Abstract
New technologies and science have contributed to improved surgical outcomes in patients with congenital cardiovascular diseases. However, current materials display shortcomings, such as risk of infection and lack of growth capacity when applied to the pediatric patient population. Tissue engineering has the potential to address these limitations as the ideal tissue engineered vascular graft (TEVG) would be durable, biocompatible, nonthrombogenic, and ultimately remodel into native tissue. The traditional TEVG paradigm consists of a scaffold, cell source, and the integration of the scaffold and cells via seeding. The subsequent remodeling process is driven by cellular adhesion and proliferation, as well as, biochemical and mechanical signaling. Clinical trials have displayed encouraging results, but graft stenosis is observed as a frequent complication. Recent investigations have suggested that a host’s immune response plays a vital role in neotissue formation. Current and future studies will focus on modulating host immunity as a means of reducing the incidence of stenosis.
Keywords: Stem cells and vascular tissue engineering, tissue-engineered vascular grafts, bone marrow-derived mononuclear cells, cell seeding, cardiac surgery, Fontan
Introduction
Congenital heart defects are the leading cause of newborn death and affects nearly one percent of all live births (1). Although new technologies and science have contributed to improved patient outcomes, nearly one quarter of these patients will require major reconstructive surgery (2). Current synthetic grafts are typically made out of non-degradable materials such as polytetrafluoroethylene (PTFE, or Gore-Tex®) and polyethylene terephthalate (PET, or Dacron®) (3). While these conduits can be successful in large diameter (>6 mm) operations, they are often susceptible to infection, thrombosis, stenosis, and ectopic calcification (4). Additionally, somatic overgrowth is often times an issue in the pediatric patient population as these grafts lack the capacity to grow, therefore necessitating multiple operations (5). Other alternatives include using autologous tissues, allografts, or xenografts. Unfortunately, all of these substitutes display insufficiencies to varying degrees. Simply put, there is a vast shortage of viable donor tissues and organs, and currently used surgical materials and devices are limited in their efficacy.
Tissue engineering is a relatively new scientific field that could potentially provide solutions to the problems that plague current conduits. Tissue engineering is defined as an interdisciplinary field that combines the principles of engineering and biomedical sciences to create materials that integrate with a patient’s native tissue to restore or improve physiologic function (6). The classic paradigm of tissue engineering has three components: (I) a tissue inducing scaffold material; (II) cells or cellular substitutes and; (III) a means of integrating the scaffold and cells via seeding (7). The three components are interdependent and indispensable to each other if organized neotissue is to be formed. Because the newly formed neotissue is comprised of autologous cells, these constructions would theoretically be more thromboresistant, less susceptible to infection, and have the capacity for growth. While there are many new paradigms and approaches that continue to form, this review will focus on the traditional role of scaffolding and cells in tissue engineered vascular grafts (TEVGs). Additionally, we present the current status of TEVGs utilized for congenital cardiac surgery and arteriovenous applications.
Scaffolding
The ideal scaffold is biocompatible and resistant to thrombosis, stenosis, ectopic calcification, and infection. Surgically, it is important that it is easily handled, sutured, and readily available “off the shelf”. Furthermore, it must have adequate mechanical properties to withstand the hemodynamic changes of its designated system. Initially, scaffolds provide a TEVG’s structural integrity, as well as the architecture to which cells adhere and remodel (8). Eventually, neotissue will assume the structural and mechanical responsibilities of a TEVG as the original scaffolding degrades. Hundreds of polymers, natural materials, and blends have been investigated in efforts to find the ideal TEVG scaffold. While it is unlikely that there will be one material that will be able to handle the variety of dynamic cases present in pediatric cardiovascular surgery, a select handful are being investigated rigorously. These materials can often be classified based on their synthetic or biological origin.
Synthetic biodegradable
The most commonly used synthetic biodegradable materials utilized for TEVGs are polyglycolic acid (PGA), polylactic acid (PLA), and Poly(ε-caprolactone) (PCL). The three materials feature a wide range of properties and have been approved by the FDA for implantation as vascular grafts and other medical applications (9). Their respective degradation rates are dependent on their molecular weight, exposed surface area, and crystallinity. The in vivo degradation times of these hydrophobic polymers have been reported to be 2–3 weeks, 6–12 months, and greater than 2–3 years respectively (10,11).
Additionally, combining homopolymers and controlling their ratios can lead to materials that exhibit multiple beneficial properties that otherwise would have been unique to each individual polymer (12). As an example, poly(l-lactide-co-ε-caprolactone) (PLCL), would potentially have the strength of PLA and elasticity of PCL (13). These co-polymers could also include natural materials, which often display better biocompatibility than synthetic polymers in vivo. However, it is important to note that linear relationships between the ratio of homopolymers and their physiomechanical properties are nonexistent. For example PGLA, a copolymer comprised of PGA and PLA homopolymers, tends to degrade faster than either individual homopolymers would by itself (14).
Standard processing of these synthetic biodegradable scaffolds include freeze drying, gas foaming, phase separation, salt leaching, three-dimensional printing, and electrospinning. Of these methods, there is considerable attention being paid to electrospun nanofibers. Electrospinning techniques can produce thin fibers that range from 3 nm to 5 µm, and mimic fibril structures found in an extracellular matrix (ECM) (15).
There are a multitude of synthetic biodegradable materials, copolymers, and processing methods that have been investigated for TEVGs. With all these variables, investigators must carefully consider scaffold properties with respect to cellular environments. Generally, slow degrading or small-fiber materials initially give a TEVG better mechanical properties, however they also simultaneously tend to inhibit cell infiltration and proliferation. Minimal cellular proliferation will often lead to poor outcomes later on in a graft’s development. Therefore, refining a material’s initial mechanical properties is often in conflict with improving cell attachment and differentiation. A balance between the two desired TEVG characteristics must be achieved in order for successful neotissue formation to occur.
Biological
ECM, is a tissue’s natural scaffolding. Biological TEVG approaches have centered on obtaining or mimicking this vitally important structure. One approach is to decellularize xenogeneic tissue. Decellularization involves removing most of a tissue’s cellular and antigenic components through a washing process that includes physical agitation and chemical removal of surfactants and nucleotides. A decellularized tissue would then theoretically leave behind an intact ECM with mechanical properties similar to that of a human. In fact, xenogenic TEVGs constructed with small intestinal submucosa have been successfully implanted in canine and ovine models with positive results (16,17). However, concerns over the risk of viral and prion transmission remain. Additionally, the decellularization process can adversely affect the graft’s biomechanical properties and make consistent reproducibility difficult. Both of these concerns need to be addressed before this method is fully translated.
The other biological approach is to create an ECM-like scaffold using ECM components such as collagen or fibrin. Weinberg and Bell are credited with reporting the first TEVG, which utilized a collagen gel seeded with smooth muscle cells (SMCs) and endothelial cells (ECs) (18). However, this construct lacked sufficient biomechanical properties and was combined with a Dacron mesh in order to evaluate its efficacy in vivo. While technologies and methods utilizing collagen gels have improved, they are yet to display adequate physiomechanical scaffold properties by themselves (19). Fibrin is another ECM component that has been investigated for its potential to induce collagen and elastin production, display high seeding efficiencies, and promote even cellular distribution (20). Moreover, fibrin constructs, supplemented with PLA and autologous arterial-derived cells, have produced positive results following successful implantation in an ovine carotid artery model (21). Both biological approaches have shown encouraging results, but still warrant further investigation before clinical translation.
Cells
SMCs and ECs
The tunica intima and media layers of a blood vessel are mainly composed of ECs and SMCs respectively. ECs, SMCs, and fibroblasts are essential to create a stable intima. Additionally, SMCs make up a large portion of an ECM, which ultimately defines a vessel’s mechanical properties. As such, early TEVG investigations looked intently at EC and SMC populations. Early TEVG research showed that seeding SMCs onto a biodegradable graft encouraged rapid neotissue formation (22), and demonstrated physiomechanical properties that were comparable to human vessels (23). However, hyper proliferative SMCs must be controlled in order to avoid neointimal hyperplasia.
ECs are responsible for a number of physiologic functions and the synthesis of many important regulatory substances and growth factors (24). Unfortunately, ECs are difficult to obtain and have a limited capacity to regenerate. However, the establishment of a confluent EC monolayer on a TEVG’s luminal surface is vital in its resistance to neointimal hyperplasia and thrombosis. In one investigation, implantation of ePTFE grafts seeded with ECs produced significantly higher patency rates when compared with an unseeded ePTFE control (25). Interestingly, another study reported that ECs in the pseudointima of a Dacron conduit function at less than 10% of physiologic levels found in native vasculature (26). Additionally, it has been reported that 95% of ECs that are seeded onto grafts are lost within 24 hours (27). Even though the limited number of ECs on a TEVG’s lumen confer beneficial resistance to neointimal hyperplasia, and appear to prevent acute thrombosis, several questions remain. “How can endothelialization be improved in quantity and speed?” Also, “where do seeded cells go?” or “what is their purpose?”
Stem cells and bone marrow mononuclear cells (BM-MNCs)
Stems cells are an exciting area of scientific research. Consequently, embryonic stem cells (ESs), induced pluripotent stem cells (iPSs), and mesenchymal stem cells (MSCs) have been recently investigated for TEVG purposes in varying degrees and models. In a mouse model, ECs have been derived from pluripotent ESs, seeded onto a synthetic biodegradable scaffold, and gone on to form a EC monolayer (28). However, there has been limited ES research done in humans as there are political and ethical concerns with obtaining ESs from the inner cell mass of a developing embryo. Fortunately, iPSs do not have to deal with these concerns as they are derived from autologous fibroblasts. However, iPS research is currently limited with respect to traditional TEVG approaches and their potential to form teratomas. MSCs are derived from the mesenchyme of mesodermal connective tissue. They are an intriguing area of TEVG research. Specifically, MSCs are being investigated for their ability to migrate to inflammatory sites, pluripotency, lack of immunogenicity, and secretion of bioactive molecules that can inhibit inflammation and stimulate cell healing (9,29).
Bone marrow contains an abundant amount of stem cells and the use of BM-MNCs has been successfully translated in human TEVG clinical trials. It was previously believed that the stem cell fraction in harvested BM-MNCs went on to eventually differentiate into the mature vascular cells of a TEVG’s neotissue. However, it was discovered that the number of seeded BM-MNCs decreases rapidly in the first few days following implantation, and eventually disappear altogether within 1 week (30). Further experiments have led us to the conclusion that seeded BM-MNCs act in a paracrine manner to recruit host cells to remodel via an inflammation mediated process (31). Investigations have demonstrated that too much inflammation can lead to occluded grafts by thrombosis or stenosis, but on the other hand, an absence of an inflammatory response leads to no neotissue formation. Though BM-MNC seeding has been successfully translated clinically, the precise mechanism of their effect on TEVGs warrants further study.
Studies and clinical trials
Arteriovenous
While going against the conventional tissue engineering paradigm, L’Heureux et al. pioneered the tissue engineering by self-assembly approach (TESA). This approach utilized cultured human skin fibroblast sheets wrapped and fused around a mandrel. Subsequently, the resulting construct’s lumen was then seeded with autologous ECs (32). Following promising animal studies, these constructs were implanted as arteriovenous grafts in end-stage renal disease (ESRD) patients. Preliminary results from human trials were reported in 2007, and followed by expanded results in 2009. Out of 9 patients, 1 died due to non-graft related complications and 3 patients experienced graft failure due to either dilation, thrombosis, or aneurysm. The remaining 5 patients were able to continue dialysis treatment past 6 months (33). In comparison to conventional ePTFE grafts, the TESA grafts displayed a 4.2 fold decrease in interventions required. However, it should be noted that the TESA approach involves complicated production methods, extensive fabrication times of greater than six months, and faces challenges with respect to costs (34).
In 2011, Niklason et al. in a baboon model, reported successful implantation of a TEVG which utilized human cadaveric SMCs seeded onto a PGA scaffold that was subsequently cultured for 8 weeks, and then decellularized of potentially antigenic components (35). These readily available “off the shelf” conduits are produced by Humacyte Inc. Phase II clinical trial results were recently published for their human acellular construct implanted as arteriovenous grafts into 60 ESRD patients. At 18 months, their constructs had a primary patency of 18% and secondary patency of 81% compared to 33% and 55% respectively in ePTFE grafts (36,37). The human acellular graft is currently in a phase III clinical trial and could potentially be a new viable option for dialysis patients in the near future.
Vein and pulmonary
Following successful TEVG implantations in large animal models, in 2001 we proceeded with the first human TEVG clinical trial focused on children with congenital heart disease in Japan (38). Between 2001 and 2004, a cohort of 25 Japanese patients underwent extracardiac total cavopulmonary connection procedures utilizing an autologous BM-MNC seeded TEVG made from PCL/PLLA polymer mixtures on a PGA or PLA backbone (Figure 1). Mindful of the challenges that are presented in small diameter and high-pressure systems, we implanted TEVGs in a modified Fontan procedure that provided an optimal balance of utility and safety by focusing on a high-flow, low-pressure vascular environment.
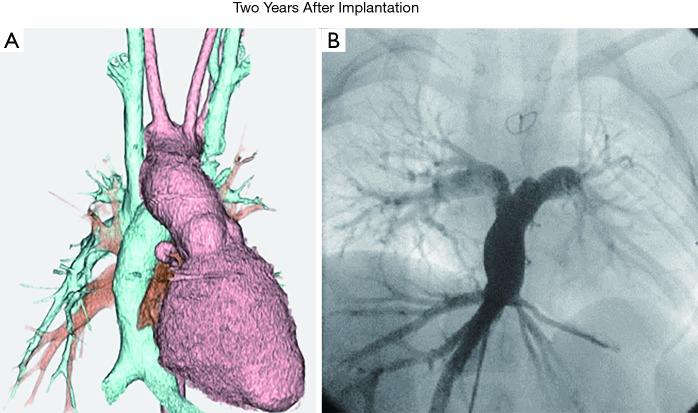
Figure 1.
Postoperative images of TEVG. (A) 3D-CT; (B) angiographic image. Adapted with permission from Hibino et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 2010. TEVG, tissue engineered vascular graft.
Follow-up data currently extends out to 9 years (Table 1). At 1-year follow-up, the cohort revealed no major graft-related complications or mortality (39). Long-term follow-up, 4 years post implantation, revealed no significant evidence of graft-related mortality, rupture, aneurysm, or ectopic calcification (40). Additionally, serial imaging demonstrated long-term growth capacity of the grafts (Figure 2). However, 6 patients developed an asymptomatic graft narrowing. Of these patients, 1 had a stent inserted at the site of stenosis and four underwent successful balloon angioplasty.
Table 1. Patient status after TEVG implantation, as of August 2016 [all grafts are patent, but seven (28%) were complicated by stenosis].
| Patient ID # | Age at surgery (years) | Graft type | Graft diameter (mm) | Patient status | Graft patency | Graft-related complications |
|---|---|---|---|---|---|---|
| 1 | 2 | PLA | 16 | Alive | Patent | None |
| 2 | 1 | PLA | 20 | Alive | Patent | None |
| 3 | 8 | PLA | 18 | Dead | Patent | Stenosis |
| 4 | 22 | PLA | 24 | Alive | Patent | None |
| 5 | 13 | PLA | 22 | Dead | Patent | None |
| 6 | 4 | PLA | 20 | Alive | Patent | Stenosis |
| 7 | 14 | PLA | 24 | Dead | Patent | None |
| 8 | 17 | PLA | 24 | Alive | Patent | None |
| 9 | 22 | PLA | 22 | Dead | Patent | None |
| 10 | 4 | PLA | 12 | Dead | Patent | Stenosis |
| 11 | 2 | PLA | 16 | Dead | Patent | None |
| 12 | 2 | PGA | 16 | Alive | Patent | Stenosis |
| 13 | 2 | PGA | 16 | Alive | Patent | Thrombosis, stenosis |
| 14 | 2 | PGA | 18 | Alive | Patent | None |
| 15 | 2 | PGA | 12 | Alive | Patent | Stenosis |
| 16 | 2 | PGA | 16 | Dead | Patent | None |
| 17 | 24 | PGA | 18 | Alive | Patent | None |
| 18 | 1 | PGA | 16 | Alive | Patent | Stenosis |
| 19 | 11 | PGA | 18 | Alive | Patent | None |
| 20 | 2 | PGA | 14 | Alive | Patent | None |
| 21 | 3 | PGA | 16 | Alive | Patent | None |
| 22 | 5 | PGA | 18 | Alive | Patent | None |
| 23 | 4 | PGA | 18 | Alive | Patent | None |
| 24 | 13 | PGA | 16 | Alive | Patent | None |
| 25 | 2 | PGA | 18 | Dead | Patent | None |
TEVG, tissue engineered vascular graft; PLA, polylactic acid; PGA, polyglycolic acid.
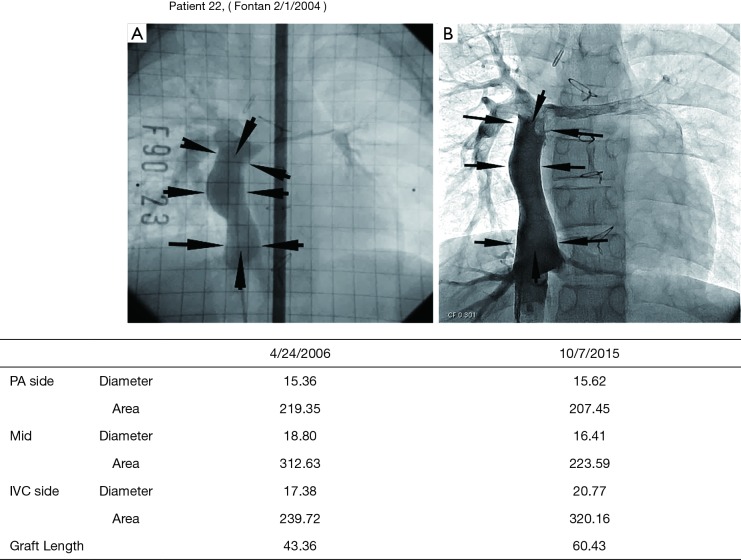
Figure 2.
Postoperative growth of a TEVG. A TEVG was implanted in a 5-year-old patient undergoing a Fontan procedure. Angiography 2 years (A) and 11 years (B) after implantation demonstrate growth, with length of the graft increasing from 43.4 to 60.4 cm. Reused with permission from Shinoka T. What is the best materials for extracardiac Fontan operation? J Thorac Cardiovasc Surg 2017. TEVG, tissue engineered vascular graft.
Upon autopsy of a patient who died due to non-graft related issues, gross and histologic examination of the TEVG revealed an appearance similar to that of native vasculature (Figure 3). The current iteration of our work continues in Columbus, Ohio as a phase I clinical trial investigating the use of TEVGs in congenital heart surgery. Stenosis of the TEVG remains a valid concern and continues to be a focus of investigation. However, our current and past pediatric patients continue to do well and display signs of robust TEVG growth and remodeling.
Figure 3.
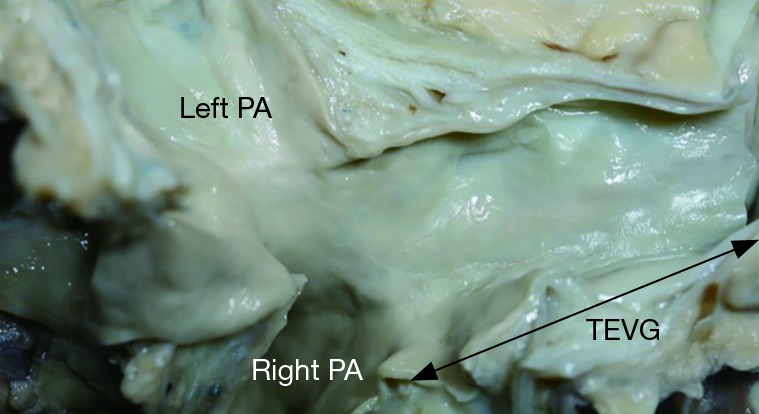
Gross image of a TEVG 13 years after implantation. The appearance is similar to native vein. Reused with permission from Shinoka T. What is the best materials for extracardiac Fontan operation? J Thorac Cardiovasc Surg 2017. PA, pulmonary artery; TEVG, tissue engineered vascular graft.
Conclusions
TEVGs have been successfully implanted in arteriovenous and large, low-pressure vascular systems. In the classic tissue engineering model, it is vital that a scaffold be biocompatible and present adequate mechanical properties to maintain a vessel’s structural integrity as host cells adhere to it and remodel. The ideal TEVG is resistant to thrombosis, stenosis, ectopic calcification, and infection, while also being easily handled, cost effective, and readily available “off the shelf”. Keeping that in mind, current TEVG studies have focused on synthetic biodegradable and biological material approaches. Both approaches are apparent in the two TEVG clinical trials that are currently ongoing. Although the methodologies utilized in both clinical trials appear promising, the exact mechanisms of tissue formation and TEVG pathologies must be further elucidated. While there are challenges ahead, further investigations to optimize scaffold neotissue formation will broaden the clinical utility of TEVGs. Even though they are relatively new areas of study, the prospects of tissue engineering and TEVGs is exciting and hold immense promise for the future of pediatric surgery.
Acknowledgements
Funding: This work is supported by Grant # R01 HL128847 and Grant from Gunze Limited.
Footnotes
Conflicts of Interest: Dr. Shinoka has received grant support from Gunze Ltd. Toshihiro Shoji has no conflicts of interest to declare.
References
- 1.van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241-7. 10.1016/j.jacc.2011.08.025 [DOI] [PubMed] [Google Scholar]
- 2.Simeone RM, Oster ME, Cassell CH, et al. Pediatric inpatient hospital resource use for congenital heart defects. Birth Defects Res A Clin Mol Teratol 2014;100:934-43. 10.1002/bdra.23262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayers RD, Raptis S, Berce M, et al. Long-term results of femorotibial bypass with vein or polytetrafluoroethylene. Br J Surg 1998;85:934-8. 10.1046/j.1365-2168.1998.00765.x [DOI] [PubMed] [Google Scholar]
- 4.Drews JD, Miyachi H, Shinoka T. Tissue-engineered vascular grafts for congenital cardiac disease: Clinical experience and current status. Trends Cardiovasc Med 2017;27:521-31. 10.1016/j.tcm.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurobe H, Maxfield MW, Breuer CK, et al. Concise review: tissue-engineered vascular grafts for cardiac surgery: past, present, and future. Stem Cells Transl Med 2012;1:566-71. 10.5966/sctm.2012-0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999;354 Suppl 1:SI32-4. 10.1016/S0140-6736(99)90247-7 [DOI] [PubMed] [Google Scholar]
- 7.Langer R, Vacanti JP. Tissue engineering. Science 1993;260:920-6. 10.1126/science.8493529 [DOI] [PubMed] [Google Scholar]
- 8.Rosso F, Marino G, Giordano A, et al. Smart materials as scaffolds for tissue engineering. J Cell Physiol 2005;203:465-70. 10.1002/jcp.20270 [DOI] [PubMed] [Google Scholar]
- 9.Hashi CK, Zhu Y, Yang GY, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A 2007;104:11915-20. 10.1073/pnas.0704581104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue L, Greisler HP. Biomaterials in the development and future of vascular grafts. J Vasc Surg 2003;37:472-80. 10.1067/mva.2003.88 [DOI] [PubMed] [Google Scholar]
- 11.Sun H, Mei L, Song C, et al. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006;27:1735-40. 10.1016/j.biomaterials.2005.09.019 [DOI] [PubMed] [Google Scholar]
- 12.Roh JD, Nelson GN, Brennan MP, et al. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials 2008;29:1454-63. 10.1016/j.biomaterials.2007.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Mo XM, Jiang BJ, et al. Fabrication of small-diameter vascular scaffolds by heparin-bonded P(LLA-CL) composite nanofibers to improve graft patency. Int J Nanomedicine 2013;8:2131-9. 10.2147/IJN.S44956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pachence JK. Biodegradable Polymers. In: Lanza R, Langer R, Vacanti J. editors. Principles of Tissue Engineering. San Diego, CA: Academic Press, 2000:263-77. [Google Scholar]
- 15.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006;7:2796-805. 10.1021/bm060680j [DOI] [PubMed] [Google Scholar]
- 16.Hiles MC, Badylak SF, Lantz GC, et al. Mechanical properties of xenogeneic small-intestinal submucosa when used as an aortic graft in the dog. J Biomed Mater Res 1995;29:883-91. 10.1002/jbm.820290714 [DOI] [PubMed] [Google Scholar]
- 17.Row S, Peng H, Schlaich EM, et al. Arterial grafts exhibiting unprecedented cellular infiltration and remodeling in vivo: the role of cells in the vascular wall. Biomaterials 2015;50:115-26. 10.1016/j.biomaterials.2015.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science 1986;231:397-400. 10.1126/science.2934816 [DOI] [PubMed] [Google Scholar]
- 19.Charulatha V, Rajaram A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 2003;24:759-67. 10.1016/S0142-9612(02)00412-X [DOI] [PubMed] [Google Scholar]
- 20.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol 2005;288:H1451-60. 10.1152/ajpheart.00479.2004 [DOI] [PubMed] [Google Scholar]
- 21.Koch S, Flanagan TC, Sachweh JS, et al. Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials 2010;31:4731-9. 10.1016/j.biomaterials.2010.02.051 [DOI] [PubMed] [Google Scholar]
- 22.Yue X, van der Lei B, Schakenraad JM, et al. Smooth muscle cell seeding in biodegradable grafts in rats: a new method to enhance the process of arterial wall regeneration. Surgery 1988;103:206-12. [PubMed] [Google Scholar]
- 23.Niklason LE, Gao J, Abbott WM, et al. Functional arteries grown in vitro. Science 1999;284:489-93. 10.1126/science.284.5413.489 [DOI] [PubMed] [Google Scholar]
- 24.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med 1990;323:27-36. 10.1056/NEJM199007053230106 [DOI] [PubMed] [Google Scholar]
- 25.Meinhart JG, Deutsch M, Fischlein T, et al. Clinical autologous in vitro endothelialization of 153 infrainguinal ePTFE grafts. Ann Thorac Surg 2001;71:S327-31. 10.1016/S0003-4975(01)02555-3 [DOI] [PubMed] [Google Scholar]
- 26.Walles T, Gorler H, Puschmann C, et al. Functional neointima characterization of vascular prostheses in human. Ann Thorac Surg 2004;77:864-8. 10.1016/j.athoracsur.2003.08.048 [DOI] [PubMed] [Google Scholar]
- 27.Rosenman JE, Kempczinski RF, Pearce WH, et al. Kinetics of endothelial cell seeding. J Vasc Surg 1985;2:778-84. 10.1016/0741-5214(85)90122-3 [DOI] [PubMed] [Google Scholar]
- 28.Shen G, Tsung HC, Wu CF, et al. Tissue engineering of blood vessels with endothelial cells differentiated from mouse embryonic stem cells. Cell Res 2003;13:335-41. 10.1038/sj.cr.7290178 [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol 2012;5:19. 10.1186/1756-8722-5-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roh JD, Sawh-Martinez R, Brennan MP, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 2010;107:4669-74. 10.1073/pnas.0911465107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hibino N, Yi T, Duncan DR, et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J 2011;25:4253-63. 10.1096/fj.11-186585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chlupac J, Filova E, Bacakova L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol Res 2009;58 Suppl 2:S119-39. [DOI] [PubMed] [Google Scholar]
- 33.McAllister TN, Maruszewski M, Garrido SA, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 2009;373:1440-6. 10.1016/S0140-6736(09)60248-8 [DOI] [PubMed] [Google Scholar]
- 34.Peck M, Gebhart D, Dusserre N, et al. The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs 2012;195:144-58. 10.1159/000331406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahl SL, Kypson AP, Lawson JH, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 2011;3:68ra9. 10.1126/scitranslmed.3001426 [DOI] [PubMed] [Google Scholar]
- 36.Lawson JH, Glickman MH, Ilzecki M, et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet 2016;387:2026-34. 10.1016/S0140-6736(16)00557-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber TS, Carter JW, Carter RL, et al. Patency of autogenous and polytetrafluoroethylene upper extremity arteriovenous hemodialysis accesses: a systematic review. J Vasc Surg 2003;38:1005-11. 10.1016/S0741-5214(03)00426-9 [DOI] [PubMed] [Google Scholar]
- 38.Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med 2001;344:532-3. 10.1056/NEJM200102153440717 [DOI] [PubMed] [Google Scholar]
- 39.Shin'oka T, Matsumura G, Hibino N, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg 2005;129:1330-8. 10.1016/j.jtcvs.2004.12.047 [DOI] [PubMed] [Google Scholar]
- 40.Hibino N, McGillicuddy E, Matsumura G, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 2010;139:431-6, 436.e1-2. [DOI] [PubMed]