 minute skin exposure of 10 μg Alt and 100 μg PNE every 3-4 days, which is within range for skin exposures in the household environment, and developed anaphylaxis after oral challenge. At these doses, there was no synergy of Alt with HDM (Fig. 1), suggesting that these doses found in house dust provide maximal sensitization through either Alt or HDM in the presence of skin barrier mutations.
In infants and children, loss-of-function mutations in skin barrier mutations associate with peanut allergy36, 90, 92, 93. Also, IgE-mediated food allergy is observed in up to 35% in children affected with atopic dermatitis29. Other reports predict that there are additional factors involved in development of food allergy92. Another factor may be soap components such as sodium lauryl sulfate, which is SDS, in cleansing wipes which may facilitate allergen uptake during feeding and cleaning of infants with skin barrier mutations. We report that other factors such as skin exposure of environmental allergens along with application of SDS can readily sensitize food allergen responsiveness in neonatal mice. In our studies, SDS facilitated absorption of the topical application of the allergens whereas without the SDS the liquid droplet containing allergen remained as a droplet and the droplet rolled off of the skin. To translate our findings to humans and their environment, for an infant that has skin barrier mutations, food allergen sensitization may occur very early in life by skin exposure to food allergens and concurrent skin exposure to soap in cleansing wipes and to house dust mites, Alternaria alternata and food allergens in house dust, bedding, blankets and flooring, and when handled by family members that are preparing or eating food. Moreover, these skin exposures occur early in infancy before clinical manifestations of atopic dermatitis. In children with Flg mutations, barrier defects precede clinical eczema diagnosis94. Interestingly, in our studies, skin sensitization occurred in the neonatal mice with skin barrier mutations well before the development of spontaneous atopic dermatitis skin lesions that develop months later in FT−/− mice38. Furthermore, early pre-exposure to oral allergen before exposure to environmental allergy blocked development of anaphylaxis. This is consistent with a randomized, double-blind, placebo-controlled clinical trial that demonstrated that egg allergy is prevented by restoration of skin barrier function and early oral introduction of egg95. Future clinical food allergy studies in neonates and infants are needed to determine effects of food allergen when stratifying individuals by loss of skin barrier function before their development of atopic dermatitis94.
In summary, these studies demonstrate mechanisms for development of food allergy driven by skin sensitization with environmental allergens in house dust and by skin barrier mutations. This is consistent with many features of early life exposures and genetics in clinical food allergy. Moreover, offspring of allergic mothers had elevated responses to a limited number of skin exposures indicating that there is a maternal factor contributing to the development of food allergy. These studies of mechanisms for development of food allergen sensitization provide a basis towards designing studies to test interventions that more effectively modulate these pathways in allergic disease. Currently, avoidance of triggering food allergens is a main treatment, with a profound negative impact on quality of life96. More studies are needed in humans to examine mechanisms for development of food allergy and mechanisms for induction of tolerance to food allergens early in life.
Supplementary Material
Key Messages.
In neonatal mice, concurrent skin exposure with food and environmental allergens leads to induction of food allergy before clinical manifestations of atopic dermatitis.
Neonatal pups with heterozygous skin barrier mutations from WT allergic mothers, have elevated food allergen sensitization, inflammatory mediators, and anaphylaxis to food allergen challenge, compared to offspring of non-allergic mothers or offspring without skin barrier mutations.
Blockade of ST2 during skin sensitization inhibited anaphylaxis and inflammatory mediators.
In neonates, tolerance to food allergens is blunted by concurrent pre-exposure of the skin to environmental allergen while oral pre-exposure to food allergen.
Capsule summary.
Food allergy develops in neonates with skin barrier mutations and brief concurrent cutaneous exposure to food and environmental allergens and this was inhibited by blockade of ST2. In neonates with skin barrier mutations, oral food allergen-induced tolerance is blunted by cutaneous exposure to environmental allergens.
Acknowledgments
We thank Derek Carter for preparing and staining the skin tissue sections. These studies were supported by National Institutes of Health Grants R01 HL124120 and U01 AI131337 (J.M.C-M), and R01 AI095282 (MHK).
Abbreviations
- Alt
Alternaria alternata extract
- CT
cholera toxin (CT)
- Flg
filaggrin
- FT+/−
Flaky tail mice heterozygous for filaggrin mutation and heterozygous for mattrin mutation
- FT−/−
Flaky tail mice with homozygous filaggrin mutation and homozygous mattrin mutation
- HDM
house dust mite
- LEAP
Learning Early about Allergy to Peanut trial
- Matt
mattrin
- OVA
chicken egg ovalbumin fraction V
- PND
postnatal day
- PNE
peanut extract
- SEB
staphylococcal enterotoxin B
- ST2
IL-33 receptor
- TSLP
thymic stromal lymphopoietin
- WT
wild type
- 3ss
3 skin sensitizations
- 6ss
6 skin sensitizations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Matthew Walker, Jeremy Green, Ryan Ferrie, and Ashley Queener participated in performing and interpreting experiments. Joan M. Cook-Mills conceived of the study design and participated in performing experiments, statistical analysis, interpretations, and manuscript preparation. Mark Kaplan provided the flaky tail mice and participated in discussions and manuscript preparation.
References
- 1.Wang J, Sampson HA. Food allergy. J Clin Invest. 2011;121:827–35. doi: 10.1172/JCI45434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DaVeiga SP. Epidemiology of atopic dermatitis: a review. Allergy Asthma Proc. 2012;33:227–34. doi: 10.2500/aap.2012.33.3569. [DOI] [PubMed] [Google Scholar]
- 3.Shaker M. New insights into the allergic march. Curr Opin Pediatr. 2014;26:516–20. doi: 10.1097/MOP.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 4.Makhija MM, Robison RG, Caruso D, Cai M, Wang X, Pongracic JA. Patterns of allergen sensitization and self-reported allergic disease in parents of food allergic children. Ann Allergy Asthma Immunol. 2016;117:382–6. doi: 10.1016/j.anai.2016.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousquet J, Anto J, Auffray C, Akdis M, Cambon-Thomsen A, Keil T, et al. MeDALL (Mechanisms of the Development of ALLergy): an integrated approach from phenotypes to systems medicine. Allergy. 2011;66:596–604. doi: 10.1111/j.1398-9995.2010.02534.x. [DOI] [PubMed] [Google Scholar]
- 6.Lim RH, Kobzik L. Maternal transmission of asthma risk. Am J Reprod Immunol. 2009;61:1–10. doi: 10.1111/j.1600-0897.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, et al. Allergen-independent maternal transmission of asthma susceptibility. J Immunol. 2003;170:1683–9. doi: 10.4049/jimmunol.170.4.1683. [DOI] [PubMed] [Google Scholar]
- 8.Fedulov AV, Leme AS, Kobzik L. Duration of allergic susceptibility in maternal transmission of asthma risk. Am J Reprod Immunol. 2007;58:120–8. doi: 10.1111/j.1600-0897.2007.00496.x. [DOI] [PubMed] [Google Scholar]
- 9.Hubeau C, Apostolou I, Kobzik L. Targeting of CD25 and glucocorticoid-induced TNF receptor family-related gene-expressing T cells differentially modulates asthma risk in offspring of asthmatic and normal mother mice. J Immunol. 2007;178:1477–87. doi: 10.4049/jimmunol.178.3.1477. [DOI] [PubMed] [Google Scholar]
- 10.Hubeau C, Apostolou I, Kobzik L. Adoptively transferred allergen-specific T cells cause maternal transmission of asthma risk. Am J Pathol. 2006;168:1931–9. doi: 10.2353/ajpath.2006.051231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herz U, Joachim R, Ahrens B, Scheffold A, Radbruch A, Renz H. Allergic sensitization and allergen exposure during pregnancy favor the development of atopy in the neonate. Int Arch Allergy Immunol. 2001;124:193–6. doi: 10.1159/000053708. [DOI] [PubMed] [Google Scholar]
- 12.Herz U, Joachim R, Ahrens B, Scheffold A, Radbruch A, Renz H. Prenatal sensitization in a mouse model. Am J Respir Crit Care Med. 2000;162:S62–5. doi: 10.1164/ajrccm.162.supplement_2.ras-1. [DOI] [PubMed] [Google Scholar]
- 13.Fedulov AV, Kobzik L. Allergy Risk is Mediated by Dendritic Cells with Congenital Epigenetic Changes. Am J Respir Cell Mol Biol. 2011;44:285–92. doi: 10.1165/rcmb.2009-0400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdala-Valencia H, Berdnikovs S, Soveg F, Cook-Mills JM. alpha-Tocopherol Supplementation of Allergic Female Mice Inhibits Development of CD11c+CD11b+ Dendritic Cells in Utero and Allergic Inflammation in Neonates. Am J Physiol Lung Cell Mol Physiol. 2014;307:L482–96. doi: 10.1152/ajplung.00132.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdala-Valencia H, Soveg F, Cook-Mills JM. γ-Tocopherol Supplementation of Allergic Female Mice Augments Development of CD11c+CD11b+ Dendritic Cells in Utero and Allergic Inflammation in Neonates. Am J Physiol Lung Cell Mol Physiol. 2016;310:L759–L71. doi: 10.1152/ajplung.00301.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smaldini PL, Orsini Delgado ML, Fossati CA, Docena GH. Orally-Induced Intestinal CD4+ CD25+ FoxP3+ Treg Controlled Undesired Responses towards Oral Antigens and Effectively Dampened Food Allergic Reactions. PLoS One. 2015;10:e0141116. doi: 10.1371/journal.pone.0141116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivastava KD, Siefert A, Fahmy TM, Caplan MJ, Li XM, Sampson HA. Investigation of peanut oral immunotherapy with CpG/peanut nanoparticles in a murine model of peanut allergy. J Allergy Clin Immunol. 2016;138:536–43. doi: 10.1016/j.jaci.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 18.Frossard CP, Zimmerli SC, Rincon Garriz JM, Eigenmann PA. Food allergy in mice is modulated through the thymic stromal lymphopoietin pathway. Clin Transl Allergy. 2015;6:2. doi: 10.1186/s13601-016-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, et al. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity. 2014;41:141–51. doi: 10.1016/j.immuni.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009;123:231–8. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO. Cholera in 1994. Part I. Wkly Epidemiol Rec. 1995;70:201–8. [PubMed] [Google Scholar]
- 22.Bousquet J, Bousquet PJ, Godard P, Daures JP. The public health implications of asthma. Bull World Health Organ. 2005;83:548–54. [PMC free article] [PubMed] [Google Scholar]
- 23.Vollmer WM, Osborne ML, Buist AS. 20-year trends in the prevalence of asthma and chronic airflow obstruction in an HMO. Am J Respir Crit Care Med. 1998;157:1079–84. doi: 10.1164/ajrccm.157.4.9704140. [DOI] [PubMed] [Google Scholar]
- 24.Friebele E. The attack of asthma. Environ Health Perspect. 1996;104:22–5. doi: 10.1289/ehp.9610422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Schayck CP, Smit HA. The prevalence of asthma in children: a reversing trend. Eur Respir J. 2005;26:647–50. doi: 10.1183/09031936.05.00019805. [DOI] [PubMed] [Google Scholar]
- 26.Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42:512–23. doi: 10.1016/j.immuni.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlsten C, Dimich-Ward H, Ferguson A, Watson W, Rousseau R, Dybuncio A, et al. Atopic dermatitis in a high-risk cohort: natural history, associated allergic outcomes, and risk factors. Ann Allergy Asthma Immunol. 2013;110:24–8. doi: 10.1016/j.anai.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Kelleher MM, Dunn-Galvin A, Gray C, Murray DM, Kiely M, Kenny L, et al. Skin barrier impairment at birth predicts food allergy at 2 years of age. J Allergy Clin Immunol. 2016;137:1111–6. doi: 10.1016/j.jaci.2015.12.1312. [DOI] [PubMed] [Google Scholar]
- 29.Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8. doi: 10.1542/peds.101.3.e8. [DOI] [PubMed] [Google Scholar]
- 30.Alduraywish SA, Lodge CJ, Campbell B, Allen KJ, Erbas B, Lowe AJ, et al. The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy. 2016;71:77–89. doi: 10.1111/all.12784. [DOI] [PubMed] [Google Scholar]
- 31.Li M. Current evidence of epidermal barrier dysfunction and thymic stromal lymphopoietin in the atopic march. Eur Respir Rev. 2014;23:292–8. doi: 10.1183/09059180.00004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunders SP, Goh CS, Brown SJ, Palmer CN, Porter RM, Cole C, et al. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. J Allergy Clin Immunol. 2013;132:1121–9. doi: 10.1016/j.jaci.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–54. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ. 2009;39:b2433. doi: 10.1136/bmj.b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandilands A, Terron-Kwiatkowski A, Hull PR, O’Regan GM, Clayton TH, Watson RM, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007;39:650–4. doi: 10.1038/ng2020. [DOI] [PubMed] [Google Scholar]
- 36.Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127:661–7. doi: 10.1016/j.jaci.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asai Y, Greenwood C, Hull PR, Alizadehfar R, Ben-Shoshan M, Brown SJ, et al. Filaggrin gene mutation associations with peanut allergy persist despite variations in peanut allergy diagnostic criteria or asthma status. J Allergy Clin Immunol. 2013;132:239–42. doi: 10.1016/j.jaci.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sehra S, Krishnamurthy P, Koh B, Zhou HM, Seymour L, Akhtar N, et al. Increased Th2 activity and diminished skin barrier function cooperate in allergic skin inflammation. Eur J Immunol. 2016;46:2609–13. doi: 10.1002/eji.201646421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leyva-Castillo JM, Hener P, Jiang H, Li M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol. 2013;133:154–63. doi: 10.1038/jid.2012.239. [DOI] [PubMed] [Google Scholar]
- 40.Bantz SK, Zhu Z, Zheng T. The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma. J Clin Cell Immunol. 2014;5(2):202. doi: 10.4172/2155-9899.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ewald DA, Noda S, Oliva M, Litman T, Nakajima S, Li X, et al. Major differences between human atopic dermatitis and murine models, as determined by using global transcriptomic profiling. J Allergy Clin Immunol. 2017;139:562–71. doi: 10.1016/j.jaci.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Fukutomi Y, Taniguchi M. Sensitization to fungal allergens: Resolved and unresolved issues. Allergol Int. 2015;64:321–31. doi: 10.1016/j.alit.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Zock JP, Brunekreef B. House dust mite allergen levels in dust from schools with smooth and carpeted classroom floors. Clin Exp Allergy. 1995;25:549–53. doi: 10.1111/j.1365-2222.1995.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Xiong L, Yin X, Wang J, Zhang Q, Yu Z, et al. House dust mite allergen levels in households and correlation with allergic rhinitis symptoms. Am J Rhinol Allergy. 2014;28:193–6. doi: 10.2500/ajra.2014.28.4095. [DOI] [PubMed] [Google Scholar]
- 45.Trendelenburg V, Ahrens B, Wehrmann AK, Kalb B, Niggemann B, Beyer K. Peanut allergen in house dust of eating area and bed–a risk factor for peanut sensitization? Allergy. 2013;68:1460–2. doi: 10.1111/all.12226. [DOI] [PubMed] [Google Scholar]
- 46.Salo PM, Arbes SJ, Jr, Crockett PW, Thorne PS, Cohn RD, Zeldin DC. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J Allergy Clin Immunol. 2008;121:678–84. doi: 10.1016/j.jaci.2007.12.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salo PM, Arbes SJ, Jr, Sever M, Jaramillo R, Cohn RD, London SJ, et al. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006;118:892–8. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang E, Tanaka T, Tajima M, Tsuboi R, Nishikawa A, Sugita T. Characterization of the skin fungal microbiota in patients with atopic dermatitis and in healthy subjects. Microbiol Immunol. 2011;55:625–32. doi: 10.1111/j.1348-0421.2011.00364.x. [DOI] [PubMed] [Google Scholar]
- 49.Glatz M, Bosshard PP, Hoetzenecker W, Schmid-Grendelmeier P. The Role of Malassezia spp. in Atopic Dermatitis. J Clin Med. 2015;4:1217–28. doi: 10.3390/jcm4061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harada K, Saito M, Sugita T, Tsuboi R. Malassezia species and their associated skin diseases. J Dermatol. 2015;42:250–7. doi: 10.1111/1346-8138.12700. [DOI] [PubMed] [Google Scholar]
- 51.Mittermann I, Wikberg G, Johansson C, Lupinek C, Lundeberg L, Crameri R, et al. IgE Sensitization Profiles Differ between Adult Patients with Severe and Moderate Atopic Dermatitis. PLoS One. 2016;11:e0156077. doi: 10.1371/journal.pone.0156077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hedayati MT, Arabzadehmoghadam A, Hajheydari Z. Specific IgE against Alternaria alternata in atopic dermatitis and asthma patients. Eur Rev Med Pharmacol Sci. 2009;13:187–91. [PubMed] [Google Scholar]
- 53.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–27. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 54.Koppelman SJ, Knol EF, Vlooswijk RA, Wensing M, Knulst AC, Hefle SL, et al. Peanut allergen Ara h 3: isolation from peanuts and biochemical characterization. Allergy. 2003;58:1144–51. doi: 10.1034/j.1398-9995.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 55.Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, et al. Allergen-independent maternal transmission of asthma susceptibility. J Immunol. 2003;170:1683–9. doi: 10.4049/jimmunol.170.4.1683. [DOI] [PubMed] [Google Scholar]
- 56.Muto T, Fukuoka A, Kabashima K, Ziegler SF, Nakanishi K, Matsushita K, et al. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int Immunol. 2014;26:539–49. doi: 10.1093/intimm/dxu058. [DOI] [PubMed] [Google Scholar]
- 57.Wavrin S, Bernard H, Wal JM, Adel-Patient K. Cutaneous or respiratory exposures to peanut allergens in mice and their impacts on subsequent oral exposure. Int Arch Allergy Immunol. 2014;164:189–99. doi: 10.1159/000363444. [DOI] [PubMed] [Google Scholar]
- 58.Noti M, Kim BS, Siracusa MC, Rak GD, Kubo M, Moghaddam AE, et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immunol. 2014;133:1390–9. doi: 10.1016/j.jaci.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monteiro RC. Role of IgA and IgA fc receptors in inflammation. J Clin Immunol. 2010;30:1–9. doi: 10.1007/s10875-009-9338-0. [DOI] [PubMed] [Google Scholar]
- 60.Leme AS, Hubeau C, Xiang Y, Goldman A, Hamada K, Suzaki Y, et al. Role of breast milk in a mouse model of maternal transmission of asthma susceptibility. J Immunol. 2006;176:762–9. doi: 10.4049/jimmunol.176.2.762. [DOI] [PubMed] [Google Scholar]
- 61.Hamada K, Suzaki Y, Leme A, Ito T, Miyamoto K, Kobzik L, et al. Exposure of pregnant mice to an air pollutant aerosol increases asthma susceptibility in offspring. J Toxicol Environ Health A. 2007;70:688–95. doi: 10.1080/15287390600974692. [DOI] [PubMed] [Google Scholar]
- 62.van Gool CJ, Thijs C, Dagnelie PC, Henquet CJ, van Houwelingen AC, Schrander J, et al. Determinants of neonatal IgE level: parity, maternal age, birth season and perinatal essential fatty acid status in infants of atopic mothers. Allergy. 2004;59:961–8. doi: 10.1111/j.1398-9995.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- 63.Matsuoka S, Nakagawa R, Nakayama H, Yamashita K, Kuroda Y. Prevalence of specific allergic diseases in school children as related to parental atopy. Pediatr Int. 1999;41:46–51. doi: 10.1046/j.1442-200x.1999.01011.x. [DOI] [PubMed] [Google Scholar]
- 64.Pohlabeln H, Muhlenbruch K, Jacobs S, Bohmann H. Frequency of allergic diseases in 2-year-old children in relationship to parental history of allergy and breastfeeding. J Investig Allergol Clin Immunol. 2010;20:195–200. [PubMed] [Google Scholar]
- 65.Marenholz I, Esparza-Gordillo J, Ruschendorf F, Bauerfeind A, Strachan DP, Spycher BD, et al. Meta-analysis identifies seven susceptibility loci involved in the atopic march. Nat Commun. 2015;6:8804. doi: 10.1038/ncomms9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmitz R, Atzpodien K, Schlaud M. Prevalence and risk factors of atopic diseases in German children and adolescents. Pediatr Allergy Immunol. 2012;23:716–23. doi: 10.1111/j.1399-3038.2012.01342.x. [DOI] [PubMed] [Google Scholar]
- 67.Alexander G. Body temperature control in mammalian young. Br Med Bull. 1975;31:62–8. doi: 10.1093/oxfordjournals.bmb.a071243. [DOI] [PubMed] [Google Scholar]
- 68.Waterhouse J, Weinert D, Nevill A, Atkinson G, Reilly T. Some factors influencing the sensitivity of body temperature to activity in neonates. Chronobiol Int. 2000;17:679–92. doi: 10.1081/cbi-100101074. [DOI] [PubMed] [Google Scholar]
- 69.Smith J, Alcock G, Usher K. Temperature measurement in the preterm and term neonate: a review of the literature. Neonatal Netw. 2013;32:16–25. doi: 10.1891/0730-0832.32.1.16. [DOI] [PubMed] [Google Scholar]
- 70.Lunze K, Hamer DH. Thermal protection of the newborn in resource-limited environments. J Perinatol. 2012;32:317–24. doi: 10.1038/jp.2012.11. [DOI] [PubMed] [Google Scholar]
- 71.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng G, Mu Z, Cui L, Liu P, Wang Y, Wu W, et al. Anti-IL-33 Antibody Has a Therapeutic Effect in an Atopic Dermatitis Murine Model Induced by 2, 4-Dinitrochlorobenzene. Inflammation. 2018;41:154–63. doi: 10.1007/s10753-017-0673-7. [DOI] [PubMed] [Google Scholar]
- 73.Taracanova A, Alevizos M, Karagkouni A, Weng Z, Norwitz E, Conti P, et al. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc Natl Acad Sci U S A. 2017;114:E4002–E9. doi: 10.1073/pnas.1524845114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saluja R, Khan M, Church MK, Maurer M. The role of IL-33 and mast cells in allergy and inflammation. Clin Transl Allergy. 2015;5:33. doi: 10.1186/s13601-015-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie ANJ, et al. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol. 2016;138:1356–66. doi: 10.1016/j.jaci.2016.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenhawt M, Fleischer D, Chan ES, Venter C, Stukus D, Gupta R, et al. LEAPing Through the Looking Glass: Secondary Analysis of the Effect of Skin Test Size and Age of Introduction on Peanut Tolerance after Early Peanut Introduction. Allergy. 2016;29:13100. doi: 10.1111/all.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–13. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Gramberg JL, de Veer MJ, O’Hehir RE, Meeusen EN, Bischof RJ. Use of animal models to investigate major allergens associated with food allergy. J Allergy (Cairo) 2013;2013:635695. doi: 10.1155/2013/635695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartnikas LM, Gurish MF, Burton OT, Leisten S, Janssen E, Oettgen HC, et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol. 2013;131:451–60. doi: 10.1016/j.jaci.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–8. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Noh JY, Shin JU, Park CO, Lee N, Jin S, Kim SH, et al. Thymic stromal lymphopoietin regulates eosinophil migration via phosphorylation of l-plastin in atopic dermatitis. Exp Dermatol. 2016;25:880–6. doi: 10.1111/exd.13111. [DOI] [PubMed] [Google Scholar]
- 83.Li X, Huang CK, Schofield BH, Burks AW, Bannon GA, Kim KH, et al. Strain-dependent induction of allergic sensitization caused by peanut allergen DNA immunization in mice. J Immunol. 1999;162:3045–52. [PubMed] [Google Scholar]
- 84.Jarrett E, Hall E. Selective suppression of IgE antibody responsiveness by maternal influence. Nature. 1979;280:145–7. doi: 10.1038/280145a0. [DOI] [PubMed] [Google Scholar]
- 85.Lopez-Exposito I, Song Y, Jarvinen KM, Srivastava K, Li XM. Maternal peanut exposure during pregnancy and lactation reduces peanut allergy risk in offspring. J Allergy Clin Immunol. 2009;124:1039–46. doi: 10.1016/j.jaci.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gerhold K, Avagyan A, Reichert E, Seib C, Van DV, Luger EO, et al. Prenatal allergen exposures prevent allergen-induced sensitization and airway inflammation in young mice. Allergy. 2012;67:353–61. doi: 10.1111/j.1398-9995.2011.02775.x. [DOI] [PubMed] [Google Scholar]
- 87.Jarvinen KM, Westfall J, De Jesus M, Mantis NJ, Carroll JA, Metzger DW, et al. Role of Maternal Dietary Peanut Exposure in Development of Food Allergy and Oral Tolerance. PLoS One. 2015;10:e0143855. doi: 10.1371/journal.pone.0143855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baek JO, Roh JY, Jung Y. Oral tolerance inhibits atopic dermatitis-like type 2 inflammation in mice by modulating immune microenvironments. Allergy. 2017;72:397–406. doi: 10.1111/all.12960. [DOI] [PubMed] [Google Scholar]
- 89.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123:417–23. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 90.Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol. 2014;134:867–75. doi: 10.1016/j.jaci.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977–85. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 92.Tan HT, Ellis JA, Koplin JJ, Matheson MC, Gurrin LC, Lowe AJ, et al. Filaggrin loss-of-function mutations do not predict food allergy over and above the risk of food sensitization among infants. J Allergy Clin Immunol. 2012;130:1211–3. doi: 10.1016/j.jaci.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 93.Ashley SE, Tan HT, Vuillermin P, Dharmage SC, Tang ML, Koplin J, et al. The skin barrier function gene SPINK5 is associated with challenge proven IgE-mediated food allergy in infants. Allergy. 2017;18:13143. doi: 10.1111/all.13143. [DOI] [PubMed] [Google Scholar]
- 94.Flohr C, England K, Radulovic S, McLean WH, Campbel LE, Barker J, et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br J Dermatol. 2010;163:1333–6. doi: 10.1111/j.1365-2133.2010.10068.x. [DOI] [PubMed] [Google Scholar]
- 95.Natsume O, Kabashima S, Nakazato J, Yamamoto-Hanada K, Narita M, Kondo M, et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:276–86. doi: 10.1016/S0140-6736(16)31418-0. [DOI] [PubMed] [Google Scholar]
- 96.Greenhawt M. Food allergy quality of life and living with food allergy. Curr Opin Allergy Clin Immunol. 2016;16:284–90. doi: 10.1097/ACI.0000000000000271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


