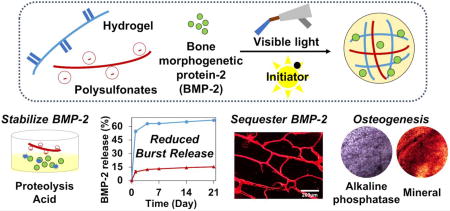
Abstract
Although bone morphogenetic protein-2 (BMP-2) is known to be the most potent stimulator available for bone formation, a major barrier to widespread clinical use is its inherent instability and absence of an adequate delivery system. Heparin is being widely used in controlled release systems due to its strong binding ability and protective effect for many growth factor proteins. In this work, we developed a hydrogel surface that can mimic heparin to stabilize BMP-2 and to enhance osteogenesis by introducing heparin-mimicking sulfonated molecules such as poly-vinylsulfonic acid (PVSA) or poly-4-styrenesulfonic acid (PSS), into photo-crosslinkable hydrogel. Bioactivity of BMP-2 was well preserved in the presence of polysulfonates during exposure to various therapeutically relevant stressors. The heparin-mimicking sulfonated hydrogels were effective to bind BMP-2 compared to unmodified MeGC hydrogel and significantly enhanced osteogenic differentiation of encapsulated bone marrow stromal cells (BMSCs) without the addition of exogenous BMP-2. The sulfonated hydrogels were effective in delivering exogenous BMP-2 with reduced initial burst and increased BMSCs osteogenesis induced by BMP-2. These findings suggest a novel hydrogel platform for sequestering and stabilizing BMP-2 to enhance osteoinductive activity in bone tissue engineering.
Keywords: Heparin, Hydrogel, Bone Morphogenetic Protein-2, Mesenchymal stem cells, Osteogenesis
Graphical abstract
1. Introduction
Bone formation requires a proper commitment of mesenchymal stem cells (MSCs) to an osteoprogenitor lineage mediated by various osteoinductive factors, particularly bone morphogenetic protein-2 (BMP) signaling[1, 2]. Recombinant human BMP-2 (rhBMP-2) demonstrated extraordinary potential in bone formation and has been widely employed for bone repair[3, 4]. However, clinical applications of rhBMP-2 require supraphysiological milligram-level dosages due to its intrinsic instability and fast enzymatic degradation in vivo[5–11]. The premature release of such high dose rhBMP-2 from conventional collagen carriers may lead to unwanted ectopic bone formation and numerous unpredictable side effects such as well-documented soft tissue swelling, osteoclastic bone resorption, and inappropriate adipogenesis [12–18].
The extracellular matrix (ECM) is a natural reservoir of many growth factors and potentiates their bioactivities[19–21]. In particular, sulfated polysaccharides such as heparin possess structural domains exhibiting affinity to various growth factors including BMPs and have been shown to form stable complexes with rhBMP-2[22–24]. Therefore, heparin is often used to immobilize rhBMP-2 onto biomaterials for a controlled protein delivery with prolonged bioactivity[25–27]. However, heparin itself suffers from natural variability in structure, difficulty in modification, non-targeting bioactivities, anticoagulant activity, and unknown physiological roles[28–33]. Thus, the direct use of native heparin in tissue engineering is not desirable. Similar biological activities to heparin have been observed in small compounds that contain groups similar to those in the heparin, in particular, sulfates or sulfonates[34, 35].
We have previously developed an injectable hydrogel system composed of visible light crosslinkable chitosan (methacrylated glycol chitosan, MeGC) and riboflavin as a photoinitiator [36–38]. The MeGC hydrogel well supported proliferation of encapsulated MSCs and their commitment to an osteo- and chondroprogenitor lineage[38–40]. However, the hydrogel system was not efficient to deliver growth factor proteins and the majority of the initially loaded protein was released in one day from the hydrogel [41].
Here, we report a hydrogel surface that can mimic a natural protector of BMPs, heparin, to stabilize rhBMP-2 and enhance osteogenesis by incorporating heparin-mimicking polysulfonates, poly-vinylsulfonic acid (PVSA) or poly-4-styrenesulfonic acid (PSS), into MeGC hydrogel. The protective effect of PVSA or PSS on BMP stability was evaluated in various therapeutically relevant environments in comparison to that of natural heparin. The ability of the sulfonated hydrogels to bind or deliver rhBMP-2 was evaluated by incubating the hydrogels in a rhBMP-2 solution or loading rhBMP-2 into the hydrogels during photocrosslinking. We also determined the ability of the developed hydrogels to enhance BMP signaling and osteoinductive activity by encapsulating bone marrow stromal cells (BMSCs) into the hydrogels with or without the addition of exogenous rhBMP-2.
2. Materials and methods
2.1. Materials
Glycol chitosan (GC, ~ 100 kDa) was supplied from Wako Chemical USA, Inc. (Richmond, VA). Glycidyl methacrylate, poly-vinylsulfonic acid (PVSA, ~ 2 kDa), poly-4-styrenesulfonic acid (PSS, ~ 75 kDa), heparin sodium salt from porcine intestinal mucosa (heparin, ~ 18 kDa), Tween-20, p-nitrophenol phosphate, β-glycerophosphate, dexamethasone, nitro blue tetrazolium (NBT) and alizarin red S were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human bone morphogenetic protein-2 (rhBMP-2) was purchased from R&D Systems (Minneapolis, MN). Mouse bone marrow stromal cells (BMSCs, D1 ORL UVA, CRL-12424) and mouse myoblast (C2C12, CRL-1772) cells were obtained from American Type Culture Collection (ATCC, Manassas, VA).
2.2. Polysulfonates cytotoxicity
Each polysulfonate was dissolved in phosphate buffered saline (PBS) at final concentrations of 65, 2.6, and 29 µM for PVSA, PSS, and Hep, respectively (This will be referred to as 1× polysulfonate solution), to present the same number of sulfonate groups per polysulfonate molecule. BMSCs were cultured in culture medium (CM) with high glucose Dulbecco’s Modified Eagle’s Medium (DMEM, Life Technologies, Grand Island, NY), 10% (v/v) Fetal Bovine Serum (FBS, Mediatech Inc, Manassas, VA) and 1% (v/v) antibiotic-antimycotic (AA, Life Technologies) at 37 °C with a 5% CO2 humidified atmosphere. BMSCs at passages 3–6 were used and stemness of early passage cells were confirmed by monitoring cell morphology and/or expression of MSC surface markers as previously described[42]. Then, BMSCs were seeded in a 96-well culture plate at 2 × 104 cells per well. After 24 h, the medium was replaced with the mixture of CM (180 µL) and 1× polysulfonate solution (20 µL). The polysulfonates were tested in various concentrations ranging from 0.1× (6.5, 0.26, and 2.9 µM for PVSA, PSS, and Hep, respectively) to 10× (655, 26, and 290 µM for PVSA, PSS, and Hep, respectively) The cell viability was measured by following equation (1) after alamarBlue (Thermo Fisher Scientific, CA) assay as previously described[43]. In brief, BMSCs were cultured in CM containing polysulfonate 1× solution for 24 h and the medium was replaced with 10% (v/v) alamarBlue reagent in CM. Following 3 h incubation, alamarBlue fluorescence was measured.
| (1) |
where Fe, Fc, and Fb refers fluorescence value read at 585 nm and excited at 570 nm of experimental, control and blank groups, respectively.
2.3. BMP-2 stabilizing effect of polysulfonates
rhBMP-2 (1 µg) and 100 µL of polysulfonate 1× solution was dissolved in 1 mL of PBS. The rhBMP-2 and polysulfonate mixture was incubated in PBS at 37 °C for 7 days, 0.5% trypsin at 37 °C for 24 h, or pH 4.5 buffer at 37 °C for 24 h. Then, 20 µL of the treated mixture was combined with at 180 µL of osteogenic media (OM) including DMEM, 10% (v/v) FBS, 1% (v/v) AA, 10 mM β-glycerophosphate, 50 mg/mL L-ascorbic acid, and 100 nM dexamethasone. The bioactivity of rhBMP-2 in the treated mixture was determined by assessing its ability to enhance expression of alkaline phosphatase (ALP) in BMSCs. After incubation for 4 days, cells were fixed with 10% neutral buffered formalin (NBF) and incubated in a solution consisting of NBT and 5-bromo-4-chloro-3-indoxylphosphate solutions in AP buffer (100 mM Tris pH 8.5, 50 mM MgCl2, and 100 mM NaCl) for 3 h. In addition, ALP activity was measured by p-nitrophenol phosphate test with the same method from a previous study[38].
2.4. Preparation of heparin-mimicking sulfonated hydrogels
Photo-crosslinkable methacrylated glycol chitosan (MeGC) was prepared by following the previously developed methods[36, 44]. Briefly, GC was dissolved in distilled water (DW) (2% w/v) and functionalized with GMA (1:1.1 molar ratio) at pH 9.0 for 40 h. After the purification and lyophilization step, MeGC was rehydrated at 2% (w/v) in PBS solution. The sulfonated hydrogels were formed by 40 s irradiation of a mixture of MeGC, 1× polysulfonates solution, and 6 µM riboflavin initiator (100:10:0.5 volume ratio) with visible blue light (VBL, 400–500 nm, 300 mW/cm2, Bisco Inc., Schaumburg, IL).
2.5. Characterization of the sulfonated hydrogels
Sulfonated hydrogels were incubated in PBS for 21 days and collected at predetermined time points (0 h, 24 h, 7 days, 14 days, and 21 days). The incubating solution was used for 1,9-dimethyl-methylene blue (DMB, Sigma-Aldrich) assay to quantify polysulfonate released in PBS[39–41]. The collected hydrogels were stained with 1% (w/v) toluidine blue (Sigma-Aldrich, MO) solution in PBS for 5 min and washed with PBS for 1 h. Mechanical strength was assessed by measuring compressive modulus with an indentation experiment setting using Instron Electro-Mechanical Testing Machines (Instron, Model 5564, Norwood, MA). The compressive modulus was calculated using a Poisson’s ratio of 0.25 as described from the previous protocol[36, 44]. The water contents of hydrogels were calculated by the following equation (2).
| (2) |
where Ww and Wd, refers wet weight of a hydrogel and dry weight of the same hydrogel after lyophilization, respectively. The morphology of hydrogels was observed using scanning electron microscopy (SEM, FEI Nova NanoSEM 230, Hillsboro, OR). All hydrogels were frozen in liquid nitrogen for 5 min, lyophilized overnight, and gold-coated with a sputter coater at 20 mA under 70 mTorr for 1 min.
2.6. Cell proliferation in the sulfonated hydrogels
BMSCs were encapsulated in the sulfonated hydrogels at 2 × 106 cells/mL concentration and cultured in CM for 14 days. The live/dead staining image was obtained on an Olympus IX71 fluorescence microscope (Olympus, Tokyo, Japan) after calcein/ethidium homodimer staining for 15 min. The viability of cells was quantified by ImageJ software (NIH, Bethesda, Maryland) analysis measuring the ratio of live cells (green) to total cells (green + red). BMSCs proliferation was measured using alamarBlue assay following the manufacturer’s protocol.
2.7. BMP-2 release from sulfonated hydrogels
rhBMP-2 was encapsulated as 10 µg/mL in the mixture of MeGC, 1× polysulfonates, and 6 µM riboflavin initiator (100:10:0.5 volume ratio) and crosslinked under 40 s VBL irradiation. rhBMP-2 encapsulated hydrogels were incubated in 1 mL PBS for 21 days at 37 °C. The incubating solution was replaced with fresh PBS twice a week. At predetermined time points, the released rhBMP-2 in supernatant was quantified using BMP-2 ELISA kit (R&D Systems, MN) as per manufacturer’s protocol. The released rhBMP-2 was collected at day 7, 14, and 21 and its bioactivity was assessed by monitoring ALP expression in C2C12 cells.
2.8. Zeta potential of sulfonated hydrogels
The electrostatic potential on the hydrogel surface was evaluated by measuring the zeta potential measurement of the hydrogel. Briefly, samples were prepared according to the previous protocol[45]. Briefly, freeze-dried powders of the sulfonated hydrogels were suspended in DW to generate 5% (w/w) stock solution and centrifuged at 12,000 g for 2 min. The supernatant was used for the zeta potential measurement at 25 °C using a Malvern Zetasizer (Worcestershire, UK).
2.9. BMP-2 binding of sulfonated hydrogels
The sulfonated hydrogels were incubated in PBS with 1 µg/mL rhBMP-2 for 7 days. Then, the hydrogels were placed in optimal cutting temperature (OCT, Thermo Fisher Scientific, CA) medium and frozen at −80 °C. The frozen samples were cryosectioned at a thickness of 20 µm and mounted on glass slides. The sections were incubated with primary antibody against rhBMP-2 (R&D Systems, MN) and Alexa Flour 633-tagged secondary antibody (Invitrogen, CA). Images were obtained using an Olympus IX71 microscope (Olympus, Tokyo, Japan). The amounts of rhBMP-2 in the hydrogel and incubating supernatant were quantified by ELISA. The absorbed rhBMP-2 was collected by digesting the hydrogel in 10 mg/mL lysozyme solution at 37 °C for 16 h.
2.10. Osteogenic differentiation of BMSCs in the sulfonated hydrogels
BMSCs were encapsulated in sulfonated hydrogels loaded with PBS or rhBMP-2 (1 µg/mL) and cultured in OM for 21 days. Osteogenic ability of BMSCs in sulfonated hydrogels was determined using real-time polymerase chain reaction (qRT-PCR) of different osteogenic gene markers along with ALP and alizarin red S staining. Samples were collected at day 4 for measuring Runx2 expression and day 21 for Osteocalcin (OCN) expression. Total RNAs inside hydrogels were extracted by TRIzol (Invitrogen, CA) and RNeasy Mini kit (Qiagen, CA). The RNAs were reversely transcribed to cDNAs using cDNA transcription kit (Invitrogen) and the products were carried out in a LightCycler 480 PCR system (Indianapolis, IN) with 20 µL SYBR Green. The cDNAs were amplified for 45 cycles and the GAPDH expression was used for normalization. The primer sequence of GAPDH, Runx2, and OCN were listed in Table S1. All experiments were run in multiplicate (n=6). Hydrogels were collected at day 4 for ALP staining and at day 21 for alizarin red S staining. The collected hydrogel samples were fixed in NBF for 16 h. For alizarin red S staining, the fixed samples were incubated in 2% alizarin red S staining solution for 5 min and washed in PBS for 16 h. The stained samples were imaged with the Olympus SZX16 Stereomicroscope (Olympus, Tokyo, Japan).
2.11. BMP-2 stabilizing effect of sulfonated hydrogels
For BMP-2 stabilizing test in a hydrogel system, rhBMP-2 was encapsulated into sulfonated hydrogels during hydrogel crosslinking at 10 µg/mL. The hydrogel loaded with rhBMP-2 was incubated in PBS at 37 °C for 3 days, 0.5% trypsin at 37 °C for 5 h, or at pH 4.5, 37 °C for 5 h. Then, the treated hydrogel was digested with lysozyme (10 mg/mL in PBS) at 37 °C for 16 h. After enzymatic degradation, the hydrogel digest was centrifuged for 2 min at 10,000 g and the supernatant was collected. rhBMP-2 concentration in the supernatant was quantified using BMP-2 ELISA and the final concentration of 100 ng/mL was used for ALP activity test using BMSCs.
2.12. Statistical analysis
All experiments were performed in triplicate unless otherwise stated. The values represent the average and the error bars are the standard deviation. Statistical analysis was performed using one-way analysis of variance (ANOVA) with Tukey’s post hoc test. A value of p < 0.05 was considered as significant.
3. Results
3.1. Polysulfonates cytotoxicity
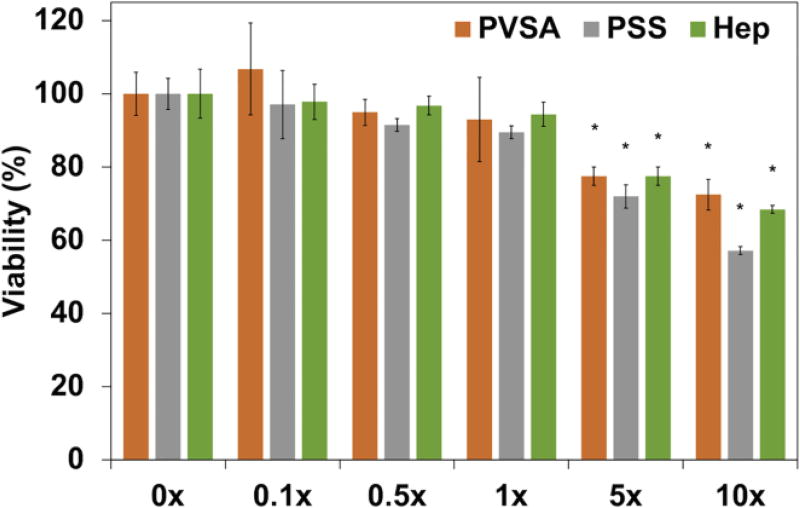
Since polysulfonates may be potentially toxic to cells, the cytotoxicity of polysulfonates was evaluated in BMSCs culture at various polysulfonate concentrations of 0, 0.1, 0.5, 1, 5, and 10× (Fig. 1). The addition of polysulfonates supported high cell viability over 90% up to 1× (65, 2.6, and 29 µM for PVSA, PSS, and Hep, respectively) polysulfonate. Increasing the polysulfonate concentration from 1× to 5× (325, 18, and 145 µM for PVSA, PSS, and Hep, respectively) led to a decrease in BMSCs viability to approximately 80%. Significantly decreased cell viability (< 70%) was observed with 10× (650, 26, and 290 µM for PVSA, PSS, and Hep, respectively) polysulfonate and PSS led to the lowest cell viability among the experiment groups with values lower than 60%.
Figure 1.
Viability of BMSCs after 24 h culture at various polysulfonate concentrations (0, 0.1, 0.5, 1, 5, and 10×) measured by alamarBlue assay (1× polysulfonate: 65, 2.6, and 29 µM for PVSA, PSS, and Hep, respectively) (*: p < 0.05 compared with 0×).
3.2. BMP-2 stabilizing effect of polysulfonates
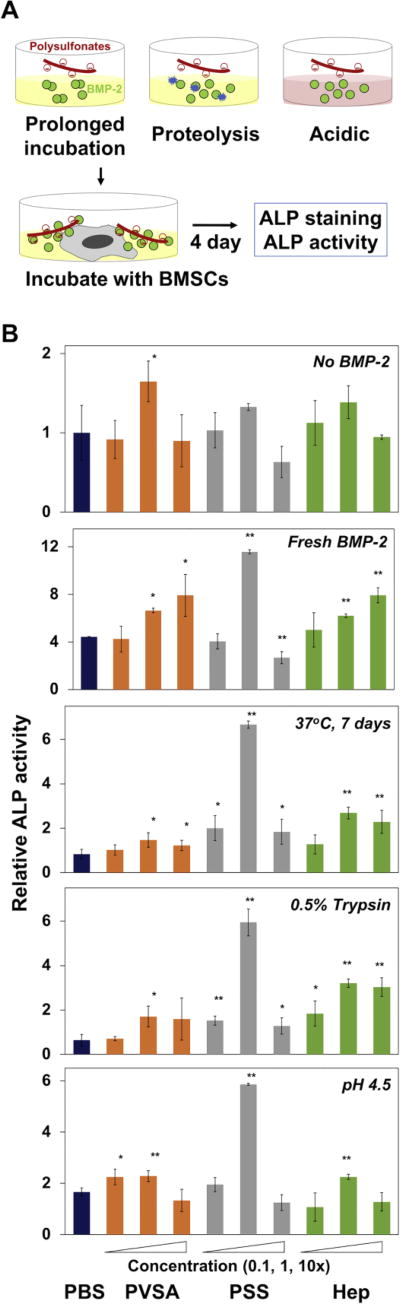
In order to evaluate the ability of heparin mimicking polysulfonate molecules to protect the bioactivity of rhBMP-2, rhBMP-2 was incubated with various concentrations of PVSA, PSS or natural heparin in various therapeutically relevant environments (Fig. 2A). rhBMP-2 was incubated with 0.5% trypsin or pH 4.5 buffer for 16 h to mimic the proteolytic or mild acidic conditions present in bone fracture healing. The bioactivity of treated rhBMP-2 was determined by monitoring its ability to increase ALP expression in BMSCs at day 4 post stimulation. Freshly thawed rhBMP-2 served as a positive control. rhBMP-2 in the treatment with trypsin or pH 4.5 buffer as well as in the incubation for 7 days in PBS at 37 °C did not significantly increase ALP activity of BMSCs, indicating that rhBMP-2 lost its bioactivity during the stressor treatments. In contrast, rhBMP-2 exposed to the stressors in the presence of polysulfonates, especially with PSS, significantly enhanced ALP expression as shown by ALP staining (Fig. S1). In particular, 1× PSS exhibited higher protective effects compared to heparin as quantified by ALP colorimetric assay (Fig. 2B). Further increase in PSS concentration to 10×, however, greatly reduced ALP activity in comparison to heparin. This is mostly due to high cytotoxicity of 10× PSS as shown in cell viability study (Fig. 1).
Figure 2.
BMP-2 stabilizing effect of polysulfonates. (A) The scheme of BMP-2 stabilizing test. BMP-2 was incubated in various therapeutically relevant environments (37 °C for 7 days, 0.5% trypsin for 16 h, and pH 4.5 for 16 h) in the presence of PVSA, PSS or heparin (Hep). BMSCs were cultured with the treated BMP-2 for 4 days and ALP production was measured by ALP staining and ALP activity test. (B) ALP activity of BMSCs measured by a colorimetric assay after 4 days of culture with BMP-2 samples treated in the presence of various concentrations of polysulfonates (0.1, 1, and 10×) (*: p < 0.05, **: p < 0.01).
3.3. Preparation and characterization of heparin-mimicking sulfonated hydrogels
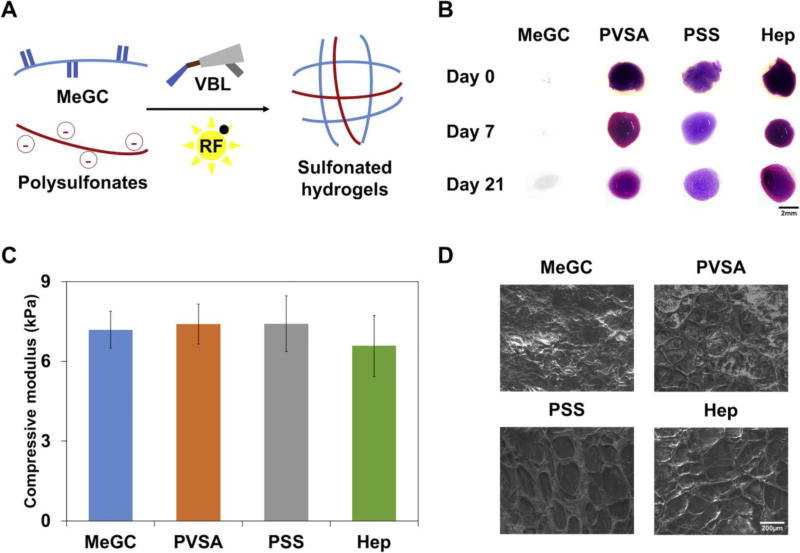
In order to prepare heparin-mimicking sulfonated hydrogels, MeGC solution was mixed with 1× polysulfonates and polymerized with a riboflavin (RF) initiator under visible blue light (VBL) irradiation (Fig. 3A). The homogenous distribution and stable incorporation of polysulfonates in hydrogels was verified using toluidine blue staining and DMB assay during a 21-day incubation. Blue color indicated incorporation of negatively charged polysulfonates in hydrogels and no significant color change or indication of polysulfonates release for three weeks was observed (Fig. 3B). High polysulfonate loading efficiency (>98%) was observed. DMB assay was performed to quantify the amount of polysulfonates released from hydrogels, and negligible quantity was detected for 21 days (Fig. S2). The mechanical strength of the sulfonated hydrogels was characterized by indentation measurements. The addition of polysulfonates or heparin did not significantly change the modulus of the hydrogel (Fig. 3C). No significant difference was observed in the equilibrium water content (>97%) among all four experimental groups (Fig. S3). The morphology of the hydrogels was characterized by SEM (Fig. 3D). Interpenetrating network was observed in sulfonated hydrogels, indicating the retention of polysulfonates or heparin added.
Figure 3.
Characterization of the sulfonated hydrogels. (A) The scheme of sulfonated hydrogels preparation. The mixture of MeGC, 1× polysulfonates, and riboflavin (RF) was crosslinked under 40 s VBL irradiation. (B) Toluidine blue staining of MeGC and sulfonated hydrogels after incubation up to 21 days. (C) Compressive modulus of hydrogels. (D) Morphological characterization of sulfonated hydrogels using SEM.
3.4. Cell viability and proliferative potential in sulfonated hydrogels
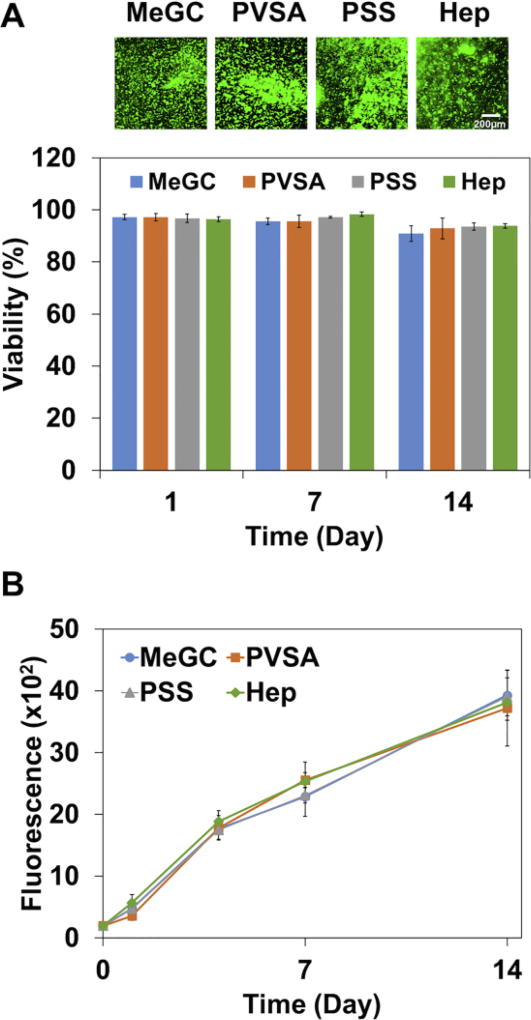
BMSCs were encapsulated in sulfonated hydrogels and viability of BMSCs was characterized during a 14-day culture. Live/dead fluorescence staining demonstrated that BMSCs in sulfonated hydrogels maintained cell viability above 90% over the 14 days culture period, indicating biocompatible nature of the hydrogel system (Fig. 4A and Fig. S4). The alamarBlue assay was employed to evaluate the proliferative potential of encapsulated cells in sulfonated hydrogels. The alamarBlue fluorescence intensity increased over time up to day 14, reflecting proliferative capacity of BMSCs, and there was no significant difference in fluorescence intensity among the hydrogels (Fig. 4B).
Figure 4.
Viability and proliferative potential of BMSCs encapsulated in MeGC and sulfonated hydrogels for 14 days. (A) Live/dead staining images were obtained and cell viability was quantified by ImageJ analysis. (B) Proliferative potential of BMSCs was evaluated by alamarBlue assay.
3.5. BMP-2 release from sulfonated hydrogels
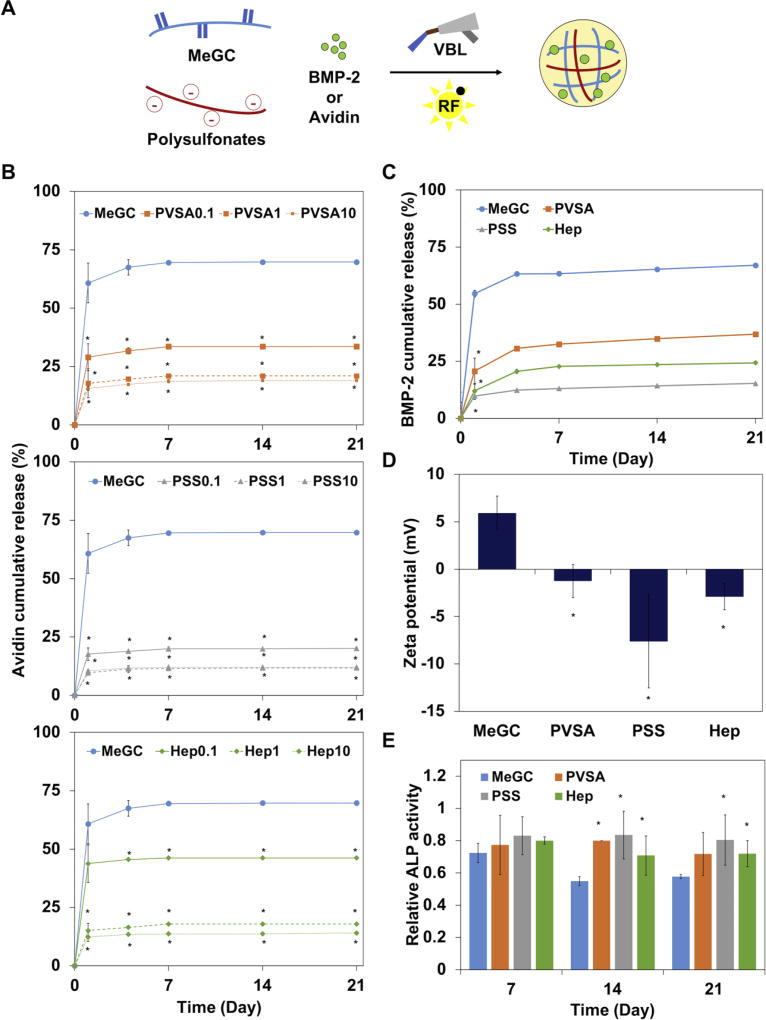
In order to investigate the ability of sulfonated hydrogels to deliver growth factors, we determined the effective concentration range of polysulfonates to reduce initial burst release using avidin, a basic glycoprotein that binds heparin[46], as a model protein. Fluorescently labeled avidin was mixed with MeGC solutions and various concentrations of polysulfonates (0.1, 1 and 10×) and polymerized under VBL irradiation with RF (Fig. 5A). The release profile of avidin encapsulated in MeGC hydrogels demonstrated a rapid burst release of 60% within one day (Fig. 5B). The addition of PVSA or PSS into hydrogels reduced the release rate of avidin in a dose-dependent manner. Similar trend of reduced burst release was observed with the addition of heparin. Since there was no significant difference in release profile between 1× and 10× polyfulsulfontes, rhBMP-2 was encapsulated in MeGC hydrogels modified with 1× polysulfonates and rhBMP-2 release was observed by BMP-2 ELISA (Fig. 5C and Fig. S5). The high loading efficiency of rhBMP-2 was observed in all hydrogels (MeGC 95%, PVSA 97%, PSS 98%, and Hep 97%). The addition of polysulfonates or heparin into MeGC hydrogels significantly reduced the burst release of loaded rhBMP-2, consistent with the model protein observations. In particular, PSS was the most effective one to lower rhBMP-2 burst release. The zeta potential of MeGC hydrogels was 6 ± 1 mV, while the modification of the hydrogels with PVSA, PSS, and heparin greatly reduced to the negative zeta potentials of −2, −8, and −3 mV respectively (Fig. 5D). This result indicates that negatively charged sulfonate groups incorporated in MeGC led to hydrogel surfaces providing stronger interactions with rhBMP-2. The bioactivity of released rhBMP-2 was evaluated by examining ALP expression in C2C12 cells (Fig. 5E). The rhBMP-2 release media collected from sulfonated hydrogels at day 14 and 21 significantly increased ALP activity compared to that of MeGC.
Figure 5.
(A) The scheme of protein (BMP-2 or avidin) encapsulation during photocrosslinking of hydrogels. (B) The release profile of a model protein, avidin, from MeGC hydrogels containing various concentrations (0.1, 1, and 10×) of PVSA, PSS or heparin (Hep) in PBS at 37 °C. (C) BMP-2 release profile from MeGC hydrogels containing 1x polysulfonates in PBS at 37 °C for 21 days. (D) Zeta potential of MeGC and sulfonated hydrogels. (E) Bioactivity of BMP-2 released from sulfonated hydrogels as assessed by measuring ALP activity in C2C12 cells (*: p < 0.05).
3.6. BMP-2 binding ability of sulfonated hydrogels
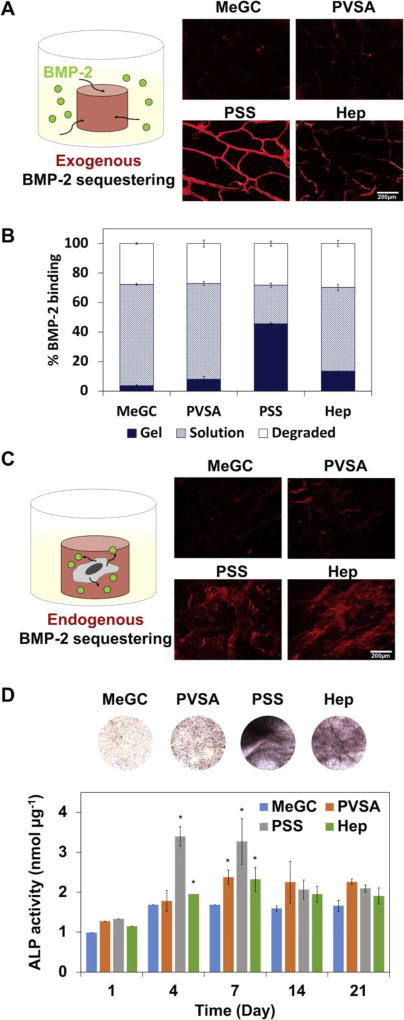
In order to evaluate the ability of rhBMP-2 binding to sulfonated hydrogels, hydrogels were incubated in a rhBMP-2 solution for a week and bound rhBMP-2 was observed after immunofluorescent staining (Fig. 6A). PSS modified hydrogels were found to bind BMP-2 while MeGC showed no significant staining of the protein, indicating successful sequestering of rhBMP-2 in sulfonated hydrogels through polysulfonates functionalization. Higher amount of rhBMP-2 was detected in the sulfonated hydrogels, especially in the PSS modified hydrogels, compared with unmodified MeGC hydrogels as measured by ELISA (Fig. 6B). To investigate the ability of sulfonated hydrogels to bind with endogenous BMP-2 produced by cells, BMSCs were encapsulated in sulfonated hydrogels and cultured in OM without rhBMP-2 supplementation (Fig. 6C). At day 7, highly intense rhBMP-2 staining was observed in PSS or heparin modified hydrogels compared to unmodified MeGC groups.
Figure 6.
BMP-2 binding to sulfonated hydrogels. (A) BMP-2 immunostaining of hydrogels after 7-day incubation in BMP-2 solution. (B) Amount of BMP-2 in sulfonated hydrogels and incubating supernatant quantified by ELISA. (C) BMP-2 immunostaining of hydrogels encapsulated with BMSCs after 7-day culture in OM. (D) ALP staining of hydrogels encapsulated with BMSCs after 4-day culture in OM. ALP activity was measured by ALP colorimetric assay (*: p < 0.05).
In order to test the intrinsic effects of sulfonated hydrogels on osteogenesis, BMSCs were encapsulated in sulfonated hydrogels in the absence of rhBMP-2 and expression of ALP was monitored. The addition of polysulfonates into MeGC hydrogels significantly increased the expression of ALP as observed by ALP staining and quantified by ALP colorimetric assay (Fig. 6D). The findings are consistent with the observation that sulfonated hydrogels bind more exogenous (Fig. 6A, B) or cell-secreted BMP-2 (Fig. 6C)
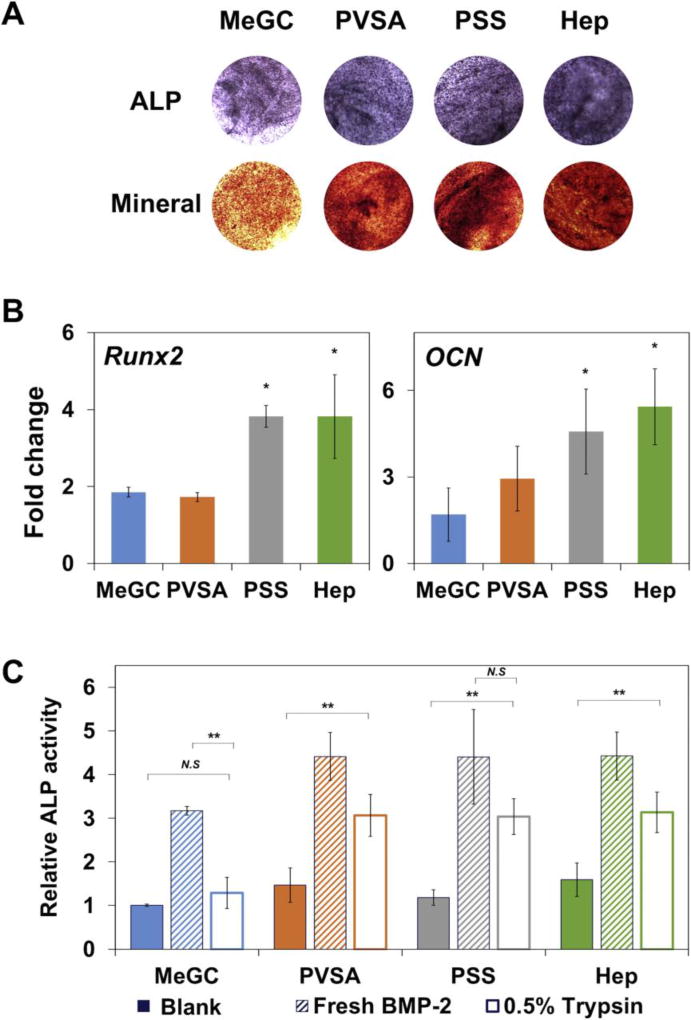
3.7. Osteogenic efficacy of BMP-2 in sulfonated hydrogels
In order to evaluate the efficacy of sulfonated hydrogels delivering rhBMP-2 to enhance osteogenesis, BMSCs and rhBMP-2 were co-encapsulated in the hydrogels and osteogenic differentiation of BMSCs was evaluated by both ALP and alizarin red S staining (Fig. 7A). The rhBMP-2 loaded hydrogels modified with polysulfonates showed highly intense positive ALP and alizarin red S staining, suggesting strong ALP expression and mineral deposition compared to unmodified MeGC hydrogels. The sulfonated hydrogels also significantly increased osteogenic differentiation of encapsulated BMSCs as observed by upregulation of Runx2, a key regulator of osteogenesis as well as OCN, a late osteogenic marker (Fig. 7B). To evaluate the maintenance of BMP-2 bioactivity in the sulfonated hydrogels during exposure to stressors, hydrogels loaded with rhBMP-2 were treated under various stress conditions first and the bioactivity of the rhBMP-2 extracted from the hydrogels was assessed in BMSCs by ALP assay (Fig. 7C and Fig. S6). Significantly increased ALP expression was observed in BMSCs cultured with rhBMP-2 recovered from MeGC or polysulfonates modified hydrogels with no stressor treatment. After exposure to stressors, MeGC hydrogels without polysulfonates exhibited low rhBMP-2 bioactivity. In contrast, sulfonated hydrogels provided better stabilizing effect during the treatment.
Figure 7.
Osteogenic differentiation of encapsulated BMSCs in sulfonated hydrogels loaded with BMP-2. (A) ALP (top) and mineral deposition (bottom) of BMSCs in hydrogels at day 4 (ALP) or day 21 (mineral) after culture. (B) Osteogenic gene expression of BMSCs analyzed by qRT-PCR at day 4 (Runx2) or day 21 (OCN) after culture. (C) BMP-2 stabilizing effect of sulfonated hydrogels. BMP-2 was loaded into sulfonated hydrogels and incubated in 0.5% trypsin for 5 h. Solution obtained after hydrogel degradation was collected and its bioactivity was determined by measuring ALP activity of BMSCs compared with hydrogels loaded with BMP-2 without trypsin treatment (Fresh BMP-2) or hydrogels loaded with PBS (Blank). (*: p < 0.05, **: p < 0.01, N.S: not significant).
4. Discussion
In this study, we seek to increase biological activity of BMP-2 for osteogenesis by binding with heparin-mimicking molecules in MeGC hydrogels. Heparin is a polysaccharide with a highly negatively charged density and is being widely used in tissue engineering scaffolds and controlled release systems for many growth factors including BMP-2 due to its strong binding property and protective effect for the proteins from degradation[47, 48]. We investigated here whether the heparin-mimicking sulfonated polymers could stabilize rhBMP-2 and enhance MSCs osteogenic differentiation induced by rhBMP-2.
We evaluated the protective effect of polysulfonates under various physiological stressors found in bone fracture healing. Fracture healing is a cascade of osteoblastic new bone formation and osteoclastic resorption events. The bone remodeling process is related to proteolytic degradation of organic extracellular compartment through enzyme activity such as matrix metalloproteinase (MMPs), cathepsin K, and trypsin[49, 50]. Osteoclasts created mild acidic extracellular environment (pH 4–5) by secreting acid to dissolve bone mineral. Our results showed that the bioactivity of rhBMP-2 was well maintained with the addition of polysulfonates during exposure to enzyme or acidic stress conditions, especially with PSS. The acidic condition decreased the rhBMP-2 bioactivity to a lesser extent probably due to increased protein solubility at the experimental pH 4.5[10, 51]. Incubation of rhBMP-2 at physiological pH for 7 days greatly reduced the protein bioactivity. Given that rhBMP-2 reduces solubility at pH above 6.5, the increase in test pH might cause protein aggregation which could affect the bioactivity of rhBMP-2[52, 53]. Interestingly, the bioactivity of freshly reconstituted rhBMP-2 was significantly increased in the presence of polysulfonates or heparin. It is demonstrated that the BMP-2 dimer contains two heparin binding sites on N-terminal domains which modulate its bioactivity[24]. The cell surface heparan sulfate played a direct and stimulatory role in BMP-2 signaling. Specifically, heparan sulfate serves as a catalyst to form signaling complexes by enhancing the recruitment of type II receptor although it does not directly affect BMP binding to type I receptor[54]. Heparin also reduced BMP-2 interaction with its antagonist noggin by modulating its distribution on the cell surface with prolonged half-life[55]. Extracellular regulation of BMP activity by other ECM proteins was also studied that BMP-7 underwent conformational change from bioactive open V-shape to latent closed ring shape upon binding to fibrillin-1[56].
rhBMP-2 binding ability of sulfonated hydrogels was explored after incorporation of the polysulfonates into MeGC. The sulfonated hydrogels were found to bind higher amount of rhBMP-2 compared with unmodified MeGC hydrogels as shown by rhBMP-2 ELISA assay. This may be attributed to strong electrostatic interactions between sulfonate residues on the hydrogel surface and positively charged rhBMP-2 (isoelectric point: 8.5) [57, 58]. Specifically, PSS-functionalized hydrogels exhibited the highest sequestering ability. This may be due to decreased (more negative) zeta potential with PSS which created higher affinity binding of rhBMP-2. Moreover, the observed low levels of protein release in sulfonated hydrogels may be associated with high levels of protein absorption.
Sulfonated hydrogels significantly increased osteogenic differentiation of encapsulated BMSCs without addition of exogenous rhBMP-2 compared with unmodified MeGC hydrogels as observed by increased ALP expression (Fig. 6D). Matrix mechanical properties such as elastic modulus and stress relaxation are known to regulate osteogenic differentiation of MSCs[59, 60]. The addition of polysulfonates into MeGC did not have significant effect on hydrogel mechanical properties as well as cell viability and proliferation. Thus, the observed increase in osteogenesis is likely due to sulfonated hydrogel surfaces that may sequester BMPs secreted by entrapped BMSCs as validated by immunostaining for BMP-2. BMP-2 stabilizing effects of sulfonated hydrogels were verified after stress exposures (Fig. 7C). These findings suggest a promising hydrogel surface that could augment endogenous BMP activity by localizing the cell-produced BMPs. Moreover, the addition of polysulfonates reduced initial burst and further increased rhBMP-2 induced osteogenesis differentiation of encapsulated BMSCs, suggesting efficient protein delivery systems. In addition, the therapeutic efficacy of using large amount of heparin in tissue engineering scaffolds or drug delivery microparticles is limited due to its cytotoxicity and high charge density limiting BMP-2 release. Our study here incorporated heparin-mimicking sulfonated molecules into biodegradable bulk hydrogels to sequester, stabilize and release BMP-2 as the hydrogel degrades in vivo. Future study will modulate hydrogel degradation properties for tunable BMP-2 release kinetics and in vivo performance will be evaluated in a more challenging bone fracture healing model for clinical translation.
5. Conclusions
We developed a heparin-mimicking hydrogel surface that can stabilize BMP activity to enhance osteogenesis by incorporating sulfonated molecules into photocrosslinkable MeGC hydrogel. The sulfonated molecules exhibited protecting effects on BMP-2 bioactivity against various therapeutically relevant stressors. The sulfonated hydrogel was not only effective in delivering exogenous BMP-2, but also potentiated endogenous BMP signaling mediated by cells. This work suggests a promising hydrogel system to improve clinical efficacy of BMP-2 and other heparin-binding growth factors and also provides basis for future development of material-based therapeutics for tissue engineering.
Supplementary Material
Statement of significance.
Although bone morphogenetic protein-2 (BMP-2) is believed to be the most potent cytokine for bone regeneration, its clinical applications require supraphysiological BMP dosage due to its intrinsic instability and fast enzymatic degradation, leading to worrisome side effects. This study demonstrates a novel hydrogel platform that mimics a natural protector of BMPs, heparin, to sequester and stabilize BMP-2 for increased osteoinductive signaling. This study will achieve the stabilization of BMPs with prolonged bioactivity by a synthetic heparin mimic that has not been examined previously. Moreover, the heparin mimetic hydrogel surface can augment endogenous BMP activity by sequestering and localizing the cell-produced BMPs. The additional knowledge gained from this study may suggest basis for future development of material-based therapeutics for tissue engineering.
Acknowledgments
This work was supported by the National Institutes of Health grants R01 AR060213 and R21 DE021819.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wozney JM. The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev. 1992;32(2):160–7. doi: 10.1002/mrd.1080320212. [DOI] [PubMed] [Google Scholar]
- 2.Okubo Y, Bessho K, Fujimura K, Konishi Y, Kusumoto K, Ogawa Y, Iizuka T. Osteoinduction by recombinant human bone morphogenetic protein-2 at intramuscular, intermuscular, subcutaneous and intrafatty sites. International Journal of Oral and Maxillofacial Surgery. 2000;29(1):62–66. [PubMed] [Google Scholar]
- 3.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T, Grp BS. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures - A prospective, controlled, randomized study of four hundred and fifty patients. Journal of Bone and Joint Surgery- American. 2002;84A(12):2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, He TC. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11(17):1312–20. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 5.Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: an update. Pharm Res. 2010;27(4):544–75. doi: 10.1007/s11095-009-0045-6. [DOI] [PubMed] [Google Scholar]
- 6.Hawe A, Wiggenhorn M, van de Weert M, Garbe JH, Mahler HC, Jiskoot W. Forced degradation of therapeutic proteins. J Pharm Sci. 2012;101(3):895–913. doi: 10.1002/jps.22812. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi MV, Laurence JS, Siahaan TJ. The role of thiols and disulfides on protein stability. Curr Protein Pept Sci. 2009;10(6):614–25. doi: 10.2174/138920309789630534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Bialy I, Jiskoot W, Reza Nejadnik M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm Res. 2017;34(6):1152–1170. doi: 10.1007/s11095-017-2147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kisiel M, Ventura M, Oommen OP, George A, Walboomers XF, Hilborn J, Varghese OP. Critical assessment of rhBMP-2 mediated bone induction: an in vitro and in vivo evaluation. J Control Release. 2012;162(3):646–53. doi: 10.1016/j.jconrel.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Luca L, Capelle MA, Machaidze G, Arvinte T, Jordan O, Gurny R. Physical instability, aggregation and conformational changes of recombinant human bone morphogenetic protein-2 (rhBMP-2) Int J Pharm. 2010;391(1–2):48–54. doi: 10.1016/j.ijpharm.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Ohta H, Wakitani S, Tensho K, Horiuchi H, Wakabayashi S, Saito N, Nakamura Y, Nozaki K, Imai Y, Takaoka K. The effects of heat on the biological activity of recombinant human bone morphogenetic protein-2. J Bone Miner Metab. 2005;23(6):420–5. doi: 10.1007/s00774-005-0623-6. [DOI] [PubMed] [Google Scholar]
- 12.James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang XL, Ting K, Soo C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Engineering Part B-Reviews. 2016;22(4):284–297. doi: 10.1089/ten.teb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2) Spine Journal. 2008;8(6):1011–1018. doi: 10.1016/j.spinee.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Irie K, Alpaslan C, Takahashi K, Kondo Y, Izumi N, Sakakura Y, Tsuruga E, Nakajima T, Ejiri S, Ozawa H, Yajima T. Osteoclast differentiation in ectopic bone formation induced by recombinant human bone morphogenetic protein 2 (rhBMP-2) J Bone Miner Metab. 2003;21(6):363–9. doi: 10.1007/s00774-003-0430-x. [DOI] [PubMed] [Google Scholar]
- 15.van den Bergh JPA, ten Bruggenkate CM, Groeneveld HHJ, Burger EH, Tuinzing DB. Recombinant human bone morphogenetic protein-7 in maxillary sinus floor elevation surgery in 3 patients compared to autogenous bone grafts - A clinical pilot study. Journal of Clinical Periodontology. 2000;27(9):627–636. doi: 10.1034/j.1600-051x.2000.027009627.x. [DOI] [PubMed] [Google Scholar]
- 16.Tang TT, Xu XL, Dai KR, Yu CF, Yue B, Lou JR. Ectopic bone formation of human bone morphogenetic protein-2 gene transfected goat bone marrow-derived mesenchymal stem cells in nude mice. Chin J Traumatol. 2005;8(1):3–7. [PubMed] [Google Scholar]
- 17.Fan J, Pi-Anfruns J, Guo M, Im DCS, Cui ZK, Kim S, Wu BM, Aghaloo TL, Lee M. Small molecule-mediated tribbles homolog 3 promotes bone formation induced by bone morphogenetic protein-2. Sci Rep. 2017;7(1):7518. doi: 10.1038/s41598-017-07932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poynton AR, Lane JM. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine (Phila Pa 1976) 2002;27(16 Suppl 1):S40–8. doi: 10.1097/00007632-200208151-00010. [DOI] [PubMed] [Google Scholar]
- 19.Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. FASEB J. 1997;11(1):51–9. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 20.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair and Regeneration. 2009;17(2):153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 21.Macri L, Silverstein D, Clark RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59(13):1366–81. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002;41(3):391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 23.Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, Ogamo A, Kamijo R. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278(44):43229–35. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- 24.Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. European Journal of Biochemistry. 1996;237(1):295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- 25.Seto SP, Casas ME, Temenoff JS. Differentiation of mesenchymal stem cells in heparin-containing hydrogels via coculture with osteoblasts. Cell and Tissue Research. 2012;347(3):589–601. doi: 10.1007/s00441-011-1265-8. [DOI] [PubMed] [Google Scholar]
- 26.Seto SP, Miller T, Temenoff JS. Effect of Selective Heparin Desulfation on Preservation of Bone Morphogenetic Protein-2 Bioactivity after Thermal Stress. Bioconjugate Chemistry. 2015;26(2):286–293. doi: 10.1021/bc500565x. [DOI] [PubMed] [Google Scholar]
- 27.Simann M, Schneider V, Le Blanc S, Dotterweich J, Zehe V, Krug M, Jakob F, Schilling T, Schutze N. Heparin affects human bone marrow stromal cell fate: Promoting osteogenic and reducing adipogenic differentiation and conversion. Bone. 2015;78:102–113. doi: 10.1016/j.bone.2015.04.039. [DOI] [PubMed] [Google Scholar]
- 28.Mulloy B, Forster MJ. Conformation and dynamics of heparin and heparan sulfate. Glycobiology. 2000;10(11):1147–56. doi: 10.1093/glycob/10.11.1147. [DOI] [PubMed] [Google Scholar]
- 29.Linhardt RJ, Gunay NS. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost. 1999;25(Suppl 3):5–16. [PubMed] [Google Scholar]
- 30.Linhardt RJ, Loganathan D, al-Hakim A, Wang HM, Walenga JM, Hoppensteadt D, Fareed J. Oligosaccharide mapping of low molecular weight heparins: structure and activity differences. J Med Chem. 1990;33(6):1639–45. doi: 10.1021/jm00168a017. [DOI] [PubMed] [Google Scholar]
- 31.Battistelli S, Genovese A, Gori T. Heparin-induced thrombocytopenia in surgical patients. Am J Surg. 2010;199(1):43–51. doi: 10.1016/j.amjsurg.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 32.Schindewolf M, Lindhoff-Last E, Ludwig RJ, Boehncke WH. Heparin-induced skin lesions. Lancet. 2012;380(9856):1867–79. doi: 10.1016/S0140-6736(12)60409-7. [DOI] [PubMed] [Google Scholar]
- 33.Le Templier G, Rodger MA. Heparin-induced osteoporosis and pregnancy. Curr Opin Pulm Med. 2008;14(5):403–7. doi: 10.1097/MCP.0b013e3283061191. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen TH, Kim SH, Decker CG, Wong DY, Loo JA, Maynard HD. A heparin-mimicking polymer conjugate stabilizes basic fibroblast growth factor. Nature Chemistry. 2013;5(3):221–227. doi: 10.1038/nchem.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paluck SJ, Nguyen TH, Lee JP, Maynard HD. A Heparin-Mimicking Block Copolymer Both Stabilizes and Increases the Activity of Fibroblast Growth Factor 2 (FGF2) Biomacromolecules. 2016;17(10):3386–3395. doi: 10.1021/acs.biomac.6b01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu J, Hou Y, Park H, Choi B, Hou S, Chung A, Lee M. Visible light crosslinkable chitosan hydrogels for tissue engineering. Acta Biomater. 2012;8(5):1730–8. doi: 10.1016/j.actbio.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 37.Hyejin P, Bogyu C, Junli H, Min L. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomaterialia. 2013;9(1):4779–86. doi: 10.1016/j.actbio.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Cui ZK, Fan JB, Fartash A, Aghaloo TL, Lee M. Photocrosslinkable chitosan hydrogels functionalized with the RGD peptide and phosphoserine to enhance osteogenesis. Journal of Materials Chemistry B. 2016;4(31):5289–5298. doi: 10.1039/C6TB01154C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi B, Kim S, Lin B, Wu BM, Lee M. Cartilaginous Extracellular Matrix-Modified Chitosan Hydrogels for Cartilage Tissue Engineering. ACS Appl Mater Interfaces. 2014 doi: 10.1021/am505723k. [DOI] [PubMed] [Google Scholar]
- 40.Choi B, Kim S, Lin B, Li K, Bezouglaia O, Kim J, Evseenko D, Aghaloo T, Lee M. Visible-light-initiated hydrogels preserving cartilage extracellular signaling for inducing chondrogenesis of mesenchymal stem cells. Acta Biomater. 2015;12:30–41. doi: 10.1016/j.actbio.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Choi B, Kim S, Fan J, Kowalski T, Petrigliano F, Evseenko D, Lee M. Covalently conjugated transforming growth factor-beta 1 in modular chitosan hydrogels for the effective treatment of articular cartilage defects. Biomaterials Science. 2015;3(5):742–752. doi: 10.1039/c4bm00431k. [DOI] [PubMed] [Google Scholar]
- 42.Mussano F, Lee KJ, Zuk P, Tran L, Cacalano NA, Jewett A, Carossa S, Nishimura I. Differential effect of ionizing radiation exposure on multipotent and differentiation-restricted bone marrow mesenchymal stem cells. J Cell Biochem. 2010;111(2):322–32. doi: 10.1002/jcb.22699. [DOI] [PubMed] [Google Scholar]
- 43.Cui ZK, Kim S, Baljon JJ, Doroudgar M, Lafleur M, Wu BM, Aghaloo T, Lee M. Design and Characterization of a Therapeutic Non-phospholipid Liposomal Nanocarrier with Osteoinductive Characteristics To Promote Bone Formation. ACS Nano. 2017 doi: 10.1021/acsnano.7b02702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amsden BG, Sukarto A, Knight DK, Shapka SN. Methacrylated glycol chitosan as a photopolymerizable biomaterial. Biomacromolecules. 2007;8(12):3758–66. doi: 10.1021/bm700691e. [DOI] [PubMed] [Google Scholar]
- 45.Betz M, Hormansperger J, Fuchs T, Kulozik U. Swelling behaviour, charge and mesh size of thermal protein hydrogels as influenced by pH during gelation. Soft Matter. 2012;8:2477–2485. [Google Scholar]
- 46.Kett WC, Osmond RI, Moe L, Skett SE, Kinnear BF, Coombe DR. Avidin is a heparin-binding protein. Affinity, specificity and structural analysis. Biochim Biophys Acta. 2003;1620(1–3):225–34. doi: 10.1016/s0304-4165(02)00539-1. [DOI] [PubMed] [Google Scholar]
- 47.Rider CC. Heparin/heparan sulphate binding in the TGF-beta cytokine superfamily. Biochemical Society Transactions. 2006;34:458–460. doi: 10.1042/BST0340458. [DOI] [PubMed] [Google Scholar]
- 48.Wood MD, Hunter D, Mackinnon SE, Sakiyama-Elbert SE. Heparin-Binding-Affinity- Based Delivery Systems Releasing Nerve Growth Factor Enhance Sciatic Nerve Regeneration. Journal of Biomaterials Science-Polymer Edition. 2010;21(6–7):771–787. doi: 10.1163/156856209X445285. [DOI] [PubMed] [Google Scholar]
- 49.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175(2):266–76. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 50.Teti A. Mechanisms of osteoclast-dependent bone formation. Bonekey Rep. 2013;2:449. doi: 10.1038/bonekey.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sigurdsson TJ, Nygaard L, Tatakis DN, Fu E, Turek TJ, Jin L, Wozney JM, Wikesjo UME. Periodontal repair in dogs: Evaluation of rhBMP-2 carriers. International Journal of Periodontics & Restorative Dentistry. 1996;16(6):525–537. [PubMed] [Google Scholar]
- 52.Sola RJ, Griebenow K. Effects of Glycosylation on the Stability of Protein Pharmaceuticals. Journal of Pharmaceutical Sciences. 2009;98(4):1223–1245. doi: 10.1002/jps.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm Res. 2003;20(9):1325–36. doi: 10.1023/a:1025771421906. [DOI] [PubMed] [Google Scholar]
- 54.Kuo WJ, Digman MA, Lander AD. Heparan sulfate acts as a bone morphogenetic protein coreceptor by facilitating ligand-induced receptor hetero-oligomerization. Mol Biol Cell. 2010;21(22):4028–41. doi: 10.1091/mbc.E10-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bramono DS, Murali S, Rai B, Ling L, Poh WT, Lim ZX, Stein GS, Nurcombe V, van Wijnen AJ, Cool SM. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2) Bone. 2012;50(4):954–64. doi: 10.1016/j.bone.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wohl AP, Troilo H, Collins RF, Baldock C, Sengle G. Extracellular Regulation of Bone Morphogenetic Protein Activity by the Microfibril Component Fibrillin-1. J Biol Chem. 2016;291(24):12732–46. doi: 10.1074/jbc.M115.704734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhakta G, Rai B, Lim ZXH, Hui JH, Stein GS, van Wijnen AJ, Nurcombe V, Prestwich GD, Cool SM. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials. 2012;33(26):6113–6122. doi: 10.1016/j.biomaterials.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kisiel M, Klar AS, Ventura M, Buijs J, Mafina MK, Cool SM, Hilborn J. Complexation and Sequestration of BMP-2 from an ECM Mimetic Hyaluronan Gel for Improved Bone Formation. Plos One. 2013;8(10) doi: 10.1371/journal.pone.0078551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim WS, Alim K, Mammoto A, Ingber DE, Duda GN, Mooney DJ. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater. 2015;14(12):1269–77. doi: 10.1038/nmat4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee H-p, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature Materials. 2015;15:326. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.