Abstract
The mouse bioassay for diarrhetic shellfish poisoning toxins has been used worldwide. In this study, dinophysistoxin-1 (DTX-1) and okadaic acid (OA) were compared for toxicity. The lethality rate increased and the median survival time decreased in a dose-dependent manner in both DTX-1 and OA. The median lethal dose value was 150.4 µg/kg (95% confidence interval=130.1–171.2 µg/kg) for DTX-1 and 185.6 µg/kg (95% confidence interval=161.2–209.6 µg/kg) for OA. The toxicity equivalent factor 1:1 has been used for OA and DTX-1 in the EU and Japan. Thus, it may be considered that toxicity potential of DTX-1 has remained underestimated as compared to that of OA and DTX-1 might be more toxic than OA.
Keywords: diarrhetic shellfish poisoning (DSP) toxin, dinophysistoxin-1 (DTX-1), mouse bioassay, okadaic acid (OA), survival curve
Diarrhetic shellfish poisoning (DSP) is a gastrointestinal illness characterized by typical symptoms such as diarrhea, nausea, and vomiting. It is caused by the consumption of shellfish contaminated with algal toxins such as okadaic acid (OA) and dinophysistoxins (DTXs), which are produced by marine dinoflagellates [18, 21]. DSP was first reported at Tohoku, Japan, by Yasumoto et al. Since then, it has been recognized as a public health and shellfish industry problem worldwide [23]. The mouse bioassay (MBA), also known as Yasumoto’s method, had been used as the official method for DSP toxin detection in Japan since 1981 [19, 20]. Since then, it has been widely used in many countries of the world [5]. However, the European Commission authorized the use of the MBA until December 31, 2014 [16], and the Japanese Government also decided to replace the MBA with LC-MS/MS for OA group toxins [6].
In the former Japanese official method [19, 20], the extract from the shellfish sample is injected intraperitoneally (i.p.) into 3 male ddY or ICR strain mice weighing 16–20 g. The sample is deemed positive (contaminated) if at least 2 of the 3 mice die within 24 hr of the injection. Humane endpoints were not applied in the method, as with the mouse bioassay for other marine biotoxins [13, 14].
In this study, we aimed to examine the time course of death of the mice inoculated with different doses of DTX-1 and OA, which were two major components of DSP toxins, for further study to consider about the humane endpoints, and to compare the toxicity of DTX-1 and OA on the MBA platform.
Specific-pathogen-free male mice (4 weeks old) of ICR strain were purchased from Japan SLC Inc. (Shizuoka, Japan). The mice were adapted to our animal facility for a few days and used at 18–20 g body weight. The room lighting was 12 hr light (09:00–21:00) −12 hr dark (21:00–09:00) cycle. The mice were housed in plastic cages with wood chip bedding and provided commercial pellets (CRF-1; Charles River Japan Inc., Yokohama, Japan) and tap water ad libitum. All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee.
DTX-1 (purity 87.2%) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), and OA (purity >98%) was purchased from LC Laboratories (Woburn, MA, U.S.A.). DTX-1 and OA inocula were prepared as described previously [9,10,11,12]. Briefly, each toxin was first dissolved in acetone and then mixed with soybean oil. Soybean oil was used as a vehicle, instead of shellfish extracts or organic solvents such as dimethyl sulfoxide, for avoiding the effects of uncontrollable ingredients or toxicity. Acetone was removed by evaporation and the residue was suspended in 1% Tween 60 saline and sonicated. The final inocula contained 10% soybean oil. An inoculum was mixed well before each injection. The amounts of both toxins were calculated and expressed by multiplying the crude weights by purity.
The mice were randomly divided into groups of 10 mice each. For each toxin, 5 dose groups were created based on the lethal doses of the toxins previously reported [4, 22], i.e., 2.62, 3.05, 3.45, 3.92 and 4.36 µg/mouse for DTX-1, and 3.43, 3.92, 4.41, 4.90 and 5.39 µg/mouse for OA. One ml of the inoculum was injected i.p. into each mouse. The mice were observed every 10 min until 12 hr after injection, and then every 30 min until 24 hr after injection. During the dark period, red light was used for observation. No negative (vehicle) control (injected only 1% Tween 60 saline contained 10% soybean oil) was examined in this experiment as no death was observed in the negative control group in our previous experiments. The experiment design basically followed the former official MBA method for DSP toxins in Japan and the EU.
Lethality was statistically compared among the groups of each toxin by using Fisher’s exact test. Survival analysis was conducted using log-rank test and Gehan-Wilcoxon test. The median lethal dose (LD50) for each toxin was determined by probit analysis. All of the above analyses were performed using the statistical package R version 2.14.0 [7].
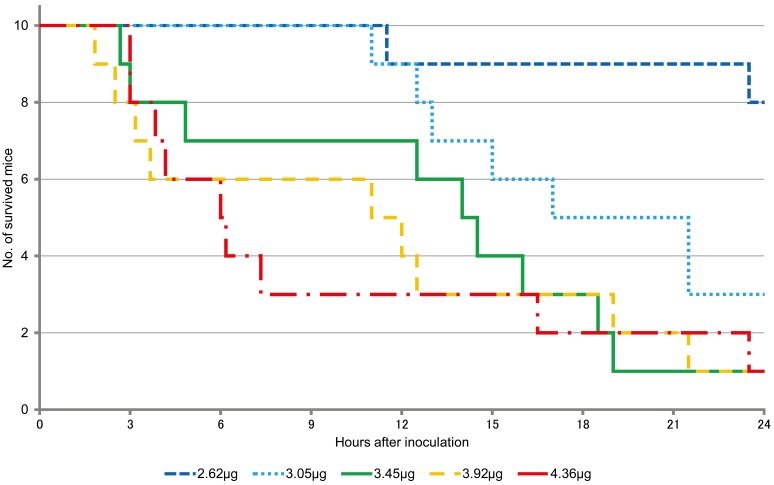
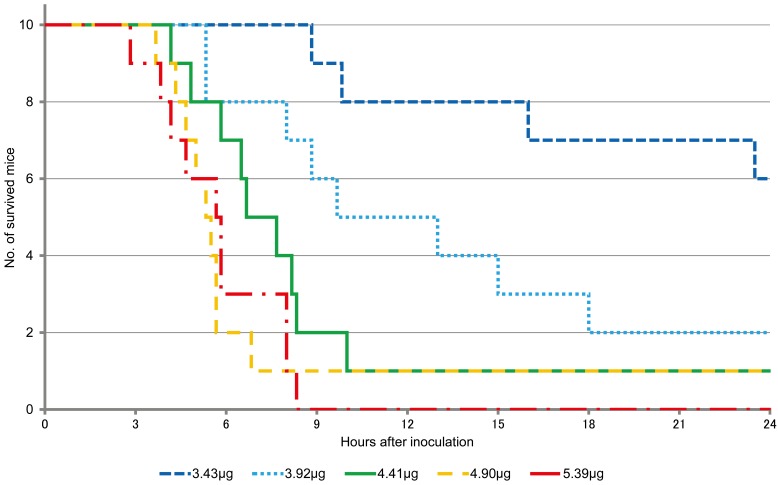
The lethality was 20, 70 and 90% in mice inoculated with 2.62 µg; 3.05 µg; and 3.45, 3.92 and 4.36 µg of DTX-1, respectively, at 24 hr after injection (Table 1). On the other hand, at the same time point, lethality was 40, 70, 90 and 100% in mice inoculated with 3.43, 3.92, 4.41 and 4.90 µg, and 5.39 µg of OA, respectively (Table 2). Fisher’s exact test showed significant differences among all the groups (P=0.0014 for DTX-1 groups and P=0.012 for OA groups).
Table 1. Lethality and survival analysis of the mice after DTX-1 inoculation.
| Dose (µg) | Lethality(%) | Survived | Dead | Median survival time (hr) | Log-Rank testa) | Gehan-Wilcoxon testa) |
|---|---|---|---|---|---|---|
| 2.62 | 20 | 8 | 2 | Inf | a | a |
| 3.05 | 70 | 3 | 7 | 19.25 | b | b |
| 3.45 | 90 | 1 | 9 | 14.25 | b,c | b |
| 3.92 | 90 | 1 | 9 | 11.50 | c | b |
| 4.36 | 90 | 1 | 9 | 6.08 | c | b |
Inf: infinite. a) the different alphabets in the column mean significantly differences in the survival curves.
Table 2. Lethality and survival analysis of the mice after OA inoculation.
| Dose (µg) | Lethality (%) | Survived | Dead | Median survival time (hr) | Log-Rank testa) | Gehan-Wilcoxon testa) |
|---|---|---|---|---|---|---|
| 3.43 | 40 | 6 | 4 | Inf | a | a |
| 3.92 | 70 | 3 | 7 | 11.33 | b | b |
| 4.41 | 90 | 1 | 9 | 7.58 | b,c | b,c |
| 4.90 | 90 | 1 | 9 | 5.42 | c | c |
| 5.39 | 100 | 0 | 10 | 5.75 | c | c |
Inf: infinite. a) the different alphabets in the column mean significantly differences in the survival curves.
The survival curves of the mice after DTX-1 and OA inoculation are shown in Figs. 1 and 2, respectively. For survival analysis, both log-rank test and Gehan-Wilcoxon test were used. It is generally believed that the log-rank test is more standard, and the Gehan–Wilcoxon test gives more weight to deaths at earlier time points. Significant differences were seen between the lower and higher dose groups in for both DTX-1 and OA (Tables 1 and 2).
Fig. 1.
Survival curves of mice after DTX-1 inoculation.
Fig. 2.
Survival curves of mice after OA inoculation.
The LD50 values for DTX-1 and OA were 2.89 µg/18–20 g mouse (95% confidence interval=2.50–3.29 µg/18–20 g mouse) and 3.57 µg/18–20 g mouse (95% confidence interval=3.10–4.03 µg/18–20 g mouse), respectively. The average body weight of the mice in the DTX-1 and OA groups were 19.22 ± 0.36 g and 19.23 ± 0.58 g, respectively. The corresponding LD50 values based on body weight were 150.4 µg/kg (95% confidence interval=130.1–171.2 µg/kg) for DTX-1 and 185.6 µg/kg (95% confidence interval=161.2–209.6 µg/kg) for OA.
The lethality rate increased and the median survival time decreased in a dose-dependent manner in both DTX-1 and OA experiments. However, survival times varied in individual mice, and it is considered difficult to estimate dose of toxins inoculated from survival time. The mice inoculated with the toxins showed immobility, lethargy, and dyspnea, however, it is concluded that the exact death time of the dying mice cannot be predicted, either within 24 hr or over 24 hr after inoculation and the humane endpoints cannot be applied from the symptoms.
There are several reports on the LD50 values of OA after intraperitoneal injection in mice. The LD50 values for intraperitoneally injected OA have been reported as 192 µg/kg [15], 210 µg/kg [2], and 225 µg/kg [17]. Aune et al. [1] compared the toxicity between OA and DTX-2 and reported that the LD50 values for OA and DTX-2 were 204 µg/kg and 352 µg/kg, respectively. In contrast, there are no reports concerning the LD50 values for DTX-1. However, minimum lethal dose value for DTX-1 has been reported to be 160 µg/kg [22]. Based on the previous reports, the EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC-MS/MS [3] described the toxicity equivalent factors (TEFs) for OA:DTX-1:DTX-2 as 1:1:0.6. The same TEFs are also applied in the present Japanese official method [6].
In the previous reports referred in the EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC-MS/MS, the mice of different strains, different sexes, and different body weight ranges were used for determining the LD50 values. However, we previously reported that the strains, sexes, and body weights of the mice affected the susceptibility to OA [9,10,11,12]. And, as to DTX-1, there was only 1 report describing the minimum lethal dose value, not the LD50 values. It is thought that the comparison of toxicity between OA and DTX-1 should be more precisely determined by using the same protocol.
In this study, the calculated LD50 values for DTX-1 and OA were 150.4 µg/kg (95% confidence interval=130.1–171.2 µg/kg) and 185.6 µg/kg (95% confidence interval=161.2–209.6 µg/kg), respectively. From the data, it may be interpreted that DTX-1 is more toxic than OA after i.p. inoculation in mice.
This study reports for the first time the comparison of toxicity between OA and DTX-1 using the same MBA platform. Earlier the TEF for DTX-1 and OA was considered as 1:1 in the EU and Japan [3, 6]. However, the results obtained in this study indicate that toxicity potential of DTX-1 is higher than that of OA. These findings may have more impact in Japan as DTX-1 is more prevalent in the marine areas of Japan, Canada, and Norway as compared to the prevalence of OA, which is more prevalent in Europe [8].
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS) under Grant-in-Aid for Scientific Research (C) (KAKENHI) Grant No. 25450437.
REFERENCES
- 1.Aune T., Larsen S., Aasen J. A., Rehmann N., Satake M., Hess P.2007. Relative toxicity of dinophysistoxin-2 (DTX-2) compared with okadaic acid, based on acute intraperitoneal toxicity in mice. Toxicon 49: 1–7. doi: 10.1016/j.toxicon.2006.07.033 [DOI] [PubMed] [Google Scholar]
- 2.Dickey R. W., Bobzin S. C., Faulkner D. J., Bencsath F. A., Andrzejewski D.1990. Identification of okadaic acid from a Caribbean dinoflagellate, Prorocentrum concavum. Toxicon 28: 371–377. doi: 10.1016/0041-0101(90)90074-H [DOI] [PubMed] [Google Scholar]
- 3.European Union Reference Laboratory for Marine Biotoxins (EURLMB)2013. EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Miotoxins in Molluscs by LC-MS/MS Version 5. http://aesan.msssi.gob.es/CRLMB/docs/docs/metodos_analiticos_de_desarrollo/EU-Harmonised-SOP-LIPO-LCMSMS_Version5.pdf [accessed on Mar 29, 2017].
- 4.Fernández M. L., Richard D. J. A., Cembella A. D.2004. In vivo assays for phycotoxins. pp. 347–380. In: Manual in Harmful Marine Microalgae. (Hallegraeff, G. M., Anderson, D. M. and Cembella, A. D. eds.), UNESCO Publishing, Paris. [Google Scholar]
- 5.Food and Agriculture Organization of the United Nations (FAO) 2004. Marine Biotoxins. 3. Diarrhoeic Shellfish Poisoning (DSP). pp. 53–95. http://www.fao.org/docrep/007/y5486e/y5486e00.htm [accessed on Mar 29, 2017].
- 6.Oshiro N.2015. Determination of diarrhetic shellfish poisoning toxins, okadaic acid group toxins. Food Sanit. Res. 65: 515–522(in Japanese). [Google Scholar]
- 7.R Development Core Team 2011. R Foundation for Statistical Computing. Vienna, Austria. R: A language and environment for statistical computing. http://www.R-project.org/ [accessed on Mar 29, 2017].
- 8.Rodríguez L. P., González V., Martínez A., Paz B., Lago J., Cordeiro V., Blanco L., Vieites J. M., Cabado A. G.2015. Occurrence of lipophilic marine toxins in shellfish from Galicia (NW of Spain) and synergies among them. Mar. Drugs 13: 1666–1687. doi: 10.3390/md13041666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki H.2012. Susceptibility of different mice strains to okadaic acid, a diarrhetic shellfish poisoning toxin. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 29: 1307–1310. doi: 10.1080/19440049.2012.685892 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H.2012. Differences in susceptibility to okadaic acid, a diarrhetic shellfish poisoning toxin, between male and female mice. Toxins (Basel) 5: 9–15. doi: 10.3390/toxins5010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H.2014. Influence of body weight of mice on the susceptibility to okadaic acid, a diarrhetic shellfish poisoning toxin. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 31: 719–722. doi: 10.1080/19440049.2014.886133 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H.2014. Strain- and sex-differences in susceptibility in the mouse bioassay for diarrhetic shellfish poisoning toxins. pp. 399–411. In: Shellfish: Human Consumption, Health Implications and Conservation Concerns (Hay, R. M. ed.), Nova Science Publishers, Inc., New York. [Google Scholar]
- 13.Suzuki H.2016. Differences in susceptibility of mouse strains to tetrodotoxin. Toxicon 119: 168–170. doi: 10.1016/j.toxicon.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H., Machii K.2014. Comparison of toxicity between saxitoxin and decarbamoyl saxitoxin in the mouse bioassay for paralytic shellfish poisoning toxins. J. Vet. Med. Sci. 76: 1523–1525. doi: 10.1292/jvms.14-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tachibana K., Scheuer P. J., Tsukitani Y., Kikuchi H., Van Engen D., Clardy J., Gopichand Y., Schmitz F. J.1981. Okadaic acid, a cytotoxic polyether from two marine sponges of the genus Halichondria. J. Am. Chem. Soc. 103: 2469–2471. doi: 10.1021/ja00399a082 [DOI] [Google Scholar]
- 16.The European Commission2011. Commission regulation (EU) No 15/2011of 10 January 2011amending Regulation (EC) No 2074/2005 as regards recognised testing methods for detecting marine biotoxins in live bivalve molluscs. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:006:0003:0006:EN:PDF [accessed on Mar 29, 2017].
- 17.Tubaro A., Sosa S., Carbonatto M., Altinier G., Vita F., Melato M., Satake M., Yasumoto T.2003. Oral and intraperitoneal acute toxicity studies of yessotoxin and homoyessotoxins in mice. Toxicon 41: 783–792. doi: 10.1016/S0041-0101(03)00032-1 [DOI] [PubMed] [Google Scholar]
- 18.Tubaro A., Sosa S., Bornancin A., Hungerford J.2008. Chapter 11 pharmacology and toxocology of diarrheic shellfish toxins. pp. 229–253. In: Seafood and Freshwater Toxins Pharmacology, Physiology, and Detection, 2nd ed. (Botana, L. M. ed.), CRC Press, Boca Raton. [Google Scholar]
- 19.Yasumoto T.1981. Method for the bioassay of diarrhetic shellfish toxin. Food Sanit. Res. 31: 515–522(in Japanese). [Google Scholar]
- 20.Yasumoto T.2005. Chapter 7. Shizendoku (natural toxins) 4. Gerisei kaidoku (kouteihou) (diarrhetic shellfish poisoning toxins (official method). pp.680–685. In: Shokuhin Eisei Kensa Shishin (Standard Methods of Analysis in Food Safety Regulation), (Ministry of Health, Labour and Welfare ed.), Japan Food Hygiene Association, Tokyo. [Google Scholar]
- 21.Yasumoto T., Murata M., Oshima Y., Matsumoto G. K.1984. Diarrhetic shellfish poisoning. pp. 207–214. In: Seafood Toxins. (Ragelis, E. P. ed.), American Chemical Society, Washington, D.C. [Google Scholar]
- 22.Yasumoto T., Murata M.1990. Polyester toxins involved in seafood poisoning. pp. 120–132. In: Marine Toxins. (Hall, S. and Strichartz, G. eds.), American Chemical Society, Washington, D.C. [Google Scholar]
- 23.Yasumoto T., Oshima Y., Yamaguchi M.1978. Occurrence of a new type of shellfish poisoning in the Tohoku district. Bul. Jpn. Soc. Sci. Fish. 44: 1249–1255. doi: 10.2331/suisan.44.1249 [DOI] [Google Scholar]