Abstract
Objective
To assess impact of Direct Acting Antiviral (DAA) therapies for treatment of Hepatitis C Virus (HCV) genotypes 1, 3 and 4 in a real-world cohort from India.
Methods
Adults with chronic HCV infection treated with Sofosbuvir (SOF) and Ledipasvir (LDV) (genotypes 1 and 4) or SOF and Daclatasvir (DCV) (genotype 3), with or without Ribavirin (RBV) between December 2015 and December 2016 were included. The primary endpoint was Sustained Virological Response at Post-treatment Week 12 (SVR12).
Results
Of the 648 patients, 181 received SOF/LDV (65 with RBV) and 467 received SOF/DCV (135 with RBV). Most patients were males (65.4%), aged 41–60 years (49.4%) and treatment-naïve (92.6%). Genotype 3 (72.1%) was most common, followed by genotypes 1 (22.4%) and 4 (5.6%). Forty two percent patients (n = 271) had cirrhosis (112 patients were decompensated). SVR12 (modified intention-to-treat) was achieved by 98.1% of patients (512/522) (100% in genotypes 1 and 4, and 97.3% (362/372) in genotype 3). On intention to treat analysis, SVR12 was 88.1% (512/581) [genotype 1—96.8% (121/125), genotype 3—85.2%, genotype 4—93.5% (29/31)]. Seventy patients had treatment failure (non response in 6, virological breakthrough in 2, 10 patients relapsed, 2 died and 50 were lost to follow up). High SVR was observed regardless of HCV genotype, presence of cirrhosis or past history of treatment. No major adverse events warranting discontinuation of treatment were noted.
Conclusions
DAA therapy for HCV genotypes 1, 3 and 4 achieves high SVR rates in all patients, including those with cirrhosis and previous non-responders.
Abbreviations: DAA, Direct Acting Antiviral; DCV, Daclatasvir; EASL, European Association of Study of the Liver (EASL); ETR, End of Treatment Response; HBV, Hepatitis B; HCC, Hepatocellular Carcinoma; HCV, Hepatitis C Virus; HIV, Human Immunedeficiency Virus; ITT, intention to treat; LDV, ledipasvir; mITT, modified Intention-to-Treat; RBV, Ribavirin; RVR, Rapid Virological Response; SOF, Sofosbuvir; SVR, Sustained Virological Response; SVR12, sustained virological response at Post-treatment Week 12
Keywords: hepatitis C, direct acting antivirals, real life experience
The introduction of oral combinations of Direct Acting Antivirals (DAAs) has quickly changed the landscape of Hepatitis C Virus (HCV) treatment in the last few years. Due to higher efficacy, safety and fewer side effects than interferon-based regimes, these DAAs have now become the standard of care for treatment of chronic HCV infection.1, 2, 3
Various clinical trials have shown high rates of Sustained Virological Response (SVR) after treatment with DAAs. In a Phase III open label study (ION-1) involving treatment naïve genotype 1 patients with or without cirrhosis, combination therapy with Sofosbuvir (SOF) and Ledipasvir (LDV) with or without Ribavirin (RBV) achieved SVR rates between 97% and 99%. ION-2 trial included genotype 1 patients who were treatment experienced and those with cirrhosis (20%) and SVR rates were 94% and 96% with SOF/LDV without and with RBV, respectively. The SVR rates increased to 99% in both groups when treatment duration was increased to 24 weeks.4 In patients with genotype 4, the SYNERGY trial showed SVR rates of 95% with SOF/LDV and RBV.5
Genotype 3 patients have been reported to have a relatively lower response rate with the DAAs. In a Phase III trial ALLY-3, SOF and Daclatasvir (DCV) given without RBV in treatment naïve patients achieved SVR rates of 97% and 58% in non-cirrhotic and cirrhotic patients, respectively. In patients who were treatment experienced, SVR rates were 94% and 69% in non-cirrhotic and cirrhotic patients, respectively.6 In the ALLY 3+ trial, treatment naïve and experienced patients with advanced fibrosis (METAVIR F3) and cirrhosis were treated with SOF, DCV and RBV for 12 and 16 weeks. SVR rates in patients with advanced fibrosis were 100% both after 12 and 16 weeks of therapy, while in cirrhotics SVR rates were 83% and 88% with 12- and 16-week therapy, respectively.7
As the clinical trials are conducted in controlled settings, the results derived from these may not be generalizable to the real world settings. The efficacy of the drugs in a real world setting is influenced by several factors like drug compliance, adverse events leading to discontinuation and other factors like cost and availability of the drugs. The present study was done to assess the efficacy and safety of DAAs and assess the impact of therapy in a real world setting in the northern state of Punjab, India.
Methods
Setting
This was a single centre, prospective, observational study evaluating the treatment outcomes in a real-world cohort of patients with chronic HCV genotypes 1, 3 and 4 who were treated with DAAs between December 2015 and December 2016.
Patients and Treatment
All patients diagnosed as chronic hepatitis C with or without cirrhosis, including those who were treatment naïve or previously exposed to interferon based regimens and were considered for all oral direct acting antiviral therapy were included in the present study. Patients who had co-infections with Hepatitis B (HBV) or Human Immune deficiency Viruses (HIV) were also included. Patients with chronic kidney disease were not offered therapy as there was a lack of data on safety of DAAs in this group during the study period. Other exclusion criteria were advanced liver disease (Child-Turcotte-Pugh (CTP) >13 or Model for End-stage Liver Disease (MELD) >20), portal vein thrombosis and patients with Hepatocellular Carcinoma (HCC) unfit for liver transplantation or locoregional therapies.
A detailed history and clinical examination was undertaken for all the patients. Anti-HCV antibody was tested for all patients by ELISA (ELISCAN HCV; RFCL Limited, Dehradun, India), quantification of HCV RNA was done by RT-PCR (COBAS TaqMan HCV Test 2.0; Roche Diagnostics Corporation, Indianapolis, IN, USA) and determination of genotype by real time PCR sequencing. High viral load was defined as HCV RNA >600,000 IU/ml. Complete blood counts, liver and renal biochemical tests, thyroid function tests, prothrombin time index, fasting blood sugar, α-fetoprotein, and ultrasound abdomen and fibroscan were also done. Cirrhosis was diagnosed on the basis of clinical, laboratory, radiological, endoscopic and/or histological criteria (where available). Liver stiffness values of >14.6 kPa (FibroScan®; Echosens, France) were considered to represent cirrhosis. CTP and MELD scores were calculated for patients with cirrhosis. Decompensated cirrhosis was defined as patients with CTP B/C or patients presenting with jaundice, ascites, encephalopathy and/or variceal bleeding.
The European Association of Study of the Liver (EASL) guidelines (2015) were followed for treatment and the regimes followed are summarized in Table 1.8 The patients were considered compliant if the antiviral drugs were not missed for 2 or more consecutive days. Drug list of all the patients was reviewed by two dedicated counsellors who explained about the possible interactions of DAA based therapy with other drugs before the start of treatment. Patients were advised to discontinue proton pump inhibitors (or take them with SOF) and consult the counsellor before starting any other treatment.
Table 1.
Regimens of Direct Acting Antivirals for Treatment of HCV Infection.
| Patients | Genotypes 1 and 4 | Genotype 3 | |
|---|---|---|---|
| Chronic hepatitis | Treatment naïve | SOFa/LDVb for 12 weeks | SOFa + DCVc for 12 weeks |
| Treatment experienced | SOFa/LDVb for 12 weeks | SOFa + DCVc + RBVd for 12 weeks | |
| SOFa + DCVc for 24 weeks if contraindications for use of RBV | |||
| Compensated cirrhosis (CTP A) | Treatment naïve | SOFa/LDVb for 12 weeks | SOFa + DCVc + RBVd for 24 weeks |
| Treatment experienced | SOFa/LDVb + RBVd for 24 weeks if negative predictors of response | ||
| Decompensated cirrhosis (CTP B, C up to 12 points) | SOFa/LDVb + RBVd for 12 weeks | SOFa + DCVc + RBVd for 24 weeks | |
| SOFa/LDVb for 24 weeks (if contraindications to use of RBV) |
SOF, Sofosbuvir 400 mg; LDV, Ledipasvir 90 mg; DCV, Daclatasvir 60 mg; RBV, Ribavirin 200 mg. a + b: fixed dose combination once a day; a + c: one tablet each daily; d: RBV 1000 mg/day if <75 kg, 1200 mg/day if >75 kg, in divided doses.
This study was approved by the Institutional Review Board at Dayanand Medical College and Hospital, Ludhiana (India).
Efficacy Assessment
The treatment efficacy was monitored at 4 weeks, at the end of therapy (12 or 24 weeks) and 12 weeks after the end of treatment for Rapid Virological Response (RVR), End of Treatment Response (ETR) and SVR, respectively. Non-response was defined as failure to achieve ETR, breakthrough as reappearance of HCV RNA at any time during treatment after a negative result or increase of 1 log 10 IU/ml from nadir and virological relapse as undetectable HCV RNA at the end of treatment, but failure to achieve SVR.
Safety Assessment
Patients were followed up on regular intervals for any adverse events or abnormal findings on physical examination and clinical laboratory tests. For patients who were treated with RBV based regimes, haemoglobin was monitored according to the CTCAE (Common Terminology Criteria for Adverse Events) and dose reduced by 200 mg each week if Hb was <10 g/dl, till the value rose to 10 g/dl or greater. RBV was withheld in patients with Hb <8.5 g/dl. The dose of DAAs remained unchanged.
Statistical Analysis
Patients were enrolled based on the clinical need for treatment and not the statistical considerations. The primary population for efficacy analysis [modified Intention-to-Treat (mITT)] included patients who started treatment and received at least one dose of the medicine and had no virological failure; those who were lost to follow up or had missing SVR data were excluded. In addition, efficacy was analyzed based on Intention to Treat (ITT) population which included all patients who received at least one dose of the planned regimen and had HCV RNA at 12 weeks post treatment (SVR12); patients who were still on treatment at the time of analysis were excluded. The data was analyzed using descriptive and inferential statistics. The measures of descriptive statistics used in this study were frequency and percentage distribution tables. Chi-square test was used as inferential statistics in the present study.
Results
Baseline Characteristics and Demographics
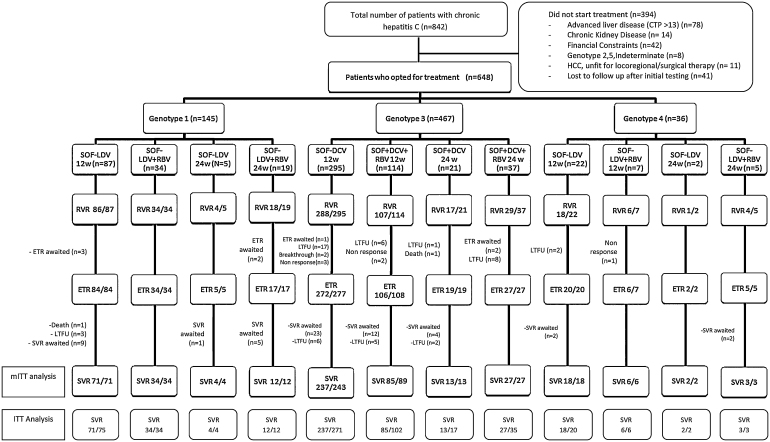
A total of 842 patients were diagnosed with HCV infection during the study period, of which 648 (77%) opted for treatment with DAAs (Figure 1). The mean age was 43.7 ± 14.1 years and a majority of patients were males (n = 424, 65.4%), in the age group of 41–60 years (n = 320, 49.4%). Forty-eight patients (7.4%) were treatment experienced. Patients were most commonly infected with genotype 3 (n = 467, 72.1%) and genotype 1 (n = 145, 22.4%) and 49.7% (n = 320) had high viral load. Several concurrent medical conditions were noted (Table 2), and 25.6% of the patients had history of concurrent significant alcohol intake. History of intravenous drug abuse was noted in 51 (7.9%) of the patients. Cirrhosis was present in 271 patients (41.8%), and among them a majority (n = 159, 58.7%) were compensated. HCC was noted in 11 patients, all of whom were eligible for locoregional therapies and thus offered DAAs with intent of reducing liver related morbidity and mortality and preventing recurrence of HCC after treatment. Coinfection with HBV was noted in 5 patients and three patients were post renal transplant recipients.
Figure 1.
Flow of patients with chronic hepatitis C treated with direct acting antivirals. mITT: all treated patients, except for those who were lost to follow-up, withdrew informed consent or withdrew for undocumented reasons; ITT: all patients who received ≥1 dose of the programme regimen, excluding those where ETR/SVR was awaited due to ongoing treatment; CTP, Child Turcotte Pugh; DCV, Daclatasvir; ETR, End of Treatment Response; HCC, Hepatocellular Carcinoma; ITT, Intention-to-Treat; LDV, Ledipasvir; LTFU, Lost to Follow up; mITT, Modified Intention-to-Treat; RBV, Ribavirin; RVR, Rapid Virological Response; SOF, Sofosbuvir; SVR12, Sustained Virological Response at Post-treatment Week 12.
Table 2.
Demographic Profile of Patients who Opted for Treatment (n = 648).
| Total patients | 648 |
| Age in years, mean (SD) | 43.7 ± 14.1 |
| <20 | 15 (2.31%) |
| 21–40 | 228 (35.19%) |
| 41–60 | 320 (49.38%) |
| >60 | 85 (13.12%) |
| Gender (males:females) | 424:224 |
| BMI | |
| <23 | 245 (37.8%) |
| 23–25 | 190 (29.3%) |
| >25 | 213 (32.9%) |
| Comorbidities/addictions | |
| Diabetes mellitus | 56 (8.6%) |
| Hypertension | 28 (4.3%) |
| Hypothyroidism | 7 (1.1%) |
| Bronchial asthma | 4 (0.6%) |
| Alcohol use | 166 (25.8%) |
| Smoker | 28 (4.3%) |
| IVDU | 51 (7.9%) |
| Coinfections | |
| HIV/HCV | 00 (0%) |
| HBV/HCV | 05 (0.8%) |
| Family history of CHC | 143 (22.1%) |
| Genotype | |
| Genotype 1 | 145 (22.4%) |
| 1a | 18 |
| 1b | 64 |
| Subtype not specified | 63 |
| Genotype 3 | 467 (72.1%) |
| 3a | 286 |
| 3b | 04 |
| Subtype not specified | 177 |
| Genotype 4 | 36 (5.6%) |
| Viral load | |
| <600,000 IU/l | 328 (50.6%) |
| >600,000 IU/l | 320 (49.4%) |
| Liver status | |
| Chronic hepatitis | 377 (58.2%) |
| Cirrhosis | 271 (41.8%) |
| HCC | 11 (1.7%) |
| CTP | |
| A | 159 (24.8%) |
| B | 92 (14.2%) |
| C | 20 (3.1%) |
| Treatment status | |
| Naïve | 600 (92.6%) |
| Experienced | 48 (7.4%) |
| Interferon based | 40 (6.2%) |
| SOF + RBV | 08 (1.2%) |
BMI: Body Mass Index; IVDU: Intravenous Drug Abuse; CHC: Chronic Hepatitis C; HIV: Human Immunodeficiency Virus; HBV: Hepatitis B Virus; HCC: Hepatocellular Carcinoma; CTP: Child Turcotte Pugh Score; SOF: Sofosbuvir; RBV: Ribavirin.
Efficacy Assessment
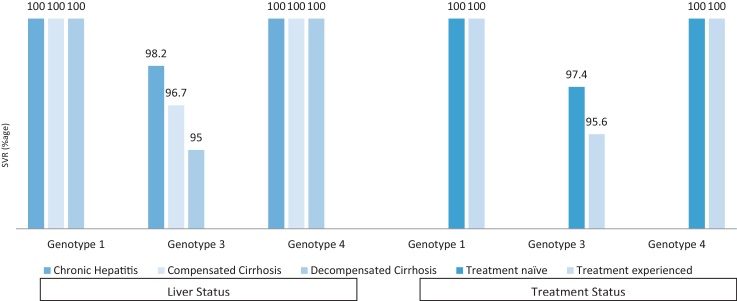
Of the 648 patients who started therapy, RVR was achieved in 612/648 (94.4%) patients and ETR in 597/605 (98.7%) patients (Figure 1). SVR (mITT) in genotypes 1 and 4 (treated with SOF/LDV with or without RBV) was 100% while in genotype 3 (treated with SOF/DCV with or without RBV) 97.3% of the patients achieved SVR. On ITT analysis overall SVR 12 was achieved in 512/581 (88.1%) patients. SVR 12 in genotypes 1, 3 and 4 on ITT analysis were 96.8% (121/125), 85.2% (362/425) and 93.5% (29/31), respectively. In patients with genotype 3, SVR rates were significantly higher in younger patients (age <40), those who achieved RVR and among the non-cirrhotic patients when compared to those with cirrhosis (Table 3, Figure 2). As compared to the patients who were previously treated with interferon-based regimes, treatment naïve patients had a higher SVR, though the difference was not statistically significant (Table 3, Figure 2). SVR rates were 100% in patients with genotypes 1 and 4, irrespective of the liver status and history of previous treatment (Table 3, Figure 2). SVR rates were similar among patients with low and high viral load, irrespective of the genotype.
Table 3.
Factors Determining SVR (Total = 512).
| Factors | Genotype 1 and 4 SOF + LDV ± RBV (n = 150) |
P value | Genotype 3 SOF + DCV ± RBV (n = 362) | P value |
|---|---|---|---|---|
| Viral factors | ||||
| Viral load | 0.359 | 0.515 | ||
| Low | 74 | 180 | ||
| High | 76 | 182 | ||
| Host factors | ||||
| Age | 0.846 | 0.016 | ||
| <40 years | 56 | 215 | ||
| >40 years | 94 | 147 | ||
| Gender | 0.310 | 0.880 | ||
| Male | 103 | 235 | ||
| Female | 47 | 127 | ||
| BMI | 0.555 | 0.648 | ||
| <23 | 57 | 136 | ||
| 23–25 | 19 | 80 | ||
| >25 | 74 | 146 | ||
| Alcohol use | 0.089 | 0.541 | ||
| Present | 47 | 86 | ||
| Absent | 103 | 276 | ||
| IVDU | 0.327 | 0.259 | ||
| Present | 13 | 29 | ||
| Absent | 137 | 333 | ||
| Comorbidities | 0.746 | 0.508 | ||
| Present | 30 | 59 | ||
| Absent | 120 | 303 | ||
| Status of liver | 0.841 | 0.016 | ||
| Chronic hepatitis | 90 | 217 | ||
| Cirrhosis | 60 | 145 | ||
| Treatment details | 0.370 | 0.742 | ||
| Naïve | 132 | 341 | ||
| Experienced | 18 | 21 | ||
| RVR 4 weeks | 0.727 | 0.013 | ||
| Present | 145 | 347 | ||
| Absent | 05 | 15 | ||
| Coinfection | 0.648 | 0.279 | ||
| HIV | 00 | 00 | ||
| HBV | 01 | 04 |
IVDU: Intravenous Drug Abuse; SOF: Sofosbuvir; LDV: Ledipasvir; DCV: Daclatasvir; RBV: Ribavirin; RVR: Rapid Virological Response; HIV: Human Immunodeficiency Virus; HBV: Hepatitis B Virus.
Figure 2.
Rates of sustained virological response (SVR 12) according to genotypes and liver status.
Treatment Failure
Virological failure was noted in 70 patients (2.8%). A majority of these (n = 50) were the patients who were lost to follow up during or after treatment. Six patients had non-response, 2 had breakthrough and 10 relapsed. Treatment failures were more commonly seen in genotype 3 (non response in 5, both patients with breakthrough and all 10 patients with relapse). Two patients died due to massive variceal bleed and intracranial bleed, respectively. Among these, one patient had achieved ETR and the other died while on treatment, after achieving RVR.
Adverse Events
Though a few patients experienced minor side effects, none of them had to stop therapy due to drug intolerance or adverse events. The side effects like fatigue and anaemia [seen in 53 patients (8.3%)] were more common in patients where RBV was added to the DAAs and improved with dose reduction.
Discussion
The introduction of DAAs has revolutionized the treatment of HCV infection over the last few years. Though various Phase III trials have reported high efficacy and safety of these all oral therapies for chronic hepatitis C, these outcomes need to be assessed in the real world where patient compliance, comorbidities and other factors can affect the SVR rates. We report here a real life experience from India with DAA-based regimes for treatment genotypes 1, 3 and 4 chronic hepatitis C.
A large number of patients diagnosed with hepatitis C (77%) opted for treatment. Overall, SVR 12 was achieved in 98.1% on modified ITT analysis (88.1% on ITT analysis). SVR rate in genotypes 1 and 4 was 100% (mITT) and that in genotype 3 was 97.3% (mITT). These SVR rates were higher than those in the Phase III trials and other real life studies. The response rates were consistently high in all patients, irrespective of their liver status or the regimen used. There were no major adverse effects warranting discontinuation of therapy though addition of RBV in patients with advanced liver disease caused anaemia in 53 patients (8.3%) requiring dose reduction.
Real world experiences have shown high SVR 12 rates (91–98%) in patients treated with SOF/LDV with or without RBV in patients with genotype 1, including patients with cirrhosis and those who were treatment experienced.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 However, most of these studies are from the developed countries like United States, United Kingdom, Italy, Spain and Germany.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 There are no real world studies from the Asian subcontinent and developing countries like India. Real world data for efficacy of SOF/DCV in patients with genotypes 3 and 4 are limited. In a recent French open-label, multicenter trial, SVR 12 rate in patients with genotype 3 treated with SOF/DCV with or without RBV was noted to be 89%.12 In the European compassionate use programme conducted in a real world setting before approval of DCV, 485 patients were included from 100 centres in Germany, Austria, the Netherlands, Sweden and Norway between April 2014 and April 2015.19 One hundred and two patients had genotype 3, 85% of whom had cirrhosis (52% with decompensation) and after treatment with SOF and DCV with or without RBV, SVR 12 was achieved in 88% of the patients. Another multicenter trial from Spain showed SVR rate of 94% with SOF/DCV with or without RBV in patients with genotype 3 who had cirrhosis.20 In a recent study from Spain, treatment of patients with genotype 4 with SOF/LDV ± RBV (n = 130) yielded high SVR rate (95.4%).21 Preliminary results from a study being conducted in Egypt on 170 patients with genotype 4 have shown high SVR 4 rates, however SVR 12 data were not available.22
Our study showed higher rates of SVR in genotypes 1, 3 and 4 when compared to the Phase III trials and the real world experiences from the West. The possible reasons for higher SVR could be related to both viral and host related factors. There were a fewer number of patients with HBV coinfection and none with HIV coinfection in our study. A larger number of patients with genotype 1b, which is considered to have a better therapeutic response with DAA therapy could also have contributed to higher rates of response. We also observed a higher prevalence of sub-genotype 3a, as noted from the neighbouring areas in Pakistan and these patients had an excellent response to treatment.23, 24 High SVR could also be attributed to host factors like a larger number of treatment naïve and non-cirrhotic patients.
The lower cost of SOF based protocols (as compared to Western world) and fewer side effects have increased the acceptability of therapy in developing nations like India. Our previous real life experience with pegylated interferon and RBV showed a much lower (one-third) acceptance for initiation of treatment and low SVR rates due to poor tolerability (6%) and non response (1%).25 In the present cohort of patients treated with DAAs, 77% of the patients diagnosed with hepatitis C infection opted for treatment and the therapy was well tolerated even in difficult to treat groups of patients like those with cirrhosis and past history of treatment failure. There were no major adverse events requiring discontinuation of therapy. Six patients (0.9%) had non response to therapy. None of these had achieved RVR and the duration of therapy in all these patients was 12 weeks (Figure 1). Extension of therapy to 24 weeks after a failed RVR in these patients may have increased the SVR rate.
Our study also presents the first real world experience of treatment of genotype 4 with SOF/LDV with or without RBV from Asia. SVR 12 rate in this group of patients (including cirrhotics and treatment experienced patients) was 100%. Though the number of patients with genotype 4 was small, these results support the efficacy of these regimens in patients with genotype 4 infection as shown in clinical trials.
The study had a few limitations. It was a single centre study. Subtyping into genotype 1a and 1b was not done in 43% of the patients. The number of patients with genotype 4 was small (though expected due to low prevalence of genotype 4 in Asia).
In conclusion, our data show that DAA-based regimens are safe and have a high success rate in the treatment of patients with genotypes 1, 3 and 4 HCV infection in real-life setting. This is independent of the liver status and past history of treatment. The availability of the dual-DAA regimens at low cost or free of cost at some areas under health care schemes, lower rates of major adverse events, ease of oral dosing has lead to increasing acceptability of treatment. Development of comprehensive strategies to improve awareness of hepatitis C, provision of universal screening with confidential testing for all, provision of drugs at low cost and counselling to reduce spread and reinfection in populations at high risk can help us achieve a world free of HCV.
Funding
None.
Conflicts of Interest
The authors have none to declare.
Patient Consent
Obtained.
Ethics Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 as revised in 2008. The study was approved by the institutional ethics committee.
Acknowledgements
The authors thank Mr Jatinder Negi for maintaining the database.
References
- 1.European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66(1):153–194. doi: 10.1016/j.jhep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 2.AASLD/IDSA HCV Guidance Panel Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 3.Majumdar A., Kitson M.T., Roberts S.K. Systematic review: current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol Ther. 2016;43(12):1276–1292. doi: 10.1111/apt.13633. [DOI] [PubMed] [Google Scholar]
- 4.Afdhal N., Reddy K.R., Nelson D.R. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor R., Kohli A., Sidharthan S. All oral treatment for genotype 4 chronic hepatitis C infection with sofosbuvir and ledipasvir: interim results from the NIAID Synergy Trial. Hepatology. 2014:60. http://insights.ovid.com/hepatology/hepa/2014/10/001/oral-treatment-genotype-chronic-hepatitis/240/01515467 [Accessed 12 February 2017] [Google Scholar]
- 6.Nelson D.R., Cooper J.N., Lalezari J.P. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61(4):1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leroy V., Angus P., Bronowicki J.-P. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY-3+) Hepatology. 2016;63(5):1430–1441. doi: 10.1002/hep.28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Association for Study of Liver EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63(1):199–236. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 9.No effect of proton pump inhibitor (PPI) use on SVR with ledipasvir/sofosbuvir (LDV/SOF): real-world data from 2034 genotype 1 patients in the TRIO Network. http://natap.org/2016/EASL/EASL_60.htm [Accessed 12 March 2017].
- 10.Treatment outcomes with 8, 12 and 24 week regimens of ledipasvir/sofosbuvir for the treatment of hepatitis C infection: analysis of a multicenter prospective, observational study. http://www.natap.org/2015/AASLD/AASLD_04.htm [Accessed 12 March 2017].
- 11.Latt N.L., Gevorkyan R., Yanny B.T., Sahota A. Ledipasvir/sofosbuvir for 8 weeks in non-cirrhotic, treatment naive patients with genotype 1 hepatitis C infection: real life experience in a community setting. J Hepatol. 2016;64(2):S802–S803. [Google Scholar]
- 12.Pol S., Corouge M., Vallet-Pichard A. Daclatasvir–sofosbuvir combination therapy with or without ribavirin for hepatitis C virus infection: from the clinical trials to real life. Hepatic Med Evid Res. 2016;8:21–26. doi: 10.2147/HMER.S62014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Backus L.I., Belperio P.S., Shahoumian T.A., Loomis T.P., Mole L.A. Comparative effectiveness of ledipasvir/sofosbuvir ± ribavirin vs. ombitasvir/paritaprevir/ritonavir + dasabuvir ± ribavirin in 6961 genotype 1 patients treated in routine medical practice. Aliment Pharmacol Ther. 2016;44(4):400–410. doi: 10.1111/apt.13696. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs M., Forte V., Tassone D. Effectiveness of ledipasvir/sofosbuvir and ombitasvir/paritaprevir/ritonavir/dasabuvir in treatment-naïve and -experienced U.S. veterans with genotype 1 hepatitis C infection. J Hepatol. 2016;64(2):S797–S798. [Google Scholar]
- 15.Cheung M.C.M., Foster G.R., Irving W.L. Antiviral treatment in patients with advanced HCV cirrhosis using sofosbuvir and ledipasvir/daclatasvir with or without ribavirin – 6 and 12 month outcomes compared to untreated patients. J Hepatol. 2016;64(2):S185–S186. [Google Scholar]
- 16.Aghemo A.M., Cologni G., Maggiolo F. Safety and efficacy of directly acting antivirals in 2432 HCV patients with advanced fibrosis: an interim analysis of the Lombardia Regional Network for Viral Hepatitis. J Hepatol. 2016;64(2):S213. [Google Scholar]
- 17.Crespo J., Fenandez I., Cabezas J. Effectiveness and safety of sofosbuvir/ledipasvir treatment for monoinfected genotype 1 HCV patients in real-life clinical practice: results from Spanish Hepa-C Cohort. J Hepatol. 2016;64(2):S217–S218. [Google Scholar]
- 18.Buggisch P., Böker K.H.W., Günther R. Ledipasvir/sofosbuvir treatment for 8 weeks in treatment-naïve HCV genotype 1 infected patients under real life conditions: data from the German Hepatitis C-Registry (DHC-R) J Hepatol. 2016;64(2):S810. [Google Scholar]
- 19.Welzel T.M., Petersen J., Herzer K. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016;65(11):1861–1870. doi: 10.1136/gutjnl-2016-312444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso S., Riveiro-Barciela M., Fernandez I. Effectiveness and safety of sofosbuvir-based regimens plus an NS5A inhibitor for patients with HCV genotype 3 infection and cirrhosis. Results of a multicenter real-life cohort. J Viral Hepat. 2017;24(4):304–311. doi: 10.1111/jvh.12648. [DOI] [PubMed] [Google Scholar]
- 21.Crespo J., Calleja J.L., Fernández I. Real-world effectiveness and safety of oral combination antiviral therapy for hepatitis C virus genotype 4 infection. Clin Gastroenterol Hepatol. 2017;15(6):945–949. doi: 10.1016/j.cgh.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Inc MG. Ledipasvir/sofosbuvir in Egyptian patients with chronic genotype 4 HCV infection. By Prof. Gamal Shiha. http://liverlearning.aasld.org/aasld/2016/thelivermeeting/143774/gamal.shiha.ledipasvir.sofosbuvir.in.egyptian.patients.with.chronic.genotype.4.html?f=p16m2t1434 [Accessed 12 March 2017].
- 23.Ali S., Ahmad A., Khan R.S. Genotyping of HCV RNA reveals that 3a is the most prevalent genotype in Mardan, Pakistan. Adv Virol. 2014;2014:e606201. doi: 10.1155/2014/606201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attaullah S., Khan S., Ali I. Hepatitis C virus genotypes in Pakistan: a systemic review. Virol J. 2011;8:433. doi: 10.1186/1743-422X-8-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sood A., Midha V., Goyal O., Hissar S., Sharma S.K., Khanna P. Treatment of chronic hepatitis C with pegylated interferon plus ribavirin in treatment-naïve “real-life” patients in India. Indian J Gastroenterol. 2014;33(4):343–349. doi: 10.1007/s12664-014-0451-5. [DOI] [PubMed] [Google Scholar]