Abstract
In the several types of amyloidoses, participation of oxidative stresses in the pathogenesis and the effect of antioxidants on amyloidosis have been reported. Meanwhile, the relationship between oxidative stresses and pathogenesis of amyloid A (AA) amyloidosis is still unclear. In this study, we used an antioxidant, Brazilian propolis, to investigate the inhibitory effects on AA amyloidosis. The results showed that AA deposition was inhibited by administration of propolis. Increased expression of antioxidant markers was detected in molecular biological examinations of mice treated with propolis. Although serum amyloid A (SAA) levels were strongly correlated with the immunoreactive area of AA deposits in the control group, the correlation was weaker in the propolis-treated groups. In addition, there were no changes in SAA levels between the control group and the propolis-treated groups. The results indicate that propolis, an antioxidant, may induce inhibitory effects against AA amyloidosis.
Keywords: AA amyloidosis, antioxidant, propolis
Introduction
Amyloid A (AA) amyloidosis is a fatal disease characterized by deposition of AA fibrils in systemic organs including the spleen, liver, and kidneys. The precursor protein of AA is serum amyloid A (SAA), which is synthesized in the liver during chronic inflammation that occurs in conditions such as rheumatoid arthritis1. In experimental animals, AA amyloidosis can be induced by the administration of amyloid fibrils as an amyloid enhancing factor (AEF) with concurrent inflammatory stimulation2. This model is known as a transmission model of AA amyloidosis, and it has been used in research on the condition3, 4. However, the pathogenesis of AA amyloidosis has not been elucidated, and there is no standardized treatment for the disease5, 6.
In the current study, the effects of an antioxidant on an experimental AA amyloidosis model were investigated. In the case of amyloid β (Aβ) amyloidosis, various antioxidants have been shown to reduce oxidative stress caused by the deposition of Aβ7, and it is known that antioxidants like vitamin E or curcumin suppress the progression of Aβ deposition in mice8, 9, 10. Furthermore, inhibitory effects of antioxidants on the formation of Aβ in vitro have been reported11. In a mouse model of familial amyloid polyneuropathy, administration of drugs with strong antioxidant properties decreased the deposition of transthyretin12. In a study on islet amyloidosis, DNA damage was induced by oxidative stress resulting from islet amyloid polypeptide deposition13. In the case of AA amyloidosis, detection of lipoperoxidation in the organs of disease patients has been reported14. However, the relationship between oxidative stress and the pathogenesis of AA amyloidosis as well as the effects of antioxidants on the deposition of AA fibrils have remained unclear.
Propolis is a resinous substance produced by honeybees from plants. It has been shown to possess various biological properties including antimicrobial activity, anti-inflammatory effects, and antioxidative effects, which have made it a popular ingredient in health foods15. Previous research shows that the active components of propolis include flavonoid-like compounds called phenylpropanoids, which protect against Aβ-induced toxicity and exhibit inhibitory effects against Aβ formation16, 17. Brazilian green propolis contains cinnamic acid derivatives and flavonoids, which possess antioxidant properties18.
In this study, we administered propolis to mice by mixing it in their diet and examined the inhibitory effects on AA amyloidosis.
Materials and Methods
Animals
Twenty male C57BL/6J mice, 7 weeks of age, were purchased from Charles River Laboratories (Tokyo, Japan). All mice were maintained under conventional conditions. Tap water from a bottle and powder feed (CRF-1, Charles River Laboratories) were supplied without restriction. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Approval from an animal care and use committee (27-96) was obtained for the research program at the Tokyo University of Agriculture and Technology.
Experimental design
Ethanol extract of Brazilian green propolis was obtained from API Co., Ltd. (Gifu, Japan). A 20-fold dilution of spleens from mice with AA amyloidosis in saline was prepared as AEF. For administration of propolis, 20 mice were divided into 4 groups, each consisting of 4-6 mice, and administered propolis at 0, 200, 1,000, or 5,000 ppm with powder feed, respectively, by mixing the appropriate volumes in their diet. Following 7 days of propolis administration, all mice were subcutaneously inoculated with 0.5 mL of 2.0% AgNO3 solution as an inflammatory stimulus and intraperitoneally inoculated with 0.3 mL of AEF. On the 10th day following this injection, all mice were euthanized under anesthesia and necropsied, upon which their spleens and livers were collected. A portion of livers was preserved by freezing for molecular biological analysis, and the rest of the livers and whole spleens were fixed in Methacarn liquid for histological analysis. In addition, sera were collected from the mice for the measurement of SAA at the following time points: 3 days after the inflammatory stimulus and at the time of necropsy. These were stored at −20°C.
Histological and immunohistochemical examinations
Fixed organs were embedded in paraffin and cut into 2-μm sections. Subsequently, they were stained with hematoxylin and eosin (H&E). Immunohistochemistry was conducted using anti-mouse SAA goat monoclonal antibody (1:160, R&D Systems, Minneapolis, MN, USA).
Quantification of AA deposition was performed by ImageJ software (National Institutes of Health, Bethesda, MD, USA), with immunohistochemical analysis conducted in three regions of the spleen, and a low-powered image of the liver was obtained from each mouse. The immunoreactive area was measured in these regions for each mouse, and in the case of the spleen, the average of the values for the three regions examined was identified as the individual score.
The individual scores for AA deposition obtained from the mouse spleens were used to compare the treated animals, and linear regression analysis was performed using scatter diagrams.
Measurement of serum SAA concentration
The SAA concentrations in mouse sera were measured using an enzyme-linked immunosorbent assay (ELISA) kit for murine SAA (Tridelta Development Limited, Maynooth, County Kildare, Ireland). ELISA was performed according to the manufacturer’s instructions. Data for SAA concentration at 3 days following inflammatory stimulation and at the time of necropsy and for AA deposition in livers and spleens were used to construct scatter diagrams for linear regression analysis. Because 1 serum sample at 3 days following inflammatory stimulation and 2 serum samples at the time of necropsy could not be analyzed due to hemolysis, the remaining 19 serum samples at 3 days following inflammatory stimulation and 18 serum samples at the time of necropsy were used for ELISA.
mRNA transcript expression of inflammatory markers and antioxidant markers in the liver
Total RNA was extracted from the liver of each mouse using an RNeasy Mini Kit (Qiagen, Venlo, Netherlands). The concentrations of total RNA samples were measured using Gen5 2.0 (BioTek Instruments, Inc., Winooski, VT, USA). Subsequently, cDNA samples were prepared from 500 ng of total RNA using PrimeScript RT Master Mix (Takara Bio Inc., Kusatsu, Shiga, Japan) in a Life ECO Thermal Cycler (Hangzhou Bioer Co., Ltd, Hangzhou, China) (37°C for 15 min and 85°C for 5 s). Real-time PCR was performed using SYBR Premix Ex Taq II (Takara Bio Inc.) in a Thermal Cycler Dice Real Time System II (Takara Bio Inc.) (95°C for 30 s followed by 40 cycles of 95°C for 5 s, 60°C for 30 s, 95°C for 15 s, 60°C for 30 s, and 95°C for 15 s). For marker analysis, three targets for inflammatory cytokines [interleukin (IL)-6, IL-1β, monocyte chemotactic protein (MCP)-1] and six targets for antioxidant-related factors [catalase (CAT), glutathione peroxidase (GPx) 1, peroxiredoxin (Prdx) 1, superoxide dismutase (SOD) 1, SOD 2, glutathione reductase (GSR)] were investigated. Primer sequences are listed in Supplementary Table S1: online only. The relative values of gene expression as compared with a control were calculated using standard curve values normalized against those of the endogenous control gene ß-actin in the same sample.
Statistical analysis
Significant differences among the experimental groups were determined by calculating P values using Student’s t-test or Dunnett’s test. Correlations were determined by calculating Spearman’s rank correlation coefficient.
Results
Histological and immunohistochemical examinations
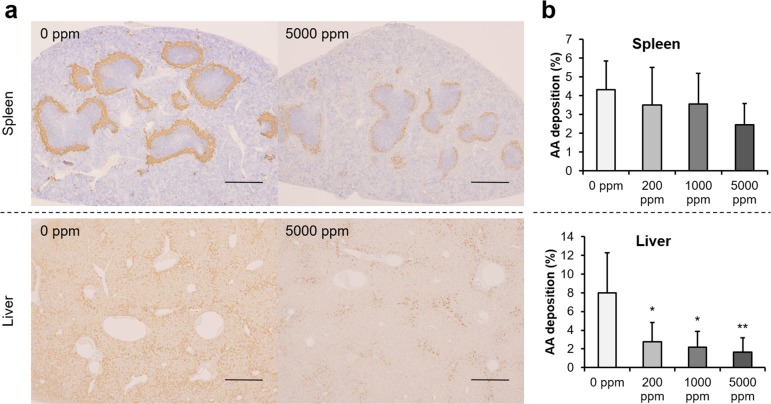
Histologically, amyloid deposits were observed around the white pulp of spleens and Disse’s spaces of livers in mice of all experimental groups. Amyloid deposits were immunopositive for mouse SAA (Fig. 1a). ImageJ analysis revealed a downward trend in the immunoreactive area of splenic AA deposits in mice treated with propolis and a significant decline in hepatic AA deposits in mice treated with 200, 1,000, and 5,000 ppm propolis (Fig. 1b), when compared with control values.
Fig. 1.
Immunohistochemical analysis of amyloid deposits and immunoreactive area data. (a) Images of immunohistochemistry with anti-mouse SAA antibody in spleens and livers; bars = 500 µm. (b) ImageJ analysis revealed a downward trend for splenic AA deposition in the propolis treatment group and significant decline in hepatic AA deposition in the 200, 1,000, and 5,000 ppm propolis treatment groups. *p<0.05 and **p<0.01 when compared with the untreated control using Dunnett’s test.
Measurement of serum SAA concentration
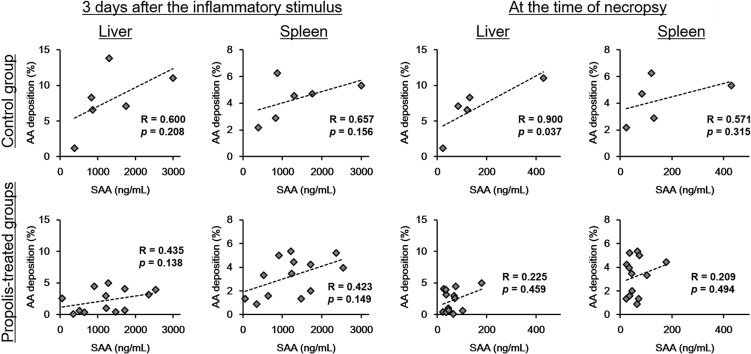
There were no significant differences in serum SAA concentrations between mouse groups (Fig. 2). In the control group, moderate to strong positive correlation was observed between the serum SAA concentration on the third day following inflammatory stimulation and at the time of necropsy and the individual scores of hepatic and splenic AA deposition (Fig. 3). But, in propolis-treated groups, the correlation coefficients were lower than those in the control groups.
Fig. 2.
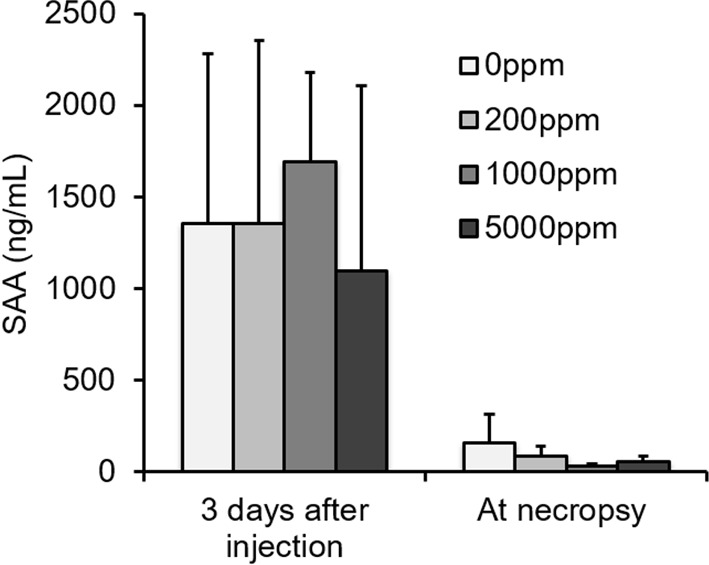
Serum SAA concentrations in the experiment. There were no significant differences in serum SAA concentration between mouse groups.
Fig. 3.
Correlation analysis between serum SAA concentration and degree of AA deposition. In the control group, there were moderate to strong positive correlations between the serum SAA concentration and the degrees of hepatic and splenic AA deposition both in the acute phase (3 days after the inflammatory stimulus) and at necropsy. In contrast, the correlation coefficient decreased in propolis-treated groups.
mRNA transcript expression of inflammatory markers and antioxidant markers in the liver
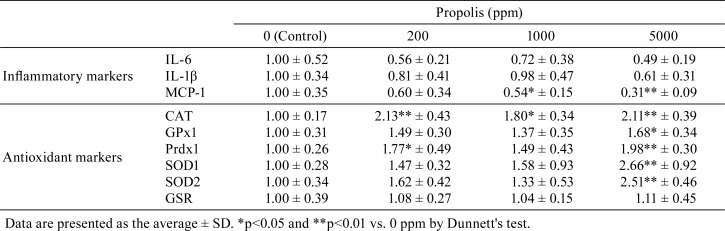
The mRNA levels of IL-6 and IL-1β were suppressed in the 5,000 ppm group, while the mRNA level of MCP-1 was significantly reduced in the 1,000 and 5,000 ppm groups. The mRNA levels of CAT, GPx1, Prdx1, SOD1, and SOD2 were significantly elevated in the 5,000 ppm group, but that of GSR was not found to be affected by propolis (Table 1).
Table 1. Comparisons of Transcript Expression of Inflammatory Markers and Antioxidant Markers among Groups.
Discussion
In this study, AA deposition in the spleen and liver of a mouse model of AA amyloidosis was inhibited by propolis administration. Propolis administration elevated the expression of antioxidant-related genes, CAT, GPx1, Prdx1, SOD1, and SOD2. Additionally, it was previously reported that propolis also induced the expression of antioxidant-related genes such as Heme oxygenase-1, Glutamine-cysteine ligase catalytic subunit, Glutamine-cysteine ligase modifier subunit, and thioredoxin reductase-1 and inhibited ROS production in vitro19. All of these antioxidant-related genes were under-controlled by NF-E2-related factor 2 (NRF2), and activation of NRF2 led to elevated antioxidant activity20. Therefore, propolis may have enhanced the expression of antioxidant-related genes, and antioxidant activity may have increased in accordance with propolis administration. Generally, a high serum SAA concentration reflects the severity of amyloid deposition2. In this study, the serum SAA levels in the control group were strongly correlated with the degree of AA deposits both in the acute phase and at necropsy, but these correlations were weaker in the propolis-treated groups. Further, there were no changes in SAA levels upon intake of propolis. These results suggest that the propolis had antioxidant effects rather than anti-inflammatory effects in this model. Furthermore, it is possible that the decrease in AA levels was caused not so much by anti-inflammatory effects as by the antioxidant activity of propolis.
The expression levels of MCP-1 were significantly reduced by propolis. MCP-1 is a chemokine produced by macrophages under the influence of an inflammatory stimulus, and in promotes the chemotaxis and activation of monocytes21. Although the detailed mechanisms are not clear, macrophages are known to be involved in both the formation and resolution of AA fibrils22. Furthermore, in AA amyloidosis, it was reported that lipoperoxidation was detected in macrophages present around amyloid deposits14. The observed decrease in MCP-1 levels in amyloid-reduced mice also suggests that activated macrophages may contribute to amyloid formation.
In conclusion, we showed that administration of propolis resulted in inhibition of AA amyloidosis. Detection of inhibitory effects on AA amyloidosis caused by antioxidants points towards the development of therapeutic strategies to prevent or treat AA amyloidosis.
Disclosure of Potential Conflict of Interest
The authors declare that they have no conflicts of interest.
Supplementary Material
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers 16H05027 and 17K17702.
References
- 1.Real de Asúa D, Costa R, Galván JM, Filigheddu MT, Trujillo D, and Cadiñanos J. Systemic AA amyloidosis: epidemiology, diagnosis, and management. Clin Epidemiol. 6: 369–377. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murakami T, Ishiguro N, and Higuchi K. Transmission of systemic AA amyloidosis in animals. Vet Pathol. 51: 363–371. 2014. [DOI] [PubMed] [Google Scholar]
- 3.Murakami T, Inoshima Y, and Ishiguro N. Systemic AA amyloidosis as a prion-like disorder. Virus Res. 207: 76–81. 2015. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K, Uchida K, Chambers JK, Tei M, Shoji A, Ushio N, and Nakayama H. Experimental transmission of AA amyloidosis by injecting the AA amyloid protein into interleukin-1 receptor antagonist knockout (IL-1raKO) mice. Vet Pathol. 52: 505–512. 2015. [DOI] [PubMed] [Google Scholar]
- 5.Obici L, and Merlini G. AA amyloidosis: basic knowledge, unmet needs and future treatments. Swiss Med Wkly. 142: w13580 2012. [DOI] [PubMed] [Google Scholar]
- 6.Shtrasburg S, Pras M, Rabinovich E, Gal R, Livneh A, and Lidar M. Attempts at suppression of amyloidogenesis in a mouse model by a variety of anti-inflammatory agents. Autoimmun Rev. 12: 18–21. 2012. [DOI] [PubMed] [Google Scholar]
- 7.Jesudason EP, Masilamoni JG, Ashok BS, Baben B, Arul V, Jesudoss KS, Jebaraj WC, Dhandayuthapani S, Vignesh S, and Jayakumar R. Inhibitory effects of short-term administration of DL-alpha-lipoic acid on oxidative vulnerability induced by Abeta amyloid fibrils (25-35) in mice. Mol Cell Biochem. 311: 145–156. 2008. [DOI] [PubMed] [Google Scholar]
- 8.Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, Trojanowski JQ, Lee VM, McIntosh TK, and Praticò D. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J Neurochem. 90: 758–764. 2004. [DOI] [PubMed] [Google Scholar]
- 9.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, and Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 21: 8370–8377. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Browne A, Child D, and Tanzi RE. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J Biol Chem. 285: 28472–28480. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ono K, Hasegawa K, Naiki H, and Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res. 75: 742–750. 2004. [DOI] [PubMed] [Google Scholar]
- 12.Macedo B, Magalhães J, Batista AR, and Saraiva MJ. Carvedilol treatment reduces transthyretin deposition in a familial amyloidotic polyneuropathy mouse model. Pharmacol Res. 62: 514–522. 2010. [DOI] [PubMed] [Google Scholar]
- 13.Kamata K, Mizukami H, Inaba W, Tsuboi K, Tateishi Y, Yoshida T, and Yagihashi S. Islet amyloid with macrophage migration correlates with augmented β-cell deficits in type 2 diabetic patients. Amyloid. 21: 191–201. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ando Y, Nyhlin N, Suhr O, Holmgren G, Uchida K, el Sahly M, Yamashita T, Terasaki H, Nakamura M, Uchino M, and Ando M. Oxidative stress is found in amyloid deposits in systemic amyloidosis. Biochem Biophys Res Commun. 232: 497–502. 1997. [DOI] [PubMed] [Google Scholar]
- 15.Silva-Carvalho R, Baltazar F, and Almeida-Aguiar C. Propolis: A complex natural product with a plethora of biological activities that can be explored for drug development. Evid Based Complement Alternat Med. 2015: 206439 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R, Wu CX, Zhou D, Yang F, Tian S, Zhang L, Zhang TT, and Du GH. Pinocembrin protects against β-amyloid-induced toxicity in neurons through inhibiting receptor for advanced glycation end products (RAGE)-independent signaling pathways and regulating mitochondrion-mediated apoptosis. BMC Med. 10: 105 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyamae Y, Han J, Sasaki K, Terakawa M, Isoda H, and Shigemori H. 3,4,5-tri-O-caffeoylquinic acid inhibits amyloid β-mediated cellular toxicity on SH-SY5Y cells through the upregulation of PGAM1 and G3PDH. Cytotechnology. 63: 191–200. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima Y, Shimazawa M, Mishima S, and Hara H. Neuroprotective effects of Brazilian green propolis and its main constituents against oxygen-glucose deprivation stress, with a gene-expression analysis. Phytother Res. 23: 1431–1438. 2009. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Shen X, Wang K, Cao X, Zhang C, Zheng H, and Hu F. Antioxidant activities and molecular mechanisms of the ethanol extracts of Baccharis propolis and Eucalyptus propolis in RAW64.7 cells. Pharm Biol. 54: 2220–2235. 2016. [DOI] [PubMed] [Google Scholar]
- 20.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 53: 401–426. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz Silva M, van der Ende-Metselaar H, Mulder HL, Smit JM, and Rodenhuis-Zybert IA. Mechanism and role of MCP-1 upregulation upon chikungunya virus infection in human peripheral blood mononuclear cells. Sci Rep. 6: 32288 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundmark K, Vahdat Shariatpanahi A, and Westermark GT. Depletion of spleen macrophages delays AA amyloid development: a study performed in the rapid mouse model of AA amyloidosis. PLoS One. 8: e79104 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.