Abstract
Background
Antibiotic resistance is one of the most serious health threats to modern medicine. The lack of potent antibiotics puts us at a disadvantage in the fight against infectious diseases, especially those caused by antibiotic-resistant microbial strains. To this end, an urgent need to search for alternative antimicrobial approaches has arisen. In the last decade, light-based therapy has made significant strides in this fight to combat antibiotic resistance among various microbial strains. This method includes utilizing antimicrobial blue light, antimicrobial photodynamic therapy, and germicidal ultraviolet irradiation, among others. Light-based therapy is advantageous over traditional antibiotics in that it eradicates microbial cells rapidly and the likelihood of light-resistance development by microbes is low.
Methods
This review highlights the patents on light-based therapy that were filed approximately within the last decade and are dedicated to eradicating pathogenic microorganisms. The primary database that was used for the search was Google Patents. The searches were performed using the keywords including blue light, antimicrobial photodynamic therapy, ultraviolet irradiation, antibiotic resistance, disinfection, bacterium, fungus, and virus.
Results
Forty-five patents were obtained in our search: 9 patents for the antimicrobial blue light approach, 21 for antimicrobial photodynamic therapy, 11 for UV irradiation, and lastly 4 for other light-based anti-infective approaches. The treatments and devices discussed in this review are interestingly enough able to be used in various different functions and settings, such as dental applications, certain eye diseases, skin and hard surface cleansing, decontamination of internal organs (e.g., the stomach), decontamination of apparel and equipment, eradication of pathogenic microorganisms from buildings and rooms, etc. Most of the devices and inventions introduce methods of destroying pathogenic bacteria and fungi without harming human cells and tissues.
Conclusions
Light-based antimicrobial approaches hold great promise for the future in regards to treating antibiotic-resistant infections and related diseases.
Keywords: light-based therapy, antimicrobial blue light, antimicrobial photodynamic therapy, germicidal UV irradiation, disinfection, antibiotic resistance, bacterium, fungus, virus
1. INTRODUCTION
In 2013, the Center for Disease Control and Prevention (CDC) released a detailed report titled Antibiotic Resistance Threats in the United States [1]. The report highlights the significance of current antibiotic-resistant threats in the U.S. (classified by microorganisms), distinguishes between the different degrees of severity, and prioritizes the pathogenic microorganisms into one of three categories: urgent, serious, and concerning threats.
The CDC estimates that in the US, over 2 million people are sickened every year due to antibiotic-resistant infections, with at least 23,000 dying as a result [1]. The most staggering fact regarding this is the total economic cost of antibiotic resistance to the U.S. economy, which has been difficult to calculate. Estimates have varied, but have ranged to as high as $20 billion in excess direct healthcare costs, with additional costs to society for lost productivity as high as $35 billion per year [2].
The transmission of antibiotic-resistance among microorganisms has arisen due to the excessive use and abuse of antibiotics [2,3]. As a response to this, researchers and scientists have begun to consider alternative treatments, such as light-based antimicrobial therapy. This form of therapy can act as a treatment for a vast array of antibiotic-resistant infections. This review highlights the patents on light-based therapy that were filed approximately within the last decade and are dedicated to eradicating pathogenic microorganisms. These approaches include antimicrobial blue light (aBL), antimicrobial photodynamic therapy (aPDT), germicidal ultraviolet (UV) irradiation, etc.
2. METHODS
This review highlights the patents on light-based therapy that were filed approximately within the last decade and are dedicated to eradicating pathogenic microorganisms. The primary database that was used for the search was Google Patents. The searches were performed using the keywords including blue light, antimicrobial photodynamic therapy, ultraviolet irradiation, antibiotic resistance, disinfection, bacterium, fungus, and virus.
3. RECENT PATENTS ON LIGHT-BASED ANTI-INFECTIVE APPROACHES
3.1. Antimicrobial Blue Light
Blue light within the range of 400–470 nm excites the endogenous photosensitizing chromophores in microbial cells, leading to the generation of reactive oxygen species (ROS) that are toxic to the microbial cells (Fig. 1) [4]. An advantage of treating microbial infections locally with aBL is that there are little or no side effects on humans or animals [5–7]. In addition to treating infections, aBL can be utilized for many other purposes, such as air disinfection, surface disinfection of materials, and most importantly wound protection and tissue disinfection. Table 1 summarizes the recent patents pertaining to aBL as discussed in details below, most of which are on the development of devices for the delivery of aBL.
Fig. 1.
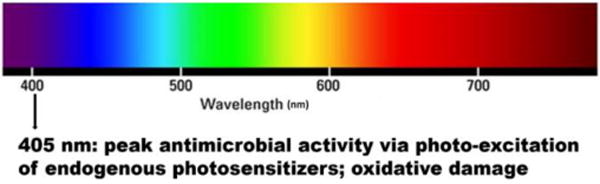
Antimicrobial blue light.
Table 1.
Recent Patents on Antimicrobial Blue Light.
| Inventors | Patent No. | Title |
|---|---|---|
| Osnat Feuerstein, Ervin I. Weiss, Perez Davidi | US20060085052A1 [8] | Method and means for exerting a phototoxic effect of visible light of microorganisms |
| John G. Alexander, Michelle Maclean, Gerald A. Woolsey, Scott J. MacGregor | US9039966 [9] | Inactivation of Gram-positive bacteria |
| Bo Zhou | US20120310307A1 [10] | Treatment of fungal infection by light irradiation |
| Bernhard Banowski, Florian Garnich, Rainer Simmering | DE102013202122A1 [11] | Device for performing deodorizing medical treatment |
| Bernhard Banowski, Florian Garnich, Rainer Simmering | DE102012224183A1 [12] | Device for cosmetic and/or medical treatment |
| J. Max Goodson, Nikos Soukos | US8021148 [14] | Intraoral light-emitting device |
| Curt Binner, Megha Reddy | US8186997 [15] | Method for cleaning the oral cavity |
| Yosef Krespi, Ashutosh Kacker | US20100076526 [16] | Control of halitosis-generating and other microorganisms in the non-dental upper respiratory tract |
| Taro Kanno, Keisuke Nakamura, Yoshimi Niwano, Minoru Kanno | WO2014136255A1 [33] | Peri-implantitis therapy device |
Patent US20060085052A1 describes an apparatus of using aBL for treating local microbial infections [8]. It includes a lamp which emits blue light between 400 and 550 nm. The temperature of the exposed tissue and/or organ is ≤ 42 °C (the threshold temperature of tissue damage) during the treatment or immediately after the treatment, which enables a phototoxic effect on microbial cells to be exerted. This apparatus is low-cost and has been proven to be especially useful for dental and skin surface applications.
In patent US9039966, an approach is provided for inhibiting Gram-positive bacteria using aBL [9]. These bacteria include Staphylococcus (MRSA), Streptococcus, Enterococcus, and Clostridium species, and are selectively inactivated by aBL. It is reported that light between 400 and 500 nm is preferable, with optimal inactivation at 405 nm. In addition, some bacteria are commensal in that they colonize and live on healthy human tissues without harming them, but become pathogenic once the host’s immune system is compromised. Therefore, the system also takes into account “potentially pathogenic” bacteria and eradicates them as well by inhibiting the growth ability of the bacteria.
Patent US20120310307 introduces an approach for the treatment of fungal infections using aBL [10]. The apparatus includes one or more blue light-emitting diodes (LEDs), which are applied externally to the infection zone periodically at scheduled times with continuous or pulsed irradiation. A sufficient irradiance and exposure time can produce a sufficient level of ROS, which is lethal to the fungal cells (conidia or hyphae). However, the tissue or organ around the infected zone is not adversely impacted by aBL under the therapeutic radiant exposures for treating fungal infections. aBL is able to penetrate the skin or nail and reach the infected zone depending on the scattering and absorption within the human tissue or organ. Therefore, the approach makes it possible to treat fungal infections underneath the skin or nail plate without having to remove the surface.
To perform deodorizing medical treatments, a device is introduced by patent DE102013202122A1 with antimicrobial function for cosmetic treatment of human tissue and skin [11]. The apparatus is assembled using a handset, a light source and a positioning device. The independent manageable handset unit comprises a container. The light source includes an optical fiber which delivers aBL between 400 and 410 nm. The positioning device adjusts the irradiance of aBL, while a control unit controls the radiant exposure.
A similar device for cosmetic and medical treatments, such as antimicrobial treatment of the human skin, is described in patent DE102012224183A1 [12]. Like the device discussed above, this device also includes a unit to control the radiant exposure of aBL. In addition, it includes an actuator for adjusting the irradiance. The device also has an independently manageable handset unit provided with a power supply. An LED is provided for the emission of light between 250 and 700 nm.
aBL has also been proven to be successfully useful for dental applications. Peri-implantitis is the destructive inflammatory process that affects the soft and hard tissues surrounding dental implants. Traditional treatments include removing dead tissue, antibiotics, and improved dental hygiene such as using mouthwash and washing with chlorhexidine. Patent WO2014136255A1 introduces an aBL therapy device for peri-implantitis [13]. The device is cylindrical with the bottom unit placed around the implant. Provided on the inner surface is an opening for light irradiation, while on the bottom surface is a fungicide outlet. It also includes a wave-generating source, a sterilization apparatus, and a material supply source. The light wavelength is between 400 and 500 nm.
Another patent (US8021148) on dental application is the invention of an intraoral LED used to treat intraoral infections [14]. The device is composed of a light source, a power source, and a bite-activated power switch. The size and shape of the device fits comfortably within an individual’s oral cavity. The light source emits wavelengths of 350–700 nm, irradiance ranging from 1 to 1000 mW/cm2 and therefore radiant exposures between 0.1 to 1000 J/cm2. The entire device is encapsulated by a waterproof case. The light source is positioned so as to emit aBL to a portion of the tongue, teeth, gums, and to one or more lingual, buccal, palatal or facial surface. Additionally, the device includes a compartment for storing an antimicrobial agent or drug that enhances the therapeutic effect of the intraoral device and an optional timer.
Also essential to dental health is the cleanliness of the oral cavity. To address this issue, a device is invented in patent US8186997 [15]. The device can detect and remove plaque from the oral cavity as well as clean and irradiate the surface of a tooth in the oral cavity. During the treatment, a fluorescent agent is applied onto the tooth and then excited by incidental irradiation of a suitable wavelength to provide a fluorescent emission. A portion of the fluorescent emission can be collected over a time period before the initiation of the aBL exposure, determining a first average plaque value (APV1), and over a second-time period after the aBL exposure, determining a second average plaque value (APV2). APV1 and APV2 are then compared to determine the amount of plaque present before and after fluorescent emission and whether the treatment is effective or not. The light source typically emits wavelengths of 450 to 500 nm, although the range may vary depending on the particular fluorescent drug applied to the surface of the oral cavity.
Patent US20100076526 introduces a light-based personal care device [16]. It includes a method for a safe and simple broad-spectrum treatment for halitosis (the technical term for “bad breath”) and other bacterial infections of the non-dental upper respiratory tract. This invention utilizes pulses of photothermal energy rich in blue light wherein at least 70% of the pulse energy in the visible spectrum is polychromatic and is within the blue light region of 400 to 500 nm. Light is topically applied to the internal surfaces of the upper respiratory tract to destroy or inhibit the bacteria without the use of antibiotics. The apparatus is a handheld energy applicator composed of a light output head suitable for treating the back of the tongue and the tonsils, which may be interchangeably provided with extensions to reach the sinuses. The energy applicator can be supported and guided by a mounting device held between a patient’s teeth. The device also includes preparative treatment of the target surfaces with a photosensitizing agent. Optionally, a pre-treatment procedure may be employed to remove detritus and microfloral overgrowths that may mask deeply-seated microorganisms.
There have been a substantial number of pre-clinical studies of aBL have been carried out [4]. It has been shown that the majority of the important bacteria and fungi are sensitive to aBL [4]. aBL is much less harmful to microbes than to host cells [17–20], therefore, there is a therapeutic window where the microbes can be selectively inactivated over host cells by aBL. Some pre-clinical studies also showed that no aBL-resistance development was observed by microbes after up to 10 cycles successive sub-lethal aBL inactivation [17,18,21]. In addition, aBL was also found to have synergistic effect with other antimicrobials, such as medicinal plants and their extracts [22–25], antibiotics [26,27], and disinfectants [28–30]. In contrast to pre-clinical studies, very few clinical studies have been reported so far. Two clinical studies of dental application demonstrated that aBL significantly reduced the bacterial burden of human dental plaque [31, 32]. No aBL-induced adverse effect was observed [31].
3.2. Antimicrobial Photodynamic Therapy
Photodynamic therapy (PDT) involves the use of nontoxic photosensitizers (PSs) combined with harmless visible light of the suitable wavelength in order to excite the PS. During PDT, PSs in the excited triplet state transfer energy directly (type II reaction) or electrons indirectly (type I reaction) to the ground state molecular oxygen in order to produce ROS to inactivate target cells (e.g., bacteria, fungi, etc.) and tissues (e.g. vascular closure) (Fig. 2) [34]. Although the effect of PDT against microorganisms were found in the early stages of PDT, its potential against microbial diseases was not fully exploited due to the discovery of antibiotics, which made people once believe that microbial diseases would never threaten the health of human beings.
Fig. 2.
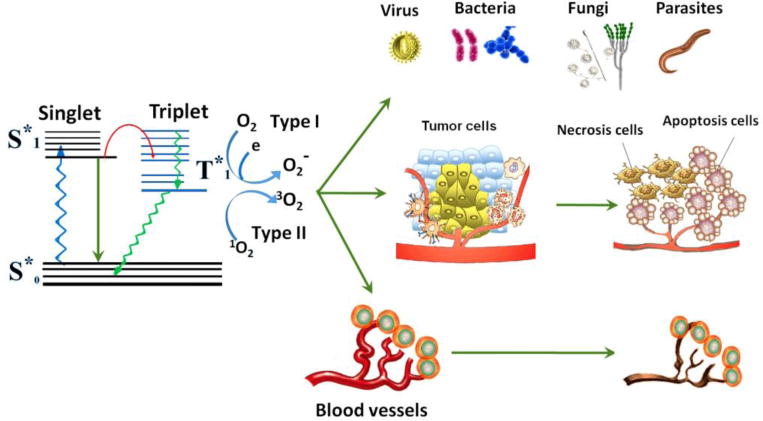
Schematic illustration of photodynamic action [35].
However, with the rapid emergence of antibiotic resistance, antimicrobial photodynamic therapy (aPDT) or photodynamic inactivation (PDI) has been proposed as an alternative method to eradicate pathogenic microorganisms, such as bacteria and fungi. Because of the rapid and effective actions of ROS as well as its multi-targeted nature, resistance to aPDT is less likely to be induced by microorganisms. Until now, aPDT has been utilized for the disinfection of blood products, especially the inactivation of virus in frozen plasma [36–42]. In addition, it is especially useful for the treatment of infections in the mouth (oral), orthopedics, and dermatology [34,43–48].
In recent years, studies of aPDT were mainly focused on the design of devices or accessories to deliver light and PSs suitably (Table 2) [57–61], the optimization of light exposure and PS dose for clinical treatment (Table 2) [62], and the design and synthesis of PSs or photosensitive formula to screen infections quickly and eradicate pathogenic microorganisms effectively (Table 2) [63–77]. Among these focuses, the most common issue is how to efficiently photo-inactivate microbial cells while minimizing the side effects on host cells and tissues, and one of the crucial points is the design of PSs. An ideal PS should selectively bind microbial cells over normal host cells. Until now, a variety of PSs such as porphyrin derivatives, transition metal complexes, conjugated polymers, nanoparticles, and novel organic chromophores have been studied for aPDT (Fig. 3) [49–56].
Table 2.
| Inventors | Patent Number | Description |
|---|---|---|
| Peter Paterok | US20120310310 [57] | Materials and accessories for the application of aPDT |
| Nikolaos S. Soukos et al | US20150030989 [58] | Handheld dental device for PDT and aPDT |
| Andrew Wescott et al | US20160059031 [59] | Catheter/Stent System to activate PDT |
| Gerhard D. Wieland et al | CN102802694 [61] | A novel method for microbial depletion in human blood and blood products using antimicrobial photodynamic therapy |
| Zewen Lin | CN103610464 [62] | Method and device for carrying out oral-cavity photodynamics therapy on patient suffering from periodontitis through LED |
| Shu Wang et al | CN102731405 [63] | A medical composition which consists of a photosensitizer and a chemical activator for the treatment of tumors and pathogenic bacteria infection |
| Graefe Susanna et al | EP2429498 [64] | New oral formulations for tetrapyrrole derivatives |
| Marrugat S. Nonell, et al | WO2012001194 [65] | Cation derivatives of 2,7,12,17-aryl porphycenes, preparation method thereof and use of same as photosensitisers in antimicrobial photodynamic therapy |
| Fan Lin | CN103143015 [66] | Application of photosensitizer for treating acne |
| Fedele Rosalisa et al | EP2616062 [67] | Use of derivatives of pentaphyrine as antimicrobial and desinfectant agents |
| Dei Donata | US20140163218 [68] | Novel phthalocyanine derivatives for therapeutic use |
| Tianjun Liu et al | CN103601727 [69] | Use of novel amine compound modified protoporphyrin |
| Gary W. Jones et al | US20140296524 [70] | Halogenated compounds for photodynamic therapy |
| Chunying Shu et al | CN103724356 [71] | Fullerene-porphyrin derivate photosensitizer can produce ROS at a low oxygen concentration condition, and has a efficient and broad spectrum antibacterial ability |
| Peng Zhang et al | WO2015134204 [72] | Silver nanoparticle-enhanced photosensitizers for inactivation of bacteria |
| Arkady Mandel | WO2016116859 [73] | Metal-glycoprotein complexes for treating cancer and destroying microbial cells, such as bacteria, fungi, and protozoa, and destroying viruses |
| Daniela Vecchio, et al | WO2016081594 [74] | System and method for photo-dynamic procedure |
| Benzhong Tang and Engui Zhao | WO2016078603 [75] | Aie luminogens for bacteria imaging, killing, photodynamic therapy and antibiotics screening, and their methods of manufacturing |
| George V. Garner and Marianne Fuierer | WO2016039812 [76] | Compositions for photodynamic control of infection |
| Sherri A. McFarland | EP2976347 [77] | Metal-based coordination complexes are designed as therapeutic & diagnostic agents for preventing or treating cancers and microbial cells |
| Min Kyu Oh et al | KR1020120090317 | A microorganism-killing microsphere containing porphyrin/phthalocyanine and a target agent for aPDT |
| Aicher Daniel et al | EP2616065 | Connecting dihydroxychlorins or β-functionalized chlorins to carbohydrate moieties to combat standard strains and resistant strains of bacteria |
Fig. 3.
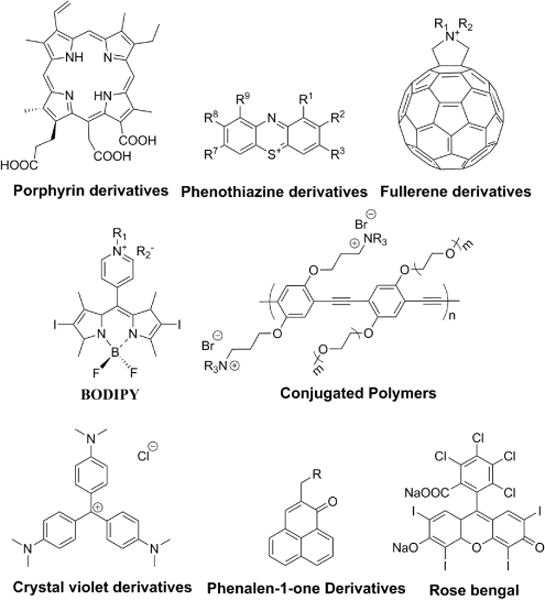
Chemical structures of the most commonly used photosensitizers [49–56].
Combining a PS with a pathogen-targeting agent or a carrier is one prospective approach for the selective binding of microorganisms [78,79]. In patent US6462070, PSs are conjugated to a small antimicrobial peptide (SAMP) for targeting microorganisms [80]. The SAMP can be histatins, defensins, cecropins, magainins, Gram-positive bacteriocins and peptide antibiotics. As shown in Fig. 4, after combing PS chlorin e6 with SAMP polylysine, the cationic conjugate was selectively phototoxic to Porphyromonas gingivalis compared to mammalian cells, and 99.9% of the bacteria were killed while the viability loss of HCPC-1 cells was less than 2%.
Fig. 4.
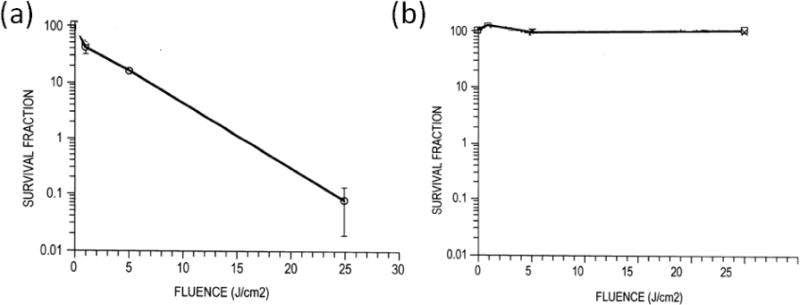
Survival fractions of (a) P. gingivalis and (b) HCPC-1 cells following light exposure of wavelength 630–710 nm, with the cells previously having taken up chlorin e6 conjugates [80].
In patent US6977075, PS conjugates are invented specifically to treat mycobacterial infections or any disease that is caused or aggravated by an intracellular pathogen [81]. In general, mycobacteria are phagocytosed by macrophages where they are “sheltered” from many antibiotic drugs and from the subject’s immune system. In this patent, PSs are featured as being conjugated to a targeting moiety, which can be a polypeptide or a SAMP that targets macrophages or interacts with a microbe.
The term “carbohydrate receptor” means a carbohydrate compound or carbohydrate moiety of a compound which selectively binds to microorganisms. The carbohydrate receptor may have as few as one sugar unit or several sugar units [82–85]. In patent US20120263625A1, dihydroxychlorins or β-functionalized chlorins are conjugated to carbohydrate moieties [86]. These conjugates exhibit effective antibacterial abilities against both Gram-positive and Gram-negative bacteria, including their resistant strains. Since the aPDT effects could be decreased remarkably while blood serum or blood exists, this patent also highlights the excellent photo-inactivation abilities of these conjugates in complex environments, including blood, serum and other body fluids present in a patient’s body.
Conjugating PSs with siderophores to construct a transportation system is another example [87–90]. Almost all microorganisms require iron (Fe) as an essential nutrient. For example, micromolar concentration of Fe is important for enteric bacteria, such as Salmonella and Escherichia coli, in order to replicate and colonize in the vertebrate host. Bacteria are able to biosynthesize and export the iron-chelating compound siderophore to scavenge Fe from the host. After conjugating PSs with siderophores, the conjugates are able to specifically target bacteria or fungi and are not taken up by mammalian cells, thus exhibiting selective photo-inactivation ability against pathogenic microorganisms over host cells.
Aside from conjugating PSs to a target moiety, researchers are also exploring the possibility of designing special PSs that could bind more efficiently with microorganisms than mammalian cells [91–93]. To this end, efforts have been made to discover the differences of 3D structures between bacterial and mammalian cells. In general, the structures of Gram-positive and Gram-negative bacteria are quite different (Fig. 5) [94]. Both types of bacteria contain an outer cell wall. Specifically, the outer wall of Gram-positive bacteria is about 15–80 nm thick and contains up to 100 layers of peptidoglycan in which lipoteichoic and negatively-charged teichuronic acids intervene. This wall is relatively porous and allows various macromolecules (molecular weight [Mw] 30–60 kDa) [95,96] to readily diffuse towards the inner plasma membrane and thus does not significantly affect the diffusion of commonly used PSs as long as the Mw does not exceed 1500–1800 Da. For the outer wall of Gram-negative bacteria, the peptidoglycan layer is relatively thin (~7 nm). However, there is an additional outer layer that is densely packed and highly negatively-charged (10–15 nm) and surrounds the peptidoglycan layer. Due to this permeability barrier, Gram-negative bacteria can inhibit the penetration of host cellular and humoral defense factors and exhibit more resistance against antibiotics. It is reported that only relatively hydrophilic compounds (Mw lower than 600–700 Da) are able to diffuse through some of the porin channels of Gram-negative bacteria [97,98].
Fig. 5.
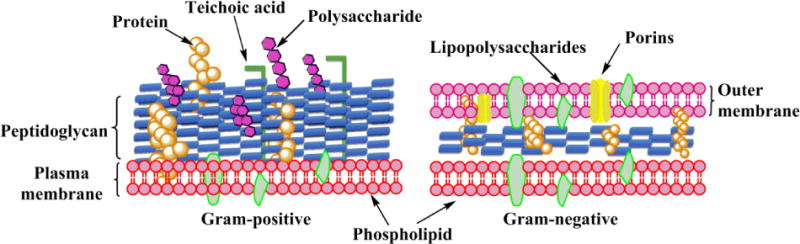
Schematic representation of the outer wall and cytoplasmic membrane structure in Gram-positive and Gram-negative bacteria [34].
Studies have revealed that the negative transmembrane potential in bacterial cells is much higher than that in mammalian cells. More importantly, the negative charges are located on the outer wall of bacteria but within the inner leaflets of mammalian cells. Zhu et al. reported that a cationic conjugated polymer with a relatively high quaternary ammonium ratio selectively combined with bacterial cells but did not bind to Jurkat T cells, indicating that the membrane charge difference between bacteria and mammalian cells was large enough to be clearly distinguished by cationic PSs [91,92]. Additionally, positively-charged PSs can bind to negatively-charged bacterial cell walls via electrostatic interaction and efficiently inactivate both Gram-positive and Gram-negative bacteria by not only the destructive effects of 1O2, but also by the destabilization and interruption of native organized bacterial cell walls [97,98].
One issue especially of note is that, as stated above, Gram-negative bacteria are more resistant to commonly used antibiotics and antibacterial photodynamic processes because of the additional outer layer. Therefore, to efficiently photo-inactivate Gram-negative bacteria, strategies to improve the penetration of PSs through the bacterial cell wall have been widely explored [99]. Obviously, cationic PSs possess advantages in this issue based on electrostatic interactions with the negatively-charged bacterial cell wall. Besides this, another method is coupling or combing PSs with positively-charged entities, such as EDTA, poly-L-lysine, polyethylenimine, and polymyxin B nonapeptide (PMBN) [100–102]. Moreover, PSs can also be combined with surfactant materials, such as sodium dodecyl sulfate (SDS), cetrimide, or benzalkonium chloride in order to mediate aPDT against bacteria, fungi, and their biofilms [99].
Based on the promising in vitro studies of aPDT, scientists have begun investigating the in vivo effects of aPDT. Dai et al. developed a burn wound infection model utilizing a Gram-negative bacterium, Acinetobacter baumannii [103]. A polyethylenimine chlorine (e6) (PEI-ce6) conjugate was applied as the PS, and a 660 nm non-coherent red light was set up as the light source. Results revealed that the aPDT that started on Day 0 was able to reduce the bacterial load in mice by about 3-log10 CFU, indicating the effectiveness of this application of aPDT. Due to the rapid emergence of antibiotic-resistant microbial strains, alternative antimicrobial approaches such as aPDT are drawing more attention.
Clinical applications of aPDT has been mainly in dermatology for acne using 5-aminolevulanic acid (ALA) and in dentistry using phenothiazinium dyes [47]. A recent study conducted ALA-aPDT in 13 Korean patients with inflammatory acne vulgaris, and found it to be effective and with no adverse effects [104]. In another study, Braun et al. reported that in patients with chronic periodontitis, clinical outcomes of conventional subgingival debridement were improved by adjunctive aPDT using toluidine blue ortho as the photosensitizer [105]. aPDT administered after the scaling and rooting planning treatment in patients with periodontitis infected with Fusobacterium nucleatum reduced periodontal inflammatory symptom and successfully eradicated the infection [106].
3.3. Germicidal Ultraviolet Irradiation
Germicidal ultraviolet (UV) irradiation has been found to have significantly positive effects on treating microbial infections. The mechanism of action of UV inactivation of microorganisms is to damage the genetic material in the nucleus of the microbial cell or nucleic acids in the virus [107]. The ultraviolet C (UVC) spectrum, especially in the range of 250–270 nm, is strongly absorbed by the nucleic acids of a microorganism and, therefore, is the most lethal range of wavelengths for microorganisms (Fig. 6). The penetration depth of UVC radiation into human skin is very limited and therefore the risk of skin cancer is very low, even when unprotected body parts are exposed to UVC.
Fig. 6.
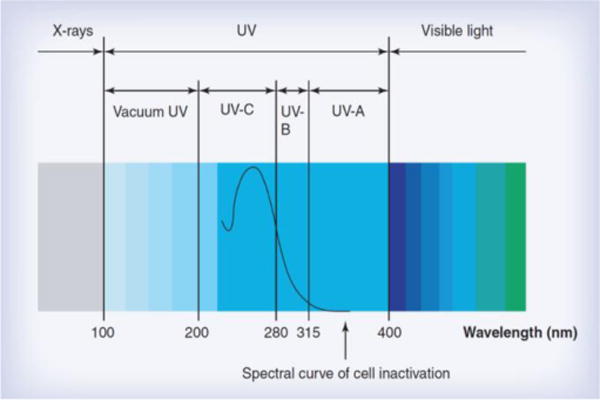
Germicidal UV irradiation spectrum [107].
Like aBL and aPDT, germicidal UVC irradiation has been found to be highly applicable for dental applications. Patent US20070099154A1 introduces the application of UVC to a patient’s mouth in order to eliminate bacteria during a dental procedure [108]. Through this method, the risk of bacterial infection to both the patient and the oral surgeon is greatly reduced. The instrument comprises a handle, a tool portion attached to the handle that can be applied to the mouth of the patient, and a UVC lamp mounted on the handle to irradiate a portion of the patient’s mouth during a dental surgery.
In addition to dental applications, UV irradiation has also been found to be able to treat human eye infections. In patent US9474811B2, infections of body tissues, specifically those in the eye, are treated with low radiant exposures of UVC [109]. The treatment device contains a LED which produces UVC at about 265 nm with a power output of 5 mW. The UVC irradiation is directed onto a zone of tissue that is approximately 4 mm in diameter. Exposure time of 1 s has been found to be effective, which is equivalent to a radiant exposure of 4 mJ/cm2. Longer exposure times and higher radiant exposures may be used for more resistant infections, but can be harmful if the exposure time is overlong. The irradiation can be delivered endoscopically to treat internal infections or to prevent infection during eye surgery. The device can be handheld or mounted onto a support.
In patent US20080065175A1, a handheld UV irradiation device is introduced to treat bacterial, viral, fungal and parasitic infections found in many of the body’s anatomical orifices [110]. Such infections include MRSA colonies that may be present in the nose or on the skin, as well as viral infections such as avian influenza, with the goal of eliminating or significantly reducing the infectious colonies without damaging the underlying tissue of the patient. Another aspect of the patent is to provide a device for treating various sexually transmitted diseases (STDs) such as Gonococcal and Non-gonococcal urethritis. It is also stated that the device can be used to treat common yeast and fungal infections in the vagina.
Patent US20090143842A1 demonstrates a light-based therapy device used to treat infections, diseases, and disorders such as cancerous cells or parasites in humans and animals [111]. It can be specifically employed to treat fungal and bacterial infections within the nails of the hands and the feet, which are normally difficult to treat using oral or topical drugs. This invention employs a variety of germicidal lights, such as continuous or pulsed UVC irradiation, and contains synergistic wavelengths, which are visible or infrared wavelengths that have germicidal characteristics. The device can accomplish the following goals: either inactivate or destroy pathogenic microorganisms or cells that are unwanted, condition the treated area so that it automatically resists or destroys microorganisms or unwanted cells, or can be combined with other treatments such as medications for enhanced antimicrobial effect.
In addition to external applications, germicidal UV has also been found to be effective within the human body. Helicobacter pylori is a Gram-negative bacterium that is found within the stomach. H. pylori infections are the cause of several gastrointestinal disorders associated with an increased secretion of gastric acid. So far, the eradication of this bacterium is completed by the combination of several expensive antibiotics that have strong side effects, and a number of increasingly resistant H. pylori strains have been found. Therefore, patent DE102010010763A1 provides an alternative method of using UVC irradiation for eradication of H. pylori [112]. In this process, a gastric tube is inserted through the mouth and into the stomach (similar to a feeding tube) for a prolonged period of time, while a small light source is embedded in the tube. The UVC irradiation is then generated using LED or gas discharge lamps.
UV irradiation has also been proven to be successful for disinfection or sterilization purposes. Patent US20110215261A1 describes a UV system to disinfect the hard surfaces in a room while minimizing missed areas due to shadows [113]. This is accomplished by providing multiple UV irradiation towers that can be placed in several areas of a room such that the shadowed areas are eliminated. The towers can be transported by a cart that is low to the ground such that the towers may be loaded and unloaded easily by a single operator. The system is able to be controlled remotely so that during activation of the system, no operator needs to be present, and the power can automatically be cut off in case another person enters the room.
In another patent (US20120126134A1), a UV area sterilizer or disinfector is designed so that it can be incorporated into a building structure where concern exists regarding the presence of pathogenic bacteria on environmental surfaces [114]. UVC is directed to architectural partitions of an enclosed area, and the architectural partitions reflect UVC to inactivate pathogens in the area. The device transmits a calculated radiant exposure of UVC from a fixture that is mounted to an architectural partition in the enclosed area. Once an effective cumulative exposure of UVC has been reflected to radiation sensors, the device shuts down. The device also allocates power to specific UVC emitters so as to direct UVC irradiation more uniformly throughout the area.
Patent US20120223216A1 reports a sterilization system for eradicating biological contaminants [115]. The system includes a self-propelled robotic mobile platform for locating and eradicating pathogenic bacteria and virus on floors, objects, walls, cabinets, angled structures, etc., using one or multiple UV irradiation sources. A controller allows the system to adjust the radiant exposure of UV irradiation received by a surface by changing the intensity of energy input to a UV source, the distance between a UV irradiation source and a surface being irradiated, the speed of the platform to affect the time of exposure, and by returning to contaminated areas for additional exposure. The platform also includes a sensor that is capable of detecting the fluorescence of biological contaminants irradiated with UV to locate the contaminated areas. The system is thus capable of a “seek and destroy” functionality by navigating towards the contaminated areas and irradiating such areas with UV.
Another decontamination invention involves an apparatus for disinfecting an object by removing a biologically-active contaminant from the object (patent US8710460) [116]. The apparatus includes a housing which encloses a disinfection chamber, where a portion of the object is inserted into the chamber in order for it to be disinfected. A UV source emits UV irradiation to be imparted on the portion of the object that is inserted into the chamber for inactivating at least a portion of the biologically-active contaminant(s) present on the object.
Disinfection and decontamination of human body parts is considered a hot topic that is highly investigated by many researchers and scientists. Patent US9095704 introduces a method of mitigating the effect of pathogenic microorganisms found in the body, including providing a light applicator with a housing chamber, a power supply, and at least one light source configured to emit UV irradiation [117]. It directs the applicator towards a body surface or body part so as to directly irradiate the surface for a certain period of time.
Aside from body parts, medical apparel also requires significant decontamination due to constant presence in hospitals and laboratories around bacteria. Patent US9162001 provides an apparatus for disinfecting and sterilizing medical or laboratory apparel, accessories, equipment, etc. It comprises a cabinet, a disinfecting system housed within the cabinet, a disinfecting agent, and an embedded computer or a programmable logic controller (PLC) housed within the cabinet. The PLC controls one or more systems of the apparatus. The cabinet has advanced tracking features that can track the apparel or accessories being disinfected. The PLC is also capable of sending electronic messages to users or additional computers for further transmission of information at regular intervals.
A large number of studies regarding UV inactivation of microbes have been carried out [107]. In vitro studies demonstrated that UV, especially, UVC irradiation is highly germicidal [107]. Animal studies showed UVC irradiation significant reduced bacterial burden in mouse wounds [107]. The results from the animal studies were endorsed by clinical studies, which indicated that UVC irradiation efficiently eradicated pathogenic microbes in infected chronic wounds [121,122] and toenails [107]. One of the concern of using UV irradiation is its genotoxic effect on host cells. However, UV irradiation should be an appropriate approach for the disinfection of environment and equipment.
3.4. Other Light-Based Anti-Infective Approaches
2.4.1. Dual Wavelength Irradiation Antimicrobial Treatment
A dual-wavelength irradiation system for endodontic treatment is described in patent WO2014116659A1 [123]. In the system, a special fluid is placed within a root canal. The fluid absorbs irradiation of a first wavelength between 1,500 and 3,000 nm, and is transparent to irradiation of a second wavelength between 700 and 1,500 nm. Irradiation of the first wavelength is applied inside a pulp chamber just above the root canal, or at a depth inside the fluid-filled canal. The irradiation at the first wavelength is in short pulses with the durations ranging from 1 ns to 1 ms and the pulse energy within 1 mJ to 600 mJ. The irradiation of the first wavelength induces pressure waves in the fluid. These pressure waves may be generated at a single frequency or mixed frequency ranging from the audible range, 20 Hz, to ultrasound and up to 20 MHz. The pressure waves may also include a shockwave, which is a pressure wave traveling at or faster than the speed of sound in a fluid medium. The pressure waves eradicate bacteria by damaging the cell membrane and facilitate the removal of soft tissue and smear layer. Bacteria damaged by the effects of the first wavelength are more susceptible to antimicrobial treatments, including chemical and thermal methods. Irradiation of the second wavelength is applied inside the pulp chamber just above the root canal, or at a depth inside the fluid-filled canal, in long pulses with the durations ranging from 1 ns to 1 s and an average power ranging from 1 mW to 10 W. The irradiation of the second wavelength causes thermal disinfection. The combined irradiation of the first and the second wavelength enables a synergistic effect, where the pressure waves resulting from the irradiation of the first wavelength increase the efficacy of the thermal disinfection resulting from the irradiation of the second wavelength.
Another duel-wavelength irradiation system using two distinct near-infrared wavelengths ranging from 865–875 nm and 925–935 nm is reported in patent US20080021370A1 [124]. The purpose of the invention is to destruct bacteria off-site and on-site and, more particularly, destruct bacteria in vivo in medical, dental, and veterinary surgical sites, as well as other sites in biological or related systems. Because of the low absorption of near infrared energy in water, the penetration of near infrared irradiation in biological tissue is far greater than that of far infrared wavelengths. Therefore, with near infrared diode lasers, heat deposition is much deeper in biological tissue and more therapeutic and beneficial in eradicating bacterial infections. During the procedure, a significant temperature increase should preferably occur for a given period of time in the infected site, and subsequently bacteria are killed by photothermolysis. With traditional near infrared diode optical energy, bacteria can be killed selectively without causing irreversible heat-induced damage to host tissues and cells. The laser combination, which emits the two wavelengths either alternately, continuously, intermittently, or simultaneously, preferably incorporates at least one ultra-short pulse laser oscillator that is composed of titanium-doped sapphire.
2.4.2. Selective Inactivation of Microorganisms with a Femtosecond Laser
Tsen and his collaborators developed a method for selectively inactivating pathogenic microorganisms with a femtosecond pulsed laser (Patent US20100136646A1) [125]. The method employs: (a) a light source emitting a wavelength transparent to water, (b) a process which produces significantly large vibrations on the outer structure of microorganisms through scattering and not via absorption of light, and (c) a process which targets the microorganisms but leaves mammalian cells unharmed. The method accomplishes these goals through proper manipulation and control of the femtosecond pulsed laser via an impulsive stimulated Raman scattering process.
2.4.3. Laser-Induced Shockwave for Treatment of Infected Wounds
An invention using laser-induced shockwave for treatment of infected wounds is described by patent WO2014089552A1 [126]. The system includes the methods that apply a nanosecond pulsed laser energy to an infected wound, e.g., a wound infected with biofilm, pre-treated with nano-encapsulated time-released antibiotic-containing compounds and covered with a laser absorbing composition. The laser-induced shockwave breaks up the bacterial biofilm while simultaneously stimulating antibiotic penetration of the biofilm. In addition, the methodological parameters can be manipulated so that the shockwave stimulates the generation of collagen and other growth factors in the host cells, and subsequently helps facilitate wound healing. Depending upon the severity of the infection, the process can be repeated multiple times. Similarly, the process can also be tailored to facilitate wound debridement.
CONCLUSION
The widespread growth of antibiotic resistance among pathogenic microorganisms has forced researchers and scientists to search for alternative antimicrobial methods that do not rely on traditional antibiotics. The field of light-based therapeutics has made significant progress in this battle to eliminate antibiotic-resistant infections. Many of the common methods and devices include using aBL, aPDT, and germicidal UV irradiation, while dual-wavelength infrared irradiation, femtosecond laser irradiation and laser-generated shockwaves have been implemented in other inventions. Among the three principle light-based antimicrobial approaches, UVC and aBL are simpler to operate than is aPDT, as no additional PS is required. UVC is much more efficient than aBL in inactivating microorganisms. aBL, however, is thought to have the advantage of fewer side effects on mammalian cells.
Forty-five patents were obtained in our search: 9 patents for the antimicrobial blue light approach, 21 for antimicrobial photodynamic therapy, 11 for UV irradiation, and lastly 4 for other light-based anti-infective approaches. The treatments and devices discussed in the review above are interestingly enough able to be used in various different functions and settings, such as dental applications, certain diseases in the eye, skin and hard surface cleansing, decontamination of internal organs such as the stomach, decontamination of apparel and equipment, elimination of bacteria from buildings and rooms, etc. Most of the devices and inventions introduce methods of destroying bacteria and fungi without harming human cells and tissues. This suggests that light-based therapy has an advantage over traditional antibiotics, and it can be concluded that light-based antimicrobial approaches hold great promise for the future in regards to eliminating antibiotic-resistant infections and diseases.
Table 3.
Recent Patents on Germicidal Ultraviolet Irradiation.
| Inventors | Patent No. | Title |
|---|---|---|
| Robert G. Johnson | US20070099154A1 [108] | Method of treating dental patients with ultraviolet C range light |
| Anant Sharma | US9474811B2 [109] | Method of treating an eye infection using electromagnetic radiation in the UVC |
| William E. Cumbie, Douglas B. Juanarena | US20090143842A1 [111] | A process for the eradication of Helicobacter pylori |
| Joachim C. Arnold, Rainer Kuth, Karl-Heinz Maier | DE102010010763A1 [112] | Sterilization system with ultraviolet emitter for eradicating biological contaminants |
| Jeffery L. Deal, Philip J. Ufkes | US20120126134A1 [114] | Decontamination apparatus and method |
| Patrick Flaherty, Bruce L. Winkler, Robert J. Gold | US20120223216A1 [115] | Ultraviolet light applicator system and method |
| Roderick M. Dayton | US8710460 [116] | Therapeutic radiation device |
| Kevin McGuire | US9095704 [117] | Hard-surface disinfection system |
| Russell J. Redmond, Claude Vidal | US20080065175A1 [118] | Method and apparatus for the disinfection or sterilization of medical apparel and accessories |
| Waldemar J.Lyslo, Mark H.Schwartz, Stephen B. Pettis | US20110215261A1[119] | Hard-Surface Disinfection System |
| Naresh Sunkara, Ricardo R. Garcia, Nikhil S. Naikal, Jaya Sunkara | US9162001 [120] | Method and apparatus for the disinfection or sterilization of medical apparel and accessories |
Acknowledgments
Research in TD laboratory is supported by the National Institutes of Health (R21AI109172 and R01AI123312), Department of Defense (FA9550-16-1-0479 and FA9550-17-1-0277), Center for Integration of Medicine and Innovative Technology ((#13-1021, #13-1033, and #14-1894), Orthopaedic Trauma Association (#16 and #181), Airlift Research Foundation (#109421), American Society for Laser Medicine & Surgery (BS.F04.14), Executive Committee On Research at Massachusetts General Hospital, and Smith Infectious Diseases Foundation. We are grateful to Tayyaba Hasan, PhD, at The Wellman Center for her co-mentorship of Yanyan Fang.
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
References
- 1.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (Accessed on: October 12, 2017)
- 2.Hampton T. Report reveals scope of US antibiotic resistance threat. JAMA. 2013;310(16):1661–3. doi: 10.1001/jama.2013.280695. [DOI] [PubMed] [Google Scholar]
- 3.Cowen LE, Sanglard D, Howard SJ, Rogers PD, Perlin DS. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb Perspect Med. 2014;5(7):a019752. doi: 10.1101/cshperspect.a019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP, Hamblin MR. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat. 2012;15(4):223–36. doi: 10.1016/j.drup.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramakrishnan P, Maclean M, MacGregor SJ, Anderson JG, Grant MH. Differential sensitivity of osteoblasts and bacterial pathogens to 405-nm light highlighting potential for decontamination applications in orthopedic surgery. J Biomed Opt. 2014;19(10):105001. doi: 10.1117/1.JBO.19.10.105001. [DOI] [PubMed] [Google Scholar]
- 6.McDonald RS, Gupta S, Maclean M, Ramakrishnan P, Anderson JG, MacGregor SJ, et al. 405 nm Light exposure of osteoblasts and inactivation of bacterial isolates from arthroplasty patients: potential for new disinfection applications? Eur Cell Mater. 2013;25:204–14. doi: 10.22203/ecm.v025a15. [DOI] [PubMed] [Google Scholar]
- 7.Kleinpenning MM, Smits T, Frunt MH, van Erp PE, van de Kerkhof PC, Gerritsen RM. Clinical and histological effects of blue light on normal skin. Photodermatol Photoimmunol Photomed. 2010;26:16–21. doi: 10.1111/j.1600-0781.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 8.Feuerstein O, Weiss EI, Davidi MP. US20060085052A1. Method and means for exerting a phototoxic effect of visible light on microorganisms. 2006
- 9.Anderson JG, Maclean M, Woolsey GA, Macgregor SJ. US9039966. Inactivation of gram-positive bacteria. 2015
- 10.Zhou B. US20120310307. Treatment of fungal infection by light irradiation. 2012
- 11.Banowski B, Garnich F, Simmering R. DE102013202122. Device for performing deodorizing medical treatment e.g. Acne treatment, of human skin, has dosing device including source of radiation for creation and emission of electromagnetic radiation with wavelength between specific ranges. 2014
- 12.Banowski B, Garnich F, Simmering R. DE102012224183. Device for cosmetic and/or medical treatment such as antimicrobial treatment of human skin, has actuating device which is provided for adjusting radiation intensity, and control unit is provided for controlling emission of radiation. 2014
- 13.Kanno T, Nakamura K, Niwano Y, Kanno M. WO2014136255 A1. Periimplantitis therapy device. 2014
- 14.Goodson MJ, Soukos NI. US8021148. ntraoral light-emitting device. 2011
- 15.Binner C, Reddy M. US8186997. Method for cleaning the oral cavity. 2012
- 16.Krespi Y, Kacker A. US20100076526. Control of halitosis-generating and other microorganisms in the non-dental upper respiratory tract. 2010
- 17.Zhang Y, Zhu Y, Chen J, Wang Y, Sherwood ME, Murray CK, et al. Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence. 2016;7:536–45. doi: 10.1080/21505594.2016.1155015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, et al. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. J Infect Dis. 2014;209:1963–71. doi: 10.1093/infdis/jit842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai T, Gupta A, Huang YY, Sherwood ME, Murray CK, Vrahas MS, et al. Blue light eliminates community-acquired methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomed Laser Surg. 2013;31:531–8. doi: 10.1089/pho.2012.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai T, Gupta A, Huang YY, Yin R, Murray CK, Vrahas MS, et al. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother. 2013;57:1238–45. doi: 10.1128/AAC.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amin RM, Bhayana B, Hamblin MR, Dai T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg Med. 2016;48:562–8. doi: 10.1002/lsm.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K, Yamada Y, Ikai H, Kanno T, Sasaki K, Niwano Y. Bactericidal action of photoirradiated gallic acid via reactive oxygen species formation. J Agric Food Chem. 2012;60:10048–54. doi: 10.1021/jf303177p. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Ishiyama K, Sheng H, Ikai H, Kanno T, Niwano Y. Bactericidal activity and mechanism of photoirradiated polyphenols against Gram-positive and -negative bacteria. J Agric Food Chem. 2015;63:7707–13. doi: 10.1021/jf5058588. [DOI] [PubMed] [Google Scholar]
- 24.Neelakantan P, Cheng CQ, Ravichandran V, Mao T, Sriraman P, Sridharan S, et al. Photoactivation of curcumin and sodium hypochlorite to enhance antibiofilm efficacy in root canal dentin. Photodiagnosis Photodyn Ther. 2015;12:108–14. doi: 10.1016/j.pdpdt.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Diao WR, Hu QP, Feng SS, Li WQ, Xu JG. Chemical composition and antibacterial activity of the essential oil from green huajiao (Zanthoxylum schinifolium) against selected foodborne pathogens. J Agric Food Chem. 2013;61:6044–9. doi: 10.1021/jf4007856. [DOI] [PubMed] [Google Scholar]
- 26.Fila G, Kawiak A, Grinholc MS. Blue light treatment of Pseudomonas aeruginosa: Strong bactericidal activity, synergism with antibiotics and inactivation of virulence factors. Virulence. 2016:1–21. doi: 10.1080/21505594.2016.1250995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasukawa H, Konno N, Haneda Y, Yamamori B, Iseki M, Shibusawa M, et al. Photomanipulation of antibiotic susceptibility and biofilm formation of Escherichia coli heterologously expressing photoactivated adenylyl cyclase. J Gen Appl Microbiol. 2012;58:183–90. doi: 10.2323/jgam.58.183. [DOI] [PubMed] [Google Scholar]
- 28.Moorhead S, Maclean M, Coia JE, MacGregor SJ, Anderson JG. Synergistic efficacy of 405 nm light and chlorinated disinfectants for the enhanced decontamination of Clostridium difficile spores. Anaerobe. 2016;37:72–7. doi: 10.1016/j.anaerobe.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Feuerstein O, Moreinos D, Steinberg D. Synergic antibacterial effect between visible light and hydrogen peroxide on Streptococcus mutans. J Antimicrob Chemother. 2006;57:872–6. doi: 10.1093/jac/dkl070. [DOI] [PubMed] [Google Scholar]
- 30.Lagori G, Fornaini C, Rocca JP, Merigo E. Use of photo-Fenton’s reaction by 400-nm LED light for endodontic disinfection: A preliminary in vitro study on Enterococcus faecalis. J Photochem Photobiol B, Biology. 2017;171:85–9. doi: 10.1016/j.jphotobiol.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 31.Soukos NS, Stultz J, Abernethy AD, Goodson JM. Phototargeting human periodontal pathogens in vivo. Lasers Med Sci. 2015;30:943–52. doi: 10.1007/s10103-013-1497-9. [DOI] [PubMed] [Google Scholar]
- 32.Genina EA, Titorenko VA, Belikov AV, Bashkatov AN, Tuchin VV. Adjunctive dental therapy via tooth plaque reduction and gingivitis treatment by blue light-emitting diodes tooth brushing. J Biomed Opt. 2015;20:128004. doi: 10.1117/1.JBO.20.12.128004. [DOI] [PubMed] [Google Scholar]
- 33.Kanno T, Nakamura K, Niwano Y, Kanno M. CA2904102 A1. Periimplantitis therapy device. 2013
- 34.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections–state of the art. Photodiagnosis Photodyn Ther. 2009;6:170–88. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin R, Dai T, Avci P, Jorge AES, de Melo WCMA, Vecchio D, et al. Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr Opin Pharmacol. 2013;13:731–62. doi: 10.1016/j.coph.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sellera FP, Nascimento CL, Ribeiro MS. Photodynamic therapy in veterinary medicine: From basics to clinical practice. Springer International Publishing; Cham: 2016. [Google Scholar]
- 37.Mohr H, Lambrecht B, Selz A. Photodynamic virus inactivation of blood components. Immunol Invest. 1995;24:73–85. doi: 10.3109/08820139509062763. [DOI] [PubMed] [Google Scholar]
- 38.Rywkin S, Ben-Hur E, Malik Z, Prince AM, Li YS, Kenney ME, et al. New phthalocyanines for photodynamic virus inactivation in red blood cell concentrates. Photochem Photobiol. 1994;60:165–70. doi: 10.1111/j.1751-1097.1994.tb05085.x. [DOI] [PubMed] [Google Scholar]
- 39.Lavie G, Mazur Y, Lavie D, Prince AM, Pascual D, Liebes L, et al. Hypericin as an inactivator of infectious viruses in blood components. Transfusion. 1995;35:392–400. doi: 10.1046/j.1537-2995.1995.35595259149.x. [DOI] [PubMed] [Google Scholar]
- 40.Santus R, Grellier P, Schrevel J, Maziere JC, Stoltz JF. Photode-contamination of blood components: advantages and drawbacks. Clin Hemorheol Microcirc. 1998;18:299–308. [PubMed] [Google Scholar]
- 41.Allen CM, Weber JM, van Lier JE. Sulfophthalocyanines for photodynamic inactivation of viruses in blood products: effect of structural modifications. Photochem Photobiol. 1995;62:184–9. doi: 10.1111/j.1751-1097.1995.tb05256.x. [DOI] [PubMed] [Google Scholar]
- 42.Zupan K, Egyeki M, Toth K, Fekete A, Herenyi L, Modos K, et al. Comparison of the efficiency and the specificity of DNA-bound and free cationic porphyrin in photodynamic virus inactivation. J Photochem Photobiol B, Biology. 2008;90:105–12. doi: 10.1016/j.jphotobiol.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Dos Santos LFM, Melo NB, de Carli ML, Mendes A, Bani G, Verinaud LM, et al. Photodynamic inactivation of Paracoccidioides brasiliensis helps the outcome of oral paracoccidiodomycosis. Lasers Med Sci. 2017;32:921–30. doi: 10.1007/s10103-017-2193-y. [DOI] [PubMed] [Google Scholar]
- 44.Freire F, Ferraresi C, Jorge AO, Hamblin MR. Photodynamic therapy of oral Candida infection in a mouse model. J Photochem Photobiol B, Biology. 2016;159:161–8. doi: 10.1016/j.jphotobiol.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dos Reis JA, Jr, Dos Santos JN, Barreto BS, de Assis PN, Almeida PF, Pinheiro AL. Photodynamic Antimicrobial Chemotherapy (PACT) in osteomyelitis induced by Staphylococcus aureus: Microbiological and histological study. J Photochem Photobiol B, Biology. 2015;149:235–42. doi: 10.1016/j.jphotobiol.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 46.dos Reis JA, Jr, de Carvalho FB, Trindade RF, de Assis PN, de Almeida PF, Pinheiro AL. A new preclinical approach for treating chronic osteomyelitis induced by Staphylococcus aureus: in vitro and in vivo study on photodynamic antimicrobial therapy (PAmT) Lasers Med Sci. 2014;29:789–95. doi: 10.1007/s10103-013-1422-2. [DOI] [PubMed] [Google Scholar]
- 47.Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. Photodynamic therapy for infections: clinical applications. Lasers Surg Med. 2011;43:755–67. doi: 10.1002/lsm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossi R, Bruscino N, Ricceri F, Grazzini M, Dindelli M, Lotti T. Photodynamic treatment for viral infections of the skin. Giornale italiano di dermatologia e venereologia: organo ufficiale, Societa italiana di dermatologia e sifilografia. 2009;144:79–83. [PubMed] [Google Scholar]
- 49.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother. 2005;49:2822–7. doi: 10.1128/AAC.49.7.2822-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wainwright M. Phenothiazinium salts as antimicrobial photosensitizing agents. In: Hamblin MR, Jori G, Hader DP, editors. Photodynamic inactivation of microbial pathogens: Medical and environmental applications. Cambridge: The Royal Society of Chemistry; 2011. pp. 19–43. [Google Scholar]
- 51.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chemistry & Biology. 2005;12:1127–35. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caruso E, Banfi S, Barbieri P, Leva B, Orlandi VT. Synthesis and antibacterial activity of novel cationic BODIPY photosensitizers. J Photochem Photobiol B, Biology. 2012;114:44–51. doi: 10.1016/j.jphotobiol.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Lu L, Rininsland FH, Wittenburg SK, Achyuthan KE, McBranch DW, Whitten DG. Biocidal activity of a light-absorbing fluorescent conjugated polyelectrolyte. Langmuir. 2005;21:10154–9. doi: 10.1021/la046987q. [DOI] [PubMed] [Google Scholar]
- 54.Li K, Zhang Y-Y, Jiang G-Y, Hou Y-J, Zhang B-W, Zhou Q-X, et al. A bivalent cationic dye enabling selective photo-inactivation against Gram-negative bacteria. Chem Commun. 2015;51:7923–6. doi: 10.1039/c5cc00174a. [DOI] [PubMed] [Google Scholar]
- 55.Späth A, Leibl C, Cieplik F, Lehner K, Regensburger J, Hiller K-A, et al. Improving photodynamic inactivation of bacteria in dentistry: Highly effective and fast killing of oral key pathogens with novel tooth-colored Type-II photosensitizers. J Med Chem. 2014;57:5157–68. doi: 10.1021/jm4019492. [DOI] [PubMed] [Google Scholar]
- 56.Rossoni RD, Junqueira JC, Santos ELS, Costa ACB, Jorge AOC. Comparison of the efficacy of Rose Bengal and erythrosin in photodynamic therapy against Enterobacteriaceae. Lasers Med Sci. 2010;25:581–6. doi: 10.1007/s10103-010-0765-1. [DOI] [PubMed] [Google Scholar]
- 57.Paterok P. US20120310310. Materials and accessories for the application of antibacterial photodynamic therapy. 2012
- 58.Soukos NS, Stashenko PP. US20150030989. Handheld device for delivering photodynamic therapy. 2015
- 59.Wescott A, Teng Y, Zheng T, Zhang X, Foster TH. US20160059031. Catheter/stent system for activation of photodynamic therapy within the catheter/stent system. 2016
- 60.Paterok P. EP2528658 B1. Device for use in antibacterial photodynamic therapy. 2015 inventor.
- 61.Wieland GD, Albrecht V, Voepel K-H, Gitter B. EP2459248 A2. A novel method for microbial depletion in human blood and blood products using antimicrobial photodynamic therapy. 2012
- 62.Lin Z, Zhou X, Liu M, Yu L, Wu X, Xu M. CN103610464 A. Method and device for carrying out oral-cavity photodynamics therapy on patient suffering from periodontitis through LED. 2014
- 63.Wang S, Yuan H, Liu L. CN201210234471. A medical composition which consists of a photosensitizer and a chemical activator for the treatment of tumors and pathogenic bacteria infection. 2014
- 64.Graefe S, Nifantiev N, Albrecht V, Neuberger W, Scheglmann D, Gerhard W, et al. WO2010129337 A2. New oral formulations for tetrapyrrole derivatives. 2010
- 65.Nonell MS, Sanchez GD, Borrell BJI, Agut BM, Ragas AX. WO2012001194 A1. Cation derivatives of 2,7,12,17-aryl porphycenes, preparation method thereof and use of same as photosensitisers in antimicrobial photodynamic therapy. 2012
- 66.Lin F. CN103143015 B. Application of photosensitizer for treating acne. 2015
- 67.Fedele R, Comuzzi C, Rossi G, Goi D. US8940775 B2. Use of derivatives of pentaphyrine as antimicrobial and desinfectant agents. 2015
- 68.Dei D, Roncucci G, Soldaini G, Nistri D, Chiti G, Municchi M, et al. US20140163218 A1. Novel phthalocyanine derivatives for therapeutic use. 2014
- 69.Liu T, Zhao Z, Guo J. CN103601727 A. Use of novel amine compound modified protoporphyrin. 2014
- 70.Jones GW, Tatarets AL, Patsenker LD. US9572881 B2. Halogenated compounds for photodynamic therapy. 2017
- 71.Shu C, Guan M, Wang C. CN103724356 B. One kind of fullerene porphyrin derivatives and preparation method and application photosensitizer. 2015
- 72.Zhang P, Hu B, Tang H. US20170071210 A1. Silver nanoparticle-enhanced photosensitizers. 2017
- 73.Mandel A. US9737565 B2. Metal-glycoprotein complexes and their use as chemotherapeutic compounds. 2017
- 74.Vecchio D, Hamblin MR, Huang Y, Huang L, Landi G, Gelfand G. WO2016081594 A1. Système et procédé pour protocole photodynamique. 2016
- 75.Tang B, Zhao E. WO2016078603 A1. Aie luminogens for bacteria imaging, killing, photodynamic therapy and antibiotics screening, and their methods of manufacturing. 2016
- 76.Garner GV. WO2016039812 A1. An aqueous composition (PurePurge) can be utilized for the disinfection and sterilization of materials, commodities and surfaces contaminated with micro-organisms. 2016
- 77.Mcfarland SA. US9737565 B2. Metal-based coordination complexes as photodynamic compounds and their use. 2017
- 78.Brevet D, Hocine O, Durand J-o, Maillard P, Morere A, Garcia M, et al. US8425879 B2. Metalloporphyrin derivatives, nanoparticles comprising the same, and use thereof for photodynamic therapy. 2013
- 79.Albrecht V, Wiehe A, Roeder B, Neuberger W. US20050281777 A1. Photo-triggered release of active substances from dendrimer-photosensitizer complexes. 2005
- 80.Hasan T, Hamblin MR, Soukos N. US6462070 B1. Photosensitizer conjugates for pathogen targeting. 2002
- 81.Hasan T, Gross J, Nau GJ. US6977075 B2. Photosensitizer conjugates for targeting intracellular pathogens. 2005
- 82.Ginsburg V, Krivan HC, Roberts DD. WO1991006007 A1. Diagnostic kit and diagnostic method utilizing carbohydrate receptors. 1991
- 83.Nifantiev N. US20030176326 A1. Photosensitzers for photodynamic therapy of microbial infections. 2003
- 84.Aicher D, Albrecht V, Gitter B, Stark CBW, Wiehe A. US9308185 B2. Glyco-substituted dihydroxychlorins and ß-functionalized chlorins for anti-microbial photodynamic therapy. 2016
- 85.Krivan HC, Blomberg ALI. US5466681 A. Receptor conjugates for targeting penicillin antibiotics to bacteria. 1995
- 86.Aicher D, Albrecht V, Gitter B, Stark CBW, Wiehe A. WO2013015774 A1. Glyco-substituted dihydroxy-chlorins and beta-functionalized chlorins for anti-microbial photodynamic therapy. 2012
- 87.Grafe S, Gebhardt P, Albrecht V. US20040186087 A1. Siderophore conjugates of photo-active dyes for photodynamic therapy. 2004
- 88.Nolan EM, Chairatana P, Raffatellu M, Sassone Corsi M, Perez-lopez A. US20160022794 A1. Siderophore-based immunization against Gram-negative bacteria. 2016 doi: 10.1073/pnas.1606290113. [DOI] [PMC free article] [PubMed]
- 89.Miller MJ, Ji C, Miller PA. EP2986617 A1. Anti-bacterial siderophore-aminopenicillin conjugates. 2016
- 90.Broenstrup M, Hu H, Sergeev G. EP2987792 A1. 14,7,10-Tetrazacyclododecane based agents to target bacteria and its use. 2016
- 91.Zhu C, Yang Q, Liu L, Lv F, Li S, Yang G, et al. Adv Mater. Vol. 23. Deerfield Beach, Fla: 2011. Multifunctional cationic poly(p-phenylene vinylene) polyelectrolytes for selective recognition, imaging, and killing of bacteria over mammalian cells; pp. 4805–10. [DOI] [PubMed] [Google Scholar]
- 92.Fang Y, Liu T, Zou Q, Zhao Y, Wu F. Cationic benzylidene cyclopentanone photosensitizers for selective photodynamic inactivation of bacteria over mammalian cells. RSC Adv. 2015;5:56067–74. [Google Scholar]
- 93.Benhamou RI, Shaul P, Herzog IM, Fridman M. Di-N-Methylation of anti-Gram-positive aminoglycoside-derived membrane disruptors improve antimicrobial potency and Broadens spectrum to Gram-negative bacteria. Angew Chem Int Ed Engl. 2015;54:13617–21. doi: 10.1002/anie.201506814. [DOI] [PubMed] [Google Scholar]
- 94.Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, et al. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg Med. 2006;38:468–81. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 95.Friedrich CL, Moyles D, Beveridge TJ, Hancock REW. Antibacterial action of structurally diverse cationic peptides on Gram-positive bacteria. Antimicrob Agents Chemother. 2000;44:2086–92. doi: 10.1128/aac.44.8.2086-2092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nikaido H. Prevention of drug access to bacterial targets: Permeability barriers and active efflux. Science. 1994;264:382–8. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 97.Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974;235:109–29. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- 98.Fang Y, Liu T, Zou Q, Zhao Y, Wu F. Water-soluble benzylidene cyclopentanone based photosensitizers for in vitro and in vivo antimicrobial photodynamic therapy. Sci Rep. 2016;6:28357. doi: 10.1038/srep28357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sperandio FF, Huang Y-Y, Hamblin MR. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat Antiinfect Drug Discov. 2013;8:108–20. doi: 10.2174/1574891x113089990012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hamblin MR, O’Donnell DA, Murthy N, Rajagopalan K, Michaud N, Sherwood ME, et al. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49:941–51. doi: 10.1093/jac/dkf053. [DOI] [PubMed] [Google Scholar]
- 101.Tegos GP, Anbe M, Yang C, Demidova TN, Satti M, Mroz P, et al. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob Agents Chemother. 2006;50:1402–10. doi: 10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem Photobiol. 1992;55:89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 103.Dai T, Tegos GP, Lu Z, Huang L, Zhiyentayev T, Franklin MJ, et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009;53:3929–34. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.An JS, Kim JE, Lee DH, Kim BY, Cho S, Kwon IH, et al. 0.5% Liposome-encapsulated 5-aminolevulinic acid (ALA) photodynamic therapy for acne treatment. J Cosmet Laser Ther. 2011;13:28–32. doi: 10.3109/14764172.2011.552613. [DOI] [PubMed] [Google Scholar]
- 105.Braun A, Dehn C, Krause F, Jepsen S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. J Clin Periodontol. 2008;35:877–84. doi: 10.1111/j.1600-051X.2008.01303.x. [DOI] [PubMed] [Google Scholar]
- 106.Sigusch BW, Engelbrecht M, Volpel A, Holletschke A, Pfister W, Schutze J. Full-mouth antimicrobial photodynamic therapy in Fusobacterium nucleatum-infected periodontitis patients. J Periodontol. 2010;81:975–81. doi: 10.1902/jop.2010.090246. [DOI] [PubMed] [Google Scholar]
- 107.Dai T, Vrahas MS, Murray CK, Hamblin MR. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev Anti Infect Ther. 2012;10:185–95. doi: 10.1586/eri.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnson RG. US20070099154 A1. Method of treating dental patients with ultraviolet C range light. 2007
- 109.Sharma A. US9474811 B2. assignee Method of treating an eye infection using electromagnetic radiation in the UVC. 2016 inventor.
- 110.Dungan TE. EP0829272 A1. Radiation device for therapeutic use. 1998
- 111.Cumbie WE, Juanarena DB. WO2009059270 A1. Phototherapy treatment and device for infections, diseases, and disorders. 2009
- 112.Arnold JC, Kuth R, Maier KH. DE102010010763 A1. Verfahren zur Eradikation von Helicobacter pylori. 2011
- 113.Flaherty P, Winkler BLM, Gold RJ. US20120223216 A1. Sterilization system with ultraviolet emitter for eradicating biological contaminants. 2012
- 114.Deal JL, Ufkes PJ. US20120126134. Ultraviolet disinfection device and method. 2012
- 115.Flaherty P, Winkler BL, Gold RJ. US20120223216 A1. Sterilization system with ultraviolet emitter for eradicating biological contaminants. 2012
- 116.Dayton RM. US9675721 B2. Decontamination apparatus and method. 2016
- 117.Mcguire K. US9095704 B2. Ultraviolet light applicator system and method. 2015
- 118.Redmond RJ, Vidal C. US20080065175 A1. Therapeutic radiation device. 2008
- 119.Lyslo WJ, Schwartz MH, Pettis SB. US20110215261 A1. Hard-surface disinfection system. 2011
- 120.Sunkara N, Garcia RR, Naikal NS, Sunkara J. US9522201 B2. Method and apparatus for the disinfection or sterilization of medical apparel and accessories. 2015
- 121.Thai TP, Keast DH, Campbell KE, Woodbury MG, Houghton PE. Effect of ultraviolet light C on bacterial colonization in chronic wounds. Ostomy Wound Manage. 2005;51:32–45. [PubMed] [Google Scholar]
- 122.Thai TP, Houghton PE, Campbell KE, Woodbury MG. Ultraviolet light C in the treatment of chronic wounds with MRSA: a case study. Ostomy Wound Manage. 2002;48:52–60. [PubMed] [Google Scholar]
- 123.Boutoussov D, Sivriver A, Netchiailo V. US20140205965 A1. Dual wavelength endodontic treatment. 2014
- 124.Bornstein E. US20080159345 A1. Near infrared microbial elimination laser system. 2008
- 125.Tsen KT, Tsen SWD, Kiang JG. US20100136646 A1. Selective inactivation of microorganisms with a femtosecond laser. 2010
- 126.Haake DA, Bhupathy V, Navarro A, Grundfest WS, Beenhouwer DO, Gupta V, et al. WO2014089552 A1. Laser-based bacterial disruption for treatment of infected wounds. 2014


