Abstract
Thyroid nodules are a common incidental finding during a routinely ultrasound (US) exam unrelated to the thyroid gland in the healthy adult population with a prevalence of 20–76%. As treated before with surgery, in the last years new minimally invasive techniques have been developed as an alternative to surgery. The aim of this review, based on newly revised guidelines, is to provide some information regarding the basic principles, indications, materials, techniques, and results of mini-invasive procedures or treatments for thyroid nodules. We performed a narrative review including both newest and representative papers and guidelines based on the different procedures of ablation techniques developed in the last years for the diagnosis and the treatment of thyroid nodules. All examined papers referred very good results in term of volume nodule reduction, improvement in related symptoms and cosmetic problems, with a very low rate of complications and side effects for all the minimally invasive technique analyzed. Obviously, some differents between technique based on different kind of thyroid nodules and different indication were found. In conclusion, many thyroid nodules nowadays could be treated thanks to the advent of new mini-invasive technique that are less expensive and present a lower risk of major complications and side effects compared to surgery.
Keywords: Thyroid nodule, ablation technique, ethanol injection, laser, radiofrequency, microwave, high intensity focused ultrasound (HIFU)
Introduction
Palpable thyroid nodules are found in about 5% of world population in non-endemic areas with right iodine supplement, with a higher correlation with sex (prevalence in women), age, abnormal intake of iodine and ionizing radiation exposure (1-3). Thyroid nodules are a common incidental finding during routinely ultrasound (US) exams unrelated to thyroid gland in the healthy adult population with a prevalence of 20–76%. The majority of thyroid nodules are benign (>95%) and as long as they do not cause associated symptoms like dyspnea, dysphagia, hoarseness, hyperthyroidism or cosmetic problems, they do not need any treatment (1,4). Beside malignant nodules, treatment should be considered even for growing or large nodules (>4 cm) and in presence of clinical symptoms or cosmetic problems. Surgical procedures such as radical thyroidectomy or hemithyroidectomy have been considered as the gold standard, even though associated with high, sometimes permanent, risks of complications (bleeding, infection, lesion of recurrent laryngeal nerve, large neck scar, and hypothyroidism), leading patients to a long-term drug treatment with controversial efficacy. In addition, due to the request of general anesthesia, surgery may not be indicated for all patients (2,5-7). More recently, in the radiological field, new non-invasive procedures have been developed as alternative to surgery (8-19). The aim of this review, based on newly revised guidelines, is to provide some information regarding the basic principles, indications, materials, techniques and results of mini-invasive procedures or treatments for thyroid nodules.
Thyroid fine-needle aspiration biopsy (FNAB)
Today, FNAB represents the gold standard for the diagnosis of the nature of thyroid nodules due to its safety, reliability, low costs and high tolerability by the patients (3,20). According to the newly reviewed guidelines for thyroid nodules (<5 mm), US monitoring is always recommended. FNAB is indicated in presence of large nodules (≤5–10 mm), with dubious US features associated to pathologic lymphnodes, extrathyroidal growth, personal or familiar history of thyroid cancer, and dubious clinical or imaging findings. In addition, FNAB should be performed in case of (I) nodules (>10 mm) showing frankly benign features; (II) nodules (≥20 mm) with spongiform, iso-hyperechoic or predominant (>50%) cystic aspect in absence of suspicious US findings; (III) in presence of progressive increase in size. Exclusion criteria include presence of hot nodules on scintigraphy and/or patients with severe coagulation disorders. US guidance makes FNAB safer and is now considered mandatory, allowing real-time control of the tip of the needle when approaching the target areas avoiding the vascular structures (1,3,21-23). Five cytologic diagnostic categories are considered for the diagnosis of thyroid nodules (1): nondiagnostic, benign, indeterminate, suspicious for malignancy, and malignant.
Technique and complications
For all interventional procedures on the thyroid gland, the patient is on the prone position, with a pillow under the shoulders allowing hyperextension of the neck. The neck is cleansed with betadine or alcohol. Subsequently, local anesthetic is administered. FNAB is performed using needles of variable caliber (ranging from 21 to 27 G) usually attached to a 10 cc syringe. In rare cases of cystic nodules, where the colloid content is too dense, thicker needles are employed (up to 18 G). While entering into the nodule, the tip of the needle is continuously visualized by the operator under US-guidance. With or without aspiration, depending on the structure and vascularization of the nodules, the needle is moved to and fro of some millimeters to remove cells and, in the end, the material extracted is fixed in alcohol or dried air and sent to the cytopathologist (1-3) (Figure 1). Low rates of complications are described in literature, when the procedure is performed by expert operators, and include bleeding with risk of cervical hematoma, local infections and vasovagal syncope. Proper use of antiseptics, US assistance (for identification of intra and extra-gland vascular structures) and the manual compression of the entry site may help reduce the likelihood of complications (3,24-29). Still controversial is the use of antithrombotic agents to reduce the risk of post-procedural bleeding. The cause is that the antithrombotic agents can probably lead to major cardiac and cerebrovascular accidents (3,27-29). Severe coagulative deficits may represent a contraindication to FNAB. Despite Abu-Yousef et al. reported only 2 hematomas in 593 patients submitted to antithrombotic therapy vs. 4 events of bleeding in 449 patients not taking antithrombotic medications, it is suggested, whenever applicable, to suspend or modify the antithrombotic medication after consulting with the clinician (3,26,27).
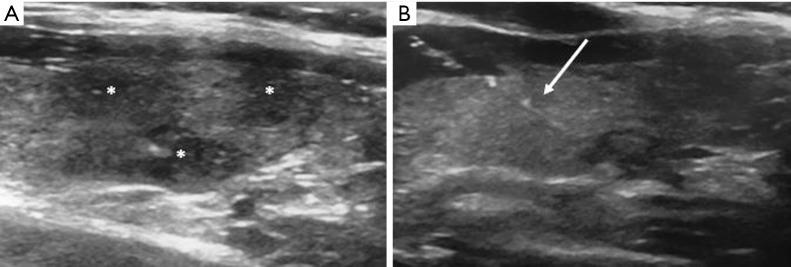
Figure 1.
FNAB procedure in a patient with multinodular goitre. (A) US images of thyroid show three hypo-echogenic nodules (*); (B) US-guided FNAB of one nodule. The needle (white arrow) with its tip perfectly inserted inside the lesion is shown. FNAB, fine-needle aspiration biopsy; US, ultrasound.
Results
The superiority of US-guided FNAB in terms of adequacy of the cytological material obtained, possibility of false negative results, sensitivity and specificity has been demonstrated in several studies. In 9,683 patients, Danese et al. found low rates of cytologically inadequate material (3.5% vs. 8.7%) using US-guided FNAB, experiencing an increase in the percentage of sensitivity, specificity, and global diagnostic accuracy (97.1% vs. 91.8%, 70.9% vs. 68.8% and 75.9% vs. 72.6%, respectively). They reported only 1% of false-negative results vs. 2.3% without US assistance (30). Another study carried out by Can et al. demonstrated a statistically relevant low rate of inadequate cytologic material after US-guided FNAB (P<0.01). In fact they observed 27.2% of inadequate cytologic results in nodules aspirated by palpation (in 202 patients) vs. 12.5% in those aspirated under US-guidance (184 patients) with a sensitivity of 100% for both procedures (31). In their experience, Deandrea et al. demonstrated that 52% of histologically malignant nodules could be detected only thanks to US assistance, concluding that manually guided FNAB is not feasible in non-palpable nodules and not so accurate in a multinodular goiter (32). Another study conducted by Wu presented satisfying results in terms of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy (100% vs. 96%, 86% vs. 50%, 97% vs. 98, 100% vs. 33% and 97% vs. 94%, respectively). The author reported 1% of non-diagnostic material vs. 7% in 100 patients submitted to US-guided FNAB vs. 100 patients who underwent manual FNAB. All procedures had been performed by the same pathologist (33). Overall, US-guided FNAB provides satisfying results in terms of sensitivity, specificity, PPV, NPV and accuracy with low rates of complications, particularly when performed by expert operators (20,21,23-35). Moudgil et al. performed US-FNAB on 86 thyroid nodules in 70 children. Ninty point seven percent of the diagnostic procedures showed good correlation between cytopathology and pathology in patients undergoing surgery (93.1%), without false positive or false negative results for malignancy. These results are quite satisfying when we consider that the rates of malignant nodules in children are higher than in adults (35).
Percutaneous ethanol injection (PEI)
PEI is a mini-invasive technique consisting in the introduction of ethanol into the tissues. The consequence is a thrombosis of small vessels associated at inflammatory reaction. The resulting coagulative necrosis followed by fibrosis leads to reduction of the dimension of the treated lesion (36-38). According to the new guidelines, PEI represents the first-line treatment for relapsing and symptomatic benign cystic lesions and for nodules with an important fluid component. This is due to its safety, tolerability, effectiveness in volume reduction, cost effectiveness, low rates of recurrence, and short- and long- term complications (1,36,39-43). PEI was initially used also to treat hyper-functioning nodules or nodular goiters. However, compared to surgical treatment or radioiodine therapy, high recurrence rates of hyperthyroidism, increase in complications, and risk of progressive regrowth make this technique indicated only for the treatment of hot nodules that cause compressing symptoms. PEI is also used in those patients presenting contraindications to or no benefits from more effective alternative treatments (1,36,44,45). The nodule decrease in volume, albeit of lower entity than in cystic nodules, is also described in literature. However, the need of repeat treatments brings the potential risk of fibrosis of the cervical structures surrounding the treated area due to the diffusion of ethanol, making a possible surgery treatment more difficult. For all these reasons, PEI should be considered for the treatment of benign solid nodules only when alternative and more effective modalities are not applicable (1,36,46-48).
Technique and complications
A unique protocol to perform PEI has not been defined, yet. Still under way are the studies of the right amount of ethanol to be injected, the number of repeat treatments and the time lapse between them, to achieve maximum positive effects with minor number of complications (40). The patient positioning is the same as during FNAB. After skin sterilization, variable size needles (usually ranging from 20 to 25 G) are inserted in the center of the lesion under US-guidance with or without previous injection of local anesthesia at the entrance site. The cyst or the fluid parts of the nodule are almost completely drained by aspiration. Ethanol (usually with a concentration ranging from 95% to 99%) is slowly injected in the residual cavity through a syringe, filling up about 30–50% of the previously aspired volume. The procedure lasts about 2 minutes and is always monitored with US (ethanol shows an intense echogenicity). The solid part of the nodule is avoided. The procedure stops in case ethanol leakage is observed out of the nodule or if the patient refers severe pain. Some authors inject a saline solution or lidocaine during final withdrawal of the needle at the end of the procedure (36,40-50). Usually, PEI is a well-tolerated and safe technique. Diffusely described and not common complications are represented by local pain, dizziness, local hematoma, or complications related to ethanol extravasation from the nodule (recurrent laryngeal nerve palsy, peri-glandular fibrosis). More severe but rare complications include ethanol-related larynx or skin necrosis, Graves’ disease, Graves’ orbitopathy, Horner’s syndrome. One case of Plummer adenoma has also been described in literature (36,40,50-53).
Results
The effectiveness of PEI in the treatment of symptomatic cystic or relapsing cystic lesions and of mixed nodules with a predominant fluid content has been largely described, so much to be considered by the guidelines the first-line option due to its safety, cost-effectiveness (1,36,39-43) and low potential risk of impairing the thyroid function. In a preliminary randomized trial (including 20 patients with predominantly cystic thyroid nodules divided into two groups) and in a prospective study (including 32 patients with the same nodule characteristics), Verde et al. demonstrated significant effectiveness in nodule volume reduction (>50%), at 12-month follow-up, in 80% patients treated with ethanol injection after fine needle aspiration of the fluid component, without relevant side effects (54). Zingrillo et al. treated 43 patients with relapsed cyst after two aspirations or patients with an inefficient aspiration procedure due to the viscosity of the fluid material. They showed an impressive reduction in the volume of the cyst (91.9%±11.4%) in 93% of patients at 5-year follow-up with an additional 5% increased rate of success after one retreating procedure. The patients reported disappearance of related symptomatology and only mild pain was described as complication (42). Also Bennedbaek et al. described “curative” volume reduction in recurrent thyroid cyst treated with PEI equal to 82% (vs. 48% treated only with cyst aspiration and isotonic saline solution). They reported a lower number of sessions (single session in 64% vs. 18%) and low rates of side effects consisting only in moderate/severe pain and one case of transient dysphonia (48). Several studies correlate the number of PEI sessions with the dimension of the cystic volume. For example, a recent work by Negro et al. demonstrates a significantly (P<0.05) higher number of PEI in the group with larger cystic lesions (>30.0 mL) with the smallest percentage in volume reduction after the first treatment (P<0.05). Conversely, the smallest cystic lesions (<10.0 mL) had a significant volume reduction only after one PEI session in a high number of cases (85.7%). Even in these cases, the rate of side effects consisting of mild/moderate pain was very low (17.4% and 4.1%, respectively) (50,55,56). Baek et al. did not find significant differences in volume reduction, symptomatology and cosmetic scores comparing the treatment with PEI and radiofrequency ablation (RFA) in cystic nodules, and considered the first as a better technique due to cost-effectiveness and low risks of major complications (40). The same considerations are shared by other authors (Sung et al.) and can be found in the revised guidelines (1,39,56,57).
Concerning the treatment of solid thyroid nodules, though many non-randomized and a few randomized trials demonstrate a decrease in nodule volume after PEI treatment, an increased rate of complications is described, with only a limited improvement observed for repeated treatments (44,58,59).
Using PEI, Kim et al. found statistically significant differences (P<0.01) between nodule volume reduction in cystic lesions and solid lesions (59). For this reason, PEI is not the first choice in the treatment of solid thyroid nodules and is reserved to those patients who present contraindications to other more effective treatments (1,44,46-48,60-62).
PEI can find indication in hyperfunctioning or goitre thyroid nodules. As secondary treatment choice, it is employed in patients with solid lesions, due to short-term volume reduction and thyroid-stimulating hormone (TSH) serum suppression in many cases, with an high rate of recurrence (1,44,61,62).
Some authors conducted meticulous search on the effectiveness of PEI in the treatment of recurrent papillary thyroid carcinoma, but their investigations are limited by too short-term follow-up studies. A recent research published in the current year by Kim et al., based on the treatment with PEI of 41 recurrent lesions that could not undergo repeat surgery, and long-term follow-up (minimum 60 months), shows a good rate of size reduction (58.5%) and statistically significant index of no-response lesions depending on age and size. Finally, the authors conclude considering PEI as a secondary treatment option with some limitations, to be used for patients who cannot be submitted to or refuse repeat surgery, and in lesions with a diameter >10 mm (51).
Laser thermal ablation (LTA)
LTA is a quite new mini-invasive technique that, causing coagulative necrosis, can significantly reduce thyroid nodule volume as well as symptoms and cosmetic problems. The technique uses heat generated from laser light. The resulting increase in local temperature (>60 °C) leads to necrosis, followed by fibrosis and consequent reduction of the lesion size. The new guidelines provide evidence of the effectiveness, safety and well tolerability of this technique as alternative to surgery. LTA reduces large benign nodules that cause symptoms or cosmetic problems without determining changes in thyroid function or autoimmunity (1,35,63-67).
Technique and complications
After putting the patient in the typical position for thyroid procedures (hyperextension of the neck with a pillow under the shoulder), skin disinfection is performed, followed by local anesthetic injection into the subcutaneous tissue and the thyroid capsule. Silica optical fibers are inserted, under US-guidance, into the lesion through one to four 21 G needles positioned along the longitudinal major nodule axis, at a distance of 10–15 mm from each other depending on the nodule size. Energy is generated by Nd:YAG (neodymium: yttrium aluminum garnet) or by laser diode and delivered into the lesion for 5–15 minutes, until an inhomogeneous hyperechogenic area appears in place of the nodule. In case of large nodules, the “pull back technique” is employed; consisting in the retraction of the fibers of 1.0–1.5 cm. LTA is a safe procedure with low rate of side effects. Minor complications include cervical swelling and pain, local bleeding, hematomas and fever. More rarely, major side effects were observed such as changes in thyroid function or autoimmunity, laryngeal dysfunction, vocal cord palsy, recurrent nerve lesion or cervical structure injuries (1,35,45,65-75).
Results
In agreement with the guidelines in endocrinology, non-randomized, randomized and multicentre retrospective studies confirm the effectiveness, safety and clinical efficacy of LTA in the treatment of symptomatic cold thyroid nodules in terms of volume reduction and improvement in symptoms and cosmetic problems (1,35,62,64,65,72). Valcavi et al. reported a reduction of about 50% (47.8%) in nodule volume at 3-year follow-up studies in 122 patients. Changes in thyroid function were not observed and symptoms and cosmetic signs improved in 73.0% and 71.3%, respectively, with a worsened condition for both inferior to 5%. Only 9% of nodule regrowth and low rates of side effects were observed (2 patients showed delayed laryngeal dysfunction; alterations of the thyroid function were observed in 4 patients) (68). Similar results were reported also in a randomized trial work by Døssing et al. carried out in 78 euthyroid patients at 1 year follow-up, with a 51% rate of decreasing nodules, disappearance of symptoms and cosmetic complaints in 84% and 72% of patients. Only moderate pain, lasting 4 days, was reported as side effect with no modification in thyroid function (69). Gharib et al. produced two prospective randomized studies where patients with cold thyroid nodules, treated with two different kinds of laser source (Nd:YAG and diode), showed similar and significant reduction in nodule volume (44% and 43%, respectively). They also observed reduction of local symptoms in most of the patients at 6- and 12-month follow-up. These studies were compared with LT4 suppression therapy and with a control group and both did not present significant changes in nodule diameters and symptoms (46). A recent study conducted by Pacella in 1,871 patients showed statistically relevant (P<0.001) improvement after LTA treatment in terms of volume reduction (mean 72.11%, more significant in mixed nodules where it reached 79.7%), related symptoms (from 49% to 10%) and cosmetic signs (from 86% to 8%). The complication rate was low (only 0.9%) and no thyroid function or autoimmunity changes were reported (64). Concerning hyperfunctioning thyroid nodules and multinodular goitre, LTA does not represent the first choice treatment because radioiodine therapy and surgery provide better results. Although some investigations carried out on small series observed recovery of the physiological thyroid function and disappearance of hyperfunctioning areas at radioisotope scan in patients treated with LTA, in other studies the use of this technique was deemed unsatisfactory. Repeat treatments were necessary to normalize the TSH serum levels and the technique did not prove as cost-effective as radiation therapy (35,44,62,69,73-75). In a randomized trial, Døssing et al. compared the results between a single LTA treatment and radioiodine therapy on 30 solitary hot nodules. Even though LTA showed similar volume nodule reduction, the normalization of serum TSH was obtained in 50% of patients alone (73). In conclusion, LTA may be considered as a therapeutic option in small, solitary and small hyperfunctioning nodules in patients with contraindication or pharmacological interaction to radioiodine therapy (1,35,44,62). Several studies compared LTA to RFA and provided different results on their efficacy in the treatment of benign thyroid nodules. In favour of LTA is a recent study performed by Pacella et al. where in 601 nodules (449 treated with LTA and 152 with RFA), a slightly superior nodule shrinkage was observed in the LTA group, particularly in large nodules. The same incidence of complications was observed (74). The treatment of unresectable or recurrent malignant thyroid cancer is poorly described in literature. The few investigations available are based on low numbers of patients and short-term follow-up studies. Radioiodine therapy and surgery remain the gold standard in these cases. LTA can be considered as palliative in primary thyroid carcinoma and as an alternative in the local control of small recurrent papillary carcinoma (44). Zhou et al. have recently published a work where 27 recurrent papillary carcinoma lesions (with diameter <15 mm) were treated with LTA. In their experience, the lesions showed great reduction in volume (7.5±2.8 mm and 105.4±114 mm3 to 0.4±1 mm and 0.8±2.4 mm3 at the final follow-up). Three patients needed a second treatment session due to incomplete ablation detected by contrast-enhanced ultrasound (CEUS). No patients presented vascular signals at color-Doppler exam. Absence of vascularization was also appreciated at CEUS examination. No major complications were described (75).
RFA
RFA is a new minimally invasive technique developed in the last years. Applied also for the treatment of bone and abdominal tumors, RFA is based on the movement of ions determined by an electric field, which in turn is produced by an external radiofrequency generator. The latter is connected to an electrode needle that determines an increase in local temperature (between 60 and 100 °C). The result is a coagulative necrosis of the area around the needle. RFA can be performed in the treatment of benign thyroid nodules which cause symptomatology and cosmetic problems. It can also be applied in patients with recurrent thyroid cancers who present contraindications or simply do not acconsent undergoing surgery (1,2,4,35,44,76-81). At present time, this technique is not recommended for the treatment of primary thyroid cancer because so far there is not scientific evidence on its effectiveness (1,4,82,83).
Technique and complications
With the patient in the supine position and hyperextended neck, the skin is disinfected and local anesthesia performed. The latter is preferred to general anesthesia because the patient must be constantly monitored. Onsets of pain or voice alterations, in fact, are events that require interruption of the procedure. Local anesthesia is injected in sub-cutaneous tissues and under the thyroid capsule. RFA can be carried out with two different techniques both under US-guidance, using two different types of needles. The “fixed ablation technique” consists in the introduction of a 14 G multitined expandable needle (with 4–9 expandable hooks) along the long axis of the nodule (cranio-caudal approach). The “moving shot technique” consists in the introduction of an internally cooled needle with variable diameter (17–19 G), length (7–15 cm) and active tips (ranging from 0.5 mm to 2 cm). The needle is inserted through the isthmus (trans-isthmic approach), starting from the middle to the lateral direction, to reach the nodule which is divided in different small hypothetical zones, each ablated by moving the tip of the needle from the deepest position upwards to the most superficial part of the nodule. The “moving shot technique” presents some advantages over the “fixed ablation technique”. The latter provides a spheroidal ablation area, while the “moving shot” technique allows treatment of ellipsoidal areas (that represent the most likely form of thyroid nodule in routine practice). Moreover reduces the risk of complications and side effects, due to the minor exposure and the constant US monitoring of the laryngeal recurrent nerve that runs in the “danger triangle” situated between trachea, esophagus and thyroid gland. In addition, the trans-isthmic approach allows stability of the needle and prevents its involuntary movement even if the patient talks or coughs. Finally, the ablation of the lesion is confirmed by the US appearance of a hyperechogenic area associated to a sudden increase of impedence registered by the external generator (1,4,35,44,84-90) (Figure 2). Complications and side effects are similar to those observed during other interventional procedures. Low rates of major complications are described when the procedure is performed by an expert team. Several sensitive structures surround the thyroid gland: recurrent laryngeal and vagus nerve, cervical ganglion, esophagus, trachea and important vessels. However, so far, no injury of vessels, esophagus and trachea has been described in literature. Hydrodissection, consisting of 5% dextrose solution injection between the peripheral nodule area and surrounding critical structure, has been demonstrated effective to reduce clinical complications (91). Major complications were represented by voice changes determined by an injury of the laryngeal or vagus nerve (usually transient and not lasting more than 3 months), brachial plexus damage, nodule rupture and rare changes in thyroid function (above all transient). Minor complications included bleeding and subsequent hematoma (lasting few weeks and reduced by post-procedural neck-compression), pain (it is the most frequent side effect and was generally controlled by stopping the ablation or using pain-killers for 2–3 days post-treatment), skin burns around the puncture sites, and infection (for this reason, antibiotics must always be administered before RFA), cough and vomiting (4,60,91-94).
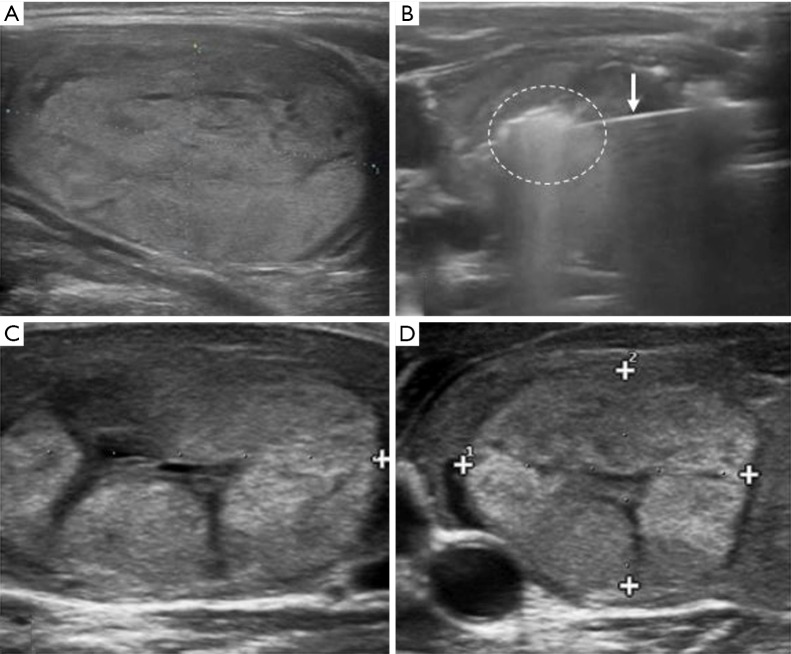
Figure 2.
RFA procedure of a benign thyroid nodule. (A) US image of a thyroid nodule of the right lobe in a 47-year old woman; (B) RFA of the nodule: the needle inside the lesion (white arrow) with the appearance of a hyper echogenic area represented the ablated area; (C) US appearance of thyroid nodule after RFA treatment at 1 month follow-up. Reduction in nodule volume can be appreciated; (D) US image shows a significant shrinkage of the benign nodule compared to image (A) and (C) at 6-month follow-up study. +, the cursors to measure the lesion diameter. US, ultrasound; RFA, radiofrequency ablation.
Results
Several studies demonstrate the effectiveness, safety and clinical efficacy of RFA in terms of nodule volume reduction, improvement in symptoms and cosmetics in the treatment of both benign cold and hyperfunctioning thyroid nodules (resolving thyrotoxic status in hot nodules). The newly reviewed guidelines also confirm RFA as an alternative to surgery and radioiodine therapy. A systematic review carried out by Fuller et al. in 2014 analyzed nine studies inclusive of 292 nodules (both cold and hot) in 284 patients with a mean of 1.05 session treatment performed for each nodule. The reviewed studies included 3 single arm observational investigations employing RFA alone, 2 single arm observational studies performing RFA before or after PEI, 2 randomized controlled trials comparing RFA to a control group, 1 randomized prospective trial comparing the effectiveness of one to two RFA sessions treatments and 1 randomized, prospective trial confronting RFA to PEI. The longest follow-up was 12 months. These studies demonstrated a statistically significant important reduction in mean volume of cold (−9.67 mL, ranging from −15.04 to −4.30 mL) and autonomously functioning nodules (−16.14 mL, ranging from −24.28 to −7.99 mL) after RFA. After the treatment, symptoms and cosmetic problems had a statistically significant improvement as well. Four trials using VAS for symptoms showed a mean improvement of −2.89 points (ranging from −2.51 to −3.28 points) and five trials presenting objective four-point scale for cosmetic problems showed a mean improvement of −2.02 points (ranging from −1.69 to −2.35 points). Even in those studies, using a combined symptom-cosmetic 0 to 6 point scale, changes were statistically significant, with a mean improvement of −2.96 points (ranging from −2.66 to −3.25 points) (95). In benign hyperfunctioning thyroid nodules, improvement in gland function was also appreciated and associated to reduction of antithyroid medication. These studies showed low rate of complications and side effects (3.9%). Two major complications (1 vocal cord palsy lasting 1 month after treatment and 1 diffuse glandular haemorrhage) and 11 minor complications (hematoma, slight-moderate pain, fever, edema and low-grade skin burn at the site of electrode insertion) were noticed (4,57,88-90,92-97) and were comparable to the rate of complications observed in another large study performed by Baek et al. (3.3%) (91). Lim et al. showed an important shrinkage of nodules with a mean of 93.5%±11.7% in 111 patients at longer follow-up series (about 4 years) with a mean session treatment of 2.2±1.4. They noticed a faster and better reduction in volume in predominantly cystic, compared to solid nodules, and obtained a statistically significant improvement in symptoms and cosmetics (P<0.001) (96). The rate of regrowth superior to 50% was low (5.6%) depending on an undertreating of the peripheral nodule portion that could lead to more sessions of treatments and a relapse of increase in thyroid function (according to other authors) (85,98,99). Deandrea et al. (98) showed at 6-month follow-up study, a significant volume reduction in patients treated with RFA (15.1±3.1 vs. 4.2±2.7 mL; P<0.0001), compared to patients who underwent observation alone (14.4±3.3 vs. 15.2±3.5 mL). Furthermore, both cosmetic and compressive symptoms had a statistically significant (P<0.001) improvement (3.6±0.5 vs. 1.7±0.4 and 3.6±1.9 vs. 0.4±0.7, respectively) compared to the other group without reporting any complications or changes in thyroid function (100). In a recent study in 108 patients with single or multiple nodules, Tang et al., showed a statistically significant decrease in nodule volume (P<0.05) after RFA with volume reduction ratio (VRR) at 1- and 3-month follow-up of 64.12% and 85.54%, respectively (2). Sung et al. treated 44 hot nodules (23 with a toxic nodule and 21 with a pretoxic nodule) that refused or presented contraindication to surgery or radioiodine therapy (99). The mean follow-up was 19.9±12.6 months. They observed an important shrinkage of nodules already at 1 month and even greater during the last follow-up passing from a mean volume of 18.5±30.1 mL to a mean volume of 4.5±9.8 mL (P<0.001). A significant improvement of thyroid serum hormon was also observed and, at scintigraphy, 35 nodules showed loss of overall uptake. Nine nodules showed decreased uptake at the last follow-up. Related symptoms and cosmetic problems were also found significantly improved during the last follow-up study. No major side effects were found. Different studies compared RFA with LTA resulting in higher efficacy of RFA in providing nodule volume decrease (1,100,101). However,-recent studies performed on a number superior to 1,500 patients reported that both techniques had similar uptake decrease (1,98). RFA can be used for locoregional control of cancer or improvement of cancer-related symptoms in patients with recurrent thyroid cancer who present high surgical risk and refuse repeat surgery. RFA of recurrent thyroid cancer in the neck resulted in a mean volume reduction of 56% to 93% with 42% to 58% of nodules completely disappearing and 64% of patients experiencing symptom improvement and serum thyroglobulin concentration decrease. Long-term follow-up data have not been published, yet (88,102,103). According to the Korean Society of Thyroid Radiology and confirmed by a 2015 Italian report, even in absence of long-term follow-up studies, RFA is indicated in the treatment of recurrent thyroid cancers in patients with high surgical risk or refusing repeat surgery. In literature, the mean tumor volume reduction described presented a variable rate ranging from 50.9% to 8.4%. The rate of tumor complete disappearance ranged from 25% to 94% of cancers with an improvement in therapeutic success and symptoms ranging from 75% to 97% and 64%, respectively. Moreover, in many patients a decrease in serum thyroglobulin concentration was described (88,102-111). A recent meta-analysis made by Suh et al. compared the effectiveness and safety of RFA and PEI for the treatment of total sample size of recurrent thyroid cancers in 270 patients with a VRR ≥50% rate of 100.0% after RFA and of 89.5% after PEI associated to a rate of complete disappearance of the nodules of 68.8% and 53.4%, respectively. The RFA rate of recurrence of 0.0% and 2.4% concerning PEI and also the number of sessions were lower in patients treated with RFA (less than 1.3 vs. greater than 2 in PEI). All this rates were however no statistically significant (110). Based on the guidelines, RFA is not indicated as first-line therapeutic option due to a low number of evidence (1,92). Despite this, a recent study by Zhang et al. reported no residual or recurrent tumor after RFA at 12- and 18-month follow-up in 92 patients with low-risk papillary thyroid micro-carcinomas (PTMC). In addition, no suspicious metastatic lymphnodes were detected and US-guided biopsy confirmed the absence of residual or recurrent cancer areas. No major complications were described during this study (111).
Percutaneous microwave ablation (PMWA)
PMWA is a new technique used to treat thyroid nodules. Due to its novelty, the technique is still scarcely described in literature. Used for the treatment of tumors of other nature, it uses the rotation of molecules produced from microwave energy to increase the local kinetic energy thus producing a rapid increase of local temperature inside the tissues. The result is the ablation of the target tissue (112-119).
Technique and complications
The patient is in the supine position with neck extension. The material needed is a microwave generator, a flexible low-loss coaxial cable and a cooled shaft antenna. The generator produces 1–100 W of power at 2,450 MHz, releasing energy in pulse or continuous way. The antenna is a 16 G needle and is composed by polytetrafluoroethylene. It is 3–5 mm long. Distilled water is conveyed inside the antenna shaft to cool it. After skin disinfection and local anesthesia the needle antenna is positioned along the long axis of the nodules under US-guidance. Delivered energy usually ranges from 30 to 50 W and the ablation zone appears like a hyperechoic area, similarly to RFA. The complete ablation is obtained moving the tip of the needle. Several authors recommend compression of the neck to avoid the formation of hematoma. Hydrodissection techniques can also be used to increase the distance between the target lesions and the critical structures surrounding the thyroid (112,113). Complications and side effects are the same as in the other ablation techniques and include hematomas, different degrees of pain intensity, transient voice changes, recurrent laryngeal nerve injury, skin burns and thyroid dysfunction (115-119).
Results
Only a few studies report the effectiveness of PMWA in the treatment of benign solid thyroid nodules. The described volume reduction rate ranges from 45% to 65% in follow-up studies lasting up to 12 months. Improvement of symptoms and cosmetic problems and no significative changes in thyroid function were observed (116,117). Recent studies included an important number of patients. Liu et al. treated with PMWA 474 benign thyroid nodules in 435 patients and observed a significant decrease in volume (90% is the mean rate of final volume reduction at 1-year follow-up). No major complications were described (112). Wu et al. treated 121 benign thyroid nodules and observed statistically significant (P<0.001) improvement in the decrease of nodule volume, symptoms and cosmetic problems at 1-year follow-up with a low number of complications (2 patients had hoarseness recovered within 2 months; 2 patients showed a slight skin burns; 1 case developed Horner syndrome, recovered within 2 months) (113). Yue et al. reported a significant shrinkage of nodules treated with PMWA in 110 patients at 1 year (ranging from 12.6±15.1 to 3.2±5.7 mL). Sixteen patients had recurrence 12 months after treatment and the authors put this in relation to initial large volume of the nodule, presence of more irregular blood vessels and lower energy delivered (116). Only one study describes the use of PMWA in the treatment of 17 patients with 23 recurrent thyroid cancers with high rate of mean tumour volume reduction (91%) at 18-month follow-up. Thirty point four percent of patients showed complete disappearance of tumors and 52.2% residual small scar-like lesions (119).
High intensity focused ultrasound (HIFU)
HIFU is a new mini-invasive technique that induces thermal coagulative necrosis inside the tissues due to high intensity US beam focalized into a target lesion, without skin penetration by devices. Several significations are required to perform the complete ablation of the lesions considered the small ablative volumes provided by each single sonication. This may lead to prolonged duration of the treatment (120-126).
Technique and complications
Under conscious sedation, the patient is placed in a supine position with hyperextended neck. According to literature, only one US-guided device for the treatment of thyroid nodules is commercially available, namely the EchoPulse® (Theraclion SA, Malakoff, France). This device has two independent US systems, one for US-guidance and the other attached to the transducer generating the focalised US beam. The focal point is always put in the centre of the US image where the target area always appears as hyperechoic. The device is setted to limit automatically its power when approaching the sensible structures around the nodule. Maintenance of safety margins is always recommended to avoid complications as described in literature. The lesion is divided into different voxel areas of treatment. Each sonication lasts from 4 to 8 seconds with 20 to 40 seconds of cooling interval (7,123-126).
Results
A low number of patients were treated with HIFU and a recent systematic review presented by Lang et al. identified five original studies which showed nodule volume reduction rate ranging between 45% and 68%, after a single session of HIFU ablation, with varying results depending on nodule size and length of follow-up (7). An investigation carried out in 2017 by Sennert et al. on 19 benign nodules showed volume reduction rate of 58% at 3-month follow-up, with 10 out of 19 patients showing therapeutic success (defined as a volume reduction ≥50%) (126). No studies have been published, yet, describing the treatment of primary of recurrent thyroid cancer.
Conclusions
Treated by surgery alone during the past decades, many thyroid nodules nowadays can be treated by means of new mini-invasive techniques that are less expensive and present lower risks of major complications and side effects over surgery. In particular, PEI is in presence of cystic thyroid nodules, LTA and RFA in the treatment of both cold and hyperfunctioning benign nodules in patients who refuse or have contraindications to surgery or radioiodine therapy. In presence of recurrent papillary thyroid cancer, PEI, LTA and above all RFA can be considered as a valid option in patients who cannot undergo surgery. PMWA and HIFU show less evidence of effectiveness in the treatment of this kind of nodules even if the results published so far are encouraging. During the last years, PMWA has importantly developed and the last studies have demonstrated similar effects compared to RFA. So, PMWA can be considered as a valid alternative to the other ablation techniques. Further studies are needed instead to confirm the effectiveness of HIFU as treatment of choice. At present, the complication rate of HIFU is lower than in other techniques, but the rate of volume reduction of the nodules is lower. There are also less therapeutic indications which represent a limit to its employment.
Acknowledgements
We thank Dr. Angela Martella for her contribution in the language revision of the manuscript.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Gharib H, Papini E, Garber JR, et al. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS, AMERICAN COLLEGE OF ENDOCRINOLOGY, AND ASSOCIAZIONE MEDICI ENDOCRINOLOGI MEDICAL GUIDELINES FOR CLINICAL PRACTICE FOR THE DIAGNOSIS AND MANAGEMENT OF THYROID NODULES--2016 UPDATE. Endocr Pract 2016;22:622-39. 10.4158/EP161208.GL [DOI] [PubMed] [Google Scholar]
- 2.Tang X, Cui D, Chi J, et al. Evaluation of the safety and efficacy of radiofrequency ablation for treating benign thyroid nodules. J Cancer 2017;8:754-60. 10.7150/jca.17655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozkurt H, Irkorücü O, Aziret M, et al. Comparison of 1869 thyroid ultrasound-guided fine-needle aspiration biopsies between general surgeons and interventional radiologists. Ann Med Surg (Lond) 2016;10:92-102. 10.1016/j.amsu.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Bernardi IC, Floridi C, Muollo A, et al. Vascular and interventional radiology radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: literature review. Radiol Med 2014;119:512-20. 10.1007/s11547-014-0411-2 [DOI] [PubMed] [Google Scholar]
- 5.Sui WF, Li JY, Fu JH. Percutaneous laser ablation for benign thyroid nodules: A meta- analysis. Oncotarget 2017;8:83225-36. 10.18632/oncotarget.17928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang B, Han ZY, Yu J, et al. Factors related to recurrence of the benign non-functioning thyroid nodules after percutaneous microwave ablation. Int J Hyperthermia 2017. [Epub ahead of print]. 10.1080/02656736.2016.1274058 [DOI] [PubMed] [Google Scholar]
- 7.Lang BH, Wu AL. High intensity focused ultrasound (HIFU) ablation of benign thyroid nodules – a systematic review. J Ther Ultrasound 2017;5:11. 10.1186/s40349-017-0091-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floridi C, Reginelli A, Capasso R, et al. Percutaneous needle biopsy of mediastinal masses under C-arm cone-beam CT guidance: diagnostic performance and safety. Med Oncol 2017;34:67. 10.1007/s12032-017-0911-8 [DOI] [PubMed] [Google Scholar]
- 9.Arrigoni F, Barile A, Zugaro L, et al. Intra-articular benign bone lesions treated with Magnetic Resonance-guided Focused Ultrasound (MRgFUS): imaging follow-up and clinical results. Med Oncol 2017;34:55. 10.1007/s12032-017-0904-7 [DOI] [PubMed] [Google Scholar]
- 10.Barile A, Arrigoni F, Zugaro L, et al. Minimally invasive treatments of painful bone lesions: state of the art. Med Oncol 2017;34:53. 10.1007/s12032-017-0909-2 [DOI] [PubMed] [Google Scholar]
- 11.Cazzato RL, Garnon J, Ramamurthy N, et al. Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol 2016;33:140. 10.1007/s12032-016-0848-3 [DOI] [PubMed] [Google Scholar]
- 12.Barile A, La Marra A, Arrigoni F, et al. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol 2016;89:20150355. 10.1259/bjr.20150355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ierardi AM, Floridi C, Fontana F, et al. Microwave ablation of liver metastases to overcome the limitations of radiofrequency ablation. Radiol Med 2013;118:949-61. 10.1007/s11547-013-0968-1 [DOI] [PubMed] [Google Scholar]
- 14.Di Zazzo E, Porcile C, Bartollino S, et al. Critical Function of PRDM2 in the Neoplastic Growth of Testicular Germ Cell Tumors. Biology (Basel) 2016;5(4). 10.3390/biology5040054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cappabianca S, Colella G, Pezzullo MG, et al. Lipomatous lesions of the head and neck region: imaging findings in comparison with histological type. Radiol Med 2008;113:758-70. 10.1007/s11547-008-0258-5 [DOI] [PubMed] [Google Scholar]
- 16.Caranci F, Napoli M, Cirillo M, et al. Basilar artery hypoplasia. Neuroradiol J 2012;25:739-43. 10.1177/197140091202500613 [DOI] [PubMed] [Google Scholar]
- 17.Caranci F, Tedeschi E, Leone G, et al. Errors in neuroradiology. Radiol Med 2015;120:795-801. 10.1007/s11547-015-0564-7 [DOI] [PubMed] [Google Scholar]
- 18.Muccio CF, Di Blasi A, Esposito G, et al. Perfusion and spectroscopy magnetic resonance imaging in a case of lymphocytic vasculitis mimicking brain tumor. Pol J Radiol 2013;78:66-9. 10.12659/PJR.884011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierot L, Söderman M, Bendszus M, et al. Statement of ESMINT and ESNR regarding recent trials evaluating the endovascular treatment at the acute stage of ischemic stroke. Neuroradiology 2013;55:1313-8. 10.1007/s00234-013-1249-3 [DOI] [PubMed] [Google Scholar]
- 20.Theoharis CG, Schofield KM, Hammers L, et al. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid 2009;19:1215-23. 10.1089/thy.2009.0155 [DOI] [PubMed] [Google Scholar]
- 21.Baskin HJ. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules and multinodular goitres. Endocr Pract 2004;10:242-5. 10.4158/EP.10.3.242 [DOI] [PubMed] [Google Scholar]
- 22.Zoccali C, Rossi B, Zoccali G, et al. A new technique for biopsy of soft tissue neoplasms: A preliminary experience using MRI to evaluate bleeding. Minerva Medica 2015;106:117-20. [PubMed] [Google Scholar]
- 23.Manning AM, Yang H, Falciglia M, et al. Thyroid Ultrasound-Guided Fine-Needle Aspiration Cytology Results: Observed Increase in Indeterminate Rate over the Past Decade. Otolaryngol Head Neck Surg 2017;156:611-5. 10.1177/0194599816688190 [DOI] [PubMed] [Google Scholar]
- 24.Karadeniz Cakmak G, Emre AU, Tascılar O, et al. Diagnostic adequacy of surgeon-performed ultrasound-guided fine needle aspiration biopsy of thyroid nodules. J Surg Oncol 2013;107:206-10. 10.1002/jso.23212 [DOI] [PubMed] [Google Scholar]
- 25.Seiberling KA, Dutra JC, Gunn J. Ultrasound-guided fine needle aspiration biopsy of thyroid nodules performed in the office. Laryngoscope 2008;118:228-31. 10.1097/MLG.0b013e318157465d [DOI] [PubMed] [Google Scholar]
- 26.Oktay I, Reyhan E, Kamuran CD. Surgeon-performed ultrasound-guided thyroid fine-needle aspiration biopsy: evaluation of 470 biopsies. Hell J Surg 2013;85:380-5. 10.1007/s13126-013-0070-0 [DOI] [Google Scholar]
- 27.Abu-Yousef MM, Larson JH, Kuehn DM, et al. Safety of ultrasound-guided fine needle aspiration biopsy of neck lesions in patients taking antithrombotic/anticoagulant medications. Ultrasound Q 2011;27:157-9. 10.1097/RUQ.0b013e31822b5681 [DOI] [PubMed] [Google Scholar]
- 28.Lee YH, Baek JH, Jung SL, et al. Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the Korean Society of Thyroid Radiology. Korean J Radiol 2015;16:391-401. 10.3348/kjr.2015.16.2.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hor T, Lahiri SW. Bilateral thyroid hematomas after fine-needle aspiration causing acute airway obstruction. Thyroid 2008;18:567-9. 10.1089/thy.2007.0363 [DOI] [PubMed] [Google Scholar]
- 30.Danese D, Sciacchitano S, Farsetti A, et al. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid 1998;8:15-21. 10.1089/thy.1998.8.15 [DOI] [PubMed] [Google Scholar]
- 31.Can AS, Peker K. Comparison of palpation-versus ultra- sound-guided fine-needle aspiration biopsies in the evaluation of thyroid nodules, BMC Res Notes 2008;1:12. 10.1186/1756-0500-1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deandrea M, Mormile A, Veglio M, et al. Fine-needle aspiration biopsy of the thyroid: comparison between thyroid palpation and ultrasonography. Endocr Pract 2002;8:282-6. 10.4158/EP.8.4.282 [DOI] [PubMed] [Google Scholar]
- 33.Wu M. A comparative study of 200 head and neck FNAs performed by a cytopathologist with versus without ultrasound guidance: evidence for improved diagnostic value with ultrasound guidance. Diagn Cytopathol 2011;39:743-51. 10.1002/dc.21460 [DOI] [PubMed] [Google Scholar]
- 34.Leung VA, Kirpalani A, Mnatzakanian G, et al. Effect of a Biopsy Center on Adequacy Rates of Thyroid Nodule Fine-Needle Aspiration. AJR Am J Roentgenol 2017;209:358-62. 10.2214/AJR.16.17157 [DOI] [PubMed] [Google Scholar]
- 35.Moudgil P, Vellody R, Heider A, et al. Ultrasound-guided fine-needle aspiration biopsy of pediatric thyroid nodules. Pediatr Radiol 2016;46:365-71. 10.1007/s00247-015-3478-6 [DOI] [PubMed] [Google Scholar]
- 36.Papini E, Gugliemi R, Pacella CM. Laser, radiofrequency, and ethanol ablation for the management of thyroid nodules. Curr Opin Endocrinol Diabetes Obes 2016;23:400-6. 10.1097/MED.0000000000000282 [DOI] [PubMed] [Google Scholar]
- 37.Crescenzi A, Papini E, Pacella CM, et al. Morphological changes in a hyperfunctioning thyroid adenoma after percutaneous ethanol injection: histological, enzymatic and sub-microscopical alterations. J Endocrinol Invest 1996;19:371-6. 10.1007/BF03344972 [DOI] [PubMed] [Google Scholar]
- 38.Pomorski L, Bartos M. Histologic changes in thyroid nodules after percuta- neous ethanol injection in patients subsequently operated on due to new focal thyroid lesions. APMIS 2002;110:172-6. 10.1034/j.1600-0463.2002.100208.x [DOI] [PubMed] [Google Scholar]
- 39.Monzani F, Caraccio N, Basolo F, et al. Surgical and pathological changes after percutaneous ethanol injection therapy of thyroid nodules. Thyroid 2000;10:1087-92. 10.1089/thy.2000.10.1087 [DOI] [PubMed] [Google Scholar]
- 40.Baek JH, Ha EJ, Choi YJ, et al. Radiofrequency versus Ethanol Ablation for Treating Predominantly Cystic Thyroid Nodules: A Randomized Clinical Trial. Korean J Radiol 2015;16:1332-40. 10.3348/kjr.2015.16.6.1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felício JS, Conceição AM, Santos FM. Ultrasound-guided Percutaneous ethanol injection Protocol to Treat solid and Mixed Thyroid nodules. Front Endocrinol (Lausanne) 2016;7:52. 10.3389/fendo.2016.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zingrillo M, Torlontano M, Chiarella R, et al. Percutaneous ethanol injection may be a definitive treatment for symptomatic thyroid cystic nodules not treatable by surgery: five-year follow-up study. Thyroid 1999;9:763-7. 10.1089/thy.1999.9.763 [DOI] [PubMed] [Google Scholar]
- 43.Bennedbaek FN, Hegedüs L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab 2003;88:5773-7. 10.1210/jc.2003-031000 [DOI] [PubMed] [Google Scholar]
- 44.Kim DW, Rho H, Park J, et al. Ultrasonography-guided ethanol ablation of predominantly solid thyroid nodules: a preliminary study for factors that predict the outcome. Br J Radiol 2012;85:930-6. 10.1259/bjr/81849588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papini E, Pacella CM, Misischi I, et al. The advent of ultrasound-guided ablation techniques in nodular thyroid disease: towards a patient-tailored approach, Best Pract Res Clin Endocrinol Metab 2014;28:601-18. 10.1016/j.beem.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 46.Gharib H, Hegedüs L, Pacella CM, et al. Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab 2013;98:3949-57. 10.1210/jc.2013-1806 [DOI] [PubMed] [Google Scholar]
- 47.Zingrillo M, Collura D, Ghiggi MR, et al. Treatment of large cold benign thyroid nodules not eligible for surgery with percutaneous ethanol injection. J Clin Endocrinol Metab 1998;83:3905-7. 10.1210/jcem.83.11.5257 [DOI] [PubMed] [Google Scholar]
- 48.Bennedbaek FN, Nielsen LK, Hegedüs L. Effect of per- cutaneous ethanol injection therapy versus suppressive doses of L-thyroxine on benign solitary solid cold thyroid nodules: a randomized trial. J Clin Endocrinol Metab 1998;83:830-5. 10.1210/jcem.83.3.4673 [DOI] [PubMed] [Google Scholar]
- 49.Guglielmi R, Pacella CM, Bianchini A, et al. Percutaneous ethanol injection treatment in benign thyroid lesions: role and efficacy. Thyroid 2004;14:125-31. 10.1089/105072504322880364 [DOI] [PubMed] [Google Scholar]
- 50.Negro R, Colosimo E, Greco G. Outcome, Pain Perception, and Health-Related Quality of Life in Patients Submitted to Percutaneous Ethanol Injection for Simple Thyroid Cysts. J Thyroid Res 2017;2017:9536479. [DOI] [PMC free article] [PubMed]
- 51.Kim SY, Kim SM, Chang H, et al. Long-term outcomes of ethanol injection therapy for locally recurrent papillary thyroid cancer. Eur Arch Otorhinolaryngol 2017;274:3497-501. 10.1007/s00405-017-4660-2 [DOI] [PubMed] [Google Scholar]
- 52.Regalbuto C, Le Moli R, Muscia V, et al. Severe graves’ ophthalmopathy after percutaneous ethanol injection in a nontoxic thyroid nodule. Thyroid 2012;22:210-3. 10.1089/thy.2011.0315 [DOI] [PubMed] [Google Scholar]
- 53.Cesareo R, Naciu AM, Pasqualini V, et al. A Rare Complication following Thyroid Percutaneous Ethanol Injection: Plummer Adenoma. Case Rep Endocrinol 2017;2017:1026139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verde G, Papini E, Pacella CM, et al. Ultrasound guided percutaneous ethanol injection in the treatment of cystic thyroid nodules. Clin Endocrinol (Oxf) 1994;41:719-24. 10.1111/j.1365-2265.1994.tb02785.x [DOI] [PubMed] [Google Scholar]
- 55.Sung JY, Kim YS, Choi H, et al. Optimum first-line treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? AJR Am J Roentgenol 2011;196:W210-4. 10.2214/AJR.10.5172 [DOI] [PubMed] [Google Scholar]
- 56.Sung JY, Baek JH, Kim KS, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radio-frequency ablation: a prospective randomized study. Radiology 2013;269:293-300. 10.1148/radiol.13122134 [DOI] [PubMed] [Google Scholar]
- 57.Bennedbaek FN, Hegedus L. Percutaneous ethanol injection therapy in benign solitary solid cold thyroid nodules: a randomized trial comparing one injection with three injections. Thyroid 1999;9:225-33. 10.1089/thy.1999.9.225 [DOI] [PubMed] [Google Scholar]
- 58.Bennedbaek FN, Hegedus L. Alcohol sclerotherapy for benign solitary solid cold thyroid nodules. Lancet 1995;346:1227. 10.1016/S0140-6736(95)92930-4 [DOI] [PubMed] [Google Scholar]
- 59.Kim JH, Lee HK, Lee JH, et al. Efficacy of sonographically guided percutaneous ethanol injection for treatment of thyroid cysts versus solid thyroid nodules. AJR Am J Roentgenol 2003;180:1723-6. 10.2214/ajr.180.6.1801723 [DOI] [PubMed] [Google Scholar]
- 60.Lippi F, Ferrari C, Manetti L, et al. Treatment of solitary autonomous thyroid nodules by percutaneous ethanol injection: results of an Italian multicenter study. The Multicenter Study Group. J Clin Endocrinol Metab 1996;81:3261-4. [DOI] [PubMed] [Google Scholar]
- 61.Papini E, Guglielmi R, Bizzarri G, et al. Ultrasound-guided laser thermal ablation for treatment of benign thyroid nodules. Endocr Pract 2004;10:276-83. 10.4158/EP.10.3.276 [DOI] [PubMed] [Google Scholar]
- 62.Papini E, Rago T, Gambelunghe G, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules: results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab 2014;99:3653-9. 10.1210/jc.2014-1826 [DOI] [PubMed] [Google Scholar]
- 63.Pacella CM, Bizzarri G, Spiezia S, et al. Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology 2004;232:272-80. 10.1148/radiol.2321021368 [DOI] [PubMed] [Google Scholar]
- 64.Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab 2015;100:3903-10. 10.1210/jc.2015-1964 [DOI] [PubMed] [Google Scholar]
- 65.Døssing H, Bennedbaek FN, Karstrup S, et al. Benign solitary solid cold thyroid nodules: US-guided interstitial laser photocoagulation – initial experience. Radiology 2002;225:53-7. 10.1148/radiol.2251011042 [DOI] [PubMed] [Google Scholar]
- 66.Døssing H, Bennedbaek FN, Hegedus L. Ultrasound-guided interstitial laser photocoagulation of an autonomous thyroid nodule: the introduction of a novel alternative. Thyroid 2003;13:885-8. 10.1089/105072503322401104 [DOI] [PubMed] [Google Scholar]
- 67.Stafford RJ, Fuentes D, Elliott AA, et al. Laser- induced thermal therapy for tumor ablation. Crit Rev Biomed Eng 2010;38:79-100. 10.1615/CritRevBiomedEng.v38.i1.70 [DOI] [PubMed] [Google Scholar]
- 68.Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid 2010;20:1253-61. 10.1089/thy.2010.0189 [DOI] [PubMed] [Google Scholar]
- 69.Døssing H, Bennedbæk FN, Hegedüs L. Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol 2011;165:123-8. 10.1530/EJE-11-0220 [DOI] [PubMed] [Google Scholar]
- 70.Spiezia S, Vitale G, Di Somma C, et al. Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid 2003;13:941-7. 10.1089/105072503322511346 [DOI] [PubMed] [Google Scholar]
- 71.Cakir B, Gul K, Ugras S, et al. Percutaneous laser ablation of an autonomous thyroid nodule: effects on nodule size and histopathology of the nodule 2 years after the procedure. Thyroid 2008;18:803-5. 10.1089/thy.2007.0316 [DOI] [PubMed] [Google Scholar]
- 72.Barbaro D, Orsini P, Lapi P, et al. Percutaneous laser ablation in the treatment of toxic and pretoxic nodular goiter. Endocr Pract 2007;13:30-6. 10.4158/EP.13.1.30 [DOI] [PubMed] [Google Scholar]
- 73.Døssing H, Bennedbaek FN, Bonnema SJ, et al. Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol 2007;157:95-100. 10.1530/EJE-07-0094 [DOI] [PubMed] [Google Scholar]
- 74.Pacella CM, Mauri G, Cesareo R., et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia 2017;33:911-9. [DOI] [PubMed] [Google Scholar]
- 75.Zhou W, Zhang L, Zhan W, et al. Percutaneous laser ablation for treatment of locally recurrent papillary thyroid carcinoma <15 mm. Clin Radiol 2016;71:1233-9. 10.1016/j.crad.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 76.Masciocchi C, Zugaro L, Arrigoni F, et al. Radiofrequency ablation versus magnetic resonance guided focused ultrasound surgery for minimally invasive treatment of osteoid osteoma: a propensity score matching study. Eur Radiol 2016;26:2472-81. 10.1007/s00330-015-4111-7 [DOI] [PubMed] [Google Scholar]
- 77.Arrigoni F, Barile A, Zugaro L, et al. CT-guided radiofrequency ablation of spinal osteoblastoma: treatment and long-term follow-up. Int J Hyperthermia 2017;9:1-7. 10.1080/02656736.2017.1334168 [DOI] [PubMed] [Google Scholar]
- 78.Masciocchi C, Arrigoni F, La Marra A, et al. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br J Radiol 2016;89:20150356. 10.1259/bjr.20150356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow up in 236 patients. Eur Radiol 2008;18:1244-50. 10.1007/s00330-008-0880-6 [DOI] [PubMed] [Google Scholar]
- 80.Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: com- plications encountered in a multicenter study. Radiology 2003;226:441-51. 10.1148/radiol.2262012198 [DOI] [PubMed] [Google Scholar]
- 81.Shin JH, Baek JH, Ha EJ, et al. Radiofrequency ablation of thyroid nodules: Basic principles and clinical application. Int J Endocrinol 2012;2012:919650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baek JH, Moon WJ, Kim YS, et al. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg 2009;33:1971-7. 10.1007/s00268-009-0130-3 [DOI] [PubMed] [Google Scholar]
- 83.Baek JH, Lee JH, Valcavi R, et al. Thermal ablation for benign thyroid nodules: Radiofrequency and laser. Korean J Radiol 2011;12:525-40. 10.3348/kjr.2011.12.5.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deandrea M, Limone P, Basso E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol 2008;34:784-91. 10.1016/j.ultrasmedbio.2007.10.018 [DOI] [PubMed] [Google Scholar]
- 85.Spiezia S, Garberoglio R, Di Somma C, et al. Efficacy and safety of radiofrequency thermal ablation in the treatment of thyroid nodules with pressure symptoms in elderly patients. J Am Geriatr Soc 2007;55:1478-9. 10.1111/j.1532-5415.2007.01306.x [DOI] [PubMed] [Google Scholar]
- 86.Faggiano A, Ramundo V, Assantiand AP, et al. Thyroid nodules treated with percutaneous radiofrequency thermal ablation: a comparative study. J Clin Endocrinol Metab 2012;97:4439-45. 10.1210/jc.2012-2251 [DOI] [PubMed] [Google Scholar]
- 87.Park HS, Baek JH, Park AW, et al. Thyroid Radiofrequency Ablation: Updates on Innovative Devices and Techniques. Korean J Radiol 2017;18:615-23. 10.3348/kjr.2017.18.4.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology 2012;262:335-42. 10.1148/radiol.11110416 [DOI] [PubMed] [Google Scholar]
- 89.Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol 2012;13:117-25. 10.3348/kjr.2012.13.2.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jang SW, Baek JH, Kim JK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol 2012;81:905-10. 10.1016/j.ejrad.2011.02.039 [DOI] [PubMed] [Google Scholar]
- 91.Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol 2010;194:1137-42. 10.2214/AJR.09.3372 [DOI] [PubMed] [Google Scholar]
- 92.Kim YS, Rhim H, Tae K, et al. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 2006;16:361-7. 10.1089/thy.2006.16.361 [DOI] [PubMed] [Google Scholar]
- 93.Kim DW. Sonography-guided ethanol ablation of a remnant solid component after radiofrequency ablation of benign solid thyroid nodules: a preliminary study. Am J Neuroradiol 2012;33:1139-43. 10.3174/ajnr.A2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huh JY, Baek JH, Choi H, et al. Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session—prospective randomized study. Radiology 2012;263:3:909-16. 10.1148/radiol.12111300 [DOI] [PubMed] [Google Scholar]
- 95.Fuller CW, Nguyen SA, Lohia S, et al. Radiofrequency ablation for treatment of benign thyroid nodules: systematic review. Laryngoscope 2014;124:346-53. 10.1002/lary.24406 [DOI] [PubMed] [Google Scholar]
- 96.Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol 2013;23:1044-9. 10.1007/s00330-012-2671-3 [DOI] [PubMed] [Google Scholar]
- 97.Baek JH, Jeong HJ, Kim YS, et al. Radiofrequency ablation for an autonomously functioning thyroid nodule. Thyroid 2008;18:675-6. 10.1089/thy.2007.0274 [DOI] [PubMed] [Google Scholar]
- 98.Deandrea M, Sung JY, Limone P, et al. Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid 2015;25:890-6. 10.1089/thy.2015.0133 [DOI] [PubMed] [Google Scholar]
- 99.Sung JY, Baek JH, Jung SL, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid 2015;25:112-7. 10.1089/thy.2014.0100 [DOI] [PubMed] [Google Scholar]
- 100.Ha EJ, Baek JH, Kim KW, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J Clin Endocrinol Metab 2015;100:1903-11. 10.1210/jc.2014-4077 [DOI] [PubMed] [Google Scholar]
- 101.Monchik JM, Donatini G, Iannuccilli J, et al. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg 2006;244:296-304. 10.1097/01.sla.0000217685.85467.2d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garberoglio R, Aliberti C, Appetecchia M, et al. Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound 2015;18:423-30. 10.1007/s40477-015-0169-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 2016;17:370-95. 10.3348/kjr.2016.17.3.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park KW, Shin JH, Han BK, et al. Inoperable symptomatic recurrent thyroid cancers: preliminary result of radiofrequency ablation. Ann Surg Oncol 2011;18:2564-8. 10.1245/s10434-011-1619-1 [DOI] [PubMed] [Google Scholar]
- 105.Baek JH, Kim YS, Sung JY, et al. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol 2011;197:W331-6. 10.2214/AJR.10.5345 [DOI] [PubMed] [Google Scholar]
- 106.Radzina M, Cantisani V, Rauda M, et al. Update on the role of ultrasound guided radiofrequency ablation for thyroid nodule treatment. Int J Surg 2017;41 Suppl 1:S82-93. 10.1016/j.ijsu.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 107.Jeong SY, Baek JH, Choi YJ, et al. Ethanol and thermal ablation for malignant thyroid tumours. Int J Hyperthermia 2017;33:938-45. [DOI] [PubMed] [Google Scholar]
- 108.Dupuy DE, Monchik JM, Decrea C, et al. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery 2001;130:971-7. 10.1067/msy.2001.118708 [DOI] [PubMed] [Google Scholar]
- 109.Lee SJ, Jung SL, Kim BS, et al. Radiofrequency ablation to treat loco-regional recurrence of well-differentiated thyroid carcinoma. Korean J Radiol 2014;15:817-26. 10.3348/kjr.2014.15.6.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid 2016;26:420-8. 10.1089/thy.2015.0545 [DOI] [PubMed] [Google Scholar]
- 111.Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid 2016;26:1581-7. 10.1089/thy.2015.0471 [DOI] [PubMed] [Google Scholar]
- 112.Liu YJ, Qian LX, Liu D, et al. Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients. Exp Biol Med (Maywood) 2017;242:1515-23. 10.1177/1535370217727477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu W, Gong X, Zhou Q, et al. US-guided percutaneous microwave ablation for the treatment of benign thyroid nodules. Endocr J 2017;64:1079-85. 10.1507/endocrj.EJ17-0152 [DOI] [PubMed] [Google Scholar]
- 114.Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound 2016;20:11-22. 10.1007/s40477-016-0221-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feng B, Liang P, Cheng Z, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol 2012;166:1031-7. 10.1530/EJE-11-0966 [DOI] [PubMed] [Google Scholar]
- 116.Yue W, Wang S, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol 2013;82:e11-6. 10.1016/j.ejrad.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 117.Heck K, Happel C, Grünwald F. Percutaneous microwave ablation of thyroid nodules: effects on thyroid function and antibodies. Int J Hyperthermia 2015;31:560-7. 10.3109/02656736.2015.1032371 [DOI] [PubMed] [Google Scholar]
- 118.Korkusuz H, Nimsdorf F, Happel C. Percutaneous microwave ablation of benign thyroid nodules. Functional imaging in comparison to nodular volume reduction at a 3-month follow-up. Nuklearmedizin 2015;54:13-9. 10.3413/Nukmed-0678-14-06 [DOI] [PubMed] [Google Scholar]
- 119.Yue W, Chen L, Wang S, et al. Locoregional control of recurrent papillary thyroid carcinoma by ultrasound-guided percutaneous microwave ablation: a prospective study. Int J Hyperthermia 2015;31:403-8. 10.3109/02656736.2015.1014433 [DOI] [PubMed] [Google Scholar]
- 120.Masciocchi C, Conchiglia A, Gregori LM, et al. Critical role of HIFU in musculoskeletal interventions. Radiol Med 2014;119:470-5. 10.1007/s11547-014-0414-z [DOI] [PubMed] [Google Scholar]
- 121.Ferrari F, Arrigoni F, Miccoli A, et al. Effectiveness of Magnetic Resonance-guided Focused Ultrasound Surgery (MRgFUS) in the uterine adenomyosis treatment: technical approach and MRI evaluation. Radiol Med 2016;121:153-61. 10.1007/s11547-015-0580-7 [DOI] [PubMed] [Google Scholar]
- 122.Masciocchi C, Arrigoni F, Ferrari F, et al. Uterine fibroid therapy using interventional radiology mini-invasive treatments: current perspective. Med Oncol 2017;34:52. 10.1007/s12032-017-0906-5 [DOI] [PubMed] [Google Scholar]
- 123.Esnault O, Franc B, Ménégaux F, et al. High-intensity focused ultrasound ablation of thyroid nodules: first human feasibility study. Thyroid 2011;21:965-73. 10.1089/thy.2011.0141 [DOI] [PubMed] [Google Scholar]
- 124.Korkusuz H, Fehre N, Sennert M, et al. Early assessment of high-intensity focused ultrasound treatment of benign thyroid nodules by scintigraphic means. J Ther Ultrasound 2014;2:18. 10.1186/2050-5736-2-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Korkusuz H, Fehre N, Sennert M, et al. Volume reduction of benign thyroid nodules 3 months after a single treatment with high- intensity focused ultrasound (HIFU). J Ther Ultrasound 2015;3:4. 10.1186/s40349-015-0024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sennert M, Happel C, Korkusu Y, et al. Further Investigation on High-intensity Focused Ultrasound (HIFU) Treatment for Thyroid Nodules: Effectiveness Related to Baseline Volumes. Acad Radiol 2018;25:88-94. 10.1016/j.acra.2017.07.011 [DOI] [PubMed] [Google Scholar]