Abstract
It has been suggested that an increase in the use of pesticides affects neurodevelopment, but there has been no animal experiment showing a causal relation between neonicotinoid pesticides (NNs) and depression. We examined whether dinotefuran (DIN), the most widely used NN in Japan, induces depression. Male mice were administered DIN between 3 and 8 weeks of age, referring to the no-observed-effect level (NOEL). The mice were then subjected to a tail suspension test (TST) and a forced swimming test (FST). After these tests, their brains were dissected for immunohistochemical analyses of serotonin (5-HT). Antidepressant activity in TST and no decrease in 5-HT-positive cells were observed. The subchronic exposure to DIN alone in juvenile male mice may not cause depression-like indication.
Keywords: behavioral test, depression, neonicotinoid, pesticide, serotonin
Endocrine disrupters including insecticides induce neurodevelopmental effects in humans and wildlife [38], and early-life exposure to pesticides is associated with adverse effects on the neurodevelopment and behavior of children [32]. Neonicotinoid pesticides (NNs), one of the newly developed pesticides, are now the most widely used pesticides in the world. They are a class of neuroactive insecticides chemically similar to nicotine. Currently there are seven major NNs in the market: imidacloprid (IMI), nitenpyram (NTP), acetamiprid (ACE), thiamethoxam (TMX), thiacloprid (THI), clothianidin (CTD) and dinotefuran (DIN). Although these NNs were developed as selective agonists to insect nicotinic acetylcholine receptor (nAChR), an in vitro study showed that IMI, ACE and nicotine cause similar excitatory effects through mammalian nAChRs [22], and several in vivo studies revealed reproductive and neurobehavioral effects of CTD [17,18,19, 40]. Triggered by one case report of a 31-year-old depressed male who had been exposed to NNs due to the termite control of his dwelling [35], we focused on depression among neurodevelopmental disorders.
The total number of people with depression in the world increased by 18.4% between 2005 and 2015 [12]. In Japan, the number of patients with depression has also been increasing over the last two decades [26]. Although the exact cause(s) of depression and susceptibility to depression are not fully understood, many genetic and environmental factors are suspected; for example, a polymorphism in the promoter region of the serotonin (5-HT) transporter gene [5] and occupational pesticide exposure [4] have been described. Moreover, depression is associated with sex differences [1] and interactions between genes and the environment [34]. Based on the above, it is apparent that various factors can be associated with depression.
The ‘monoamine hypothesis’ was proposed as one of the explanations of causes of depression. According to this hypothesis, depression can be induced by a depletion of monoamine neurotransmitters: 5-HT, dopamine and noradrenaline [7]. The involvement of the alteration in 5-HT neural function in particular in the pathophysiology of depression is supported by considerable evidence [27]. The 5-HT neurons are controlled through nAChRs. The release of 5-HT is facilitated by α7 nAChR activation [3], and 5-HT neuron excitability is increased by α4β2 nAChRs in the dorsal raphe nucleus (DRN) in which most of the 5-HT neurons are located [11]. Many studies on cholinergic signaling or smoking suggest the modulation of depression through nAChRs [24, 29] and an association between nicotine and depression [9, 13, 25].
Two tests, the tail suspension test (TST) and forced swim test (FST), are widely used for the evaluation of antidepressants in rodent models [6]. In these tests, the efficacy of drugs is evaluated by the length of the animal’s immobility time, which is thought to reflect behavioral despair. The length of immobility time is decreased by many types of antidepressants, including selective 5-HT reuptake inhibitors.
DIN, which was developed at Mitsui Chemicals, is the most widely used pesticide in Japan among the NNs for the control of insect pests on leafy vegetables, in residential and commercial buildings, and for professional turf management and so on. However, there has been no animal experimental study on the involvement of DIN in depression. We conducted the present study to investigate the relationship between subchronic exposures to DIN and a depression-related phenotype by using behavioral tests such as TST and FST, and immunohistochemical analysis.
Three-week-old male C57BL/6NCrSlc mice were purchased from Japan SLC (Hamamatsu, Japan) and maintained as described elsewhere [18]. This study was approved by the Institutional Animal Care and Use Committee (Permission number: 26-05-07) and carried out according to the Kobe University Animal Experimental Regulations.
Assuming the exposure situation in agricultural land, Water-soluble Arubarin® (contains 20% DIN; Mitsui Chemical Co., Ltd., Tokyo) was administered to mice in their drinking water for 5 weeks from the age of 3 weeks. We divided the mice into four groups (n=6 mice in each): DIN-0 (vehicle as Control), DIN-100 (100 mg/kg/day), DIN-500 (500 mg/kg/day) and DIN-2500 (2,500 mg/kg/day) with reference to the no-observed-effect level (NOEL) of 550 mg/kg/day in the ICR mouse [10]. Twice a week, we determined the body weights of individual mice and calculated the water intakes from the decrement of the water weights placed in the bottle of each group.
On the last day of the 5 weeks of exposure to DIN, the TST and FST were performed as described elsewhere [30, 33] with some modification. In the TST, the mouse was suspended from a hook of a white box 60 cm above the surface of a table, by a plastic tape set 1 cm away from the tip of the mouse’s tail. The mouse was considered “immobile” when it was completely motionless. In the FST, the mouse was placed in an acrylic cylinder (40 cm height ×20 cm dia.) containing 20-cm-deep water kept at 23–25°C. The mouse was considered “immobile” when it remained floating in the water, except for movements to keep its head above the water. In both tests, after a 2-min acclimatization period, the immobility time was recorded from a side view by a video camera for 4 min. The percentage of immobility time during this 4-min period was calculated.
Next day, all mice were deeply anesthetized with isoflurane by an inhalation anesthesia apparatus (BS-400T; Brain Science idea, Osaka, Japan) and transcardially perfused with 0.9% normal saline, followed by perfusion with ice-cold 4% paraformaldehyde in phosphate buffer. The brains were excised, weighed and postfixed with the same fixative overnight at 4°C. The brains were then dehydrated through a graded series of ethanol followed by xylene, and embedded in paraffin. Serial sections of each brain were then cut at 10-µm thickness on a sliding microtome (SM2000R; Leica Microsytems, Wetzlar, Germany) and mounted on slide glasses (Platinum Pro; Matsunami Glass, Kishiwada, Japan). All sections were stored at −30°C until use for the following steps.
To detect 5-HT on the DRN, we performed the immunohistochemistry protocol similar to that described [18]. Briefly, the rabbit polyclonal anti-5-HT antibody (20080, ImmunoStar, Hudson, WI, U.S.A.) diluted 1:80,000 in phosphate-buffered saline with 0.05% Tween-20 (PBST; pH 7.4) was used as the primary antibody, and goat anti-rabbit immunoglobulins conjugated to peroxidase-labeled dextran polymer in tris (hydroxymethyl) aminomethan-HCl buffer (EnVision®+; Dako, Glostrup, Denmark) was used as the secondary antibody. Finally, the brain sections were counterstained with hematoxylin, dehydrated with absolute ethanol, cleared by xylene and coverslipped with Eukitt® (O. Kindler GmbH, Freiburg, Germany). We counted the 5-HT-positive cells using three sections of the DRN: −4.48, −4.60 and −4.72 mm from the bregma according to the brain atlas [28], and the average of these values was determined for each mouse.
Statistical analyses were performed with Excel Statistics 2012 (SSRI version 1.00, Tokyo, Japan). All data were analyzed by one-way ANOVA followed by Dunnett’s test. The results were considered significant when the P-value was <0.05.
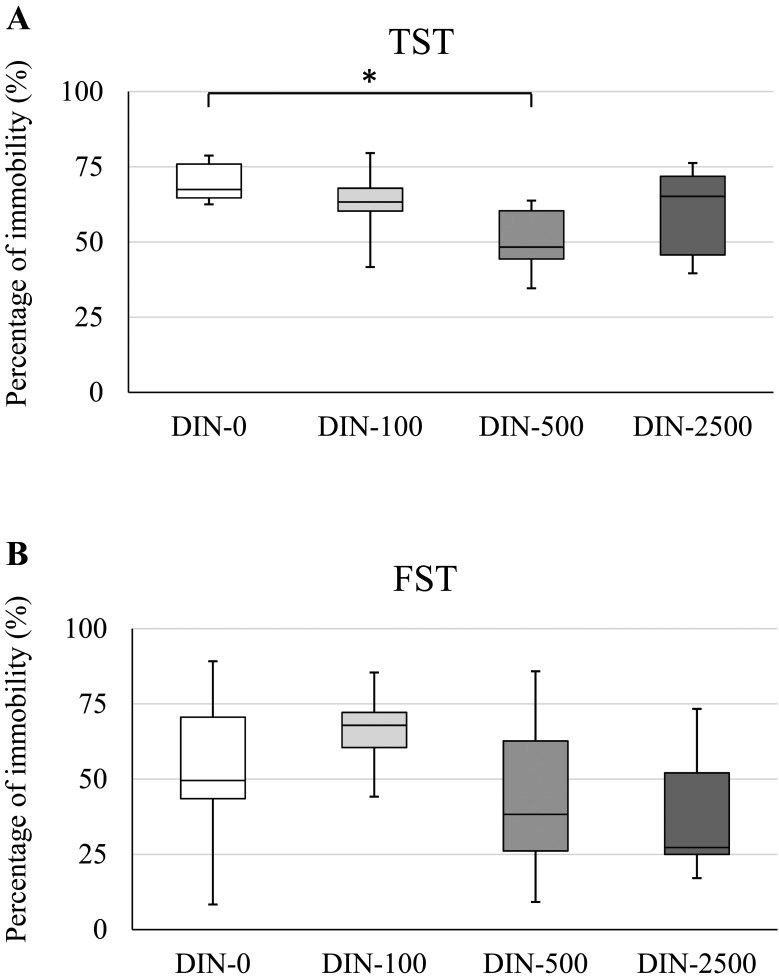
The body weight, brain weight and water intake data are shown in Table 1. DIN did not significantly suppress these three parameters in all of the DIN-administered groups compared to the control groups. In the TST, the immobility time was significantly decreased in the DIN-500 group, and the median immobility time was lower in the DIN-100, DIN-500 and DIN-2500 groups compared to the DIN-0 group (Fig. 1A). In the FST, no significant difference in the immobility time by DIN administration was observed compared with the DIN-0 group (Fig. 1B). Compared to the DIN-0 group, the median immobility time was higher in the DIN-100 group but lower in the DIN-500 and DIN-2500 groups (Fig. 1B). In both the TST and FST, DIN administration did not significantly increase the immobility time, which increases when mice show behavioral despair (Fig. 1A and 1B). An antidepressant-like effect of both acute and chronic nicotine treatment was suggested in studies using the TST and FST [2, 36, 37], and these previous studies support our findings of DIN, which is chemically similar to nicotine.
Table 1. Body weight, brain weight and water intake.
| Groups | ||||
|---|---|---|---|---|
| DIN-0 | DIN-100 | DIN-500 | DIN-2500 | |
| Body weight (g) | 25.16 ± 1.86 | 25.73 ± 1.57 | 25.54 ± 1.71 | 23.96 ± 0.93 |
| Brain weight (g) | 0.425 ± 0.022 | 0.439 ± 0.014 | 0.433 ± 0.012 | 0.427 ± 0.015 |
| Water intake (g/day) | 4.31 ± 0.59 | 4.74 ± 0.99 | 4.08 ± 0.58 | 3.41 ± 0.56 |
Mean ± SD, n=6.
Fig. 1.
Effects of DIN exposure on the immobility time in the TST (A) and FST (B). Data are reported as a box-and-whisker plot. The bottom and top of the box are 25th and 75th quartiles respectively and the band inside the box is the median. The whiskers extend to the highest and lowest value. A: In the DIN-500 mice, the immobility times were significantly decreased (Dunnett’s test, *P<0.05). The medians of the immobility time were lower in the DIN-100, DIN-500 and DIN-2500 groups compared to the DIN-0 group. B: The medians of immobility time were higher in the DIN-100 group and lower in DIN-500 and DIN-2500 groups than the DIN-0 group.
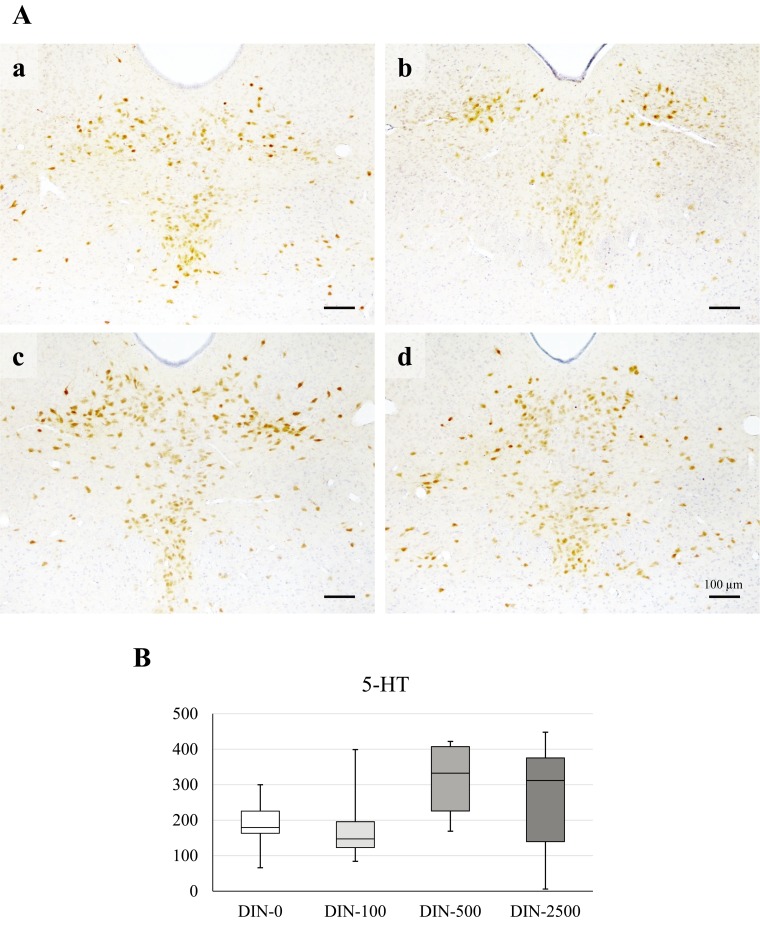
The immunohistochemical detection of 5-HT in the DRN is illustrated in Fig. 2A. DIN administration did not significantly decrease the number of 5-HT-positive cells which decreases when mice are depressed. The median number of 5-HT-positive cells was lower in the DIN-100 group but higher in the DIN-500 and DIN-2500 groups compared to the DIN-0 group (Fig. 2B).
Fig. 2.
Representative immunohistochemistry for 5-HT of the DRN in the mice of the DIN-0 (A-a), DIN-100 (A-b), DIN-500 (A-c) and DIN-2500 (A-d) groups. B: The numbers of 5-HT-positive cells. Data are reported as a box-and-whisker plot. The bottom and top of the box are 25th and 75th quartiles respectively and the band inside the box is the median. The whiskers extend to the highest and lowest value. The median numbers of 5-HT positive cells were lower in DIN-100 and DIN-2500 groups and higher in the DIN-500 group compared to the DIN-0 group. The between-group differences were not significant.
Regarding the phenotype of depression, in the present study the difference in the number of 5-HT-positive cells depending on the DIN dose showed the same trend as the immobility time in the FST, but not the same trend as the immobility time in the TST. Although both the TST and FST are used to study depression, they involve different neuronal mechanisms; monoamine metabolism changes follow the FST but not the TST [31]. This difference in neuronal mechanisms could cause the different trends between the TST and FST. We also observed that the tendency of the change in immobility time in the DIN-100 and DIN-2500 groups differed between the TST and FST. Further studies should focus on the difference in the effects on depression-like behavior that is dependent on the DIN dose.
The 5-HT neurons project to many areas of brain (e.g., the substantia nigra, amygdala and hippocampus). The 5-HT2C receptor controls dopaminergic system in the brain [8]. A disturbance of dopamine induces hyperlocomotion [16]. The release of 5-HT from DRN terminals in the amygdala may enhance conditioned fear [14]. Postsynaptic 5-HT1A receptors in the hippocampus participate in the development of tolerance to aversive events [15]. A change in the number of 5-HT-positive cells in the DRN can disturb the activities of these destinations, which could cause behavioral changes. The behavioral tests focusing on these abnormalities are required to be conducted.
Tryptophan hydroxylase (TPH) is a rate-limiting enzyme of the biosynthesis of 5-HT. Nicotine administration inhibits TPH expression in dorsal and median raphe nuclei [20]. Moreover, the administration of nicotine to adolescent rats altered the concentrations and functions of 5-HT receptors [39], and the transcription of the 5HT1A receptor in the cerebral cortex and dorsal hippocampus was increased by nicotine administration [21]. We thus need to research the effects of DIN on the 5-HT system, including TPH and 5-HT receptors.
Our present analyses did not confirm that DIN alone cause depression-like indication. However, this study was performed under short-term conditions that may not reflect depression closely. The pathogenic mechanism of depression is still unknown and is assumed to be due to a combination of genetic, environmental and psychosocial factors. For example, chronic stress is a risk factor for depression [23]. Further investigations are needed to clarify the effects of DIN on mice exposed to stressful events.
Acknowledgments
This work was partly supported by a Grant-in-Aid for Scientific Research B (#24310046) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y. Tabuchi and N. Hoshi).
REFERENCES
- 1.Altemus M., Sarvaiya N., Neill Epperson C.2014. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 35: 320–330. doi: 10.1016/j.yfrne.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreasen J. T., Redrobe J. P.2009. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav. Pharmacol. 20: 286–295. doi: 10.1097/FBP.0b013e32832c713e [DOI] [PubMed] [Google Scholar]
- 3.Andreasen J. T., Redrobe J. P., Nielsen E. Ø.2012. Combined α7 nicotinic acetylcholine receptor agonism and partial serotonin transporter inhibition produce antidepressant-like effects in the mouse forced swim and tail suspension tests: A comparison of SSR180711 and PNU-282987. Pharmacol. Biochem. Behav. 100: 624–629. doi: 10.1016/j.pbb.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 4.Beard J. D., Umbach D. M., Hoppin J. A., Richards M., Alavanja M. C. R., Blair A., Sandler D. P., Kamel F.2014. Pesticide exposure and depression among male private pesticide applicators in the agricultural health study. Environ. Health Perspect. 122: 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspi A., Sugden K., Moffitt T. E., Taylor A., Craig I. W., Harrington H., McClay J., Mill J., Martin J., Braithwaite A., Poulton R.2003. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301: 386–389. doi: 10.1126/science.1083968 [DOI] [PubMed] [Google Scholar]
- 6.Castagné V., Moser P., Roux S., Porsolt R. D.2010. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Pharmacol. Chapter 5: Unit 5. 8. [DOI] [PubMed] [Google Scholar]
- 7.Coppen A.1967. The biochemistry of affective disorders. Br. J. Psychiatry 113: 1237–1264. doi: 10.1192/bjp.113.504.1237 [DOI] [PubMed] [Google Scholar]
- 8.Di Matteo V., De Blasi A., Di Giulio C., Esposito E.2001. Role of 5-HT2C receptors in the control of central dopamine function. Trends Pharmacol. Sci. 22: 229–232. doi: 10.1016/S0165-6147(00)01688-6 [DOI] [PubMed] [Google Scholar]
- 9.Dierker L., Rose J., Selya A., Piasecki T. M., Hedeker D., Mermelstein R.2015. Depression and nicotine dependence from adolescence to young adulthood. Addict. Behav. 41: 124–128. doi: 10.1016/j.addbeh.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food Safety Commission of Japan 2016. Agricultural Chemicals and Animal Drug Evaluation Form. 6th ed., pp. 62–64. http://www.fsc.go.jp/fsciis/attachedFile/download?retrievalId=kai20161031no1&fileId=120 [accessed on January 14, 2018].
- 11.Garduño J., Galindo-Charles L., Jiménez-Rodríguez J., Galarraga E., Tapia D., Mihailescu S., Hernandez-Lopez S.2012. Presynaptic α4β2 nicotinic acetylcholine receptors increase glutamate release and serotonin neuron excitability in the dorsal raphe nucleus. J. Neurosci. 32: 15148–15157. doi: 10.1523/JNEUROSCI.0941-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators 2016. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goesling J., Brummett C. M., Meraj T. S., Moser S. E., Hassett A. L., Ditre J. W.2015. Associations between pain, current tobacco smoking, depression, and fibromyalgia status among treatment-seeking chronic pain patients. Pain Med. 16: 1433–1442. doi: 10.1111/pme.12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graeff F. G., Viana M. B., Mora P. O.1997. Dual role of 5-HT in defense and anxiety. Neurosci. Biobehav. Rev. 21: 791–799. doi: 10.1016/S0149-7634(96)00059-0 [DOI] [PubMed] [Google Scholar]
- 15.Guimarães F. S., Del Bel E. A., Padovan C. M., Netto S. M., de Almeida R. T.1993. Hippocampal 5-HT receptors and consolidation of stressful memories. Behav. Brain Res. 58: 133–139. doi: 10.1016/0166-4328(93)90098-B [DOI] [PubMed] [Google Scholar]
- 16.Hagino Y., Kasai S., Fujita M., Setogawa S., Yamaura H., Yanagihara D., Hashimoto M., Kobayashi K., Meltzer H. Y., Ikeda K.2015. Involvement of cholinergic system in hyperactivity in dopamine-deficient mice. Neuropsychopharmacology 40: 1141–1150. doi: 10.1038/npp.2014.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano T., Yanai S., Omotehara T., Hashimoto R., Umemura Y., Kubota N., Minami K., Nagahara D., Matsuo E., Aihara Y., Shinohara R., Furuyashiki T., Mantani Y., Yokoyama T., Kitagawa H., Hoshi N.2015. The combined effect of clothianidin and environmental stress on the behavioral and reproductive function in male mice. J. Vet. Med. Sci. 77: 1207–1215. doi: 10.1292/jvms.15-0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano T., Yanai S., Takada T., Yoneda N., Omotehara T., Kubota N., Minami K., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Hoshi N.2018. NOAEL-dose of a neonicotinoid pesticide, clothianidin, acutely induce anxiety-related behavior with human-audible vocalizations in male mice in a novel environment. Toxicol. Lett. 282: 57–63. doi: 10.1016/j.toxlet.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 19.Hoshi N., Hirano T., Omotehara T., Tokumoto J., Umemura Y., Mantani Y., Tanida T., Warita K., Tabuchi Y., Yokoyama T., Kitagawa H.2014. Insight into the mechanism of reproductive dysfunction caused by neonicotinoid pesticides. Biol. Pharm. Bull. 37: 1439–1443. doi: 10.1248/bpb.b14-00359 [DOI] [PubMed] [Google Scholar]
- 20.Jang M. H., Shin M. C., Lee T. H., Kim Y. P., Jung S. B., Shin D. H., Kim H., Kim S. S., Kim E. H., Kim C. J.2002. Alcohol and nicotine administration inhibits serotonin synthesis and tryptophan hydroxylase expression in dorsal and median raphe of young rats. Neurosci. Lett. 329: 141–144. doi: 10.1016/S0304-3940(02)00622-5 [DOI] [PubMed] [Google Scholar]
- 21.Kenny P. J., File S. E., Rattray M.2001. Nicotine regulates 5-HT1A receptor gene expression in the cerebral cortex and dorsal hippocampus. Eur. J. Neurosci. 13: 1267–1271. doi: 10.1046/j.0953-816x.2001.01501.x [DOI] [PubMed] [Google Scholar]
- 22.Kimura-Kuroda J., Komuta Y., Kuroda Y., Hayashi M., Kawano H.2012. Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS ONE 7: e32432. doi: 10.1371/journal.pone.0032432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahar I., Bambico F. R., Mechawar N., Nobrega J. N.2014. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 38: 173–192. doi: 10.1016/j.neubiorev.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 24.Mineur Y. S., Picciotto M. R.2010. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol. Sci. 31: 580–586. doi: 10.1016/j.tips.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mineur Y. S., Fote G. M., Blakeman S., Cahuzac E. L. M., Newbold S. A., Picciotto M. R.2016. Multiple nicotinic acetylcholine receptor subtypes in the mouse amygdala regulate affective behaviors and response to social stress. Neuropsychopharmacology 41: 1579–1587. doi: 10.1038/npp.2015.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ministry of Health, Labour and Welfare 2014. Patient Survey. http://www.mhlw.go.jp/toukei/saikin/hw/kanja/10syoubyo/dl/h26syobyo.pdf [accessed on January 14, 2018].
- 27.Owens M. J., Nemeroff C. B.1994. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin. Chem. 40: 288–295. [PubMed] [Google Scholar]
- 28.Paxinos G., Franklin K. B. J.2001. The Mouse Brain in Stereotaxic Coordinates, 2nd ed., Academic Press, Hong Kong. [Google Scholar]
- 29.Picciotto M. R., Lewis A. S., van Schalkwyk G. I., Mineur Y. S.2015. Mood and anxiety regulation by nicotinic acetylcholine receptors: A potential pathway to modulate aggression and related behavioral states. Neuropharmacology 96Pt B: 235–243. doi: 10.1016/j.neuropharm.2014.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porsolt R. D., Bertin A., Jalfre M.1977. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 229: 327–336. [PubMed] [Google Scholar]
- 31.Renard C. E., Dailly E., David D. J. P., Hascoet M., Bourin M.2003. Monoamine metabolism changes following the mouse forced swimming test but not the tail suspension test. Fundam. Clin. Pharmacol. 17: 449–455. doi: 10.1046/j.1472-8206.2003.00160.x [DOI] [PubMed] [Google Scholar]
- 32.Roberts J. R., Karr C. J.Council On Environmental Health2012. Pesticide exposure in children. Pediatrics 130: e1765–e1788. doi: 10.1542/peds.2012-2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steru L., Chermat R., Thierry B., Simon P.1985. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl.) 85: 367–370. doi: 10.1007/BF00428203 [DOI] [PubMed] [Google Scholar]
- 34.Strachan E., Duncan G., Horn E., Turkheimer E.2017. Neighborhood deprivation and depression in adult twins: genetics and gene×environment interaction. Psychol. Med. 47: 627–638. doi: 10.1017/S0033291716002622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taira K.2014. Human neonicotinoids exposure in Japan. Jpn. J. Clin. Ecol. 23: 14–24. [Google Scholar]
- 36.Tizabi Y., Overstreet D. H., Rezvani A. H., Louis V. A., Clark E., Jr, Janowsky D. S., Kling M. A.1999. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl.) 142: 193–199. doi: 10.1007/s002130050879 [DOI] [PubMed] [Google Scholar]
- 37.Vázquez-Palacios G., Bonilla-Jaime H., Velázquez-Moctezuma J.2004. Antidepressant-like effects of the acute and chronic administration of nicotine in the rat forced swimming test and its interaction with flouxetine. Pharmacol. Biochem. Behav. 78: 165–169. doi: 10.1016/j.pbb.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization (WHO) and United Nations Environment Programme (UNEP) 2012. State of the Science of Endocrine Disrupting Chemicals 2012, an assessment of the state of the science of endocrine disruptors prepared by a group of experts for the UNEP and WHO. (Bergman, Å., Heindel, J. J., Jobling, S., Kidd, K. A. and Zoeller, R. T. eds.), UNEP: Nairobi, Kenya; WHO: Geneva. [Google Scholar]
- 39.Xu Z., Seidler F. J., Cousins M. M., Slikker W., Jr, Slotkin T. A.2002. Adolescent nicotine administration alters serotonin receptors and cell signaling mediated through adenylyl cyclase. Brain Res. 951: 280–292. doi: 10.1016/S0006-8993(02)03174-8 [DOI] [PubMed] [Google Scholar]
- 40.Yanai S., Hirano T., Omotehara T., Takada T., Yoneda N., Kubota N., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Hoshi N.2017. Prenatal and early postnatal NOAEL-dose clothianidin exposure leads to a reduction of germ cells in juvenile male mice. J. Vet. Med. Sci. 79: 1196–1203. doi: 10.1292/jvms.17-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]