Abstract
Thyroid nodules are a common finding in general population, with a prevalence of 20% to 70% at ultrasound (US) examination. Many of them are benign but treatment can be necessary to relief compressive symptoms. In the last years, percutaneous ablations have achieved amazing development in the treatment of thyroid nodules as they provide a minimally invasive but effective approach. We aimed to summarize the main aspects related to treatment of thyroid nodules with radiofrequency ablation (RFA), focusing on the use of different types of needles. A narrative review was performed and all papers analyzed reported good results in terms of nodule’s size reduction and symptoms relief. No major complications have been reported, even though needles of bigger size seemed related with major risks of post-procedural local edema. Thus, thinner internally cooled multi tined needles [18–19 Gauge (G)] rather than larger needles (14 G) seem to have better results and less complications.
Keywords: Ablation techniques, radiofrequency ablation (RFA), thyroid nodule, needle
Introduction
Thyroid nodules are a common clinical finding, with a prevalence at physical examination of 1% to 5% (1-5), and of 20% to 70% at ultrasound (US) examination (2,3,6-9). The prevalence is higher in women and it tends to increase with age, impaired iodine intake or ionizing radiation exposure (8).
As less of 10% of detected nodules are malignant, the vast majority of cases can be managed conservatively (i.e., clinical and US follow up). Nevertheless, a growing nodule can determine compression on structures around the thyroid gland (i.e., esophagus, trachea), leading to hoarseness, dyspnea, discomfort or cosmetic issues. This is why a debulking treatment can be proposed, even in benign cases (8).
Surgery is a valuable treatment, but it carries a number of possible complications, such as postoperative hypothyroidism, compressive hematomas, recurrent laryngeal nerve injuries as well as risks typically connected with general anesthesia (2). Furthermore, quality of life of patients who undergo surgical treatment may be impaired by life-long dependence of thyroxine substitutive therapy, subtle metabolic changes and presence of a neck scar (6).
For these reasons, in the last decade, percutaneous ablation techniques [i.e., laser therapy and radiofrequency ablation (RFA)] have been proposed to treat thyroid nodules. They aimed to reduce invasiveness, morbidity, complication rate, hospitalization time and patient’s discomfort, if compared to surgery (10,11).
Year by year, different authors proposed various percutaneous approaches to thyroid lesions (12,13). Our aim is to focus on different types of needles that can be used to perform RFA because, even if based on common principles, they all have specifics and it is still under debate whether different materials can modify the outcome of the procedure.
RFA
RFA of thyroid lesions is usually performed under local anesthesia, with patient in a supine position and with neck extension (3,14). Local anesthesia has to be preferred to general anesthesia because it allows a real time monitoring of sudden pain and/or voice changes that should induce the operator to stop the procedure (15). RFA is carried out with an external radiofrequency generator probe, combined with an electrode needle placed in the target tissue. The ablation is due to high frequency electric current ranging between 200 and 1,200 KHz and producing an alteration on tissue ions, that leads to protein denaturation, blood coagulation and final coagulative necrosis of the target area (16,17).
Energy delivered during ablation is responsible for the temperature reached in the tissue and consequently for the extension of necrosis. Thus, the generator has to be set in relation to the presence of vulnerable structures near the target lesion, considering that the higher is the temperature reached by the tip, the larger will be the area of necrosis around the needle. Nevertheless, a minimum temperature of 50–80 °C for a number of minutes is generally needed to achieve a satisfactory result (18-23).
Different techniques have been proposed to perform RFA on a thyroid lesion. The needle’s tip can be positioned along the long axis of the nodule (craniocaudal approach) or from the medial to the lateral part of the nodule with transverse US image (trans-isthmic approach). The second one is usually preferred because it allows a better control of the needle’s tip and it reduces risk of complications, as laryngeal recurrent nerve and esophagus are less exposed (10,24) (Figures 1-3).
Figure 1.
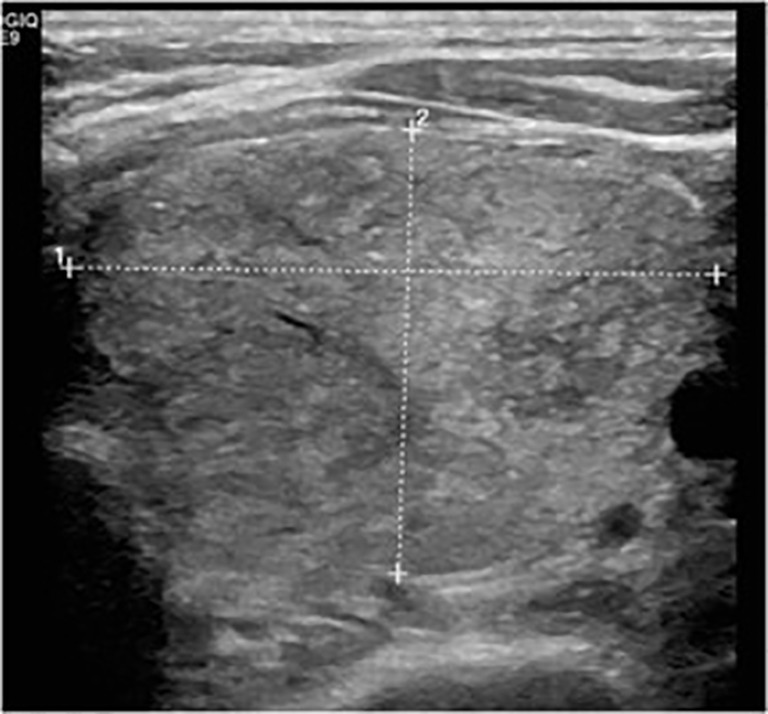
Ultrasound appearance of a massive nodule of the left thyroid lobe that replaces all the normal glands.
Figure 2.
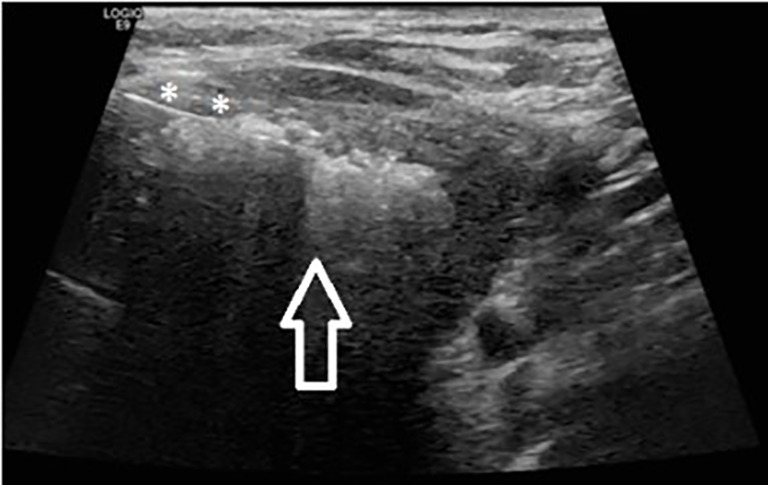
RFA of the thyroid nodule with a trans-isthmic approach. The needle (asterisks) is inserted into the lesion. The delivered energy creates a hyperechoic shadow due to microbubbles (arrow).
Figure 3.
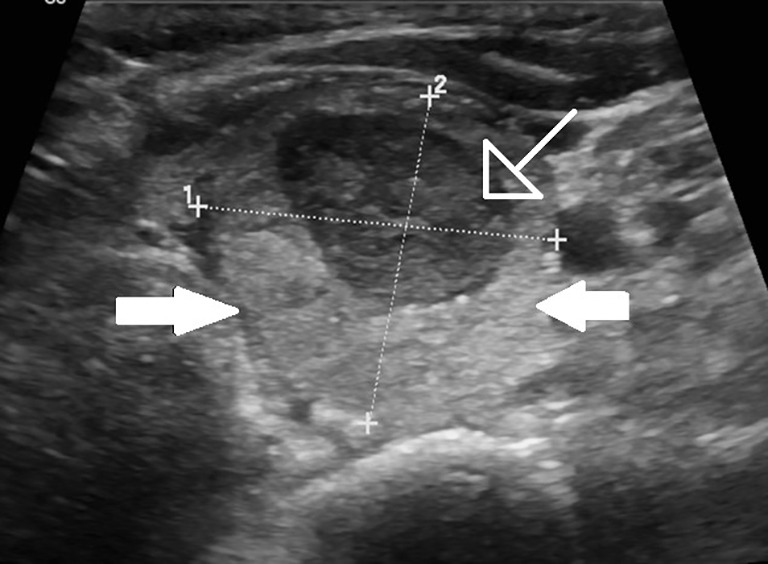
One month follow up shows the normal thyroid lobe (solid arrows) with the residual nodule decreasing in size (open arrow).
Treatment with RFA is mainly proposed for benign nodules even though, in recent years, the possibility of treating malignant lesions has been considered, especially for patients at high risk of surgical complications or that decline surgery (25). Good results have been achieved with RFA performed in patients with tumor persistence or recurrence after initial surgical treatment (26).
Needles
Different approaches have been proposed to carry out RFA of thyroid nodules. Two main options can be considered: the one with thinner needles (18–19 G) (27) and the one, less used, with needles of 14 to 17 G (28).
Considering the shape and dimensions of the thyroid, specific thinner internally cooled multi tined needles (18–19 G) have been developed to minimize tissue injury and to easily move the needle itself within the lesion (Figure 4). Nevertheless, thin needles have been often addressed to have low visibility under US guidance, leading to difficulties in controlling position and deepness of the distal tip (14). This limitation has to be considered when nodules are deep or close to other structures not intended to be involved in the ablation (such as vessels, laryngeal nerve, oesophagus).
Figure 4.

Needle for thyroid RFA: 7 cm shaft (white line), 18 Gauges of diameter and 1 cm tip (asterisks). RFA, radiofrequency ablation.
On the other hand, larger needles (14–17 G), with 1-cm straight active internally cooled electrode, can be better visualized during the procedure and they allow ablation of wider areas, without moving the needle during the procedure. Nevertheless, the ablation zone is less controllable with this needle, so it should be preferred for isolated and bigger lesions (28).
In the last years, many papers regarding RFA on thyroid nodules have been produced. They mainly dealt with ablation of benign nodules, even though some series regarding ablation of malignant lesions have been presented.
After preliminary experiences with 14 G needles, the main part of literature focused on thinner and shorter instruments as they are considered more suitable to approach the thyroid gland.
Sung et al. (29) reported 44 patients with single thyroid nodules (23 toxic and 21 pre-toxic) ablated with a 18-gauge needle with an internally cooled electrode with active tips of various size (5, 10, 15 mm). The needle was 7 cm long as, considering the subcutaneous position of the thyroid gland, no further length is needed. Moreover, a short shaft allows a better control of the instrument during the procedure. To overcome the low visibility of the needle, the operator started the ablative procedure from the deepest part of the nodule, in order to decrease the interference on the US signal due to microbubbles produced during ablation. The mean number of radiofrequency sessions for each patient was 1.8±0.9 and the authors did not report any characteristic of the lesion that let the radiologist choose for one tip or another, even though different amount of energy (watts) are reported for any tip. We can suppose that the choice depended on the size and composition (solid or cystic) of the nodule. Moreover, the authors did not report major complications and the procedure was safely ended in every case, even though each patient experienced a certain grade of pain or sensation of heat on the neck and in the surrounding structures. Despite that, all patients were discharged after a couple of hours of observation and 24-month follow up showed a mean nodule volume reduction of 79.4%.
As previously mentioned, the first to describe the use of a modified needle for RFA of thyroid was Baek in 2009 (30), who developed a 7 cm-shaft/18 G-diameter needle. This electrode, shorter and thinner than the ones used before, allows a better control of the procedure but reduces the ablated area. So, the author himself proposed the so called moving-shot technique, by radially moving the electrode inside the lesion, in order to create multiple spots of ablation and to reach the whole nodule from a single puncture site. In the first presented series (25), the technique, which Baek reported to last no more than 22 minutes, induced a statistically significant reduction of the dimension of the nodules, without major complication. All patients went home after 2 hours of supervision.
More recently, Lim et al. (26) treated patient with recurrent papillary carcinomas of the thyroid. As the lesion were generally small in size (mean diameter 0.79±0.43 cm), the authors modified the commercial needles, creating an internally cooled electrode of 7 cm of shaft, with 19 G of diameter and various dimensions of the tip (0.5, 0.7 and 1 cm). The tip was chosen in relation to the size of the lesion, being certain to avoid damage on any surrounding structure. Moreover, when the distance between the lesion and any vital structure was less than 5 mm, a dextrose solution was injected with a 23 G needle, in order to prevent unwanted damages. All procedures were carried out under conscious sedation. Furthermore, the ablation always began with 10 W of power and it was increased only if no hyperechoic images appeared within 10 seconds from the beginning of the procedure. Conversely, the power was reduced in case of pain or discomfort for the patient. Also in this case, the moving shot technique was applied when appropriate (i.e., when a single focus of ablation was not sufficient to destroy the entire lesion). Clinical results were excellent, as 82% of lesions disappeared completely after treatment. Nevertheless, the author reported 3 patients (7.7%) who had voice changes within 24 hours after the procedure (but resolved spontaneously within 2 months). Even though the percentage and entity of complications is still low, it appears higher than the already cited series, demonstrating a certain criticism in treating this specific type of lesions.
On the other side, some groups have reported series of patients treated with needles of a greater diameter (up to 14 G). Deandrea et al. (28) reported their experience with a 14 G needle used to treat 33 benign nodules of 31 patients not suitable for surgery. In this case, the approach to the lesion was slightly different and some issues should be discussed. First of all, as a 14 G needle is too traumatic to pass through the skin, all patients needed a surgical incision of about 5 mm to let the needle reach the nodule. The patient has to be previously informed about the cosmetic issues related with this type of needle as, even though of little concern, can be cause of discomfort in some cases. Secondly, due to needle dimensions, the moving shot technique could not be used. So, a single access was chosen case by case, depending on the position of the lesion and, when it was too big in size and one single point of ablation was not sufficient to treat the entire nodule, the needle had to be retracted and reinserted cranially, to end the ablation.
Another issue is related to the structure of the needle. In fact, it was created with nine prongs that allowed the operator to reach a wider portion of the gland, ensuring a better distribution of heat in the nodule. Nevertheless, the distribution of each prong within the gland had to be carefully checked with US, as the operator had to be sure that extensions were far enough from thyroid’s capsule (i.e., 10 mm) and from vital surrounding structures (i.e., 15 mm) to avoid any unwanted ablation. Almost all patient experienced mild edema after the procedure, but steroids were administered only three times and all other cases resolved spontaneously. At follow up, the mean reduction of volume was 50.7% with a general improvement of symptoms.
This kind of needle presents advantages and disadvantages when compared to smaller ones (i.e., 18–19 G). In fact, smaller needles can be moved easier within the lesion and they can be better monitored if compared to the prongs. So, thinner needles can be used for smaller lesions but, conversely, 14 G needles allow a faster and more effective ablation of bigger. In the presented paper, nodules’ size ranged between 20 and 73 mm in diameter. This range of dimensions justifies the use of larger needles. Nevertheless, in common clinical practice, the operators may need to be more precise in order to deal with smaller nodules. In fact, as a consequence, the use of 14 G needles tends to decrease and few series have been reported in the last years.
More recently, Faggiano et al. (31) reported a series of 40 patients with compressive benign thyroid nodules, dividing the population in two groups: the first one was treated with RFA, using a 14 G needle, and the second one was only observed. The study confirmed that group treated with RFA had a significant reduction of nodule volume (from 13.3±1.8 to 1.8±0.3 mL at 12 months follow up) and improvement of symptoms. In this case, the needle had only 4 prongs, with an expansion of 4 cm opened within the lesion under US control. Each session of treatment lasted 5–7 minutes and it was well tolerated in every case, as patients reported only a moderate sense of heat in the neck during the procedure.
Conclusions
RFA is nowadays an established treatment to manage patients with thyroid nodules. RFA has shown excellent results and few complications compared with surgery, and today it represents an excellent option. RFA can be used to treat both benign thyroid nodules and recurrent thyroid cancers but it is not yet considered as a first line treatment for thyroid malignancies (32-35).
To summarize the result of our review, thinner internally cooled multi tined needles (18–19 G) carry less complications and excellent results, even though the procedure is longer and the needle is less visible, especially when dealing with deep caudal nodules.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf) 1977;7:481-93. 10.1111/j.1365-2265.1977.tb01340.x [DOI] [PubMed] [Google Scholar]
- 2.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am 2007;36:707-35, vi. 10.1016/j.ecl.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 4.Guth S, Theune U, Aberle J, et al. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest 2009;39:699-706. 10.1111/j.1365-2362.2009.02162.x [DOI] [PubMed] [Google Scholar]
- 5.Rossi UG, Cariati M. Images in endocrinology: multinodular goitre. Arq Bras Endocrinol Metabol 2014;58:873-4. 10.1590/0004-2730000003549 [DOI] [PubMed] [Google Scholar]
- 6.Papini E, Pacella CM, Hegedus L. Diagnosis of endocrine disease: thyroid ultrasound (US) and US-assisted procedures: from the shadows into an array of applications. Eur J Endocrinol 2014;170:R133-46. 10.1530/EJE-13-0917 [DOI] [PubMed] [Google Scholar]
- 7.Brander AE, Viikinkoski VP, Nickels JI, et al. Importance of thyroid abnormalities detected at US screening: a 5-year follow-up. Radiology 2000;215:801-6. 10.1148/radiology.215.3.r00jn07801 [DOI] [PubMed] [Google Scholar]
- 8.Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med 2004;351:1764-71. 10.1056/NEJMcp031436 [DOI] [PubMed] [Google Scholar]
- 9.Filetti S, Durante C, Torlontano M. Nonsurgical approaches to the management of thyroid nodules. Nat Clin Pract Endocrinol Metab 2006;2:384-94. 10.1038/ncpendmet0215 [DOI] [PubMed] [Google Scholar]
- 10.Baek JH, Lee JH, Valcavi R, et al. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol 2011;12:525-40. 10.3348/kjr.2011.12.5.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid 2010;20:1253-61. 10.1089/thy.2010.0189 [DOI] [PubMed] [Google Scholar]
- 12.Pacella CM. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound 2017;20:347-9. 10.1007/s40477-017-0269-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound 2016;20:11-22. 10.1007/s40477-016-0221-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin JH, Baek JH, Ha EJ, et al. Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol 2012;2012:919650. 10.1155/2012/919650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen F, Tian G, Kong D, Zhong L, et al. Radiofrequency ablation for treatment of benign thyroid nodules: A PRISMA-compliant systematic review and meta-analysis of outcomes. Medicine (Baltimore) 2016;95:e4659. 10.1097/MD.0000000000004659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia 2016:1-5. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Mauri G, Sconfienza LM. Percutaneous ablation holds the potential to substitute for surgery as first choice treatment for symptomatic benign thyroid nodules. Int J Hyperthermia 2016:1-2. [DOI] [PubMed] [Google Scholar]
- 18.Cesareo R, Palermo A, Pasqualini V, et al. Radiofrequency ablation for the management of thyroid nodules: A critical appraisal of the literature. Clin Endocrinol (Oxf) 2017;87:639-48. 10.1111/cen.13422 [DOI] [PubMed] [Google Scholar]
- 19.Cesareo R, Palermo A, Pasqualini V, et al. Efficacy and safety of a single radiofrequency ablation of solid benign non-functioning thyroid nodules. Arch Endocrinol Metab 2017;61:173-9. 10.1590/2359-3997000000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrara V, Buonomenna C, Mauri G. Image-guided ablations in patients with thyroid tumors. J Cancer Res Clin Oncol 2017;143:2637-9. 10.1007/s00432-017-2503-6 [DOI] [PubMed] [Google Scholar]
- 21.Franz AM, Seitel A, Bopp N, et al. First clinical use of the EchoTrack guidance approach for radiofrequency ablation of thyroid gland nodules. Int J Comput Assist Radiol Surg 2017;12:931-40. 10.1007/s11548-017-1560-2 [DOI] [PubMed] [Google Scholar]
- 22.Park HS, Baek JH, Choi YJ, et al. Innovative Techniques for Image-Guided Ablation of Benign Thyroid Nodules: Combined Ethanol and Radiofrequency Ablation. Korean J Radiol 2017;18:461-9. 10.3348/kjr.2017.18.3.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang X, Cui D, Chi J, et al. Evaluation of the safety and efficacy of radiofrequency ablation for treating benign thyroid nodules. J Cancer 2017;8:754-60. 10.7150/jca.17655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baek JH, Kim YS, Sung JY, et al. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol 2011;197:W331-6. 10.2214/AJR.10.5345 [DOI] [PubMed] [Google Scholar]
- 25.Park KW, Shin JH, Han BK, et al. Inoperable symptomatic recurrent thyroid cancers: preliminary result of radiofrequency ablation. Ann Surg Oncol 2011;18:2564-8. 10.1245/s10434-011-1619-1 [DOI] [PubMed] [Google Scholar]
- 26.Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol 2015;25:163-70. 10.1007/s00330-014-3405-5 [DOI] [PubMed] [Google Scholar]
- 27.Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol 2010;194:1137-42. 10.2214/AJR.09.3372 [DOI] [PubMed] [Google Scholar]
- 28.Deandrea M, Limone P, Basso E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol 2008;34:784-91. 10.1016/j.ultrasmedbio.2007.10.018 [DOI] [PubMed] [Google Scholar]
- 29.Sung JY, Baek JH, Jung SL, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid 2015;25:112-7. 10.1089/thy.2014.0100 [DOI] [PubMed] [Google Scholar]
- 30.Baek JH, Moon WJ, Kim YS, et al. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg 2009;33:1971-7. 10.1007/s00268-009-0130-3 [DOI] [PubMed] [Google Scholar]
- 31.Faggiano A, Ramundo V, Assanti AP, et al. Thyroid nodules treated with percutaneous radiofrequency thermal ablation: a comparative study. J Clin Endocrinol Metab 2012;97:4439-45. 10.1210/jc.2012-2251 [DOI] [PubMed] [Google Scholar]
- 32.Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia 2017;33:920-30. [DOI] [PubMed] [Google Scholar]
- 33.Dupuy DE, Monchik JM, Decrea C, et al. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery 2001;130:971-7. 10.1067/msy.2001.118708 [DOI] [PubMed] [Google Scholar]
- 34.Ha SM, Sung JY, Baek JH, et al. Radiofrequency ablation of small follicular neoplasms: initial clinical outcomes. Int J Hyperthermia 2017;33:931-7. [DOI] [PubMed] [Google Scholar]
- 35.Papini E, Guglielmi R, Gharib H, et al. Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid 2011;21:917-20. 10.1089/thy.2010.0447 [DOI] [PMC free article] [PubMed] [Google Scholar]


