Abstract
PURPOSE
Geographic atrophy (GA) is a sequelae of macular degeneration. Automated inner retinal analysis using optic coherence tomography (OCT) is flawed because segmentation software is calibrated for normal eyes. The purpose of this study is to determine if ganglion cell layer (GCL) volume is reduced in GA using manual analysis.
METHODS
Nineteen eyes with subfoveal GA and 22 controls were selected for morphometric analyses. Heidelberg scanning laser ophthalmoscope OCT images of the optic nerve and macula were obtained, and the Viewing module was used to manually calibrate retinal layer segmentation. Retinal layer volumes in the central 3 mm and surrounding 6 mm diameter were measured. Linear mixed models were used for statistics.
RESULTS
The GCL volume in the central 3 mm of the macula is less (p = 0.003) and retinal nerve fiber layer (RNFL) volume is more (p=0.02) in GA patients when compared to controls. GCL volume positively correlated with ONL volume (p=0.020).
CONCLUSIONS
GA patients have a small significant loss of the GCL. Ganglion cell death may precede axonal loss, and increased macular RNFL volumes are not indicative of GCL volume. Residual ganglion cell stimulation by interneurons may enable vision in GA patients.
Keywords: age-related macular degeneration, ganglion cell, ganglion cell complex, geographic atrophy, manual segmentation, optical coherence tomography, segmentation errors
Introduction
Geographic atrophy (GA) is a highly prevalent, devastating end-stage result of long standing age-related macular degeneration (AMD) characterized by loss of the retinal pigment epithelium and retinal photoreceptors.1 Histopathologic studies have reported an additional loss of ganglion cells when significant outer nuclear layer (ONL) thinning is present.2 On spectral domain-optical coherence tomography (SD-OCT), ganglion cell deterioration can be grossly tracked by measuring ganglion cell volume with automated semi-quantitative methods.3 Individual retinal layers have been studied on the Cirrus machine (Carl Zeiss Meditec Inc) in GA with automated segmentation, confirming decreased ganglion cell thicknesses.4 On the Cirrus, macular ganglion cell and inner plexiform layer measurements have been made using automated segmentation in patients with and without glaucoma, but ganglion cell layer volume has not been isolated in the macula.5,6 However, SD-OCT image analysis automated segmentation software is error-prone and non-repeatable in eyes with retinal pathology.7
Thus far, only automated segmentation algorithms have been used to evaluate the macular GCL and retinal nerve fiber layer (RNFL) volumes in patients with GA. This study specially utilized manual correction of automatic segmentation in Heidelberg SD-OCT images by retinal specialists to reduce computational errors. Our GA patients mainly have disease affecting the central 3 mm of the macula, so we decided to look at the inner circle of the ETDRS map and use the 3–6 mm circle volumes as an internal control to see if the findings in the affected areas of GA are different than those in the area without this pathology.
We expect that loss of the GCL would be associated with pseudoexpansion of the RNFL, as previously reported,8 and we decided to evaluate the entire macula to assess for local (3mm) involvement using the 3–6 mm unaffected zones as a negative control. It is important to know if there is damage to the inner retina to know if replacing the RPE and/or photoreceptor layer can restore vision in patients with GA. With a small hypothesized decrease in macular GCL volume, we do not expect to see a significant decrease in circumpapillary RNFL measurements. However, confirming this in patients with subfoveal GA is important to ensure sufficient residual RNFL is present to stimulate with future therapies. Circumpapillary RNFL scans were also obtained to determine if the RNFL in the more affected quadrants is reduced in patients with asymmetric GA.
We designed this study to accurately assess the volume of the macula’s inner retinal layers to determine if ganglion cells are lost in patients with GA when compared to healthy controls.
Methods
Data Collection
This HIPAA compliant retrospective study was approved by the Institutional Review Board at the University of California, San Diego and performed in compliance with the Declaration of Helsinki. A computer-based data search of patients who had retinal imaging at the Jacobs Retina Center at the Shiley Eye Institute between 2014 and 2016 yielded records for 43 patients with subfoveal geographic atrophy and 38 healthy controls who underwent OCT with Spectralis (HRA2/Spectralis acquisition module 6.3.2.0, Heidelberg Engineering, Heidelberg, Germany). Medical charts were reviewed for patient’s sex, date of birth, Snellen visual acuity, early treatment diabetic retinopathy study (ETDRS) letters seen, lens status, intraocular pressure, and ocular history. This data was recorded, and eligibility for the study was determined.
Inclusion criteria were: age over 50 and diagnosis of GA involving the fovea from dry age-related macular degeneration. GA was defined based on an area of RPE loss and punched out atrophy on clinical exam that corresponded with an area of hypoautofluorescence and outer nuclear layer loss on OCT within the macula. Exclusion criteria were: intraocular surgery other than cataract surgery, intravitreal injection treatment, and history of laser photocoagulation or photodynamic therapy. Patients who had ocular hypertension (IOP > 25) at any clinical visit or a diagnosis of glaucoma in the included eye were excluded. Included eyes had OCT images with sufficient quality for manual segmentation with at least a 37 line horizontal raster of the macula. Circumpapillary RNFL scans without artifact on the topographic maps were included.
Image analysis
The scan pattern used to measure central macular thicknesses (CMT) and GCL thickness was a dense high speed scan pattern. Each image had 7 μm axial and 14 μm transverse resolutions in tissue and consisted of a 768 by 241 volume cube (30° horizontal × 25° vertical, 241 horizontal lines of 768 A-scans each) with frame averaging set to 8 scans yielding a sampling or pixel size of 11 μm in horizontal direction and 30 microns in vertical direction. The patients were scanned with the high-resolution scan pattern volume scan (20° horizontal × 15° vertical, at least 37 horizontal lines of 1,536 A-scans each) with frame averaging set to 8 scans. The OCT images were viewed on the Heidelberg Eye Explorer (Heyex version 1.9.10.0, Heidelberg Engineering, Heidelberg, Germany), and the built-in software (HRA/Spectralis viewing module 6.3.4.0) was used to manually delineate the internal limiting membrane, retinal nerve fiber layer, ganglion cell layer, inner plexiform layer, outer plexiform layer, and the external limiting membrane (Figure 1). To control for grader bias, two image readers performed manual correction of segmentation, after which two more retina specialists reviewed and edited segmentation, if necessary. If they were any disputes, a third reader (WF) broke the tie. The graders that did the initial segmentation did not have access to volumetric data or perform data extraction. The layer thicknesses were measured in the central 1 mm and superior, inferior, nasal, and temporal regions, up to 6 mm from the center.
Figure 1. Methodology for manual correction of segmentation.
A. Single line optical coherence tomography (OCT) scan through the fovea in a patient with Geographic Atrophy (GA) demonstrating altered retinal layers
B. Line scan after applying the Heidelberg software automated retinal layer lines, demonstrating incorrect segmentation at the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL) (arrow) and inner plexiform layer (IPL), as well as at the outer plexiform layer (OPL) and external limiting membrane (ELM).
C. Line scan after manual correction of retinal layers using Heidelberg software.
D. Composite retinal thickness topographic map of the RNFL after manual correction of retinal segmentation layers (left) with the RNFL retinal thicknesses (right).
Patients with poor OCT data (signal quality score ≤15) or images with non-segmentable retinal layers because of a discontinuous vessel pattern or haze were excluded from this study. The Q score for the scans was collected and reported for circumpapillary scans. The Q score is not reported for the volume scans because there are 241 individual Q scores per volume scan, and the machine does not provide the average Q score. We used the frame averaging technique of the Heidelberg SLO to improve the visibility of retinal layers.
Once manually corrected segmentation was accomplished, the volume (V) of the retinal layers was calculated using the following formulae:
In addition, circumpapillary scans were performed with a diameter of 12° and each scan was the composite of 100 individual scans using the automatic retinal tracker in the high-speed mode. The retinal thicknesses of the circle scans were measured using the Heidelberg Eye-explorer software. Images were examined by the investigator. Eleven eyes with geographic atrophy without glaucoma were included for circumpapillary scans. Normal eyes were not scanned because the Heidelberg software includes a normative age-adjusted database as a control, and the control values in each quadrant for each scan were recorded and used for reference.
GA image analysis
The HRA2 (Heidelberg Retina Angiograph 2, Heidelberg Engineering, Germany) was used to acquire the FAF images. Standard protocol for image acquisition involved excitation at the argon blue wavelength 488 nm with an optically pumped solid-state laser, and emission detected above 500 nm with a barrier filter. Typically 9–15 images were averaged to acquire a mean image. Either the Automatic Real Time (ART) mode or the mean image mode was used for all FAF image acquisition. The illumination beam is 3 mm in diameter, and the aperture of the dilated eye is used to collect light from the posterior pole.
Two separate graders calculated total GA area on the digital images using the Heidelberg imaging analysis software. Each grader used a computer mouse to manually trace all areas of GA within the vascular arcades. GA was recognized as well-demarcated black areas corresponding to dead or absent retinal pigment epithelium (RPE). In normal eyes, there is hypofluorescence of the fovea from the luteal pigment and melanin. Given that this area is variable in person to person, the normal area of macular hypoautofluorescence was measured in controls and reported (Table 1).
Table 1.
Demographic and Baseline Ocular characteristics of Control and Geographic Atrophy patients for Macular and Circumpapillary Analyses
| MACULAR ANALYSES | CIRCUMPAPILLARY RNFL ANALYSES | ||
|---|---|---|---|
| Group | Control | Geographic Atrophy | Geographic Atrophy |
| N (eyes) | 22 | 19 | 11 |
| Right, n (%) | 12 (54%) | 9 (47%) | 5 (45%) |
| Age (y) (mean ± Stdev) | 70.9 ± 10.7 | 78.0 ± 9.4 * | 83.4 ± 3.9 |
| Sex | |||
| Male, n (%) | 5 (22.7%) | 9 (47%) | 3 (27%) |
| Female, n (%) | 17 (77.2%) | 10 (52%) | 8 (73%) |
| ETDRS letters (mean ± Stdev) | 64.3 ± 6.7 | 41.6 ± 16.2 | 34.0 ± 19.8 |
| Macular hypoautofluorescence area (mm2) | 0.4 ± 0.1 | 10.8 ± 9.7 ** | 8.4 ± 8.0 |
| Location of GA | - | ||
| Central | 14 (73.7%) | 3 (27.2%) | |
| Superior | 3 (15.8%) | 5 (45.5%) | |
| Inferior | 2 (10.5%) | 3 (27.2%) | |
| Pseudophakia, n (%) | 6 (27%) | 11 (57%) | 10 (90%) |
| Q value score | - | - | 23.4 ± 5.9 |
P<0.05
P<0.001
y: years; Stdev: standard deviation
Peripapillary atrophy was included in the tracing only if it was confluent with macular GA. Areas of intact autofluorescence within GA were also traced, but with a different color to signify sparing of RPE. Areas of atrophy smaller than 0.02 mm2 were excluded. Graders assessing total GA area were masked to each other. The Heidelberg imaging analysis software automates conversion of pixels to mm2 based on the magnification factors of the imaging device.
The location of geographic atrophy in the macula was measured by placing an ETDRS grid from the Heidelberg imaging analysis software centered on fovea using the fundus autofluorescence image. Then, the topographic areas of fundus hypoautofluorescence were tabulated in a binary fashion (central 1 mm, central 3, 6 mm circle in the superior, inferior, temporal, and nasal locations). The symmetry of each autofluorescence image associated with a circumpapillary RNFL scan was graded by a masked readers using the ETDRS circle overlay on the autofluorescence image. If the measured area of hypofluorescence in the 3–6 mm circle in the superior quadrant was within 10% of the area of hypofluorescence in the 3–6 mm circle in the inferior quadrant, the image was graded as central. If the value was at least 10% greater in the superior or inferior image, it was graded as superior or inferior, respectively.
The ONL volume was also calculated by manually correcting the segmentation lines of the outer plexiform later and external limiting membrane for all scans and using Heidelberg imaging analysis software to generate a volume based on the same aforementioned algorithm for the other retinal volumes.
Using these methods, the amount of GA was defined by both area of fundus hypoautofluorescence and ONL volume. The location(s) and symmetry of GA involvement is described using the ETDRS circle and area mapping (Figure 2).
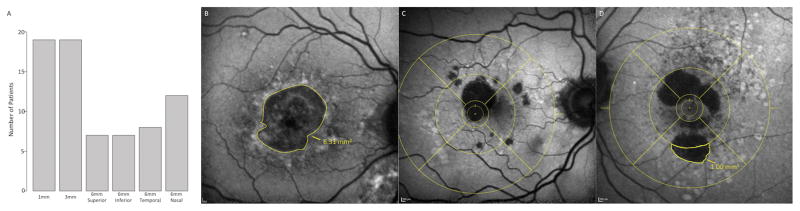
Figure 2. Methodology of Geographic Atrophy Localization and Symmetry.
A. Barplot demonstrating the ETDRS quadrants affected in our GA population. All patients had involvement of the central 1 mm and central 3 mm, while some had mild involvement in the 6 mm circles.
B. Autofluorescence image of a GA patient with the quantified area of macular hypoautofluorescence using the Heidelberg software tool.
C. Autofluorescence image with the ETDRS grid overlay, demonstrating mild involvement of the nasal and superior 3–6 mm areas.
D. Autofluorescence image with the ETDRS grid overlay and the area of macular hypoautofluorescence drawn in the inferior 3–6 mm using the Heidelberg software tool, demonstrating asymmetric inferior GA.
Statistical Analyses
To evaluate differences in measurements between study groups, linear mixed models were fitted with the measurement as the response and the patient diagnosis as a grouping factor using study subject as a random effect to account for the fact some participants had two eyes included in the study. Age and lens status were also included in the model to account for the effect of these variables. Linear mixed modelling was used to assess associations between measurement variables. Eyes with missing data for either variable being analyzed were dropped from that part of the analysis. Correlation coefficients were calculated using Spearman’s Rank correlation coefficient, and the relationship between the lens and grouping was evaluated using a chi-squared test. In this analysis, a linear mixed model was fit with subject ID as random effect to account for the fact that some subjects had two eyes in the study. In the plots, raw data and plotted lines and p-values from the linear mixed lens- and age-corrected models are shown. A t-test was used to analyze the difference in the RNFL quadrants in patients with superior and inferior GA. All analyses were undertaken using R statistical software.10
Results
Nineteen eyes with GA and 22 control eyes met the inclusion and exclusion criteria of the study and were analyzed for macular volumes. Eleven eyes with GA were analyzed with circumpapillary RNFL scans. The patients in the GA group had a similar sex, eye laterality, and lens status when compared with the control population (Table 1). GA patients (mean age 78.4 ± 9.4 yrs) were slightly older than the control patients (70.9 ±10.7 yrs, p=0.04), so this was accounted for in future analyses.
GA patients had a greater area of macular hypoautofluorescence (p < 0.001) and lower vision as measured by ETDRS letters (p < 0.001) than the control patients. The area of macular hypoautofluorescence negatively correlated with outer nuclear layer volume (Spearman’s Rho = −0.436, p = 0.003). ONL volume (μm3) in the central 3 mm is significantly less in patients with GA (0.33 ± 0.05) when compared to controls (0.50± 0.07; p < 0.001). ONL volume was preserved in the 6 mm circle, as most GA lesions were isolated to the central 3 mm (Figure 2).
Quantitative measurements of the macular inner retinal volumes in the central 3 mm and 6 mm are presented (Table 2). The ganglion cell volume (μm3) is significantly less in the central 3 mm in patients with GA (0.256 ± 0.048) when compared with controls (0.303 ± 0.036, p = 0.007). There is a positive correlation between GCL volume in the central 3 mm of the macula and ONL volume (p=0.020) and GCL volume and area of macular hypoautofluorescence (p=0.0112), (Figure 3).
Table 2.
Macular Retinal Volumes in Geographic Atrophy patients and Controls
| GCL volume (mm3) | RNFL volume (mm3) | IPL volume (mm3) | GCC complex volume (mm3) | Outer nuclear layer volume (mm3) | |
|---|---|---|---|---|---|
| Mean ± Stdev | Mean ± Stdev | Mean ± Stdev | Mean ± Stdev | Mean ± Stdev | |
| Geographic Atrophy | |||||
| 3 mm | 0.253 ± 0.039 (p=0.003)† | 0.158 ± 0.025 (p=0.024)† | 0.257± 0.044 (p=0.833)† | 0.667 ± 0.077 (p=0.395)† | 0.33 ± 0.10 (p < 0.001)† |
| 6 mm | 0.920 ±0.150 (p=0.146)† | 0.935 ± 0.158 (p=0.242)† | 0.858 ± 0.130 (p=0.637)† | 2.714 ± 0.348 (p=0.932)† | 1.46 ± 0.38 (p=0.129)† |
| Controls | |||||
| 3 mm | 0.302 ± 0.035 | 0.143 ± 0.014 | 0.259 ± 0.027 | 0.705 ± 0.071 | 0.50 ± 0.07 |
| 6 mm | 1.03 ± 0.132 | 0.882 ± 0.121 | 0.856 ± 0.098 | 2.77 ± 0.300 | 1.71± 0.22 |
GCL: Ganglion cell layer; RNFL: Retinal nerve fiber layer; IPL: Inner plexiform layer; GCC: ganglion cell complex (comprised of the sum of the IPL, GCL, and RNFL); Stdev: standard deviation; 3 mm and 6 mm refers to the ETDRS circle diameter represented
p value compares geographic atrophy group to controls
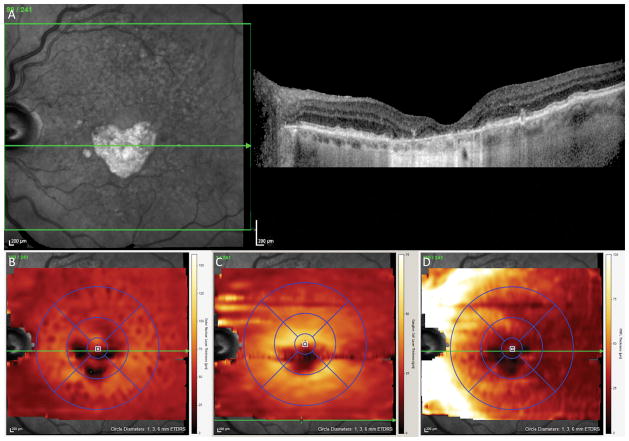
Figure 3. Topographic maps of the inner and outer retinal layers in a Geographic Atrophy (GA) patient.
A. Infrared image demonstrating a well circumscribed area of GA involving the inferior 3 mm of the central macula (left) with a corresponding single line optical coherence tomography (OCT) scan through the fovea
B. Outer Nuclear Layer (ONL) thickness map after manual correction of segmentation demonstrating ONL loss in only the inferior central 3 mm
C. Ganglion Cell Layer (GCL) thickness map after manual correction of segmentation demonstrating GCL loss in only the inferior central 3 mm
D. Retinal Nerve Fiber Layer (RNFL) thickness map after manual correction of segmentation demonstrating focal RNFL loss and adjacent thickening in the inferior central 3 mm
There is a significantly greater RNFL volume at 3 mm (p=0.024) but not at 6 mm in GA patients compared to controls. We also found a significant correlation between RNFL volume at 3 mm and the log of the hypoautofluorescence area (p=0.028).
When the circumpapillary RNFL was analyzed in patients with asymmetric GA, mean superior RNFL thickness was less in superior GA patients compared to inferior (p=0.084) and central (p=0.079) GA patients, but these findings were not significant (Table 3). Similar findings were seen in inferior GA patients, and no difference was seen in temporal RNFL. The global circumpapillary RNFL in similar to controls in all groups.
Table 3.
Thickness of Circumpapillary Retinal Nerve Fiber Layer in Geographic Atrophy patients with asymmetric and symmetric disease
| Superior GA | Inferior GA | Central GA | |
|---|---|---|---|
|
Superior RNFL Mean ± stdev (μm) |
208 ± 10.2 | 234.8 ± 20.2 | 249.3 ± 14.6 |
|
Δ Mean ± stdev (μm) |
−22.3 ± 27.6 | 4.8 ± 20.2 | 19.3 ± 14.6 |
|
Inferior RNFL Mean ± stdev (μm) |
255.6 ± 10.1 | 252.2 ± 26.4 | 248.7 ± 32.2 |
|
Δ Mean ± stdev (μm) |
−0.6 ± 10.1 | −13.8 ± 26.4 | 11.7 ± 32.2 |
|
Global RNFL Mean ± stdev (μm) |
94 ± 3.6 | 100.2 ± 7.2 | 100.0 ± 1.6 |
|
Δ Mean ± stdev (μm) |
0.0 ± 3.6 | 6.2 ± 7.2 | 6.0 ± 1.6 |
|
Temporal RNFL Mean ± stdev (μm) |
328.7 ± 40.8 | 338.4 ± 28.0 | 341.3 ± 10.0 |
|
Δ Mean ± stdev (μm) |
−3.3 ± 40.8 | 10.7 ±27.9 | 10.0 ± 10.0 |
RNFL: Retinal nerve fiber layer; GA: geographic atrophy
Superior RNFL: Sum of circumpapillary retinal nerve fiber layer thicknesses in the superotemporal and superonasal quadrants;
Inferior RNFL: Sum of circumpapillary retinal nerve fiber layer thicknesses in the inferotemporal and inferonasal quadrants
Temporal RNFL: Sum of circumpapillary retinal nerve fiber layer thicknesses in the superotemporal, inferotemporal, and temporal quadrants
Superior GA: defined as having asymmetrically more GA involvement in the superior 3–6 mm of the macula based on the ETDRS overlay circle.
Inferior GA: defined as having asymmetrically more GA involvement in the superior 3–6 mm of the macula based on the ETDRS overlay circle.
Central GA: symmetric GA not involving 3–6 mm ETDRS ring or involving it equally superiorly and inferiorly.
Δ: Column above minus the normative control value of the same area generated by Heidelberg software
stdev: standard deviation
There is no significant difference in IPL or ganglion cell complex (GCC) volumes at 3 mm or 6 mm in patients with GA when compared with controls. GCL volume was not significantly associated with ETDRS letters seen (p>0.1).
Discussion
While it is well-known that dry AMD- associated subfoveal geographic atrophy results in photoreceptors loss11 in the outer retina, very few studies have looked at the inner retina in this population.2,4,12 In this study, we used manual correction of automated segmentation to quantify the macular RNFL, GCL, IPL, and ONL in the central 3 mm of the macula in GA patients and healthy controls. We quantified the extent and location of GA involvement and used the 3 to 6 mm quadrants of the ETDRS circle as an internal control to determine if the volumetric changes found were localized to the affected area of GA. Circumpapillary RNFL scans were also performed in GA patients to confirm that no significant RNFL loss results from these focal lesions. We used multiple graders to manually calibrate segmentation and calculated inner and outer retinal volumes. Patients with a history of ocular hypertension or glaucoma were excluded. To our knowledge, this is the first study to evaluate the ganglion cell layer separate from the ganglion cell complex using manual calibration of segmentation with SD-OCT in patients with non-exudative geographic atrophy. It is also the first study to compare circumpapillary RNFL findings with location of GA. We found a small (16%) significant decrease in the ganglion cell layer (GCL) volume in patients with geographic atrophy with an associated local increase in the macular RNFL volume with preservation of the circumpapillary RNFL.
The authors believe that the increased macular RNFL volumes in GA patients is due to pseudoexpansion of the RNFL into the lost GCL, similar to what is seen in cotton wool spots with pseudoexpansion of the outer nuclear layer once the RNFL develops atrophy.8 While macular RNFL values were increased, the circumpapillary RNFL values were similar to the age-matched controls, indicating this phenomenon may only be happening in the area adjacent to the area of outer retinal atrophy (Figure 3). We noted more GCL loss in the central 3 mm with preservation of the 6 mm area, which served as our internal control because the GA lesions in our population affected the central 3 mm of the macula much more than the 6 mm area.
Histopathologic studies with a 77% loss of the ONL had 74% preservation of the GCL.2 Compared to this study, our GA patients only had a 16.2% loss of the ONL at 3 mm with preservation of 83.7% of the GCL. The histopathologic study was performed on 10 eyes (mean age 80.2 years) compared to our study on 22 eyes (mean age 77.4 years). Other histopathologic reports13 that demonstrated preservation of the GCL in AMD were in patients with early dry AMD without advanced subfoveal GA. In a study by our group, we found no significant difference in the mean RNFL and GCL thicknesses in patients with other stages of dry AMD but increased RNFL and GCL in patients with wet AMD using similar methods.14 In this study, GCL loss is linearly associated with ONL loss and hypoautofluorescence area, two separate measures of GA severity (Figure 4). This result was confirmed in our population and correlates with clinical expectations.
Figure 4. Decreased Ganglion Cell Layer (GCL) Volume with increased Geographic Atrophy (GA) severity.
A. Scatter plot demonstrating a significant correlation between GCL volume and macular hypoautofluorescence area, p=0.0112.
B. Scatter plot demonstrating a significant correlation between GCL volume and Outer Nuclear Layer (ONL) volume, p = 0.0201.
C. Scatter plot demonstrating a significant correlation between Retinal Nerve Fiber Layer (RNFL) volume and macular hypoautofluorescence area, p = 0.00284.
D. Scatter plot demonstrating a significant correlation between Outer Nuclear Layer (ONL) volume and macular hypoautofluorescence area, p = 0.00240.
Directional OCT increases the contrast of the ONL in one half of the image at a time. While this technique is helpful for single image acquisition on the Cirrus OCT,9 Heidelberg software’s frame averaging and built-in hardware eye tracker allow for excellent detail of retinal layers. Using automated segmentation on the Cirrus HD OCT, Zucchiatti et al.4 reported that the GCC of GA patients was significantly thinner than controls. In this study, we found no difference in the GCC and only a difference in the GCL layer. We believe our findings differ from the previous report because our findings are more accurate given manual correction of segmentation by multiple masked image graders and superior raw images with better contrast between retinal layers with Heidelberg SLO when compared to the Cirrus OCT. Both of our reports show that there is more preservation of the inner retina in GA from AMD than in retinitis pigmentosa.15
One hypothesis for GCL loss in geographic atrophy is ganglion cell apoptosis from transneuronal degeneration over time. In monkeys exposed to surgical ablation of visual input from the fovea, significant GCL loss developed from retrograde transneuronal degeneration.16 In advanced geographic atrophy, visual input to the GCL is reduced from loss of retinal photoreceptors, and this may result in a similar phenomenon of retrograde transneuronal degeneration. A recent study in mice with retinal degeneration demonstrated that photoreceptor loss resulted in decreased synaptic input to retinal ganglion cells and loss of peripheral ganglion cells.17 Another hypothesis is that the retinal photoreceptors as well as inner nuclear layer cells are chronically hypoperfused and ischemic from microvascular choroidal damage from AMD.18 It has recently been shown that GCL loss in the macula can temporally precede circumpapillary RNFL loss in the same location in patients with early stage glaucoma.19 While this may be due to cell loss preceding axonal loss, the RNFL measurement also includes glial cells and blood vessels, which are included in the RNFL thickness measurement along with axons.20,21
An interesting finding in both our studies and the Zucchiatti paper4 is that the macular RNFL is increased while the GCL is decreased. The topographic map in Figure 3D, demonstrates that macular RNFL is still reduced over the focal lesion, but the cumulative measurement in the central 3 mm is increased (Table 2). One hypothesis is that preserved islands of photoreceptors adjacent to the area of atrophy may still be providing visual input to peripapillary nerve fibers.11 It has been shown that photoreceptors in uninvolved areas may stimulate the GCL via retinal interneurons such as amacrine cells with very long processes.22 After retinal injury, Müller glial cells proliferate and dedifferentiate into horizontal cells with wide receptive fields,23 and this may preserve and amplify signals from the remaining photoreceptors to the GCL. The effect of RNFL pseudoexpansion is limited to the macula and the global circumpapillary RNFL values are similar to age-matched controls. We measured the macular and circumpapillary RNFL. As expected, the small focal area of GCL loss does not cause a significant loss of the circumpapillary RNFL, confirming our hypothesis that significant residual RNFL is present in patients with GA. While there is a trend toward superior GA causing more superior RNFL loss and inferior GA resulting in more inferior RNFL loss, these results were not significant. From the glaucoma literature19,21 we know that inferior macular GCL loss is associated with inferior circumpapillary RNFL loss. In this population, further studies are necessary to evaluate these findings over time.
One of the limitations of this study is that the sample size was moderate, but the study was adequately powered to detect a clinically significant difference in retinal volumes. Given the high quality of the data collected and the highly significant difference in GCL volume between the groups, enrolling additional patients was not necessary to draw a clear conclusion. Another potential limitation would be grader bias with manual segmentation as GCL thickness in GA is often apparent on OCT. Given manual segmentation is much more accurate than automated segmentation in pathologic eyes, retina specialists comfortable with the anatomic layers of the retina on OCT are the best candidates for manual segmentation. Furthermore, although the specialists involved with segmentation in this study were aware of the study purposes, it would be difficult to have naïve retinal specialists who would not recognize the classic repeated layer patterns of GA. To account for this, multiple graders masked to the volume measurements performed the segmentation to avoid biasing the results.
There was a slight difference in age between the groups. While there is an age-associated decrease in RNFL,24 GCL does not change with age.25 Therefore, the age difference does not influence our main conclusion. This was a retrospective study without long-term follow-up. While we saw a significant correlation between GCL volume and ONL volume and macular hypoautofluorescence, it is important in future studies to prospectively evaluate the GCL volume over years to see if progression of ONL atrophy is associated with decreased GCL volume and circumpapillary RNFL thickness.
Others have shown that the optic disc of eyes with large areas of advanced AMD (with either a disciform scar or geographic atrophy) are more likely to be classified as glaucomatous by glaucoma specialists when compared to the fellow eye.26 While we did encounter these patients in the screening group, if a glaucoma specialist determined glaucoma was present (not just suspect), the patient was excluded from analysis in this study. It is possible that some patients without glaucoma but with nerves that appeared glaucomatous secondary to GA were excluded from this study for this reason, but because the clinical distinction is difficult this was done to keep a homogenous study population. Future studies evaluating the effect of glaucoma on inner retinal volumes in GA patients are important to understand the effects on GCL volume and would require large numbers and longitudinal follow-up.
This study makes a significant contribution to the literature by increasing our understanding of inner retinal anatomy in patients with subfoveal geographic atrophy from dry macular degeneration. The significant but small loss of the GCL overlying the area of geographic atrophy is important to take into consideration when planning therapeutic intervention. The majority of the GCL and RNFL remain intact even with significant outer retinal loss, so stimulation of the GCL with retinal prosthetics in patients with GA may be clinically useful. Future prospective longitudinal studies could better demonstrate the long-term inner retinal changes in GA. It is important to know if replacing the RPE and/or photoreceptor layer can restore vision in patients with mild GCL loss. The ability to study the inner retinal structures in eyes with GA will give us important information when selecting possible therapies for patients with advanced outer retinal disease.
SUMMARY STATEMENT.
With manual segmentation of retinal layers in optical coherence tomography images, we found that the macular ganglion cell volume is significantly reduced in patients with geographic atrophy compared to healthy controls.
Acknowledgments
Financial Support: Supported in part by UCSD Vision Research Center Core Grant P30EY022589 (WRF), NIH grant EY016323 (DUB) and an unrestricted grant from Research to Prevent Blindness, NY (WRF)
Footnotes
Presented at ARVO in San Francisco, CA May 2016
None of the authors have any financial/conflicting interests to disclose.
References
- 1.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SY, Sadda S, Humayun MS, de Juan E, Melia BM, Green WR. Morphometric analysis of the macula in eyes with geographic atrophy due to age-related macular degeneration. [Accessed May 24, 2014];Retina. 2002 22(4):464–470. doi: 10.1097/00006982-200208000-00011. http://www.ncbi.nlm.nih.gov/pubmed/12172114. [DOI] [PubMed] [Google Scholar]
- 3.Holz FG, Strauss EC, Schmitz-Valckenberg S, Van Lookeren Campagne M. Geographic atrophy: Clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079–1091. doi: 10.1016/j.ophtha.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Zucchiatti I, Parodi MB, Pierro L, et al. Macular ganglion cell complex and retinal nerve fiber layer comparison in different stages of age-related macular degeneration. Am J Ophthalmol. 2015;160(3):602–607. e1. doi: 10.1016/j.ajo.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Garvin MK, Lee K, Burns TL, Abràmoff MD, Sonka M, Kwon YH. Reproducibility of SD-OCT-based ganglion cell-layer thickness in glaucoma using two different segmentation algorithms. Invest Ophthalmol Vis Sci. 2013;54(10):6998–7004. doi: 10.1167/iovs.13-12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mwanza J, Budenz DL, Godfrey DG, et al. Diagnostic Performance of Optical Coherence Tomography Ganglion Cell e Inner Plexiform Layer Thickness Measurements in Early Glaucoma. Ophthalmology. 2013;121(4):849–854. doi: 10.1016/j.ophtha.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Lee H-J, Kim M-S, Jo Y-J, Kim J-Y. Macular Ganglion Cell Complex and Retinal Nerve Fiber Layer Comparison in Different Stages of Age-Related Macular Degeneration. Am J Ophthalmol. 2016;161:214. doi: 10.1016/j.ajo.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Gomez ML, Mojana F, Bartsch D-U, Freeman WR. Imaging of Long-term Retinal Damage after Resolved Cotton Wool Spots. Ophthalmology. 2009;116(12):2407–2414. doi: 10.1016/j.ophtha.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lujan BJ, Roorda A, Croskrey JA, et al. DIRECTIONAL OPTICAL COHERENCE TOMOGRAPHY PROVIDES ACCURATE OUTER NUCLEAR LAYER AND HENLE FIBER LAYER MEASUREMENTS. Retina. 2015;35(8):1511–1520. doi: 10.1097/IAE.0000000000000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.R Development Core Team. R: A Language and Environment for Statistical Computing. R Found Stat Comput; Vienna Austria: 2016. [Google Scholar]
- 11.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Investig Ophthalmol Vis Sci. 1996;37(7):1236–1249. [PubMed] [Google Scholar]
- 12.Lee HJ, Kim MS, Jo YJ, Kim JY. Ganglion Cell-Inner Plexiform Layer Thickness in Retinal Diseases: Repeatability Study of Spectral-Domain Optical Coherence Tomography. Am J Ophthalmol. 2015;160(2):283–289.e1. doi: 10.1016/j.ajo.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Medeiros NE, Curcio Ca. Preservation of ganglion cell layer neurons in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42(3):795–803. [PubMed] [Google Scholar]
- 14.Muftuoglu IK, Bartsch DRHFW. Quantitative Analyses of Macular Ganglion cell complex using Accurate Segmentation in Age-related Macular Degeneration. Retina. 2017 [Google Scholar]
- 15.Stone JL. Morphometric Analysis of Macular Photoreceptors and Ganglion Cells in Retinas With Retinitis Pigmentosa. Arch Ophthalmol. 1992;110(11):1634. doi: 10.1001/archopht.1992.01080230134038. [DOI] [PubMed] [Google Scholar]
- 16.Hendrickson A, Warner CE, Possin D, Huang J, Kwan WC, Bourne JA. Retrograde transneuronal degeneration in the retina and lateral geniculate nucleus of the V1-lesioned marmoset monkey. Brain Structure and Function. 2013:1–10. doi: 10.1007/s00429-013-0659-7. [DOI] [PubMed] [Google Scholar]
- 17.Saha S, Greferath U, Vessey KA, Grayden DB, Burkitt AN, Fletcher EL. Changes in ganglion cells during retinal degeneration. Neuroscience. 2016;329:1–11. doi: 10.1016/j.neuroscience.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Feigl B, Brown B, Lovie-Kitchin J, Swann P. Functional loss in early age-related maculopathy: the ischaemia postreceptoral hypothesis. Eye. 2007;21(6):689–696. doi: 10.1038/sj.eye.6702389. [DOI] [PubMed] [Google Scholar]
- 19.Kim YK, Ha A, Na KI, Kim HJ, Jeoung JW, Park KH. Temporal Relation between Macular Ganglion Cell?Inner Plexiform Layer Loss and Peripapillary Retinal Nerve Fiber Layer Loss in Glaucoma. Ophthalmology. 2017;124(7):1056–1064. doi: 10.1016/j.ophtha.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hood DC, Raza AS, de Moraes CGV, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1–21. doi: 10.1016/j.preteyeres.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dacey DM. The dopaminergic amacrine cell. J Comp Neurol. 1990;301(3):461–489. doi: 10.1002/cne.903010310. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Zhou G-M. Interneuron regeneration after ouabain treatment in the adult mammalian retina. Neuroreport. 2015;26(12):712–717. doi: 10.1097/WNR.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 24.Parikh RS, Parikh SR, Sekhar GC, Prabakaran S, Babu JG, Thomas R. Normal Age-Related Decay of Retinal Nerve Fiber Layer Thickness. Ophthalmology. 2007;114(5):921–926. doi: 10.1016/j.ophtha.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Demirkaya N, van Dijk HW, van Schuppen SM, et al. Effect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomography. Investig Ophthalmol Vis Sci. 2013;54(7):4934–4940. doi: 10.1167/iovs.13-11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law SK, Sohn YH, Hoffman D, Small K, Coleman AL, Caprioli J. Optic disk appearance in advanced age-related macular degeneration. Am J Ophthalmol. 2004;138(1):38–45. doi: 10.1016/j.ajo.2004.02.021. [DOI] [PubMed] [Google Scholar]