Abstract
Accumulating evidence confirmed that many dysregulated signaling pathways and aberrant genetic alterations contribute to the oncogenesis and heterogeneity of lymphoid malignancies. Therapeutically targeting dysregulating signaling pathways and their hidden oncogenic biomarkers are becoming available, but did not show desired therapeutic effect in current clinical practice. It is meaningful to further understand the underlying mechanisms of the dysregulated signaling pathways and to address the potential utility of pathway-related biomarkers. To precisely identify the dysregulation of signaling pathways and the “driver” oncogenic biomarkers, as well as to develop reliable and reproducible risk-stratification based on biomarkers will be challenging. Nevertheless, pathway-based targeted therapy will raise the hope to improve the outcomes of the patients with lymphoid malignancies, especially with aggressive types, and the efficient utility of pathway-related biomarkers in diagnosis, prognosis, prediction of lymphoid malignancies may also be able to power precision medicine.
Keywords: Lymphoma, Signaling pathway, Biomarker, Therapeutic target
Introduction
Lymphoid malignancies are known for a wide variety of types and molecular and clinical heterogeneity. Increasing evidence supported that many dysregulated oncogenic signaling pathways and aberrant genetic alterations have contributed to the oncogenesis and heterogeneity.1, 2 The most frequently dysregulated signaling pathways involved in lymphoid malignancies include B-cell receptor (BCR) pathway, nuclear factor-kappa B (NF-κB) pathway, phosphoinositide-3-kinase/v-akt murine thymoma viral oncogene homolog/mechanistic target of rapamycin (PI3K/AKT/mTOR) pathway, the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, apoptosis pathway, and programmed death-1/programmed death-ligands (PD-1/PD-Ls) pathway.
In the current era of precision medicine, therapeutically targeting dysregulated signaling pathways and their hidden oncogenic biomarkers are becoming hot topics in the field of cancer research and translational medicine worldwide. Meanwhile, progress has been made in risk-stratification of patients based on the targeted biomarkers, accordingly providing optimal intervention for different risk groups. Many preclinical and clinical trials demonstrated that targeted therapies have clinical activity against a broad spectrum of lymphoid malignancies.3, 4
In this review, to comprehensively understand the detailed mechanisms underlying the development of lymphoid malignancies and the potential of targeted therapy, we summarized several key dysregulated signaling pathways involved in oncogenesis and heterogeneity of lymphoid malignancies. The utility of pathway-related biomarkers for diagnostic, predictive, and therapeutic usage is also included.
BCR signaling pathway
BCR is a transmembrane receptor whose membrane-bound immunoglobulin can bind to extracellular antigen. Correspondingly, immunoglobulin-linked heterodimer of cluster of differentiation (CD)79A/CD79B can deliver the antigen stimulatory signals from outside to inside the cell. Following a series of molecules activation, BCR signaling and its downstream signaling cascades consequently control precise function of normal B cells.5
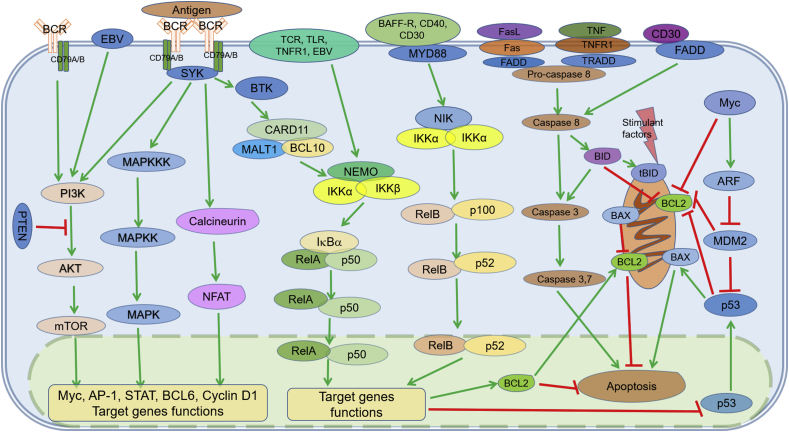
In the three BCR signaling pathways (Fig. 1), the chronic active BCR signaling pathway is classical and antigen-dependent. With the antigen-mediated BCR clustering towards cell membrane, the cytoplasmic tail of BCR, especially immune receptor tyrosine-based activation motifs (ITAM) domain of CD79A and CD79B, becomes phosphorylated by Src family members. Being subsequently recruited and activated by phosphorylated ITAM, spleen associated tyrosine kinase (SYK) thereby activates downstream signals including PI3K/AKT/mTOR signaling, mitogen-activated protein kinase (MAPK) signaling, NF-κB signaling, and nuclear factor of activated T cells (NF-AT) signaling through phosphorylating bruton tyrosine kinase (BTK) and B-cell linker (BLNK). As linker molecules, AKT and BTK are key components to deliver multiple signals.2, 6
Fig. 1.
Illustration of cross-communication network of BCR, PI3K, apoptosis, and NF-κB signaling pathways. BCR, PI3K/AKT/mTOR, apoptosis, and NF-κB signaling pathways are independent but interconnected and may form complex crosstalk network. One pathway may act as upstream or downstream of other pathways and some molecular targets function as key points and players involved in several pathways. These key molecules have been shown promise to be a good therapeutic target for effective treatment in lymphoid malignancies. The green arrows indicate direction of activating signaling steps; the red bars indicate inhibitory signaling steps. AKT: v-akt murine thymoma viral oncogene homolog; ARF: alternative reading frame; AP-1: activator protein-1; BAFF-R: B-cell activating factor receptor; BAX: BCL2-associated X protein; BCL: B-cell leukemia/lymphoma; BCR: B-cell receptor; BID: BH3 interacting death agonist; BTK: Bruton's tyrosine kinase; CARD11: caspase recruitment domain family, member 11; CD: cluster of differentiation; EBV: Epstein–Barr virus; FADD: Fas associated via death domain; FasL: Fas ligand; IKK: IκB kinase; IκB: inhibitor of nuclear factor κB; MALT1: mucosa-associated lymphoid tissue lymphoma translocation protein 1; MAPK: mitogen-activated protein kinase; MAPKK: mitogen-activated protein kinase kinase; MAPKKK: mitogen-activated protein kinase kinase kinase; MDM2: mouse double minute-2 homolog; mTOR: mechanistic target of rapamycin; MYD88: myeloid differentiation primary response 88; NEMO: NF-κB essential modifier; NFAT: nuclear factor of activated T-cells; NF-κB: nuclear factor kappa B; NIK: NF-κB-inducing kinase; PI3K: phosphoinositide-3-kinase; PTEN: phosphatase and tensin homolog; STAT: signal transducer and activator of transcription; SYK: spleen associated tyrosine kinase; tBID: truncated BID; TCR: T-cell receptor; TLR: Toll-like receptor; TNF: tumor necrosis factor; TNFR1: tumor necrosis factor receptor 1; TRADD: TNFRSF1A associated via death domain.
The tonic BCR signaling pathway and autonomous BCR signaling pathway exist, depending on the interaction between BCR and Lyn/SYK or the two neighboring BCRs rather than external antigenic stimulation.6
Normal BCR signaling has been proven to be functional in B-cell proliferation, survival, apoptosis, and differentiation; aberrantly activated BCR pathway is related to oncogenesis of several types of B-cell hematologic malignancies, especially in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), active B-cell-like diffuse large B-cell lymphoma (ABC-DLBCL), and mucosa associated lymphoid tissue (MALT) lymphoma.2, 7 Furthermore, several key components in these pathways are potential therapeutic targets or diagnostic biomarkers.
CD79A/B
CD79A, a transmembrane protein with a cytoplasmic ITAM, forms a CD79A/B heterodimer with CD79B, which is required for the BCR aggregation to induce signal-initiating. In about 20% of ABC-DLBCL cases, CD79A and CD79B mutations can be observed, suggesting their oncogenic roles in dysregulation of BCR signaling pathway in specific ABC subtype.7
BTK
BTK is fundamental to the function of BCR signaling pathway and its downstream signaling. As a member of Tec family, BTK is by far the most studied cytoplasmic tyrosine kinase. It is restrictedly expressed in B cells and plays an important role in the differentiation and activation of B cells.8 It is also related to immune function, transcription regulation, and apoptosis modulation due to its function in Toll-like receptor (TLR) pathway and cytokine receptor signaling pathway.9 In BCR signaling pathway, BTK is responsible for receiving signals from SYK and transducing signals to initiate downstream signaling pathway.10
Given the key function of BTK in BCR pathway and downstream NF-κB pathway, an inhibitor against BTK, ibrutinib, showed encouraging efficacy on patients with untreated and relapsed/refractory CLL, ABC-DLBCL, follicular lymphoma (FL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), and lymphoplasmacytic lymphoma (LPL). The inhibition against BTK induces downstream kinase inactivation and cell apoptosis through binding to BTK at the C481 residue irreversibly.11, 12
NF-κB pathway
NF-κB pathway is one of the most common signaling pathways (Fig. 1). In the classical NF-κB pathway that is downstream of BCR and some other pathways, caspase recruitment domain family, member 11/mucosa-associated lymphoid tissue lymphoma translocation protein 1/B-cell leukemia/lymphoma 10 (CARD11/MALT1/BCL10) complex is specific for IκB kinase (IKK) phosphorylation which directly activates IκBα. Subsequently, following activation by active IκBα, RelA/p50 heterodimers translocate into nucleus, executing many functions such as cell survival/anti-apoptosis, proliferation, inflammation, and innate immunity (Fig. 1).13, 14, 15
In the alternative NF-κB pathway, preferentially, the extracellular initiator is B-cell activating factor receptor (BAFF-R), and the activator of IKK complex that includes two IKKα is NF-κB-inducing kinase (NIK). Additionally, the heterodimer RelB/p50 and RelB/p52 are the major types of dimer. The final effector function of alternative NF-κB pathway mainly involves in lymphoid organogenesis, adaptive immunity, anti-inflammatory properties, and B cell maturation.15, 16
These two NF-κB signaling pathways can be seen constitutively activated in almost all types of lymphoma. In ABC-DLBCL, MALT, Hodgkin's lymphoma (HL), Burkitt lymphoma (BL), and some other lymphomas, constitutively activated NF-κB signaling pathway may mostly or partly result in the oncogenic events, mediated by specific mutation of CARD11, CD79A/B, and myeloid differentiation primary response 88 (MYD88), or chromosomal translocations and Epstein–Barr virus (EBV) infections.7, 17, 18, 19
The integrated mediators of NF-κB signaling cascades include MYD88, p50, p52, c-Rel, CD30, and CD40, etc.
MYD88
MYD88 participates in the activation of both NF-κB and JAK/STAT signaling pathways through TLR/ interleukin-1 (IL-1) mediation.20 MYD88L265P mutations have not only been identified as the hallmark of CLL and LPL/Waldenstrom's macroglobulinemia (WM), but also have been found to be related to poor outcome of a subset of DLBCL patients.19 Therefore, it is reasonable to believe that patients with MYD88L265P mutations and dysregulated NF-κB pathways may benefit from drugs inhibiting interleukin-1 receptor associated kinase 4 (IRAK4) that is downstream of MYD88 and is closely related to the function of MYD88, or interrupting NF-κB pathway, TLR/IL-1 pathway, and other related pathways.20, 21
CD30
CD30, a transmembrane cell-surface member of the tumor necrosis factor receptor superfamily, is one of the activators of the NF-κB pathway, and is highly restrictedly expressed in tumor cells in classical HL (cHL), anaplastic large cell lymphoma (ALCL), and a subset of DLBCL and EBV-driven lymphoproliferative disorders.22 In clinical practice, CD30 was mainly used as a valuable diagnostic biomarker to diagnose cHL and ALCL. It was also showed in a study that soluble CD30 in serum could predict poor outcomes and disease progression in CD30-positive lymphomas. Apart from as a diagnostic and predictive biomarker, CD30 has been utilized as a target to develop inhibitor agents, typically like brentuximab vedotin, to treat lymphomas with CD30 high expression, such as HL and ALCL.22
PI3K-AKT signaling pathway
Following several upstream pathways like BCR pathway, the PI3K-AKT pathway is an important intracellular signaling pathway in regulating cell cycle and is directly related to cellular quiescence, proliferation, cancer, and longevity.23 PI3K receives upstream signals and then actives AKT via phosphatidylinositol-3,4,5-triphosphate (PIP3). As an oncogenic factor, activated AKT can target many molecules, indirectly activating mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) and increasing mRNA translation, protein synthesis, and cellular proliferation as a result.23, 24, 25 During this PI3K-AKT signaling process, phosphatase and tensin homolog (PTEN) acts as a negative regulator (Fig. 1).26
Considering the oncogenicity conferred by PI3K/AKT/mTOR pathway in several types of lymphomas, the main drivers have been explored in numerous studies. The oncogenic activation may be driven by PIK3CD mutation and copy number increase of PIK3CA in DLBCL and MCL, respectively. In germinal center B-cell-like (GCB)-DLBCL, loss of function of PTEN may be the main driven cause.27, 28, 29
PI3Ks
PI3K family includes three classes of kinase on the basis of their different structures and functions. Isoforms p110α, p110β, p110δ, and p110γ belong to Class I and are more frequently related to cancer. Among them, p110δ and p110γ are related closely to B-cell, T-cell, and natural killer (NK) cell development, proliferation, cytokine secretion, and other cellular functions.30, 31
Therapeutically, as a representative inhibitor of p110δ isoform, idelalisib has exhibited clinical activity in relapsed CLL, MCL, FL, and DLBCL. In addition to its direct inhibitory effect on p110δ, the efficacy of idelalisib may also be due to the indirect regulation of cytokines and chemokines secretion in the microenvironment.31, 32, 33 Other inhibitors, such as buparlisib against pan class-I PI3K and duvelisib against p110δ/p110γ isoform, also have showed efficacy in both T- and B-cell lymphomas.34, 35
AKT and PTEN
AKT and PTEN act as positive and negative mediator of downstream PI3K pathway, respectively, regulating biological processes like cell survival, growth, proliferation, and angiogenesis.36 Owing to some alterations at genetic and epigenetic level, as well as protein–protein interaction, loss-of-function of tumor suppressor PTEN protein may cause the increase of AKT and mTOR activity, which promotes tumor growth and other pathological changes.37, 38 GCB-DLBCL and MCL are the major lymphoma types involved in functional deficiency of PTEN.39, 40
Since the inactivation of PTEN is closely associated with the abnormal activation of PI3K/AKT/mTOR pathway, therapeutically targeting PI3K/AKT/mTOR pathway to treat malignancies with PTEN deficiency may be rational.37, 38, 41 For instance, MK-2206 as a second-generation AKT inhibitor has been used in patients with relapsed/refractory lymphoma in clinical trials.42 Pan-PI3K inhibitor like buparlisib, p110δ-specific PI3K inhibitor like idelalisib, mTOR inhibitor like everolimus, are all in clinical evaluation.33, 43, 44 Additionally, the immunohistochemical expression of phosphorylated-AKT protein can be used to assess outcome or treatment response in some lymphoid malignancies.39
mTOR
Serine/threonine-protein kinase mTOR is a key protein kinase in the PI3K/AKT/mTOR signaling pathway which is closely related to cellular metabolism, survival, and growth through targeting several types of proteins. mTOR is the structural unit for the function of mTORC1 and mTORC2. After receiving phosphorylation signals from upstream AKT, activated mTORC1 and mTORC2 execute their corresponding function, positively regulating mRNA translation, cell survival, cytoskeleton organization, and negatively regulating autophagy, respectively.23, 24
Several mTORC1 inhibitors have shown preclinical or clinical efficacy in DLBCL, HL, MCL, FL, CLL/SLL, and even T-cell lymphomas. Temsirolimus and everolimus are the typical mTORC1 inhibitors. They are also expected to have good efficacy with minimized toxicity, either alone or in combination with rituximab.43, 45, 46
Apoptosis signaling pathway
Apoptosis is a genetically programmed process of cell death and is essential for organism development and environmental homeostasis, which involves many complex molecular mechanisms. There are at least two distinct but interconnected pathways in the process of apoptosis: extrinsic and intrinsic apoptosis pathways (Fig. 1).
Extrinsic apoptotic pathway is initiated when ligands bind to a specific subset of death receptors, such as Fas and tumor necrosis factor receptor 1 (TNFR1). Fas firstly binds to Fas associated via death domain (FADD) and subsequently unites with caspase 8 to form a death inducing signaling complex, promoting apoptosis downstream caspase 3 and caspase 7 directly, or activating caspase cascades via mitochondrial-dependent pathway.47, 48 Compared with Fas-mediated way, apoptosis mediated by TNFR1 more likely integrates complex TNFRSF1A associated via death domain (TRADD) (death domain-containing proteins like TRADD) and TNF receptor associated factor 2 (TRAF2), inducing cell death or activating NF-κB function through IKK recruitment.49, 50
In the intrinsic apoptosis pathway, apoptosis is triggered immediately more frequently owing to oncogenic stress, DNA damage and hypoxia. The signals in this pathway are transduced and controlled through interacting with p53, DNA checkpoint proteins, and BCL2 family members located in their upstream or downstream pathways, thereby finally activating caspase 3 and caspase 7 to produce effects like extrinsic pathway.47, 51
While normal apoptosis pathway is disturbed by multiple cellular and extracellular factors, abnormal proliferation finally results in the oncogenesis of hematologic malignancies. Meaningfully, it is worth exploring the diagnostic, predictive, and therapeutic values of BCL2, Myc, p53 and other molecules involved in apoptosis pathways.
BCL2
BCL2 is one of the members of BCL2 family that programmatically control cell death through anti-apoptotic and pro-apoptotic function. BCL2 suppresses apoptotic death in combination with caspases and other BCL2 family members.52, 53
Owing to its translocation with juxtaposed immunoglobulin heavy locus (IgH), t(14; 18)/IgH/BCL2 is a molecular hallmark of FL and can be also detected in some GCB-DLBCL.54, 55 By contrast, in ABC-DLBCL subtype, BCL2 is more often amplified. Apart from its diagnostic value in lymphoma, both translocation and overexpression of BCL2 can be used to predict aggressive clinical features and adverse outcome in some types of lymphoma.40, 56
As for the therapeutic implication, BCL2 appears suitable to develop its inhibitors based on the special structure of BCL2 homology domain 3 (BH3) that can be mimicked through binding to BCL2 or BCL-XL.57 Obatoclax and venetoclax (ABT199) are BCL2 inhibitors which can be used for the treatment of relapsed/refractory HL and previously untreated FL while in combination with rituximab through restoring normal apoptosis.58, 59, 60
p53
p53, a well-known nuclear transcription factor and tumor suppressor protein, targets a broad spectrum of functional genes regulating physiological cellular functions and mediates cell apoptosis and growth arrest with the interaction with BCL2 family members and other molecules.61, 62 As a result, p53 inactivation or the dysregulation of p53-involved signaling pathway has been implicated to be oncogenic event for cancers, including lymphomas.61, 63
Theoretically speaking, it seems rational to restore tumor suppressor function of p53, to interrupt mouse double minute-2 homolog (MDM2)-p53 interaction, and to reactivate p53-mediated apoptosis pathways for the development of therapeutics against p53-related malignancies. For example, as a MDM2 inhibitor, APG-115 is such an agent which showed antitumor activities in multiple human cancer xenograft models. It may be able to show activity and safety in human cancer.61, 64, 65, 66
Myc
Myc is well known as a representative pleiotropic transcription factor and an oncogene protein, targeting numerous genes and regulating multiple processes of cell biology and oncology.67, 68 Myc has also been associated with oncogenesis, malignant transformation, and aggressive clinical features in many aggressive cancers including aggressive lymphomas. In BL and a subset of DLBCL, chromosomal translocation of MYC like t(8; 14)(q24; q32) is considered as typical oncogenic event and aggressive feature. MYC translocation accompanied by BCL2 or BCL6 is termed as double-hit or triple-hit lymphoma with relatively unfavorable prognosis. A similar situation also occurs in the double-expression lymphoma with Myc and BCL2 co-overexpression. Therefore, both MYC translocation and Myc overexpression have reference value for diagnosis and prognosis of lymphoma in clinical practice.67, 69
As for the development of Myc targeting therapeutics, JQ1, a type of small molecule inhibitor against bromodomain-containing protein 4 (BRD4), showed encouraging efficacy in patients with hematologic or some other Myc-driven malignancies through downregulating Myc function and expression.70, 71 Another Myc inhibitor against bromodomain and extra-terminal domain (BET)/BRD4 is AZD5153, which showed activity for hematologic malignancies by transcriptionally affecting Myc, E2 promoter binding factor (E2F), and mTOR.72 Other strategies for developing Myc-targeted therapeutics may be established in reducing Myc/Myc associated factor X (MAX) hetero-dimerization and DNA binding, affecting MYC-associated chromatin modification, targeting cell cycle kinases, and interfering with its downstream target genes.73
PD-1/PD-Ls signaling pathway
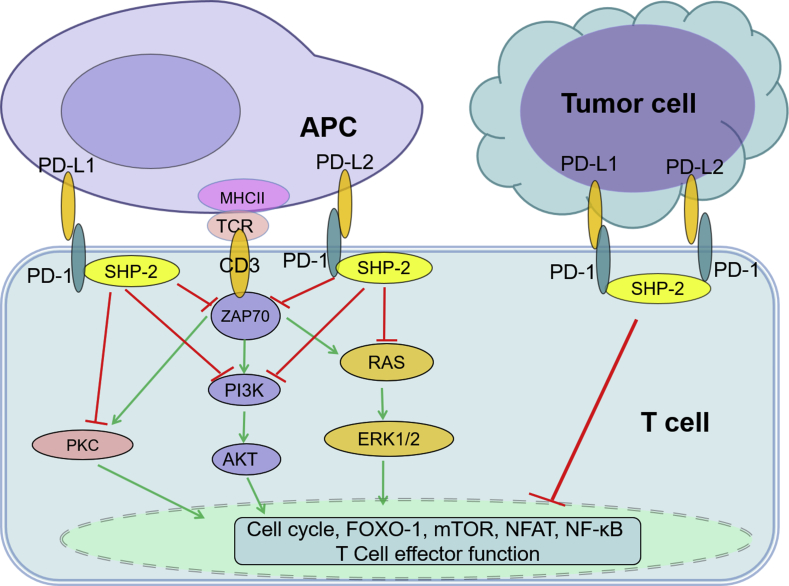
As a pair of co-inhibitory molecules, normal PD-1 and PD-Ls are employed by immune system to balance immune function. Normally, PD-1 negatively regulates effector T-cell functions with the inhibition of T-cell receptor (TCR)/CD3/zeta chain of T cell receptor associated protein kinase 70 (ZAP70) signaling mediated by Src homology region 2 domain-containing phosphatase-1/2 (SHP-1/2) (Fig. 2).74, 75 However, under the triggering of genetic alteration, virus infection, or other conditions in the pathological process, overexpressed PD-Ls in tumor cells and infiltrating cells lead to exhaustion of effector T cell and final immune escape. Such an immune escape signaling pathway contributes to oncogenesis, tumor aggression and metastasis in many types of malignancies.74, 76
Fig. 2.
Overview of PD-1/PD-Ls signaling pathway. Upon binding to its ligands PD-Ls, PD-1 delivers co-inhibitory signals, negatively regulating T-cell function to maintain immune balance. In the cases of tumor, overexpressed PD-Ls activate PD-1 signaling, leading to exhaustion of effector T cell after interaction of PD-1 and PD-Ls. AKT: v-akt murine thymoma viral oncogene homolog; APC: antigen presenting cell; CD: cluster of differentiation; ERK: extracellular signal-regulated kinase; FOXO-1: forkhead box 1; MHCII: major histocompatibility complex, class II; mTOR: mechanistic target of rapamycin; NFAT: nuclear factor of activated T cells; NF-κB: nuclear factor-kappa B; PD-1: programmed death-1; PD-L: programmed death-ligand; PI3K: phosphoinositide-3-kinase; PKC: protein kinase C; SHP-2: Src homology region 2 domain-containing phosphatase-2; TCR: T-cell receptor; ZAP70: zeta chain of T cell receptor associated protein kinase 70.
Many other immune checkpoint pathways and molecules involved in lymphoma can also function like PD-1/PD-Ls signaling. Such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-40)/CD86/CD80, B and T lymphocyte attenuator (BTLA)/herpes virus entry mediator (HVEM), lymphocyte-activation gene 3 (LAG-3)/major histocompatibility complex class II (MHCII), and T cell immunoglobulin mucin-3 (TIM-3)/galectin-9, which are paired respectively to form co-stimulatory signals and co-inhibitory signals, participate in these pathways.4 Encouragingly, targeting and blocking PD-1/PD-Ls pathway by monoclonal antibodies has been approved to apply in clinical immunotherapy management for cHL, DLBCL, and FL.4, 74
PD-1 and PD-Ls
PD-1, as an inhibitory cell surface receptor, is encoded by PDCD1 at locus 2q37.3 and mainly expressed on the surface of activated T-cells. PD-L1 and PD-L2, encoded by CD274 and PDCD1LG2 are generally expressed on antigen presenting cells, activated dendritic cells and some macrophages, respectively. Under some pathological conditions, like CD274 amplification or translocation at 9p24.1, as well as oncogenic activation of JAK/STAT or AP-1 pathways or viral infection, PD-L1 has been seen to be overexpressed in lymphoma cells and stromal cells, particularly in cHL, primary mediastinal B-cell lymphoma (PMBL), and a small part of ABC-DLBCL and anaplastic lymphoma kinase (ALK)-positive ALCL.74, 75, 77, 78 In FL and CLL/SLL, PD-1 overexpressed in infiltrating T-cells in follicles of lymph node or tumor cells of CLL cases.79, 80 Interestingly, both PD-L1 and PD-1 overexpression was observed in virus-associated lymphomas.78, 81, 82
Importantly, accumulating studies have proved that prediction for patient outcome and treatment response is feasible according to the aberrant expression status of PD-1 and PD-L1. Therefore, detection of PD-1 and PD-L1 immunohistochemically will be useful to guide the selection of lymphoma patients.79, 83, 84 Additionally, PD-1 combined with other follicular T-helper cell markers like chemokine (C-X-C motif) ligand 13 (CXCL-13) are the robust diagnostic biomarkers for angioimmunoblastic T-cell lymphoma (AITL) diagnosis.85
More excitingly, monoclonal antibodies targeting PD-1, pidilizumab, nivolumab and pembrolizumab, have been approved to be used in hematologic malignancies, including cHL, PMBL, FL, CLL/SLL, particularly in relapsed/refractory patients or in combination with autologous hematopoietic stem cell transplantation.86, 87, 88, 89 Some inhibitors against PD-L1, durvalumab, atezolizumab, and avelumab, have also been approved by FDA for the treatment of some solid tumors. Many other agents against PD-1 and PD-L1 are also under investigation or clinical evaluation in patients with solid tumor and hematologic malignancies.90, 91
JAK/STAT signaling pathway
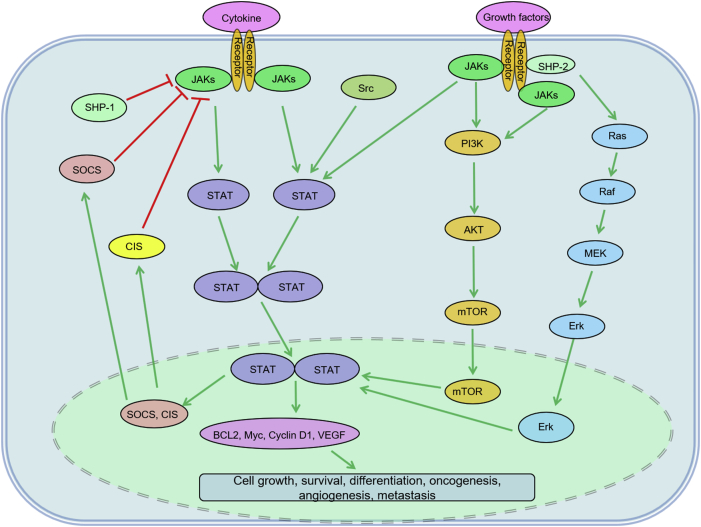
JAK/STAT signaling pathway essentially mediates cytokine signaling and growth factor signaling, involving a network of molecules with different functions. Upon cytokine receptors-cytokine binding, JAKs and STATs become active sequentially. After that, phosphorylated STATs are dimerized and thereby transfer to nucleus from cytoplasm, causing transcriptional activation of the target genes and eventually regulating apoptosis, proliferation, angiogenesis, and metastasis, etc. In turn, this pathway is negatively regulated by suppressor of cytokine signaling (SOCS) and cytokine-induced STAT inhibitor (CIS) (Fig. 3).92, 93, 94
Fig. 3.
Illustration of JAK/STAT signaling pathway. AKT: v-akt murine thymoma viral oncogene homolog; BCL: B-cell leukemia/lymphoma; CIS: cytokine-induced STAT inhibitor; Erk: extracellular signal-regulated kinase; JAK: Janus kinase; mTOR: mechanistic target of rapamycin; MEK: methyl ethyl ketone; PI3K: phosphoinositide-3-kinase; SHP: Src homology region 2 domain-containing phosphatase; SOCS: suppressor of cytokine signaling; STAT: signal transducer and activator of transcription; VEGF: vascular endothelial growth factor.
The dysregulation of JAK/STAT pathway can be seen in lymphomas such as PMBL, cHL, and ABC-DLBCL. The oncogenic events may result from loss-of-function of positive regulator JAK2, STAT3, STAT5, and Jumonji domain-containing protein 2C (JMJD2C), as well as negative regulator SOCS1 and protein tyrosine phosphatase non-receptor type 2 (PTPN2).92, 93, 94, 95, 96
JAKs
Four JAKs (JAK1, JAK2, JAK3, and tyrosine kinase 2) generally involve in hematopoiesis, host defense, and immune response. Several abnormalities of JAKs have been identified as specific signatures in lymphomas. Aberrant amplification of JAK2 and JMJD2C with loss of function of SOCS1 and PTPN2 have been shown in cHL and PMBL; JAK3 mutation in NK T-cell lymphoma and adult T-cell leukemia/lymphoma, JAK2/SEC31A fusion involving t(4; 9)(q21; p24) in cHL, and JAK2/PCM1 fusion deriving from t(8; 9)(p22; p24) in T-cell lymphoma, all have been identified.97, 98, 99, 100
Inhibitor against JAK2/Fms-like tyrosine kinase 3 (FLT3) fusion, pacritinib (SB1518), has been developed for treatment of relapsed/refractory cHL, FL, and DLBCL, demonstrating clinical safety and efficacy.101 Meanwhile, tofacitinib, JAK3 inhibitor, has therapeutic efficacy for EBV-associated NK- and T-cell lymphoma, probably due to its ability to decrease STAT5 phosphorylation, to inhibit proliferation, and to reduce EBV latency.102
STATs
STATs family consist of seven members: STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6. STAT3 expression increased significantly in ABC-DLBCL and ALCL patients; in the latter, nucleophosmin 1 (NPM-1)/ALK fusion was proposed to be related to increased STAT3.93, 103, 104 Additionally, STAT3 and STAT5 activation can be observed in cutaneous T-cell lymphoma, and STAT6 mutation occurs in PMBL and FL.105, 106
Therapeutically, a STAT3 inhibitor, pyrimethamine, for the treatment of relapsed CLL/SLL, is being estimated in phase I/II clinical trial (Clinical Trials Registration: NCT01066663).
Remarks and conclusion
As noted above, it has been well recognized that complicate network of dysregulated signaling pathways and their hidden oncogenic alterations contribute a lot to lymphoma oncogenesis and heterogeneity. In the era of precision medicine, it is imperative to precisely identify the dysregulation of these signaling pathways and the “driver” oncogenic biomarkers; it is meaningful to further understand the underlying mechanisms of the dysregulated signaling pathways and to address the potential utility of pathway-related biomarkers. Table 1 summarized the pathway-related biomarkers and their clinical utilities in lymphoid malignancies.
Table 1.
Pathway-related biomarkers and their clinical utilities in lymphoid malignancies.
| Oncogenic biomarkers | Related signaling pathways | Functions | Clinical utilities | Targeted agents | Diseases applied | Detection approaches |
|---|---|---|---|---|---|---|
| BTK | BCR, NF-κB signaling pathway | Modulates B-cell development, immune function, transcription, and apoptosis | Therapeutic | BTK inhibitor: Ibrutinib (PCI-32765), BGB-3111, AVL-292 cc-292, M7583, Acalabrutinib (ACP-196) | CLL/SLL, R/R MCL, ABC-DLBCL, FL, MZL, T-cell lymphoma | Sequencing, PCR, IHC |
| MYD88 | NF-κB, IL-1, TLR signaling pathway | Acts as a signal transducer in the IL-1, IL-18, and TLR signaling pathways; involves in innate and adaptive immunity | Diagnostic: MYD88L256P mutation for LPL/WM, HCL; prognostic: ABC-DLBCL | TLRs ligands inhibitor: IMO-8400; IRAK4 inhibitor; ND-2158, ND-2110 | ABC-DLBCL with MYD88L256P mutation, other malignancies harboring aberrant MYD88, LPL/WM | Sequencing, PCR, IHC |
| CD30 | NF-κB pathway | Diagnostic and differential diagnostic: CD30 overexpression in cHL, ALCL; Therapeutic | Anti-CD30 antibody: Brentuximab vedotin | cHL and ALCL | IHC | |
| PI3Ks | PI3K/AKT/mTOR, BCR, TLR/MYD88 pathway | PI3Kδ involves in cell growth, proliferation, survival, and morphology; PI3Kγ involves in B-cell, T-cell, and NK cell development, proliferation, migration, and cytokine production | Therapeutic | PI3K inhibitor: SAR 245408 (XL147); PI3Kδ/γ inhibitor: Duvelisib (IPI-145, INK1197); PI3Kδ inhibitor: Idelalisib (CAL-101, GS-1101), AMG 319, Acalisib (GS-9820) (CAL-120); PI3Kγ inhibitor: Buparlisib (BKM120) | R/R NHL, advanced hematologic malignancies | Sequencing, PCR, IHC |
| AKT | PI3K/AKT/mTOR, BCR, TLR/MYD88 pathway | Regulates cell survival, growth, proliferation, and angiogenesis | Prognostic and predictive: abnormal phosphorylated-AKT expression; Therapeutic | Akt inhibitor: Perifosine (KRX-0401), MK-2206, GSK690693 | Hematologic malignancies, Lymphomas | IHC, Sequencing, PCR |
| mTOR | PI3K/AKT/mTOR, BCR, TLR/MYD88 pathway | Regulates cellular metabolism, survival, growth; mTORC1 regulates mRNA translation, protein synthesis, and autophagy; mTORC2 regulates cell survival and cytoskeleton organization | Therapeutic | mTOR inhibitor: Temsirolimus (CCI-779), Rapamycin (Sirolimus), Ridaforolimus (AP23573, MK-8669); mTORC1/mTORC2 inhibitor: Everolimus (RAD001), AZD2014 | Lymphoma, multiple myeloma, hematologic malignancies | Sequencing, PCR, IHC |
| BCL2 | Apoptosis pathway | Suppresses apoptotic death | Diagnostic: Overexpression and translocation in FL, double-expression or double-hit lymphoma; amplification in ABC-DLBCL; Therapeutic | BCL2 inhibitor: Venetoclax (ABT199), Obatoclax Mesylate (GX15-070MS) | FL, DLBCL, R/R lymphoid malignancies | IHC, ISH, PCR, sequencing |
| p53 | Apoptosis pathway | Regulates transcription; regulates cell cycle; induces growth arrest or apoptosis | Diagnostic: overexpression or TP53 mutation; Therapeutic | MDM2 inhibitor: APG-115, DS-3032 | Advanced solid tumors or lymphomas | IHC, PCR, sequencing |
| Myc | Apoptosis pathway | Involves in cell cycle progression, apoptosis and cellular transformation | Diagnostic and prognostic: Overexpression and translocation in BL, double-expression or double-hit lymphoma; Therapeutic | BET inhibitor: JQ1, CPI-0610, AZD5153, AZD5153; Aurora A inhibitor: Alisertib; c-Myc-Max dimerization inhibitor: 10058-F4 | BL, DLBCL, hematologic malignancies | IHC, ISH |
| PD-1 | Immune system pathway, TCR pathway | Negatively regulates effector T-cell functions | Predictive: overexpression; diagnostic: expression pattern in AITL; Therapeutic |
Anti-PD-1 antibody: Pidilizumab (CT-011), Nivolumab (BMS-936558, MDX-1106, ONO-4538), Pembrolizumab (lambrolizumab, MK-3475) | Aggressive B-cell lymphomas; advanced malignancies; T-cell or NK-cell lymphomas | IHC, ISH |
| PD-L1 | Immune system pathway, TCR pathway | Inhibits T-cell activation and cytokine production upon interaction with PD-1 | Predictive: overexpression; Therapeutic | Anti-PD-L1 antibody: Durvalumab (MEDI4736), Avelumab (MSB0010718C), Atezolizumab (MPDL3280A) (RG7446) | Lymphomas, solid tumors; Advanced cHL; R/R PTCL | IHC, ISH |
| JAKs | JAK/STAT pathway | Involves in cell growth, development, and differentiation; mediates adaptive and innate immunity | Diagnostic: JAK2/SEC31A fusion in cHL; JAK2/PCM1 fusion in T-cell lymphoma; JAK2/FLT3 fusion in cHL, FL, and DLBCL; Aberrant JAK2 and JMJD2C amplification in cHL and PMBL; Therapeutic | JAK3 inhibitor: Tofacitinib (CP-690550); JAK2 inhibitor: Pacritinib (SB1518), Ruxolitinib (INCB018424) | cHL, EBV-related NK/T cell lymphoma; R/R cHL, FL, MCL, and DLBCL; R/R T-cell or NK cell lymphoma, R/R PMBL | ISH, sequencing, PCR, IHC |
| STATs | JAK/STAT pathway | STAT3 involves in cell growth and apoptosis; STAT6 activates transcription, involves in IL-4 signaling, induces anti-apoptotic activity | Therapeutic | STAT3 inhibitor: Pyrimethamine, IONIS-STAT3Rx (ISIS 481464) | Relapsed CLL/SLL; DLBCL, lymphomas, advanced cancers | Sequencing, PCR, IHC |
ABC: active B-cell; ALCL: anaplastic large cell lymphoma; AITL: angioimmunoblastic T-cell lymphoma; AKT: v-akt murine thymoma viral oncogene homolog; BCL2: B-cell leukemia/lymphoma 2; BCR: B-cell receptor; BET: bromodomain and extra-terminal proteins; BL: Burkitt lymphoma; BTK: bruton tyrosine kinase; CD: cluster of differentiation; cHL: classical Hodgkin lymphoma; CLL: chronic lymphocytic leukemia; DLBCL: diffuse large B-cell lymphoma; EBV: Epstein–Barr virus; FL: follicular lymphoma; HCL: Hairy lymphoma; HL: Hodgkin's lymphoma; IHC: immunohistochemistry; IL: interleukin; IRAK4: interleukin-1 receptor associated kinase 4; ISH: in situ hybridization; JAK: Janus kinase; LPL: lymphoplasmacytic lymphoma; MALT: mucosa associated lymphoid tissue lymphoma; MCL: mantle cell lymphoma; MDM2: mouse double minute 2 protein; MM: multiple myeloma; mTOR: mechanistic target of rapamycin; mTORC: mTOR complex; MYD88: myeloid differentiation primary response 88; MZL: marginal zone lymphoma; NF-κB: nuclear factor-kappa B; NHL: non-Hodgkin's lymphoma; NK: natural killer; PCNSL: primary central nervous system lymphoma; PCR: polymerase chain reaction; PD-1: programmed death-1; PD-Ls: programmed death-ligands; PI3K: phosphoinositide-3-kinase; PMBL: primary mediastinal B-cell lymphoma; PTCL: peripheral T-cell lymphoma; R/R: refractory / relapsed; SLL: small lymphocytic lymphoma; STAT: signal transducer and activator of transcription; TCR: T-cell receptor; TLR: Toll-like receptor; WM: Waldenstrom's macroglobulinemia.
With the progress of biotechnologies and the application of advanced detection platform, identifying pathway-based biomarkers becomes available, providing good opportunities to utilize these biomarkers reasonably and feasibly. The two most promising utilizations lie in precise diagnosis and treatment. Instead of relying solely on morphologic and histologic features and clinical experience, molecular classification based on specific biomarker has almost become a requirement for diagnosis, risk-stratification, and treatment-guidance in some lymphoid malignancies.
These pathway-based biomarkers may include gene rearrangement, gene amplification, specific tumor-driving mutations, epigenetic alterations, unusual expressions, as well as other abnormal genotypes and phenotypes. Therefore, faced with so many complicate alterations, there are big challenges to utilize these biomarkers reasonably and efficiently.
In biomarkers screening and detecting, reliable and reproducible biomarkers accurately conveying activated oncogenic pathways in patients will be needed. For example, PD-1 and PD-L1 are not only the therapeutically targeted biomarkers but also predictive biomarkers to guide patients who may benefit from immunotherapy. Moreover, PD-1 is useful for AITL diagnosis. In practice, the robustness of PD-1 and PD-L1 detection may depend on the specificity and sensitivity of the antibody, the platform or method used, the status of intra-tumor heterogeneity, and other factors.
Additionally, in front of enormous amount of information triggered by complex techniques, especially by next generation sequencing (NGS), we are confronted with many problems in the discovery and application of new biomarkers. For instance, despite NGS is becoming more and more popular and the cost is getting lower and lower, in clinical practice, a panel sequencing focusing on a limited number of genes is still a better option than whole genome sequencing. Nevertheless, it will be a trend to apply whole genome sequencing and transcriptome sequencing in routine clinical practice in the future with the advances of technology and increased availability. Another focus is that in addition to single specific diagnostic or prognostic biomarker, rational algorithms or some grouped gene signatures are of great value for routine diagnosis, classification, prognosis, prediction, or monitoring in lymphomas. In fact, a lot has been put into practice.
As for precision treatment, many arguments have emerged. Are patients with malignancies matched with drugs for mutations or “pathway” mutations? Why did the current targeted drugs not achieve desired therapeutic effect in clinical practice? How do clinicians choose the best treatment regimens? Single targeted agent alone? Or in combination with multiple pathway inhibitors? We do need to notice and address these issues. It will be difficult to assess the effect of combination regimens using predictive biomarkers from individual patient. Probably a long-time and large population validation is required for the effect of predictive biomarkers and targeted therapies. In addition, the side effects of these targeted therapies in lymphomas are also of great concern. Here, we summarized the side effects of agents targeting pathway-related biomarkers in Table 2.
Table 2.
Therapeutics targeting pathway-related biomarkers and their side effects in lymphomas.
| Biomarker | Therapeutics | Agent | Involved lymphomas | Phase | Common side effects |
|---|---|---|---|---|---|
| BTK | BTK inhibitor | Ibrutinib (PCI-32765) | Recurrent B-cell lymphoma, CLL/SLL, R/R MCL, ABC-DLBCL, FL, MZL, T-cell lymphoma | Phase1/2 | Mild diarrhea, nausea, fatigue, upper respiratory tract infections, rash, dyspnea |
| BTK inhibitor | BGB-3111 | R/R B-cell malignancies | Phase1 | Minimal side effects | |
| BTK inhibitor | Acalabrutinib (ACP-196) | R/R ABC-DLBCL; CLL/SLL; MCL | Phase1/2 | Comparatively less toxicity, including rash, major bleeding and atrial fibrillation | |
| PI3Ks | PI3Kδ/γ inhibitor | Duvelisib (IPI-145, INK1197) | R/R NHL, advanced hematologic malignancies | Phase1/2 | Tansient cytopenias, febrile neutropenia and pneumonia |
| PI3Kδ inhibitor | Idelalisib | R/R MCL, FL, SLL, LPL, MZL | Phase 1/2 | Fatigue, diarrhea, nausea, rash, chills, and pyrexia | |
| PI3Kγ inhibitor | Buparlisib (BKM120) | PCNSL, SCNSL, CLL/SLL | Phase 2 | Neuropsychiatric symptoms such as mood alteration, suicidal ideation, and altered mental status associated with its use | |
| PI3Kα/δ inhibitor | Copanlisib (Bay 80-6946) | NHL, aggressive B-cell lymphomas | Phase 3 | Hypertension, neutropenia, hyperglycemia, diarrhea, and fatigue | |
| AKT | Akt inhibitor | Perifosine (KRX-0401) | Hematologic malignancies, Lymphomas | Phase 1/2 | Nausea, vomiting, diarrhea, and fatigue |
| Akt1/2/3 inhibitor | MK-2206 | Relapsed lymphoma, R/R DLBCL | Phase 2 | Dehydration, hyperglycemia, rash and neutropenia | |
| mTOR | mTOR inhibitor | Temsirolimus (CCI-779) | R/R HL; R/R PCNSL; FL, CLL/SLL, R/R MCL | Phase 1/2/4 | Thrombocytopenia |
| mTORC1/mTORC2 inhibitor | Everolimus (RAD001) | R/R NHL, R/R MCL, R/R cutaneous T-cell lymphoma | Phase 2 | Neutropenia, anemia, and thrombocytopenia in DLBCL | |
| BCL2 | BCL-2, BCL-XL, BCL-W inhibitor | Navitoclax (ABT263) | R/R lymphoid malignancy | Phase 2 | Diarrhea, nausea, vomiting, fatigue and dose-dependent thrombocytopenia |
| BCL2 inhibitor | Venetoclax (ABT199) | NHL, CLL/SLL, MM, R/R NHL | Phase 1/2/3 | Diarrhea, neutropenia, fatigue, upper respiratory tract infection, and cough | |
| BCL2 inhibitor | Obatoclax Mesylate (GX15-070MS) | R/R HL | Phase 2 | Neurologic toxicity | |
| P53 | P53-MDM2 blockade | ALRN-6924 | Advanced solid tumors or lymphomas | Phase 1/2 | GI side effects, fatigue, anemia, and headache |
| Myc | Aurora A inhibitor | Alisertib | Myc-positive aggressive B-cell lymphomas | Phase 1 | Myelosuppression alopecia, mucositis and fatigue |
| BET inhibitor | JQ1 | Hematologic malignancies | Preclinical | Pre-clinical development, no serious side effect was reported | |
| Syk | Syk inhibitor | Fostamatinib (R788) | CLL/SLL, DLBCL, MCL, FL, T-cell lymphoma | Phase1/2 | Fatigue, diarrhea, cytopenias, and hypertension |
| PD-1 | Anti-PD-1 antibody | Pidilizumab (CT-011) | DLBCL and PMBL after ASCT; Stage III-IV DLBCL | Phase 2 | Mild fatigue, rash, pruritus, diarrhea, and colitis |
| Anti-PD-1 antibody | Nivolumab (BMS-936558, MDX-1106, ONO-4538) | R/R DLBCL, PCNSL, PTL, FL, PTCL | Phase 2 | Mild fatigue, rash, pruritus, diarrhea, and colitis | |
| Anti-PD-1 antibody | Pembrolizumab (lambrolizumab, MK-3475) | R/R FL; HL, DLBCL and T-NHL after ASCT; T-cell or NK-cell lymphomas; R/R HL; recurrent PCNSL; R/R PMBL | Phase 2 | Mild fatigue, rash, pruritus, diarrhea, and colitis | |
| PD-Ls | Anti-PD-L1 antibody | Durvalumab (MEDI4736) | R/R lymphoma, solid tumor | Phase 1 | Fatigue, muscle and bone pain, constipation, decreased appetite, nausea, swelling, and urinary tract infections |
| Anti-PD-L1 antibody | Avelumab (MSB0010718C) | Advanced cHL; R/R PTCL | Phase1/2 | Immune-mediated adverse reactions (pneumonitis, hepatitis, colitis, adrenal insufficiency, hypo- and hyperthyroidism, diabetes mellitus, and nephritis) and life-threatening infusion reactions; fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, and peripheral edema | |
| JAKs | JAK3 inhibitor | Tofacitinib (CP-690550) | EBV-related NK/T cell lymphoma | Preclinical | pre-clinical development |
| JAK2 inhibitor | Pacritinib (SB1518) | R/R cHL, FL, MCL, and DLBCL | Phase 1/2 | Diarrhea, nausea, vomiting, and abdominal pain | |
| JAK2 inhibitor | Ruxolitinib (INCB018424) | R/R T-cell or NK cell lymphoma, R/R HL and PMBL | Phase 2 | Thrombocytopenia and anemia |
ABC: active B-cell; AKT: v-akt murine thymoma viral oncogene homolog1; ASCT: autologous stem cell transplantation; BET: bromodomain and extra-terminal proteins; BCL: B-cell leukemia/lymphoma; BL: Burkitt lymphoma; BTK: bruton tyrosine kinase; cHL: classical Hodgkin lymphoma; CLL: chronic lymphocytic leukemia; DLBCL: diffuse large B-cell lymphoma; EBV: Epstein–Barr virus; FL: follicular lymphoma; GI: gastrointestinal; HL: Hodgkin's lymphoma; JAK: Janus kinase; LPL: lymphoplasmacytic lymphoma; MCL: mantle cell lymphoma; MDM2: mouse double minute-2 homolog; MM: multiple myeloma; mTOR: mechanistic target of rapamycin; mTORC: mTOR complex; MYC: v-Myc avian myelocytomatosis viral oncogene homolog; MYD88: myeloid differentiation primary response 88; MZL: marginal zone lymphoma; NK: natural killer; NHL: non-Hodgkin's lymphoma; PCNSL: primary central nervous system lymphoma; PD-1: programmed death-1; PD-Ls: programmed death-ligands; PI3K: phosphoinositide-3-kinase; PMBL: primary mediastinal B-cell lymphoma; PTCL: peripheral T-cell lymphoma; PTL: primary testicular lymphoma; R/R: refractory / relapsed; SCNSL: secondary central nervous system lymphoma; SLL: small lymphocytic lymphoma; SYK: spleen associated tyrosine kinase; T-NHL: T-cell NHL.
In conclusion, despite the many problems encountered, pathway-based targeted therapy in combination with traditional chemotherapy, single specific targeted antibody, and immunotherapy will raise the hope for the patients with lymphoid malignancies. The rational and efficient utilization of pathway-related biomarkers in diagnosis, prognosis, prediction, and treatment selection in lymphoid malignancies will power precision medicine.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgements
Rui-Fang Sun is the recipient of professorship scholarship award and supported by Shanxi Scholarship Council of China (2017-128) and Health and Family Planning Commission of Shanxi Province (201601058). This work was supported by National Cancer Institute/National Institutes of Health grants R01CA138688, R01CA187415 and 1RC1CA146299 to Ken H. Young. Ken H. Young is supported by The University of Texas MD Anderson Cancer Center Institutional Research and Development Fund, Gundersen Lutheran Medical Foundation Award, Hagemeister Lymphoma Foundation Award, and the University Cancer Foundation via the Sister Institution Network Fund at The University of Texas MD Anderson Cancer Center. Ken H. Young also receives research support from Roche Molecular System, Gilead Sciences, Seattle Genetics, Dai Sanyo, Adaptive Biotechnology, Incyte Pharmaceutical, HTG Molecular Diagnostics and Perfectgen Diagnostics. This work was also partially funded by National Cancer Institute and National Institutes of Health grants P50CA136411 and P50CA142509, and by MD Anderson's Cancer Center Support Grant CA016672.
Edited by Pei-Fang Wei
Footnotes
Peer review under responsibility of Chinese Medical Association.
Contributor Information
Rui-Fang Sun, Email: srf491@163.com.
Ken H. Young, Email: khyoung@mdanderson.org.
References
- 1.Swerdlow S.H., Campo E., Pileri S.A. The 2016 revision of World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaffer A.L., 3rd, Young R.M., Staudt L.M. Pathogenesis of human B cell lymphomas. Annu Rev Immunol. 2012;30:565–610. doi: 10.1146/annurev-immunol-020711-075027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younes A. Beyond chemotherapy: new agents for targeted treatment of lymphoma. Nat Rev Clin Oncol. 2011;8:85–96. doi: 10.1038/nrclinonc.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P., Allison J.P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger J.A., Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34:592–601. doi: 10.1016/j.it.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young R.M., Staudt L.M. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013;12:229–243. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis R.E., Ngo V.N., Lenz G. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niemann C.U., Wiestner A. B-cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol. 2013;23:410–421. doi: 10.1016/j.semcancer.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwood N.J., Page T.H., McDaid J.P. Bruton's tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J Immunol. 2006;176:3635–3641. doi: 10.4049/jimmunol.176.6.3635. [DOI] [PubMed] [Google Scholar]
- 10.Buggy J.J., Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol. 2012;31:119–132. doi: 10.3109/08830185.2012.664797. [DOI] [PubMed] [Google Scholar]
- 11.Advani R.H., Buggy J.J., Sharman J.P. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M.L., Rule S., Martin P. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staudt L.M. Oncogenic activation of NF-kappaB. Cold Spring Harb Perspect Biol. 2010;2:a000109. doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagel D., Vincendeau M., Eitelhuber A.C., Krappmann D. Mechanisms and consequences of constitutive NF-κB activation in B-cell lymphoid malignancies. Oncogene. 2014;33:5655–5665. doi: 10.1038/onc.2013.565. [DOI] [PubMed] [Google Scholar]
- 15.Yu L., Li L., Medeiros L.J., Young K.H. NF-κB signaling pathway and its potential as a target for therapy in lymphoid neoplasms. Blood Rev. 2017;31:77–92. doi: 10.1016/j.blre.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarnegar B.J., Wang Y., Mahoney D.J. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenz G., Davis R.E., Ngo V.N. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319:1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- 18.Davis R.E., Brown K.D., Siebenlist U., Staudt L.M. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngo V.N., Young R.M., Schmitz R. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loiarro M., Ruggiero V., Sette C. Targeting the Toll-like receptor/interleukin 1 receptor pathway in human diseases: rational design of MyD88 inhibitors. Clin Lymphoma Myeloma Leuk. 2013;13:222–226. doi: 10.1016/j.clml.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Milhollen M.A., Traore T., Adams-Duffy J. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. 2010;116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 22.Sotomayor E.M., Young K.H., Younes A. Clinical roundtable monograph: CD30 in lymphoma: its role in biology, diagnostic testing, and targeted therapy. Clin Adv Hematol Oncol. 2014;12:1–22. [PubMed] [Google Scholar]
- 23.Westin J.R. Status of PI3K/Akt/mTOR pathway inhibitors in lymphoma. Clin Lymphoma Myeloma Leuk. 2014;14:335–342. doi: 10.1016/j.clml.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay N., Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 25.Manning B.D., Cantley L.C. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cully M., You H., Levine A.J., Mak T.W. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 27.Abubaker J., Bavi P.P., Al-Harbi S. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007;21:2368–2370. doi: 10.1038/sj.leu.2404873. [DOI] [PubMed] [Google Scholar]
- 28.Baohua Y., Xiaoyan Z., Tiecheng Z., Tao Q., Daren S. Mutations of the PIK3CA gene in diffuse large B cell lymphoma. Diagn Mol Pathol. 2008;17:159–165. doi: 10.1097/PDM.0b013e31815d0588. [DOI] [PubMed] [Google Scholar]
- 29.Psyrri A., Papageorgiou S., Liakata E. Phosphatidylinositol 3'-kinase catalytic subunit alpha gene amplification contributes to the pathogenesis of mantle cell lymphoma. Clin Cancer Res. 2009;15:5724–5732. doi: 10.1158/1078-0432.CCR-08-3215. [DOI] [PubMed] [Google Scholar]
- 30.Swat W., Montgrain V., Doggett T.A. Essential role of PI3Kdelta and PI3Kgamma in thymocyte survival. Blood. 2006;107:2415–2422. doi: 10.1182/blood-2005-08-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim N., Saudemont A., Webb L. The p110delta catalytic isoform of PI3K is a key player in NK-cell development and cytokine secretion. Blood. 2007;110:3202–3208. doi: 10.1182/blood-2007-02-075366. [DOI] [PubMed] [Google Scholar]
- 32.Cheah C.Y., Fowler N.H. Idelalisib in the management of lymphoma. Blood. 2016;128:331–336. doi: 10.1182/blood-2016-02-702761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert J.A. Idelalisib: targeting PI3Kδ in B-cell malignancies. Lancet Oncol. 2014;15:e108. doi: 10.1016/s1470-2045(14)70052-x. [DOI] [PubMed] [Google Scholar]
- 34.Vangapandu H.V., Jain N., Gandhi V. Duvelisib: a phosphoinositide-3 kinase δ/γ inhibitor for chronic lymphocytic leukemia. Expert Opin Investig Drugs. 2017;26:625–632. doi: 10.1080/13543784.2017.1312338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balakrishnan K., Peluso M., Fu M. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (Duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia. 2015;29:1811–1822. doi: 10.1038/leu.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maehama T., Dixon J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 37.Sansal I., Sellers W.R. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 38.Wang X., Huang H., Young K.H. The PTEN tumor suppressor gene and its role in lymphoma pathogenesis. Aging (Albany NY) 2015;7:1032–1049. doi: 10.18632/aging.100855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudelius M., Pittaluga S., Nishizuka S. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006;108:1668–1676. doi: 10.1182/blood-2006-04-015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenz G., Wright G.W., Emre N.C. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008;105:13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu G., Zhang W., Bertram P., Zheng X.F., McLeod H. Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR pathway in common human tumors. Int J Oncol. 2004;24:893–900. [PubMed] [Google Scholar]
- 42.Oki Y., Fanale M., Romaguera J. Phase II study of an AKT inhibitor MK2206 in patients with relapsed or refractory lymphoma. Br J Haematol. 2015;171:463–470. doi: 10.1111/bjh.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witzig T.E., Reeder C.B., LaPlant B.R. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011;25:341–347. doi: 10.1038/leu.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maira S.M., Pecchi S., Huang A. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 45.Witzig T.E., Geyer S.M., Ghobrial I. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 46.Witzig T.E., Reeder C., Han J.J. The mTORC1 inhibitor everolimus has antitumor activity in vitro and produces tumor responses in patients with relapsed T-cell lymphoma. Blood. 2015;126:328–335. doi: 10.1182/blood-2015-02-629543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muñoz-Pinedo C. Signaling pathways that regulate life and cell death: evolution of apoptosis in the context of self-defense. Adv Exp Med Biol. 2012;738:124–143. doi: 10.1007/978-1-4614-1680-7_8. [DOI] [PubMed] [Google Scholar]
- 48.Yin X.M. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000;10:161–167. doi: 10.1038/sj.cr.7290045. [DOI] [PubMed] [Google Scholar]
- 49.Lüschen S., Falk M., Scherer G., Ussat S., Paulsen M., Adam-Klages S. The Fas-associated death domain protein/caspase-8/c-FLIP signaling pathway is involved in TNF-induced activation of ERK. Exp Cell Res. 2005;310:33–42. doi: 10.1016/j.yexcr.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Baud V., Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 51.Fridman J.S., Lowe S.W. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 52.Gross A., McDonnell J.M., Korsmeyer S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 53.Martinou J.C., Youle R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freedman A. Follicular lymphoma: 2014 update on diagnosis and management. Am J Hematol. 2014;89:429–436. doi: 10.1002/ajh.23674. [DOI] [PubMed] [Google Scholar]
- 55.Iqbal J., Sanger W.G., Horsman D.E. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol. 2004;165:159–166. doi: 10.1016/s0002-9440(10)63284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kendrick S.L., Redd L., Muranyi A. BCL2 antibodies targeted at different epitopes detect varying levels of protein expression and correlate with frequent gene amplification in diffuse large B-cell lymphoma. Hum Pathol. 2014;45:2144–2153. doi: 10.1016/j.humpath.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fesik S.W. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 58.Oki Y., Copeland A., Hagemeister F. Experience with obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma. Blood. 2012;119:2171–2172. doi: 10.1182/blood-2011-11-391037. [DOI] [PubMed] [Google Scholar]
- 59.Goy A., Berger M., Ford P. Sequential single-agent obatoclax mesylate (GX15-070MS) followed by combination with rituximab in patients with previously untreated follicular lymphoma. Leuk Lymphoma. 2014;55:2932–2934. doi: 10.3109/10428194.2014.900760. [DOI] [PubMed] [Google Scholar]
- 60.King A.C., Peterson T.J., Horvat T.Z., Rodriguez M., Tang L.A. Venetoclax: a first-in-class oral BCL-2 inhibitor for the management of lymphoid malignancies. Ann Pharmacother. 2017;51:410–416. doi: 10.1177/1060028016685803. [DOI] [PubMed] [Google Scholar]
- 61.Xu-Monette Z.Y., Medeiros L.J., Li Y. Dysfunction of the TP53 tumor suppressor gene in lymphoid malignancies. Blood. 2012;119:3668–3683. doi: 10.1182/blood-2011-11-366062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boehme K.A., Blattner C. Regulation of p53–insights into a complex process. Crit Rev Biochem Mol Biol. 2009;44:367–392. doi: 10.3109/10409230903401507. [DOI] [PubMed] [Google Scholar]
- 63.Dai C., Gu W. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med. 2010;16:528–536. doi: 10.1016/j.molmed.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cillessen S.A., Meijer C.J., Notoya M., Ossenkoppele G.J., Oudejans J.J. Molecular targeted therapies for diffuse large B-cell lymphoma based on apoptosis profiles. J Pathol. 2010;220:509–520. doi: 10.1002/path.2670. [DOI] [PubMed] [Google Scholar]
- 65.Chipuk J.E., Maurer U., Green D.R., Schuler M. Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell. 2003;4:371–381. doi: 10.1016/s1535-6108(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 66.Prabhu V.V., Allen J.E., Hong B., Zhang S., Cheng H., El-Deiry W.S. Therapeutic targeting of the p53 pathway in cancer stem cells. Expert Opin Ther Targets. 2012;16:1161–1174. doi: 10.1517/14728222.2012.726985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyer N., Penn L.Z. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 68.Dang C.V. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu S., Xu-Monette Z.Y., Tzankov A. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from the International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021–4031. doi: 10.1182/blood-2012-10-460063. quiz 4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trabucco S.E., Gerstein R.M., Evens A.M. Inhibition of bromodomain proteins for the treatment of human diffuse large B-cell lymphoma. Clin Cancer Res. 2015;21:113–122. doi: 10.1158/1078-0432.CCR-13-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zuber J., Shi J., Wang E. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rhyasen G.W., Hattersley M.M., Yao Y. AZD5153: a novel bivalent BET bromodomain inhibitor highly active against hematologic malignancies. Mol Cancer Ther. 2016;15:2563–2574. doi: 10.1158/1535-7163.MCT-16-0141. [DOI] [PubMed] [Google Scholar]
- 73.Yin X., Giap C., Lazo J.S., Prochownik E.V. Low molecular weight inhibitors of Myc-Max interaction and function. Oncogene. 2003;22:6151–6159. doi: 10.1038/sj.onc.1206641. [DOI] [PubMed] [Google Scholar]
- 74.Xia Y., Jeffrey Medeiros L., Young K.H. Signaling pathway and dysregulation of PD1 and its ligands in lymphoid malignancies. Biochim Biophys Acta. 2016;1865:58–71. doi: 10.1016/j.bbcan.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Francisco L.M., Sage P.T., Sharpe A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Green M.R., Monti S., Rodig S.J. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamamoto R., Nishikori M., Tashima M. B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci. 2009;100:2093–2100. doi: 10.1111/j.1349-7006.2009.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen B.J., Chapuy B., Ouyang J. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carreras J., Lopez-Guillermo A., Roncador G. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol. 2009;27:1470–1476. doi: 10.1200/JCO.2008.18.0513. [DOI] [PubMed] [Google Scholar]
- 80.Brusa D., Serra S., Coscia M. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica. 2013;98:953–963. doi: 10.3324/haematol.2012.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paydas S., Bağır E., Seydaoglu G., Ercolak V., Ergin M. Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol. 2015;94:1545–1552. doi: 10.1007/s00277-015-2403-2. [DOI] [PubMed] [Google Scholar]
- 82.Ni L., Ma C.J., Zhang Y. PD-1 modulates regulatory T cells and suppresses T-cell responses in HCV-associated lymphoma. Immunol Cell Biol. 2011;89:535–539. doi: 10.1038/icb.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muenst S., Hoeller S., Dirnhofer S., Tzankov A. Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol. 2009;40:1715–1722. doi: 10.1016/j.humpath.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 84.Kiyasu J., Miyoshi H., Hirata A. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126:2193–2201. doi: 10.1182/blood-2015-02-629600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roncador G., García V.J.F., Tedoldi S. Expression of two markers of germinal center T cells (SAP and PD-1) in angioimmunoblastic T-cell lymphoma. Haematologica. 2007;92:1059–1066. doi: 10.3324/haematol.10864. [DOI] [PubMed] [Google Scholar]
- 86.Ansell S.M., Lesokhin A.M., Borrello I. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Armand P., Nagler A., Weller E.A. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31:4199–4206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lesokhin A.M., Ansell S.M., Armand P. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase ib study. J Clin Oncol. 2016;34:2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Armand P., Shipp M.A., Ribrag V. Programmed Death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after Brentuximab Vedotin failure. J Clin Oncol. 2016;34:3733–3739. doi: 10.1200/JCO.2016.67.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guibert N., Mazières J. Nivolumab for treating non-small cell lung cancer. Expert Opin Biol Ther. 2015;15:1789–1797. doi: 10.1517/14712598.2015.1114097. [DOI] [PubMed] [Google Scholar]
- 91.Kotsakis A., Georgoulias V. Avelumab, an anti-PD-L1 monoclonal antibody, shows activity in various tumour types. Lancet Oncol. 2017;18:556–557. doi: 10.1016/S1470-2045(17)30227-9. [DOI] [PubMed] [Google Scholar]
- 92.Ilangumaran S., Rottapel R. Regulation of cytokine receptor signaling by SOCS1. Immunol Rev. 2003;192:196–211. doi: 10.1034/j.1600-065x.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 93.Ding B.B., Yu J.J., Yu R.Y. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111:1515–1523. doi: 10.1182/blood-2007-04-087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi S., Calhoun H.C., Xia F., Li J., Le L., Li W.X. JAK signaling globally counteracts heterochromatic gene silencing. Nat Genet. 2006;38:1071–1076. doi: 10.1038/ng1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kleppe M., Tousseyn T., Geissinger E. Mutation analysis of the tyrosine phosphatase PTPN2 in Hodgkin's lymphoma and T-cell non-Hodgkin's lymphoma. Haematologica. 2011;96:1723–1727. doi: 10.3324/haematol.2011.041921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weniger M.A., Melzner I., Menz C.K. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Verh Dtsch Ges Pathol. 2006;90:210–215. [PubMed] [Google Scholar]
- 97.Koo G.C., Tan S.Y., Tang T. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012;2:591–597. doi: 10.1158/2159-8290.CD-12-0028. [DOI] [PubMed] [Google Scholar]
- 98.Elliott N.E., Cleveland S.M., Grann V., Janik J., Waldmann T.A., Davé U.P. FERM domain mutations induce gain of function in JAK3 in adult T-cell leukemia/lymphoma. Blood. 2011;118:3911–3921. doi: 10.1182/blood-2010-12-319467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Roosbroeck K., Cox L., Tousseyn T. JAK2 rearrangements, including the novel SEC31A-JAK2 fusion, are recurrent in classical Hodgkin lymphoma. Blood. 2011;117:4056–4064. doi: 10.1182/blood-2010-06-291310. [DOI] [PubMed] [Google Scholar]
- 100.Adélaïde J., Pérot C., Gelsi-Boyer V. A t(8;9) translocation with PCM1-JAK2 fusion in a patient with T-cell lymphoma. Leukemia. 2006;20:536–537. doi: 10.1038/sj.leu.2404104. [DOI] [PubMed] [Google Scholar]
- 101.Hart S., Goh K.C., Novotny-Diermayr V. SB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the treatment of myeloid and lymphoid malignancies. Leukemia. 2011;25:1751–1759. doi: 10.1038/leu.2011.148. [DOI] [PubMed] [Google Scholar]
- 102.Ando S., Kawada J.I., Watanabe T. Tofacitinib induces G1 cell-cycle arrest and inhibits tumor growth in Epstein-Barr virus-associated T and natural killer cell lymphoma cells. Oncotarget. 2016;7:76793–76805. doi: 10.18632/oncotarget.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han Y., Amin H.M., Franko B., Frantz C., Shi X., Lai R. Loss of SHP1 enhances JAK3/STAT3 signaling and decreases proteosome degradation of JAK3 and NPM-ALK in ALK+ anaplastic large-cell lymphoma. Blood. 2006;108:2796–2803. doi: 10.1182/blood-2006-04-017434. [DOI] [PubMed] [Google Scholar]
- 104.Mughal T.I., Girnius S., Rosen S.T. Emerging therapeutic paradigms to target the dysregulated Janus kinase/signal transducer and activator of transcription pathway in hematological malignancies. Leuk Lymphoma. 2014;55:1968–1979. doi: 10.3109/10428194.2013.863307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Springuel L., Renauld J.C., Knoops L. JAK kinase targeting in hematologic malignancies: a sinuous pathway from identification of genetic alterations towards clinical indications. Haematologica. 2015;100:1240–1253. doi: 10.3324/haematol.2015.132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Q., Nowak I., Vonderheid E.C. Activation of Jak/STAT proteins involved in signal transduction pathway mediated by receptor for interleukin 2 in malignant T lymphocytes derived from cutaneous anaplastic large T-cell lymphoma and Sezary syndrome. Proc Natl Acad Sci U S A. 1996;93:9148–9153. doi: 10.1073/pnas.93.17.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]