Abstract
Bartonella bovis is a small Gram-negative bacterium recognized as an etiological agent for bacteremia and endocarditis in cattle. As few reports are available on the taxonomic position of B. bovis and its mechanism of virulence, this study aims to resolve the phylogeny of B. bovis and investigate putative virulence genes based on whole genome sequence analysis. Genome-wide comparisons based on single nucleotide polymorphisms (SNP) and orthologous genes were performed in this study for phylogenetic inference of 27 Bartonella species. Rapid Annotation using Subsystem Technology (RAST) analysis was used for annotation of putative virulence genes. The phylogenetic tree generated from the genome-wide comparison of orthologous genes exhibited a topology almost similar to that of the tree generated from SNP-based comparison, indicating a high concordance in the nucleotide and amino acid sequences of Bartonella spp. The analyses show consistent grouping of B. bovis in a cluster related to ruminant-associated species, including Bartonella australis, Bartonella melophagi and Bartonella schoenbuchensis. RAST analysis revealed genes encoding flagellar components, in corroboration with the observation of flagella-like structure of BbUM strain under negative straining. Genes associated with virulence, disease and defence, prophages, membrane transport, iron acquisition, motility and chemotaxis are annotated in B. bovis genome. The flagellin (flaA) gene of B. bovis is closely related to Bartonella bacilliformis and Bartonella clarridgeiae but distinct from other Gram-negative bacteria. The absence of type IV secretion systems, the bona fide pathogenicity factors of bartonellae, in B. bovis suggests that it may have a different mechanism of pathogenicity.
Keywords: Bartonella bovis, genome-based phylogeny, putativevirulence genes
Bartonella species are small, fastidious Gram-negative bacteria which are responsible for a wide variety of clinical syndromes in humans and animals [9,10,11]. The bacteria infect mammalian erythrocytes and endothelial cells and are usually transmitted through the bites of hematophagous arthropods, such as fleas, lice, flies, and ticks [11]. Humans acquire Bartonella henselae or Bartonella clarridgeiae infections following scratches or bites by cats infested with flea (Ctenocephalides felis). Bartonella bacilliformis, the etiologic agent of Carrion’s disease or Oroya fever, is transmitted by the sandflies of the genus Lutzomyia. The transmission of trench fever (caused by Bartonella quintana) occurs through the bites of infected human louse (Pediculus humanus) [2]. Rodent-borne Bartonella spp., such as Bartonella elizabethae, Bartonella tamiae, Bartonella rattimassiliensis, and Bartonella tribocorum have been associated with zoonotic diseases [31, 32].
Several Bartonella spp. including Bartonella bovis, Bartonella melophagi, Bartonella schoenbuchensis, and Bartonella australis have been isolated from ruminants. Cattle have been reported as the main reservoir for B. bovis. The prevalence of B. bovis in cattle is generally high but varies widely across studies from different continents [5]. Long-term persistence of B. bovis and adaptation to cattle has been postulated to mimic Bartonella infection in cats and mice [37]. As the potential role of B. bovis as a zoonotic pathogen has been suggested [13, 43], further studies on the genomic and virulence of this bacterium is required. Several B. bovis strains have been identified using multilocus sequence typing (MLST) approach in a Malaysian study [27]. One of the strains (herein referred to as BbUM) isolated from a dairy cow, has been identified as MLST ST27 and proposed under a new lineage (IIa) of B. bovis [27].
In this study, the draft genome sequence of the BbUM strain was determined to enable further study on the phylogeny and prediction of virulence genes. To verify the species status of BbUM strain, average nucleotide identity (ANI) and tetranucleotide usage patterns (TETRA) analyses were performed [20, 28, 54]. ANI determination is based on pair-wise comparisons of sequences shared between two strains. The approach has been used widely for classification of bacterial species [24]. For species definition, ANI values >94% correspond to 70% DNA–DNA re-association standard [20, 29, 30]. TETRA analyzes tetranucleotide usage patterns in genomic fragments, and a correlation co-efficient value of above 0.99 suggests the probability of two strains as the same species [51]. Previously, the genetic classification of Bartonella spp. has been based on sequence analysis of 16S rDNA and several protein-encoding genes [25, 39, 42]. However, the high degree of 16S rDNA sequence similarity provides very little statistical support for the determination of phylogenetic relationship within the genus Bartonella [42]. Additionally, the prediction of phylogenetic relationship between species can be confusing when two or more protein genes are analysed [25].
With the availability of whole genome sequences for most Bartonella spp., comparative genome-wide analyses based on single nucleotide polymorphisms (SNP) and orthologous genes are now possible. In a previous study [23], the phylogenetic relationship of 16 Bartonella species has been analyzed based on orthologous core genes. This study reports the phylogenetic analyses of 27 Bartonella species based on SNP and orthologous genes. Additionally, putative virulence genes are identified by using Rapid Annotation using Subsystem Technology (RAST) analysis.
MATERIALS AND METHODS
Growth conditions and whole genome sequencing of BbUM strain
BbUM strain was cultured on fresh Columbia agar supplemented with 5% sheep blood (Isolab, Shah Alam, Malaysia) at 37°C in 5% CO2 atmosphere for four days. After Gram staining, the strain was observed under light microscopy. Bacterial morphology was observed using transmission electron microscopy (EM JEM1200EX; JEOL, Tokyo, Japan) at 100 kV with negative staining. The colonies were suspended in lysis buffer for DNA extraction using a QIAmp DNA mini kit (Qiagen, Hilden, Germany). The concentration and purity of the eluted DNA were measured using a NanoDrop™ 2000 Spectrophotometer (Thermo Scientific, Wilmington, DE, U.S.A.) and a Qubit fluorometer (Invitrogen Corp., Carlsbad, CA, U.S.A.). The DNA sample was sequenced using Illumina platform with 100 bp paired-end reads. The reads were then subjected to adapters and low quality reads trimming based on Q25 using Trimmomatics [8] and Sycthe (https://github.com/vsbuffalo/scythe). String Graph Assembler (SGA) [50] was used for error correction. After pre-analysis, the reads were subjected to de novo genome assembly, using IDBA-UD, a de novo assembler for sequencing data with highly uneven depth [41]. Only contigs larger than 200bp were used for downstream analysis. The assembled contigs were subjected to left over adapters and contaminant filter against NCBI univec database via seq clean (default parameter) (http://hpc.ilri.cgiar.org/seqclean/seqclean). The whole genome sequencing project has been submitted to the GenBank database (accession no: MWVG00000000).
Inter-strain comparisons with ANI and TETRA analyses
Analyses to determine average nucleotide identity (ANI) [20] and tetra-nucleotide usage patterns TETRA (http://www.megx.net/tetra) [54] were performed by using JSpecies v1.2.1 [44], using 27 Bartonella genome sequences available in the GenBank database. Table 1 is the list of Bartonella species included in this analysis.
Table 1. List of Bartonella species and outgroup selected for SNP and orthologous gene analysis.
| Genome | Accession number | Source | Cluster (SNP)a) | Cluster (Orthologous gene)b) |
|---|---|---|---|---|
| B. quintana RM-11 | NC_018533 | Monkey (Rhesus macaques) | 1 | I |
| B. henselae MVT02 | NZ_LN879429 | Human (Homo sapiens) | 1 | I |
| B. henselae str. Houston-1 | NC_005956 | Human (Homo sapiens) | 1 | I |
| B. koehlerae C-29 | NZ_AHPL00000000 | Cat (Felis domesticus) | 1 | I |
| B. senegalensis OS02 | NZ_CALV00000000 | Soft tick (Ornithodoros sonrai) | 1 | I |
| B. elizabethae (BeUM) | LFMF00000000 | Wild rat (Rattus diardii) | 2 | II |
| B. elizabethae F9251 | NZ_AIMF00000000 | Human (Homo sapiens) | 2 | II |
| B. elizabethae Re6043vi | NZ_AILW00000000 | Polynesian rat (Rattus exulans) | 2 | II |
| B. queenslandensis AUST/NH15 | NZ_CALX00000000 | Wild rat (Rattus leucopus) | 2 | II |
| B. rattimassiliensis 15908 | NZ_AILY00000000 | Wild rat (Rattus norvegicus) | 2 | II |
| B. tribocorum BM1374166 | NZ_HG969192 | Wild rat (Rattus norvegicus) | 2 | II |
| B. tribocorum CIP 105476 | NC_010161 | Wild rat (Rattus norvegicus) | 2 | II |
| B. alsatica IBS 382 | NZ_AIME00000000 | Wild rabbit (Oryctolagus cuniculus) | 2 | IV |
| B. vinsonii spp. berkhoffii | NC_020301 | Dog (Canis lupus) | 2 | IV |
| B. birtlesii IBS 325 | NZ_CM001557 | Mouse (Apodemus spp.) | 3 | IV |
| B. taylorii 8TBB | NZ_AIMD00000000 | Vole (Microtus agrestis) | 3 | IV |
| B. washoensis Sb944nv | NZ_AILU00000000 | California ground squirrel(Spermophilus beecheyi) | 3 | I |
| B. doshiae NCTC 12862 | NZ_AILV00000000 | Vole (Microtus agrestis) | 3 | - |
| B. australis Aust/NH1 | NC_020300 | Kangaroo (Macropus giganteus) | 3 | III |
| B. bacilliformis KC583 | NC_008783 | Human (Homo sapiens) | 3 | III |
| B. bovis (BbUM) | MWVG00000000 | Crossbred cattle (Mafriwal) | 3 | III |
| B. bovis 91–4 | NZ_CM001844 | Cattle (Bos taurus) | 3 | III |
| B. bovis m02 | NZ_AGWB00000000 | Moose (Alces alces) | 3 | III |
| B. clarridgeiae 73 | NC_014932 | Cat (Cattus felis) | 3 | III |
| B. melophagi K-2C | NZ_AIMA00000000 | Sheep ked (Melophagus ovinus) | 3 | III |
| B. rochalimae ATCC BAA-1498 | NZ_AHPK00000000 | Human (Homo sapiens) | 3 | III |
| B. schoenbuchensis m07a | NZ_AGWC00000000 | Moose (Alces alces) | 3 | III |
| Brucella abortus 2308 | NC_007618 (Chr 1), NC_007624 (Chr 2) | Aborted fetus of a cow | N/A | N/A |
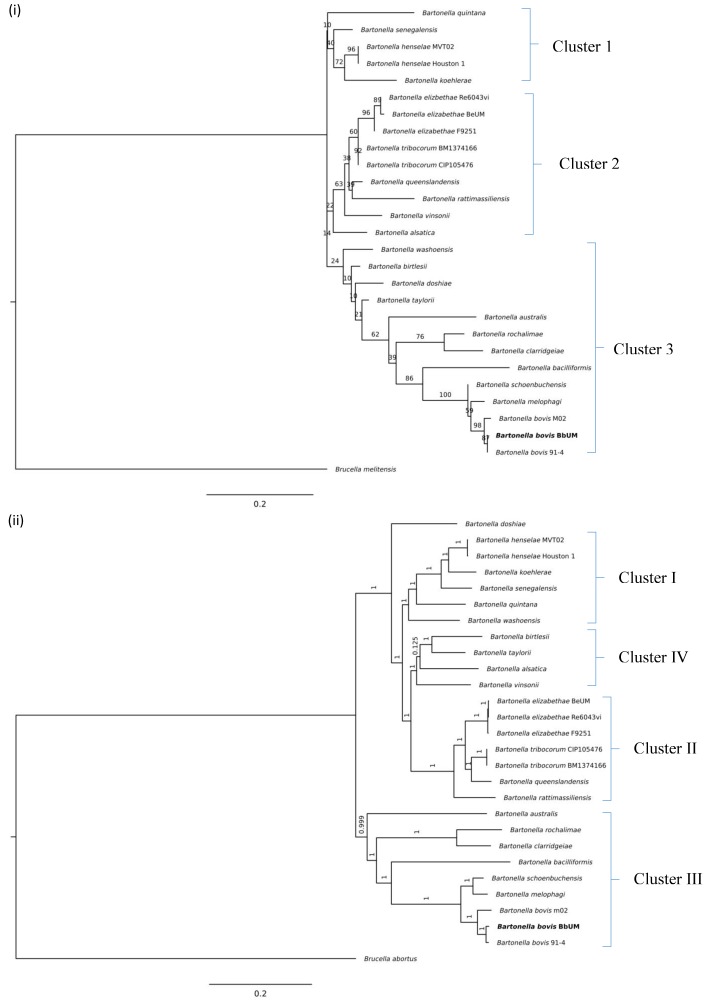
a) refer to Fig. 2 (i); b) refer to Fig. 2 (ii); N/A, not available.
Investigation of phylogenetic relationships amongst Bartonella species
In this study, genome-wide SNP and orthologous gene analyses were performed for inference of phylogenetic relationship amongst Bartonella species. i) Genome-wide SNP-based comparison. Completed and draft sequences downloaded from NCBI (Table 1) were subjected to a series of pre-processing to generate a FASTA input file compatible with the analytic software. The analysis was carried out using the software package kSNP3.0, in which SNP discovery was based on k-mer analysis, using single variant position within sequences of nucleotide length k [19]. The kchooser script was used to select an optimum value of k-mer size for the data set. Jellyfish [38] in the kSNP3.0 package was used to enumerate a list of k-mers at optimum k-value for each genome in the data set. The sequence variation of the data set was evaluated and the fraction of core-k-mers at optimum k-value was identified. kSNP was used to search for putative SNP loci in the data set and the identified SNP alleles were concatenated into a string. Multiple sequence alignment was produced for each nucleotide in the SNP matrix using MUSCLE (version 3.8.31) [18]. Best fit nucleotides substitution model was selected using jModelTest [17, 21] and trees of different models were assessed using Akaike Information Criterion framework (AIC) [1]. PhyML 3.0 [22] was used to generate maximum likelihood tree with 100 bootstrap replicates using the jModelTest suggested model. ii) Genome-wide comparison of orthologous genes. The predicted proteins from BbUM genome were compared to proteins from other Bartonella species and Brucella abortus (Table 1) using OrthoMCL (version 2.0.9) [14, 35]. Subsequently, shared proteins (core proteins) of three B. bovis strains (B. bovis m02, 91-4 and BbUM) were analysed. A Venn diagram was drawn to show the distribution of genes amongst three B. bovis strains. Orthologue groups with single-copy genes in all species in the study were identified. For each orthologue group, the protein sequences for all species were extracted and aligned using MUSCLE (version 3.8.31) [18]. Each multiple sequence alignment was trimmed using trimAl (version 1.4) [12] before merging into one long sequence alignment of orthologous proteins using a custom script. The alignment was then subjected to substitution model tests using ProtTest (version 3.4 release 20140123) [16] and phylogeny estimation was performed using PhyML (release 20111216) [21]. Owing to the large alignment size, branch support for each node in the protein evolution estimation was tested with the approximate likelihood ratio test (aLRT) [3] instead of bootstrap. Trees were generated separately using three supported aLRTs in PhyML, i.e., aLRT, Chi2-based aLRT and SH-like aLRT.
Annotation and gene prediction
The contigs were subjected to gene prediction using Prokaryotic Dynamic Programming Genefinding Algorithm (PRODIGAL) Version 2.60 [26]. Annotation of predicted coding sequences was performed by homology search against the nr database. tRNA and rRNA were predicted using ARAGORN [34] and RNAmmer [33], respectively. The assembled contigs were annotated using RAST (Rapid Annotation using Subsystem Technology) pipeline [4].
Analysis of flagella (flaA) gene
Two flaA sequences (type 1 and 2) of BbUM strain were retrieved based on the data generated from RAST analysis and compared for similarity with other flagella sequences in the GenBank database using BioEdit Sequence Alignment Editor Software (Version 7.0.5.3). A dendrogram was constructed based on the sequences using neighbour-joining method of the MEGA software and bootstrap analysis with 1,000 re-samplings [52]. E. coli flagellin (fliC, NC000913) was used as an outgroup for comparison.
RESULTS
B. bovis BbUM strain demonstrated small (1–2 mm in diameter), smooth and greyish colonies upon culturing on blood agar after four days of incubation. Electron microscopy of negative staining reveals flagella-like structure at one pole and a thin slime layer surrounding the cell body of the BbUM strain (measuring 1.45 µm in length and 0.59 µm in width) (Fig. 1).
Fig. 1.
Transmission electron micrograph showing the presence of a flagella-like structure (arrow) at the polar region and slime layer surrounding the cell body of B. bovis BbUM strain.
The assembly from the sequencing reads of BbUM strain generated 331 contigs with an average length of 4,977 bp (200–406,386 bp). N50, the length of a collection of contigs that covers at least 50% of the genome assembly, was 158,089 bp. The draft genome of BbUM strain has a total length of 1,647,450 bp and a GC content of 37.7%. BbUM strain shares the highest values of ANI (98.12%) and TETRA (0.99438) with B. bovis 91–4 type strain, followed by B. bovis m02 (Suppl Table 1), thus placing the strains under the same species. The next genetically closely related species with B. bovis were B. melophagi K-2C, and B. schoenbuchensis m07a (Suppl. Table 1), two Bartonella species originated from sheep ked and moose, respectively.
Figure 2 (i) shows the SNP-based phylogenetic tree of Bartonella spp. using B. abortus 2308 as an outgroup. The SNP-based phylogenetic tree shows the differentiation of Bartonella spp. into three distinct clusters (1–3) (Table 1). Cluster 1 consists of two cat-associated species, i.e., B. henselae and B. koehlerae, B. quintana and a tick-derived B. senegalensis. Cluster 2 is mainly comprised of rodent-associated Bartonella spp. i.e., B. elizabethae, B. tribocorum, B. queenslandensis, and B. rattimassiliensis, a dog-derived (B. vinsonii) and a rabbit-derived Bartonella spp. (B. alsatica). Cluster 3 has the largest number of Bartonella spp., with four ruminant-associated species (B. australis, B. bovis, B. melophagi and B. schoenbuchensis), four rodent-associated species (B. washoensis, B. birtlesii, B. doshiae and B. taylorii) and three human pathogens (B. rochalimae, B. clarridgeiae, and B. bacilliformis).
Fig. 2.
(i) SNP-based phylogenetic tree with Brucella abortus 2308 as an outgroup. (ii) Construction of dendrogram based on protein evolution of Bartonella species. The analysis indicates that BbUM strain is originated from the same cluster with B. bovis type strain 91-4.
In the analysis of orthologous genes, a total of 2,690 orthologue groups were identified, of which, 1,299 groups contained at least one B. bovis (B. bovis m02, B. bovis 91–4 and BbUM) protein. There was a high number of shared orthologue groups (1,092), suggesting the high degree of similarity amongst B. bovis strains. The protein evolution estimation using three different aLRTs produced the same tree with different branch-support values. Figure 2 (ii) shows the dendrogram constructed based on SH-like aLRT branch support. Four clusters (I-IV) were obtained. The cat-associated species (B. henselae and B. koehlerae), B. quintana and a tick-derived B. senegalensis are grouped in the same phylogenetic group (cluster I) with B. washoensis. Cluster II is mainly comprised of rodent-associated Bartonella spp. (B. elizabethae, B. tribocorum, B. queenslandensis, and B. rattimassiliensis). BbUM strain is placed in the same phylogenetic group (cluster III) with B. bovis 91–4 strain and other ruminant-associated Bartonella spp. (B. australis, B. bovis, B. melophagi, and B. schoenbuchensis) and three human pathogens (B. rochalimae, B. clarridgeiae, B. bacilliformis), similar to the results derived from SNP-based phylogenetic analysis.
The taxonomic positions of B. washoensis, B. doshiae, B. birtlesii, B. taylorii, B. vinsonii and B. alsatica were inconsistent by SNP and orthologous gene analyses. Three rodent-associated Bartonella spp. i.e., B. birtlesi, B. taylorii, B. alsatica and a dog-derived Bartonella spp. (B. vinsonii) previously grouped in cluster 3 in the SNP-based phylogeny tree formed a new cluster (IV) in the orthologous gene analysis (Fig. 2 i and ii).
Figure 3 shows the distribution of BbUM genes in different functional categories assigned by the RAST pipeline. These genes include those involved in the basic biological functions such as protein metabolism (199 genes), amino acid and derivatives (128 genes), cofactors, vitamins, prosthetic group pigments (99 genes), RNA metabolism (97 genes), DNA metabolism (71 genes), carbohydrates (58 genes), and fatty acids, lipids and isoprenoids (50 genes) and motility and chemotaxis (50 genes). Based on RAST analysis, 10 genes associated with phages (encoding phage major tail tube, sheath proteins, and the large subunit of phage terminase in the subsystem of phage packaging machinery) were annotated in the BbUM genome (Table 2).
Fig. 3.
Subsystem statistics (based on functional categories) of the RAST-predicted genes of BbUM strain.
Table 2. RAST prediction of genes in BbUM, classified under various functional categories.
| Virulence, disease and defense (n=27) |
Phages, prophages, transposable elements, plasmids (n=10) |
Membrane transport (n=45) |
Motility and chemotaxis (n=50) |
|---|---|---|---|
| Bacteriocins, ribosomally synthesized antibacterial peptides (6)
-Colicin V and bacteriocin adhesion (0) -Toxins and superantigens (0) -Production cluster (6) Resistance to antibiotics and toxic compounds (12) -Lysozyme inhibitors (1) -Cobalt-zinc-cadmium resistance (4) -Resistance to fluoroquinolones (4) -Copper homeostasis: copper tolerance (2) -Beta-lactamase (1) Invasion and intracellular resistance (9) -Mycobacterium virulence operon involved in protein synthesis (SSU ribosomal proteins) (4) -Mycobacterium virulence operon involved in DNA transcription (2) -Mycobacterium virulence operon involved in protein synthesis (LSU ribosomal proteins) (3) |
Phages, prophages (10) Pathogenicity islands (0) Gene transfer agent (GTA) (0) Plasmid related functions (0) Phage family-specific subsystems (0) Transposable elements (0) |
Protein translocation across cytoplasmic membrane (3) -ABC transporters (14) -Cation transporters (2) -Uni-sym and antiporters (8) Membrane transport-no subcategory (18) Protein secretion system, Type I, II, III, IV, V, VI (0) Protein secretion system, Type II (0) Protein secretion Protein secretion system, Type VII (chaperone/usher pathway, CU) (0) Protein secretion system, Type VIII (Extracellular nucleation/precipitation pathway, ENP) (0) TRAP transporters (0) Sugar phosphotransferase systems, PTS (0) |
Flagellar motility in Prokaryota (50) Magnetotaxis (0) Motility and chemotaxis- no subcategory (0) Social motility and nonflagellar swimmingin bacteria (0) |
The numbers in parenthesis=No. of genes.
The RAST prediction of genes associated with virulence, disease and defence, phages, prophages, transposable elements, plasmids; membrane transport, and motility and chemotaxis is shown in Table 2. The genes associated with the virulence, defence and intracellular resistance of BbUM strains including colicin V and bacteriocin production cluster, lysozyme inhibitors, cobalt-zinc-cadmium resistance, resistance to fluoroquinolones, copper homeostasis: copper tolerance, and beta-lactamase, and Mycobacterium virulence operons involved in protein synthesis (SSU and LSU ribosomal proteins) and DNA transcription are annotated in the BbUM genome (Table 2). In the membrane transport category, type IV secretion systems (T4SSs), VirB/VirD4, Vbh/TraG and Trw, and T4SS-translocated effector proteins were not annotated in the BbUM strain. Additionally, the adhesins in bartonellae, the orthologous trimeric autotransporter adhesins, BadA in B. henselae and the Vomp family in B. quintana [56] are also not annotated.
Several genes encoding protein translocation across the cytoplasmic membrane, ABC transporters, cation transporters and Uni-Sym- and Antiporters are annotated in BbUM genome (Table 2). Various components of iron acquisition metabolism including iron compound ABC uptake transporter substrate-binding proteins PiuA and PiuC, hemin transport protein HmuS, periplasmic hemin-binding protein, ABC-type hemin transport system, ATPase component, electron transfer flavoprotein, beta subunit, ferric siderophore transport system, periplasmic binding protein TonB, TonB-dependent hemin, and ferrichrome receptor are also annotated in the BbUM genome.
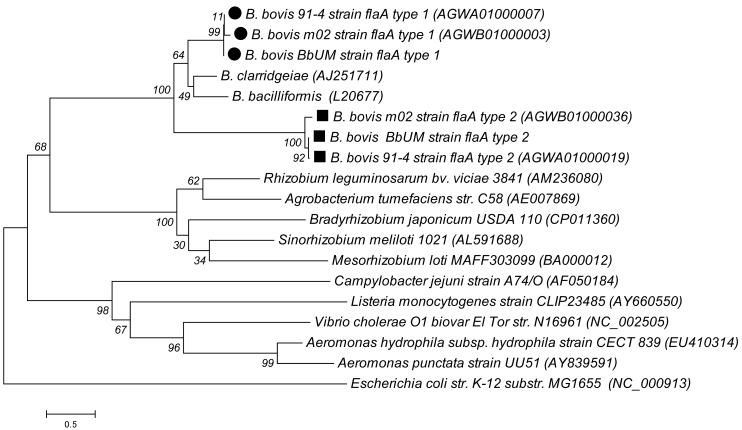
Since flagella-like structure was observed under electron microscopy (Fig. 1), the presence of flagellin gene in BbUM genome was investigated. Based on RAST analysis, 50 genes associated with flagellar motility were annotated under the motility and chemotaxis category (Table 2). The genes included flaA, flgB, flgC, flgD, flgE, flgF, flgG, flgH, flgI, flgK, flgL, flhA, flhB, fliE, fliF, fliG, fliI, fliL, fliM, fliP, fliQ, fliR, motA and motB. Additionally, the genes for flagellar protein FlgJ and flagellar hook-associated switch protein (FliN) were also annotated. Two flagellin genes (labelled as flaA sequence types 1 and 2) sharing the highest sequence similarities (60.6 and 73.0% nucleotide similarity, respectively) with that of B. bacilliformis, and 59.8 and 73.6% nucleotide similarity, respectively, with that of B. clarridgeiae, were retrieved for further analysis. The flaA type 1 gene of BbUM strain was also detected in B. bovis 91-4 and m02 strains with 99.6 and 94.2% sequence similarities, while the type 2 gene was detected in B. bovis 91-4 and m02 strains with 99.2 and 90.7% sequence similarities, with the BbUM strain. The similarity between the flaA type 1 and type 2 sequences for BbUM strain was 55.5%. Figure 4 shows the comparison of flaA sequence types 1 and 2 with those of B. clarridgeiae and B. bacilliformis, and several other published bacterial flaA genes. All Bartonella flaA genes formed a cluster (with 100% bootstrap value) separated from the plant-associated soil bacteria (Agrobacteriun tumefaciens and Sinorhizobium meliloti), Campylobacter jejuni, Listeria monocytogenes, Vibrio cholerae, Aeromonas hydrophila and A. punctata (Fig. 4).
Fig. 4.
Phylogenetic tree constructed based on flaA genes of BbUM strain, and others available in the GenBank database.
DISCUSSION
The high values obtained from the interstrain comparison using ANI and TETRA analyses in this study (ANI=98.12%, TETRA=0.99438 with reference to B. bovis 91-4 type strain) confirms the high degree of genetic relatedness amongst B. bovis strains. Taken together, the phylogenetic tree generated from the genome-wide comparison of orthologous genes from 27 Bartonella species exhibited a topology almost similar to that of the phylogenetic tree generated from SNP-based comparison, indicating a high concordance in the nucleotide and amino acid sequences of Bartonella spp. Two cat-associated Bartonella spp. (B. henselae and B. koehlerae) and a tick-derived Bartonella sp. (B. senegalensis) are grouped in the same phylogenetic group (Cluster I) of the SNP and orthologous gene-based phylogenetic trees (Table 1). All members in Cluster 2 (B. elizabethae, B. queenslandensis, B. rattimassiliensis and B. tribocorum) are associated with the isolation from wild rodents. B. bovis is grouped in cluster 3 in the SNP phylogenetic tree (Fig. 2i), together with three ruminant-associated Bartonella spp., i.e., B. australis (isolated from kangaroo), B. melophagi (isolated from sheep ked) and B. schoenbuchensis (isolated from moose, Alces alces), and three human pathogens, B. rochalimae, B. clarridgeiae and B. bacilliformis. The clustering of some cat- or rodent-associated Bartonella spp. has led to the hypothesis that these bacteria and their reservoir hosts might have co-evolved [25]. The observation of the clustering of cat- (cluster 1/I), rodent- (cluster 2/II), and ruminant-associated Bartonella spp. (cluster 3/III) in the phylogenetic trees constructed from both SNP and orthologous genes in this study further strengthens the hypothesis. This study also confirms the finding of a previous study [23] that B. bacilliformis and B. australis are related to ruminant-associated Bartonella species.
The ability of Bartonella spp. to adapt to specific vectors and reservoirs has been recognized as a common strategy of bartonellae transmission [15]. Variable gene pool in bartonellae, as observed in B. henselae, has been reported to play a key role in the establishment of persistent infection in the natural host [36]. The host adaptation of Bartonella spp. has also been linked to the run-off replication with gene transfer agents [7].
Similar to B. bacilliformis, T4SSs which are the bona fide pathogenicity factors of bartonellae in mediating interbacterial DNA-transfer, and secretion of virulence factors into eukaryotic target cells [45, 48, 49, 53] are not annotated in BbUM genome (Table 2). The absence of virB, vbh or trw gene clusters has also been reported in B. bovis strain m02 [23]. Based on genomic analysis, it is speculated that B. bovis may have a different mechanism of pathogenicity which merits further investigation.
Flagellum-mediated motility has been postulated as a major virulence factor of B. bacilliformis for host invasion [6, 47]. Benson et al. [6] observed that B. bacilliformis was able to deform erythrocyte membrane in an irreversible fashion while nonmotile variants bound poorly and did not invade erythrocytes. The flagellin of B. clarridgeiae has been characterized and is closely related to the flagellin of B. bacilliformis [46]. In this study, two sequence types (types 1 and 2) of flagellin genes of BbUM, 91-4 and m02 strains were described (Fig. 4). It is yet to be investigated whether flaA gene is a major component of flagellar filament of B. bovis, as has been reported to be essential for motility of Campylobacter jejuni [40] and H. pylori [55].
In summary, comparative genome-wide SNP and orthologous gene analyses confirm the taxonomic placement of B. bovis with other ruminant-associated Bartonella spp. i.e., B. australis, B. melophagi and B. schoenbuchensis. The genome-based phylogeny study also strengthens the hypothesis on co-evolution of Bartonella species in specific animal hosts. The analysis of flagellin and other putative virulence genes provides the basis for further study of the pathogenicity of B. bovis as an etiological agent of bacteremia and endocarditis in cattle. With the availability of whole genome sequences, functional characterisation of certain genes is now possible to enhance research in the diagnosis and vaccine development for B. bovis.
Supplementary Material
Acknowledgments
This project was funded by the University of Malaya Research Grant (UMRG no. RP013-2012A), High Impact Research Grant (UM.C/625/1/HIR-MOHE/CHAN/11) and the Ministry of Science, Technology and Innovation E-Science Fund (SF014-2015).
REFERENCES
- 1.Akaike H.1974. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19: 716–723. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 2.Alsmark C. M., Frank A. C., Karlberg E. O., Legault B. A., Ardell D. H., Canbäck B., Eriksson A. S., Näslund A. K., Handley S. A., Huvet M., La Scola B., Holmberg M., Andersson S. G.2004. The louse-borne human pathogen Bartonella quintana is a genomic derivative of the zoonotic agent Bartonella henselae. Proc. Natl. Acad. Sci. U.S.A. 101: 9716–9721. doi: 10.1073/pnas.0305659101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anisimova M., Gascuel O.2006. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst. Biol. 55: 539–552. doi: 10.1080/10635150600755453 [DOI] [PubMed] [Google Scholar]
- 4.Aziz R. K., Bartels D., Best A. A., DeJongh M., Disz T., Edwards R. A., Formsma K., Gerdes S., Glass E. M., Kubal M., Meyer F., Olsen G. J., Olson R., Osterman A. L., Overbeek R. A., McNeil L. K., Paarmann D., Paczian T., Parrello B., Pusch G. D., Reich C., Stevens R., Vassieva O., Vonstein V., Wilke A., Zagnitko O.2008. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75. doi: 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai Y., Malania L., Alvarez Castillo D., Moran D., Boonmar S., Chanlun A., Suksawat F., Maruyama S., Knobel D., Kosoy M.2013. Global distribution of Bartonella infections in domestic bovine and characterization of Bartonella bovis strains using multi-locus sequence typing. PLoS ONE 8: e80894. doi: 10.1371/journal.pone.0080894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson L. A., Kar S., McLaughlin G., Ihler G. M.1986. Entry of Bartonella bacilliformis into erythrocytes. Infect. Immun. 54: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berglund E. C., Frank A. C., Calteau A., Vinnere Pettersson O., Granberg F., Eriksson A. S., Näslund K., Holmberg M., Lindroos H., Andersson S. G.2009. Run-off replication of host-adaptability genes is associated with gene transfer agents in the genome of mouse-infecting Bartonella grahamii. PLoS Genet. 5: e1000546. doi: 10.1371/journal.pgen.1000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolger A. M., Lohse M., Usadel B.2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. doi: 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breitschwerdt E. B., Maggi R. G., Chomel B. B., Lappin M. R.2010. Bartonellosis: an emerging infectious disease of zoonotic importance to animals and human beings. J. Vet. Emerg. Crit. Care (San Antonio) 20: 8–30. doi: 10.1111/j.1476-4431.2009.00496.x [DOI] [PubMed] [Google Scholar]
- 10.Breitschwerdt E. B., Sontakke S., Cannedy A., Hancock S. I., Bradley J. M.2001. Infection with Bartonella weissii and detection of Nanobacterium antigens in a North Carolina beef herd. J. Clin. Microbiol. 39: 879–882. doi: 10.1128/JCM.39.3.879-882.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitschwerdt E. B., Linder K. L., Day M. J., Maggi R. G., Chomel B. B., Kempf V. A.2013. Koch’s postulates and the pathogenesis of comparative infectious disease causation associated with Bartonella species. J. Comp. Pathol. 148: 115–125. doi: 10.1016/j.jcpa.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capella-Gutiérrez S., Silla-Martínez J. M., Gabaldón T.2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25: 1972–1973. doi: 10.1093/bioinformatics/btp348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang C. C., Chomel B. B., Kasten R. W., Heller R. M., Kocan K. M., Ueno H., Yamamoto K., Bleich V. C., Pierce B. M., Gonzales B. J., Swift P. K., Boyce W. M., Jang S. S., Boulouis H. J., Piémont Y.2000. Bartonella spp. isolated from wild and domestic ruminants in North America. Emerg. Infect. Dis. 6: 306–311. doi: 10.3201/eid0603.000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen F., Mackey A. J., Stoeckert C. J., Jr, Roos D. S.2006. OrthoMCL-DB: querying a comprehensive multi-species collection of ortholog groups. Nucleic Acids Res. 34: D363–D368. doi: 10.1093/nar/gkj123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chomel B. B., Boulouis H. J., Breitschwerdt E. B., Kasten R. W., Vayssier-Taussat M., Birtles R. J., Koehler J. E., Dehio C.2009. Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet. Res. 40: 29. doi: 10.1051/vetres/2009011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darriba D., Taboada G. L., Doallo R., Posada D.2011. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27: 1164–1165. doi: 10.1093/bioinformatics/btr088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darriba D., Taboada G. L., Doallo R., Posada D.2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9: 772. doi: 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgar R. C.2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797. doi: 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner S. N., Slezak T., Hall B. G.2015. kSNP3.0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics 31: 2877–2878. doi: 10.1093/bioinformatics/btv271 [DOI] [PubMed] [Google Scholar]
- 20.Goris J., Konstantinidis K. T., Klappenbach J. A., Coenye T., Vandamme P., Tiedje J. M.2007. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 57: 81–91. doi: 10.1099/ijs.0.64483-0 [DOI] [PubMed] [Google Scholar]
- 21.Guindon S., Delsuc F., Dufayard J. F., Gascuel O.2009. Estimating maximum likelihood phylogenies with PhyML. Methods Mol. Biol. 537: 113–137. doi: 10.1007/978-1-59745-251-9_6 [DOI] [PubMed] [Google Scholar]
- 22.Guindon S., Dufayard J. F., Lefort V., Anisimova M., Hordijk W., Gascuel O.2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59: 307–321. doi: 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 23.Guy L., Nystedt B., Toft C., Zaremba-Niedzwiedzka K., Berglund E. C., Granberg F., Näslund K., Eriksson A. S., Andersson S. G.2013. A gene transfer agent and a dynamic repertoire of secretion systems hold the keys to the explosive radiation of the emerging pathogen Bartonella. PLoS Genet. 9: e1003393. doi: 10.1371/journal.pgen.1003393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han N., Qiang Y., Zhang W.2016. ANItools web: a web tool for fast genome comparison within multiple bacterial strains. Database (Oxford) 2016: 1. doi: 10.1093/database/baw084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houpikian P., Raoult D.2001. Molecular phylogeny of the genus Bartonella: what is the current knowledge? FEMS Microbiol. Lett. 200: 1–7. doi: 10.1111/j.1574-6968.2001.tb10684.x [DOI] [PubMed] [Google Scholar]
- 26.Hyatt D., Chen G. L., Locascio P. F., Land M. L., Larimer F. W., Hauser L. J.2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11: 119. doi: 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kho K. L., Koh F. X., Jaafar T., Nizam Q. N. H., Tay S. T.2015. Prevalence and molecular heterogeneity of Bartonella bovis in cattle and Haemaphysalis bispinosa ticks in Peninsular Malaysia. BMC Vet. Res. 11: 153. doi: 10.1186/s12917-015-0470-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M., Oh H. S., Park S. C., Chun J.2014. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 64: 346–351. doi: 10.1099/ijs.0.059774-0 [DOI] [PubMed] [Google Scholar]
- 29.Konstantinidis K. T., Tiedje J. M.2005. Genomic insights that advance the species definition for prokaryotes. Proc. Natl. Acad. Sci. U.S.A. 102: 2567–2572. doi: 10.1073/pnas.0409727102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konstantinidis K. T., Ramette A., Tiedje J. M.2006. The bacterial species definition in the genomic era. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361: 1929–1940. doi: 10.1098/rstb.2006.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosoy M., Morway C., Sheff K. W., Bai Y., Colborn J., Chalcraft L., Dowell S. F., Peruski L. F., Maloney S. A., Baggett H., Sutthirattana S., Sidhirat A., Maruyama S., Kabeya H., Chomel B. B., Kasten R., Popov V., Robinson J., Kruglov A., Petersen L. R.2008. Bartonella tamiae sp. nov., a newly recognized pathogen isolated from three human patients from Thailand. J. Clin. Microbiol. 46: 772–775. doi: 10.1128/JCM.02120-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosoy M., Bai Y., Sheff K., Morway C., Baggett H., Maloney S. A., Boonmar S., Bhengsri S., Dowell S. F., Sitdhirasdr A., Lerdthusnee K., Richardson J., Peruski L. F.2010. Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am. J. Trop. Med. Hyg. 82: 1140–1145. doi: 10.4269/ajtmh.2010.09-0778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagesen K., Hallin P., Rødland E. A., Staerfeldt H. H., Rognes T., Ussery D. W.2007. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35: 3100–3108. doi: 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laslett D., Canback B.2004. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 32: 11–16. doi: 10.1093/nar/gkh152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L., Stoeckert C. J., Jr, Roos D. S.2003. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 13: 2178–2189. doi: 10.1101/gr.1224503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindroos H., Vinnere O., Mira A., Repsilber D., Näslund K., Andersson S. G.2006. Genome rearrangements, deletions, and amplifications in the natural population of Bartonella henselae. J. Bacteriol. 188: 7426–7439. doi: 10.1128/JB.00472-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maillard R., Grimard B., Chastant-Maillard S., Chomel B., Delcroix T., Gandoin C., Bouillin C., Halos L., Vayssier-Taussat M., Boulouis H. J.2006. Effects of cow age and pregnancy on Bartonella infection in a herd of dairy cattle. J. Clin. Microbiol. 44: 42–46. doi: 10.1128/JCM.44.1.42-46.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marçais G., Kingsford C.2011. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 27: 764–770. doi: 10.1093/bioinformatics/btr011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marston E. L., Sumner J. W., Regnery R. L.1999. Evaluation of intraspecies genetic variation within the 60 kDa heat-shock protein gene (groEL) of Bartonella species. Int. J. Syst. Bacteriol. 49: 1015–1023. doi: 10.1099/00207713-49-3-1015 [DOI] [PubMed] [Google Scholar]
- 40.Neal-McKinney J. M., Samuelson D. R., Eucker T. P., Nissen M. S., Crespo R., Konkel M. E.2014. Reducing Campylobacter jejuni colonization of poultry via vaccination. PLOS ONE 9: e114254. doi: 10.1371/journal.pone.0114254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng Y., Leung H. C., Yiu S. M., Chin F. Y.2012. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 28: 1420–1428. doi: 10.1093/bioinformatics/bts174 [DOI] [PubMed] [Google Scholar]
- 42.Pitulle C., Strehse C., Brown J. W., Breitschwerdt E. B.2002. Investigation of the phylogenetic relationships within the genus Bartonella based on comparative sequence analysis of the rnpB gene, 16S rDNA and 23S rDNA. Int. J. Syst. Evol. Microbiol. 52: 2075–2080. [DOI] [PubMed] [Google Scholar]
- 43.Rudoler N., Rasis M., Sharir B., Novikov A., Shapira G., Giladi M.2014. First description of Bartonella bovis in cattle herds in Israel. Vet. Microbiol. 173: 110–117. doi: 10.1016/j.vetmic.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 44.Richter M., Rosselló-Móra R.2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. U.S.A. 106: 19126–19131. doi: 10.1073/pnas.0906412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saenz H. L., Engel P., Stoeckli M. C., Lanz C., Raddatz G., Vayssier-Taussat M., Birtles R., Schuster S. C., Dehio C.2007. Genomic analysis of Bartonella identifies type IV secretion systems as host adaptability factors. Nat. Genet. 39: 1469–1476. doi: 10.1038/ng.2007.38 [DOI] [PubMed] [Google Scholar]
- 46.Sander A., Zagrosek A., Bredt W., Schiltz E., Piémont Y., Lanz C., Dehio C.2000. Characterization of Bartonella clarridgeiae flagellin (FlaA) and detection of antiflagellin antibodies in patients with lymphadenopathy. J. Clin. Microbiol. 38: 2943–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scherer D. C., DeBuron-Connors I., Minnick M. F.1993. Characterization of Bartonella bacilliformis flagella and effect of antiflagellin antibodies on invasion of human erythrocytes. Infect. Immun. 61: 4962–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schröder G., Dehio C.2005. Virulence-associated type IV secretion systems of Bartonella. Trends Microbiol. 13: 336–342. doi: 10.1016/j.tim.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 49.Schulein R., Dehio C.2002. The VirB/VirD4 type IV secretion system of Bartonella is essential for establishing intraerythrocytic infection. Mol. Microbiol. 46: 1053–1067. doi: 10.1046/j.1365-2958.2002.03208.x [DOI] [PubMed] [Google Scholar]
- 50.Simpson J. T., Durbin R.2012. Efficient de novo assembly of large genomes using compressed data structures. Genome Res. 22: 549–556. doi: 10.1101/gr.126953.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi M., Kryukov K., Saitou N.2009. Estimation of bacterial species phylogeny through oligonucleotide frequency distances. Genomics 93: 525–533. doi: 10.1016/j.ygeno.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 52.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S.2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tay S. T., Kho K. L., Wee W. Y., Choo S. W.2016. Whole-genome sequence analysis and exploration of the zoonotic potential of a rat-borne Bartonella elizabethae. Acta Trop. 155: 25–33. doi: 10.1016/j.actatropica.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 54.Teeling H., Waldmann J., Lombardot T., Bauer M., Glöckner F. O.2004. TETRA: a web-service and a stand-alone program for the analysis and comparison of tetranucleotide usage patterns in DNA sequences. BMC Bioinformatics 5: 163. doi: 10.1186/1471-2105-5-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe S., Takagi A., Tada U., Kabir A. M., Koga Y., Kamiya S., Osaki T., Miwa T.1997. Cytotoxicity and motility of Helicobacter pylori. J. Clin. Gastroenterol. 25Suppl 1: S169–S171. doi: 10.1097/00004836-199700001-00027 [DOI] [PubMed] [Google Scholar]
- 56.Zhang P., Chomel B. B., Schau M. K., Goo J. S., Droz S., Kelminson K. L., George S. S., Lerche N. W., Koehler J. E.2004. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc. Natl. Acad. Sci. U.S.A. 101: 13630–13635. doi: 10.1073/pnas.0405284101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.