Abstract
Background
Three years treatment with either sublingual or subcutaneous allergen immunotherapy has been shown to be effective and to induce long-term tolerance. The GRASS* trial demonstrated that two years treatment via either route was effective in suppressing the response to nasal allergen challenge, although was insufficient for inhibition one year after discontinuation.
Objective
To examine in the GRASS trial the time-course of immunologic changes during two years sublingual and subcutaneous immunotherapy and for one year after treatment discontinuation.
Methods
We performed multi-modal immunomonitoring to assess allergen-specific CD4 T cell properties, in parallel with analysis of local mucosal cytokine responses induced by nasal allergen exposure and humoral immune responses that included IgE-dependent basophil activation and measurement of serum inhibitory activity for allergen-IgE binding to B cells (IgE-Facilitated Allergen Binding).
Results
All three of these distinct arms of the immune response displayed significant and coordinate alterations during 2 years allergen desensitization, followed by reversal at 3 years, reflecting a lack of a durable immunological effect. Whereas frequencies of antigen-specific Th2 cells in peripheral blood determined by HLA class II tetramer analysis most closely paralleled clinical outcomes, IgE-antibody dependent functional assays remained partially inhibited one year following discontinuation.
Conclusion
Two years of allergen immunotherapy were effective but insufficient for long-term tolerance. Allergen-specific Th2 cells most closely paralleled the transient clinical outcome and it is likely that recurrence of the T cell ‘drivers’ of allergic immunity abrogated the potential for durable tolerance. On the other hand, persistence of IgE-blocking antibody one year after discontinuation may be an early indicator of a pro-tolerogenic mechanism.
Keywords: Allergy, Immunotherapy, Immune tolerance, Allergen desensitization, Th2 cells
Introduction
Allergen immunotherapy is an effective treatment option for patients with allergic rhinitis who do not respond adequately to usual anti-histamine and topical corticosteroid medications (1). Subcutaneous immunotherapy involves weekly administration of incremental doses of allergenic material by injection followed by monthly maintenance injections for several years (2–4). Immunotherapy has been associated with overall changes in T cell function with cytokine changes that suggest a shift from Th2 cells towards Th1 phenotypes or induction of regulatory T cells (5, 6). These alterations are accompanied by decreases in recruitment and/or activation of allergic effector cells including mast cells, eosinophils and basophils in target organs (7, 8). Measurement of serum immunoglobulins directed against the allergen in such immunotherapy studies indicates that specific IgG, particularly of the IgG4 subclass, can be induced by therapy and is presumed to be mechanistically linked to clinical benefit by virtue of competitive inhibition of allergic responses triggered by specific IgE directed to the same allergens (9–12). Alternative routes of allergen administration for immunotherapy are now under active investigation, including sublingual (13–15), and epicutaneous routes (16, 17). For food allergens, the oral route has also shown promising results (18, 19). Since immunological properties at each of these sites differ, the mechanisms through which these forms of allergen immunotherapy exert their therapeutic effects may differ, as well.
The GRASS (Long-Term Effects of Sublingual Grass Therapy) clinical trial was a randomized, placebo-controlled, double-blind study of 106 adults with a clinical history of moderate to severe seasonal allergic rhinitis due to grass pollen. Study participants received two years of subcutaneous immunotherapy, sublingual immunotherapy or placebo and were extensively studied over three years for clinical and immunological parameters of response (20). Clinical assessments in this trial were recently reported, demonstrating successful suppression of the nasal response to allergen challenge after two years of therapy for both the subcutaneous and sublingual routes, with lack of sustained benefit in the subsequent untreated third year (20). We now report immunological findings from this trial, including peripheral blood cellular and humoral assessments, as well as local tissue responses to allergen: evaluation of antigen-specific CD4+ T cells in peripheral blood, functional outcomes from changes in the humoral response detected in serum and peripheral IgE-dependent basophil assays and cytokine responses to allergen challenge in the nasal mucosa.
Methods
Sample collection
Clinical characteristics of the subjects in the GRASS study and details of the protocol have been previously reported (20). Subcutaneous alum-adsorbed grass pollen immunotherapy (Alutard SQ Grass Pollen®, ALK, Horsholm, Denmark) or matched placebo subcutaneous injections were given weekly for 15 weeks followed by monthly maintenance injections until 2 years. Freeze-dried grass pollen (Phleum Pratense) sublingual tablets (Grazax®, ALK, Horsholm, Denmark) or matched placebo sublingual tablets were self-administered daily for 2 years. Timothy grass-specific IgE and specific IgG4 were quantified using the CAP FEIA system (Phadia, Uppsala, Sweden). Peripheral blood lymphocytes were collected and prepared for cryopreservation as previously described (20). Coded samples were provided to the operator.
Tetramer assays and flow cytometry analysis
Timothy grass specific CD4+ T cell epitopes were identified by Tetramer Guided Epitope Mapping (21, 22). Epitope specific pMHC tetramer reagents were generated by loading specific peptides onto biotinylated soluble DR monomers, and subsequently conjugated with PE-streptavidin (23). These included HLA-DR04:01, DR03:01, DR04:01, DR07:01, DR10:01 and DR11:01 tetramer reagents. For ex vivo tetramer staining, 20 to 40 million frozen PBMC from subjects with HLA genotypes corresponding to these tetramers were thawed and re-suspended in 200 μl of T cell culture medium and, in order to enhance tetramer staining, were treated with dasatinib (Sigma-Aldrich) for 10 minutes at 37°C before tetramer staining (24). PE-labeled, pooled tetramers were then added to a final concentration of 20 μg/ml, and the staining was carried out for 100 minutes at room temperature. 1/100 fraction of the cells were saved and the rest of the PE-tetramer positive cells were then enriched by the anti-PE bead enrichment protocol through a magnetic column according to the manufacturer’s protocol (Miltenyi Biotec) (22, 25). Cells in both the enriched fraction and the pre-column fraction were stained with a panel of antibodies of interest, including CD14 (HCD14, Biolegend), CD19 (HIB19, Biolegend), CD45RA (HI100, BD Biosciences), CD4 (RPA-T4, BD Biosciences), CRTH2 (CRTH2, BM16, BD Biosciences), CD161 (PK136, Biolegend) and CD27 (O323, Biolegend); and were further treated with BD Via-Probes™ (BD Biosciences), before flow cytometry. Frequencies of tetramer positive cells were calculated by the formula n/N, where n is the number of tetramer positive cells in the enriched fraction, and N is the total number of cells in the sample, which can be calculated by counting the number of cells in the pre-column fraction × 100. Efficiency of recovery was optimized by using less than 30 million cells as starting material on samples with less than 300 tetramer-positive cells per million, capturing greater than 95% of the PE-tetramer-stained populations.
Isolation of grass pollen allergen-reactive T cells with CD154 upregulation assay
Global grass pollen-reactive CD4+ T cells were tracked using the CD154 assay (26, 27). Briefly, frozen/thawed PBMC were cultured at a density of 106/ml with 1 μl/ml Timothy grass pollen crude extract and 1 μg/ml of anti-CD40 blocking mAb (HB14, Miltenyi Biotec). After 18 hour stimulation at 37°C, cells were harvested and labeled with PE-Conjugated anti-CD154 mAb for 10 minutes at 4°C. Cells were then washed, labeled with anti-PE magnetic beads and enriched by using a magnetic column, according to the manufacturer’s instructions (Miltenyi Biotec). Magnetically enriched cells were next stained with antibodies against markers of interest and analyzed on a FACSAria™ II flow cytometer (BD). Live memory CD45RO+ CD154+ CD4+ T cells were sort-purified for subsequent transcript analysis.
Real-time PCR expression analysis
The Fluidigm BioMark 96.96 Dynamic Array (28) was used to measure the gene expression in small cell populations. Ten cells per well were sorted by FACS in quadruplicate into 96-well plates containing a reaction mix for reverse transcription (CellsDirect One-Step qRT-PCR kit; Invitrogen) and pre-amplification with 96 selected gene primer pairs (DELTAgene assays, Fluidigm). After sorting, samples were reverse-transcribed and pre-amplified for 18 cycles. Primers and dNTPs were removed by incubation with ExonucleaseI (NE Biolabs), and samples were diluted (5×) with TE buffer and stored at −20°C. Samples and assays (primer pairs) were prepared for loading onto 96.96 Fluidigm Dynamic arrays according to the manufacturer’s recommendations. The 96.96 Fluidigm Dynamic Arrays (Fluidigm Corp.) were primed and loaded on an IFC Controller HX (Fluidigm Corp.) and real-time PCR was run on a BiomarkHD (Fluidigm Corp.). Data were collected and analyzed using Fluidigm Real-Time PCR Analysis software (v4.1.2).
Measurement of nasal cytokines
Nasal challenge was performed using Aquagen® (ALK) Phleum Pratense (Timothy grass) extract as described previously (20). Challenge dose was determined according to a dose-titration challenge at screening. The same dose was then used at the baseline (pre-treatment) nasal challenge visit and at each subsequent challenge visit. Dose range was 1,500 BU/ml (equivalent to 1.0 μg/ml major allergen) to 30,000 BU/ml (equivalent to 20.2 μg/ml major allergen).
Nasal secretions were collected using synthetic polyurethane sponges pre-cut to 20 × 15 × 15 mm (RG 27 grau; Gummi-Welz GmbH & Co., Neu-Ulm, Germany) and sterilized by autoclaving. A single sponge was inserted into each of the participant’s nostrils, posterior to the muco-cutaneous junction, by a study physician under direct vision using croc forceps and a nasal speculum (Phoenix Surgical Instruments Ltd, Hertfordshire, UK). Sponges were left in place for 2 minutes before removal and then added to 2-ml centrifuge tubes with indwelling 0.22 μm cellulose acetate filters (Costar Spin-X; Corning, Corning, NY, USA). Tubes were kept briefly on ice before being centrifuged. At baseline, sponges were centrifuged ‘neat’ without adding an elution buffer. At years 2 and 3, 75 μl of elution buffer [Milliplex Assay Buffer; Millipore, Darmstadt, Germany; PBS pH 7.4, BSA (1%), Tween-20 (0.05%), sodium azide (0.05%)] was added to sponges within their centrifuge tubes before being centrifuged. The isolated fluid was then pipetted into Eppendorf tubes and stored at −80°C.
After thawing, nasal fluid was analyzed for cytokines in yearly batches. Measurements of IL-4, IL-5, IL-10, IL-13 and IFN-γ, were performed using MSD Human TH1/TH2 7-Plex, Ultra-Sensitive Kit according to the manufacturer’s instructions (MS6000 7 spot; Meso Scale Discovery, Maryland, USA). Briefly, after incubation of plates with diluent, 25 μl of samples, calibrators, and high and low standards were added to appropriate wells and incubated on a plate-shaker for 2 hours. Plates were then washed in PBS plus 0.05% Tween-20 using an automated washer (Aquamax 2000). Twenty-five microliters of detection antibody at 1 μg/ml was added to wells, followed by incubation on a plate-shaker for 2 hours in the dark. Plates were then washed 3 times as before. One hundred and fifty microliters of Read Buffer T were then added to each well before plates were read on an MSD SECTOR® 6000 instrument. All measurements were performed in duplicate and reported as mean values per standardized volume. The assay was validated for analysis of nasal secretion and the level of quantification was 5–5000 pg/ml for all cytokines.
IgE-FAB and basophil activation
Serum inhibitory activity for IgE-facilitated allergen binding and presentation was measured by FAB assay (10, 29, 30). Briefly, an indicator serum containing high concentration of Timothy grass pollen (P. pratense)-specific IgE (>100 IU/mL), was pre-incubated with 1 μg/mL allergen at 37°C for 1 hour to allow formation of allergen-IgE complexes. To test for inhibition of facilitated allergen binding, indicator serum and test serum (baseline, year 1, year 2 and year 3) or RPMI alone as a control was mixed. During this step, CD23-enriched EBV-transformed B cells were washed three times by centrifugation in RPMI-1640 at 423 × g for 7 minutes at 4°C. Cells were then re-suspended in FAB buffer (138.60 mM NaCL, 1.12 mM NaH2PO4, 8.16 mM Na2HPO4 and 0.1% bovine serum albumin dissolved in 1 liter of distilled H2O, adjusted pH to 7.2) at 2×107 cells/ml. 1×105 EBV-transformed B cells were added to the IgE serum/allergen complexes and incubated for 1 hour at 4°C on ice. Cells were then washed twice to remove any unbound allergen-IgE complexes and immunostained with PE-labelled anti-human IgE (Miltenyi; Biotech, Woking, UK) for 45 minutes at 4°C on ice. The cells were then washed and re-suspended in FAB buffer and the percentage of cells bound by allergen-IgE complexes was assessed by flow cytometry (BD FACSCanto II; BD Biosciences, San Jose, CA) and data analyzed with FACS DIVA software (BD Biosciences, San Jose, CA). Five thousand gated cells were analyzed and all samples were measured in triplicate.
Assessment of ex-vivo allergen-induced basophil responsiveness by flow cytometry was performed on heparinized whole blood (47). Briefly, whole blood was incubated with or without 100 ng/ml of P. pratense extract (ALK-Abelló) in a 37°C water bath for 15 minutes. Cells were immunostained with anti-human CD3, CD303, CD294 (CRTh2), CD63 (all BD Biosciences, San Jose, CA). Erythrocytes from whole blood were lysed with BD lysing solution (BD Biosciences, San Jose, CA) for 10 minutes at room temperature in the dark, samples were centrifuged (5 min, 200 × g) and the supernatants discarded. The resulting cell pellets were washed in 3 ml PBS (without Ca2+ and Mg2+) and re-suspended in 450 μl ice-cold fixative solution (CellFix, BD Biosciences, San Jose, CA) prior to acquisition on the BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA). Activated cells were also identified as CD63+CRTh2+ basophils. Analyses were performed using FlowJo v10.2 (FlowJo, LLC, Oregon).
Statistics
In the GRASS trial, the intention to treat population included 106 randomized participants of whom 92provided an evaluable primary endpoint at 3 years. (20). The per-protocol (PP) population included 84 participants who remained in the study 3 years, were compliant with study medications, defined as taking 50% or more of their study medication for the duration of the study, and had an evaluable primary endpoint. All mechanistic data were assessed in the PP population using a linear mixed model adjusted for baseline values. To be consistent, clinical endpoints, nasal challenge induced total nasal symptom score (TNSS) area under curve (AUC) and peak nasal inspiratory flow (PNIF) change from pre-challenge AUC, were re-analyzed in the PP population using a linear mixed model adjusted for baseline values. The threshold for significance was p<0.05 (two-sided). Since all analyses were considered exploratory, p-values were not adjusted for multiple comparisons. Analyses were performed with SAS Version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.2.4 (R Foundation for Statistical Computing).
Data and materials availability
Datasets and Figures from this study, along with clinical data from the GRASS trial, are available on TrialShare, the Immune Tolerance Network data visualization portal, at: https://www.itntrialshare.org/GRASSmech.url.
Study approval
Written informed consent was received from participants prior to inclusion in the study. The study was approved by the National Research Ethics Committee in the UK.
Results
Peripheral antigen-specific T cells
Allergen-specific T cell responses were assessed by direct ex vivo HLA class II tetramer staining of CD4+ lymphocytes from peripheral blood. Peptide epitopes from the major Timothy grass pollen allergens Phl p 1 and Phl p 5 that were used for tetramer production are shown in Table I; HLA genotypes in the Table were matched for each subject analyzed. Figure 1 shows representative flow cytometry analysis of these allergen-specific T cells from a study participant receiving subcutaneous grass allergen immunotherapy. Detection of lymphocytes that bind the tetramers—consisting of timothy grass peptides bound to HLA class II molecules—identifies CD4 T cells specific for the grass pollen allergen. As shown in Figure 1A, flow cytometry profiles prior to therapy identify grass pollen-specific T cells in peripheral blood that display a typical allergic profile, with expression of CRTH2, CD161 and CCR4 surface markers characteristic of Th2 lymphocytes involved in allergic diseases, and few cells expressing CD27. Two years after continuous subcutaneous immunotherapy (SCIT), profiles of remaining allergen-specific T cells have shifted as characterized by reduced frequencies of the CCR4/CRTH2/CD161-positive cells, and increased frequencies of CD27-positive cells. This altered pattern of cell-surface markers indicates a phenotypic change consistent with a loss of Th2 cells in the circulating allergen-specific compartment. However, after one additional year off therapy, this phenotypic profile has reverted back towards the original distribution of allergic markers, once again displaying increased frequencies of CRTH2- and CD161-positive cells and reduced frequency of CD27-positive cells in the allergen-specific CD4 T cell compartment.
Table I.
Peptide epitopes from the major grass allergens used for HLA class II tetramer production.
| HLA | Class II tetramers | AA sequences |
|---|---|---|
| DRB1*0101 | Phl p 1 153–172 | KGSNPNYLALLVKYVNGDGD |
| Phl p 5b 26–45 | KLIEDINVGFKAAVAAAASV | |
| DRB1*0301 | Phl p 1 169–188 | GDGDVVAVDIKEKGKDKWIE |
| DRB1*0401 | Phl p 1 97–116 | EEPIAPYHFDLSGHAFGAMA |
| Phl p 1 221–240 | TEAEDVIPEGWKADTSYESK | |
| DRB1*0701 | Phl p5a 119–138 | PEAKYDAYVATLSEALRIIA |
| Phl p5b 90–109 | ATPEAKFDSFVASLTEALRV | |
| DRB1*1001 | Phl p5a 32–51 | GKATTEEQKLIEKINAGFKA |
| Phl p5a 103–122 | LDAAYKLAYKTAEGATPEAK | |
| Phl p5a 167–186 | VDAAFKVAATAANAAPANDK | |
| DRB1*1101 | Phl p 1 153–172 | KGSNPNYLALLVKYVNGDGD |
| Phl p 1 185–204 | KWIELKESWGAIWRIDTPDK | |
| Phl p5a 79–98 | FAEGLSGEPKGAAESSSKAA |
Fig. 1.
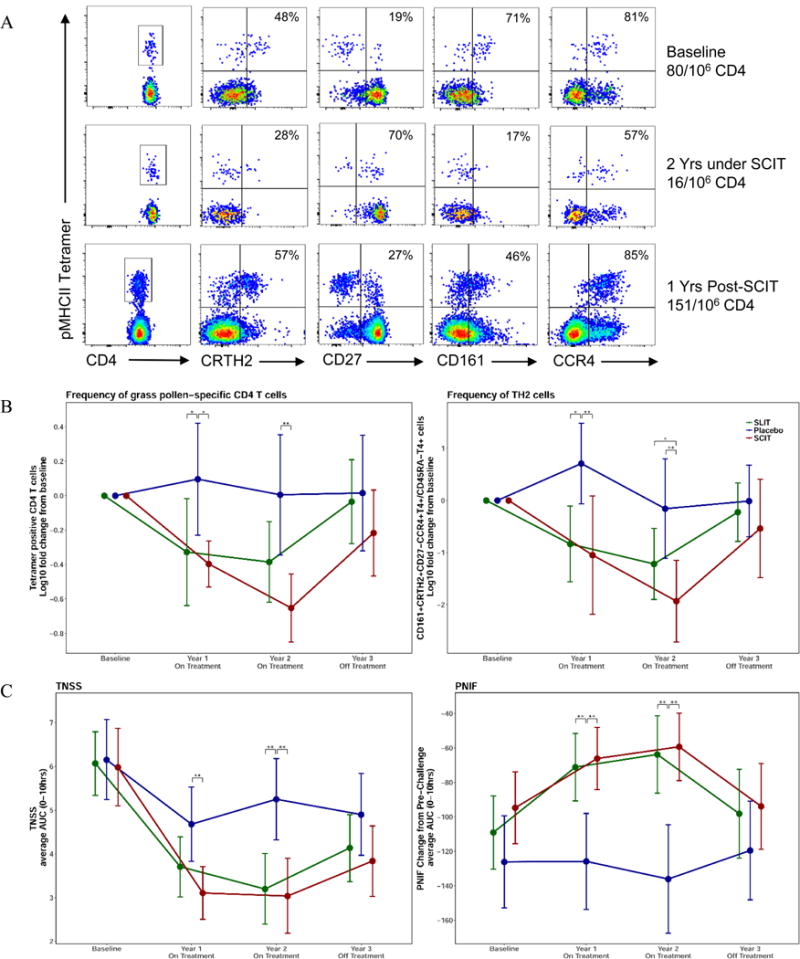
Analysis of allergen-specific CD4 T cells and relationship to the clinical parameters measured during the GRASS trial. (A) Representative flow cytometry analysis of tetramer bound allergen-specific T cells from a study subject receiving subcutaneous allergen immunotherapy. Lymphocytes that bind the pooled tetramers are displayed on the y axis and expression of phenotypic cell surface markers CD4, CRTH2, CD27, CD161 and CCR4 are displayed on the x axis at baseline, at 2 years after continuous SCIT therapy and at 3 years after one year off therapy. (B) Frequencies of allergen-specific CD4 T cells were determined for 53 HLA-DR4 subjects in the GRASS trial, by tetramer binding (left panel). Frequencies of Th2 cells are identified by CD161+CRTH2+CD27−CCR4+T4+/CD45RA−T4+ phenotypic marker expression (right panel). (C) The total nasal symptom score (TNSS) average AUC (left panel) and the peak nasal inspiratory flow (PNIF) average AUC (right panel) for 0–10 hours following allergen challenge were measured at baseline and years 1–3 for all participants treated with sublingual immunotherapy (green), subcutaneous immunotherapy (red), and placebo (blue). Significant differences are indicated by * (p<.05), ** (p<.01). Data are shown as means with 95% confidence intervals, for the 84 per-protocol subjects enrolled in the GRASS trial.
Figure 1B summarizes these profiles for 53/84 Per Protocol population (SCIT: 16, SLIT: 21, placebo: 16) enrolled in the GRASS clinical trial in whom there was adequate HLA-matching for tetramer-profiling of allergen-specific T cells. In placebo-treated participants, allergen-specific CD4 T cell frequencies were stable over 3 years. Participants in both the subcutaneous and the sublingual therapy arms of the trial showed significant decreases compared to placebo in the frequencies of allergen-specific cells, during the first year of treatment, a timepoint where clinical parameters in the two arms were also similar (Fig 1C). This downward trend continued in the group receiving a 2nd year of subcutaneous therapy and remained significant compared to placebo, while the decline appeared to plateau in the sublingual group compared to placebo at year 2 (Fig. 1B) The decrease in overall CD4 antigen-specific cells (Fig. 1B [left panel]) reflects the specific decrease in Th2 cells (Fig. 1B [right panel]). After two years of therapy, subjects receiving subcutaneous therapy had fewer allergen-specific Th2 cells in the peripheral blood compared to the sublingual therapy group. Notably, one year after discontinuation of the allergen therapy, specific CD4 T cell numbers in both treatment groups returned to the baseline frequencies, indicating a lack of a durable immunological effect.
These antigen-specific T cell profiles are remarkably concordant with the clinical parameters measured in the GRASS trial (20). This is illustrated in Figure 1C for symptom-related outcomes of the per-protocol subjects studied in the trial, with clinical outcomes measured on the same day as collection of samples used for the T cell analysis: the total nasal symptom score (TNSS), a composite clinical index (scale 0–12) of the severity of nasal symptoms after nasal allergen challenge (left panel), and the peak nasal inspiratory flow (PNIF), an objective measurement of nasal airflow obstruction (right panel), for the same subjects and time-points. Clinical improvement was seen with both forms of immunotherapy over the two year period of treatment, but reverted back to the baseline allergic parameters at year 3, one year after discontinuation of treatment. Thus, the allergen-specific CD4+ T cell frequencies measured by flow cytometry of tetramer-binding cells closely paralleled clinical outcomes.
Effects of therapy on local Th2 cytokines
Nasal allergen challenges were performed before treatment and at yearly intervals, with collection and cytokine analysis of nasal fluids. Nasal allergen challenge-induced increases in interleukin (IL)-4, IL-5 and IL-13 in nasal fluids were significantly suppressed after 2 years treatment with either subcutaneous or sublingual immunotherapy, as shown in Figure 2. There was no treatment effect on IFN-γ or IL-10 responses, indicating that both treatment modalities selectively reduced local Th2 cytokines. Similar to what we observed with allergen specific Th2 cells in peripheral blood, and in parallel with clinical outcomes in Figure 1C, suppression of these local Th2 cytokine responses to nasal allergen provocation was not maintained one year after therapy cessation at year 3.
Fig. 2.
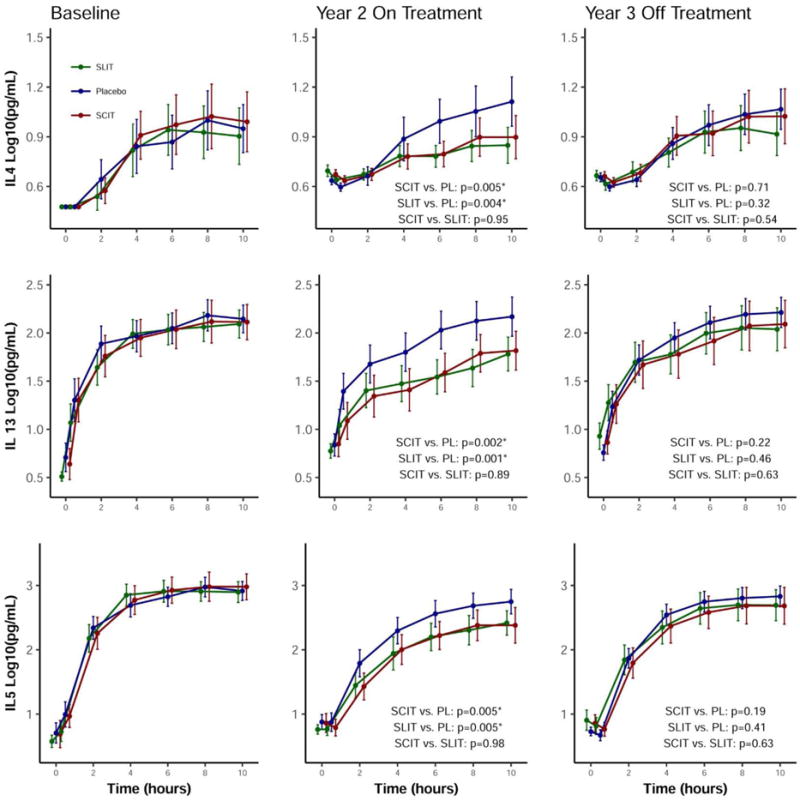
Cytokine levels of nasal fluids following nasal allergen challenge. The levels of cytokines IL-4, IL-5 and IL-13 in nasal fluids for 10 hours following nasal allergen challenge are displayed from study subjects at baseline, after 2 years of desensitization and at 3 years, one year after discontinuation of desensitization therapy. Participants treated with sublingual immunotherapy are displayed in green, subcutaneous immunotherapy in red and placebo in blue. Data are shown as means with 95% confidence intervals.
These changes in mucosal cytokines together with fewer circulating allergen-specific Th2 cells, suggested an overall decrease of Th2 immunity in desensitized subjects. We confirmed this interpretation through transcriptional analysis of antigen-reactive memory CD4+ T cells at baseline and at year 2 (Supplemental Figure 1A) (27, 31). For these experiments, cryopreserved PBMC were stimulated with Timothy grass extract overnight in the presence of anti-CD40, and upregulation of CD154 on memory CD4+ T cells was used to identify allergen-reactive T cells. Similar to the tetramer assay, both subcutaneous and sublingual treatment modalities reduced the frequency of allergen-reactive memory CD4+ T cells in peripheral blood at year 2, but this reduction was only statistically significant for subcutaneous immunotherapy. These CD154-positive cells were isolated using fluorescence-activated cell sorting and RNA extracted for transcriptional analysis; as shown in Supplemental Figure 1B. The CD27-negative population of allergen-reactive cells displayed a characteristic Th2 transcript profile with increased IL-4, IL-5, IL-13, IL-31 and ST2 expression, consistent with a Th2-predominant phenotype of the allergen-specific CD4+ T cells impacted by specific immunotherapy.
Humoral immunological outcomes
As previously reported, Timothy grass pollen-specific IgG4 levels increased during allergen immunotherapy, in parallel with the therapeutic response (9, 10). In order to assess the functional capacity of this induced IgG, we measured the ability of post-immunotherapy serum to inhibit the binding of allergen-IgE complexes to B cells (IgE-FAB), an in vitro surrogate of IgE-facilitated antigen presentation. In this assay, serum from patients who have received allergen-specific immunotherapy was evaluated for its ability to inhibit this allergen-IgE complex binding (10). As shown in Figure 3A, there was a marked decrease in IgE-FAB during allergen immunotherapy, for both subcutaneous and sublingual-treated subjects. Interestingly, after 2 years therapy, the changes in allergen specific IgG4/IgE ratios were 10-fold higher after subcutaneous compared to sublingual immunotherapy (Fig. 3B), whereas the changes in IgG-associated inhibitory activity for IgE-FAB were comparable for the 2 groups. At 3 year follow up, one year off therapy, the IgE-FAB binding trended back towards baseline values, but remained significantly depressed relative to placebo-treated participants. This pattern was similar to the profile reflected in the allergen-specific IgG4-to-IgE ratio (Fig. 3B).
Fig. 3.
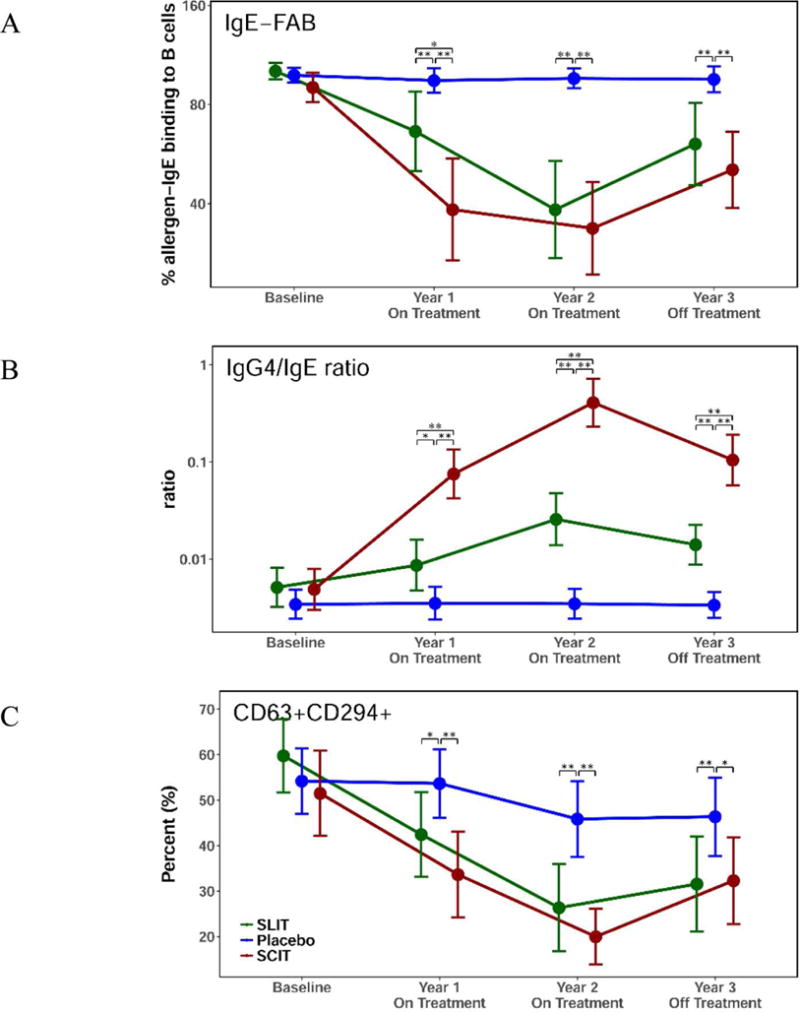
Allergen-specific IgE-dependent functional assays. (A) The impact of the IgG4 increase on the ability of allergen-IgE complexes to bind B cells was measured by IgE-FAB assay. The serum from patients was evaluated for ability to inhibit allergen-IgE complex binding at baseline and years 1–3. (B) Grass pollen-specific IgG4 and IgE were monitored and the IgG4/IgE ratio is displayed for subjects at baseline, after years 1 and 2 during desensitization therapy and at 3 years after discontinuation of therapy. (C) Basophil surface activation markers from whole blood of participants treated with sublingual immunotherapy (green), subcutaneous immunotherapy (red) and placebo (blue) at baseline and years 1–3 after incubation with grass pollen allergen. Significant differences are indicated by * (p<.05), ** (p<.01). Data are shown as means with 95% confidence intervals.
Basophil activation is another indicator of IgE-mediated allergic response that can be monitored ex vivo by detection of basophil surface activation markers following incubation of grass pollen allergen with whole blood (32, 33). Similar to what was observed for serum inhibitory activity for IgE, both therapies significantly suppressed grass pollen allergen-induced basophil hyper-responsiveness as measured by surface CD63 expression. This effect persisted at year 3 follow up, one year after withdrawal of treatment (Fig. 3C).
Relationships between local tissue and systemic immunological parameters
The overall concordance between decreased nasal Th2 cytokine measurements, lower peripheral blood antigen-specific CD4+ T cells, and antigen-specific IgE activity after 2 years of allergen desensitization therapy suggested coordinated immune mechanisms. To explore the relationship between the treatment effect on these immunological parameters, each was plotted for individual subjects as year 2 fold-change from baseline in a 3-D scatter plot. Indeed, we identified a distinct co-clustering of study participants within the immunotherapy treatment arms, well-demarcated from the placebo-treated controls (Fig. 4). Many participants cluster near the origin of the graph, indicating synchronous allergen-specific CD4+ T cell, nasal Th2 cytokine, and antibody changes, suggesting that the changes observed are reflecting the same shift in the immune responses even though differences in the significance compared to placebo are observed. There was no difference between the subcutaneous and sublingual treatment groups, which cluster together in this analysis at the end of two years of desensitization therapy.
Fig. 4.
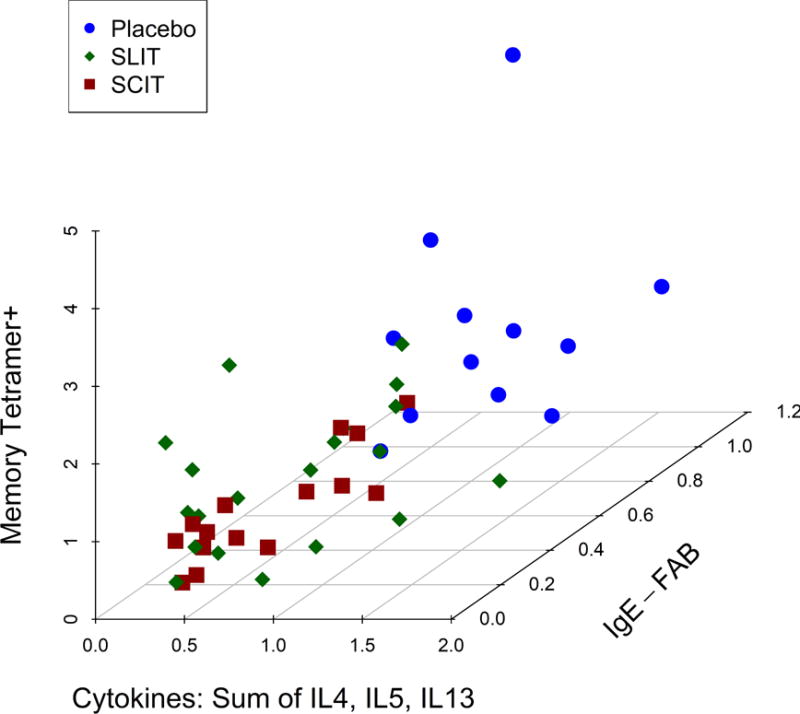
Relationship between nasal cytokine measurements and lower peripheral blood antigen-specific T cells (memory tetramer + cells) and serum antigen-specific IgE-FAB for each individual studied. Data displayed are expressed as fold changes from the baseline at year 2 (on treatment) for each immunological parameter measured for participants treated with Sublingual immunotherapy (green diamonds), Subcutaneous immunotherapy (red triangles), and placebo (blue circles), so values <1 on each axis represent reduction (improvement) in the parameters shown. Nasal cytokine measurements are the summation of area under curve (AUC) from 2 to 10 hours post-challenge for Th2 cytokines (IL-4, IL-5 and IL-13). Lower peripheral blood antigen-specific T cells are measured as the frequency of memory tetramer + cells per million CD4+ cells. Serum antigen-specific IgE-FAB is measured as the percentage of allergen-IgE binding to B cells. Cluster distributions were compared using a Hotelling T-square test, as follows: Placebo vs. SCIT: p<0.001; Placebo vs. SLIT: p<0.001; SLIT vs. SCIT: p=0.31. An online interactive version of this Figure is available at https://www.itntrialshare.org/GRASSmech_fig4.url.
Discussion
Allergic manifestations are largely driven by Th2-mediated immune mechanisms, and allergen-specific immunotherapy may inhibit, deviate and/or delete these effector responses. In the GRASS clinical trial, immunological assays were utilized to directly compare different immune modalities, monitoring subjects using a highly sensitive and specific tetramer assay to identify and phenotype allergen-specific CD4+ T cells, a sensitive and quantitative measurement of in vivo, allergen-provoked nasal cytokines to measure local mucosal immunity, and two assessments of peripheral allergen-specific IgE reactivity, namely inhibitory activity for IgE-FAB and inhibition of allergen-stimulated peripheral basophil activation. The distinctive opportunity to conduct this study in the context of a randomized, placebo-controlled trial comparing two different forms of allergen administration in humans is unique, as is the integration of immunobiology for antigen-specific peripheral blood T cells and humoral immunity with in vivo target organ immune response following allergen exposure.
The three modalities tested—specific T cells, local cytokines, and specific humoral immunity—were generally concordant while on treatment, as demonstrated by the movement toward the origin of both subcutaneous and sublingual treated populations in Figure 4. Thus, despite the fundamentally different routes of allergen administration and treatment between sublingual and subcutaneous therapy, with corresponding site-specific differences in antigen presentation, allergen immunotherapy via either route of exposure similarly reduced the immunological effectors of the allergic response in each case. However, differences were evident in the magnitude, timing and duration of effect: Allergen-specific CD4+ T cells were decreased in frequency after one year of treatment via either the subcutaneous or sublingual route, and continued to decrease during therapy for a second year only in subjects receiving subcutaneous allergen immunotherapy. This decrease occurred within the Th2 subpopulation of allergen-specific memory T cells, characterized by expression of CRTH2 and CD161, and lack of expression of CD27 (22). Transcript analysis of allergen-reactive CD4+ T cells, purified using the parameter of CD154-upregulation after allergen exposure in vitro, confirmed the loss of Th2 phenotype (Supplemental Figure 1). Similar kinetics were found in the serum immunoglobulin compartment: Therapeutic elevations of the ratio of specific IgG4/IgE, as well as decreases in facilitated IgE binding assays were seen with both forms of specific immunotherapy, but were more prominent earlier—at one year after initiation of therapy—in the subcutaneous treatment arm.
Clinical symptoms improved in the GRASS study participants during therapy, but this response was not durable. When assessed one year after discontinuation of treatment, subjects receiving either form of immunotherapy regained their responses to allergen challenge, indicating a lack of immune tolerance (20). Of the three types of immunological characteristics studied—circulating T cells, local tissue cytokines and systemic immunoglobulins—the allergen-specific CD4+ T cell frequencies and nasal Th2 cytokine levels showed a close temporal relationship with clinical outcome, reverting back to baseline values after cessation of therapy at year three. This supports the concept that future clinical studies for allergen tolerance may need to focus on more durable strategies for deleting or deviating the allergen-specific T cell compartment; an example is the current CATNIP clinical trial (NCT 02237196) combining anti-TSLP with allergen-specific immunotherapy.
IgG4/IgE and functional assays of IgE-FAB and basophil activation showed a slightly different pattern compared to the T cell responses (Fig. 3). Increases in specific IgG4/IgE ratio and inhibition of IgE-FAB (which is known to be largely IgG4-associated (34)) were less marked for sublingual compared to subcutaneous immunotherapy at year 1. In contrast to the T cell response, changes in IgG4/IgE-blocking activity in the intervention groups persisted until year 3, one year after discontinuation, although the magnitude was reduced compared to the year 2 values. This could simply reflect a slower change in immunoglobulin levels compared to the T cell profiles over time, or alternatively might indicate the potential for uncoupling of B cell associated responses—raising the possibility that B cells might be more amenable to long-term tolerance effects of specific immunotherapy.
Previous studies of allergen-responsive T cells during immunotherapy have revealed immune deviation away from Th2 in favor of Th1 responses (35, 36) whereas others have shown no change in T cell phenotype (37, 38). PBMCs harvested during immunotherapy have shown suppression of allergen-stimulated proliferation, accompanied by increases in TGF-beta (39, 40) and/or IL-10 (39, 41–43) in culture supernatants or by ELIspot assay (44). These changes were accompanied by increases in phenotypic Tregs as determined by flow cytometry (36, 39–41). In two studies, immunotherapy-induced suppression of allergen-stimulated T cell proliferation was reversed by the addition of either anti-IL-10 (36) or TGF-beta soluble receptor (40) to the cultures. Suppression of allergen-reactive Th2 cells by measurement of allergen-stimulated CD154+ CD4+ T cells has previously been shown during subcutaneous immunotherapy (45), whereas studies of class II tetramer-positive T cells have been variable, with trials suggesting either decreases (22, 46, 47) or no change (48) in tetramer-positive cells after immunotherapy. The present study demonstrates clear decreases in tetramer-positive phenotypic Th2 cells that parallel the clinical response during and following specific immunotherapy, similar for both subcutaneous and sublingual modes of allergen administration.
Transient increases in specific IgE and IgG4/IgE ratios have previously been demonstrated (9, 10) but not in a long-term comparison of sublingual and subcutaneous immunotherapy (20). In this study, while the onset of blocking antibody activity was slower for sublingual immunotherapy, it persisted at 3 years follow up and was equivalent to that observed for subcutaneous immunotherapy despite a 10-fold lower increase in specific IgG4/IgE ratio for sublingual immunotherapy after two years desensitization (Figure 3). This highlights likely differences between the two routes of delivery where local antigen processing via the sublingual route may possibly result in fewer IgG4 antibodies but with higher avidity and/or affinity and greater IgE-blocking activity.
The transient clinical benefit from two years of immunotherapy in the GRASS study stands in contrast to previous allergy clinical trials (49, 50) that demonstrated more durable benefit from three years of treatment, suggesting that 3 years of desensitization may be required for sustained effects. Our finding of a close temporal relationship between the frequency of allergen-specific circulating Th2 cells and this transient clinical outcome could represent a causal relationship, in that recurrence of the T cell ‘drivers’ of allergic immunity may have abrogated the potential for durable tolerance. On the other hand, persistence of IgE-blocking antibody (9, 20) may be an early indicator of a pro-tolerogenic mechanism. If this hypothesis is correct, then future strategies for allergen immunotherapy could be directed both at enhancing persistent depletion of the allergen-specific Th2 cell population for optimal induction of tolerance and augmenting the antigen-specific therapeutic B cell response for maintenance of long-term effect.
Supplementary Material
Fig. S1. Antigen reactive memory CD4+ T cells and mRNA expression profiles. (A) Frequency of antigen reactive cells after 2 years of desensitization treatment based on CD154 up-regulation after allergen stimulation. Participants treated with sublingual immunotherapy are displayed in green, subcutaneous immunotherapy in red and placebo in blue. An Analysis of Covariance (ANCOVA) model with baseline adjustment was used for analyzing CD154 assay data. Data are shown in the plot as means of log10 fold change from baseline with 95% confidence intervals.
(B) Transcriptional analysis of extracted RNA from CD27+CD154+ and CD27−CD154+ cells at the 2-year timepoint. Transcript levels are displayed in color scale from high (yellow) to low (blue). Genes are grouped by category with Th2 in red, apoptosis in grey and Th1 related genes in purple.
Key Messages.
Two years of grass pollen immunotherapy leads to decreased frequency of circulating allergen-specific Th2 cells, suppressed increases in nasal cytokine response to allergen challenge and decreased antigen-specific IgE activity.
Capsule Summary.
During and after grass pollen immunotherapy, changes in peripheral antigen-specific Th2 cells paralleled clinical outcomes, reflecting distinctive cellular response in concert with changes in specific IgE antibodies and local tissue cytokines, suggesting coordinated immune mechanisms.
Acknowledgments
We thank Olivia Doyle, PhD, for assistance in preparing the manuscript, and the Systems Immunology Program at Benaroya Research Institute for assistance with CD154 transcriptional profiling. Allergen extracts for immunotherapy (sublingual grass pollen tablets Grazax® and Subcutaneous alum-adsorbed grass pollen Phleum Pratense Alutard SQ®) were provided for the GRASS study free of charge by ALK, Horsholm, Denmark
We acknowledge GRASS Study Contributors: Moises Calderon, MD PhD, Arif Eifan, MD, Martin Penagos, MD, Imperial College Study Clinicians; Natalia Klimowska-Nassar, MA, Mimi Poon, MSc, Imperial College Study management; Andrea Goldstone, RN, Fotini Rozakeas, RN, Rachel Yan, RN, MS, Rachel Yan, Imperial College Nursing Staff; Delica Kit Cheung, M Sc, Constance Ito, M Sc, Janice Layhadi, PhD, Elisabeth Lemm, B Sc, Tomokasu Matsuoka, MD, PhD, Rebecca Parkin, B Sc, and Amy Switzer, M Sc, Imperial College Laboratory Projects. Nadia Tchao, MD (past), Adam Asare, PhD (past), Eduard Chani, PhD, Judith Evind, Deborah Phippard, PhD (past), Peter Sayre, MD, PhD, Maureen Sharkey, MA (past), and Don Whitehouse, MS, Immune Tolerance Network (ITN); Joy Laurienzo and Maria-Concetta Veri, NIAID; and Michelle L Sever, Rho Federal.
Funding: Research reported in this publication was sponsored by the Immune Tolerance Network and supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1AI109565 and National Institutes of Health grant R01 AI095074. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: Dr Durham reports receipt of grants from the ITN and NIAID, and nonfinancial support from ALK during the conduct of the study; and grants from Regeneron, Biotech Tools, ALK, personal fees from Anergis, Circassia, Biomay, Merck, Allergy Therapeutics, ALK, and med Update GmbH outside the submitted work. No other disclosures were reported.
Gauging Response in Allergic rhinitis to Sublingual and Subcutaneous immunotherapy
Author contributions
S.R.D, S.J.T., G.T.N., K.M.H. W.W.K. and A.T. contributed to concept development and experimental design, A.R., M.H.S, G.W.S, P.A.W. and E.W. collected data and performed experiments. T.Q. helped with data analysis and visualization. M.H.S., K.M.H, T.Q., G.W.S, A.T, G.T.N., W.W.K. and S.R.D. aided in interpretation of the data. K.M.H, G.T.N and S.R.D wrote the manuscript. All authors made contributions to the final manuscript prior to submission.
References and Notes
- 1.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. The Journal of allergy and clinical immunology. 2011;127(1 Suppl):S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. The New England journal of medicine. 1999;341(7):468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 3.Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. The Cochrane database of systematic reviews. 2007;(1):Cd001936. doi: 10.1002/14651858.CD001936.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson HS, Norman PS. Allergen-specific immunotherapy. Chemical immunology and allergy. 2014;100:333–8. doi: 10.1159/000360047. [DOI] [PubMed] [Google Scholar]
- 5.Matsuoka T, Shamji MH, Durham SR. Allergen immunotherapy and tolerance. Allergology international: official journal of the Japanese Society of Allergology. 2013;62(4):403–13. doi: 10.2332/allergolint.13-RAI-0650. [DOI] [PubMed] [Google Scholar]
- 6.Akdis CA, Akdis M. Advances in allergen immunotherapy: aiming for complete tolerance to allergens. Science translational medicine. 2015;7(280):280ps6. doi: 10.1126/scitranslmed.aaa7390. [DOI] [PubMed] [Google Scholar]
- 7.Wilson DR, Irani AM, Walker SM, Jacobson MR, Mackay IS, Schwartz LB, et al. Grass pollen immunotherapy inhibits seasonal increases in basophils and eosinophils in the nasal epithelium. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2001;31(11):1705–13. doi: 10.1046/j.1365-2222.2001.01231.x. [DOI] [PubMed] [Google Scholar]
- 8.Nouri-Aria KT, Pilette C, Jacobson MR, Watanabe H, Durham SR. IL-9 and c-Kit+ mast cells in allergic rhinitis during seasonal allergen exposure: effect of immunotherapy. The Journal of allergy and clinical immunology. 2005;116(1):73–9. doi: 10.1016/j.jaci.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 9.James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. The Journal of allergy and clinical immunology. 2011;127(2):509–16. e1–5. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 10.Shamji MH, Ljorring C, Francis JN, Calderon MA, Larche M, Kimber I, et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67(2):217–26. doi: 10.1111/j.1398-9995.2011.02745.x. [DOI] [PubMed] [Google Scholar]
- 11.Dodev TS, Bowen H, Shamji MH, Bax HJ, Beavil AJ, McDonnell JM, et al. Inhibition of allergen-dependent IgE activity by antibodies of the same specificity but different class. Allergy. 2015;70(6):720–4. doi: 10.1111/all.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James LK, Till SJ. Potential Mechanisms for IgG4 Inhibition of Immediate Hypersensitivity Reactions. Current allergy and asthma reports. 2016;16(3):23. doi: 10.1007/s11882-016-0600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durham SR, Emminger W, Kapp A, Colombo G, de Monchy JG, Rak S, et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. The Journal of allergy and clinical immunology. 2010;125(1):131–8. e1–7. doi: 10.1016/j.jaci.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Calderon MA, Penagos M, Sheikh A, Canonica GW, Durham S. Sublingual immunotherapy for treating allergic conjunctivitis. The Cochrane database of systematic reviews. 2011;(7):Cd007685. doi: 10.1002/14651858.CD007685.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Dahl R, Roberts G, de Blic J, Canonica GW, Kleine-Tebbe J, Nolte H, et al. SQ grass sublingual allergy immunotherapy tablet for disease-modifying treatment of grass pollen allergic rhinoconjunctivitis. Allergy and asthma proceedings. 2016;37(2):92–104. doi: 10.2500/aap.2016.37.3937. [DOI] [PubMed] [Google Scholar]
- 16.Senti G, von Moos S, Tay F, Graf N, Johansen P, Kundig TM. Determinants of efficacy and safety in epicutaneous allergen immunotherapy: summary of three clinical trials. Allergy. 2015;70(6):707–10. doi: 10.1111/all.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SM, Sicherer SH, Burks AW, Leung DY, Lindblad RW, Dawson P, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. The Journal of allergy and clinical immunology. 2016;139(4):1242–52. doi: 10.1016/j.jaci.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burbank AJ, Sood P, Vickery BP, Wood RA. Oral Immunotherapy for Food Allergy. Immunology and allergy clinics of North America. 2016;36(1):55–69. doi: 10.1016/j.iac.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. The Journal of allergy and clinical immunology. 2017;139(1):173–81.e8. doi: 10.1016/j.jaci.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scadding GW, Calderon MA, Shamji MH, Eifan AO, Penagos M, Dumitru F, et al. Effect of 2 Years of Treatment With Sublingual Grass Pollen Immunotherapy on Nasal Response to Allergen Challenge at 3 Years Among Patients With Moderate to Severe Seasonal Allergic Rhinitis: The GRASS Randomized Clinical Trial. Jama. 2017;317(6):615–25. doi: 10.1001/jama.2016.21040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novak EJ, Liu AW, Gebe JA, Falk BA, Nepom GT, Koelle DM, et al. Tetramer-guided epitope mapping: rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. Journal of immunology (Baltimore, Md: 1950) 2001;166(11):6665–70. doi: 10.4049/jimmunol.166.11.6665. [DOI] [PubMed] [Google Scholar]
- 22.Wambre E, DeLong JH, James EA, Torres-Chinn N, Pfutzner W, Mobs C, et al. Specific immunotherapy modifies allergen-specific CD4(+) T-cell responses in an epitope-dependent manner. The Journal of allergy and clinical immunology. 2014;133(3):872–9.e7. doi: 10.1016/j.jaci.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4+ T cells proliferating in response to influenza A antigen. The Journal of Clinical Investigation. 1999;104(12):R63–R7. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lissina A, Ladell K, Skowera A, Clement M, Edwards E, Seggewiss R, et al. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. Journal of immunological methods. 2009;340(1):11–24. doi: 10.1016/j.jim.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwok WW, Roti M, Delong JH, Tan V, Wambre E, James EA, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. The Journal of allergy and clinical immunology. 2010;125(6):1407–9.e1. doi: 10.1016/j.jaci.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nature medicine. 2005;11(10):1118–24. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 27.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nature medicine. 2005;11(10):1113–7. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 28.Warren L, Bryder D, Weissman IL, Quake SR. Transcription factor profiling in individual hematopoietic progenitors by digital RT-PCR. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(47):17807–12. doi: 10.1073/pnas.0608512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shamji MH, Wilcock LK, Wachholz PA, Dearman RJ, Kimber I, Wurtzen PA, et al. The IgE-facilitated allergen binding (FAB) assay: validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. Journal of immunological methods. 2006;317(1–2):71–9. doi: 10.1016/j.jim.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2010;40(4):598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 31.Renand A, Archila LD, McGinty J, Wambre E, Robinson D, Hales BJ, et al. Chronic cat allergen exposure induces a TH2 cell-dependent IgG4 response related to low sensitization. The Journal of allergy and clinical immunology. 2015;136(6):1627–35. e1–13. doi: 10.1016/j.jaci.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann HJ, Santos AF, Mayorga C, Nopp A, Eberlein B, Ferrer M, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. 2015;70(11):1393–405. doi: 10.1111/all.12698. [DOI] [PubMed] [Google Scholar]
- 33.Eberlein B, Hann R, Eyerich S, Pennino D, Ring J, Schmidt-Weber CB, et al. Optimizing of the basophil activation test: Comparison of different basophil identification markers. Cytometry Part B, Clinical cytometry. 2015;88(3):183–9. doi: 10.1002/cyto.b.21203. [DOI] [PubMed] [Google Scholar]
- 34.Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. Journal of immunology (Baltimore, Md: 1950) 2004;172(5):3252–9. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 35.Ebner C, Siemann U, Bohle B, Willheim M, Wiedermann U, Schenk S, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major grass pollen allergen. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 1997;27(9):1007–15. doi: 10.1111/j.1365-2222.1997.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 36.Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. The Journal of allergy and clinical immunology. 2007;120(3):707–13. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Wachholz PA, Nouri-Aria KT, Wilson DR, Walker SM, Verhoef A, Till SJ, et al. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral Th1:Th2 cytokine ratios. Immunology. 2002;105(1):56–62. doi: 10.1046/j.1365-2567.2002.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanta C, Bohle B, Hirt W, Siemann U, Horak F, Kraft D, et al. Systemic immunological changes induced by administration of grass pollen allergens via the oral mucosa during sublingual immunotherapy. International archives of allergy and immunology. 1999;120(3):218–24. doi: 10.1159/000024270. [DOI] [PubMed] [Google Scholar]
- 39.Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. European journal of immunology. 2003;33(5):1205–14. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 40.O’Hehir RE, Gardner LM, de Leon MP, Hales BJ, Biondo M, Douglass JA, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T cells. American journal of respiratory and critical care medicine. 2009;180(10):936–47. doi: 10.1164/rccm.200905-0686OC. [DOI] [PubMed] [Google Scholar]
- 41.Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. The Journal of allergy and clinical immunology. 2003;111(6):1255–61. doi: 10.1067/mai.2003.1570. [DOI] [PubMed] [Google Scholar]
- 42.Francis JN, James LK, Paraskevopoulos G, Wong C, Calderon MA, Durham SR, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. The Journal of allergy and clinical immunology. 2008;121(5):1120–5.e2. doi: 10.1016/j.jaci.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 43.Shamji MH, Kappen JH, Akdis M, Jensen-Jarolim E, Knol EF, Kleine-Tebbe J, et al. Biomarkers for Monitoring Clinical Efficacy of Allergen Immunotherapy for Allergic Rhinoconjunctivitis and Allergic Asthma: an EAACI Position Paper. Allergy. 2017;72(8):1156–73. doi: 10.1111/all.13138. [DOI] [PubMed] [Google Scholar]
- 44.Schulten V, Tripple V, Aasbjerg K, Backer V, Lund G, Wurtzen PA, et al. Distinct modulation of allergic T cell responses by subcutaneous vs. sublingual allergen-specific immunotherapy. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2016;46(3):439–48. doi: 10.1111/cea.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell JD, Buchmann P, Kesting S, Cunningham CR, Coffman RL, Hessel EM. Allergen-specific T cell responses to immunotherapy monitored by CD154 and intracellular cytokine expression. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2010;40(7):1025–35. doi: 10.1111/j.1365-2222.2010.03505.x. [DOI] [PubMed] [Google Scholar]
- 46.Swamy RS, Reshamwala N, Hunter T, Vissamsetti S, Santos CB, Baroody FM, et al. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. The Journal of allergy and clinical immunology. 2012;130(1):215–24.e7. doi: 10.1016/j.jaci.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suarez-Fueyo A, Ramos T, Galan A, Jimeno L, Wurtzen PA, Marin A, et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. The Journal of allergy and clinical immunology. 2014;133(1):130–8. e1–2. doi: 10.1016/j.jaci.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 48.Bonvalet M, Moussu H, Wambre E, Ricarte C, Horiot S, Rimaniol AC, et al. Allergen-specific CD4+ T cell responses in peripheral blood do not predict the early onset of clinical efficacy during grass pollen sublingual immunotherapy. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2012;42(12):1745–55. doi: 10.1111/cea.12015. [DOI] [PubMed] [Google Scholar]
- 49.Didier A, Malling HJ, Worm M, Horak F, Sussman GL. Prolonged efficacy of the 300IR 5-grass pollen tablet up to 2 years after treatment cessation, as measured by a recommended daily combined score. Clinical and translational allergy. 2015;5:12. doi: 10.1186/s13601-015-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durham SR, Emminger W, Kapp A, de Monchy JG, Rak S, Scadding GK, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. The Journal of allergy and clinical immunology. 2012;129(3):717–25.e5. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Antigen reactive memory CD4+ T cells and mRNA expression profiles. (A) Frequency of antigen reactive cells after 2 years of desensitization treatment based on CD154 up-regulation after allergen stimulation. Participants treated with sublingual immunotherapy are displayed in green, subcutaneous immunotherapy in red and placebo in blue. An Analysis of Covariance (ANCOVA) model with baseline adjustment was used for analyzing CD154 assay data. Data are shown in the plot as means of log10 fold change from baseline with 95% confidence intervals.
(B) Transcriptional analysis of extracted RNA from CD27+CD154+ and CD27−CD154+ cells at the 2-year timepoint. Transcript levels are displayed in color scale from high (yellow) to low (blue). Genes are grouped by category with Th2 in red, apoptosis in grey and Th1 related genes in purple.


