Abstract
Objective
To develop standardized treatment regimens for chronic nonbacterial osteomyelitis (CNO), also known as chronic recurrent multifocal osteomyelitis (CRMO) to enable comparative effectiveness treatment studies.
Methods
Virtual and face-to-face discussions and meetings were held within the CNO subgroup of the Childhood Arthritis and Rheumatology Research Alliance (CARRA). A literature search was conducted, and CARRA membership was surveyed to evaluate available treatment data and identify current treatment practices. Nominal group technique was used to achieve consensus on treatment plans for CNO refractory to non-steroidal anti-inflammatory drug (NSAID) monotherapy and/or with active spinal lesions.
Results
Three consensus treatment plans (CTPs) were developed for the first 12 months of therapy for CNO patients refractory to NSAID monotherapy and/or with active spinal lesions. The three CTPs are: (1) methotrexate or sulfasalazine, (2) tumor necrosis factor (TNF)-alpha inhibitors with optional use of methotrexate, and (3) bisphosphonates. Short courses of glucocorticoids and continuation of NSAIDs are permitted for all regimens. Consensus was achieved on these CTPs among CARRA members. Consensus was also reached on subject eligibility criteria, initial evaluations that should be conducted prior to the initiation of CTPs, and data items to collect to assess treatment response.
Conclusion
Three consensus treatment plans were developed for pediatric patients with CNO refractory to NSAIDs and/or with active spinal lesions. Use of these CTPs will provide additional information on efficacy and will generate meaningful data for comparative effectiveness research in CNO.
Keywords: Chronic nonbacterial osteomyelitis, consensus treatment plans, NSAID, spinal lesion
INTRODUCTION
Chronic nonbacterial osteomyelitis (CNO) is an autoinflammatory bone disease that mainly affects children and adolescents. Clinical presentations range from mild and sometimes limited unifocal disease to severe, chronically active or recurrent inflammation of multiple bones. The latter is referred to as chronic recurrent multifocal osteomyelitis (CRMO). Here we will use the term “CNO” to refer to the entire spectrum of this disease. CNO can be complicated by vertebral compression fractures, kyphosis, and leg length discrepancy when it is not recognized early or treated adequately. The diagnosis of CNO is made by excluding alternatives in the differential diagnosis including malignancy (leukemia, lymphoma, and primary or metastatic bone tumors), Langerhans cell histiocytosis, and infection. Clinical assessment in conjunction with serum inflammatory parameters and imaging studies, particularly magnetic resonance imaging (MRI), are crucial for the diagnosis and monitoring of disease activity of CNO (1).
Because of significant variation in clinical treatment practices among pediatric rheumatologists, standardized treatment regimens (consensus treatment plans, CTPs) have been developed within the Childhood Arthritis and Rheumatology Research Alliance (CARRA), a North American organization comprised of pediatric rheumatologists and researchers, for systemic juvenile idiopathic arthritis (JIA) (2), polyarticular JIA (3), lupus nephritis (4), juvenile localized scleroderma (5), and juvenile dermatomyositis (6). These CTPs enable progress to be made towards identifying optimal treatment for these diseases through prospective observational studies. The developed CTPs were based on best available evidence and current treatment practices, and generated through consensus methodology including nominal group techniques. The intention of these CTPs was to limit treatment practice variation to enable researchers to conduct comparative effectiveness studies. Because of the variability in second line treatment of CNO, we have worked to develop standardized treatment plans and data collection forms and measures for CNO patients with an NSAID-refractory course and/or with active spinal lesions. These CTPs will facilitate future comparative effectiveness studies for CNO. It must be noted that CTPs are not meant to be clinical care guidelines. A treating physician may deviate from the CTP at any time if it is deemed appropriate for the patient’s care.
MATERIALS AND METHODS
The CARRA CNO work group of the CARRA Scleroderma, Vasculitis, Autoinflammatory and Rare Diseases (SVARD) subcommittee consists of board-certified pediatric rheumatologists with special interest and expertise in CNO and family representatives. The CARRA CNO work group reviewed evidence published between 1966 and April 29, 2015. A literature search was conducted using Pubmed with the following MESH terms: SAPHO[All Fields] OR (Chronic[All Fields] AND nonbacterial[All Fields] AND ("osteomyelitis"[MeSH Terms] OR "osteomyelitis"[All Fields])) OR ("Chronic recurrent multifocal osteomyelitis"[Supplementary Concept] OR "Chronic recurrent multifocal osteomyelitis"[All Fields] OR "chronic recurrent multifocal osteomyelitis"[All Fields]) OR (noninfectious[All Fields] AND ("osteomyelitis"[MeSH Terms] OR "osteomyelitis"[All Fields])) AND (hasabstract[text] AND "humans"[MeSH Terms] AND English[lang]). In total, 398 articles were screened. A complete list of literature is included in Supplement 1. There was no randomized controlled study or case-control study in CNO. Therefore, case series, historical cohort, and prospective observational studies with at least 3 months of follow-up data in the pediatric population were included for review. Twenty-one articles met the criteria. Eleven additional articles published between April 29, 2015 and January 1, 2017 were later included. The group formulated clinical scenarios, analyzed survey responses from CNO workgroup members, organized consensus meetings, and finalized treatment plans. Levels evidence were graded from 2 to 5 according to guidelines established by the Oxford Centre for Evidence-Based Medicine (CEBM; online at www.cebm.net).
Planning meetings
Members of the CARRA CNO work group initiated the process to develop CTPs at the CARRA 2014 annual conference. Monthly conference calls within the work group were then used to develop a survey to CARRA members and ongoing discussion of the CTPs. The targeted population included patients refractory to NSAID monotherapy and/or with active spinal lesions because physicians perceived less favorable outcomes and need of additional treatment in such patients. At the planning meeting (Austin, TX, April 2015), a survey was sent to the SVARD subcommittee to collect responses of diagnostic, disease monitoring, and therapeutic approaches chosen by CARRA-affiliated pediatric rheumatologists. Further discussion at that meeting outlined the core substance of the planned CTPs. A detailed survey was sent to the CNO group (67% of 34 members responded) asking for comments on summarized plans and proposed options (see Supplement 2). At the American College of Rheumatology annual conference (San Francisco, CA, 2015), a second meeting was attended by 13 pediatric rheumatologists. Patient characteristics, treatment options, and imaging monitoring were discussed in depth.
Consensus meetings
At the CARRA annual conference (Toronto, 2016), a CNO meeting was attended by 6 family representatives and 30 pediatric rheumatologists, one of whom acted as the facilitator (YZ). The facilitator and family representatives participated in the discussion but were not eligible to vote.
Nominal group technique was used to achieve consensus (defined as ≥80% agreement within the group) on all questions considered during the meeting and subsequent conference calls. The facilitator framed the question to be discussed and presented data from the survey relevant to each question. Potential responses to the question were shown based on prior group discussion. Each participant had the opportunity to express his or her opinion for 1–2 minutes without interruption. Potential responses were updated accordingly.
Participants were then given the opportunity to vote for their preferred responses to the questions using a show-of-hand vote. Eighty percent or more of positive or negative votes was considered a consensus vote. If consensus was not achieved, participants were given the opportunity to speak uninterrupted for 1–2 minutes to share their thought process. After excluding answers that would not result in consensus votes, or after modifying potential responses, another vote was taken. If necessary, this process continued for two rounds on each question. If a clear consensus was not reached after two rounds, the decision was made to move to the next question.
RESULTS
What standardized disease-assessment tools of CNO have been reported?
Literature review of the clinical cohort studies on CRMO, CNO and pediatric synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome revealed a lack of agreement on a standardized evaluation tool. Two articles have reported standardized assessments with level IV evidence. Beck and colleagues used a PedCNO score (7) to assess prospectively the responses to naproxen in 37 children with CNO. After 12 months of treatment, 54% of patients achieved PedCNO 70 (at least 70% improvement in at least 3 out of 5 core variables and no more than one of remaining variables deteriorating by more than 70%). Within the core set, erythrocyte sedimentation rate (ESR), and the childhood health assessment questionnaire (CHAQ) had a floor effect by 3 months whereas the number of radiological lesions by MRI, severity of disease estimated by the physician, and severity of disease estimated by the patient or parent continued to improve over 12 months. Zhao and colleagues further described the characteristics of CNO lesions based on MRI findings using a grading system to score the severity of bone edema and soft tissue inflammation as well as the presence of periosteal reaction, hyperostosis, growth plate damage and vertebral compression (8). Applying this scoring tool to two retrospective cohorts of patients with CNO, authors found a significant decrease of number of nonvertebral lesions and maximum severity of bone edema in the group receiving aggressive treatment.
What evidence of effectiveness of second-line treatments is there in CNO?
Studies focusing on children with CNO who failed NSAID treatment are limited. Seven articles have reported treatment of pamidronate in CNO with level IV evidence. Kerrison and colleagues reported significant pain relief and improved activity and well-being with pamidronate use in seven children (three with spinal lesions) who failed NSAIDs (9). Simm et al. (10) and Miettunen et al. (11) demonstrated the effectiveness of pamidronate in children with CNO refractory to NSAIDs. Greater than 80% of patients had pain relief and more than 90% of patients in Miettunen’s study exhibited resolution of bone lesions on MRI after six months of treatment. Gleeson and colleagues reported pain relief with pamidronate in six of seven children who failed NSAIDs (12). Among five children with spinal fractures, three had follow-up x-rays showing regression of height loss in affected vertebrae in response to pamidronate therapy. Hospach et al. (13) reported complete resolution of hyperintensity signal of active spinal lesions after three to six cycles of pamidronate and a median interval of 13 months follow-up with MRI in eight of nine children with CNO refractory to NSAIDs. Roderick et al. treated 11 children with CNO refractory to NSAIDs with four cycles of pamidronate at 1 mg/kg/day on three consecutive days every three months (14). Two patients exhibited a good response, six individuals showed a moderate response, one had a mild response, while two failed to respond based on repeated whole body MRIs. Schnabel et al. described pamidronate to be highly effective in CNO patients refractory to standard treatment with NSAIDs and/or glucocorticoids (15).
Published data on the use of tumor necrosis factor (TNF)-alpha inhibitors (TNFi) in CNO are more limited. Eight articles have reported treatment of TNFi in CNO with level IV evidence. A small cohort study (n=4) reported by Eleftheriou et al. showed decreased pain in children with CNO after infliximab treatment (n=3) and anakinra (n=1, later switched to adalimumab) (16). Borzutzky et al. (17) and Wipff et al. (18) observed the highest rates of clinical remission (46%) or efficacy (89%) from TNFi compared to glucocorticoids, methotrexate, sulfasalazine, and NSAIDs. Jansson et al. (19), reported disease remission induced by infliximab in two patients who failed NSAIDs, glucocorticoids, DMARDs, and pamidronate. Recently, a combination of infliximab and methotrexate with or without zolendronic acid significantly improved clinical, laboratory, and imaging results in 9 children with CNO (8). However, Kaiser et al. showed poor response to TNFi in children with CNO in that only two of seven patients achieved remission (not defined) (20). On the other hand, etanercept was effective in all five patients in a small childhood series (21). Anti-interleukin (IL)-1 has been reported in fewer pediatric cases (20). In an adult cohort (n=6), anakinra improved the patient global assessment of disease activity within one month in five patients (22).
Most literature reported variable success of methotrexate (MTX) and sulfasalazine (SSZ) in patients with poor responses to NSAIDs or frequent relapses. Other DMARDs were rarely used. Five articles have reported treatment of DMARDs in CNO with level IV evidence. Jansson et al. (19), Catalano et al. (23), and Kaiser et al. (20) documented poor responses to SSZ, MTX, and azathioprine in children with CNO. Borzutzky et al. (17) and Wipff et al. (18) showed relatively lower remission rates (18–20%) and efficacy (38–41%) in children treated with MTX or SSZ. There was poor tolerance of MTX and dosing was not reported in most studies.
Currently, there is no consensus on subsequent treatment for patients refractory to NSAID treatment. Based on a survey sent to members of CARRA, 95% of treating physicians responded (41% response rate) use NSAIDs as first-line treatment in children with a new diagnosis of CNO (evidence level V) (24). For patients who failed NSAID treatment, the most commonly used treatments were reported as methotrexate (67%), TNFi (65%), and bisphosphonates (46%) (24). These results guided the development of consensus treatment plans (CTPs).
What patient characteristics should be included for this CTP?
Initial intent was to include all children with CNO. However, through further work group discussion, it was agreed that NSAIDs were generally considered first-line treatment for all newly diagnosed patients without active spinal lesions. Therefore, our attention turned to a more defined subset: patients refractory to NSAIDs and/or with active spinal lesions. The definition of “refractory to NSAID” was debated among group members, and the duration of initial NSAID trial was decided to be a minimum of 4 weeks. Based on the physician’s discretion and disease severity, further treatment may be initiated. The rationale of including active spinal lesions as a patient characteristic is the perceived significance of increased risk of vertebral fracture (11–13). The age limit for the CTP was set to 21 years, because children and adults younger than 21 years of age are commonly seen and followed in children’s hospitals. Patients with malignancy, infectious osteomyelitis, or other contraindications to the proposed treatment agents are not eligible for the CTP. Detailed characteristics of patients are provided in Table 1.
Table 1.
Patient characteristics for pediatric CNO refractory to NSAID monotherapy and/or with active spinal lesion
| Enrolled patients should have: |
| Age at enrollment equal to or younger than 21 years |
| Presence of bone edema on STIR or T2 fat saturation sequence on MRI within 12 weeks of enrollment |
| Whole body imaging evaluation (either WB MRI$ or bone scintigraphy) |
| Active disease* after failing at least 4 weeks of NSAIDs and/or presence of active spinal lesions& regardless of NSAID trial |
| Bone biopsy to exclude infection or malignancy unless bone lesions follow typical distribution# or there is IBD, psoriasis, or palmar plantar pustulosis |
| Enrolled patients should not have: |
| History of or current malignancy |
| Current infectious osteomyelitis |
| Contraindication to the selected treatment agent |
CNO: chronic nonbacterial osteomyelitis; NSAID: non-steroidal anti-inflammatory drug; STIR: short tau inversion recovery sequence; MRI: magnetic resonance imaging; WB: whole body; IBD: inflammatory bowel disease.
Suggested protocol includes STIR or fat saturation sequences of coronal views of whole body and sagittal view of total spine. Some patients may require dedicated views of hands or feet when lesions in these areas are present. Gadolinium is not required.
Active disease is defined as persistent pain with focal tenderness and/or warmth and/or persistence of bone edema on MRI in at least one lesion site
Active spinal lesions are defined as bone edema within at least one vertebral body of the cervical, thoracic, or lumbar spine
Typical distribution of lesions include the clavicle or symmetrical lesions in long bones at metaphysis/epiphysis
What standardized data should be collected at the initial evaluation?
Each patient should undergo a complete clinical assessment, including comprehensive musculoskeletal exam, since clinically active lesions are defined by findings of focal tenderness, and/or swelling, and/or warmth in addition to patient’s report of pain. Active joint counts with arthritis and enthesitis are important to record because of reported overlap between enthesitis-related arthritis (ERA) and CNO. Due to the lack of validated CNO-specific patient reported outcomes, both CHAQ and Patient-Reported Outcomes Measurement Information System (PROMIS) will be collected. Based on previously published diagnostic criteria of CNO (19,25) and results from physician surveys (24), a bone biopsy was recommended unless typical lesions including the clavicle or symmetrical lesions at metaphysis/epiphysis of long bones or comorbidities such as inflammatory bowel disease, palmoplantar pustulosis, or psoriasis are present. All participants agreed that whole body imaging is required to identify all bone lesions. Whole body MRI is preferred (26). A suggested protocol is included in Table 1. Bone scintigraphy was considered an adequate alternative if whole body MRI is not available. Total number of bone lesions is recorded per radiologist’s report. A baseline MRI (whole body or regional) is required to define active bone lesions based on the presence of bone marrow edema from short tau inversion recovery (STIR) or T2 fat saturation sequences, as the MRI findings are important to guide treatment decisions and to monitor disease activity (8,11,13,24,27). The normal range of bone marrow signal on MRI has not been established yet. Therefore, distinguishing abnormal marrow signal is subject to the experience of the radiologist reading the image. The size and severity of bone edema and/or soft tissue inflammation is determined by the radiologist based on previous description (8). Bony expansion, growth plate damage, and vertebral compression were considered disease damage and not active inflammation (8). In children who were treated with bisphosphonates, a linear hyperintense signal should not be mistaken as active lesions. Laboratory data including complete blood cell counts, ESR, and CRP are required for disease monitoring. Alkaline phosphatase at baseline is required to screen for metabolic bone disease. HLA-B27 has been reported with association of cutaneous diseases in CNO and strong association with ERA. Thus, participants agreed to include HLA-B27 test.
What are the most important therapies to include?
We reviewed the literature for medications with reported efficacy in CNO refractory to NSAIDs, including non-biological disease modifying anti-rheumatic drugs (DMARDs), TNFi, and bisphosphonates (8–14,17,18,20,28,29). There are no head-to-head comparisons among these treatments, even though current data suggested higher remission rates in children treated with TNFi than those with DMARDs (17,18). Among physicians in CARRA, methotrexate, TNFi, and bisphosphonates were most commonly used after children with CNO failed NSAIDs (24). Consensus was reached to include the three most commonly applied combinations of medications in final CTPs. There was a discussion on whether concomitant NSAIDs and/or oral glucocorticoid “bursts” were allowed. The group decided on the optional use of both with limits on the allowable duration of glucocorticoids due to their known side effects. Glucocorticoid “bursts” were defined as glucocorticoids (equivalent dosing of prednisone) up to 2 mg/kg/day (maximum daily dose of 60 mg) for up to a total of 6 weeks of treatment with or without tapering. Chosen strategies were in agreement with current practice echoed by participants. In patients treated with TNFi, concomitant methotrexate was allowed to suppress formation of human anti-chimeric anti-TNF antibody production (particularly with infliximab) as well as for combination therapy (Table 2).
Table 2.
Consensus treatment plans for the first 6–12 months
| Treatment A: Non-biological DMARDs |
| Methotrexate (oral or subcutaneous): 15 mg/m2 (maximum 25 mg/dose) weekly |
| OR |
| Sulfasalazine (oral): 50 mg/kg/day (maximum 1500 mg/dose) divided twice daily |
| Treatment B: TNF-alpha inhibitors (TNFi) with or without methotrexate |
| Adalimumab (subcutaneous):20 mg every other week for body weight between 15–30 kg; 40 mg every other week for body weight ≥ 30 kg. May increase to weekly. |
| OR |
| Etanercept (subcutaneous): 0.8 mg/kg (maximum 50 mg/dose) weekly. May split into twice a week. |
| OR |
| Infliximab (intravenous): 5–10 mg/kg (maximum 1000 mg/dose) at week 0,2,6 then every 4–8 weeks |
| OR |
| Other TNFi as per discretion of treating physician |
| Optional: concomitant methotrexate (do not need to follow treatment protocol, may use lower dosing, i.e. 5–10 mg/m2) |
| Treatment C: Bisphosphonate |
| Pamidronate (intravenous)*: |
| Option 1: 1 mg/kg/dose (maximum 60 mg/dose) every month |
| Option 2: 1 mg/kg/dose for 3 consecutive days every 3 months |
| OR |
| Zolendronic acid (intravenous): Initial dose 0.0125–0.025 mg/kg every 6 months. May increase dose to 0.05 mg/kg/dose (maximum 4 mg/dose) |
| All options allow concurrent use of |
| NSAIDs (see appendix for detailed dosing) |
| Glucocorticoids: Up to a total of 6 weeks of treatment with or without tapering with an upper limit of 2 mg/kg/day (equivalent dosing of prednisone, maximum 60 mg daily). |
DMARD: disease modifying anti-rheumatic drug; TNF: tumor necrosis factor; NSAID: non-steroidal anti-inflammatory drug. With the exception of bisphosphonates (3–6 months), minimum treatment duration should be 12 months.
Both options may use lower dose of 0.5 mg/kg at the initiation of the treatment. Both options should continue for a minimum duration of 3 months. Maximum cumulative dose is 11.5 mg/kg/year.
What dose/route/frequency should be used for each medication in the CTPs?
The most commonly used DMARD by physician members in the CARRA survey was methotrexate (34%) (24). However, within the CNO group, members reported that sulfasalazine was commonly used based on personal experiences. Thus, only these two DMARDs were included in the protocol. TNFi reported for use in CNO were limited to etanercept, adalimumab, and infliximab. The frequency of using these TNFi among surveyed CARRA physician members was 26% with adalimumab and infliximab, and 17% with etanercept (24). Thus, all 3 were included in the protocol. Other TNFi may be used by the treating physician with discretion. Mandatory tuberculosis screening is required prior to the initiation of a TNFi. The dosing of DMARDs and TNFi followed standard JIA treatment regimens as reported in the literature and clinical practice (Table 2). Pamidronate was the most commonly reported bisphosphonate (9–15) whereas zolendronic acid was only reported as concomitant treatment in a single study (8). However, both were used by physicians within CARRA (pamidronate 79%, zolendronic acid 21%) (24). Therefore, in the bisphosphonate arm, pamidronate and zolendronic acid were both included in the protocol. The dosing of bisphosphonates was based on the pediatric endocrinology literature and has been utilized in case series of CNO and SAPHO patients (Table 2). Suggested toxicity monitoring and immunizations are included in Appendix 1.
What criteria should be used to determine treatment failure?
Various parameters have been used to define “treatment response” in CNO, including the PedCNO score (7), total number of clinically active bone lesions, and severity of bone edema and soft tissue inflammation on MRI (8). After discussion, group consensus was reached to use a composite score similar to the JIA core set based on significant variation of symptoms and a high incidence of pain amplification (pain in the absence of inflammatory activity) among CNO patients. Various items were proposed by group members and after in-depth discussion; the group identified the top 6 individual items. These treatment response criteria are considered as expert opinion (evidence level IV). Thereby, a modified composite score was proposed by replacing CHAQ and severity of disease estimated by patient or parent with the size and severity of bone marrow edema and/or soft tissue inflammation in the MRI and the total count of clinically active lesions. These criteria have not been validated and are merely suggestions for physicians to consider during their clinical management of children with CNO. As shown in Table 3, a combination of criteria for treatment failure include: patient pain as measured by visual analogue scale (VAS), total number of clinically active lesions (defined as focal tenderness, and/or swelling, and/or warmth in addition to patient’s report of pain at a known CNO lesion site), physician evaluation of disease activity as measured on a 10 centimeter Likert scale (VAS), number of radiological lesions by whole body MRI or bone scintigraphy, maximum severity of marrow edema of CNO lesions on imaging, and abnormal ESR and/or CRP after exclusion of other potential causes. The CHAQ was not included because of its floor effect and lack of applicability to the majority of CNO patients accordingly to CNO group members’ experience. Treatment failure at 3 months was defined as: no improvement in at least 4 of the 6 criteria, or no improvement in ≥ 50% of applicable criteria if not all are available. Treatment success (complete resolution) was defined as resolution of pain, normalization of ESR and CRP, and resolution of marrow edema in MRI, reported previously (17–19).
Table 3.
Criteria for treatment failure at 3 months (when a patient fails to improve on at least 4/6 of the criteria or over 50% of applicable criteria)
|
VAS: visual analogue scale: CNO: chronic nonbacterial osteomyelitis; WBMRI: whole body magnetic resonance imaging; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein.
Clinically active lesion is defined as a body part with focal tenderness, and/or swelling, and/or warmth in addition to patient’s report of pain at a known CNO lesion site.
Abnormal ESR is defined as ≥20 mm/hr and abnormal CRP is defined as ≥1 mg/dL.
Management of CNO patients at follow-up visits will depend on the overall assessment of disease activity by the physician because there are no validated criteria at this time. At the three-month follow-up visit, an escalation of treatment such as switching medications within an arm or switching to a different arm is recommended if there is worsening of the disease or no improvement based on the treating physician’s assessment (Figure 1). Otherwise, maintaining current treatment is recommended. With the exception of bisphosphonates (3–6 months), minimum treatment duration should be 12 months based on the chronic nature of CNO, poor response to previous NSAIDs treatment, and the risk of vertebral compression fractures.
Figure 1.
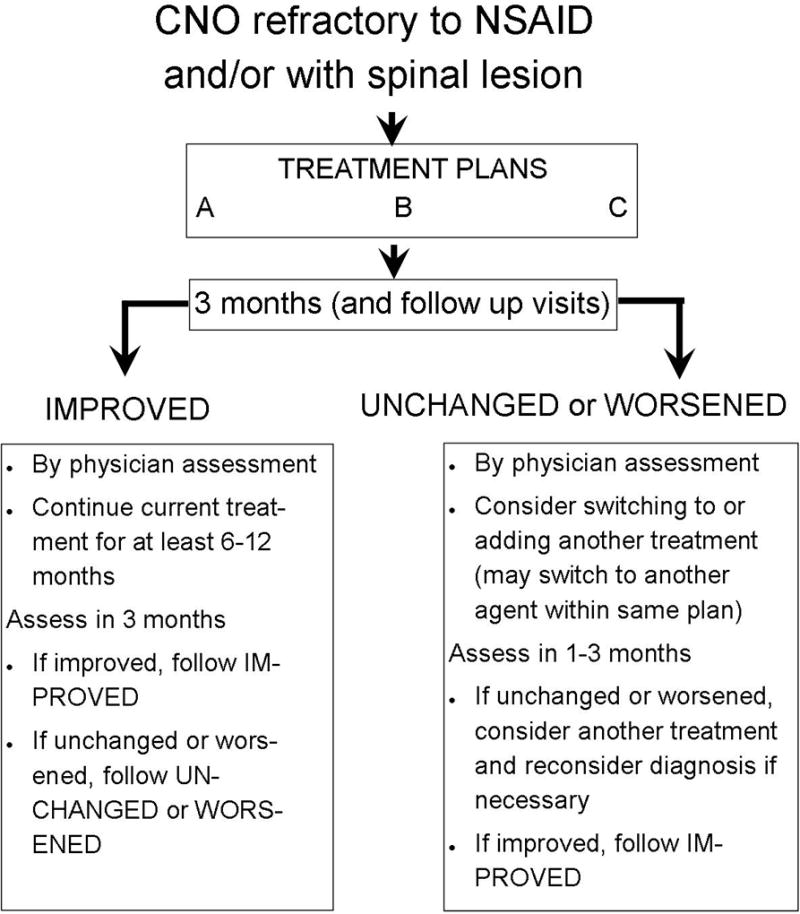
Summary of protocols
At what intervals should patients be followed for the purposes of data collection?
Consensus was reached to follow intended CNO patients a minimum of every 3 months for the first year. The follow-up visit may occur earlier if the physician has concerns about the clinical course and/or treatment response. In addition to routine history, physical exams and laboratories, MRI is strongly recommended to objectively assess disease activity at 6 and 12 months after adjusting therapy. Additional imaging is recommended in suspected disease flares or persistent activity despite treatment escalation. Whole body MRI is generally preferred, but regional MRI of known sites is acceptable in unifocal disease or if whole body MRI is not available.
What data should be collected at follow-up?
Consensus was reached to minimize data collection for practicability using a standardized form. Table 4 includes clinical parameters, imaging and laboratory tests considered essential for CNO follow-up assessment.
Table 4.
Suggested minimum data collection and assessment intervals to be used with treatment plans*
| Proposed variables | Baseline visit |
Follow up visits |
|---|---|---|
| History | ||
| Demographics | ||
| Date of birth | x | |
| Sex | x | |
| Race and ethnicity | x | |
| Clinical symptoms | ||
| Fever | x | x |
| Rash | x | x |
| Gastroenterological symptoms | x | x |
| Bone pain | x | x |
| Limitation of motor function$ | x | x |
| Pre-enrollment treatment history for CNO | x | |
| Family history of CNO-associated conditions# | x | |
| Past medical history/concurrently CNO-associated conditions# | x | x |
| Current medications and doses | x | x |
| Patient-reported outcomes and global assessments | ||
| Pain | x | x |
| Health-related quality of life | x | x |
| Physical function –CHAQ, PROMIS | x | x |
| Parent/patient global assessment of disease activity | x | x |
| Physician global assessment of disease activity | x | x |
| Physical examination | ||
| Height, weight | x | x |
| Clinically active CNO lesion count | x | x |
| Active joint counts | x | x |
| Enthesitis | x | x |
| Rash | x | x |
| Imaging findings | ||
| MRI (whole body preferred if available) | x | x |
| Bone scintigraphy if WB MRI is not available | x | |
| X ray if done | x | x |
| CT if done | x | x |
| DXA if done | x | x |
| Laboratory findings | ||
| CBC with differential | x | x |
| C-reactive protein | x | x |
| Erythrocyte sedimentation rate | x | x |
| Alkaline Phosphatase | x | |
| HLA-B27 | x | |
| Bone biopsy findings& | ||
| Bacterial, fungal and AFB culture | x | |
| Pathology | x | |
| Treatment plan–related items | ||
| Serious adverse events or important medical event | x | |
| If plan discontinued, rationale | x | |
| Number of glucocorticoid burst, if any | x |
Data are collected at baseline and at follow up visits every 2–3 months.
CNO: chronic nonbacterial osteomyelitis; MRI: magnetic resonance imaging; CT: computed tomography; DXA: dual energy X ray absorptiometry; CBC: complete blood cell count; HLA: human leukocyte antigen.
Prolonged school/daycare absences, limited use of upper limb, difficulty weight bearing, requiring crutches, bedridden from spinal/leg pain.
Psoriasis, inflammatory bowel disease, celiac disease, inflammatory arthritis, spondyloarthropathy
Needed when bone lesions do not follow typical distribution (clavicle or symmetrical lesions in long bones at metaphysis/epiphysis) in the absence of CNO-associated conditions.
Final approval of the CTP by CARRA members
A final survey (Supplement 2) was sent to 337 active voting members of CARRA in April 2017. A total of 275 responses were received with a response rate of 82%. Among responders, 254 were attending pediatric rheumatologists in North America who have cared for children with CNO. Within these 254 responders, a total of 216 (85%) completed the entire survey. Most responders (70%) had 1–4 patients with CNO who either failed NSAIDs or had active spinal lesions over the last 12 months whereas 14% had none and 16% had 5 or more.
Survey results showed that greater than 90% of responders agreed with patient characteristics, treatment plans, and definition of treatment failure. Within the treatment plans, 78%, 90%, and 50% of 228 responders use DMARDs, TNFi, or bisphosphonates, respectively, on children who fail NSAIDs; 62%, 94%, and 73% of 221 responders are willing to use these respective treatment plans on children with active spinal lesions.
DISCUSSION
To our knowledge, these are the first consensus treatment plans developed for children with CNO by members of a professional society. Our work demonstrates the feasibility of achieving consensus in treatment plans and data collection for a rare and under-studied pediatric rheumatic disease using a combination of surveys, a comprehensive literature search, and nominal group technique.
Lack of validated criteria for classification and follow-up, as well as standardized treatment, has hindered the progress of comparative effectiveness research in CNO. Current approaches are solely based on small case series, personal experience, and expert opinion (1). Our work is one step forward towards standardizing applied treatment regimens based on existing data and collection of a minimal set of data. This may allow objective evaluation of the effectiveness of different treatments. In addition, consistent imaging data collection will provide important corroboration with patient reported outcomes and the physician’s clinical assessment.
The CTPs presented here reflect current clinical practice of CARRA members. Thus, they are highly applicable and more likely to be adopted in daily practice by practitioners. The intent of these CTPs is to reduce the variation of applied treatment options so that meaningful data from as many patients as possible can be collected in an observational study.
Of note, the proposed treatment plans are standardized regimens without strong evidence of which treatment is optimal. They are not to be misinterpreted as guidelines as their intention is to enable further study to identify optimal treatment. These CTPs currently do not include any biologic treatments other than TNFi, because of the rarity of their use in CNO and a lack of support by the available literature. However, these CTPs may be revised in the future to include other potentially effective forms of treatment as more evidence becomes available.
Bone biopsy was not required for all children with CNO (18,25). However, other diagnoses must be excluded prior to using these plans based on the treating physician’s thorough evaluation. These plans should only be used when physicians are confident of the diagnosis.
Since whole body MRI offers the most thorough imaging evaluation for CNO without exposure to radiation, it should be considered the gold standard. However, regional MRIs (or a series of multiple regional MRIs) are considered reasonable when whole body MRI is not available. Other whole body imaging, such as bone scintigraphy, is considered an alternative one-time baseline assessment whenever whole body MRI is not available.
The CTP presented here has limitations. First, this CTP does not extend beyond 12 months of treatment. Second, this CTP does not include biologic treatments other than TNFi. Third, validated disease monitoring scoring tools are lacking and the proposed criteria of treatment failure need further evaluation and validation.
CONCLUSION
Three standardized consensus treatment plans were developed for patients with CNO with insufficient response to NSAIDs and/or the presence of active spinal lesions. Use of these treatment plans will provide the opportunity to generate meaningful data for future prospective observational studies to evaluate their effectiveness in children with CNO.
Supplementary Material
Significance and Innovations.
Three standardized consensus treatment plans were developed for patients with CNO who have had insufficient response to NSAIDs and/or who have active spinal lesions.
The consensus treatment plans developed by members of the Childhood Arthritis and Rheumatology Research Alliance (CARRA) are the first ever for patients with CNO.
Use of these treatment plans will allow for evaluation of these medications in patients with CNO in future comparative effectiveness research studies.
Acknowledgments
The authors would like to thank participants in CARRA who contributed in meetings of the CNO/CRMO Group from 2014–2017: Leslie Abramson, Kevin Baszis, Michal Cidon, Leslie Favier, Olha Halyabar, Norman Ilowite, Hanna Kim, Andrew Lasky, Debora Rothman, Aliese Sarkissian, Salma Siddique, Brandi Stevens, Katie Stewart, Sarah Taber, Jessica Turnier, Ali Yalcindag, Andrew Zeft, Larry Zemel, Donald Goldsmith, Stacey Tarvin, Christi J Inman, Jon M Burnham, Henner Morbach, Toni Hospach.
We appreciate the input from the following family representatives: Dr. Marie Faughnan, Rebecca Gholson, Dr. Colin McKerlie, Diane McKerlie, Elizabeth Murray, Violet Randle.
CARRA was funded by NIAMS, Friends of CARRA and the Arthritis Foundation. Christian Hedrich is supported by the Fritz Thyssen foundation, the Novartis foundation for therapeutic research, and the intramural MeDDrive program, University of Technology Dresden. Gil Amarilyo received consulting fees and research support from Novartis. Matthew Basiaga was supported by NIH T32 GM075766-09. Polly Ferguson is supported by R01AR059703 from NIH/NIAMS. Yongdong Zhao is supported by Clinical Research Scholar Program from Seattle Children’s Research Institute and CARRA.
Appendix 1
Medication Dosing and Monitoring for CNO CTPs
Nonsteroidal anti-inflammatory drugs (NSAIDs)
- Dose (1)
- Celecoxib (age 2 and older): 10–25 kg: 100 mg/dose twice daily; > 25 kg: 200 mg/dose twice daily
- Diclofenac: 2–3 mg/kg/day divided 3 times per day, maximum 150 mg/day (2)
- Ibuprofen: 30–40mg/kg/day divided 3 times per day, maximum 3200 mg/day
- Indomethacin: 1–3 mg/kg/day divided 2–3 times per day, maximum 200 mg/day (3)
- Meloxicam: 0.125–0.25 mg/kg/day once daily, maximum 15 mg/day
- Piroxicam: 0.2–0.3 mg/kg/day once daily, maximum 20 mg/day
- Toxicity monitoring
- Check CBC with differential, serum creatinine, and liver enzymes prior to initiation and every 6 months during chronic daily use (8)
- Other recommendations
- Take with food
- Do not combine with other NSAIDs
- Avoid in patients with renal dysfunction, thrombocytopenia, liver dysfunction
Side effects to capture: gastritis or gastric ulcer, hepatic or renal toxicity, pseudoporphyria
Glucocorticoids
-
Dose
Glucocorticoids (equivalent dosing of prednisone) up to 2 mg/kg/day with maximum daily dose of 60 mg for up to 6 weeks with or without tapering are allowed for flares
- Toxicity monitoring
- Not necessary unless patient has diabetes or hypertension
- Other recommendations
- Take in the morning to reduce sleep disturbance and other side effects
- Dietary guidance to reduce the risk of weight gain
Side effects to capture: weight gain, infection, acne, hirsutism, behavioral changes
Non-Biologic DMARDs (9)
Methotrexate (MTX)
- Route
- Oral or subcutaneous dosing allowed (reminder that subcutaneous route may have fewer side effects, better absorption, and improved efficacy at doses greater than 10 mg/m2)
- Dose (10)
- Recommended dosing: 15 mg/m2/week
- Maximum recommended dose at any time: 25 mg
- Toxicity monitoring
- Check CBC with differential, LFTs (AST, ALT), and creatinine prior to initiation, approximately 1 month after initiation, approximately 1–2 months after an increase in dose, repeat every 3–4 months if prior results are normal and dose is stable
- Folic acid and/or leucovorin is recommended
- Consider hepatitis A, hepatitis B, hepatitis C, and/or varicella screening at baseline
- Consider PPD or interferon gamma release assay prior to starting
- Interferon gamma release assay may not be a reliable assay in children < 2 years of age or children on glucocorticoid therapy
Side effects to capture: fatigue, headache, neurologic affects, nausea, vomiting, and abdominal pain
Sulfasalazine (SSZ)
- Dosing
- Recommended dosing: 50 mg/kg/day up to 3 grams/day
-
Toxicity monitoring:
- Check CBC with differential, LFTs (AST, ALT) and creatinine prior to initiation, approximately 1 month after initiation, approximately 1–2 months after an increase in dose, repeat every 3–4 months if prior results are normal and dose is stable
- Consider hepatitis B screening at baseline
- Reminder that hemolytic anemia (associated with glucose-6-phosphate dehydrogenase deficiency), Stevens Johnson Syndrome, and DRESS syndrome* have been reported in patients taking SSZ
*DRESS syndrome – Rash, eosinophilia, and at least one of the following: enlarged lymph nodes, hepatitis (transaminases or AST, ALT >2× upper limit of normal), interstitial nephropathy, lung disease or myocardial involvement
Tumor necrosis factor (TNF)-a inhibitors (9)
- Dosing
- Etanercept: 0.8 mg/kg SQ weekly or divided twice weekly, maximum 50 mg per week
- Adalimumab: 15–30 kg: 20 mg SQ every other week, may increase to every week; ≥ 30 kg: 40 mg SQ every other week, may increase to every week (11)
- Other TNF-α inhibitor use and dosing are subject to the discretion of treating physician
- Concomitant methotrexate (do not need to follow treatment protocol, may use lower dosing)
- Toxicity Monitoring
- Check CBC with differential, liver enzymes, serum creatinine prior to initiation, repeat approximately every 3– 6 months if prior results normal and dose stable
- PPD or interferon gamma release assay prior to initiation, repeat approximately once yearly if risk factors present. If positive, need chest X ray and treatment per infectious diseases prior to initiating treatment (usually at least 4–6 weeks of treatment)
- Interferon gamma release assay may not be a reliable assay in children < 2 years of age or children on glucocorticoid therapy
- Consider screening for histoplasmosis, blastomycosis, coccidiomycosis in endemic areas
- Other recommendations
- Avoid live virus vaccinations while on biologic agents
- Avoid combinations of biologic agents
- Recommend discussion of the FDA malignancy risk warning as part of routine counseling prior to initiation of therapy
- Methotrexate use is recommended but not required with infliximab to avoid human antichimeric antibody development
Bisphosphonates
Route – intravenous administration
- Dose
- Pamidronate:
- Option 1: 1 mg/kg/dose (maximum 60 mg/dose) every month (12)
-
Zolendronic acid:
- Toxicity monitoring
- Check electrolytes, calcium, phosphate, magnesium, and creatinine prior to each monthly infusion or the first day of three consecutive infusions
- Other recommendations
- Consider Zofran as pre-medications and use for post-infusion nausea
- Daily Calcium and vitamin D supplementation
- Baseline dental evaluation and regular dental care are recommended
- Avoid dental extraction per dentist’s recommendation
- Discussion of fetal risk with female patients (21)
- 1.Cassidy J, Petty R, Laxer R, Lindsley C. Textbook of Pediatric Rheumatology. 6. Philadelphia, PA: Saunders Elsevier; 2010. [Google Scholar]
- 2.Job-Deslandre C, Krebs S, Kahan A. Chronic recurrent multifocal osteomyelitis: five-year outcomes in 14 pediatric cases. Jt Bone Spine. 2001;68:245–251. doi: 10.1016/s1297-319x(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 3.Abril JC, Ramirez A. Successful treatment of chronic recurrent multifocal osteomyelitis with indomethacin: a preliminary report of five cases. J Pediatr Orthop. 2007;27:587–91. doi: 10.1097/BPO.0b013e318070cbd3. [DOI] [PubMed] [Google Scholar]
- 4.Girschick HJ, Krauspe R, Tschammler A, Huppertz HI. Chronic recurrent osteomyelitis with clavicular involvement in children: diagnostic value of different imaging techniques and therapy with non-steroidal anti-inflammatory drugs. Eur J Pediatr. 1998;157:28–33. doi: 10.1007/s004310050761. [DOI] [PubMed] [Google Scholar]
- 5.Girschick HJ, Raab P, Surbaum S, Trusen A, Kirschner S, Schneider P, et al. Chronic non-bacterial osteomyelitis in children. Ann Rheum Dis. 2005;64:279–85. doi: 10.1136/ard.2004.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansson A, Renner ED, Ramser J, Mayer A, Haban M, Meindl A, et al. Classification of non-bacterial osteitis: Retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatology. 2007;46:154–160. doi: 10.1093/rheumatology/kel190. [DOI] [PubMed] [Google Scholar]
- 7.Beck C, Morbach H, Beer M, Stenzel M, Tappe D, Gattenlöhner S, et al. Chronic nonbacterial osteomyelitis in childhood: prospective follow-up during the first year of anti-inflammatory treatment. Arthritis Res Ther. 2010;12:R74. doi: 10.1186/ar2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken) 2011;63:465–82. doi: 10.1002/acr.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringold S, Weiss PF, Colbert RA, DeWitt EM, Lee T, Onel K, Prahalad S, Schneider R, Shenoi S, Vehe RK KYJIARC of the CA and RRA Childhood Arthritis and Rheumatology Research Alliance Consensus Treatment Plans for New Onset Polyarticular Juvenile Idiopathic Arthritis. Arthritis Care Res (Hoboken) 2014;66:1063–72. doi: 10.1002/acr.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Chauvin NA, Jaramillo D, Burnham JM. Aggressive Therapy Reduces Disease Activity without Skeletal Damage Progression in Chronic Nonbacterial Osteomyelitis. J Rheumatol. 2015;42:1245–51. doi: 10.3899/jrheum.141138. Epub 2015 May. [DOI] [PubMed] [Google Scholar]
- 11.Eleftheriou D, Gerschman T, Sebire N, Woo P, Pilkington CA, Brogan PA. Biologic therapy in refractory chronic non-bacterial osteomyelitis of childhood. Rheumatology. 2010;49:1505–1512. doi: 10.1093/rheumatology/keq122. [DOI] [PubMed] [Google Scholar]
- 12.Gleeson H, Wiltshire E, Briody J, Hall J, Chaitow J, Sillence D, et al. Childhood chronic recurrent multifocal osteomyelitis: Pamidronate therapy decreases pain and improves vertebral shape. J Rheumatol. 2008;35:707–712. [PubMed] [Google Scholar]
- 13.Kerrison C, Davidson JE, Cleary aG, Beresford MW. Pamidronate in the treatment of childhood SAPHO syndrome. Rheumatology (Oxford) 2004;43:1246–51. doi: 10.1093/rheumatology/keh295. [DOI] [PubMed] [Google Scholar]
- 14.Simm P, Allen R, Zacharin M. Bisphosphonate Treatment in Chronic Recurrent Multifocal Osteomyelitis. J Pediatr. 2008;152:571–575. doi: 10.1016/j.jpeds.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 15.Miettunen PM, Narendran A, Jayanthan A, Behrens EM, Cron RQ. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatology (Oxford) 2011;50:417–9. doi: 10.1093/rheumatology/keq218. [DOI] [PubMed] [Google Scholar]
- 16.Roderick M, Shah R, Finn A, Ramanan AV. Efficacy of pamidronate therapy in children with chronic non-bacterial osteitis: disease activity assessment by whole body magnetic resonance imaging. Rheumatology (Oxford) 2014;53:1973–6. doi: 10.1093/rheumatology/keu226. [DOI] [PubMed] [Google Scholar]
- 17.Compeyrot-Lacassagne S, Rosenberg AM, Babyn P, Laxer RM. Pamidronate treatment of chronic noninfectious inflammatory lesions of the mandible in children. J Rheumatol. 2007;34:1585–9. [PubMed] [Google Scholar]
- 18.Hospach T, Langendoerfer M, Kalle T von, Maier J, Dannecker GE. Spinal involvement in chronic recurrent multifocal osteomyelitis (CRMO) in childhood and effect of pamidronate. Eur J Pediatr. 2010;169:1105–11. doi: 10.1007/s00431-010-1188-5. [DOI] [PubMed] [Google Scholar]
- 19.Barros ER, Saraiva GL, Oliveira TP de, Lazaretti-Castro M. Safety and efficacy of a 1-year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2012;25:485–91. doi: 10.1515/jpem-2012-0016. [DOI] [PubMed] [Google Scholar]
- 20.Vuorimies I, Toiviainen-Salo S, Hero M, Mäkitie O. Zoledronic Acid Treatment in Children with Osteogenesis Imperfecta. Horm Res Paediatr. 2011;75:346–353. doi: 10.1159/000323368. [DOI] [PubMed] [Google Scholar]
- 21.Green SB, Ashley L. Pappas. Effects of maternal bisphosphonate use on fetal and neonatal outcomes. Am J Heal Syst Pharm. 2014;71:2029–2036. doi: 10.2146/ajhp140041. [DOI] [PubMed] [Google Scholar]
Footnotes
Author contribution
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Zhao had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Dedeoglu, Ferguson, Lapidus, Laxer, Li, Wu, Zhao.
Literature review: Amarilyo, Basiaga, Cooper, Dedeoglu, Devergsten, Ferguson, Fox, Lapidus, Laxer, Lee, Li, Vora, Wu, Zhao.
Analysis and interpretation of data: all authors.
Critical review of the manuscript: Cooper, Dedeoglu, Ferguson, Girschick, Haines, Hedrich, Lapidus, Laxer, Lee, Li, Oliver, Wu, Zhao in great detail and other co-authors in lesser detail.
References
- 1.Hofmann SR, Schnabel A, Rosen-Wolff A, Morbach H, Girschick HJ, Hedrich CM. Chronic Nonbacterial Osteomyelitis: Pathophysiological Concepts and Current Treatment Strategies. J Rheumatol. 2016 doi: 10.3899/jrheum.160256. [DOI] [PubMed] [Google Scholar]
- 2.DeWitt EM, Kimura Y, Beukelman T, Nigrovic Pa, Onel K, Prahalad S, et al. Consensus treatment plans for new-onset systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2012;64:1001–10. doi: 10.1002/acr.21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringold S, Weiss PF, Colbert RA, DeWitt EM, Lee T, Onel K, Prahalad S, Schneider R, Shenoi S, Vehe RK KYJIARC of the CA and RRA Childhood Arthritis and Rheumatology Research Alliance Consensus Treatment Plans for New Onset Polyarticular Juvenile Idiopathic Arthritis. Arthritis Care Res (Hoboken) 2014;66:1063–72. doi: 10.1002/acr.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mina R, Scheven Evon, Ardoin SP, Eberhard BA, Punaro M, Ilowite N, et al. Consensus treatment plans for induction therapy of newly-diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2011;64:375–383. doi: 10.1002/acr.21558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li SC, Torok KS, Pope E, Dedeoglu F, Hong S, Jacobe HT, et al. Development of consensus treatment plans for juvenile localized scleroderma: A roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care Res. 2012;64:1175–1185. doi: 10.1002/acr.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber AM, Robinson AB, Reed AM, Abramson L, Bout-Tabaku S, Carrasco R, Curran M, Feldman BM, Gewanter H, Griffin T, Haines K, Hoeltzel MF, Isgro J, Kahn P, Lang B, Lawler P, Shaham B, Schmeling H, Scuccimarri R, Shishov M, Stringer E, Wohrley J, Ilowite N WCJDS of the CA and RRA Consensus Treatments for Moderate Juvenile Dermatomyositis: Arthritis Care Res (Hoboken) Beyond the First Two Months: Results of the Second Children’s Arthritis and Rheumatology Research Alliance Consensus Conference. Arthritis Care Res. 2012;64:546–553. doi: 10.1002/acr.20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck C, Morbach H, Beer M, Stenzel M, Tappe D, Gattenlöhner S, et al. Chronic nonbacterial osteomyelitis in childhood: prospective follow-up during the first year of anti-inflammatory treatment. Arthritis Res Ther. 2010;12:R74. doi: 10.1186/ar2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Chauvin NA, Jaramillo D, Burnham JM. Aggressive Therapy Reduces Disease Activity without Skeletal Damage Progression in Chronic Nonbacterial Osteomyelitis. J Rheumatol. 2015;42:1245–51. doi: 10.3899/jrheum.141138. Epub 2015 May. [DOI] [PubMed] [Google Scholar]
- 9.Kerrison C, Davidson JE, Cleary aG, Beresford MW. Pamidronate in the treatment of childhood SAPHO syndrome. Rheumatology (Oxford) 2004;43:1246–51. doi: 10.1093/rheumatology/keh295. [DOI] [PubMed] [Google Scholar]
- 10.Simm P, Allen R, Zacharin M. Bisphosphonate Treatment in Chronic Recurrent Multifocal Osteomyelitis. J Pediatr. 2008;152:571–575. doi: 10.1016/j.jpeds.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 11.Miettunen PM, Wei X, Kaura D, Reslan WA, Aguirre AN, Kellner JD. Dramatic pain relief and resolution of bone inflammation following pamidronate in 9 pediatric patients with persistent chronic recurrent multifocal osteomyelitis (CRMO) Pediatr Rheumatol Online J. 2009;7:2. doi: 10.1186/1546-0096-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gleeson H, Wiltshire E, Briody J, Hall J, Chaitow J, Sillence D, et al. Childhood Chronic Recurrent Multifocal Osteomyelitis: Pamidronate Therapy Decreases Pain and Improves Vertebral Shape. J Rheumatol. 2008;35:707–712. [PubMed] [Google Scholar]
- 13.Hospach T, Langendoerfer M, Kalle T von, Maier J, Dannecker GE. Spinal involvement in chronic recurrent multifocal osteomyelitis (CRMO) in childhood and effect of pamidronate. Eur J Pediatr. 2010;169:1105–11. doi: 10.1007/s00431-010-1188-5. [DOI] [PubMed] [Google Scholar]
- 14.Roderick M, Shah R, Finn A, Ramanan AV. Efficacy of pamidronate therapy in children with chronic non-bacterial osteitis: disease activity assessment by whole body magnetic resonance imaging. Rheumatology (Oxford) 2014;53:1973–6. doi: 10.1093/rheumatology/keu226. [DOI] [PubMed] [Google Scholar]
- 15.Schnabel A, Range U, Hahn G, Berner R, Hedrich CM. Treatment Response and Longterm Outcomes in Children with Chronic Nonbacterial Osteomyelitis. J Rheumatol. 2017 doi: 10.3899/jrheum.161255. jrheum.161255. [DOI] [PubMed] [Google Scholar]
- 16.Eleftheriou D, Gerschman T, Sebire N, Woo P, Pilkington CA, Brogan PA. Biologic therapy in refractory chronic non-bacterial osteomyelitis of childhood. Rheumatology. 2010;49:1505–1512. doi: 10.1093/rheumatology/keq122. [DOI] [PubMed] [Google Scholar]
- 17.Borzutzky A, Stern S, Reiff A, Zurakowski D, Steinberg Ea, Dedeoglu F, et al. Pediatric Chronic Nonbacterial Osteomyelitis. Pediatrics. 2012;130:e1190–7. doi: 10.1542/peds.2011-3788. [DOI] [PubMed] [Google Scholar]
- 18.Wipff J, Costantino F, Lemelle I, Pajot C, Duquesne A, Lorrot M, et al. A large national cohort of French patients with chronic recurrent multifocal osteitis. Arthritis Rheumatol. 2015;67:1128–1137. doi: 10.1002/art.39013. [DOI] [PubMed] [Google Scholar]
- 19.Jansson A, Renner ED, Ramser J, Mayer A, Haban M, Meindl A, et al. Classification of non-bacterial osteitis: retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatology (Oxford) 2007;46:154–60. doi: 10.1093/rheumatology/kel190. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser D, Bolt I, Hofer M, Relly C, Berthet G, Bolz D, et al. Chronic nonbacterial osteomyelitis in children: a retrospective multicenter study. Pediatr Rheumatol Online J. 2015;13:25. doi: 10.1186/s12969-015-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batu ED, Ergen FB, Gulhan B, Topaloglu R, Aydingoz U, Ozen S. Etanercept treatment in five cases of refractory chronic recurrent multifocal osteomyelitis (CRMO) Jt Bone Spine. 2015;82:471–473. doi: 10.1016/j.jbspin.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Wendling D, Prati C, Aubin F. Anakinra treatment of SAPHO syndrome: short-term results of an open study. Ann Rheum Dis. 2012;71:1098–1100. doi: 10.1136/annrheumdis-2011-200743. [DOI] [PubMed] [Google Scholar]
- 23.Catalano-Pons C, Comte A, Wipff J, Quartier P, Faye A, Gendrel D, et al. Clinical outcome in children with chronic recurrent multifocal osteomyelitis. Rheumatology (Oxford) 2008;47:1397–9. doi: 10.1093/rheumatology/ken249. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Dedeoglu F, Ferguson PJ, Lapidus S, Laxer RLS. Physicians’ Perspectives on the Diagnosis and Treatment of Chronic Nonbacterial Osteomyelitis. Int J Rheumatol. 2017 doi: 10.1155/2017/7694942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roderick MR, Shah R, Rogers V, Finn A, Ramanan AV. Chronic recurrent multifocal osteomyelitis (CRMO) – advancing the diagnosis. Pediatr Rheumatol. 2016;14(1):47. doi: 10.1186/s12969-016-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voit AM, Arnoldi AP, Douis H, Bleisteiner F, Jansson MK, Reiser MF, et al. Whole-body magnetic resonance imaging in chronic recurrent multifocal osteomyelitis: Clinical longterm assessment may underestimate activity. J Rheumatol. 2015;42:1455–1462. doi: 10.3899/jrheum.141026. [DOI] [PubMed] [Google Scholar]
- 27.Hedrich CM, Hofmann SR, Pablik J, Morbach H, Girschick HJ. Autoinflammatory bone disorders with special focus on chronic recurrent multifocal osteomyelitis (CRMO) Pediatr Rheumatol. 2013;11:47. doi: 10.1186/1546-0096-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Job-Deslandre C, Krebs S, Kahan A. Chronic recurrent multifocal osteomyelitis: five-year outcomes in 14 pediatric cases. Jt Bone Spine. 2001;68:245–251. doi: 10.1016/s1297-319x(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 29.Walsh P, Manners PJ, Vercoe J, Burgner D, Murray KJ. Chronic recurrent multifocal osteomyelitis in children: nine years’ experience at a statewide tertiary paediatric rheumatology referral centre. Rheumatology (Oxford) 2015;54:1688–1691. doi: 10.1093/rheumatology/kev013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


