Abstract
A 12-year-old female Shih-Tzu with hyperadrenocorticism and hypothyroidism developed concurrent refractory generalized demodicosis that did not respond to doramectin treatment. Although amitraz treatment was effective, the dog developed severe diabetes, which resulted in the cessation of amitraz and trilostane. Attempts to control the diabetes were unsuccessful, and its hyperadrenocorticism was left untreated, leading to the recurrence of demodicosis. However, demodicosis went into complete remission with a single dose of fluralaner. Transient erythematous papules appeared on the trunk three days after the administration of fluralaner, but no other adverse reactions were noted. We demonstrated that fluralaner is a potent treatment for demodicosis, and skin eruptions are possible after the first dose of the drug.
Keywords: canine demodicosis, fluralaner, transient eruption
Demodicosis is generally treated with macrocyclic lactone insecticides and/or the amidine insecticide amitraz [4]. The novel isoxazoline insecticide fluralaner may also be used to treat canine demodicosis; however, only a few peer reviewed studies involving a limited number of cases have examined its efficacy [3, 15]. We encountered a dog in which demodicosis recurred during treatment for hyperadrenocorticism. Treatment with doramectin, a macrocyclic lactone insecticide, was restarted, but the response was poor. Therefore, we initiated additional amitraz treatment, which was effective and eradicated most of the mites. However, the dog developed diabetes, and amitraz was withdrawn, which led to the recurrence of demodicosis. In this study, we examined the use of fluralaner to treat canine demodicosis resistant to macrocyclic lactone insecticides.
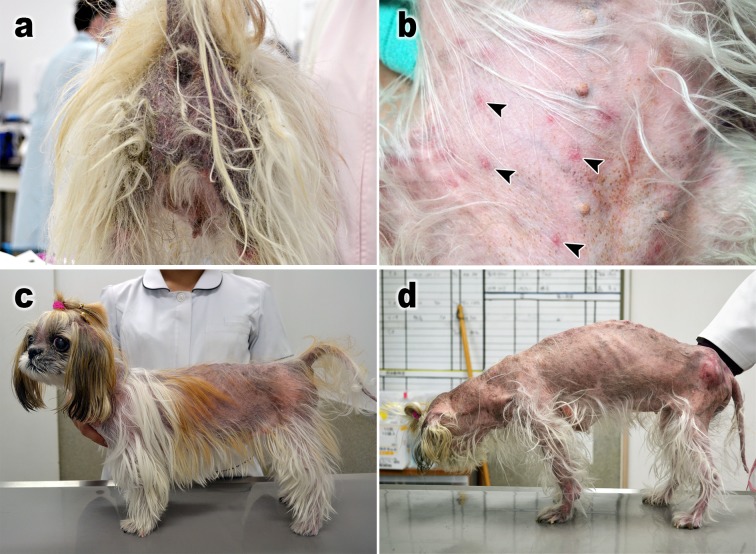
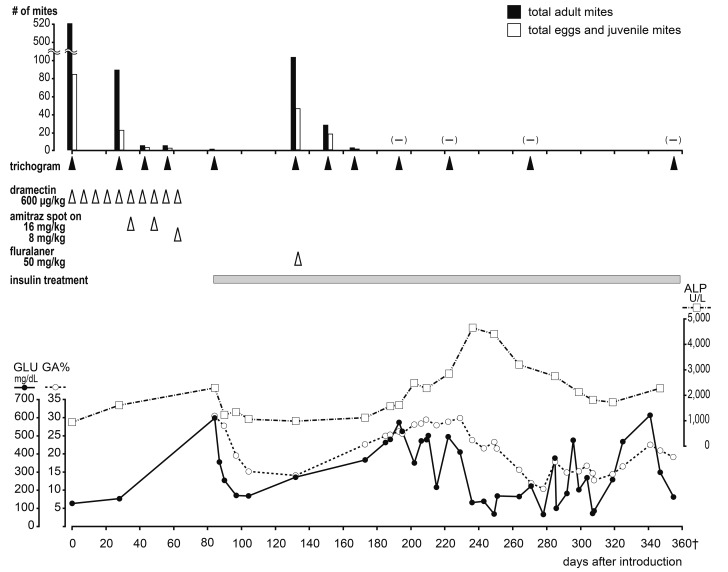
A 12-year-old female Shih Tzu presented with relapsed generalized demodicosis that was refractory to doramectin treatment (Fig. 1a). Nine months before referral, the dog developed severe generalized demodicosis. At a primary care veterinary hospital, doramectin (Dectomax, Zoetis Japan, Tokyo, Japan) was subcutaneously (SC) injected at a dose of 600 µg/kg per week [6], which was effective. At the same time, it was diagnosed with hyperadrenocorticism by the adrenocorticotropic hormone (ACTH) stimulation test, and treatment with trilostane (Adrestan, Kyoritsu Seiyaku, Tokyo, Japan) (2 mg/kg, orally (PO), every 2 days), an adrenal function inhibitor was started. However, the demodicosis recurred 2 weeks before referral. The same treatment was restarted, but no improvement was observed, and the dog was referred to our dermatology department. Figure 2 shows the dog’s clinical course (day 0: the day of the first visit). Demodex canis-induced demodicosis was diagnosed based on trichograms of the four limbs and head [9], and Malassezia dermatitis was diagnosed based on the skin tape-stripping test. The dog was also found to have hypothyroidism during a thyroid hormone test (total T4: 0.71 µg/dl, free T4: 0.30 ng/dl and canine TSH: 0.60 ng/ml). On day 5, an ACTH stimulation test performed while the dog was being treated with trilostane revealed pre- and post-ACTH serum cortisol levels of 1.81 and 8.59 µg/dl, respectively, which confirmed that the hyperadrenocorticism was under control. The ACTH level before the ACTH stimulation test was 5.0 ng/ml, suggesting non-pituitary hyperadrenocorticism. As no symptoms of hyperadrenocorticism were observed, and the size of the left adrenal gland was within normal limits (4.4 mm) and the right one was too small to observe on day 0 ultrasonography by a skilled veterinarian, a veterinarian in the clinical endocrinology department decided that trilostane should be temporarily stopped to confirm the diagnosis of hyperadrenocorticism. Treatment for demodicosis with doramectin (600 µg/kg, SC, every week) was continued, and levothyroxine (Thyradin-S, Aska Pharmaceutical Co., Ltd., Tokyo, Japan), a synthetic thyroid hormone preparation, was concurrently administered (10 µg/kg, PO, BID). Itraconazole (Nichi-Iko Pharmaceutical Co., Ltd., Toyama, Japan) (5 mg/kg, PO, SID) was initiated to treat the Malassezia dermatitis. It had no observable effects on the demodicosis; therefore, an amitraz- and fipronil-containing spot-on agent (Certifect, Merial Japan, Tokyo, Japan) was applied at the amitraz dose of 16 mg/kg every 2 weeks [2] in addition to doramectin from day 35. Marked effects were detected after one week. The dog’s clinical symptoms had resolved by the second round of amitraz treatment, and the number of mites detected on trichograms decreased markedly (Fig. 2a). However, the dog exhibited anorexia and soft stools, and polydipsia, polyuria, and congested eyes occurred after the 3rd application of amitraz even though the dose was halved (8 mg/kg) on day 63. The owner stopped applying amitraz thereafter. On day 84, the dog was examined for polydipsia, polyuria, and wobbling. Hyperglycemia (blood glucose level: 599 mg/dl) was detected during blood tests. Thus, insulin therapy was initiated. Amitraz was discontinued because it may adversely affect diabetes. An ACTH stimulation test (pre- and post-ACTH serum cortisol levels: 5.04 and 36.3 µg/dl, respectively) confirmed that the dog had hyperadrenocorticism, and trilostane treatment was restarted; however, severe adverse effects, including vomiting, developed. Thus, trilostane was discontinued after one round of treatment. On day 132 (48 days after the withdrawal of amitraz), the dog was referred back to the dermatology department with severe dermatitis symptoms and parasitism, and a large number of demodectic mites were revealed by trichograms. Therefore, 50 mg/kg of fluralaner (Bravecto, Intervet (Japanese subsidiary of MSD Animal Health), Osaka, Japan) was orally administered once on day 133. Three days after the administration of fluralaner, diffuse non-itchy erythematous papules were observed on the dog’s trunk (Fig. 1b), but they disappeared within a few days. No other adverse reactions were observed. On day 151 of the follow-up period (18 days after the administration of fluralaner), demodectic mites of all stages were detected, but only in very small numbers (Fig. 1c). On day 193 (60 days after the administration of fluralaner) the skin symptoms had healed, and no mites were detected. As the dog naturally exhibited an irregular appetite, had hyperadrenocorticism, and went into oestrus during the treatment, the insulin therapy was administered in an irregular manner, resulting in high blood glucose levels and increased glycated albumin (GA%) values [11] (Fig. 2b). The treatment for hyperadrenocorticism was suspended on day 85 after the first examination. No deep skin scraping examination was performed due to hyperadrenocorticism-induced skin weakening and sensitivity to infections. However, no mites were detected on trichograms during the approximately 5-month period from day 193 to 355, after which the dog died suddenly at home, which was attributed to complications associated with diabetes and hyperadrenocorticism (Figs. 1d and 2a).
Fig. 1.
Clinical aspects of the case. Perineal region of the dog with alopecia, scales, lichenization, and pigmentation by demodicosis on day 0 (a). Sporadic erythematous papules (arrowheads) were transiently observed on the abdominal region on day 136, three days after the administration of fluralaner (b). The appearance on day 151, 18 days after the administration of fluralaner. Although alopecia caused by hyperadrenocorticism was observed, demodicosis was improved (c). The appearance on day 322. Generalized alopecia was observed, and swollen deep pyodermal lesions accompanied by redness were seen on the lumbar femoral base region, which may have been due to severe hyperadrenocorticism. However, there were no demodectic mites from day 193 to 355 on trichograms (d).
Fig. 2.
Clinical course from the first examination (day 0). The number of mites detected on trichograms was plotted on the X-axis of the upper graph (a), and the treatments administered are shown in the rows between (a) and (b). In the lower graph (b), the changes in the blood glucose (GLU) level and glycated albumin (GA%) values are shown as indices of diabetes, and the change in the alkaline phosphate (ALP) level is shown as an index of hyperadrenocorticism.
Macrocyclic lactone insecticides are frequently used to treat demodicosis [4], but cannot be used to treat dogs that are at risk for adverse drug effects associated with ATP-binding cassette subfamily B member 1 (ABCB1) gene mutations [7], and disease recurrence results in an unfavourable treatment response [1]. On the other hand, amitraz may adversely affect the circulatory and metabolic systems, and several specific considerations need to be taken into account prior to its use [8].
In the present case, as doramectin (an avermectin), which is considered to be a more potent agent than ivermectin for generalized demodicosis by many Japanese practitioners [12], was not effective against the recurrent demodicosis, suggestive of resistance, amitraz was additionally administered and was very effective. However, because severe adverse effects were observed, amitraz was only administered three times. The dog developed diabetes 21 days after the final administration of amitraz and had to be treated with insulin. Amitraz-induced hyperglycemia was previously reported to be rare and transient [8], suggesting no causal relationship between its administration and the development of diabetes. Trichograms performed at the time of withdrawal of amitraz demonstrated that most of the mites had been eradicated, but demodicosis recurred 10 weeks after the final dose of amitraz. A few demodectic mites were detected at the final round of amitraz treatment, and they may have caused the recurrent demodicosis. Demodectic mange develops secondary to hyperadrenocorticism and thyroid hypo-function [8], for which amitraz is not necessarily safe. In the present complex case, we attempted to orally administer fluralaner, which has highly safe insecticidal effects, as a flea- and tick-preventive drug for a prolonged period (three months) [10]. Although the acaricidal effects of fluralaner were slower than those of amitraz, the dog’s clinical symptoms improved rapidly.
In previous reports involving amitraz-induced eruptions, the lesions were observed immediately after the administration of the drug [5]. However, in the present study, sporadic papules were noted on the dog’s trunk three days after the administration of fluralaner, and it is unlikely that these lesions were drug-induced eruptions. This phenomenon is infrequently encountered by practitioners in Japan when fluralaner is used to treat generalized demodicosis, suggesting that it is a biological reaction against demodectic mites that have been affected by the drug because fluralaner has been demonstrated to rapidly kill ticks and mites [13, 14]. The papules did not appear to be harmful because they were not accompanied by itching and disappeared within a few days. Clinicians should tell dog owners that such transient papules may arise before administering fluralaner treatment for demodicosis. No other adverse reactions to fluralaner were noted, suggesting that fluralaner is safe. In order to distinguish the abovementioned symptom from drug-induced eruptions, rashes of this type should be referred to as fluralaner-specific eruptions.
The progression of hyperadrenocorticism and persistent hyperglycemia triggers demodicosis [8]; however, in the present case, demodicosis did not recur during the observation period (7 months). Herein, we demonstrated that fluralaner is a safe and effective treatment for dogs with systemic demodicosis, and should be selected as a first-line treatment for demodicosis.
REFERENCES
- 1.Fondati A.1996. Efficacy of daily oral ivermectin in the treatment of 10 cases of generalized demodicosis in adult dogs. Vet. Dermatol. 7: 99–104. doi: 10.1111/j.1365-3164.1996.tb00233.x [DOI] [PubMed] [Google Scholar]
- 2.Fourie J., Dumont P., Halos L., Beugnet F., Pollmeier M.2013. Efficacy of a topical application of Certifect® (fipronil 6.26% w/v, amitraz 7.48% w/v, (S)-methoprene 5.63% w/v) for the treatment of canine generalized demodicosis. Parasite 20: 46. doi: 10.1051/parasite/2013046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fourie J. J., Liebenberg J. E., Horak I. G., Taenzler J., Heckeroth A. R., Frénais R.2015. Efficacy of orally administered fluralaner (Bravecto™) or topically applied imidacloprid/moxidectin (Advocate®) against generalized demodicosis in dogs. Parasit. Vectors 8: 187. doi: 10.1186/s13071-015-0775-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gortel K.2006. Update on canine demodicosis. Vet. Clin. North Am. Small Anim. Pract. 36: 229–241, ix. doi: 10.1016/j.cvsm.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 5.Hugnet C., Bruchon-Hugnet C., Royer H., Bourdoiseau G.2001. Efficacy of 1.25% amitraz solution in the treatment of generalized demodicosis (eight cases) and sarcoptic mange (five cases) in dogs. Vet. Dermatol. 12: 89–92. doi: 10.1046/j.1365-3164.2001.00231.x [DOI] [PubMed] [Google Scholar]
- 6.Hutt J. H. C., Prior I. C., Shipstone M. A.2015. Treatment of canine generalized demodicosis using weekly injections of doramectin: 232 cases in the U.S.A. (2002–2012). Vet. Dermatol. 26: 345–349, e73. doi: 10.1111/vde.12223 [DOI] [PubMed] [Google Scholar]
- 7.Mealey K. L.2008. Canine ABCB1 and macrocyclic lactones: heartworm prevention and pharmacogenetics. Vet. Parasitol. 158: 215–222. doi: 10.1016/j.vetpar.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 8.Miller W. H., Griffin C. E., Campbell K. L.2013. Parasitic skin disease. pp 284–342. In: Muller and Kirk’s Small Animal Dermatology. 7th ed. (Miller W. H., Griffin C. E. and Campbell K. L.). Elsevier, St. Louis. [Google Scholar]
- 9.Mueller R. S., Bensignor E., Ferrer L., Holm B., Lemarie S., Paradis M., Shipstone M. A.2012. Treatment of demodicosis in dogs: 2011 clinical practice guidelines. Vet. Dermatol. 23: 86–96, e20–e21. doi: 10.1111/j.1365-3164.2011.01026.x [DOI] [PubMed] [Google Scholar]
- 10.Rohdich N., Roepke R. K. A., Zschiesche E.2014. A randomized, blinded, controlled and multi-centered field study comparing the efficacy and safety of Bravecto (fluralaner) against Frontline (fipronil) in flea- and tick-infested dogs. Parasit. Vectors 7: 83. doi: 10.1186/1756-3305-7-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sako T., Mori A., Lee P., Sato T., Mizutani H., Takahashi T., Kiyosawa Y., Tazaki H., Arai T.2009. Serum glycated albumin: Potential use as an index of glycemic control in diabetic dogs. Vet. Res. Commun. 33: 473–479. doi: 10.1007/s11259-008-9193-0 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K., Sugimoto N., Suzuki H., Higuchi T., Takahashi K., Yuki M.2004. Two dogs with uncontrolled generalized demodicosis that improved with doramectin injection. Jpn. J. Vet. Dermatol 10: 51–54. doi: 10.2736/jjvd.10.51 [DOI] [Google Scholar]
- 13.Taenzler J., Liebenberg J., Roepke R. K. A., Frénais R., Heckeroth A. R.2016. Efficacy of fluralaner administered either orally or topically for the treatment of naturally acquired Sarcoptes scabiei var. canis infestation in dogs. Parasit. Vectors 9: 392. doi: 10.1186/s13071-016-1670-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wengenmayer C., Williams H., Zschiesche E., Moritz A., Langenstein J., Roepke R. K. A., Heckeroth A. R.2014. The speed of kill of fluralaner (Bravecto™) against Ixodes ricinus ticks on dogs. Parasit. Vectors 7: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zewe C. M., Altet L., Lam A. T. H., Ferrer L.2017. Afoxolaner and fluralaner treatment do not impact on cutaneous Demodex populations of healthy dogs. Vet. Dermatol. 28: 468–e107. doi: 10.1111/vde.12453 [DOI] [PubMed] [Google Scholar]