Abstract
Background
Previous headache trigger studies have primarily utilized checklists to measure beliefs about triggers. While this work has defined the diversity of beliefs across headache sufferers, the strength of association and the frequency at which these triggers are encountered remain unexplored.
Objective
To measure the strength of association, frequency of encounter, and influences on trigger beliefs and perceptions using a laboratory assessment task.
Methods
This cross-sectional observational study, part of the HACOGS project, included adult current migraine, tension-type, or cluster headache sufferers. Participants rated the chances they would experience a headache if they were to encounter a specific trigger and the number of days per month they experience that trigger for 33 common triggers.
Results
All 300 participants contributed data on all triggers, with little missing data (1.2%). All triggers exhibited a high degree of inter-individual variability on the strength of association and encounter perceptions. Many triggers were perceived to be encountered daily (e.g., caffeine, air conditioning), and a full range of perceptions were observed for each trigger. Stress (75% chance of headache), missing a meal (60%), and dehydration (60%) were the triggers with the greatest potency beliefs. Only 8–15% of these beliefs were related to individual differences and 26–27% to the triggers themselves.
Conclusions
Participants expressed diverse beliefs and perceptions about the strength of many common headache triggers. Variation in these beliefs was not associated with individual differences or the triggers themselves. This finding supports the importance of measuring more than just the presence-absence of trigger beliefs.
Keywords: Precipitant, potency, trigger, causality, HACOGS
Introduction
Headache is a common, disabling experience affecting a substantial portion of the population (1). Consequent is the nearly ubiquitous nature of headache trigger beliefs (2), ideas that the presence or absence of certain factors leads to headache attacks (3). Headache sufferers seek to understand precipitants of their attacks with the hope that they may be able to manage these episodes (4–6). Still, there is inadequate robust scientific evidence to facilitate these endeavors (7,8). Much has been done to assess the factors headache sufferers endorse as headache triggers, with cross-sectional checklist studies being the most commonly used study design (9). While this existing work has built the foundation for understanding triggers, it does not consider strength of association or frequency of exposure as possible influences on headache trigger beliefs. The available literature focuses on the presence or absence of triggers, and few advances in methods of assessment have occurred over many decades.
Though it is possible to gain valuable information from studies of the presence or absence of potential triggers, such methods do not account for important aspects of the trigger experience. Missing from these assessments is the perceived strength of association of triggers and headache activity. For example, what are the chances that a person will experience a headache when exposed to a potential trigger? These perceived chances may not be the same for all headache sufferers (10). The strength of association between a trigger and headache activity is a vital component in understanding the function of triggers. Similarly, the frequency with which a person encounters a trigger likely has some influence on beliefs about that trigger’s association with their headache activity (11). Further, influences of personal and psychological characteristics as well as life experiences and circumstances may affect trigger perceptions (12).
To advance understanding of headache triggers and headache sufferers’ perceptions and beliefs about triggers, it is necessary to extend the assessment of these phenomena by including additional aspects of the trigger experience. Improved methods of investigation will provide better quality information on trigger beliefs. This study aims to expand knowledge by eliciting strength of association, frequency of encounter, and influences on trigger perceptions in a laboratory setting.
Methods
This study was part of the Headache and Cognition Study (HACOGS) (13). This cross-sectional, observational study consisted of four separate sub-studies that used a computer program created specifically for this data collection effort. The HACOGS focused on cognitions related to headache sufferers’ experiences with headache activity and treatment. Participants were enrolled in this single-visit study from May 2012 to May 2014 at a single site. Approval was obtained from the Institutional Review Board of Wake Forest School of Medicine, and each participant gave written informed consent prior to beginning the study. Participants received $20 for their time. This is the second HACOGS publication.
Recruitment and eligibility criteria
Participants responded to television advertisements or were referred by their headache specialist. Adults aged 18 and older who were current migraine, tension-type, or cluster headache sufferers and who had ever experienced more than five headache attacks were eligible for participation. Participants were also required to experience headache severity that warranted the use of over-the-counter or prescription headache abortive medication. Those who were unable to read and write in English, the only language of the questionnaires, and those who could not properly complete the assessment or give informed consent (e.g. inebriation, florid psychosis) were not eligible to participate.
Trigger perceptions and beliefs task
E-Prime 2.0 software (14) was used to create and conduct the trigger perceptions and beliefs portion of the study. Prior to beginning, participants were provided with instructions and examples. During this task, 33 potential headache triggers were presented to participants in random order. These triggers were selected based on the most commonly reported triggers (3) and discussion by our team. We intended to include broadly applicable trigger candidates and to limit the total number to avoid participant burden. To limit the number of triggers that applied only to women, we included just one, hormonal birth control, out of several possible candidates (e.g. menstruation). Each screen displayed a picture and description of the trigger. Participants were asked to rate the chances they would experience a headache if they were to encounter that specific trigger by typing a response from 0% to 100%. Next, they were asked to provide the number of days per month they experience the trigger by typing a number from 0 to 30. This process was repeated for each of the 33 triggers. While using the program, participants also answered questions about their typical number of headaches per month, time since last headache, current headache pain, and preventive and abortive medication use. Because several aspects of the HACOGS assessments involved participants’ perceptions of private information, such as their satisfaction with treatment regimens, extensive demographic information was not collected to ensure anonymity and to increase candor.
Questionnaires
Additional questionnaires were administered using REDCap (15) data collection methods. Those who preferred a paper questionnaire format were provided with that alternative. To assess minor stressful events experienced in the previous week, the 25-item Weekly Stress Inventory Short Form (WSI-SF) was administered (16). The sum of perceived stress impact ratings (0 = did not occur to 7 = extremely stressful) from 25 potentially stressful events provided an impact score. The five-item Migraine Disability Scale (MIDAS) was used to measure participants’ migraine-related disability (17). To elicit depressive symptoms, the Center for Epidemiologic Studies Depression Scale (CES-D), a 20-item questionnaire, was employed (18). Finally, the 20-item State-Trait Anxiety Inventory Form Y-2 (STAI-T) questionnaire provided a measure of participants’ trait anxiety (19). Each of these questionnaires has demonstrated strong reliability and validity evidence, and all four are often used in research and clinical practice.
Internal control – the color green
Given that responding to the laboratory task could be associated with response bias (e.g. acquiescence response bias), the “color green” was included as a potential trigger candidate to assist in interpreting response patterns in the task. Because green colors are very common in nature, we anticipated that the color green should be perceived to be encountered frequently. However, as there is no evidence to support the notion that being exposed to green elicits a headache, we anticipated that green should be rated very low in terms of potency of influence. Deviations from either expectation would be interpreted as evidence of response bias in need of further scrutiny.
Statistical analyses
The original statistical power considerations for the HACOG study have been reported previously (13). The descriptive statistics are presented using methods appropriate to the distributions. Because all scaled data were skewed, the median (25th, 75th) is reported, and frequency counts (%) are used for categorical data. To examine the primary hypotheses, a pre-planned analysis was conducted according to a fixed protocol. Linear mixed models were applied using both fixed and random effects. To examine the variance components of the frequency of encounter perceptions and strength of association beliefs, both outcomes were modeled separately using random intercepts for trigger and participant ID. This approach allows the modeling of repeated measures across participants (i.e., each participant rated 33 triggers) and allows estimation of the total variance (i.e. % of total variance) in these perceptions that is due to individual-level or trigger-level sources. In a second set of models, the degree of individual-level variance was further examined using fixed effects for the individual scales (MIDAS, WSI-SF, CES-D, STAI-T). The relationship between these scales and beliefs about strength of association were plotted using a loess smoothing algorithm. A network plot was used to visualize the matrix of correlations between the trigger candidates using the qgraph package. To account for the inflated type-I error due to the very large number of correlations, the network correlations were adjusted using Holm’s method. All analyses were conducted using R 3.2 (Vienna, Austria). Where appropriate, all hypothesis tests are two-tailed with p <0.05 interpreted for statistical significance.
Results
Sample characteristics
Three hundred participants completed the questionnaires and fully participated in the laboratory task. Each participant rated all 33 triggers in terms of frequency of encounter (0–30) and potency of influence (i.e. chance of headache from 0–100%). Due to typographical errors where the participants entered implausible values into the system (e.g. frequency of encounter >30 days/month), 119 of 9,900 (1.2%) of the possible responses were excluded from analysis.
The participants were 83% female (248/300) and reported experiencing a range of headache frequencies with a median (25th, 75th) headache frequency of 7 (4, 13) days/month. All participants reported experiencing regular headaches (range: 1–30 days/month). The median MIDAS score was 19.5 (9, 44.25), the median CES-D was 13 (6, 23), the median STAI-T was 37 (30, 49), and the median WSI-SF was 37 (20, 60). The participants were taking a range of medications with 58/300 (19.3%) currently taking a prophylactic treatment for their headaches, 132/300 (44%) taking a triptan medication, and 159/300 (53%) taking an NSAID for their headaches.
Headache trigger beliefs
Participants reported a diverse set of perceptions about the frequency of exposure to the 33 headache triggers and beliefs about the chances of experiencing a headache attack. Table 1 displays the perceptions of trigger exposures and association strengths. The perceptions about trigger exposures with the greatest frequency were to air conditioning (30 [15,30] days/month), caffeine (30 [10, 30] days/month), and, as expected, the color green (30 [20,30] days/month). The least frequent perception about trigger exposure relevant to both sexes was to red wine (0 [0,1] days/month). The degree of inter-person variability in the perceived exposures was substantial, with most of the interquartile ranges for each trigger spanning 15 days or more.
Table 1.
Headache trigger perceptions.
| Name | Frequency of encounter (days/month) | Perception of strength (0–100% chance of headache) |
|---|---|---|
| Stress | 15 (10,25) | 75 (50,90) |
| Missing a meal | 5 (2,10) | 60 (30,90) |
| Dehydration | 2 (0,5) | 60 (30,90) |
| Using a computer for a long time | 20 (10,30) | 50 (20,75) |
| Bright lights | 10 (4,20) | 50 (20,75) |
| Not getting enough sleep | 12 (5,20) | 50 (30,80) |
| Neck pain | 6 (3,15) | 50 (20,90) |
| Strong perfume or cologne | 5 (2,10) | 50 (10,80] |
| Weather changes | 10 (5,19.5) | 40 (5,75) |
| Pollen | 15 (5,25) | 35 (1.5,75) |
| Bright sunlight | 15 (10,20.75) | 30 (10,60) |
| Cigarette smoke | 5 (1,15) | 30 (0,75) |
| Loud noise | 5 (2,15) | 30 (5,58.75) |
| Auto exhaust | 8 (2,20) | 25 (5,60) |
| Temperature change in your surroundings | 10 (4,20) | 22.5 (0,50) |
| Air pollution | 5 (1,20) | 20 (0,50) |
| Aerosol sprays | 5 (1,17) | 15 (0,50) |
| Gasoline odor | 4 (3,6) | 10 (0,40) |
| Heavy exercise | 4 (0,10) | 10 (0,50) |
| MSG | 2 (1,5) | 10 (0,50) |
| Caffeine | 30 (10,30) | 5 (0,26.25) |
| Red wine | 0 (0,1) | 4 (0,50) |
| Processed meats | 5 (2.5,15) | 2 (0,25) |
| Air conditioning | 30 (15,30) | 0 (0,10) |
| Artificial sweeteners | 2 (0,19) | 0 (0,25) |
| Cheese | 15 (5,20) | 0 (0,5) |
| Chocolate | 10 (4,20) | 0 (0,10) |
| Cinnamon | 2.5 (1,7.75) | 0 (0,0) |
| Fruit | 20 (15,30) | 0 (0,0) |
| Hormonal birth control | 0 (0,0) | 0 (0,25) |
| Nuts | 7 (3,15) | 0 (0,0.5) |
| Salty foods | 15 (5,20) | 0 (0,20) |
| The color green | 30 (20,30) | 0 (0,0) |
The triggers with the greatest perceived association strengths (i.e. chance of a headache given an exposure) were stress (75% [50,90]), missing a meal (60% [30,90]), and dehydration (60% [30,90]). The least perceived association strengths were for nuts (0% [0,0.5]), salty foods (0% [0,20]), and, as expected, the color green (0% [0,0]), which served as a methodology check to rule out acquiescence response bias. As with the perceptions about frequency of exposure, beliefs about association strength were extremely variable across participants, with most interquartile ranges spreading by more than 50% for each headache trigger.
There was a small inverse association between the mean encounter frequency and the mean association strength across the 33 triggers. For each additional encounter day, the perceived association strength decreased by −0.20 (95%CI: −0.27, −0.14), p <0.0001. Thus, for each 10 days of perceived exposure, the perceived association strength decreased by 2%.
Influences on trigger beliefs and perceptions
Influences on the perceptions of exposure and association strength were evaluated using variance components of mixed-effects models. For trigger exposure perceptions, 26% of the variance was attributable to differences across triggers (i.e. some triggers were perceived to be consistently more/less likely to be encountered than others) while 8% of the variance could be attributed to differences across individuals (i.e. some participants were consistently more/less likely to perceive triggers as encountered). The remaining variance (66%) was not accounted for by systematic differences attributable to individual participants or specific triggers. For trigger association strength beliefs, 27% of the variance was attributable to differences across triggers while 15% of the variance could be attributed to differences across individuals. The remaining variance (58%) was not accounted for by systematic differences attributable to individual participants or triggers.
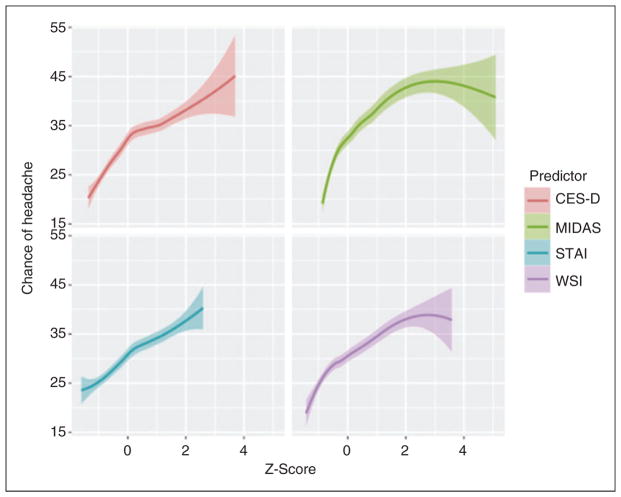
The association between participant-level predictors and perceptions about triggers were then evaluated using multivariable mixed-effect models. None of the person-level predictors were meaningfully related to the within-person differences, with about 0% of within-person variance accounted for by the MIDAS, CES-D, STAI-T, or WSI-SF. However, these person-level predictors could account for about half (7.3/15% [49.2%]) of the variance related to within-person differences in beliefs about trigger association strength. After adjusting for the other predictors in the model, scores on the CES-D: 0.20 (95%CI: 0.05, 0.35), p = 0.01; MIDAS: 0.11 (95% CI: 0.08, 0.15), p <0.001; and WSI-SF: 0.08 (0.02, 0.13), p = 0.006 were all uniquely associated with trigger perceptions. Figure 1 displays the association between the trigger beliefs and several of the psychosocial predictors.
Figure 1.
Associations between individual-level factors and headache trigger potency beliefs. The plots demonstrate that as depressive symptoms (CES-D), headache disability (MIDAS), anxiety symptoms (STAI-T) and stress (WSI-SF) increase (x axis, Z-score scale), so too do the beliefs about the average strength of headache triggers in causing headaches (y axis). Although several of these associations were statistically significant, these associations only accounted for a modest (7.3%) amount of total variation of potency beliefs.
Relationships among trigger beliefs and perceptions
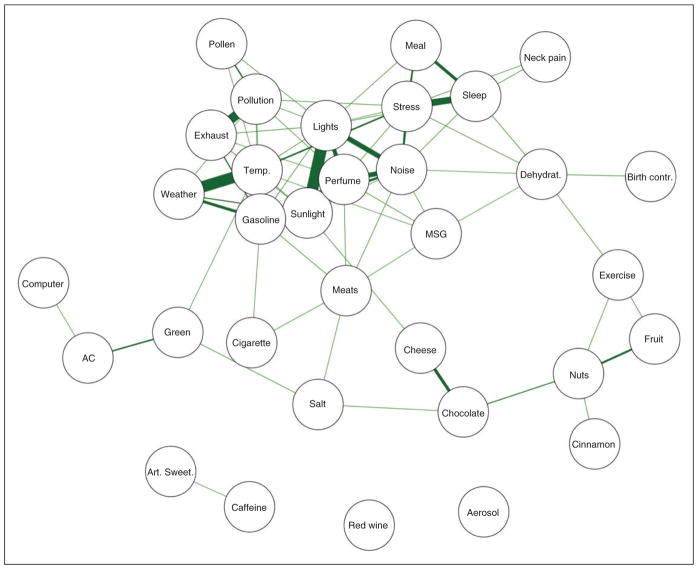
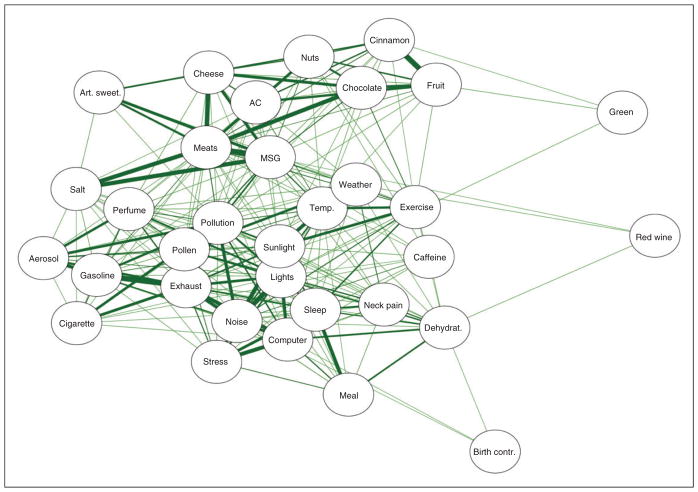
The relationships among the beliefs and perceptions were evaluated using correlations. In this way, the association between trigger beliefs could be evaluated graphically. Figures 2 and 3 display network plots that are created by displaying only the statistically-significant associations between triggers (the nodes) using edges (the lines) created by weighting the correlations between the nodes. Thicker lines correspond to greater associations, and nodes closer together are more similar to each other than nodes farther apart.
Figure 2.
A network plot illustrating the perceptions of trigger encounters. Each trigger is a node (circle) with the strength of the relationships between the triggers represented by the size of the line (edge). Only statistically-significant associations between triggers are drawn with a line (after adjustment for multiplicity). Triggers connected by lines are perceived to be similarly encountered on a daily basis.
Figure 3.
A network plot illustrating trigger potency beliefs. Each trigger is a node (circle) with the strength of the relationships between the triggers represented by the size of the line (edge). Only statistically-significant associations between triggers are drawn with a line (after adjustment for multiplicity). Triggers connected by lines are perceived to have similar potency in causing a headache attack.
Figure 2 displays the network of associations between the trigger encounter perceptions. One group of nodes near the top of the network reflects the triggers that are most often observed together (i.e. if a participant rates the frequency of encounter high on one of these nodes, they also tend to rate the nearby nodes high as well). This group appears to consist of environmental triggers and odors such as weather, temperature changes, pollen, exhaust, and gasoline. Nearby these nodes are other commonly reported triggers that are related to behavioral issues such as stress, missing a meal, sleep, and neck pain. There are many other triggers that are only loosely related to each other and are at the extremes of the network. For example, caffeine and artificial sweeteners formed their own network, given they are often encountered together, while red wine was not related to being exposed to any of the other triggers.
Figure 3 displays the network of associations between the trigger potency beliefs. This network is much more centralized than the previous one, with most of the trigger beliefs being modestly related to one another. Several nodes were particularly related to each other (i.e. thick edges), reflecting strong associations among them. For example, gasoline, auto exhaust, and loud noises were highly associated with each other, as were meats, salt, MSG, and cheese. The color green, red wine, and hormonal birth control were only weakly related to other triggers and were on the extremes of the network.
Discussion
In this study, we utilized a laboratory task to measure perceptions about headache triggers that exceed the simple presence or absence endorsement primarily used to study triggers in cross-sectional studies. Specifically, we measured both the perceived frequency of encounter and the perceived association strength of many popular triggers. The results of this assessment reveal several remarkable findings that should be considered for research and clinical purposes.
First, substantial heterogeneity was seen across perceptions about how often various headache triggers were encountered. Even when two patients had identical beliefs about how important a trigger candidate is for precipitating their headaches, they differed substantially on how often they perceive encountering this trigger. This variability is understandable for difficult to perceive triggers like “air pollution” (range: 0–30 days/month) or even for food choices such as cheese (range: 2–25 days/month). However, this level of variability was observed for nearly every trigger candidate, making the assessment of perceived encounter frequency a crucial aspect of understanding the role of these triggers in the lives of headache sufferers.
A second important finding is the confirmation that the beliefs about the association strength of headache triggers also vary substantially across individuals. In a recent meta-analysis (9), substantial heterogeneity in the beliefs about the presence or absence of triggers was observed across studies. The present findings augment this realization by supporting the idea that beliefs about association strength differ widely across individuals even for the same trigger. Certain triggers are believed to have more influence on headache activity than others (e.g. stress [75%] was believed to be twice as potent as pollen [35%]), but a full range of beliefs (0–100% association strength) were observed for nearly every trigger. Thus, far from being believed to be merely present or absent, widely variable beliefs about the association strength of triggers and headaches are endorsed by headache sufferers.
A third important finding is that approximately 60% of the variance in encounter perceptions and association strength beliefs is unexplained by any of the factors measured in this study. Measurable individual differences accounted for 8–15% of the variance in encounter and association strength perceptions. Of this modest variation, about half could be related to the individual’s self-reported affective distress on such constructs as depression or anxiety. An additional 26–27% of the variance was related to the triggers themselves. Certain triggers were more/less likely to be perceived as encountered or rated to have higher/lower association strengths. However, taken together, these variance components support the interpretation that it is extremely difficult to predict how a single individual will rate any of these trigger perceptions, or if any external predictor can account for these inter-individual differences.
The beliefs and perceptions were conceptualized as a visual network, so the correlations between these beliefs could be examined as evidence of core underlying belief systems. A belief in one trigger may commonly be associated with a belief in another trigger. Some evidence to support this notion was found for the encounter perceptions. Distinct clusters of triggers tended to be rated as more/less likely to be encountered (e.g. environmental stimuli), but many others, including food choices, were not related to other perceptions. In contrast, the network of beliefs about the potency of triggers for causing headaches was very centralized (i.e. many triggers grouped together), indicating that participants were prone to rate these beliefs similarly across triggers. Together, these findings support an initial interpretation that, at least to a modest degree (i.e. 15% of the variance), individuals can be thought of as either possessing or not possessing a group of trigger potency beliefs about a variety of triggers. The examination of trigger candidates using these methods can help us understand how individuals passively create a system that can be used heuristically to understand the personal causes of headache.
The idea of an underlying formal structure of causal beliefs about some aspect of the world has been labeled as “causal grammar” (20). This type of intuitive theory about triggers, if it exists, could greatly explain how sufferers learn, organize, and evaluate information about headache triggers (21,22). When viewed this way, the network of beliefs plotted in Figures 2 and 3 reveals a tendency (i.e. a group average) for individuals to group these triggers into a system of belief. When employed, such a system would confer several benefits for a person. First, it would allow an individual to distill large amounts of information into small amounts of memory. For example, inferring “strong odors” from the individual triggers of gasoline, bleach, auto exhaust, etc. simplifies the world for an individual and allows a better understanding of the underlying causal pathways associated with each trigger. Second, the existence of such causal grammar allows the assimilation of new information into the belief system. For example, an individual remembers smelling bananas before experiencing a headache. Although this individual does not associate bananas with “strong odors”, they can accommodate this new potential trigger into an existing system based on “odors” with only small amounts of cognitive effort. A final benefit of a system based on causal grammar is that it is easily understood by the individual and can be communicated to others (e.g. “If I avoid odors, I won’t get headaches”).
The present study has several limitations. Future research on this topic will necessarily require enhanced assessment methods of triggers (23) and underlying cognitive structures, such as causal grammar. This study illustrates the benefits of measuring more than just the presence or absence of trigger beliefs and an individualized approach to conceptualizing triggers (24). By measuring the strength of association and the belief about the frequency of encounter, we can learn a great deal about how headache sufferers organize these ideas. However, the study presented individuals with a list of triggers rather than using an open-ended assessment that could prevent biasing the individuals’ perceptions based on the information directly in front of them.
Further, ‘dose’ and timing of each individual trigger was not specified, requiring participants to rate association strength given their perception of what exposure might entail (e.g. many ‘doses’ of stress could be experienced, but this was not specified). Many trigger candidates could actually be indicative of premonitory symptoms rather than triggering factors, but this also was not studied. For example, the experience of stress often predates onset of headache (25), but sensitivity to light or odors could be a sign of the premonitory phase of an attack (26). Another limitation is that the current study was based on a convenience sample of a variety of individuals recruited from the community. The extent to which the summarized data pertains to clinic-based headache sufferers or to headache sufferers with only one type of headache (i.e. migraine) is unknown. Finally, the items utilized to measure these headache beliefs were developed de novo for this study. Although methodology checks were used to examine response bias of certain types, it is unclear how stable (i.e. reliable) the beliefs reported in this study might be over time. Additional research is needed to further explore the findings from this study.
Clinical implications.
Headache sufferers express diverse beliefs and perceptions about the strength of many common headache triggers.
Predicting how an individual will perceive any trigger factor is difficult. Neither the individual-level factors nor the triggers themselves accounted for variations in trigger beliefs.
Measuring more than just the presence or absence of trigger beliefs is an important step for assisting headache sufferers in managing their headache triggers.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH/NINDS RO1NS06525701.
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: Updated statistics from government health surveillance studies. Headache. 2015;55:21–34. doi: 10.1111/head.12482. [DOI] [PubMed] [Google Scholar]
- 2.Peroutka SJ. What turns on a migraine? A systematic review of migraine precipitating factors. Curr Pain Headache Rep. 2014;18:454. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- 3.Martin VT, Behbehani MM. Toward a rational understanding of migraine trigger factors. Med Clin North Am. 2001;85:911–941. doi: 10.1016/s0025-7125(05)70351-5. [DOI] [PubMed] [Google Scholar]
- 4.Martin PR. Behavioral management of migraine headache triggers: Learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221–227. doi: 10.1007/s11916-010-0112-z. [DOI] [PubMed] [Google Scholar]
- 5.Martin PR. Managing headache triggers: Think “coping” not “avoidance. Cephalalgia. 2010;30:634–637. doi: 10.1111/j.1468-2982.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- 6.Martin PR, Reece J, Callan M, et al. Behvavioral management of the triggers of recurrent headache: A randomized controlled trial. Behav Res Ther. 2014;61:1–11. doi: 10.1016/j.brat.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Turner DP, Smitherman TA, Martin VT, et al. Causality and headache triggers. Headache. 2013;53:628–635. doi: 10.1111/head.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houle TT, Turner DP. Natural experimentation is a challenging method for identifying headache triggers. Headache. 2013;53:636–643. doi: 10.1111/head.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walters Pellegrino AB, Davis-Martin RE, Houle TT, et al. Perceived triggers of primary headache disorders: A meta-analysis. Cephalalgia. doi: 10.1177/0333102417727535. Epub ahead of print 20 August 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothrock JF. The truth about triggers. Headache. 2008;3:499–500. doi: 10.1111/j.1526-4610.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 11.Kubik SU, Martin PR. The Headache Triggers Sensitivity and Avoidance Questionnaire: Establishing the psychometric properties of the questionnaire. Headache. 2017;57:236–254. doi: 10.1111/head.12940. [DOI] [PubMed] [Google Scholar]
- 12.Smitherman TA, Davis RE, Walters AB, et al. Anxiety sensitivity and headache: Diagnostic differences, impact, and relations with perceived headache triggers. Cephalalgia. 2015;35:710–721. doi: 10.1177/0333102414557840. [DOI] [PubMed] [Google Scholar]
- 13.Turner DP, Golding AN, Houle TT. Using a graphical risk tool to examine willingness to take migraine prophylactic medications. Pain. 2016;157:2226–2234. doi: 10.1097/j.pain.0000000000000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider W, Eschman A, Zuccolotto A. E-prime user’s guide. Pittsburgh: Psychology Software Tools Inc; 2002. [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brantley PJ, Bodenlos JS, Cowles M, et al. Development and validation of the Weekly Stress Inventory – Short Form. J Psychopathol Behav Assess. 2007;29:54–59. [Google Scholar]
- 17.Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56:S20–S28. doi: 10.1212/wnl.56.suppl_1.s20. [DOI] [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D scale, a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 19.Spielberger CD. Manual for the state-trait anxiety inventory (form Y) (“self-evaluation questionnaire”) Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 20.Tenenbaum JB, Griffiths TL, Niyogi S. Intuitive theories as grammars for causal inference. In: Gopnik A, Schulz L, editors. Causal learning: Psychology, philosophy, and computation. Oxford University Press; 2007. [Google Scholar]
- 21.Cheng PW, Novick LR. Covariation in natural causal induction. Psychol Rev. 1992;99:365–382. doi: 10.1037/0033-295x.99.2.365. [DOI] [PubMed] [Google Scholar]
- 22.McKenzie CR, Mikkelsen LA. A Bayesian view of covariation assessment. Cogn Psychol. 2007;54:33–61. doi: 10.1016/j.cogpsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Zebenholzer K, Frantal S, Pablik E, et al. Reliability of assessing lifestyle and trigger factors in patients with migraine – findings from the PAMINA study. Eur J Neurol. 2016;23:120–126. doi: 10.1111/ene.12817. [DOI] [PubMed] [Google Scholar]
- 24.Peris F, Donoghue S, Torres F, et al. Towards improved migraine management: Determining potential trigger factors in individual patients. Cephalalgia. 2017;37:452–463. doi: 10.1177/0333102416649761. [DOI] [PubMed] [Google Scholar]
- 25.Houle TT, Turner DP, Golding AN, et al. Forecasting individual headache attacks using perceived stress: Development of a multivariable prediction model for persons with episodic migraine. Headache. 2017;57:1041–1050. doi: 10.1111/head.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulte LH, Jürgens TP, May A. Photo-, osmo- and phonophobia in the premonitory phase of migraine: Mistaking symptoms for triggers? J Headache Pain. 2015;16:14. doi: 10.1186/s10194-015-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]