Abstract
Background
Post-Transplant Lymphoproliferative Disorder (PTLD) is a well-recognized complication post solid organs transplant. PTLD represents a broad spectrum of abnormalities ranging from an infectious mononucleosis like illness to malignant lymphoma.
Methods
A retrospective study was performed by collecting data of orthotopic liver transplant (OLT) patients in the National Liver Unit in Ireland from December 1993 to December 2014. Data was analyzed to identify PTLD patients and determine their demographic details, the indication for liver transplant, presenting symptoms, immunosuppression regimens, Epstein"Barr virus (EBV) status and PTLD outcome.
Results
From a total of 756 liver transplants recipients, 20 patients (2.6%) were diagnosed with PTLD. The median time from OLT to PTLD diagnosis was 83 months. The main primary indication for OLT of the PTLD cohort was Autoimmune Liver Disease (AiLD) (n = 13, 65%, mainly primary sclerosing cholangitis (PSC) n = 8, 40%). The combined group of auto-immune hepatitis, PSC and primary biliary cholangitis had a significantly higher incidence of PTLD compared to other etiologies (P < 0.01). In AiLD PTLD subgroup, 61.5% were positive for EBV. Five patients (38.5%) had extra-nodal disease and 3 patients had CNS disease. 61% of PTLD AiLD patients (n = 8) achieved complete response following their treatment.
Conclusion
PTLD has high mortality however early diagnosis and complete remission are achievable. Our study suggests that the incidence of PTLD is increased in AiLD and notably PSC patients.
Abbreviations: AiLD, Autoimmune Liver Disease; OLT, Orthotopic Liver Transplant; PTLD, Post-Transplant Lymphoproliferative Disorder
Keywords: post-transplant lymphoproliferative disorder, orthotopic liver transplant, autoimmune liver disease, primary sclerosing cholangitis Epstein"Barr virus, immunosuppressants
Post-Transplant Lymphoproliferative Disorder (PTLD) is a well recognized complication post solid organ or bone marrow transplantation develops in approximately 3% of patients following liver transplantation.1, 2, 3 It is a serious condition with mortality rates in excess of 50%.4 PTLD represents a broad heterogeneous spectrum of abnormal lymphoid proliferation. The latest World Health Organization (WHO) classification divided PTLD into early lesions which includes infectious mononucleosis like PTLD and plasmacytic hyperplasia, polymorphic lesions, monomorphic lesions which resemble frank and aggressive lymphomas that includes Diffuse B Cell Lymphoma (DBCL), Burkitts, plasma cell myeloma/plasmacytoma and T-cell neoplasm and Hodgikin's lymphoma like PTLD.5 Better outcomes have been reported especially with the early introduction of rituximab when indicated.6, 7
The incidence of PTLD is related to the organ transplant and hence the duration and doses of immunosuppressants. PTLD occurs in more than 10% of intestinal and multi-organ transplants with less incidence after heart or lung transplant (3"9%) and least with liver or kidney transplant (1"3%).8, 9 Other risk factors include presence of cytomegalovirus, pre-transplant malignancies and younger age.8, 10, 11
It has been suggested that PTLD cases are associated with Epstein"Barr (EBV) virus exposure. During primary EBV infection, T cell lymphocytes play an important role in modulation of infected B cell proliferation. Immunosuppressants, in patients post transplants, inhibit T cell function which in turn leads to lack of control of infected B-cell proliferation. This can result in free proliferation of EBV infected B cells which induce lymphoid hyperplasia and possible frank lymphomas.12
Over the last number of years, growing data has indicated a possible association of lymphocyte regulation and the development of autoimmune liver disease.13, 14, 15, 16 More recently, it has been shown that EBV is considered a trigger of autoimmune diseases based on findings indicating high titers of EBV-specific antibodies in patients compared to controls. In addition, a higher proportion of EBV-infected cells with elevated EBV loads are found in peripheral blood lymphocytes of autoimmune patients compared to other pathological or normal controls.17 Watts et al. suggested there may be an increased risk of PTLD in patients transplanted for PSC.2 The current study presents the clinical outcome of the liver transplant cohort who was diagnosed with PTLD. We aimed to determine if the rate of PTLD was increased in autoimmune liver disease compared to patients transplanted for other etiologies.
Patients and Methods
Patients
This is a retrospective study was performed by collecting data of the liver transplant patients from the National Liver Unit on the island of Ireland. The study was approved by the Ethics and Medical Research Committee (SThe EMRC) of St. Vincent's Healthcare Group (SVHG). No patient consent was required. Our study included 20 patients who developed PTLD after OLT from 1993 to the end 2014 and were treated mainly by the Medical Oncology at the same institute. Patients for this study were identified from our database, maintained within the UK NHS Blood and Transplant service (NHSBT). Patient demographics, indication for liver transplant, age at the time of OLT immunosuppression, mode of diagnosis, the time from liver transplantation to PTLD diagnosis, site of PTLD, PTLD stage, and category, EBV status whenever available, PTLD treatment modalities and clinical outcome were recorded. Several treatment modalities were utilitized as the study included patients over different timeframes although patients diagnosed after 2010 were managed as per the British society of Haematology published guidelines in 2010.18 After PTLD diagnosis all patients had initial reduction/withdrawal of immunosuppressants but maintained on steroids. Chemotherapy was started in patients with aggressive PTLD histology, EBV negative disease, CNS disease and patients with no response to reduction of immunosuppressive therapy after 2"4 weeks. Radiotherapy and surgery were used in cases of residual disease.
Response was evaluated into Complete Response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) as per RECIST 1.1 criteria.19
Methods
The Histological diagnosis was reviewed as per the WHO classification5 which includes early lesions, polymorphic PTLD, monomorphic PLTD (which includes B and T cell neoplasms) and Hodgkin's lymphoma like PTLD. Clonality was determined by the presence of kappa or lambda light chain restriction in immunohistochemistry. Flow cytometry, in situ hybridization for EBV mRNA and EBV viral loads were performed as indicated. Patients were staged as per the Ann Arbor staging system,20 computed tomography (CT) of the thorax, abdomen and pelvis was used as the primary staging modality. Imaging of the brain with CT or MRI was performed in the presence of signs and symptoms of CNS disease. All patients had baseline blood tests including complete blood count, liver function testing, kidney function testing and lactate dehydrogenase (LDH) test.
Data was analyzed using the GraphPad Prism program (GraphPad Software Inc, San Diego, CA, USA). !2 test or the Mann"Whitney test was used as appropriate to assess significance of differences.
Results
Between January 1993 and October 2014 a total of 756 adult patients had a first liver transplant in our unit. Twenty (2.6%) were subsequently diagnosed with PTLD with a median follow up of 17 months. There were more males (n = 17, 85%) than females (n = 3, 15%), with a median age of 54 years (range 22"65) at the time of liver transplant. Median time from transplant to diagnosis of PTLD was 83 months (range 19"173). The age of the patients at the time of PTLD diagnosis ranged between 29 and 75 years old with median age 61 years old. The primary indication for transplant was: PSC (n = 8) 40%, autoimmune hepatitis (AIH) (n = 3) 15%, primary biliary cholangitis (PBC) (n = 2) 10% and alcoholic liver disease (ALD) (n = 2). The total number of liver transplants secondary to AiLD (i.e. PSC, AIH cirrhosis and PBC) was 218 patients (67, 65 and 86 patients respectively). The total number of patients developed PTLD in the AiLD subgroup was thirteen patients. The majority of them were secondary to PSC (n = 8). Other causes of liver transplant are included in Table 1. The combined group of AiLD including PSC, AIH cirrhosis and PBC had a significantly higher incidence of PTLD compared to other aetiologies (13/218 vs 7/538 P < 0.01). The most commonly used immunosuppressant was tacrolimus (n = 15), followed by mycophenolate (n = 11), azathioprine (n = 9), and cyclosporine (n = 5) [used alone or with other agents]. At least 15 patients have taken more than one immunosuppressant at some point till diagnosis of PTLD and just less than half of them (n = 7) were on more than two immunosuppressants. A quarter of the patients (n = 5) of those diagnosed with PTLD were on only one immunosuppressant as per Table 1. All the patients were treated with steroids in the initial transplant period. Steroids were tapered and withdrawn except for patients with auto-immune hepatitis who were maintained on low dose prednisolone (5"10 mg daily) indefinitely.
Table 1.
PTLD Patients Clinical Characteristics.
| Gender | M:F | 17:3 | M = 85% |
|---|---|---|---|
| Age at OLT (years) | Median (range) | 54 (22"65) | |
| Age at PTLD Dx (years) | Median (range) | 61 (29"75) | |
| Interval OLT to PTLD diagnosis (months) | Median (range) | 77.5 (19"173) | |
|
1ry liver disease (n) and % |
Total number of transplant (n = 756) |
Patients with PTLD (n = 20) |
% of PTLD among each transplant pt by 1ry liver disease |
| PSC | 67 | 8 | 12% |
| AIH Cirrhosis | 65 | 3 | 4.6% |
| PBC | 86 | 2 | 2.3% |
| ALD | 208 | 2 | 1% |
| HCC | 116 | 3 | 2.6% |
| HBV | 19 | 1 | 5% |
| POD | 31 | 1 | 3% |
| Others | 164 | 0 | 0% |
| Immunosuppressant | |||
| 0two agents | N = 15 | 75% | |
| 4 agents | N = 1 | 5% | |
| 3 agents | N = 6 | 30% | |
| 2 agents | N = 8 | 40% | |
| -Tac+MMF | 4 | 20% | |
| -Tac+AZA | 3 | 15% | |
| -Cyc+AZA | 1 | 5% | |
| One agent | |||
| -Tac | 2 | 10% | |
| -Cyc | 2 | 10% | |
| -MMF | 1 | 5% | |
MMF: Mycophenolate Mofetil; Tac: Tacrolimus; Cyc: Cyclosporin; AZA: Azathioprine; PSC: Primary Sclerosing Cholangitis; AIH: Autoimmune Hepatitis; PBC: Primary Biliary Cholangitis; ALD: Alcoholic Liver Disease; HCC: Hepatocellular Carcinoma; HBV: Hepatitis B virus; POD: Paracetamol Overdose; CCA: Cholagiocarcinoma; OLT: Orthotopic Liver Transplant; PTLD: Post-Transplant Lymphoproliferative Disorder.
PTLD Characteristics
Most cases were diagnosed late after transplantation (median = 83 months) with 75% (n = 15) of the cases diagnosed after 5 years. Median age at PTLD diagnosis was 61 years (29-75yrs). Ann Arbor staging at time of diagnosis was Stage I (5 patients, 25%), Stage II (12 patients, 60%), Stage III (1 patient, 5%) and Stage IV (2 patients, 10%). Most common site of disease involvement with intra abdominal (13 patients, 68%), six patients (31%) had extra nodal disease including three patients with CNS involvement (2 patients at initial presentation and 1 patient after disease progression). The majority of patients presented with gastrointestinal symptoms (12/20 patients) and six patients reported B symptoms. PTLD patients histology consisted of fifteen (75%) monomorphic, three (15%) polymorphic disease and two patients (10%) presented with Hodgkin lymphoma-like PTLD. EBV status was available in seventeen patients with PTLD. The status was checked histologically and by PCR of whom 13/17 (76.5%) were positive for EBV, 3 patients confirmed EBV negative and another patient was indeterminate status for same.
Treatment and Outcomes
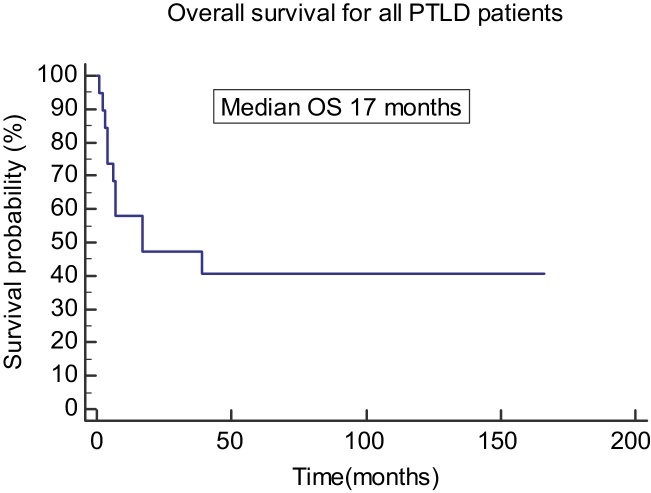
All patients had initial reduction or discontinuation of immunosuppresion, 19 patients received chemotherapy or rituximab. Ten patients treated with R-CHOP chemotherapy, two patients with R-CVP chemotherapy, two patients with CHOP, one patient with rituximab alone, two patients with Hodgkin's disease like PTLD had ABVD and CHLVPP chemotherapy and two patients with CNS PTLD had De angelis chemotherapy protocol. One patient was unwell to receive any treatment and died within 4 weeks of his diagnosis. Complete response was obtained in 11 patients (55%). Two patients had surgery for residual disease and two patients had radiotherapy after completion of treatment, one for residual disease and one for CNS disease. At the last database cut-off, 11 patients had died: five were due to treatment-related complications and three were due to progressive disease. Table 2 shows the patients histological features, treatments and outcomes. The overall median survival was 17 months as shown in the Kaplan Meier curve (Figure 1).
Table 2.
Clinical Characteristics of Patients Who Developed Post-transplant Lymphoproliferative Disorder (PTLD).
| Panel A | |||||||
|---|---|---|---|---|---|---|---|
| Age diagnosis PTLD (sex m/f) | IBD | Immuno-suppression | Interval transplant to PTLD (months) | Histology | Treatment | Outcome | Survival post PTLD diagnosis (months) |
| 61 (m) | Previous hemicolectomy (azathioprine) | TAC MMF AZA |
106 | Monomorphic large B cell | Deangelis protocol for CNS disease | Complete remission | 58 |
| 67 (m) | Mild colitis salazopyrine | TAC MMF AZA |
19 | Polymorphic | Rituximab | Complete remission | 5 |
| 63 (m) | Total colectomy | CYC | 173 | Hodgkin's disease PTLD like | ABVD | Complete remission | 20 |
| 36 (m) | Total Colectomy 15 years prior to transplant |
CYC | 72 | Monomorphic large B cell | CHOP | Died Progressive disease |
7 |
| 62 (m) | Mild colitis Salazopyrine |
TAC | 64 | Polymorphic | No chemotherapy | Died Progressive disease + sepsis |
0 |
| 42 (m) | Crohn's colitis No AZA or anti-TNF |
TAC AZA |
79 | Monomorphic large B cell | RCHOP | Died Progressive disease |
3 |
| 30 (m) | Ulcerative colitis diagnosed post transplant No AZA or anti-TNF |
TAC MMF AZA |
63 | Monomorphic large B cell | Rituximab then R CVP | Died Progressive disease |
7 |
| 69 (m) | Ulcerative colitis AZA |
TAC MMF |
87 | Monomorphic large B cell | Deangelis protocol for CNS disease | Complete remission | 19 |
| Panel B | |||||||
|---|---|---|---|---|---|---|---|
| Age diagnosis PTLD (sex m/f) | Etiology | Immuno-Suppression | Interval transplant to PTLD (months) | Histology | Treatment | Outcome | Survival post PTLD diagnosis (months) |
| 58 (f) | Autoimmune hepatitis | CYC AZA |
38 | Hodgkin's disease PTLD like | CHLVPP | Died fungal pneumonia | 55 |
| 43 (m) | Autoimmune hepatitis | TAC AZA |
22 | Monomorphic large B cell | CHOP | Complete remission | 152 |
| 66 (m) | Auto-immune hepatitis | MMF | 76 | Monomorphic large B cell | R CHOP | Died Pneumonia | 39 |
| 54 (f) | Primary biliary cirrhosis | TAC AZA |
117 | Monomorphic large B cell | R CHOP | Complete remission | 64 |
| 55 (m) | Primary biliary cirrhosis | TAC MMF |
113 | Monomorphic large B cell | R CHOP | Complete remission | 43 |
| 64 (m) | Alcoholic liver disease | MMF TAC |
93 | Monomorphic large B cell, Burkitts like | R CHOP | Died Neutropenic sepsis |
5 |
| 75 (m) | Alcoholic liver disease | TAC CYC MMF AZA |
121 | Monomorphic large B cell | R CHOP | Died Sepsis |
2 |
| 62 (m) | Alcoholic liver disease & HCC | TAC MMF AZA |
58 | Monomorphic large B cell | R CHOP | Complete remission | 29 |
| 62 (m) | Alcohol/hepatitis C HCC |
TAC MMF |
22 | Polymorphic | R CVP | Died Neutropenic sepsis |
4 |
| 64 (m) | Hepatitis C & HCC |
TAC MMF AZA |
100 | Monomorphic large B cell | R CHOP | Died Sepsis |
6 |
| 36 (m) | Fulminant paracetamol | TAC CYC MMF |
166 | Monomorphic large B cell | R CHOP | Complete remission | 65 |
| 29 (m) | Fulminant hepatitis B | TAC | 52 | Monomorphic large B cell | R CHOP | Died Sepsis | 17 |
Panel A: primary sclerosing cholangitis (PSC) patients.
Panel B: other aetiologies.
Abbreviations: R CHOP: rituximab cyclophosphamide hydroxydaunomycin Oncovin, Prednisolone. CHL VPP: chlorambucil, vinblastine, procarbazine, prednisolone.
ABVD: adriamycin, bleomycin, vinblastine, dacarbazine. CVP: cyclophosphamide, vincristine, Prednisolone, HCC: hepatocellular carcinoma.
Figure 1.
Overall survival of all PTLD patients. PTLD: post transplant lymphoproliferative disease; OS: overall survival.
PTLD in AiLD Patients
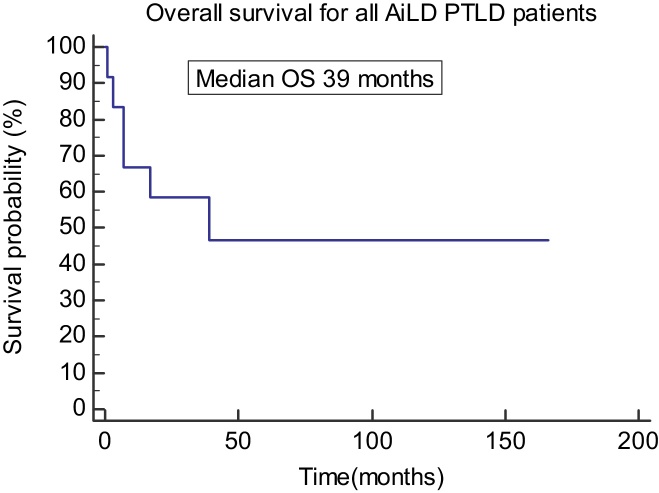
Ten male (87.5%) and three female patients had PTLD post OLT for AiLD. The median age at the time of OLT was 49 years (29"65 years). The rate of occurrence of PTLD in AiLD was significantly higher than the overall OLT patients with P value < 0.0001. Median age for the AiLD patients at development of PTLD was 58 years. Median time to diagnosis of PTLD was 74 months (19"173) post liver transplant. All of PTLD PSC patients had inflammatory bowel disease before OLT with ulcerative colitis (UC) in 75% and Crohn's disease (CD) in 25%. Of the PTLD AiLD patients, 77% were on two immunosuppressants or more either a combination of tacrolimus and mycophenolate mofetil (MMF), tacrolimus and azathioprine or cyclosporine and azathioprine. Only two patients 23% were on one immunosuppressant (one patient on tacrolimus and two on cyclosporine) alone at the time of PTLD diagnosis. Seven/ten (70%) had positive EBV status on histology while the other three patients statuses were not available. Five patients (38.5%) had extra-nodal disease and 3 patients had CNS disease (2 at diagnosis and 1 after disease progression). Nine patients (70%) had monomorphic PTLD, 2 (15%) had polymorphic PTLD and 2 (15%) had Hodgkin's disease like PTLD. 61% of PTLD AiLD patients (n = 8) achieved complete response following their treatment, of the remaining patients; 2 died due to disease progression, 2 died due to sepsis and one had residual disease/incomplete response. The median overall survival for patients with AiLD who developed PTLD was 39 months (Figure 2).
Figure 2.
Overall survival of PTLD patients secondary to AiLD. AiLD: Autoimmune Liver Disease; PTLD: post transplant lymphoproliferative disease; OS: overall survival.
Discussion
PTLD is a well established and serious complication after organ transplantation with low incidence and high mortality rate.21, 22, 23 A number of published studies share our objectives to identify reliable and reproducible pre- and post-transplant factors that may help to identify the cohort of patients who are likely to suffer PTLD.24, 25, 26 In the course of our analysis we made 3 main observations. Firstly, we report a significantly higher rate of PTLD following liver transplant in patients with autoimmune liver disease specially PSC compared to other etiologies. Secondly, we studied pre and post OLT PTLD risk factors such as EBV status and immunosuppressants. Finally, the treatment and outcome of PTLD were similar to the published literature with increasing complete responses (CR) with the use of combination chemotherapy.
In our study, PTLD was more prevalent in patients with AiLD. The autoimmune liver disease (AiLD) was the main primary indication for OLT (n = 13, 65%) in our PTLD cohort. Of interest, PTLD was developed in 11.8% of all of our PSC patients post OLT. Our study would support the previous findings of Watt et al.2 who used prospective database of liver transplant patients maintained by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), including 798 adult patients followed for a median of 10 years. There appeared to be an increased incidence of PTLD in the PSC group, although this did not reach statistical significance. Mumtaz et al. reported 32 cases of PTLD in 1372 patients following liver transplant. Seven patients had PSC and four had PBC but the total numbers of patients with PSC and PBC transplanted were not given.27
In non-transplant patients, a possible association between autoimmune liver disease and lymphoma has been described.28 We hypothesized that this subgroup had low median age that required higher doses of immunosuppressant agents to control their underlying autoimmune disease recurrence. In the mean time, patients with AiLD usually require more than one immunosuppressant agent to avoid a higher incidence of acute and chronic rejection post transplant.8, 29 Eighty five percent of our PTLD patients were on more than one immunosuppressants at the time of diagnosis. If true, this could be a factor causing increased susceptibility to PTLD. In general, PTLD has higher incidence post intestinal or multi-organ transplants with less incidence after heart or lung transplant (3"9%) and least with liver or kidney transplant (1"3%).8, 9 Moreover, data from the Collaborative Transplant Study (CTS) confirmed that the rate of NHL was high during the first year post transplant which is probably secondary to intense immunosuppressive therapy.30 Furthermore, Melosky et al.31 have shown that patients who received quadruple immunosuppressants therapy had more incidence of NHL than who received triple therapy for treatment of acute cellular rejection in renal transplant subjects.
The type of immunosuppressive agent can be an associated factor in PTLD development. The majority of our AiLD patients who developed PTLD were on tacrolimus (69%) either alone or combined with another immunosuppressant agent. Of interest, 80% of our PTLD cohort were on tacrolimus at the time of diagnosis. Tacrolimus use is associated with an increase PTLD risk compared with cyclosporine or anti IL-2 antibodies and sirolimus use.11, 32 There was no significant difference in the 5 year incidence of lymphoma between patients who received either Cyclosporine (CsA; with or without AZA and steroid) versus AZA and steroid.32 On the other hand, patients receiving MMF have reduced risk of PTLD occurrence.11, 33
Immune related mechanisms in patients with AiLD may lead to auto-reactivation of B lymphocytes. In less than one decade ago, Moritki and colleagues have discussed a possible pathogenic role of autoreactive B lymphocytes in AiLD. They have described a potential contribution of Toll-like Receptor (TLR) signaling which activate the autoimmune B cells and induce defects in regulatory T cell function.34 This is also supported by a study that described a significant reduction in the total number of circulating T cells in parallel to increased number and percentage of B cells. Moreover, alterations in the percentage of B cells correlated significantly with histologic stage and concentrations of gamma globulin, serum IgG, and bilirubin.35 Recently, Zhang et al.36 have shown an ongoing stimulated B lymphocytes which have an auto-antigen specificity in patients with PBC. These observations may link between the development and progression of AiLD and the PTLD development. While our patients did not appear to be exposed to unusually high doses of immunosuppression, a more detailed analysis including evaluation of dosage ranges and serum levels over time would be required to more adequately assess this.
Other possibilities that might explain the high incidences of PTLD among AiLD patients may be related to a possible role of EBV status. In the PTLD secondary to AiLD patients, EBV was positive in eight patients (61.5%) while two patients (15%) were negative for EBV. One of the AiLD patients status was indeterminate and two patients statuses were not available. A suggested role of EBV triggering AiLD effects was unraveled by few observations.30, 37, 38, 39, 40 An established characteristic of several autoimmune diseases is based on CD8+ T-cell deficiency. The impairment of CD8+ T cells results in the inability to control EBV infection and this ensues an accumulation of EBV-infected and autoreactive B-cells in a variety of target organs. Within a specific organ, clonal expansion of these cells, and the development of ectopic lymphoid follicles can induce pathogenic autoantibodies and provide stimulatory signals for the survival of autoreactive T cells.41 There is also growing evidence in support of infectious agents mimicking the major autoantigen in PBC, PDC-E2. This can result in loss of immunological tolerance which in turn can lead to the destruction of the bile ducts.42, 43 An increase number of EBV-infected B lymphocytes in intestinal mucosal samples from patients with ulcerative colitis compared to healthy controls has been reported.44, 45 Of interest our results showed that all of the PSC patients suffered from IBD before PTLD diagnosis.
The outcome of the PTLD patients was similar to previous reports. Two studies described overall PTLD incidence similar to ours but they did not mention a higher incidence in AiLD.46, 47 The majority of AiLD subgroup achieved complete response. Two patients died due to disease progression, two died due to sepsis and one had residual disease/incomplete response. It is worth noting that 3 patients in the AiLD had cerebral PTLD (2 at diagnosis and 1 after disease progression) which is relatively rare. In the non AiLD-PTLD patients, none of them developed CNS disease before or during the course of treatment.
In summary, PTLD has high mortality, however early diagnosis and complete remission are achievable. Our study suggests that there is an associated higher incidence of PTLD in autoimmune liver disease and notably PSC patients. Given the small number of PTLD patients though, this needs to be validated with other centers to avoid type 2 error. This finding may have implications on the management of AiLD patients post liver transplantation. While routine Ebstein Barr virus monitoring is probably not very helpful, targeted monitoring in AiLD patients along with appropriate tailoring of immunosuppressive therapy may help to reduce the incidence of this, frequently lethal, complication.
Conflicts of Interest
The authors have none to declare.
References
- 1.Gao S.-Z., Chaparro S.V., Perlroth M. Post-transplantation lymphoproliferative disease in heart and heart-lung transplant recipients: 30-year experience at Stanford University. J Heart Lung Transplant. 2003;22(5):505–514. doi: 10.1016/s1053-2498(02)01229-9. [DOI] [PubMed] [Google Scholar]
- 2.Watt K.D.S., Pedersen R., A, Kremers W.K., Heimbach J.K., Sanchez W., Gores G.J. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137(6):2010–2017. doi: 10.1053/j.gastro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrem H., Kurok M., Kaltenborn A. Incidence and long-term risk of de novo malignancies after liver transplantation with implications for prevention and detection. Liver Transpl. 2013;19(11):1252–1261. doi: 10.1002/lt.23722. [DOI] [PubMed] [Google Scholar]
- 4.Mukthinuthalapati P.K., Gotur R., Ghabril M. Incidence, risk factors and outcomes of de novo malignancies post liver transplantation. World J Hepatol. 2016;8(12):533–544. doi: 10.4254/wjh.v8.i12.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swerdlow S.H., Campo E., Harris N.L. IARC; Lyon: 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 6.Evens A.M., David K.A., Helenowski I. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010;28(6):1038–1046. doi: 10.1200/JCO.2009.25.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trappe R., Oertel S. Sequential treatment with Rituximab and CHOP chemotherapy in B-cell PTLD; moving forward to a first standard of care: results from a prospective international multicenter trial. Blood. 2010 [Google Scholar]
- 8.Cockfield S.M. Identifying the patient at risk for post-transplant lymphoproliferative disorder. Transpl Infect Dis. 2001;3(2):70–78. doi: 10.1034/j.1399-3062.2001.003002070.x. [DOI] [PubMed] [Google Scholar]
- 9.Tsai D.E., Hardy C.L., Tomaszewski J.E. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001;71(8):1076–1088. doi: 10.1097/00007890-200104270-00012. [DOI] [PubMed] [Google Scholar]
- 10.Tsai D.E., Hardy C.L., Tomaszewski J.E. Incidence of PTLD in pediatric renal transplant recipients receiving basiliximab, calcineurin inhibitor, sirolimus and steroids. Am J Transplant. 2008;8(5):984–989. doi: 10.1111/j.1600-6143.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 11.Caillard S., Dharnidharka V., Agodoa L., Bohen E., Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80(9):1233–1243. doi: 10.1097/01.tp.0000179639.98338.39. [DOI] [PubMed] [Google Scholar]
- 12.Evens A.M., Roy R., Sterrenberg D., Moll M.Z., Chadburn A., Gordon L.I. Post-transplantation lymphoproliferative disorders: diagnosis, prognosis, and current approaches to therapy. Curr Oncol Rep. 2010;12(6):383–394. doi: 10.1007/s11912-010-0132-1. [DOI] [PubMed] [Google Scholar]
- 13.Koyabu M., Uchida K., Miyoshi H. Analysis of regulatory T cells and IgG4-positive plasma cells among patients of IgG4-related sclerosing cholangitis and autoimmune liver diseases. J Gastroenterol. 2010;45(7):732–741. doi: 10.1007/s00535-010-0199-3. [DOI] [PubMed] [Google Scholar]
- 14.Sakaki M., Hiroishi K., Baba T. Intrahepatic status of regulatory T cells in autoimmune liver diseases and chronic viral hepatitis. Hepatol Res. 2008;38(4):354–361. doi: 10.1111/j.1872-034X.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- 15.Martins E.B., Graham A.K., Chapman R.W., Fleming K.A. Elevation of gamma delta T lymphocytes in peripheral blood and livers of patients with primary sclerosing cholangitis and other autoimmune liver diseases. Hepatology. 1996;23(5):988–993. doi: 10.1002/hep.510230508. [DOI] [PubMed] [Google Scholar]
- 16.Kita H., Mackay I.R., Van De Water J., Gershwin M.E. The lymphoid liver: considerations on pathways to autoimmune injury. Gastroenterology. 2001;120(6):1485–1501. doi: 10.1053/gast.2001.22441. [DOI] [PubMed] [Google Scholar]
- 17.Barzilai O., Sherer Y., Ram M., Izhaky D., Anaya J.M., Shoenfeld Y. Epstein"Barr virus and cytomegalovirus in autoimmune diseases: are they truly notorious? A preliminary report. Ann N Y Acad Sci. 2007;1108:567–577. doi: 10.1196/annals.1422.059. [DOI] [PubMed] [Google Scholar]
- 18.Parker A., Bowles K., Bradley J.A. Haemato-oncology Task Force of the British Committee for Standards in Haematology and British Transplantation Society. Br J Haematol. 2010;149(5):693–705. [Google Scholar]
- 19.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Carbone P.P., Kaplan H.S., Musshoff K., Smithers D.W., Tubiana M. Report of the Committee on Hodgkin's disease staging classification. Cancer Res. 1971;31(11):1860–1861. [PubMed] [Google Scholar]
- 21.Dror Y., Greenberg M., Taylor G. Lymphoproliferative disorders after organ transplantation in children. Transplantation. 1999;67(7):990–998. doi: 10.1097/00007890-199904150-00010. [DOI] [PubMed] [Google Scholar]
- 22.Libertiny G., Watson C.J., Gray D.W., Welsh K.I., Morris P.J. Rising incidence of post-transplant lymphoproliferative disease in kidney transplant recipients. Br J Surg. 2001;88(10):1330–1334. doi: 10.1046/j.0007-1323.2001.01924.x. [DOI] [PubMed] [Google Scholar]
- 23.Opelz G., Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342(8886"8887):1514–1516. doi: 10.1016/s0140-6736(05)80084-4. [DOI] [PubMed] [Google Scholar]
- 24.Marino D., Burra P., Boccagni P. Post-transplant lymphoproliferative disorders in liver transplanted patients: a single-centre experience. Anticancer Res. 2010;30(6):2383–2391. [PubMed] [Google Scholar]
- 25.Caillard S., Porcher R., Provot F Post-transplantation lymphoproliferative disorder after kidney transplantation: report of a nationwide French registry and the development of a new prognostic score. J Clin Oncol. 2013;31(10):1302–1309. doi: 10.1200/JCO.2012.43.2344. [DOI] [PubMed] [Google Scholar]
- 26.Knight J.S., Tsodikov A., Cibrik D.M., Ross C.W., Kaminski M.S., Blayney D.W. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009;27(20):3354–3362. doi: 10.1200/JCO.2008.20.0857. [DOI] [PubMed] [Google Scholar]
- 27.Mumtaz K., Faisal N., Marquez M., Healey A., Lilly L.B., Renner E.L. Post-transplant lymphoproliferative disorder in liver recipients: characteristics, management, and outcome from a single-centre experience with >1000 liver transplantations. Can J Gastroenterol Hepatol. 2015;29(8):417–422. doi: 10.1155/2015/517359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Man K.M., Drejet A., Keeffe E.B., Garcia-Kennedy R., Imperial J.C., Esquivel C.O. Primary sclerosing cholangitis and Hodgkin's disease. Hepatology. 1993;18(5):1127–1131. [PubMed] [Google Scholar]
- 29.Chandok N., Hirschfield G.M. Management of primary sclerosing cholangitis: conventions and controversies. Can J Gastroenterol. 2012;26(5):261–268. doi: 10.1155/2012/426430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabibi D. Autoimmune hepatitis following Epstein"Barr virus infection. BMJ Case Rep. 2008;2008 doi: 10.1136/bcr.06.2008.0071. pbcr0620080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melosky B., Karim M., Chui A. Lymphoproliferative disorders after renal transplantation in patients receiving triple or quadruple immunosuppression. J Am Soc Nephrol. 1992;2(12 suppl):S290–S294. doi: 10.1681/ASN.V212s290. [DOI] [PubMed] [Google Scholar]
- 32.Opelz G., Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2003;4:222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 33.Robson R., Cecka J.M., Opelz G., Budde M., Sacks S. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant. 2005;5(12):2954–2960. doi: 10.1111/j.1600-6143.2005.01125.x. [DOI] [PubMed] [Google Scholar]
- 34.Moritoki Y., Lian Z.-X., Ohsugi Y., Ueno Y., Gershwin M.E. B cells and autoimmune liver diseases. Autoimmun Rev. 2006;5(7):449–457. doi: 10.1016/j.autrev.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Lindor K.D., Wiesner R.H., Katzmann J.A., LaRusso N.F., Beaver S.J. Lymphocyte subsets in primary sclerosing cholangitis. Dig Dis Sci. 1987;32(7):720–725. doi: 10.1007/BF01296138. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J., Zhang W., Leung P.S.C. Ongoing activation of autoantigen-specific B cells in primary biliary cirrhosis. Hepatology. 2014;60(5):1708–1716. doi: 10.1002/hep.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rigopoulou E.I., Smyk D.S., Matthews C.E. Epstein-barr virus as a trigger of autoimmune liver diseases. Adv Virol. 2012;2012:987471. doi: 10.1155/2012/987471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vento S., Guella L., Mirandola F. Epstein"Barr virus as a trigger for autoimmune hepatitis in susceptible individuals. Lancet. 1995;346(8975):608–609. doi: 10.1016/s0140-6736(95)91438-2. [DOI] [PubMed] [Google Scholar]
- 39.Chiba T., Goto S., Yokosuka O. Fatal chronic active Epstein"Barr virus infection mimicking autoimmune hepatitis. Eur J Gastroenterol Hepatol. 2004;16(2):225–228. doi: 10.1097/00042737-200402000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Morshed S.A., Nishioka M., Saito I., Komiyama K., Moro I. Increased expression of Epstein"Barr virus in primary biliary cirrhosis patients. Gastroenterol Jpn. 1992;27(6):751–758. doi: 10.1007/BF02806528. [DOI] [PubMed] [Google Scholar]
- 41.Pender M.P. CD8+ T-cell deficiency, Epstein"Barr virus infection, vitamin D deficiency, and steps to autoimmunity: a unifying hypothesis. Autoimmune Dis. 2012;2012:189096. doi: 10.1155/2012/189096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smyk D., Rigopoulou E.I., Baum H., Burroughs A.K., Vergani D., Bogdanos D.P. Autoimmunity and environment: am I at risk? Clin Rev Allergy Immunol. 2011:1–14. doi: 10.1007/s12016-011-8259-x. [DOI] [PubMed] [Google Scholar]
- 43.Smyk D.S., Rigopoulou E.I., Lleo A. Immunopathogenesis of primary biliary cirrhosis: an old wives tale. Immun Ageing. 2011;8(1):12. doi: 10.1186/1742-4933-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanai H., Shimizu N., Nagasaki S., Mitani N., Okita K. Epstein"Barr virus infection of the colon with inflammatory bowel disease. Am J Gastroenterol. 1999;94(6):1582–1586. doi: 10.1111/j.1572-0241.1999.01148.x. [DOI] [PubMed] [Google Scholar]
- 45.Akamatsu T., Watanabe N., Chiba T. Epstein"Barr virus-associated lymphoma developed shortly after immunosuppressive treatment for ulcerative colitis. Clin Gastroenterol Hepatol. 2007;5(4):521. doi: 10.1016/j.cgh.2007.02.003. author reply 521"522. [DOI] [PubMed] [Google Scholar]
- 46.Aberg F., Pukkala E., Hockerstedt K., Sankila R., Isoniemi H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008;14(10):1428–1436. doi: 10.1002/lt.21475. [DOI] [PubMed] [Google Scholar]
- 47.Villeneuve P.J., Schaubel D.E., Fenton S.S., Shepherd F.A., Jiang Y., Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7(4):941–948. doi: 10.1111/j.1600-6143.2007.01736.x. [DOI] [PubMed] [Google Scholar]