Abstract
The structural integrity of platelet receptors is essential for platelets to play the normal hemostatic function. The high non-physiological shear stress (NPSS) commonly exists in blood contacting medical devices and has been shown to cause platelet receptor shedding. The loss of platelet receptors may impair the normal hemostatic function of platelets. The aim of this study was to quantify NPSS-induced shedding of three key receptors on the platelet surface. Human blood was subjected to the matrix of well-defined shear stresses and exposure times, generated by using a custom-designed blood shearing device. The expression of three key platelet receptors, glycoprotein (GP) Ibα, GPVI and GPIIb/IIIa in sheared blood was quantified using flow cytometry. The quantitative relationship between the loss of each of the three receptors on the platelet surface and shear condition (shear stress level and exposure time) was explored. It was found that these relationships followed well the power law functional form. The coefficients of the power law models for the shear-induced shedding of these platelet receptors were derived with coefficients of determination (R2) of 0.77, 0.73, and 0.78, respectively. The power law models with these coefficients may be potentially used to predict the shear-induced platelet receptor shedding of human blood.
Keywords: Shear stress, platelet adhesion receptors, shear-induced platelet receptor shedding, power law model
INTRODUCTION
Cardiovascular disease, lung disease and chronic renal disease affect millions of Americans [1–3]. Blood contacting medical devices (BCMDs), such as ventricular assist devices (VADs), extracorporeal membrane oxygenation (ECMO), cardiopulmonary bypass, hemodialyzers and mechanical heart valves have been commonly used to treat these diseases or to replace dysfunctional organs resulted from these diseases. The benefits with use of these devices are clearly demonstrated in clinical practice. Unfortunately, the clinical use of BCMDs is accompanied with serious adverse complications, especially thrombosis and bleeding [4–6].
Regions with high non-physiological shear stress (NPSS) often exist in BCMDs. For example, the level of shear stress at the blade tip gap of the impeller in rotary VADs is well above 100 Pascal (Pa) and even reaches 400 Pa [7, 8]. It has been demonstrated that this level of shear stress could induce a variety of damage to blood [9–11]. Platelets have long been considered as the unique cells, which mediate physiologic hemostasis and play a critical role in pathologic thrombosis [12, 13]. Platelet dysfunction will affect normal hemostasis, which may lead thrombosis or bleeding. Over the last three decades the effects of shear stress on platelet activation and aggregation have been extensively investigated [14–17]. The activation and aggregation of platelets can increase the risk of thrombotic complications. Brown et al. [18] observed that a very low shear stress level of 5 Pa resulted in the liberation of small amount of ATP, ADP and serotonin and subsequent platelet aggregation. Pathological shear stress level of 31.5 Pa, as encountered in severe atherosclerotic arteries, activated platelets and triggered platelet microparticle generation [14]. In our previous study, it had been found that NPSS (>100 Pa) even with very short exposure time (<1s) can induce platelet activation [11, 19, 20]. All the above studies focused on shear-induced platelet activation. Recently, it has been demonstrated that the elevated shear stress can also cause platelet receptor shedding [21–23]. The receptor shedding is a process of the extracellular proteolysis of transmembrane receptors at a position close to the extracellular surface of cells. This process generates a membrane-associated remnant fragment and releases a soluble ectodomain fragment. These ectodomain fragments retain the unique sequence of the receptors for which agonists bind to. The loss of these ectodomain fragments could modify the responses of platelets to agonists and stimulus, and affect the hemostatic function of platelets. There are three adhesive receptors (GPIbα, GPVI and GPIIb/IIIa) on the platelet surface, which are important for hemostasis. The binding of GPIbα with von Willebrand factor (VWF), GPVI with collagen and GPIIb/IIIa with fibrinogen and VWF can lead to platelet activation, adhesion and aggregation. If these receptors are shed from the platelet surface, the capacity of platelets for normal hemostasis could be compromised, increasing the risk of bleeding. Hu et al. [24] compared two groups (bleeding and non-bleeding) of patients supported with continuous-flow VADs, and found that bleeding was related to the shedding of the platelet GPIbα. Chen et al. [21] showed that the shedding of platelet receptors (GPIbα and GPVI) induced by NPSS could affect platelet aggregation. Other research groups also reported that the shedding of the platelet receptor GPVI was associated with bleeding [25, 26].
Since shear-induced platelet receptor shedding (SIPRS) will lead to platelet dysfunction and affect normal hemostasis, it is critical to consider both shear-induced platelet activation and SIPRS during the development of BCMDs. However, no study has been conducted to quantify the relationship between the platelet receptor shedding and the levels of NPSS and exposure time. This study aimed to quantitatively characterize the shear-induced shedding of three key platelet receptors (GPIbα, GPVI and GPIIb/IIIa) under various levels of NPSS (from 35 to 350 Pa) with short exposure time (from 0.1 to 1.5 sec). These ranges of shear stress and exposure time represent the most common shear situations which platelets would experience in BCMDs [7, 8, 27]. The quantitative relationships between shear-induced shedding of these receptors and shear stress/exposure time were derived.
MATERIALS AND METHODS
Blood-shearing device
A centrifugal flow-through Couette-type blood shearing device whose rotor was magnetically suspended with bearingless motor technology was used in this study. This device can generate unique shearing conditions (NPSS and short exposure time) similar to those observed in BCMDs. A narrow gap with a uniform width of 150 μm and a length of 2.5 mm was created between the rotor and the housing in the shearing device (Figure 1). The magnetically suspended rotor can be rotated between 500 and 5000 rpm. The shear stress generated by this device ranges from 21 to 212 Pa for a blood viscosity of 0.0036 Pa·s. But the level of shear stress can increase up to 350 Pa when the blood viscosity is higher at 0.0046 Pa·s [9]. The details of the design features and the operational principles of this device can be found in the reference [9]. Figure 1 depicts the whole blood shearing system used in this study. In this system, the syringe pump (PHD 2000, Harvard Apparatus, Holliston, MA) was used to push the blood to flow through the narrow gap in the shearing device.
Figure 1.
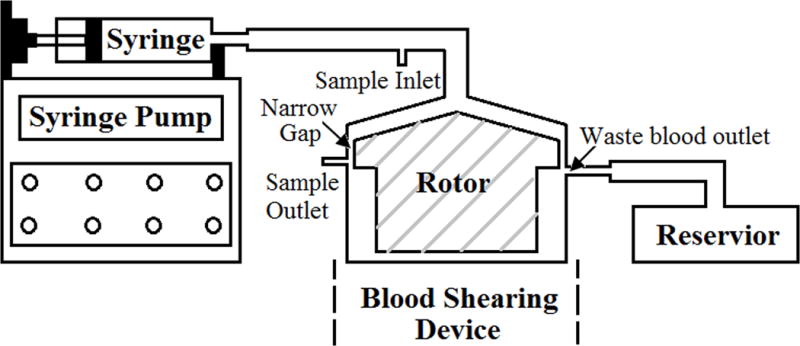
The diagram of the blood shearing system, including a syringe pump used to control the flow rate, 140ml syringe, blood shearing device (Hemolyzer-L) with 150μm narrow gap between the inner rotor and the outer housing, sample inlet, sample outlet, reservoir and tubing.
Experimental procedure
Fresh human blood was collected from 20 healthy adult donors (18 men and 2 women, 22 to 28 years old) who didn’t take any antiplatelet medication within two weeks prior to their scheduled blood donation. All the donors were informed the purpose of the study and gave informed consent. The procedures involving the blood collection were approved by the Institutional Review Board (IRB) for human subject research. For each donor, 250 ml fresh blood was collected by antecubital vena puncture and mixed with sodium citrate (9:1) within a blood bag.
Blood viscosity was measured using a semi-micro viscometer (Cannon Instrument Company, State College, PA). The required rotating speed for the rotor of the shearing device to achieve the desired shear stress was calculated based on the measured blood viscosity. The flow rate through the device was calculated according to the desired exposure time in the narrow gap of the shearing device. The uniform experimental design method was applied to choose the experimental conditions [28]. A total of 47 combinations of shear stress and exposure time were selected as the experimental conditions. The shearing experiment under each experimental condition was repeated at least 3 times. Experiments were performed in a random order on different days with different donor’s blood. The blood shearing experiments under nine experimental conditions could be performed with the 250 ml blood collected from a single donor. In each experiment, a sheared blood sample was collected from the sample outlet of the shearing device while a baseline blood sample was collected from the sample inlet (Figure 1). The nine experiments were completed within 2 hours after the blood collection.
Flow cytometry assays for quantifying the platelet receptor shedding
To quantify the shedding of the platelet GPIbα receptor in the sheared blood, platelets were stained with phycoerythrin (PE) conjugated anti-CD41 antibody (CD41-PE) (BioLegend, San Diego, CA) for identifying the platelet population and fluorescein isothiocyanate (FITC) conjugated anti-CD42b antibody (CD42b-FITC) (BioLegend, San Diego, CA) for determining the expression of GPIbα receptors in each platelet. FITC-labeled IgG1K antibody (IgG1K-FITC) was used for the negative control for CD42b. After the blood shearing experiment, the collected baseline and sheared blood samples were spun at 160 g for 15 min at 20°C for getting platelet-rich plasma (PRP). The PRP (5 μl) was incubated with 25 μl HEPES buffer mixed with 5 μl CD41-PE antibody and 5 μl CD42b-FITC antibody or 5 μl IgG1K-FITC antibody at room temperature in the dark for 30 min.
For the GPVI shedding, CD41-PE antibody, eFluor 660-labeled anti-human GPVI antibody (GPVI-eFluor 660) (eBioscience, San Diego, CA) and eFluor 660 conjugated mouse IgG1K Isotype (IgG1K-eFluor 660) were used for platelet population identification, determination of GPVI receptors on each platelet and negative control for the GPVI, respectively. The PRP (5 μl) was incubated with 25 μl HEPES buffer mixed with 5 μl CD41-PE antibody and 5 μl GPVI-eFluor 660 antibody or 5μl IgG1K-eFluor 660.
For the GPIIb/IIIa shedding, FITC conjugated anti-CD41 antibody (CD41-FITC), PerCP/Cy5.5 anti-human CD41/61 antibody (CD41/61-PerCP/Cy5.5) and PerCP/Cy5.5 Mouse IgG2a, κ Isotype antibody (IgG2ak- PerCP/Cy5.5) (BioLegend, San Diego, CA) were used for platelet population identification, determination of GPIIb/IIIa receptors on each platelet and negative control for the GPIIb/IIIa, respectively. The PRP (5 μl) was incubated with 25 μl HEPES buffer mixed with 5 μl CD41-FITC antibody and 5 μl CD41/61-PerCP/Cy5.5 antibody or 5 μl IgG2ak- PerCP/Cy5.5.
After the above labeling and incubation steps, the stained PRP samples were fixed with 1 ml 1% paraformaldehyde (PFA) for 30 min at 4 °C in the dark. The flow cytometry data for the expression of the three platelet receptors were collected with a BD™ LSR II flow cytometer (BD Bioscience, San Jose, CA) and analyzed with the FCS Express software (De Novo Software, Los Angeles, CA). In the flow cytometry data, the fluorescence intensity produced by the fluorescent-labeled antibodies bund to their specific receptors on each platelet is proportional to the number of the functional receptors. The mean fluorescence intensity (MFI) in the fluorescence channel for each of the three platelet receptors was used to represent the number of the GPIbα, GPVI and GPIIb/IIIa receptors on the platelets in the baseline and sheared blood samples.
Data analysis
In each experiment, all the MFI data were normalized with that of the baseline sample. All the data were presented in the form of mean ± standard error (n=3). Then, the experimental data were linearized with a log-scale transformation. After that, a multivariate linear regression analysis was performed to fit the power law model with the linearized data for each receptor. F-test was implemented to test the overall significance of the regression analysis. Coefficient of determination was employed to indicate the goodness of fit for model coefficients. For the representative data, statistical differences were performed with the Student’s t-test. Statistical significance was set at P < 0.05.
RESULTS
To establish the quantitative relationships between shear-induced loss of the platelet GPIbα, GPVI and GPIIb/IIIa receptors and applied high shear stress/exposure time, the power law model was used for the multivariate regression to fit the experimental data. Figures 2A, 2B and 2C exhibit the measured data points (green dots) for the shear-induced loss of the GPIbα, GPVI and GPIIb/IIIa receptors on the platelet surface under the 47 combinations of shear stress and exposure time. The mesh surfaces in the plots are generated from the fitted power law relationships between the loss of the three platelet receptors and applied shear stress/exposure time. The losses of the three platelet receptors (GPIbα, GPVI and GPIIb/IIIa) on the platelet surface under a shear condition of 300 Pa for 1 sec could be estimated at 35%, 40% and 34% from the power law model with their respective coefficients and were marked as a black dot in Figures 2A, 2B and 2C, respectively. As shown in these plots, the loss of the platelet GPIbα, GPVI and GPIIb/IIIa receptors increases with increasing level of shear stress and exposure time. Table 1 lists the three coefficients in the power law model of SIPRS for GPIbα, GPVI and GPIIb/IIIa and the coefficients of determination (R2) of fitting the experimental data with the power law models. The F values for the regression analysis were calculated by transforming the experimental data into log-scale and estimated to be 73.7, 59.5 and 82.5 for GPIbα, GPVI, and GPIIb/IIIa, respectively. These values were above the F-test acceptance criterion (F (2, 45) 0.01= 5.11). Therefore, the linearized models through log-scale transformations for SIPRS of GPIbα, GPVI and GPIIb/IIIa can be accepted. The coefficients of determination (R2) for the power law models of SIPRS for GPIbα, GPVI and GPIIb/IIIa were 0.77, 0.73 and 0.79, respectively. Thus, the power law model with the specific coefficients for each of the three platelet receptors does provide a significantly good prediction of SIPRS. The general power law model is expressed as:
Figure 2.
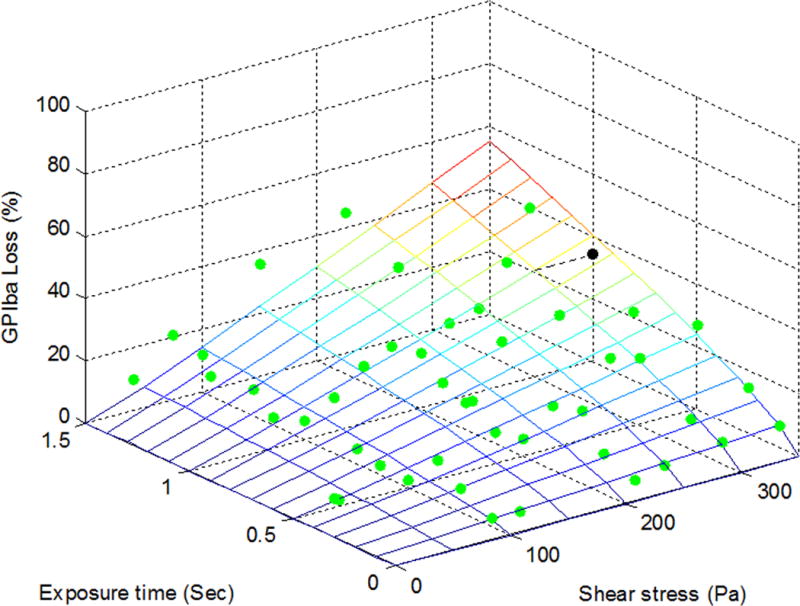
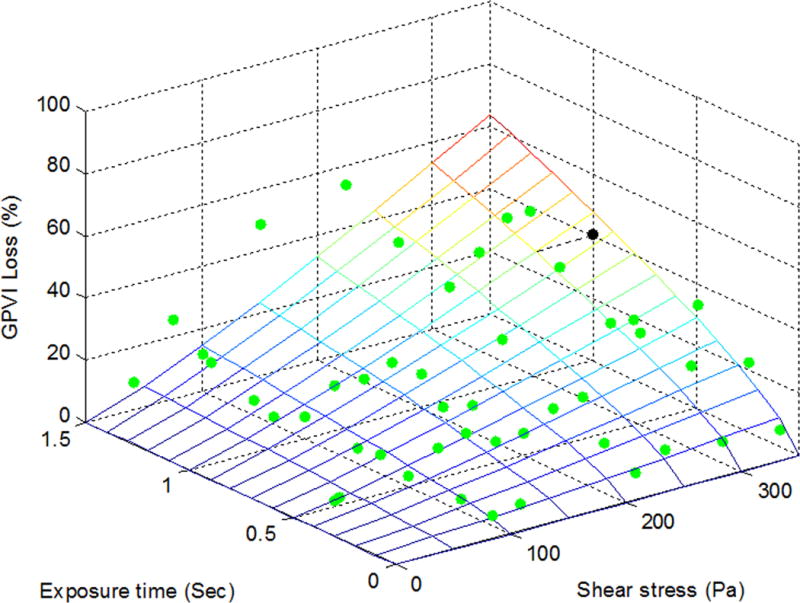
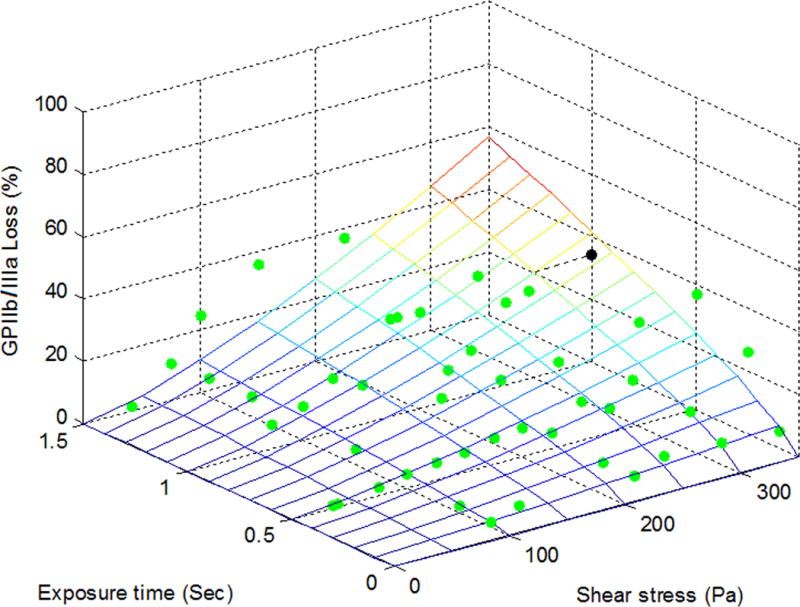
The power law representation of the loss of the shear-induced platelet (A) GPIbα, (B) GPVI and (C) GPIIb/IIIa receptors with experimental data points (green dots) under the 47 experimental conditions. The loss of the receptors is expressed as the MFI ratio of platelets in the sheared blood samples relative to the baseline blood sample.
Table 1.
Parameters of the power law models for shear-induced platelet receptor shedding of GPIbα, GPVI and GPIIb/IIIa (S=A×τα×tβ)
| Receptor | A | α | β | Coefficient (R2) | F statistic |
|---|---|---|---|---|---|
| GPIbα | 0.000934 | 1.037801 | 0.817553 | 0.77 | 73.7 |
| GPVI | 0.000503 | 1.170681 | 0.716478 | 0.73 | 59.5 |
| GPIIb/IIIa | 0.000147 | 1.356991 | 0.765028 | 0.79 | 82.5 |
Where S is the loss percentage of the platelet surface receptors (GPIbα, GPVI and GPIIb/IIIa), τ is shear stress, and t is exposure time. The values of A, α and β for GPIbα, GPVI and GPIIb/IIIa can be found in Table 1 respectively. These coefficients can be used to estimate the SIPRS for GPIbα, GPVI and GPIIb/IIIa.
The representative SIPRS under four experimental conditions were presented in Figure 3. The MFI values for the platelet receptors, GPIbα, GPVI and GPIIb/IIIa, from the sheared blood samples under four shear conditions were plotted compared with those of the baseline blood sample, respectively. The MFI values for the platelet GPIbα and GPVI receptors decreased significantly after the blood was subjected to the shear stresses of 100 Pa and 250 Pa for 0.5 sec and 1.0 sec compared to those of the baseline blood sample. The MFI value for the platelet GPIIb/IIIa receptor also decreased in the sheared blood samples compared to that of the baseline sample. However, the reduction became significant only when the blood was exposed to the shear stress of 250 Pa. The lower MFI values indicate that there was a reduction in the number of the functional GPIbα, GPVI and GPIIb/IIIa receptors on the platelets in the sheared blood samples. This reduction increased with the elevated level of high shear stress and the duration of exposure time.
Figure 3.
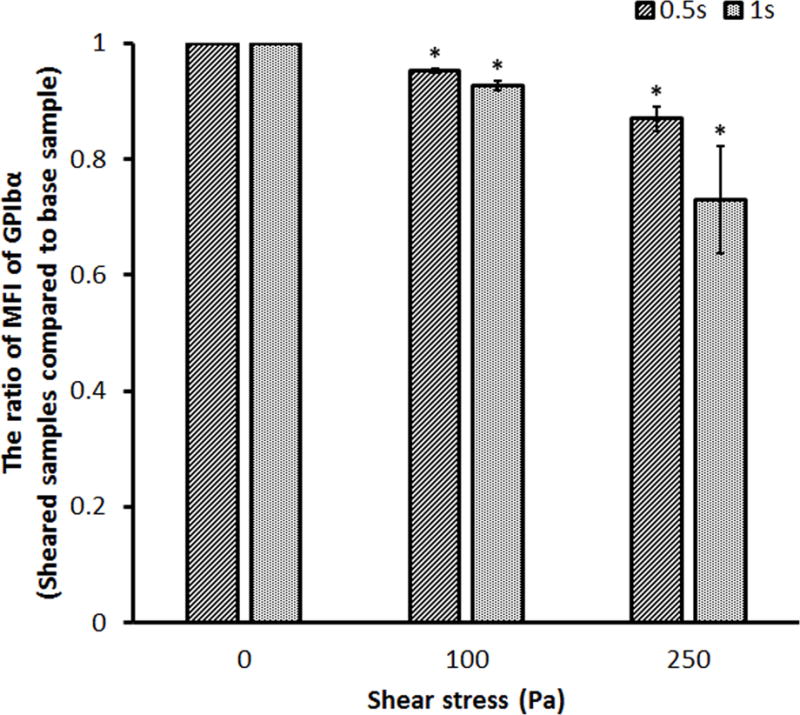
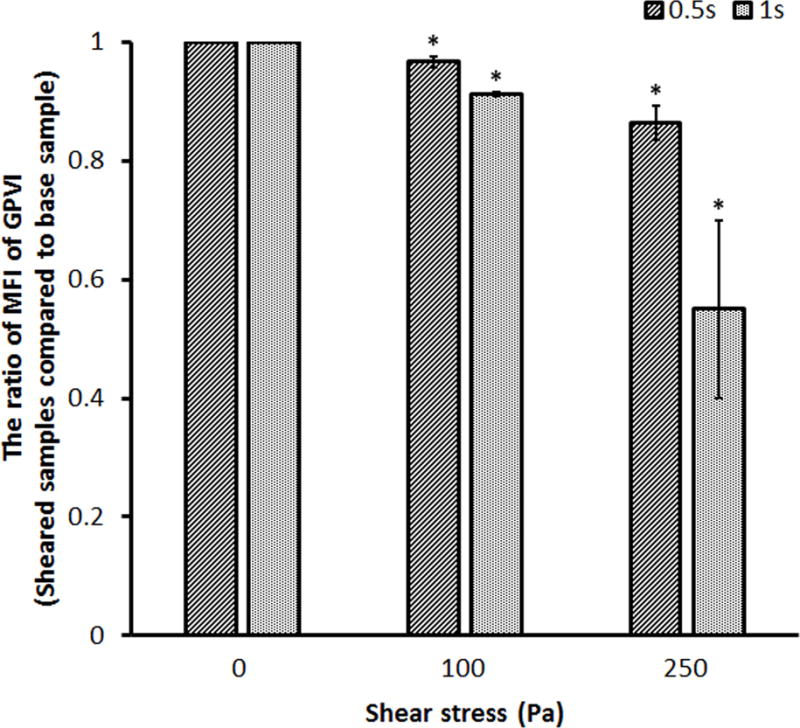
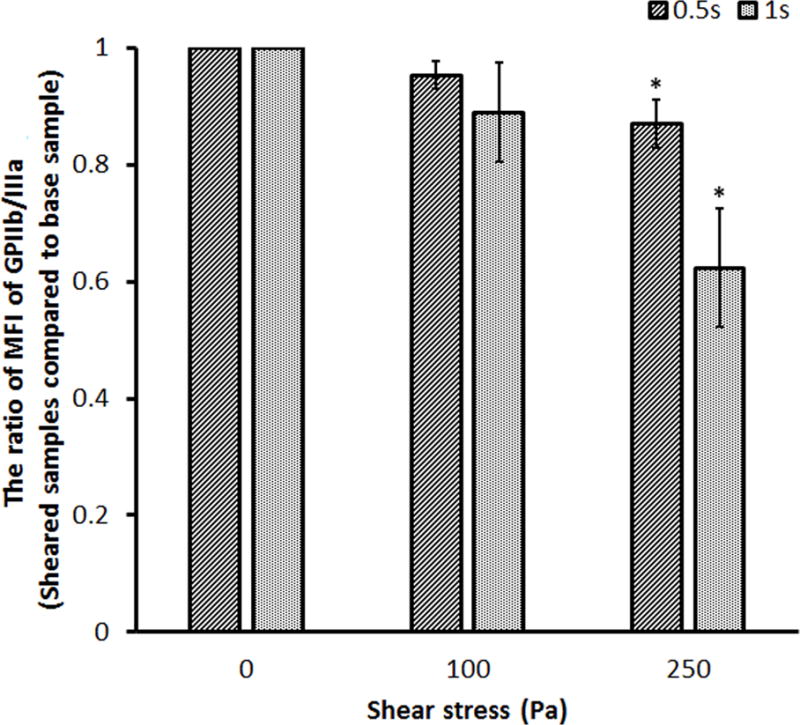
Representative mean fluorescence intensity (MFI) ratios for the platelet (A) GPIbα, (B) GPVI and (C) GPIIb/IIIa receptors in the sheared blood samples relative to the baseline blood sample (0Pa/0s and 0Pa/0s) under four shear conditions (100Pa/0.5s, 100Pa/1s, 250Pa/0.5s and 250Pa/1s).
DISCUSSION
Regions with NPSS often exist in contemporary BCMDs [7, 27]. Many studies have documented that the NPSS can cause damage to blood cells. In these previous studies, the focus and attention had been placed on shear-induced red blood cell damage (hemolysis) and platelet activation. Quantitative relationships between hemolysis and shear stress/exposure time had been established to evaluate blood trauma induced by BCMDs [9, 29]. The NPSS can also induce platelet structural damage, especially SIPRS. Previous in-vitro studies had showed that the NPSS can induce the shedding of platelet receptors [19, 21]. Lukito et al. [30] reported that the soluble GPVI level in plasma significantly elevated in patients on VAD or ECMO support. The surface expression levels of the platelet GPIbα and GPVI in these patients were significantly lower compared with healthy donors. The loss of the adhesion receptors on the platelet surface can affect platelet normal hemostatic function and increase the risk of bleeding [21–23]. Thus, it is important to consider the SIPRS during BCMD development.
To quantify the SIPRS, the blood from 20 healthy donors was exposed to a range of elevated shear stress (35 to 350Pa) for short exposure time (0.1 to 1.5s). The results showed that NPSS with short exposure time (<1s) could cause the shedding of the three key platelet receptors (GPIbα, GPVI and GPIIb/IIIa). The power law model had previously been used to describe the relationship of blood cell damage and shear stress/exposure time. For example, Giersiepen et al. [31] and Zhang et al. [9] used the power law model to relate hemolysis to shear stress and exposure time. Ding et al. [20] used the power law model to describe the relationship between the percentage of activated platelets indicated by surface P-selectin expression and shear stress/exposure time. These previous studies showed that the power law model could be useful to describe the relationship between shear-induced blood trauma and shear stress/exposure time. Therefore, we utilized the power law model to quantitatively relate the SIPRS to shear stress/exposure time and to derive the respective coefficients for the platelet GPIbα, GPVI and GPIIb/IIIa receptors. The coefficients of determination and the F-Test values for fitting the experimental data to the model indicated that the power law model was a good fit.
The shear stress and exposure time within BCMDs can be obtained by using computational fluid dynamics (CFD) modeling. The power law model and coefficients for the SIPRS can be integrated into the CFD modeling to estimate the platelet receptor shedding induced by the NPSS in a BCMD. Through the CFD modeling, areas that potentially induce severe platelet receptor shedding can be identified within BCMDs and then mitigated through the design iteration.
There are some limitations in the present study. First, the exposure time for blood subjected to NPSS to clinical BCMDs may be shorter or longer than what we used in this study. For example, the exposure time in oxygenators can be 2 to 3 seconds. Thus, the power law model for the SIPRS built in this study may be more appropriate for blood pumps or mechanical heart valves. Second, the blood samples were collected from healthy donors. The vulnerability of blood to SIPRS for these healthy donors might be different from that of patients with cardiovascular disease or other diseases involving blood cells.
CONCLUSIONS
The shear-induced shedding of three critical platelet receptors (GPIbα, GPVI and GPIIb/IIIa) was investigated. The shedding increased with increasing shear stress level and exposure time. The relationships between the loss of the platelet receptors (GPIbα, GPVI and GPIIb/IIIa) and shear stress/exposure time followed well with the power law functional form. The coefficients of the power law model for the shear-induced shedding of the platelet receptors (GPIbα, GPVI and GPIIb/IIIa) were derived with the coefficients of determination of 0.77, 0.73 and 0.79, respectively. The power law model can be used to estimate SIPRS in HF patients implanted with BCMDs and to guide the design optimization of BCMDs.
Acknowledgments
Sources of Finding: This work was partially supported by the National Institutes of Health (Grant number: R01HL 124170).
Footnotes
Conflict of Interest: All authors have no conflicts of interest with the work in the manuscript.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the american heart association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Center for Chronic Disease Prevention and Health Promotion. www.cdc.gov/ accessed on January 20, 2014.
- 3.National Kidney Foundation. The Facts About Chronic Kidney Disease. New York, NY: 2012. [Google Scholar]
- 4.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125:3038–47. doi: 10.1161/CIRCULATIONAHA.111.040246. [DOI] [PubMed] [Google Scholar]
- 5.Hering D, Piper C, Bergemann R, et al. Thromboembolic and bleeding complications following St. Jude Medical valve replacement: results of the German Experience With Low-Intensity Anticoagulation Study. Chest. 2005;127(1):53–9. doi: 10.1378/chest.127.1.53. [DOI] [PubMed] [Google Scholar]
- 6.Stulak JM, Lee D, Haft JW, et al. Gastrointestinal bleeding and subsequent risk of thromboembolic events during support with a left ventricular assist device. J Heart Lung Transplant. 2014;33(1):60–4. doi: 10.1016/j.healun.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Fraser KH, Zhang T, Taskin ME, Griffith BP, Wu ZJ. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. Journal of biomechanical engineering. 2012;134:081002. doi: 10.1115/1.4007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JB, Wood HG, Allaire PE, McDaniel JC, Olsen DB, Bearnson G. Numerical Studies of blood shear and washing in a continuous flow ventricular assist device. ASAIO J. 2000;46(4):486–94. doi: 10.1097/00002480-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T, Taskin ME, Fang HB, et al. Study of flow-induced hemolysis using novel Couette-type blood-shearing devices. Artif Organs. 2011;35(12):1180–6. doi: 10.1111/j.1525-1594.2011.01243.x. [DOI] [PubMed] [Google Scholar]
- 10.Nascimbene A, Neelamegham S, Frazier OH, Moake JL, Dong JF. Acquired von Willebrand syndrome associated with left ventricular assist device. Blood. 2016;127(25):3133–41. doi: 10.1182/blood-2015-10-636480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Wu ZJ. Paradoxical Effect of Nonphysiological Shear Stress on Platelets and von Willebrand Factor. Artif Organs. 2016;40(7):659–68. doi: 10.1111/aor.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb Res. 2004;114(5–6):447–53. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Ni H, Freedman J. Platelets in hemostasis and thrombosis: role of integrins and their ligands. Transfus Apher Sci. 2003;28(3):257–64. doi: 10.1016/S1473-0502(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 14.Holme PA, Orvim U, Hamers MJ, et al. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler Thromb Vasc Biol. 1997;17(4):646–53. doi: 10.1161/01.atv.17.4.646. [DOI] [PubMed] [Google Scholar]
- 15.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88(5):1525–1541. [PubMed] [Google Scholar]
- 16.O’Brien JR. Shear-induced platelet aggregation. Lancet. 1990;335(8691):711–3. doi: 10.1016/0140-6736(90)90815-m. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien JR, Salmon GP. An independent haemostatic mechanism: shear induced platelet aggregation. Adv Exp Med Biol. 1990;281:287–96. doi: 10.1007/978-1-4615-3806-6_30. [DOI] [PubMed] [Google Scholar]
- 18.Brown CH, 3rd, Leverett LB, Lewis CW, Alfrey CP, Jr, Hellums JD. Morphological, biochemical, and functional changes in human platelets subjected to shear stress. J Lab Clin Med. 1975;86(3):462–71. [PubMed] [Google Scholar]
- 19.Chen Z, Mondal NK, Ding J, et al. Activation and shedding of platelet glycoprotein IIb/IIIa under non-physiological shear stress. Mol Cell Biochem. 2015;409(1–2):93–101. doi: 10.1007/s11010-015-2515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding J, Chen Z, Niu S, et al. Quantification of Shear-Induced Platelet Activation: High Shear Stresses for Short Exposure Time. Artif Organs. 2015;39(7):576–83. doi: 10.1111/aor.12438. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Mondal NK, Ding J, Gao J, Griffith BP, Wu ZJ. Shear-induced platelet receptor shedding by non-physiological high shear stress with short exposure time: glycoprotein Ibα and glycoprotein VI. Thromb Res. 2015;135(4):692–8. doi: 10.1016/j.thromres.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Tamimi M, Tan CW, Qiao J, et al. Pathologic shear triggers shedding of vascular receptors: a novel mechanism for down-regulation of platelet glycoprotein VI in stenosed coronary vessels. Blood. 2012;119(18):4311–20. doi: 10.1182/blood-2011-10-386607. [DOI] [PubMed] [Google Scholar]
- 23.Cheng H, Yan R, Li S, et al. Shear-induced interaction of platelets with von Willebrand factor results in glycoprotein Ibalpha shedding. Am J Physiol Heart Circ Physiol. 2009;297(6):2128–35. doi: 10.1152/ajpheart.00107.2009. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Mondal NK, Sorensen EN, et al. Platelet glycoprotein Ibα ectodomain shedding and non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2014;33(1):71–9. doi: 10.1016/j.healun.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arthur JF, Dunkley S, Andrews RK. Platelet glycoprotein VI-related clinical defects. Br J Haematol. 2007;139(3):363–72. doi: 10.1111/j.1365-2141.2007.06799.x. [DOI] [PubMed] [Google Scholar]
- 26.Nieswandt B, Schulte V, Bergmeier W, et al. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J Exp Med. 2001;193(4):459–69. doi: 10.1084/jem.193.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song X, Throckmorton AL, Wood HG, Antaki JF, Olsen DB. Quantitative evaluation of blood damage in a centrifugal VAD by computational fluid dynamics. J Fluids Eng. 2004;126:410–8. [Google Scholar]
- 28.Wiens DP. Designs for approximately linear regression: two optimality properties of uniform designs. Statistics & probability letters. 1991;12:217–21. [Google Scholar]
- 29.Boehning F, Mejia T, Schmitz-Rode T, Steinseifer U. Hemolysis in a laminar flow-through Couette shearing device: an experimental study. Artif Organs. 2014;38(9):761–5. doi: 10.1111/aor.12328. [DOI] [PubMed] [Google Scholar]
- 30.Lukito P, Wong A, Jing J, et al. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J Thromb Haemost. 2016;14(11):2253–2260. doi: 10.1111/jth.13497. [DOI] [PubMed] [Google Scholar]
- 31.Giersiepen M, Wurzinger LJ, Opitz R, Reul H. Estimation of shear stress-related blood damage in heart valve prostheses-in vitro comparison of 25 aortic valves. Int J Artif Organs. 1990;13:300–6. [PubMed] [Google Scholar]


