Abstract
Background/aim
Post-Transarterial Chemoembolization (TACE) Liver Failure (LF) is common in patients with Hepatocellular Carcinoma (HCC). No definitive objective parameters predict its occurrence. We assessed the role of Indocyanine Green (ICG) in prediction of post-TACE LF.
Methods
Consecutive HCC patients with Child A/B class, categorized as Barcelona Clinic Liver Cancer (BCLC) staging A/B, were included between August 2012 and July 2014. All underwent ICG dynamics: Plasma Disappearance Rate (PDR) was recorded on the day of TACE. Area Under Receiver Operator Characteristic Curve (AUROC) of ICG-PDR was compared with existing prognostic scores: Model for End Stage Liver Disease (MELD), MELD-Na and Child-Turcotte-Pugh (CTP) using Hanley and McNeil method.
Results
A total of 43 patients, mean age (±sd) 55.1 ± 12.8 years were included; 35 (81.4%) patients were males. Post-TACE LF developed after 17 (28.8%) of 59 procedures. Patients with post-TACE LF had significantly elevated baseline bilirubin (P = 0.006), alkaline phosphatase (P = 0.040) and prolonged international normalized ratio (P = 0.004). The median prognostic scores were higher in patients with post-TACE LF (CTP 7 vs 6; P < 0.001 and MELD 10.5 vs 6.3; P = 0.005). There was no difference in the MELD-Na score. ICG-PDR values were lower in those patients who developed post-TACE LF (7.4%/min vs 10.6%/min; P = 0.008). AUROC for ICG-PDR was 0.72 and a cut-off value <9.25%/min predicted the development of post-TACE LF with a sensitivity, specificity, positive predictive value and negative predictive value of 64.7%, 61.9%, 40.7% and 81.2%, respectively. There were no differences in the AUROC between ICG-PDR and other prognostic markers (Hanley and McNeil, P: 0.244–0.900).
Conclusion
ICG-PDR performs similar to MELD, MELD-Na and CTP score for predicting development of post-TACE LF.
Abbreviations: AUROC, Area Under Receiver Operator Characteristic Curve; BCLC, Barcelona Clinic Liver Cancer; HCC, Hepatocellular Carcinoma; ICG, Indocyanine Green; LF, Liver Failure; MELD, model For End Stage Liver Disease; PDR, Plasma Disappearance Rate; TACE, Trans-arterial Chemoembolization
Keywords: hepatitis B virus, ACLF, prognosis, treatment
Hepatocellular Carcinoma (HCC) is an important cause of mortality across the world.1 A recent large representative survey from India reported liver cancer to be the 4th most common cause of cancer-related deaths in males, and 8th most common cause in females.2 The incidence of HCC and associated mortality are increasing across the world, and India is no exception. Large proportions of patients are diagnosed at intermediate and advanced stages, and thus cannot undergo curative treatment.3 In the absence of curative options, Transarterial Chemoembolization (TACE) is a useful palliative modality, which improves survival and quality of life in these patients.
TACE involves intra-arterial infusion of a viscous emulsion with lipiodol and chemotherapeutic drug followed by embolization of blood vessels with gelatin sponge particles or other embolic agents, resulting in a combined cytotoxic and ischemic effect on the tumor cells. Optimal patient selection is important, as the complications associated with TACE may offset the benefits in life expectancy. Post-TACE Liver Failure (LF) has been reported in up to 60% of cases.4 However, the reported mortality rates are low in large series, mainly due to the fact that advanced liver disease is usually considered as a contraindication for TACE.5 Advanced liver disease is associated with poorer outcomes post TACE.6 Hsin et al. reported Child-Turcotte-Pugh (CTP) class B and gastrointestinal bleeding to be associated with increased risk of LF after TACE.7 A large study from Korea reported elevated total bilirubin and presence of right or main PVT to be associated with LF.8
Indocyanine Green (ICG) is a synthetic dye, which is eliminated by the liver without extrahepatic metabolism and excretion. A non-invasive method similar to pulse oxymetry assesses the ICG dynamics in the circulation. Plasma Disappearance Rate (PDR) of ICG has been used as a marker for assessment of liver function and has been shown to be useful in predicting the operative risk before hepatectomy,9 perioperative evaluation of donor liver function,10 prediction of early post operative complications in liver transplant patients11, 12 predicting outcomes in acute LF,13 decompensated hepatitis B cirrhosis14 and assessment of liver function reserve in critically ill patients.15 Other tests which are used to assess the severity of underlying liver disease include CTP,16 Model for End Stage Liver Disease (MELD)17 and MELD-Na.18 There is presently no data available on the role of ICG in prediction of post-TACE LF. Therefore, the aim of the current study was to evaluate the role of ICG as an objective predictor of development of post-TACE LF, and to compare it with existing scores.
Patients and Methods
Study Design and Patient Selection
In this prospective cohort study, all consecutive patients diagnosed with HCC undergoing TACE as part of standard therapy between August 2012 and July 2014 were included. Inclusion criteria were age more than 18 years, Barcelona Clinic Liver Cancer staging classification (BCLC) stage A and B. Exclusion criteria were pregnancy, BCLC stage C and D, contraindication for ICG (iodine allergy), underlying cardiovascular and respiratory diseases, emergency TACE for tumor rupture, patients lost to follow-up and presence of renal failure.
Definitions
Cirrhosis was diagnosed in the presence of typical findings on imaging, ascites, hypersplenism, esophageal/gastric varices or hepatorenal syndrome.19 HCC was diagnosed on the basis of typical features on an imaging modality—triple phase CT or contrast enhanced MRI.5 BCLC staging20 and performance status were assessed as per the described criteria.21 Post-TACE LF was defined as occurrence of any of the following: new onset hepatic encephalopathy, increase in ascites, or increase in bilirubin >0.9 mg/dl within 2 weeks of TACE.4 CTP, MELD and MELD-Na were calculated as described previously.16, 17, 18
ICG Estimation
LiMON technology was used to calculate the ICG elimination rate. This technique involves estimation of ICG dynamics noninvasively by means of a finger probe (pulse spectrophotometry). The ICG-PDR was estimated as per the standard manufacturer's instructions. First, the ICG (each vial containing 25 mg) was reconstituted with 5 ml of sterile water. Thereafter, the ICG machine was started and ICG was injected into the patient's antecubital vein at a dose of 0.25 mg/kg, once the message “inject XX mg” was displayed on the machine. The intravenous catheter was flushed with saline solution after injection of the ICG. ICG-PDR and ICG Retention at 15 min (R15) values displayed on the ICG machine were recorded. The ICG dynamics were assessed on the same day as, and before, the TACE procedure.
All patients were admitted a day before the procedure. Super-selective TACE was performed after percutaneous access through the common femoral artery. The technique has been described elsewhere.22 During the procedure, 60 mg epirubicin mixed with lipiodol (10 ml if tumor <5 cm and 20 ml if tumor >5 cm) was injected into the feeding artery, followed by gel foam embolization. None of the patients were treated with drug-eluting beads.
Data Collection
All patients underwent blood investigations including a hemogram, International Normalized Ratio (INR), liver function tests, kidney function tests, alpha-fetoprotein and an endoscopy prior to the procedure. The etiological work up included HBsAg, total anti-HBcAb, Hepatitis B Virus (HBV) DNA, anti-HCV Ab, Hepatitis C Virus (HCV) RNA, ANA, anti-LKM1, ASMA, blood sugars, lipid profile and fasting serum insulin levels. Post-TACE, all patients were monitored for development of complications including local complications, post-TACE syndrome and LF. All procedural complications were managed as per the standard management protocols. Post-TACE liver functions, hemogram and INR estimation was done daily for 5 consecutive days. Thereafter, assessment was made at 14 days—or earlier, as clinically indicated—for development of LF. Ultrasound was done to assess for the development of ascites. All TACE procedures were assessed separately.
Statistical Analysis
The normally distributed variables were expressed as mean ± Standard Deviation (sd), and continuous variables with skewed distribution as median (range). Categorical data was presented as proportions. The qualitative data were compared using chi-square; quantitative data were compared with Student's t-tests or Mann–Whitney U test, as required. The Receiver Operating Characteristic (ROC) curves were plotted for ICG-PDR, CTP, MELD and MELD-Na with LF as outcome. The sensitivity, specificity, positive predictive value, negative predictive value and likelihood ratios were calculated. The comparison of Area Under ROC Curve (AUROC) was performed using the Hanley and McNeil method. P value of <0.05 was considered statistically significant. The relationship between ICG-PDR and other variables was assessed using Pearson's correlation coefficient. The data was analyzed using IBM SPSS Statistics software (version 20.0, Chicago, IL, USA), and MedCalc software (version 15.11.4, MedCalc Software, Ostend, Belgium).
Results
A total of 51 patients underwent 72 sessions of TACE during the study period. Of these, 43 patients were included in the study (total of 59 sessions of TACE); 8 patients were excluded: 4 patients had portal vein branch thrombosis, 3 patients were lost to follow-up and 1 patient underwent emergency TACE for tumor rupture.
Demography and Clinical Presentation
The mean age of patients was 55.1 ± 12.8 years and 35 (81.4%) were males. All patients had underlying cirrhosis. The commonest etiology of cirrhosis was hepatitis B virus in 19 (44.2%), followed by hepatitis C virus in 11 (25.6%), non-alcoholic steatohepatitis and alcohol in 4 (9.3%) each, combined HBV and alcohol in 3 (7.0%), co-infection (with HBV and HCV) and cryptogenic in 1 (2.3%) patient each. The most common clinical symptoms were pain abdomen in 46.5%, weight loss in 44.2% and fever in 16.3% (Table 1).
Table 1.
Baseline Demographic, Clinical Presentation and Laboratory Parameter of HCC Patients Managed with TACE (n = 43).
| Parameter | Value |
|---|---|
| Age (years), mean ± sd | 55.1 ± 12.8 |
| Sex (M:F) | 35:8 |
| Etiology | |
| HBV | 19 (44.2%) |
| HCV | 11 (25.6%) |
| NASH | 4 (9.3%) |
| Alcohol | 4 (9.3%) |
| HBV and alcohol | 3 (7.0%) |
| HBV and HCV co-infection | 1 (2.3%) |
| Cryptogenic | 1 (2.3%) |
| Clinical presentation | |
| Abdominal pain | 20 (46.5%) |
| Weight loss | 19 (44.2%) |
| Fever | 7 (16.3%) |
| Bilirubin (mg/dl) | 1.5 (1.0–2.5) |
| AST (IU/L) | 161 (92–254) |
| ALT (IU/L) | 107 (59–176) |
| Albumin (g/dl) | 3.4 (2.9–3.8) |
| Alkaline phosphatase (IU/L) | 285 (220–421) |
| Sodium (mEq/L) | 134 (132–138) |
| Potassium (mEq/L) | 4.1 (3.7–4.5) |
| Hemoglobin (g/dl) | 10.8 (9.6–12.0) |
| Total leukocyte count (per mm3) | 7500 (5100–9900) |
| Platelet count (×103 per mm3) | 120 (80–148) |
| Creatinine (mg/dl) | 0.9 (0.7–1.0) |
| Alpha-fetoprotein (ng/ml) | 19.1 (5.2–387) |
| INR | 1.3 (1.2–1.4) |
| CTP score | 6 (5–7) |
| MELD | 7.4 (5.4–11.2) |
| MELD-Na | 12.6 (9.2–15.5) |
| ICG-PDR (%/min) | 9.7 (6.7–12.5) |
| ICG-R15 (%) | 23.3 (15.2–36.6) |
All values are expressed as n (%) or median (IQR) unless otherwise specified. Abbreviations: HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; NASH, Non-Alcoholic Steatohepatitis; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; INR, International Normalized Ratio; CTP, Child-Turcotte-Pugh; MELD, model for end stage liver disease; MELD-Na, model for end stage liver disease-sodium; ICG-PDR, indocyanine green-plasma disappearance rate.
Child Class and Tumor Characteristics
Twenty four (55.8%) patients were Child A and 19 (44.2%) were Child B class. Single lesion was present in 21 (48.5%), 2–4 lesions in 6 (14.7%) and ≥5 in 16 (37.2%). The mean size ± sd of largest lesion was 5.9 ± 4.4 cm.
Predictors of Post-TACE LF
Of the 43 patients, 30 (69.7%) patients were BCLC stage B and 13 (30.2%) were BCLC stage A. Post-TACE syndrome developed after 46 (78.0%) out of the 59 sessions of TACE. Post-TACE LF developed after 17 (28.8%) out of the 59 sessions of TACE. None of the patients died post procedure. Patients with post-TACE LF had higher bilirubin (P = 0.006), alkaline phosphatase (P = 0.040) and prolonged prothrombin time (P = 0.004). Similarly, as expected, CTP score (P < 0.001) and MELD score (P = 0.005) were higher in patients with post-TACE LF, whereas there was no difference in the MELD-Na score. The median ICG-PDR was significantly lower in patients with post-TACE LF (7.4 vs 10.6, P = 0.008). As expected, the median ICG-R15 was higher in post-TACE LF (33.0 vs 21.4, P = 0.008). Fifteen out of the 17 episodes of post-TACE LF occurred in Child class B patients and remaining 2 episodes in Child class A patients. There were no differences in age, sex, etiology, hemoglobin, total leukocyte count, serum creatinine, sodium, potassium, albumin, aspartate aminotransferase, alanine aminotransferase and alpha-fetoprotein levels (Table 2). Also, there were no differences in the number and size of the lesions between the 2 groups. Post-TACE LF was seen to be more frequently associated with post-TACE syndrome (P = 0.084). Sixteen episodes of post-TACE LF occurred in patients who had developed post-TACE syndrome (n = 46), while only one episode occurred in a patient who did not experience post-TACE syndrome (n = 13). A significant rise in bilirubin occurred in patients who developed post-TACE LF (n = 17)—the median bilirubin (IQR) increased from 1.4 (0.9–2.5) to 2.5 (1.2–3.8), P < 0.001, and 13/17 (76.4%) had increase in ascites, but none developed hepatic encephalopathy.
Table 2.
Comparison of Demographic Profile and Laboratory Parameters of Patients With and Without Post-TACE Liver Failure.
| Parameter | Post TACE liver failure |
P value | |
|---|---|---|---|
| Present (n = 17) | Absent (n = 42) | ||
| Age (years), mean ± sd | 49.1 ± 11.4 | 54.5 ± 13.3 | 0.142 |
| Sex (M:F) | 15:2 | 35:7 | 1.0 |
| Etiology | 0.322 | ||
| HBV | 7 (41.2%) | 24 (57.1%) | |
| HCV | 7 (41.2%) | 7 (16.7%) | |
| NASH | 1 (5.9%) | 3 (7.1%) | |
| Alcohol | 2 (11.8%) | 2 (4.8%) | |
| HBV and alcohol | 0 | 4 (9.5%) | |
| HBV and HCV co-infection | 0 | 1 (2.4%) | |
| Cryptogenic | 0 | 1 (2.4%) | |
| Clinical presentation | |||
| Abdominal pain | 9 (52.9%) | 21 (50.0%) | 1.0 |
| Weight loss | 6 (35.3%) | 20 (47.6%) | 0.563 |
| Fever | 6 (35.3%) | 5 (11.9%) | 0.062 |
| Bilirubin (mg/dl) | 1.4 (1.0–2.5) | 0.9 (0.6–1.3) | 0.006 |
| AST (IU/L) | 62 (49–122) | 55 (35.5–89) | 0.388 |
| ALT (IU/L) | 33 (21–81) | 43.5 (24.7–62) | 0.586 |
| Albumin (g/dl) | 3.6 (3.1–3.7) | 3.7 (3.3–4.3) | 0.109 |
| Alkaline phosphatase (IU/L) | 461 (300–482) | 290 (207–416) | 0.040 |
| Sodium (mEq/L) | 137 (135–140.5) | 137.5 (134–140) | 0.980 |
| Potassium (mEq/L) | 4.4 (3.2–4.8) | 4.0 (3.6–4.5) | 0.574 |
| Hemoglobin (g/dl) | 10.8 (9.1–12.2) | 11.6 (10.6–12.6) | 0.238 |
| Total leukocyte count (per mm3) | 4300 (2400–7200) | 5700 (4350–7425) | 0.186 |
| Platelet count (×103 per mm3) | 97 (50–160) | 130 (76.7–171.2) | 0.389 |
| Creatinine (mg/dl) | 0.9 (0.7–1.1) | 0.8 (0.7–1.0) | 0.794 |
| Alpha-fetoprotein (ng/ml) | 164 (8.1–2290) | 78.5 (8.1–714.7) | 0.586 |
| INR | 1.4 (1.3–1.5) | 1.3 (1.2–1.4) | 0.004 |
| CTP score | 7 (7–7.5) | 6 (5–7) | <0.001 |
| MELD | 10.5 (7.4–13.8) | 6.3 (4.9–10.4) | 0.005 |
| MELD-Na | 13.7 (12.1–16.5) | 12.5 (8.8–15.0) | 0.141 |
| ICG-PDR (%/min) | 7.4 (4.8–10.7) | 10.6 (7.5–15.3) | 0.008 |
| ICG-R15 (%) | 33.0 (20.3–48.7) | 21.4 (10.3–32.6) | 0.008 |
Abbreviations: HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; NASH, non-alcoholic steatohepatitis; AST, aspartate aminotransferase; ALT, alanine transaminase; INR, international normalized ratio; CTP, Child-Turcotte-Pugh; MELD, model for end stage liver disease; MELD-Na, model for end stage liver disease-sodium; ICG-PDR, indocyanine green-plasma disappearance rate.
Prediction of Post-TACE LF with ICG and Other Prognostic Scores
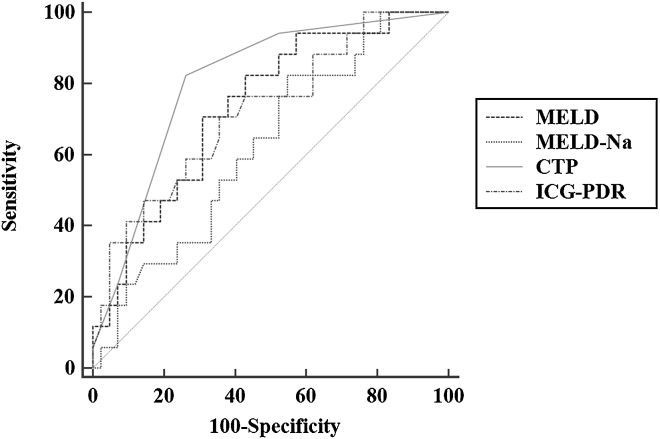
The area under the ROC curve for ICG-PDR as a predictor of post-TACE LF was 0.721 (0.589–0.830). A cut off value of 9.25 predicted the development of LF with a sensitivity of 64.3%, specificity 64.7%, positive predictive value 42.31%, negative predictive value 81.82%, likelihood ratio (+) 1.81 and likelihood ratio (−) 0.55 (Figure 1). The details of the other prognostic scores are shown in Table 3. No significant differences in AUROC were observed on comparison of ICG-PDR with other prognostic scores like MELD, MELD-Na and CTP (Hanley and McNeil P value 0.244–0.900). The Pearson correlation coefficient of ICG-PDR with CTP was −0.552, P < 0.001, with MELD −0.388, P = 0.002 and MELD-Na was −0.107, P = 0.418.
Figure 1.
Receiver operating characteristic (ROC) curves of various prognostic parameters for prediction of post-TACE liver failure. The AUROC for MELD (0.73), MELD-Na (0.62), CTP (0.80) and ICG-PDR (0.72); Hanley and McNeil P value 0.244–0.900.
Table 3.
Area Under Curve, Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value for MELD, MELD-Na, CTP and ICG-PDR for Predicting Development of Liver Failure.
| Parameter (cut-off) | AUROC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ | LR− |
|---|---|---|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||
| MELD (>8.6) | .73 | 64.7 | 69.0 | 45.8 | 82.8 | 2.09 (1.18–3.70) | 0.51 (0.26–1.0) |
| (.59–.86) | (38.3–85.8) | (52.9–82.4) | (25.5–67.2) | (66.3–93.4) | |||
| MELD-Na (>12.9) | .62 | 58.8 | 57.1 | 35.7 | 77.4 | 1.37 (0.81–2.33) | 0.72 (0.39–1.35) |
| (.47–.77) | (32.9–81.5) | (40.9–72.3) | (18.6–55.9) | (58.9–90.4) | |||
| CTP (>6) | .80 | 82.3 | 73.8 | 56.0 | 91.1 | 3.14 (1.81–5.47) | 0.24 (0.08–0.68) |
| (.68–.92) | (56.6–96.2) | (57.9–86.1) | (34.9–75.6) | (76.3–98.1) | |||
| ICG-PDR (<9.25%/min) | .72 | 64.7 | 61.9 | 40.7 | 81.2 | 1.70 (1.01–2.86) | 0.57 (0.29–1.13) |
| (.57–.86) | (38.3–85.8) | (45.6–76.4) | (22.4–61.2) | (63.6–92.8) | |||
Abbreviations: MELD, Model for End Stage Liver Disease; MELD-Na, Model for End Stage Liver Disease-Sodium; CTP, Child-Turcotte-Pugh; ICG-PDR, Indocyanine Green-Plasma Disappearance rate.
Discussion
Post-TACE LF occurs commonly in HCC patients. The questions of essence are—which patients are at risk of deterioration and what factors predict the development of LF post procedure? There is no single objective parameter to accurately predict the development of this complication. In this study, we evaluated the utility of ICG-PDR in predicting post-TACE LF in patients with HCC. We found a fair overall accuracy of ICG-PDR in predicting this outcome.
ICG is a synthetic dye, which is exclusively removed by the liver and excreted in the bile. It is not absorbed in the intestine and is excreted unchanged with no enterohepatic recirculation. This property makes it an ideal agent for assessment of liver reserve. ICG measured by pulse dye densitometry (LiMON) correlates well with the blood sample estimation.23 ICG-PDR is defined as the change of ICG concentration over time (in percent per minute), and this reflects the amount of the dye which is eliminated as a percent of the initial value. The normal range of ICG-PDR is between 18% and 25%/min. ICG has been shown to be of use in predicting operative risk before hepatectomy,9 perioperative evaluation of donor liver function,10 prediction of early post operative complications in liver transplant patients,11, 12 predicting outcomes in acute LF13 and patients with decompensated cirrhosis14 and assessment of liver function reserve in critically ill patients.15 ICG-PDR predicted post-TACE LF with low sensitivity (64.7%) and specificity (61.9%). An important observation was a high negative predictive value, suggesting that most patients with ICG-PDR value above 9.25% do not develop post-TACE LF (82% of our cases). The AUROC for ICG-PDR was 0.72, suggesting a fair accuracy in predicting LF. A prior study in patients with acute LF reported PDR ≤6.3%/min to predict the outcome with a sensitivity and specificity of 85.7% and 88.9%, respectively. On a linear scale the lower the value of PDR worse the underlying liver functional status. The lower PDR in acute LF is expected due to the underlying massive necrosis which occurs, whereas there are still functional hepatocytes in cirrhosis. It fared similar to the existing prognostic scores which are used for triaging patients at risk for developing post-TACE LF. Moreover, the liver damage grade classification proposed by the Liver Cancer Study Group of Japan (LCSGJ) includes ICGR-15, instead of hepatic encephalopathy.24 Rest of the parameters are similar to Child–Pugh scoring system. This scoring system has been shown to be useful in assessing the functional reserve capacity of liver in patients undergoing surgery. The advantages of ICG-PDR include objectivity of results and ease in measurement using the pulse oxymetry technique.
Post-TACE survival rates at 1, 3, 5 and 7 years have been reported as 82%, 47%, 26% and 16%, respectively. The reported mortality after the initial TACE procedure is low (0.5%).6 The incidence of post-TACE LF is variable and has been reported in up to 60% of patients.4 In our cohort, although the number of patients was small with relatively short follow-up, there was no post-TACE mortality. The mortality and morbidity associated with post-TACE complications like LF can be reduced by appropriate patient selection, especially in high-risk patients. The risk factors include portal vein thrombosis, high levels of aspartate aminotransferase, bilirubin, and alpha-fetoprotein, low albumin and sodium, high dosage of cisplatin, advanced stage of cirrhosis.8, 25, 26 We found bilirubin, INR, phosphatase levels to be associated with development of post-TACE LF (Table 2). In our study, the incidence of post-TACE LF was relatively low (28.8%) as compared to previous studies. This is possibly in part due to the fact that we excluded patients who are at high risk of complications i.e. patients with BCLC C (those with portal vein thrombosis) and D. Despite this, the frequency of LF was significant. Hence, there is a need for additional tests to predict outcome and triage patients effectively.
Our results indicate that ICG-PDR fairs similar to MELD and CTP scores in the prediction of LF (Table 3, Figure 1). Various quantitative liver function tests have been developed to assess the functional capacity of the liver, though not often used in routine day-to-day practice. These include galactose elimination capacity, caffeine clearance, Monoethylglycinexylidide (MEGX) and aminopyrine breath test. Among these, ICG holds promise for assessing liver function non-invasively in a clinical setup.
The present study has certain limitations, the most important of which is the small sample size. This study was exploratory to assess the role of ICG in predicting post-TACE LF. These results need to be validated at other centers. Also, we chose a select population of Child class A and B patients, who are at lower risk of development of LF. Post-TACE LF may also arise due to technical issues such as non-target embolization during TACE, which we could not account for. Future studies should include patients with Child B to assess the utility of ICG in predicting post-TACE LF. Third, while we assessed ICG at baseline, a serial assessment would probably be more beneficial in assessing the dynamic changes occurring post-TACE, and this needs to be assessed in prospectively designed studies.
In conclusion, patients with advanced liver disease and CTP class B are more predisposed to develop post-TACE LF. ICG performs similar to MELD and CTP for predicting post-TACE LF.
Funding
Grant support: All India Institute of Medical Sciences Intramural Research Grant, New Delhi, India.
Author Contributions
1. Shalimar, acquisition of data, study design and concept, data analysis, interpretation of data, drafting of manuscript.
2. Sushil Jain, acquisition of data and drafting of manuscript.
3. Shivanand Ramachandra Gamanagatti, intervention radiologist, acquisition of data and management of patients.
4. Saurabh Kedia, acquisition of data.
5. Bhaskar Thakur, analysis of data.
6. Baibaswata Nayak, acquisition of data.
7. Harpreet Kaur, acquisition of data.
8. Deepak Gunjan, acquisition of data.
9. Shashi Bala Paul, acquisition of data.
10. Subrat Kumar Acharya, study design and concept, interpretation of data, critical revision of manuscript for important intellectual content.
Conflicts of Interest
The authors have none to declare.
References
- 1.Yang J.D., Roberts L.R. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7(August (8)):448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dikshit R., Gupta P.C., Ramasundarahettige C. Cancer mortality in India: a nationally representative survey. Lancet. 2012;379(May (9828)):1807–1816. doi: 10.1016/S0140-6736(12)60358-4. [DOI] [PubMed] [Google Scholar]
- 3.Paul S.B., Chalamalasetty S.B., Vishnubhatla S. Clinical profile, etiology and therapeutic outcome in 324 hepatocellular carcinoma patients at a tertiary care center in India. Oncology. 2009;77(3–4):162–171. doi: 10.1159/000231886. [DOI] [PubMed] [Google Scholar]
- 4.Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med. 1995;332(May (19)):1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatol Baltim Md. 2011;53(March (3)):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takayasu K., Arii S., Ikai I. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131(August (2)):461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Hsin I.-F., Hsu C.-Y., Huang H.-C. Liver failure after transarterial chemoembolization for patients with hepatocellular carcinoma and ascites: incidence, risk factors, and prognostic prediction. J Clin Gastroenterol. 2011;45(Jul (6)):556–562. doi: 10.1097/MCG.0b013e318210ff17. [DOI] [PubMed] [Google Scholar]
- 8.Jeon S.H., Park K.S., Kim Y.H. Incidence and risk factors of acute hepatic failure after transcatheter arterial chemoembolization for hepatocellular carcinoma. Korean J Gastroenterol. 2007;50(September (3)):176–182. [PubMed] [Google Scholar]
- 9.Okochi O., Kaneko T., Sugimoto H., Inoue S., Takeda S., Nakao A. ICG pulse spectrophotometry for perioperative liver function in hepatectomy. J Surg Res. 2002;103(March (1)):109–113. doi: 10.1006/jsre.2001.6328. [DOI] [PubMed] [Google Scholar]
- 10.Mandell M.S., Wachs M., Niemann C.U., Henthorn T.K. Elimination of indocyanine green in the perioperative evaluation of donor liver function. Anesth Analg. 2002;95(November (5)):1182–1184. doi: 10.1097/00000539-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Levesque E., Saliba F., Benhamida S. Plasma disappearance rate of indocyanine green: a tool to evaluate early graft outcome after liver transplantation. Liver Transpl. 2009;15(October (10)):1358–1364. doi: 10.1002/lt.21805. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh C.-B., Chen C.-J., Chen T.-W. Accuracy of indocyanine green pulse spectrophotometry clearance test for liver function prediction in transplanted patients. World J Gastroenterol. 2004;10(August (16)):2394–2396. doi: 10.3748/wjg.v10.i16.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merle U., Sieg O., Stremmel W., Encke J., Eisenbach C. Sensitivity and specificity of plasma disappearance rate of indocyanine green as a prognostic indicator in acute liver failure. BMC Gastroenterol. 2009;9:91. doi: 10.1186/1471-230X-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng X.-P., Zhao J., Chen Y. Comparison of the ability of the PDD-ICG clearance test, CTP, MELD, and MELD-Na to predict short-term and medium-term mortality in patients with decompensated hepatitis B cirrhosis. Eur J Gastroenterol Hepatol. 2016;28(April (4)):444–448. doi: 10.1097/MEG.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakka S.G., van Hout N. Relation between indocyanine green (ICG) plasma disappearance rate and ICG blood clearance in critically ill patients. Intensive Care Med. 2006;32(May (5)):766–769. doi: 10.1007/s00134-006-0109-6. [DOI] [PubMed] [Google Scholar]
- 16.Pugh R.N., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(August (8)):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 17.Malinchoc M., Kamath P.S., Gordon F.D., Peine C.J., Rank J., ter Borg P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatol Baltim Md. 2000;31(April (4)):864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 18.Biggins S.W., Kim W.R., Terrault N.A. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130(May (6)):1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Brown J.J., Naylor M.J., Yagan N. Imaging of hepatic cirrhosis. Radiology. 1997;202(January (1)):1–16. doi: 10.1148/radiology.202.1.8988182. [DOI] [PubMed] [Google Scholar]
- 20.Llovet J.M., Fuster J., Bruix J., Barcelona-Clínic Liver Cancer Group The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(February (2 (suppl 1))):S115–S120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 21.Oken M.M., Creech R.H., Tormey D.C. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(December (6)):649–655. [PubMed] [Google Scholar]
- 22.Paul S.B., Gamanagatti S., Sreenivas V. Trans-arterial chemoembolization (TACE) in patients with unresectable hepatocellular carcinoma: experience from a tertiary care centre in India. Indian J Radiol Imaging. 2011;21(April (2)):113–120. doi: 10.4103/0971-3026.82294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purcell R., Kruger P., Jones M. Indocyanine green elimination: a comparison of the LiMON and serial blood sampling methods. ANZ J Surg. 2006;76(February (1–2)):75–77. doi: 10.1111/j.1445-2197.2006.03643.x. [DOI] [PubMed] [Google Scholar]
- 24.Mizuguchi T., Kawamoto M., Meguro M., Hui T.T., Hirata K. Preoperative liver function assessments to estimate the prognosis and safety of liver resections. Surg Today. 2014;44(January (1)):1–10. doi: 10.1007/s00595-013-0534-4. [DOI] [PubMed] [Google Scholar]
- 25.Min Y.W., Kim J., Kim S. Risk factors and a predictive model for acute hepatic failure after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Liver Int. 2013;33(February (2)):197–202. doi: 10.1111/liv.12023. [DOI] [PubMed] [Google Scholar]
- 26.Chan A.O., Yuen M.-F., Hui C.-K., Tso W.-K., Lai C.-L. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94(March (6)):1747–1752. doi: 10.1002/cncr.10407. [DOI] [PubMed] [Google Scholar]