Abstract
This study aimed to demonstrate the higher accuracy and reproducibility of quantitative computed tomography (QCT) compared with dual-energy X-ray absorptiometry (DXA) as a gold standard for measuring canine bone mineral density (BMD). Seven middle-aged beagle dogs underwent lumbar vertebral and bilateral femoral DXA and QCT scans. BMD (mg/cm2) was measured at the vertebral body from L2 to L6, femoral neck, and proximal and distal femoral diaphyses. The BMD values were measured 3 times and compared. The BMD value on QCT was higher than that on DXA for femoral BMD but not for vertebral BMD. The correlation was strong for the lumbar vertebrae (r=0.66) and was strongest for L3 (r=0.85). No correlation was found for the femoral neck (P=0.35), and only moderate correlations were found for the proximal and distal femoral diaphyses (r=0.43 and r=0.40, respectively). The limits of agreement were narrower for vertebral BMD than for femoral BMD, and L3 had the narrowest limits of agreement. The intraclass correlation (ICC) was higher for DXA than for QCT at all lumbar and femoral sites measured, but the ICC of QCT was higher than 0.7. In conclusion, L3 can be used to monitor changes in BMD, and relative values and sequential monitoring of femoral BMD can also be useful because of the high reproducibility of QCT measurements. QCT would be a useful technique for evaluation of BMD in veterinary practice.
Keywords: bone mineral density (BMD), dog, dual-energy X-ray absorptiometry (DXA), quantitative computed tomography (QCT)
Bone mineral density (BMD) is defined as the mineral concentration in bone. BMD is directly related to bone strength and is a useful predictor of osteoporotic fracture; it is therefore used to diagnose and monitor osteoporosis in humans [12]. In human medicine, BMD is known to be affected by numerous factors, including age, sex, endocrine disease, gastrointestinal disease, and certain drugs [33]. Osteoporosis, or low BMD, is a common condition that puts the patient at increased risk of pathologic fracture; therefore, early diagnosis, prevention, and monitoring of BMD are crucial.
Dual-energy X-ray absorptiometry (DXA) is a standard, non-invasive, and accurate method for measuring BMD and body composition in humans [18]. Typically, central DXA scans (of the lumbar vertebrae and proximal femur) are obtained, but DXA can be used to assess BMD throughout the entire body or at any specific body site [16]. This method has several advantages, including cost-effectiveness and a rapid scanning time.
Quantitative computed tomography (QCT) is also used to measure BMD in humans and has higher sensitivity than DXA, but lower specificity for diagnosing osteoporosis [15]. Although DXA is considered a standard BMD measurement technique, QCT is more sensitive than DXA for diagnosis of osteoporosis and prediction of the risk of pathologic fracture because trabecular BMD is lost more rapidly than cortical BMD when the disease progresses [6]. The decrease in BMD in patients with metabolic or endocrine disease is more obvious in trabecular bone than in cortical bone, particularly in the vertebrae [10]. Measurement of trabecular BMD is critical for early detection of decreasing bone mineral content and QCT may be preferable to DXA in some cases.
Similarly, there are many conditions in veterinary medicine that can reduce BMD, including hyperadrenocorticism, hyperparathyroidism, diabetes mellitus, renal failure, nutritional deficiency, and disuse, in addition to ovariohysterectomy and the administration of glucocorticoids [4, 5, 7, 9, 24, 32, 36]. Therefore, BMD measurements are useful for early diagnosis and treatment of osteoporosis in animals. Conventional radiographs are often used in veterinary medicine to diagnose osteopenia in dogs. However, to detect decreased bone opacity by using conventional radiographs, at least 30–40% of bone mineral content must be depleted [18]. Furthermore, conventional radiography is subjective and affected by technical factors [27]. In recent years, several studies have been conducted on BMD measurement in dogs [2, 19]. As in human medicine, several endocrine diseases are associated with decreased BMD in dogs and bone loss is most easily detected in the vertebrae [7]. Therefore, a more sensitive method for measuring BMD and evaluating osteopenia is needed in veterinary medicine.
Both QCT and DXA are presently used to measure BMD in veterinary medicine, and most studies using these techniques have evaluated BMD in the lumbar vertebrae and proximal femur, as in human medicine [5, 8, 11, 35]. Assessment of peripheral BMD is more common in veterinary medicine than in human medicine because of the greater need to monitor bone healing postoperatively [29]. However, in veterinary medicine, DXA is used less often than QCT for this purpose because veterinary DXA machines are not commercially available and scanning can be performed only under general anesthesia. QCT has been used more frequently in veterinary medicine because it has the advantages of providing anatomical information and measuring trabecular and cortical BMD separately [2, 3]. Measurement of BMD is critical for identifying factors that affect bone mineral content, but research in this regard remains rare in veterinary medicine. Furthermore, the only study that has compared measurements obtained using DXA and QCT, and assessed which is more commonly used, was performed on canine femurs ex situ [26], and to our knowledge, no studies have compared vertebral BMD measurements obtained using QCT and DXA.
The aims of this study were to demonstrate the accuracy and reproducibility of QCT compared with DXA as the gold standard and to assess the clinical usefulness of QCT for evaluating canine BMD in veterinary medicine.
MATERIALS AND METHODS
Animals
Seven middle-aged adult male beagle dogs with a mean age of 4.9 ± 0.98 years and a mean weight of 10.15 ± 0.68 kg were used in the study. All dogs were deemed to be healthy on the basis of a physical examination, complete blood count, serum chemistry, urinalysis, and radiographic and ultrasonographic examinations. The study protocol was approved by the Institutional Animal Care and Use Committee of Konkuk University (approval number KU16175).
Anesthesia
All dogs were fasted for at least 12 hr before QCT or DXA scanning was performed. The dogs were premedicated with acepromazine (Mobinul®, Myungmoon, Seoul, South Korea) at 0.02 mg/kg and glycopyrrolate (SEDAJECT®, Samu Median, Yesan, South Korea) at 0.01 mg/kg, administered via intramuscular injection before anesthesia. The dogs were anesthetized using a combination of tiletamine and zolazepam (Zoletil®, Virbac Animal Health, Carros, France) at 1.5 mg/kg and medetomidine (Domitor®, Pfizer Animal Health, Walton Oaks, U.K.) at 0.03 mg/kg, administered via intramuscular injection.
Quantitative computed tomography examination
QCT images were obtained using a 4-channel multidetector CT scanner (Light-speed Plus®, GE Healthcare, Amersham, U.K.). The lumbar vertebrae were scanned in dorsal recumbency and the femur in lateral recumbency (Fig. 1). A QCT phantom (QRM-BDC/3®, QRM GmbH, Moehrendorf, Germany) was placed under the vertebrae and femur (Fig. 1). The scanning conditions were as follows: 120 kV and 200 mA, slice thickness 1.25 mm, slice interval 1.25 mm, pitch 1.5:1, rotation time 0.6 sec, and scanning speed 7.5 mm/rotation. The phantom and femur were positioned such that their axes were perpendicular to each other and reconstructed in the transverse plane. The phantom and lumbar vertebrae were positioned such that their axes were parallel to each other. All QCT images were scanned with the bone and beam placed close to vertical without tilting of the gantry.
Fig. 1.
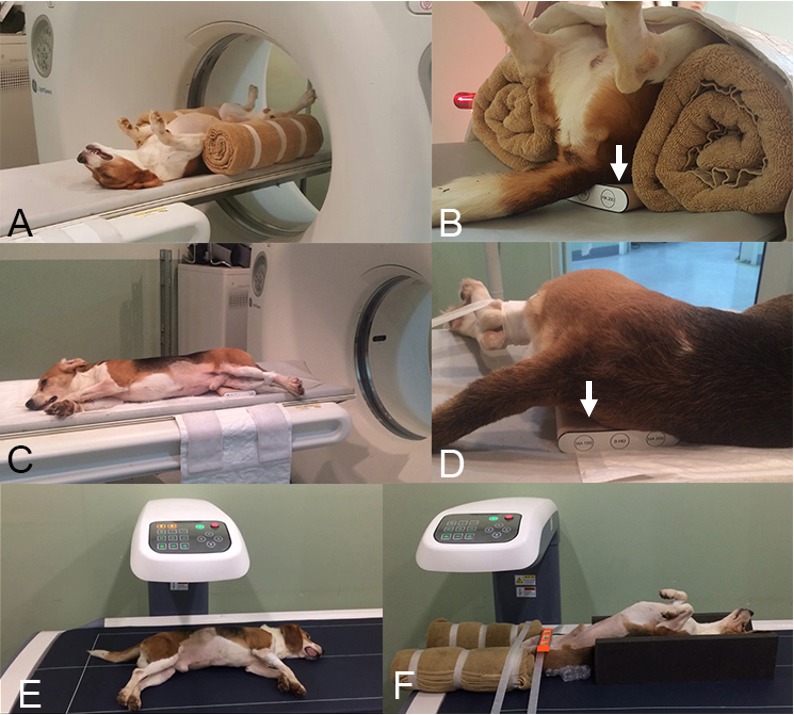
Positioning for quantitative computed tomography (QCT) and dual X-ray absorptiometry (DXA) scanning of the lumbar vertebrae and femur. Positioning of the dog and the bone density calibration phantom (arrows) when scanning the lumbar vertebrae (A and B) and femur (C and D) using QCT. Positioning of the dog and the bone density calibration phantom when scanning the lumbar vertebrae (E) and femur (F) using DXA. DXA, dual X-ray absorptiometry; QCT, quantitative computed tomography.
Dual-energy X-ray absorptiometry examination
The DXA scans were performed after the QCT scans. The stability of the DXA device (PRIMUS®, OsteoSys, Seoul, South Korea) was checked using a calibration phantom (PRIMUS Device PHANTOM®, OsteoSys) that was regularly scanned during the study period. The animals were positioned in lateral recumbency for scanning of the lumbar vertebrae and in dorsal recumbency with internal rotation of the hind limbs for scanning of the femurs (Fig. 1). The scanning conditions were 83 kV and 1.0 mA for the vertebral scans and 83 kV and 0.2 mA for the femoral scans.
Image analysis
Quantitative computed tomography: The region of interest (ROI) for QCT included only the vertebral body in the lumbar region and was measured using a slice at the origin of the transverse process (Fig. 2). The cortical and trabecular bone at all measurement sites were included in the ROI. In the femur, measurements were performed in the middle of the femoral neck, including one third of the proximal diaphysis, and one third of the distal diaphysis (Fig. 2). BMD (mg/cm2) was calculated using Hounsfield units (HU), as in prior studies [6, 14]. All ROIs were drawn by hand and measured 3 times.
Fig. 2.
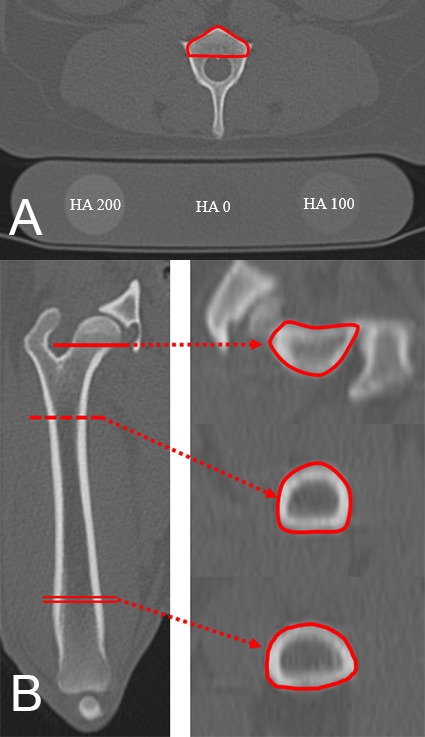
Quantitative computed tomography (QCT) images of the lumbar vertebrae and femur. Vertebral bone mineral density (BMD) (A) was measured at the origin of the transverse process. Note the different attenuation according to the HA content of the phantom placed under the dog. Femoral BMD (B) was measured at the femoral neck, the center of the proximal third of the diaphysis, and the center of the distal third of the diaphysis in transverse images. The solid line indicates the femoral neck, the dotted line indicates the proximal diaphysis, and the double line indicates the distal diaphysis. BMD, bone mineral density; HA, hydroxyapatite; QCT, quantitative computed tomography.
Dual-energy X-ray absorptiometry: The L2–L6 lumbar vertebrae, bilateral femoral neck, and bilateral proximal and distal thirds of the diaphysis were measured using QCT and DXA. To avoid interference of the transverse process and endplate, the ROI for the lumbar vertebrae when scanned using DXA was set to be trapezoidal-shaped to triangular-shaped (Fig. 3), whereas the femoral ROIs were set to be trapezoidal-shaped to rectangular-shaped (Fig. 3). The size of the ROI was set to approximately 0.55 cm2 for the lumbar vertebrae, 0.45 cm2 for the femoral neck, 0.7 cm2 for the proximal diaphysis, and 1.0 cm2 for the distal diaphysis. All ROIs were drawn by hand and measured 3 times.
Fig. 3.
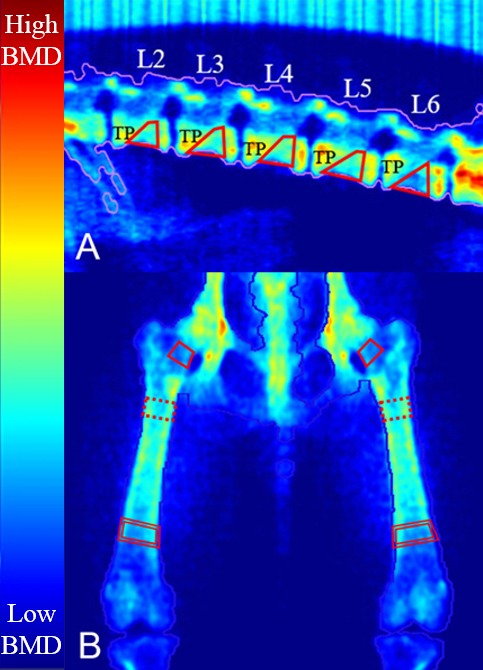
Dual X-ray absorptiometry (DXA) images of the lumbar vertebrae and femur. Vertebral bone mineral density (BMD) (A) was measured except for the transverse process and endplate. Femoral BMD (B) was measured at the femoral neck, the center of the proximal diaphysis, and the center of the distal diaphysis. The color map indicates the degree of X-ray attenuation; low X-ray attenuation appears blue, whereas high X-ray attenuation appears red. The solid line indicates the femoral neck, the dotted line indicates the proximal diaphysis, and the double line indicates the distal diaphysis. BMD, bone mineral density; DXA, dual X-ray absorptiometry; TP, transverse process.
Statistical analysis
The statistical comparisons of QCT and DXA were performed using commercially available software (IBM SPSS 24®, IBM, New York, NY, U.S.A.). The correlation and agreement between the 2 modalities were determined using Pearson correlation and Bland–Altman analysis, respectively. All the data were tested for normality before analysis. All lumbar and femoral sites scanned using QCT and DXA were compared. Interobserver agreement was assessed using 2-way random intraclass correlation coefficients (ICCs). For all comparisons, the level of significance was set at P<0.05.
RESULTS
In total, 231 lumbar and femoral BMD measurements were obtained from the 7 beagle dogs. The average BMD values obtained using QCT and DXA are shown in Table 1. With the exception of the vertebrae, the BMD values obtained using QCT were higher than those obtained using DXA, particularly in the femur. When DXA was used, femoral BMD was lower than vertebral BMD, and when QCT was used, femoral BMD was higher than vertebral BMD. In the lumbar spine, L5 had the highest BMD, and in the femur, the proximal diaphysis exhibited the highest BMD, followed by the distal diaphysis.
Table 1. Bone mineral density at the lumbar vertebrae and femur obtained using quantitative computed tomography and dual X-ray absorptiometry.
| QCT (mg/cm2) | DXA (mg/cm2) | ||
|---|---|---|---|
| Lumbar vertebrae | |||
| L2 | 0.80 ± 0.06 | 0.72 ± 0.04 | |
| L3 | 0.82 ± 0.06 | 0.79 ± 0.06 | |
| L4 | 0.84 ± 0.07 | 0.84 ± 0.09 | |
| L5 | 0.87 ± 0.06 | 0.83 ± 0.08 | |
| L6 | 0.84 ± 0.08 | 0.76 ± 0.08 | |
| Mean | 0.79 ± 0.56 | 0.79 ± 0.86 | |
| Femur | |||
| Femoral neck | 0.90 ± 0.12 | 0.50 ± 0.06 | |
| Proximal diaphysis | 1.11 ± 0.11 | 0.94 ± 0.10 | |
| Distal diaphysis | 0.90 ± 0.12 | 0.65 ± 0.11 | |
| Mean | 0.98 ± 0.14 | 0.70 ± 0.21 | |
The data are shown as the mean and standard deviation. DXA, dual X-ray absorptiometry; QCT, quantitative computed tomography.
Moderate correlation was observed between the BMD values obtained using QCT and DXA in the lumbar vertebrae, weak correlation was observed at the femoral neck, and moderate correlation was observed at the femoral diaphysis (Table 2). The correlation between QCT and DXA with regard to femoral BMD was weaker than that for vertebral BMD. The correlation for the lumbar vertebrae was the weakest for L2, and moderate to strong correlation was observed between QCT and DXA for the other vertebrae. L3 showed the strongest correlation, followed by L5, L4 and L6. In the femur, the proximal diaphysis showed the strongest correlation and the femoral neck showed the weakest correlation. No significant correlation was observed between QCT and DXA with regard to the femoral neck or L2.
Table 2. Pearson correlation between quantitative computed tomography and dual X-ray absorptiometry.
| Correlation coefficient (r) | P-value | ||
|---|---|---|---|
| Lumbar vertebrae | |||
| L2 | 0.20 | 0.38 | |
| L3 | 0.85 | <0.01 | |
| L4 | 0.63 | <0.01 | |
| L5 | 0.69 | <0.01 | |
| L6 | 0.61 | <0.01 | |
| Mean | 0.66 | <0.01 | |
| Femur | |||
| Femoral neck | 0.15 | 0.35 | |
| Proximal diaphysis | 0.43 | <0.01 | |
| Distal diaphysis | 0.40 | <0.01 | |
| Mean | 0.68 | <0.01 | |
DXA, dual X-ray absorptiometry; QCT, quantitative computed tomography.
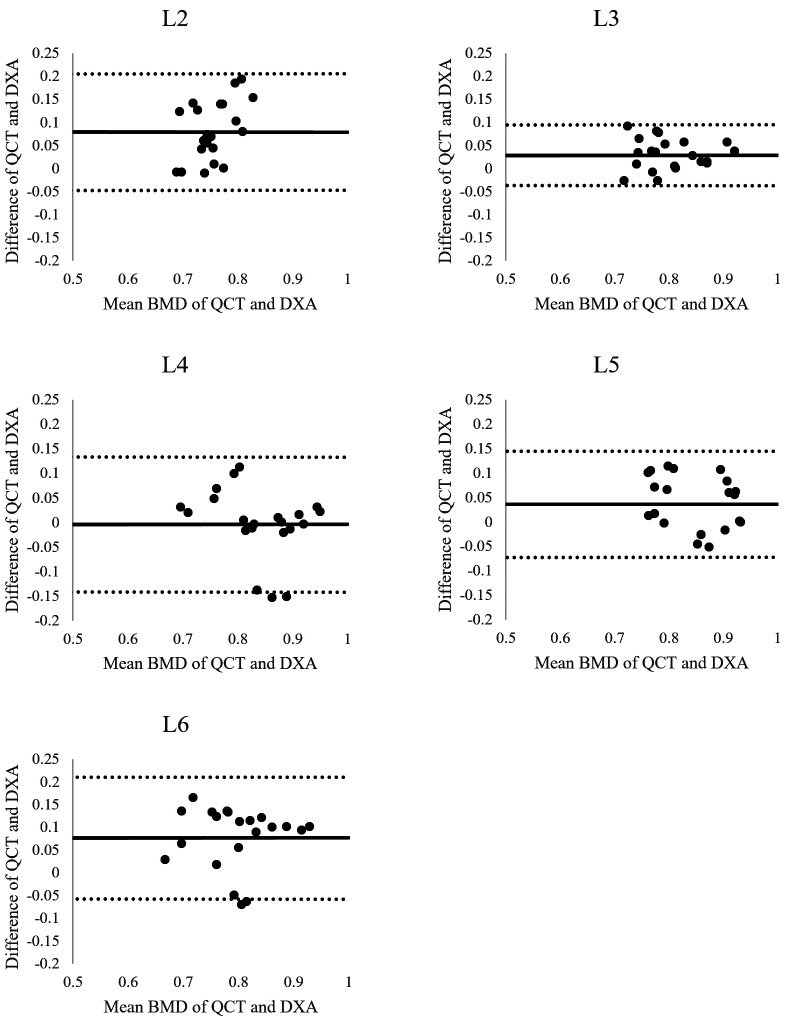
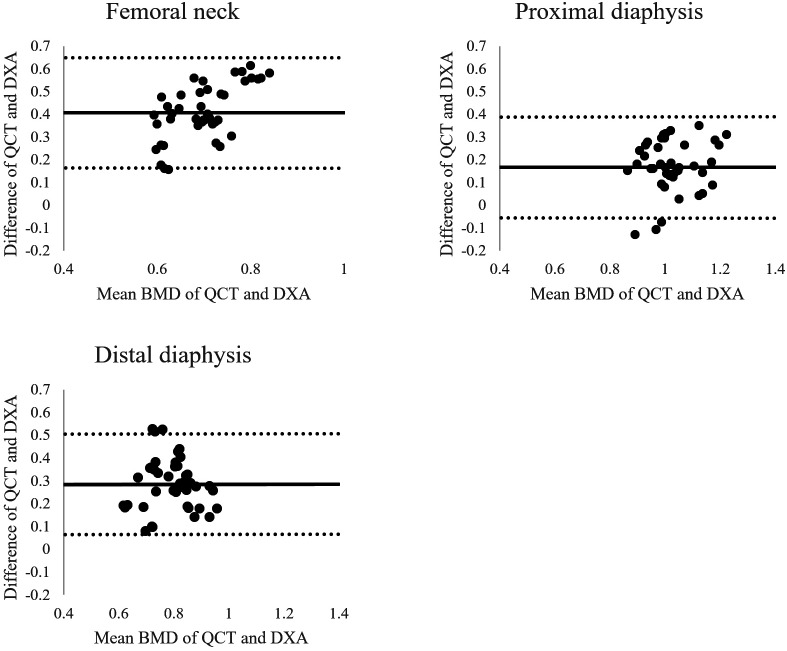
Figures 4 and 5 show the agreement of 2 methods through Bland-Altman plots. The limits of agreement for vertebral BMD were narrower than those for femoral BMD. In the lumbar vertebrae, L3 was shown to have the narrowest limits of agreement, followed by L5, and L4 had the widest limits of agreement, followed by L2. These limits of agreement were similar to those for femoral BMD.
Fig. 4.
Bland–Altman plot comparing vertebral bone mineral density (BMD) values obtained using quantitative computed tomography (QCT) and dual X-ray absorptiometry (DXA). The mean difference (solid line) and limits of agreement (dotted line) are shown. Note that the narrowest limits of agreement are at L3, followed by L5. BMD, bone mineral density; DXA, dual X-ray absorptiometry; QCT, quantitative computed tomography.
Fig. 5.
Bland–Altman plot comparing femoral bone mineral density (BMD) values obtained using quantitative computed tomography (QCT) and dual X-ray absorptiometry (DXA). The mean difference (solid line) and limits of agreement (dotted line) are shown. The limits of agreement at the 3 femoral sites were similar with each other and wider than those at the lumbar vertebrae. BMD, bone mineral density; DXA, dual X-ray absorptiometry; QCT, quantitative computed tomography.
The ICC results indicated that both QCT and DXA had high reproducibility in all parts of the lumbar vertebrae and femur (Table 3). However, the ICC for DXA was consistently higher than that for QCT at all anatomical sites measured. On QCT, the vertebral ICC was higher than the femoral ICC, and on DXA, the vertebral ICC was lower than the femoral ICC.
Table 3. Intraclass correlation of bone mineral density values at each anatomical measurement site.
| ICC for QCT | ICC for DXA | ||
|---|---|---|---|
| Lumbar vertebrae | |||
| L2 | 0.71 | 0.81 | |
| L3 | 0.91 | 0.83 | |
| L4 | 0.93 | 0.97 | |
| L5 | 0.80 | 0.93 | |
| L6 | 0.87 | 0.87 | |
| Mean | 0.84 | 0.94 | |
| Femur | |||
| Femoral neck | 0.72 | 0.99 | |
| Proximal diaphysis | 0.75 | 0.97 | |
| Distal diaphysis | 0.87 | 0.97 | |
| Mean | 0.87 | 0.99 | |
The P-value for all ICC values was <0.05. DXA, dual X-ray absorptiometry; ICC, intraclass coefficient; QCT, quantitative computed tomography.
DISCUSSION
Femoral BMD values have been compared between QCT and DXA previously, but only in an ex situ study [26]. No comparative studies of QCT and DXA have been reported, including any comparison of the femur and vertebrae. In veterinary medicine, more reports have focused on QCT than on DXA [3, 8, 19, 25, 26], and although the accessibility of QCT in veterinary clinical practice is greater than that of DXA, no reports have addressed the accuracy and usefulness of QCT. Therefore, the value of QCT in veterinary medicine requires evaluation.
The BMD values obtained using QCT were the highest at L5 in the lumbar vertebrae, which is consistent with a previous study [19]. The vertebral BMD was similar between QCT and DXA, but the femoral BMD was higher in QCT than in DXA. The reason for the difference in the BMD values at the femur may be that the BMD measurements obtained using DXA are affected by inhomogeneous fat tissue, tissue depth, and extraskeletal calcifications [17, 30, 31] in addition to the thickness, mass, and length of the bone [21, 26]. The thicker the surrounding soft tissue, the higher the attenuation, resulting in a higher BMD value. This error occurs because DXA converts 3-dimensional data to 2-dimensional data and can explain higher vertebral BMD than femoral BMD data obtained using DXA can. In addition, the ROIs were relatively small in relation to image size and limited to rectangular, trapezoidal, or triangular shapes in the DXA images. When measuring BMD on QCT images, the observers attempted to measure the entire image except for blurred areas. High image magnification was necessary to draw the ROI manually, and during this process, except for blurring, interference between surrounding artefacts and soft tissue was minimized. Beam hardening artefacts can also affect BMD measurements obtained using QCT [3]. These limitations and differences may contribute to differences in the results obtained using QCT and DXA. The cortical bone of the femur is thicker than that of the vertebrae; therefore, beam hardening artefacts are more prominent on femoral QCT images than on vertebral QCT images. These artefacts could explain why femoral BMD was higher than vertebral BMD when measured using QCT. The reason for the lower BMD value obtained by DXA at the distal diaphysis when compared with the proximal diaphysis of the femur is that the bone in the former is thinner and wider than that in the latter [22]. Furthermore, some homotypic variation may occur between the right femur and the left femur, which is more evident at the distal diaphysis than at the proximal diaphysis and femoral neck because differences in total bone diameter, medullary diameter, and cortical width are more pronounced at the distal diaphysis [26].
The correlation and agreement of BMD values between QCT and DXA were stronger in the vertebrae than in the femur, particularly at L3. This was because the observers could not measure the femoral BMD in exactly the same cross-section on QCT, and when measuring BMD in the lumbar vertebrae on DXA images, the transverse process of the next vertebra to be measured interfered with the vertebra currently being measured. This interference was the lowest at L3. By contrast, the correlation and agreement at L4–L6 were likely caused by the transverse process of the next vertebra interfering to a greater extent than occurred at L3. Furthermore, the degree of interference on DXA varies from patient to patient. On QCT images, the vertebral body becomes smaller in the caudal lumbar vertebrae, which causes the ROI to become smaller. These factors could have contributed to the weaker correlation and agreement between QCT and DXA at L4–L6. The correlation and agreement were the weakest in the vertebral body of L2 and were not statistically significant, suggesting that in some of the beagle dogs the last rib interfered with the vertebral body measurements obtained using DXA. The correlation and agreement were weaker for the femur than for the lumbar vertebral body, and were significant at all sites except at the femoral neck. The lack of significance at the femoral neck may reflect the fact that the QCT image section was not imaged at the correct transverse section. This suggests that blurring around the cortex of the femoral neck was more severe than at other parts of the femoral diaphysis on the QCT images. The BMD measurements may also have been affected by beam hardening artefacts [3].
The reproducibility of QCT was lower than that of DXA; however, QCT demonstrated high reproducibility. This is because DXA is the gold standard for the measurement of BMD. On the QCT image, vertebral BMD was measured at the origin of the transverse process and femoral BMD was measured at the center of each point. Reproducibility was particularly high in the lumbar vertebrae and at the femoral neck because, typically, only one cross-section could be taken as a reference on QCT. However, reproducibility was high at the diaphysis despite the difficulty in obtaining measurements at the same position. Therefore, it is concluded that reproducible and reliable results can be obtained if radiologists have a detailed understanding of QCT images and know exactly which sites to measure. In this study, measurement of BMD using QCT was highly reproducible, indicating its diagnostic value.
The DXA scanning protocols used in this study were based on those adopted in several prior studies [10, 13, 18]. In the veterinary literature, studies on BMD measured using DXA have been fewer than those using QCT, probably because QCT is more readily accessible than DXA in veterinary medicine. Although DXA is the more accurate method for BMD measurement, it has several limitations. First, positional errors can occur in vertebral and femoral DXA scans; in patients with rotation of the vertebrae such as scoliosis, lordosis, and kyphosis, the vertebrae cannot be positioned appropriately [33]. In human medicine, positioning devices are used when the femur is scanned [33], but no such devices were available for scanning the canine femur when we conducted the present study. Furthermore, different modes of scanning can be used according to the patient’s body habitus in human medicine [33], and various DXA scanning modes would be needed for the various canine breeds. The anterior–posterior (AP) position is the standard DXA scan view for the lumbar vertebrae in both human medicine and veterinary medicine [11, 16]. However, in the present study, lateral DXA scans were performed because they can exclude the abdominal organs and transverse processes, whereas the ventral–dorsal DXA scan cannot exclude the spinous processes when drawing the ROI. In human medicine, several studies have compared AP spine and lateral spine DXA scans [10, 34]. The lateral DXA scan is superior for diagnosing osteopenia and estimating the risk of vertebral fracture because it can avoid spinous processes and osteoarthrosis at the articular facets [10, 34]. In addition, lateral DXA BMD has a stronger correlation with QCT BMD than AP DXA BMD [10]. Despite these advantages, lateral DXA is not used as a standard position because it is difficult to maintain a position during DXA scanning, and the accuracy of predicting the risk of pathologic fracture caused by osteoporosis is similar in both lateral DXA and AP DXA [33]. However, because the dogs used in this study were a deep-chested breed, the lateral position was much easier to maintain than the AP position, and positional errors could be reduced. For these reasons, DXA scanning of the lumbar vertebrae was performed in the lateral position in our study, and additional veterinary studies on positioning and correcting positional errors are needed.
Various CT scanning conditions can affect QCT images and HU values. The scanning parameters used for QCT, such as voltage (kV) and time current product (mAs), have been investigated in previous studies [1, 19], and the protocols used in the present study followed this research. A greater slice thickness is needed to obtain superior images when using QCT because the radiopacity in thinner slices is easily affected by the inhomogeneous fat distribution in trabecular bone [20]. However, in another study [3], no significant difference was observed in CT images with various slice thicknesses, indicating that a thinner slice thickness can be used when measuring BMD by using QCT in small animals. Although QCT images can be affected by scanning conditions, QCT has relatively high accuracy for measuring BMD.
This study had several limitations. First, the study population was small, and the inclusion of only healthy dogs was likely to have affected BMD. Second, any differences in BMD measurements obtained using QCT and DXA according to sex, age group, and breed were not investigated. To minimize differences in these variables, beagle dogs of the same sex and similar age were used. Higher bone mineral content in intact male dogs compared to intact female dogs has been reported [23], and BMD has been revealed to peak at the age of 6 years and decrease gradually thereafter [19, 23, 26]. In human medicine, the Z score or T score is used to compensate for BMD differences in age, sex, and race when assessing BMD [1, 15, 28]. Third, BMD was not verified by measuring the actual mineral concentration in bone. However, DXA has been reported to be accurate and is used as a standard BMD measurement method, and studies have shown a strong correlation between the results of DXA and actual mineral concentrations [21, 35]. Therefore, DXA was used as the gold standard for comparison of the BMD measurements obtained using QCT in this study.
In conclusion, when evaluating BMD using QCT in the region of the lumbar vertebrae, L3 is considered to be the optimal site for monitoring BMD changes, showing high accuracy and reproducibility. In addition, QCT scanning of proximal femoral BMD and femoral neck BMD is considered a useful monitoring technique for evaluating relative values through comparison of the left and right sides or calibration of BMD values. Furthermore, QCT provides more detailed anatomical information in terms of ROI selection and measurement of BMD in dogs. This comparative study showed that QCT has high accuracy and reproducibility in terms of BMD measurement when DXA is used as a standard method. Therefore, QCT is a useful method for evaluation of BMD in veterinary practice.
Acknowledgments
This study was supported financially by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (#316002-05-2-SB010).
REFERENCES
- 1.Adams J. E.2009. Quantitative computed tomography. Eur. J. Radiol. 71: 415–424. doi: 10.1016/j.ejrad.2009.04.074 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong J., da Costa R. C., Martin-Vaquero P.2014. Cervical vertebral trabecular bone mineral density in Great Danes with and without osseous-associated cervical spondylomyelopathy. J. Vet. Intern. Med. 28: 1799–1804. doi: 10.1111/jvim.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae Y., Park S., Jeon S., Lee G., Choi J.2014. Effect of region of interest and slice thickness on vertebral bone mineral density measured by use of quantitative computed tomography in dogs. Am. J. Vet. Res. 75: 642–647. doi: 10.2460/ajvr.75.7.642 [DOI] [PubMed] [Google Scholar]
- 4.Bennett D.1976. Nutrition and bone disease in the dog and cat. Vet. Rec. 98: 313–321. doi: 10.1136/vr.98.16.313 [DOI] [PubMed] [Google Scholar]
- 5.Chalmers H. J., Dykes N. L., Lust G., Farese J. P., Burton-Wurster N. I., Williams A. J., Todhunter R. J.2006. Assessment of bone mineral density of the femoral head in dogs with early osteoarthritis. Am. J. Vet. Res. 67: 796–800. doi: 10.2460/ajvr.67.5.796 [DOI] [PubMed] [Google Scholar]
- 6.Cheon H., Choi W., Lee Y., Lee D., Kim J., Kang J. H., Na K., Chang J., Chang D.2012. Assessment of trabecular bone mineral density using quantitative computed tomography in normal cats. J. Vet. Med. Sci. 74: 1461–1467. doi: 10.1292/jvms.11-0579 [DOI] [PubMed] [Google Scholar]
- 7.Costa L. A. V. S., Lopes B. F., Lanis A. B., De Oliveira D. C., Giannotti J. G., Costa F. S.2010. Bone demineralization in the lumbar spine of dogs submitted to prednisone therapy. J. Vet. Pharmacol. Ther. 33: 583–586. doi: 10.1111/j.1365-2885.2010.01174.x [DOI] [PubMed] [Google Scholar]
- 8.De Decker S., Lam R., Packer R. M. A., Gielen I. M. V. L., Volk H. A.2015. Thoracic and lumbar vertebral bone mineral density changes in a natural occurring dog model of diffuse idiopathic skeletal hyperostosis. PLOS ONE 10: e0124166. doi: 10.1371/journal.pone.0124166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faugere M. C., Friedler R. M., Fanti P., Malluche H. H.1990. Bone changes occurring early after cessation of ovarian function in beagle dogs: a histomorphometric study employing sequential biopsies. J. Bone Miner. Res. 5: 263–272. doi: 10.1002/jbmr.5650050310 [DOI] [PubMed] [Google Scholar]
- 10.Finkelstein J. S., Cleary R. L., Butler J. P., Antonelli R., Mitlak B. H., Deraska D. J., Zamora-Quezada J. C., Neer R. M.1994. A comparison of lateral versus anterior-posterior spine dual energy x-ray absorptiometry for the diagnosis of osteopenia. J. Clin. Endocrinol. Metab. 78: 724–730. [DOI] [PubMed] [Google Scholar]
- 11.Grier S. J., Turner A. S., Alvis M. R.1996. The use of dual-energy x-ray absorptiometry in animals. Invest. Radiol. 31: 50–62. doi: 10.1097/00004424-199601000-00008 [DOI] [PubMed] [Google Scholar]
- 12.Hamdy R. C., Petak S. M., Lenchik L., International Society for Clinical Densitometry Position Development Panel and Scientific Advisory Committee 2002. Which central dual X-ray absorptiometry skeletal sites and regions of interest should be used to determine the diagnosis of osteoporosis? J. Clin. Densitom. 5Suppl: S11–S18. doi: 10.1385/JCD:5:3S:S11 [DOI] [PubMed] [Google Scholar]
- 13.Isola M., Zotti A., Carnier P., Baroni E., Busetto R.2005. Dual-energy X-ray absorptiometry in canine legg-Calvé-Perthes disease. J. Vet. Med. A Physiol. Pathol. Clin. Med. 52: 407–410. doi: 10.1111/j.1439-0442.2005.00746.x [DOI] [PubMed] [Google Scholar]
- 14.Kalender W. A., Suess C.1987. A new calibration phantom for quantitative computed tomography. Med. Phys. 14: 863–866. doi: 10.1118/1.596013 [DOI] [PubMed] [Google Scholar]
- 15.Kanis J. A., Glüer C. C.2000. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos. Int. 11: 192–202. doi: 10.1007/s001980050281 [DOI] [PubMed] [Google Scholar]
- 16.Kanis J.World Health Organization Scientific Group 2007. Assessment of osteoporosis at the primary health care level. Tech. Report. World Health. Organ. Collab. Cent. Metab. Bone Dis. University of Sheffield, Sheffield. [Google Scholar]
- 17.Laskey M. A., Lyttle K. D., Flaxman M. E., Barber R. W.1992. The influence of tissue depth and composition on the performance of the Lunar dual-energy X-ray absorptiometer whole-body scanning mode. Eur. J. Clin. Nutr. 46: 39–45. [PubMed] [Google Scholar]
- 18.Lauten S. D., Cox N. R., Brawner W. R., Jr, Baker H. J.2001. Use of dual energy x-ray absorptiometry for noninvasive body composition measurements in clinically normal dogs. Am. J. Vet. Res. 62: 1295–1301. doi: 10.2460/ajvr.2001.62.1295 [DOI] [PubMed] [Google Scholar]
- 19.Lee D., Lee Y., Choi W., Chang J., Kang J. H., Na K. J., Chang D. W.2015. Quantitative CT assessment of bone mineral density in dogs with hyperadrenocorticism. J. Vet. Sci. 16: 531–542. doi: 10.4142/jvs.2015.16.4.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J., Kim K., Park C.2001. Effect of the slice thickness and the size of region of interest on CT number. Korean J Oral Maxillofac Radiol 31: 85–91. [Google Scholar]
- 21.Lochmüller E. M., Miller P., Bürklein D., Wehr U., Rambeck W., Eckstein F.2000. In situ femoral dual-energy X-ray absorptiometry related to ash weight, bone size and density, and its relationship with mechanical failure loads of the proximal femur. Osteoporos. Int. 11: 361–367. doi: 10.1007/s001980070126 [DOI] [PubMed] [Google Scholar]
- 22.Markel M. D., Sielman E., Bodganske J. J.1994. Densitometric properties of long bones in dogs, as determined by use of dual-energy x-ray absorptiometry. Am. J. Vet. Res. 55: 1750–1756. [PubMed] [Google Scholar]
- 23.Martin R. K., Albright J. P., Jee W. S. S., Taylor G. N., Clarke W. R.1981. Bone loss in the beagle tibia: influence of age, weight, and sex. Calcif. Tissue Int. 33: 233–238. doi: 10.1007/BF02409442 [DOI] [PubMed] [Google Scholar]
- 24.Norrdin R. W., Carpenter T. R., Hamilton B. F., Brewster R. D.1988. Trabecular bone morphometry in beagles with hyperadrenocorticism and adrenal adenomas. Vet. Pathol. 25: 256–264. doi: 10.1177/030098588802500402 [DOI] [PubMed] [Google Scholar]
- 25.Park S., Oh J., Son K. Y., Cho K. O., Choi J.2015. Quantitative computed tomographic assessment of bone mineral density changes associated with administration of prednisolone or prednisolone and alendronate sodium in dogs. Am. J. Vet. Res. 76: 28–34. doi: 10.2460/ajvr.76.1.28 [DOI] [PubMed] [Google Scholar]
- 26.Schneider S., Breit S. M., Grampp S., Künzel W. W. F., Liesegang A., Mayrhofer E., Zentek J.2004. Comparative assessment of bone mineral measurements obtained by use of dual-energy x-ray absorptiometry, peripheral quantitative computed tomography, and chemical-physical analyses in femurs of juvenile and adult dogs. Am. J. Vet. Res. 65: 891–900. doi: 10.2460/ajvr.2004.65.891 [DOI] [PubMed] [Google Scholar]
- 27.Schwarz T., Störk C. K., Mellor D., Sullivan M.2000. Osteopenia and other radiographic signs in canine hyperadrenocorticism. J. Small Anim. Pract. 41: 491–495. doi: 10.1111/j.1748-5827.2000.tb03970.x [DOI] [PubMed] [Google Scholar]
- 28.Shin Y. L.2006. Assessment of bone mineral density. J. Korean Soc. Pediatr. Endocrinol. 11: 123–130. [Google Scholar]
- 29.Stone M. D., Alec J. T.2012. Use of dual-energy X-Ray absorptiometry (DXA) with non-human vertebrates: application, challenges, and practical considerations for research and clinical practice. In: A Bird’s-Eye View of Veterinary Medicine (Carlos, C. P. M. ed.), InTech, Rijeka. [Google Scholar]
- 30.Svendsen O. L., Hassager C., Skødt V., Christiansen C.1995. Impact of soft tissue on in vivo accuracy of bone mineral measurements in the spine, hip, and forearm: a human cadaver study. J. Bone Miner. Res. 10: 868–873. doi: 10.1002/jbmr.5650100607 [DOI] [PubMed] [Google Scholar]
- 31.Tothill P., Weir N., Loveland J.2014. Errors in dual-energy X-ray scanning of the hip because of nonuniform fat distribution. J. Clin. Densitom. 17: 91–96. doi: 10.1016/j.jocd.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 32.Verhaeghe J., van Herck E., Visser W. J., Suiker A. M. H., Thomasset M., Einhorn T. A., Faierman E., Bouillon R.1990. Bone and mineral metabolism in BB rats with long-term diabetes. Decreased bone turnover and osteoporosis. Diabetes 39: 477–482. doi: 10.2337/diab.39.4.477 [DOI] [PubMed] [Google Scholar]
- 33.Watts N. B.2004. Fundamentals and pitfalls of bone densitometry using dual-energy X-ray absorptiometry (DXA). Osteoporos. Int. 15: 847–854. doi: 10.1007/s00198-004-1681-7 [DOI] [PubMed] [Google Scholar]
- 34.Zmuda J. M., Cauley J. A., Glynn N. W., Finkelstein J. S.2000. Posterior-anterior and lateral dual-energy x-ray absorptiometry for the assessment of vertebral osteoporosis and bone loss among older men. J. Bone Miner. Res. 15: 1417–1424. doi: 10.1359/jbmr.2000.15.7.1417 [DOI] [PubMed] [Google Scholar]
- 35.Zotti A., Isola M., Sturaro E., Menegazzo L., Piccinini P., Bernardini D.2004. Vertebral mineral density measured by dual-energy X-ray absorptiometry (DEXA) in a group of healthy Italian boxer dogs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 51: 254–258. doi: 10.1111/j.1439-0442.2004.00630.x [DOI] [PubMed] [Google Scholar]
- 36.Zotti A., Caldin M., Vettorato E., Ferrari V., Cavicchioli L., Bernardini D.2006. Bone mineral density in two boxer dogs affected by moderate to end-stage chronic renal failure. Vet. Res. Commun. 30: 337–339. doi: 10.1007/s11259-006-0075-z [DOI] [Google Scholar]