Abstract
Previous reports suggest race/ethnic and sex heterogeneity in the association between the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor gamma (PPARG) gene and cognitive decline. Tests of verbal memory, processing speed, and verbal fluency and a composite global Z-score were used to assess cognitive performance longitudinally in a large (n=11,620) biracial cohort of older adults in the Atherosclerosis Risk in Communities Neurocognitive Study from mid-life to older age. Linear mixed models were used to estimate associations between the Ala12 allele and cognitive performance over 20 years of follow-up. Heterogeneity was present for rate of cognitive decline as measured by the global Z-score by race, sex and Ala12 allele status (p=0.01 for four-way interaction term: race*sex*time*Ala12 carrier status). Stratified analysis showed a significantly increased rate of global cognitive decline over the 20-year follow-up for carriers of the Ala12 allele compared to non-carriers among black males (−0.92 SD decline vs −0.57 SD; p=0.02) but not among black females, white males, or white females. Decline in global cognitive function among black male Ala12 carriers was primarily driven by decline in verbal memory. Our data underscore the context-dependent association between the Pro12Ala polymorphism and cognitive decline, specifically race/ethnic background and sex.
Keywords: aging, cognitive decline, PPARG Pro12Ala polymorphism, longitudinal, race, sex
Introduction
Reports linking metabolic dysfunction to cognitive decline and late-onset Alzheimer’s disease1 have led to the investigation of genes involved in lipid and glucose metabolism as potential susceptibility loci for cognitive decline. The peroxisome proliferator-activated receptor gamma (PPARG) gene plays a key role in lipid metabolism and insulin sensitivity and has been shown in animal models to regulate components of amyloid β metabolism2, a proposed key causative factor in Alzheimer disease. The common Pro12Ala (C→G, rs1801282) polymorphism in the PPARG gene is responsible for a Pro to Ala transition in codon 12.
Because PPARG is a master transcriptional regulator involved in the expression of numerous genes, there is potential for complex interactions between the Pro12Ala polymorphism and other genetic and/or environmental factors on cognitive decline. Two cohort studies among older adults have reported comparable ethnic- and sex-specific findings regarding the association between the Pro12Ala genotype and cognitive performance3, 4. Reports of the association of the Pro12Ala polymorphism with cognitive function that have not considered combined race/ethnic and sex interactions have yielded conflicting or non-significant results5–12.
In the current study, we examined the relationship between Ala12 allele carrier status with cognitive decline in the large, biracial Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) cohort, followed over 20 years from mid-life to older age. We hypothesized a priori that there would be significant interactions between Ala12 carrier status and cognitive decline with race and sex.
Methods
Study population
Participants were enrolled in the Atherosclerosis Risk in Communities Study (ARIC), which is a population-based, prospective cohort study of cardiovascular disease in black and white adults from four U.S. communities: Washington County MD, Forsyth County NC, Jackson MS, and suburban Minneapolis MN. Participants were seen beginning in 1987–1989. Cognitive function was evaluated at visit 2 (1990–1992), visit 4 (1996–1998), and visit 5 (2011–2013) as part of ARIC-NCS. Subsamples of the cohort also underwent cognitive testing at visit 3 (1993–95), and as part of two ancillary studies in 2004–2006 and 2005–2006, providing cognitive data at six time periods over a follow-up of 23 years. Details of the ARIC sampling frame and recruitment have been published elsewhere13.
Baseline for the present analysis was visit 2 when cognitive data were first collected. Of the 14,348 participants who attended visit 2, we excluded participants whose Pro12Ala genotyping was unavailable (n=2,574) and those missing all three standardized cognitive test scores (n=154), giving a final sample size of 11,620 participants at baseline (81% of the visit 2 sample). Compared to those included in this analysis, participants who were excluded were more likely (p<0.05 for each comparison) to be black (39% vs 22%), have prevalent diabetes (18% vs 14%), have a history of stroke (2.5% vs 1.8%), have a higher BMI (28.2 vs 27.9 kg/m2), and have less than a college education (67% vs 62%) but were not significantly different (p >0.05 for each comparison) in age (mean age = 57.1 vs 57.0 years), gender (44.7% vs 44.6% males), waist circumference (98.3 vs 97.9 cm), and APOE ε4 status (32% vs 31%). Local institutional review boards approved the ARIC protocol, and all participants gave informed consent.
Cognitive function tests
Delayed Word Recall (DWRT), Digit Symbol Substitution (DSST), and word fluency (WFT) tests were used to assess cognitive performance and were summarized with a global Z-score. Protocols for the neurocognitive test battery were standardized and administered in the same sequence by trained examiners.
The DWRT is a test of verbal learning and recent memory14. Participants were asked to learn 10 common nouns by using each in a sentence. After a five-minute filled delay, participants had 60 seconds to recall the words. The score for the DWRT is the number of words recalled.
The DSST of the Wechsler Adult Intelligence Scale-Revised15 is a test of executive function and processing speed. Participants were asked to translate numbers to symbols using a key. The score is the count of numbers correctly translated to symbols in 90 seconds.
The WFT is a test of executive function and language. Participants were asked to generate as many words as possible beginning with each of the letters F, A, and S. The WFT score is the total number of words generated across the three letters in 60 seconds16.
To facilitate comparison across cognitive tests, Z-scores standardized to visit 2 were calculated for each test by subtracting each participant’s test score at each visit from the visit 2 mean and dividing by the visit 2 standard deviation. A composite global cognitive Z-score was calculated by averaging the Z-scores of the three tests, and then standardized to visit 2 using the global Z mean and global Z standard deviation from visit 2. Thus, a Z-score of −1 would describe cognitive performance that is 1 standard deviation below the mean score at visit 2.
Pro12Ala genotyping
The Pro12Ala single nucleotide polymorphism in the study participants was genotyped at the Broad Institute (Cambridge, Massachusetts) using the Affymetrix GeneChip SNP Array 6.0® and the Birdseed calling algorithm17. These data were extensively quality-controlled according to accepted standards18, including checks for genetic outliers via average identity-by-state statistics and EIGENSTRAT-generated principal components19. The Pro12Ala (rs1801282) variant was directly measured; we imputed the genotypes for missing values (≤5% of the sample) by exploiting a scaffold containing all variants with ethnic-specific missing rates ≤0.05, minor allele frequencies≥0.01, and Hardy-Weinberg Equilibrium tests >10−5. After pre-phasing with ShapeIt (v1.r532), we employed IMPUTE2 and the 1,000 Genomes haplotypes-Phase I integrated variant set release (v3) reference panel (NCBI Build 37) to impute the missing genotypes20, 21.
Covariates
All covariates used in the regression models were assessed during visit 2 except education, race, and sex, which were assessed during ARIC visit 1. The following covariates were evaluated as confounders: age, education (<high school; high school, high school equivalent, or vocational school; college, graduate, or professional school), waist circumference (cm), history of stroke (yes/no), apolipoprotein E (APOE) ε4 genotype (0/at least 1 ε4 allele), and diabetes (self-reported physician diagnosis, diabetes medication use, or HbA1c ≥ 6.5%).
Statistical Analysis
Participants were dichotomized into those who carried at least one copy of the alanine variant (Ala12+) at rs1801282 and those who did not (Ala12−). Time was modeled as years of follow-up from the baseline visit. Linear mixed models were used to estimate associations between the Ala12 allele category and cognitive change and to account for the within-person correlations of test scores arising from the repeated measures across time. To investigate our primary aim, we tested for effect modification between Ala12 carrier status, race, and sex with cognitive decline by adding interaction terms to the models; specifically, we included a four-way interaction term (race*sex*Ala12 carrier status*time), along with the corresponding three-way and two-way interaction terms, to the model.
We also tested for interactions of Ala12 allele status with education, waist circumference, history of stroke, APOE ε4 genotype, and diabetes in relation to cognitive decline by including interaction terms to the models. Statistical analyses were performed using PC-SAS (version 9.4).
We conducted two sets of sensitivity analyses. First, although visual inspection of a superimposed LOWESS (locally weighted scatterplot smoother) curve that was fitted to the data suggested a linear relationship between cognitive decline and time, we included linear spline terms in the model and, in a separate model, included a quadratic term for time to account for potential nonlinearities. In the second sensitivity analysis, we investigated possible bias in our results due to informative dropout during the study period by incorporating joint modelling of longitudinal and survival data. Thorough methodological explanations of joint models have been previously published22, 23. Briefly, whereas traditional mixed models cannot account for survival outcomes, and survival models often do not account for outcomes with repeated measures, joint models determine the joint association of a risk factor both with survival outcomes and with repeated measurements. Applied to our analysis, such models allow us to make inferences about the association between Ala12 and longitudinal cognitive changes, while also accounting for the possibility that participants’ likelihood of not returning to later ARIC exam visits, due to death or onset of dementia, may be associated with their Ala12 carrier status.
Results
Of the 11,620 participants included in our analysis, 22% (n=2,536) were black and 45% (n=5,185) were male. Baseline age ranged from 46 to 70 years (mean=57 years; SD=5.6). Maximum follow-up was 23.5 years; of the 84.6% participants with at least one visit beyond visit 2, the median follow-up time was 19.2 (interquartile range, 6.0–21.0) years. Black study participants were much less likely to be an Ala12 carrier than white participants (3.7% vs. 22.8%, p<.0001). The Ala12 allele frequency in the black study population was 1.9% and the genotype frequencies were in agreement with Hardy-Weinberg expectations (p = 0.92). The Ala12 frequency among the white sample was 12.3%, however, there was moderate statistical support that the genotype counts differed from the expectations of Hardy-Weinberg equilibrium (p = 0.05).
Among the 915 black males, Ala12 carriers had a significantly larger waist circumference than the non-carriers but there were no differences in age, education, APOE ε4 carrier status, diabetes prevalence, history of stroke, or body mass index at the study baseline (Table 1). Among the 1,621 black females, Ala12 carriers were less likely to have diabetes than Ala12 non-carriers but there were no significant differences in age, education, APOE ε4 carrier status, history of stroke, body mass index, or waist circumference at the study baseline. Among the 4,270 white males or the 4,814 white females, there were no significant differences in baseline characteristics by Ala12 status17.
Table 1.
Baseline characteristics of study participants by race, sex, and PPARG Ala12 allele carrier status.
| Characteristic | Blacks | Whites | ||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||
| Ala12+ | Ala12− | Ala12+ | Ala12− | Ala12+ | Ala12− | Ala12+ | Ala12− | |
| N (%) | 31 (3.4) | 884 (96.6) | 64 (3.9) | 1557 (96.1) | 948 (22.2) | 3322 (77.8) | 1127 (23.4) | 3687 (76.6) |
| Age, mean (SD) | 57.5 (6.5) | 56.4 (5.8) | 54.9 (5.8) | 56.1 (5.6) | 57.5 ( 5.7) | 57.6 ( 5.7) | 57.0 (5.6) | 56.9 (5.7) |
| Education, n (%) | ||||||||
| Some high school or less | 10 (32.3) | 346 (39.2) | 23 (35.9) | 596 (38.4) | 140 (14.8) | 538 (16.2) | 168 (14.9) | 541 (14.7) |
| High school graduate/vocational school | 6 (19.3) | 239 (27.1) | 13 (20.3) | 456 (29.4) | 398 (42.0) | 1296 (39.1) | 567 (50.4) | 1913 (51.9) |
| College/graduate school | 15 (48.4) | 297 (33.7) | 28 (43.8) | 501 (32.2) | 409 (43.2) | 1482 (44.7) | 390 (34.7) | 1231 (33.4) |
| APOE ε4 carrier, n (%) | 9 (32.1) | 366 (42.3) | 24 (38.7) | 599 (39.3) | 254 (27.9) | 904 (28.3) | 301 (28.1) | 976 (27.6) |
| Diabetes, n (%) | 6 (20.7) | 205 (23.4) | 9 (14.1) | 407 (26.4)* | 127 (13.4) | 448 (13.5) | 102 (9.1) | 366 (9.9) |
| History of stroke, n (%) | 1 (3.2) | 29 (3.3) | 2 (3.1) | 40 (2.6) | 21 (2.2) | 63 (1.9) | 13 (1.2) | 38 (1.0) |
| Body mass index, kg/m2 | 30.0 (5.2) | 28.2 (4.9) | 31.5 (6.6) | 31.1 (6.6) | 27.7 (4.1) | 27.7 (4.1) | 27.2 (5.8) | 27.0 (5.5) |
| Waist circumference, cm | 104.2 (15.3) | 99.2 (12.7)* | 103.5 (14.1) | 102.3 (16.4) | 100.8 (10.8) | 100.7 (10.8) | 93.9 (16.0) | 93.6 (15.3) |
P < 0.05 for differences among Ala12 carrier status within the race/sex group.
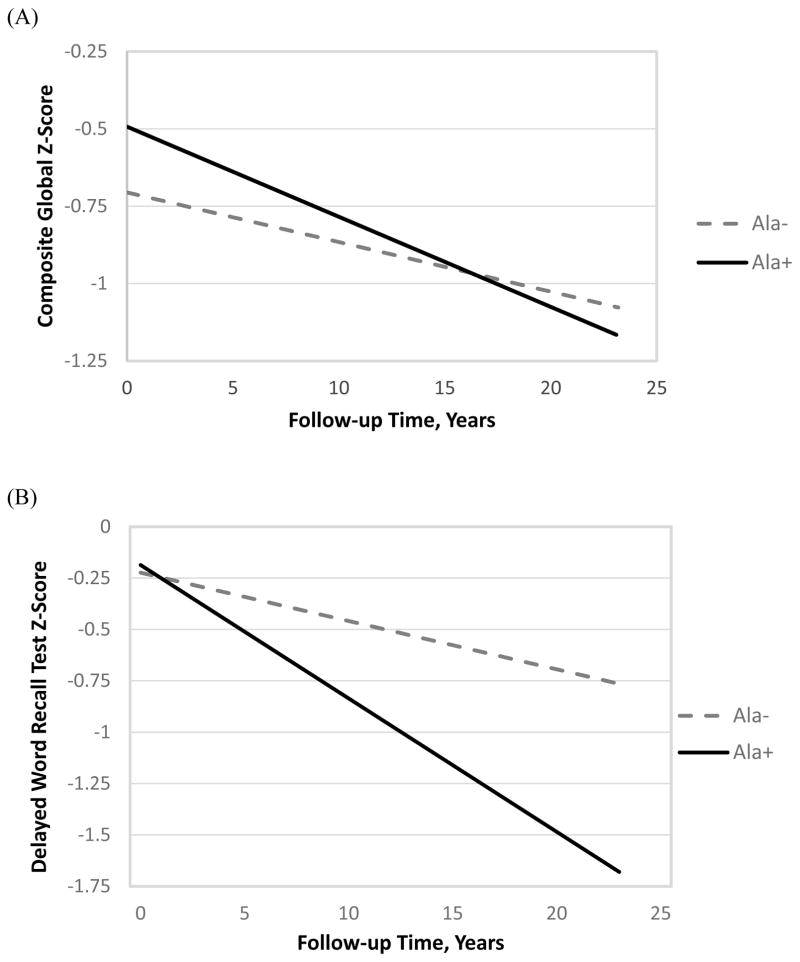
Heterogeneity was present for rate of cognitive decline as measured by changes in the global Z-score among race, sex and Ala12 allele status (p=0.01 for four-way interaction term: race*sex*time*Ala12 carrier status) and for the delayed word recall test (p=0.01 for the four-way interaction term). Table 2 shows the 20-year cognitive decline (Z-score) associated with Ala12 allele carrier status, stratified by race/ethnicity and sex for each cognitive test and adjusted for age and education. Among the black males, there was a significantly greater rate of cognitive decline in the global Z-score among Ala12+ black males compared to Ala12- black males (−0.92 SD decline vs −0.57 SD). The unadjusted rates were also significantly greater among black male Ala12 carriers and non-carriers (Figure 1A). There was no association of Ala12 carrier status with the rate of cognitive decline among black females, white males, or white females for any of the cognitive tests, with or without covariate adjustment.
Table 2.
20-year cognitive decline (Z-score) associated with PPARG Ala12 allele carrier status stratified by the cross-classification of race/ethnicity and sex for each cognitive test.
| Z-score (SE) | |||
|---|---|---|---|
| Cognitive Test/Subgroup | Ala 12+ | Ala12− | P-value for difference in rate of decline |
| Global Z-score | |||
|
| |||
| Black Males | −0.92 (0.17) | −0.57 (0.03) | .02 |
| White Males | −0.67 (0.02) | −0.69 (0.01) | .43 |
| Black Females | −0.49 (0.09) | −0.54 (0.02) | .50 |
| White Females | −0.66 (0.02) | −0.67 (0.01) | .61 |
|
| |||
| Delayed Word Recall | |||
|
| |||
| Black Males | −1.64 (0.32) | −0.95 (0.05) | .04 |
| White Males | −1.05 (0.05) | −1.10 (0.02) | .26 |
| Black Females | −0.73 (0.18) | −0.93 (0.04) | .29 |
| White Females | −0.99 (0.04) | −1.02 (0.02) | .65 |
|
| |||
| Digit Symbol Substitution | |||
|
| |||
| Black Males | −0.60 (0.21) | −0.49 (0.03) | .31 |
| White Males | −0.77 (0.01) | −0.77 (0.02) | .97 |
| Black Females | −0.49 (0.02) | −0.58 (0.10) | .31 |
| White Females | −0.84 (0.01) | −0.84 (0.02) | .86 |
|
| |||
| Word Fluency | |||
|
| |||
| Black Males | −0.24 (0.20) | −0.35 (0.03) | .42 |
| White Males | −0.20 (0.03) | −0.21 (0.01) | .76 |
| Black Females | −0.13 (0.09) | −0.25 (0.02) | .19 |
| White Females | −0.17 (0.02) | −0.18 (0.13) | .74 |
Note: Data are derived from mixed-effects models, adjusted for age and education.
SE, Standard Error.
Figure 1.
Decline in global Z-score (A) and delayed word recall (B) among black males by Ala12 status.
Among the black males, there were no significant interactions between Ala12 carrier status and education, waist circumference, history of stroke, APOE ε4 genotype, or diabetes status on global Z-score over time (p > 0.25 for each test). Because of the reduced sample size in the race- and sex-stratified analyses, we examined the potential confounders and possible mediators of APOE ε4 status, waist circumference, diabetes status, and history of stroke at baseline by adding them individually to the model which included age and education. None of these covariates altered the significantly greater rate of decline for black male Ala12 carriers compared to black male non-carriers. Similar results were also observed for black male Ala12 carriers compared to non-carriers in the DWRT scores (Figure 1B), but not for the DSST or the WFT, with or without adjustment for the potential confounders.
Sensitivity analyses from models with linear spline terms and models that included a quadratic term for time supported the significant association observed in our main analysis between Ala12 carrier status and accelerated decline among black male Ala12 carriers compared to black male non-carriers. The sensitivity analysis using joint models to account for the possibility of differential study dropout between the Ala12 carrier groups also supported the statistically significant association between Ala12 carrier status and accelerated decline in cognitive global Z-scores (p=0.03 for time*Ala12 interaction), and a marginal association in DWRT scores (p=0.07 for time*Ala12 interaction) among black males. There was no statistically significant relationship between Ala12 carrier status and cognitive decline among black females, white males, or white females in the sensitivity analyses.
Discussion
In this cohort of older black and white adults followed for 20 years with repeated cognitive assessments, there was an increased rate of cognitive decline for carriers of the Ala12 allele compared to non-carriers among black males but not among black females or among white males or females. The inclusion of standard risk factors for cognitive impairment and potential mediators in analytic models did not significantly reduce the estimated effect of the Ala12 allele. Black male Ala12 carriers experienced an additional 0.35 Z-score decline (95% CI: −0.62 to −0.08) in global cognition over the 20-year follow-up compared to black males without the allele. This estimated effect was similar to the magnitude estimated for baseline diabetes, to the extent that black males with diabetes at baseline experienced an additional 0.33 Z-score decline (95% CI: −0.46 to −0.19) in global cognition compared to black males without diabetes over the follow-up period.
The observed association of the Ala12 allele with decline in global cognitive function among the black males was primarily driven by decline in verbal memory scores, where there was an additional 0.61 Z-score decline (95% CI: −1.21 to −0.01) for verbal memory for black male Ala12 carriers compared to black male non-carriers.
Other male-restricted associations have been reported with the Pro12Ala polymorphism24–28. Recent studies suggest that genetic variation of many species affects anatomical, physiological, and metabolic traits differently in males and females, suggesting that sex might interact with genotype in a manner similar to other environmental factors29. Sex-specific effects of genes involved in the regulation of lipid and glucose metabolism such as PPARG could be attributed to hormonal differences, such as plasma estrogens. Adipose tissue is also a target for sex steroids and there is evidence to show a bidirectional signaling cross-talk between PPARG and estrogen receptors30. Integrating the evidence suggesting a role for estrogen in the regulation of adipose tissue and glucose metabolism31 with the evidence that PPARG can adapt gene expression to various lipid signals32 provides a potential biologic link to the sex-specific results of our study.
Although a phenotypic trait resulting from a specific genotype may vary between race/ethnic groups because of interactions with environmental and/or other genetic factors, we were unable to identify specific biologic mechanisms that may underlie the race-specific observations in our study.
The substitution of alanine for proline in the Pro12Ala polymorphism has been shown to moderately reduce transcriptional activity of PPARG33. It has been demonstrated in animal studies that PPARG activation promotes amyloid β clearance in the brain2. A reduction in transcription activity could potentially reduce amyloid β clearance and, as a consequence, increase amyloid β peptide deposition, which is a hallmark characteristic of Alzheimer’s dementia.
Although our study is the first, to the best of our knowledge, to investigate the cross-classification of both race and sex among a cohort of older black participants in the association of the Pro12Ala polymorphism with cognitive decline, our findings are consistent with two prior studies that have reported a similar relationship. Among a large cohort of older Mexican-Americans, an increased rate of cognitive impairment was reported only among male carriers of the Ala12 allele compared to male non-carriers (adjusted HR=2.7, 95% CI: 1.4 to 5.2)4. In a bi-ethnic cohort study, there was significantly greater cognitive decline among Mexican-American male Ala12 carriers compared to Mexican-American male non-carriers but decline did not differ between Mexican-American female, white male, or white female carriers and non-carriers3. Our data underscore the context-dependent association between the Pro12Ala polymorphism and cognitive decline, specifically race/ethnic background and sex.
The present study has several strengths including a longitudinal assessment of cognitive status in a large cohort with a long follow-up period. Examination of cognitive decline as our primary outcome as opposed to dementia or cognitive performance assessed cross-sectionally substantially reduces confounding attributable to education34, 35. Further, cognitive decline is a risk factor for incident dementia36 and is a required component of the dementia syndrome37. The sensitivity analysis suggests that our findings appear to be robust to possible bias from differential study dropout. A limitation is that the small numbers of individuals who were homozygous for the Ala12 allele restricted our ability to investigate a dose-response relationship. Selection bias due to lost to follow-up is of potential concern; however, it is known that selection bias occurs when study responses are jointly dependent on exposure and disease outcome. Although follow-up participation may be influenced by cognitive status in our study, it is unlikely that exposure status (Pro12Ala genotype) would influence participation.
In summary, our results indicate a significant association between the Pro12Ala genotype of PPARG and an increased rate of cognitive decline among older black males but not among black females, white males, or white females. The mechanisms underlying these associations will require additional study.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute contracts (grant numbers HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data was collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the National Institutes of Health. The authors thank the staff and participants of the ARIC study for their important contributions.
References
- 1.Chakrabarti S, Khemka VK, Banerjee A, et al. Metabolic Risk Factors of Sporadic Alzheimer’s Disease: Implications in the Pathology, Pathogenesis and Treatment. Aging Dis. 2015;6:282–299. doi: 10.14336/AD.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-gamma-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West NA, Baxter J, Bryant LL, Nelson TL. Cognitive decline and the PPAR-γ Pro12Ala genotype: variation by sex and ethnicity. Age and Ageing. 2017;46:96–100. doi: 10.1093/ageing/afw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West NA, Haan MN, Morgenstern H. The PPAR-gamma Pro12Ala polymorphism and risk of cognitive impairment in a longitudinal study. Neurobiol Aging. 2010;31:741–746. doi: 10.1016/j.neurobiolaging.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton G, Proitsi P, Jehu L, et al. Candidate gene association study of insulin signaling genes and Alzheimer’s disease: evidence for SOS2, PCK1, and PPARgamma as susceptibility loci. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2007;144B:508–516. doi: 10.1002/ajmg.b.30503. [DOI] [PubMed] [Google Scholar]
- 6.Johnson W, Harris SE, Starr JM, et al. PPARG Pro12Ala genotype and risk of cognitive decline in elders? Maybe with diabetes. Neurosci Lett. 2008;434:50–55. doi: 10.1016/j.neulet.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Lee YH, Song GG. COMT Val158Met and PPARgamma Pro12Ala polymorphisms and susceptibility to Alzheimer’s disease: a meta-analysis. Neurol Sci. 2014;35:643–651. doi: 10.1007/s10072-014-1645-4. [DOI] [PubMed] [Google Scholar]
- 8.Scacchi R, Pinto A, Gambina G, et al. The peroxisome proliferator-activated receptor gamma (PPAR-gamma2) Pro12Ala polymorphism is associated with higher risk for Alzheimer’s disease in octogenarians. Brain Res. 2007;1139:1–5. doi: 10.1016/j.brainres.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 9.Yaffe K, Kanaya AM, Lindquist K, et al. PPAR-gamma Pro12Ala genotype and risk of cognitive decline in elders. Neurobiol Aging. 2008;29:78–83. doi: 10.1016/j.neurobiolaging.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao L, Li K, Zhang L, et al. Influence of the Pro12Ala polymorphism of PPAR-gamma on age at onset and sRAGE levels in Alzheimer’s disease. Brain Res. 2009;1291:133–139. doi: 10.1016/j.brainres.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Yue JR, Dong BR, Huang CQ, et al. Pro12Ala polymorphism in PPAR-gamma2 and dementia in Chinese nonagenarians/centenarians. Age (Dordr) 2010;32:397–404. doi: 10.1007/s11357-010-9132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuliani G, Donnorso MP, Bosi C, et al. Plasma 24S-hydroxycholesterol levels in elderly subjects with late onset Alzheimer’s disease or vascular dementia: a case-control study. BMC neurology. 2011;11:121. doi: 10.1186/1471-2377-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 14.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141–145. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 15.Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 16.Benton AL, Hamsher K. Manual of instructions. 2. Iowa City: AJA Associates; 1989. Multilingual Aphasia Examination. [Google Scholar]
- 17.Korn JM, Kuruvilla FG, McCarroll SA, et al. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat Genet. 2008;40:1253–1260. doi: 10.1038/ng.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurie CC, Doheny KF, Mirel DB, et al. Quality control and quality assurance in genotypic data for genome-wide association studies. Genet Epidemiol. 2010;34:591–602. doi: 10.1002/gepi.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 20.Delaneau O, Zagury JF, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods. 2013;10:5–6. doi: 10.1038/nmeth.2307. [DOI] [PubMed] [Google Scholar]
- 21.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little RJA. Modeling the drop-out mechanism in repeated-measures studies. J Am Statist Assoc. 1995;90:1112–1121. [Google Scholar]
- 23.Schluchter MD. Methods for the analysis of informatively censored longitudinal data. Stat Med. 1992;11:1861–1870. doi: 10.1002/sim.4780111408. [DOI] [PubMed] [Google Scholar]
- 24.Ben Ali S, Ben Yahia F, Sediri Y, et al. Gender-specific effect of Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma-2 gene on obesity risk and leptin levels in a Tunisian population. Clin Biochem. 2009;42:1642–1647. doi: 10.1016/j.clinbiochem.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Franck N, Lanne T, Astrand O, et al. Cardiovascular risk factors related to the PPARgamma Pro12Ala polymorphism in patients with type 2 diabetes are gender dependent. Blood Press. 2012;21:122–127. doi: 10.3109/08037051.2011.623349. [DOI] [PubMed] [Google Scholar]
- 26.Mattevi VS, Zembrzuski VM, Hutz MH. Effects of a PPARG gene variant on obesity characteristics in Brazil. Braz J Med Biol Res. 2007;40:927–932. doi: 10.1590/s0100-879x2006005000114. [DOI] [PubMed] [Google Scholar]
- 27.Morini E, Tassi V, Capponi D, et al. Interaction between PPARgamma2 variants and gender on the modulation of body weight. Obesity (Silver Spring) 2008;16:1467–1470. doi: 10.1038/oby.2008.225. [DOI] [PubMed] [Google Scholar]
- 28.Rosmond R, Chagnon M, Bouchard C. The Pro12Ala PPARgamma2 gene missense mutation is associated with obesity and insulin resistance in Swedish middle-aged men. Diabetes Metab Res Rev. 2003;19:159–163. doi: 10.1002/dmrr.371. [DOI] [PubMed] [Google Scholar]
- 29.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong S, Yoon M. 17beta-Estradiol inhibition of PPARgamma-induced adipogenesis and adipocyte-specific gene expression. Acta Pharmacol Sin. 2011;32:230–238. doi: 10.1038/aps.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JH, Cho HT, Kim YJ. The role of estrogen in adipose tissue metabolism: insights into glucose homeostasis regulation. Endocr J. 2014;61:1055–1067. doi: 10.1507/endocrj.ej14-0262. [DOI] [PubMed] [Google Scholar]
- 32.Gelman L, Feige JN, Desvergne B. Molecular basis of selective PPARgamma modulation for the treatment of Type 2 diabetes. Biochim Biophys Acta. 2007;1771:1094–1107. doi: 10.1016/j.bbalip.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20:284–287. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- 34.Gottesman RF, Rawlings AM, Sharrett AR, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol. 2014;179:956–966. doi: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross AL, Mungas DM, Crane PK, et al. Effects of education and race on cognitive decline: An integrative study of generalizability versus study-specific results. Psychol Aging. 2015;30:863–880. doi: 10.1037/pag0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tifratene K, Robert P, Metelkina A, Pradier C, Dartigues JF. Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology. 2015;85:331–338. doi: 10.1212/WNL.0000000000001788. [DOI] [PubMed] [Google Scholar]
- 37.Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med. 2014;30:421–442. doi: 10.1016/j.cger.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]