Abstract
Hepatitis C Virus (HCV)-related Mixed Cryoglobulinemia (MC) is a unique condition with complex pathogenesis that involves HCV antigen-driven B-lymphocyte clonal proliferation and mutagenesis. Clinical spectrum of MC ranges from asymptomatic state to clinically-apparent vasculitis involving multiple organs. In the era of Direct-Acting Antiviral (DAA) therapy, patients with HCV-related MC achieve high rates of viral clearance that is commonly accompanied by an improvement in clinical symptoms as well as immunological profiles. Rituximab, either alone or in combination with DAA, has also been shown to be effective. Nevertheless, there have been limited and somewhat conflicting data, particularly over the long-term, regarding the rate and degree of clinical response of MC following DAA therapy. It appears that we have come quite a long way in the last decade with this condition. As with non-MC related HCV, undoubtedly long term outcome data will be forthcoming over the next few years. As we move forward successful therapy of HCV is not likely to be a challenge in contrast to access to therapy.
Abbreviations: DAA, Direct-Acting Antivirals; HCV, Hepatitis C Virus; MC, Mixed Cryoglobulinemia; NHL, Non-Hodgkin's Lymphoma; Peg-IFN, Pegylated Interferon; RBV, Ribavirin; SOF, Sofosbuvir; SVR, Sustained Virological Response
Keywords: chronic hepatitis C, mixed cryoglobulinemia, extrahepatic, direct-acting antivirals, rituximab
Hepatitis C Virus (HCV) infection affects approximately 180 million individuals worldwide and is a leading cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma. Considering that HCV is both a hepatotrophic and lymphotrophic virus, it can induce great number of extrahepatic manifestations—including lymphoproliferative and immunological disorders of various organ systems.1 Among these conditions, B-cell clonal proliferative disorders such as Mixed Cryoglobulinemia (MC) and Non-Hodgkin's Lymphoma (NHL) have been shown to be strongly linked with HCV on the basis of epidemiological and biological studies. The pathogenetic insights behind HCV-related Lymphoproliferative Disorders (LPD) have been investigated extensively, but are not yet completely elucidated. Recent data suggests that HCV-induced B-lymphocyte clonal proliferation and mutagenesis play an important role in the pathogenesis of MC. Alas, in the era of Direct-Acting Antiviral (DAA) therapy, patients with MC-vasculitis achieve high rates of viral clearance in addition to improvement in clinical symptoms. Nevertheless, among patients with late-stage, multi-phase cryoglobulinemia vasculitis, a longer follow-up time may be needed to assess clinical responses to DAA therapy. Rituximab, either alone or in combination with antiviral therapy, has been shown to be effective in the management of HCV-related MC.
Pathogenesis
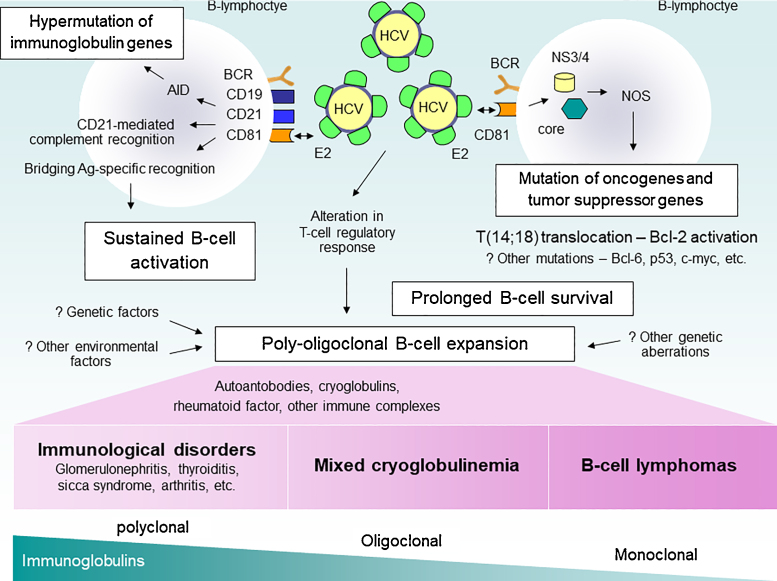
The pathogenesis of HCV-induced cryoglobulinemia and vasculitis is a complex, multistep process. Despite extensive investigation, the exact mechanisms are not clearly understood. HCV is recognized to be lymphotropic and its replication in peripheral blood mononuclear cells may be etiologically implicated in HCV-related lymphoproliferative and immunological disorders.2, 3 Several studies have highlighted the importance of sustained antigenic stimulation in promoting B-cell clonal proliferation. The binding of the HCV E2 surface protein to the CD81 molecule on the B-lymphocyte surface seems to be crucial for HCV-driven autoimmunity. CD81 is complexed with CD21, CD19, and Leu13, and this complex can reduce the threshold for B-cell activation by bridging antigen-specific recognition and CD21-mediated complement recognition.4, 5 After strong and persistent antigenic stimulation, B-cells accumulate genetic lesions through inherent genomic instability during Activation-Induced Deaminase (AID)-mediated Variable-Diversity-Joining (VDJ) class recombination6 and/or somatic hypermutations. Both of these reactions have been shown to produce DNA aberration, which can lead to overexpression of immunoglobulin genes and potential oncogenes7, 8, 9, 10 (Figure 1).
Figure 1.
Pathogenesis of HCV-related lymphoproliferative disorders. HCV, Hepatitis C Virus; NOS, Nitric Oxide Synthase; ROS, Reactive Oxygen Species; AID, activation-induced deaminase.
Apart from these mechanisms, HCV itself appears to have a mutagenic effect on host genes. The expression of HCV core protein (C) and non-structural protein 3 (NS3) are associated with the induction of Nitric Oxide Synthase (NOS), DNA damage, and subsequent mutations in oncogenes and tumor suppressor genes.11, 12 Moreover, the expression of HCV core protein contributes to B cell proliferation and survival through the upregulation of IL-10 and Bcl-2 proteins.13 Bcl-2 proto-oncogene is able to inhibit apoptosis, leading to extended cell survival.14, 15 Multiple studies have shown a significant association between Bcl-2 rearrangement (14;18 translocation) and HCV infection, especially in those who develop MC,16, 17, 18 and lymphoma.19, 20
The consequent B-lymphocyte expansion is responsible for the wide autoantibody production observed in HCV-infected individuals, including cryo- and non-cryoprecipitable immune complexes.21 Eventually, persistent B-cell activation results in MC and a variety of immunological disorders. The prolonged B-cell survival can expose these cells to other genetic aberrations, leading in some individuals to overt malignant lymphoma.22 The mechanisms of progression from B-cell proliferative state into certain overt disorders remain unclear. Factors, such as low stimulation threshold of RF-B cell,22, 23 HCV-induced alteration in regulatory and effecter T-cell function,24, 25, 26, 27 and other viral or environmental factors might play a role in this step (Figure 1).
Since a significant concentration of HCV-RNA has been found in the cryoprecipitate, HCV may play some role in facilitating cryoglobulin precipitation.28, 29 The Fc gamma-receptor like activity on HCV core30 could possibly lead to enhanced HCV-immune complex generation between IgG, IgM, and complements. This circulating HCV-immune complex can deposit in blood vessels causing vasculitis syndrome. Specifically, cryoglobulins have a high affinity for plasma C1q which allows the complex to bind to C1q receptors found on endothelial cells.31
Epidemiology
Cryoglobulinemia is a chronic systemic disease characterized by the presence of serum immunoglobulins that reversibly precipitate at temperature <37 °C, but mostly at 0–4 °C. Cryoglobulinemia is conventionally classified into 3 subgroups by Brouet et al.32: type I, composed of a monoclonal immunoglobulin and associated mainly with overt LPDs (most commonly—multiple myeloma or Waldenstrom's macroglobulinemia), and types II and III MC, composed of polyclonal IgG and monoclonal IgM (type II) or polyclonal IgG and polyclonal IgM (type III) with Rheumatoid Factor (RF). MC can be associated with infectious, immunological, and neoplastic disease. In general, the analysis of the cryoprecipitate is carried out by means of immunoelectropheresis or immunofixation, and MC accounts for the majority of patients with cryoglobulinemia (type II 62.4%; type III 31.7%).33
The strong association between HCV and MC type II and III has been supported by several epidemiological studies. HCV appears to have an important etiologic role in MC, since HCV infection (serum anti-HCV antibody or HCV-RNA) can be found in 76–95% of patients with MC.33, 34, 35 On the other hand, serum cryoglobulins can be identified in 19–54% of patients with chronic HCV infection, depending on the geographical area, population selection, diagnostic method of MC, and lead-time biases.36, 37, 38 However, serum cryoglobulins are generally asymptomatic and present at low levels. Cryoglobulinemia can be detected in all HCV genotypes, without a clear and particular genotype preponderance, is more common in females, and in those with a long duration of chronic HCV infection.36, 37, 39, 40, 41 The clinically overt MC syndrome appears to be evident in 10–30% of MC individuals and in 5–10% of all HCV-infected patients.22, 36, 37, 42 The presence of symptomatic and persistent MC is associated with advanced age, longer duration of HCV infection, type II MC, and a higher MC serum level.43 In a systematic review of 21 studies (included 1530 cases of MC in 4145 HCV individual), the pooled prevalence estimate of asymptomatic and symptomatic MC was 30.1% (95% CI: 21.4–38.9%) in HCV-infected population, while the prevalence in non-HCV population (7 studies included 204 healthy controls and 381 patients with HBV) was 1.9% (95% CI: 0.4–3.4%).44 In addition, a large retrospective cohort of 160,875 HCV-infected veterans has demonstrated that the risk of developing MC (adjusted HR = 0.61; 95% CI: 0.39–0.94) and glomerulonephritis (adjusted HR = 0.62; 95% CI: 0.48–0.79) were reduced in the SVR group compared with untreated patients. Risk reductions were also observed when patients with SVR were compared with treated patients without SVR.45
The prevalence of MC seems to be geographically heterogeneous; MC is more prevalent in Southern Europe (up to 60% of HCV-infected individuals) than in Northern Europe, North America, and Asia.46, 47, 48, 49 It is unclear whether this variation is due to unidentified genetic or environmental factors. Several studies have been conducted to identify a linkage between MC and HLA alleles. Despite the small size and heterogeneity among studies, MC has been found to be associated with HLA DRB1*11 alleles and DR2, DR3, DR5, and DR6 serological clusters.50, 51, 52, 53, 54, 55, 56 A genome-wide association study identified SNPs within NOTCH4 and in between HLA-DRB1 and HLA-DQA1 genes on chromosome 6 as significantly associated with MC and vasculitis in HCV-infected individuals.57 In addition, certain polymorphic variants of BAFF promoter and Fcγ receptors have been found more commonly in HCV patients with MC than those without (FcγR variants also seem to be crucial to the effectiveness of rituximab therapy).58
Clinical Manifestations
MC can present with different clinical/serological patterns, varying from subclinical isolated cryoglobulinemia, to complete MC syndrome. The clinical syndrome of MC is caused by the deposition of circulating immune complexes in small to medium-sized blood vessels in multiple organs, eventually leading to systemic vasculitis. The major manifestations include palpable purpura (67–98%), arthralgia (47–98%), weakness (80–100%), peripheral neuropathy (50–86%), and hypocomplementemia (particularly C4). Palpable purpura is generally localized to the lower extremities and sometimes associated with ulceration (10–25%). Peripheral neuropathy is typically of a sensory or sensory-motor axonal pattern, and can manifest as symmetrical distal neuropathies, mononeuritis multiplex, or mononeuropathies. Pure motor neuropathy and central nervous system involvement are unusual.33, 35, 43, 59 Approximately one third of patients with MC report sicca symptoms or dryness.33, 35, 59 These patients show a low rate (23%) for typical antibodies of primary Sjögren's syndrome (anti-Ro/SSA and anti-La/SSB).60 The combination of MC and Sjögren's syndrome seems to be related to poor prognosis and evolution to malignant lymphomas.61, 62, 63
Renal involvement is present in up to one third of patients and represents a strong negative prognostic factor.35, 59, 64 Nephropathy is observed in 20% of patients at the diagnosis of MC, and in 35–60% during follow up (mostly over the course of a few years).35, 65, 66 Clinically, MC-associated glomerulonephritis may range from asymptomatic abnormal urinalysis (microscopic hematuria, or sub-nephrotic range proteinuria with normal, or mildly impaired, renal function) to overt nephritis (20–25%) and nephrotic syndrome (20%), with variable progression to end-stage renal disease in 10–33% of patients.33, 35, 65, 66 The typical renal histopathologic pattern is type I Membranoproliferative Glomerulonephritis (MPGN), which can be differentiated from idiopathic MPGN by the presence of capillary thrombi, composed of precipitated cryoglobulins, and diffuse IgM deposition in the capillary loops.67, 68
Mild to moderate chronic hepatitis has been reported in two thirds of patients with MC and is generally caused by HCV. Cryoglobulinemia has been found in association with steatosis and fibrosis progression in HCV patients.46, 59, 69 In a meta-analysis of 2323 patients with chronic HCV (1022 subjects with detectable cryoglobulins), MC was found to be significantly associated with cirrhosis (occurred in 40% of patients with MC) after adjustment of age, gender, and estimated duration of disease.46
Considering the intimate pathogenetic linkage between HCV and LPD several types of lymphoid malignancies are more frequently observed in HCV patients in epidemiological studies.47, 70, 71 HCV-related B-cell derived NHL can occur during the course of MC or as a non-MC related form. Up to 10% of MC patients developed NHL during long-term follow up.35 Results from a large retrospective study of 1255 HCV patients with MC show that NHL was diagnosed at an estimated rate of 660 new cases per 100,000 patient-years, which is about 35 times higher than the general population (12 times higher if only aggressive lymphomas are included). The median time from the diagnosis of MC to the clinical onset of NHL was 6.26 years.71
The occurrence of MC generally has a great impact on the quality of life and survival. After adjustment for age, lower survival rates were observed in males and in subjects who had renal involvement, cutaneous ulcers, advanced liver disease, and immunosuppressive treatment.35, 43, 59 The causes of death reported from 2 series include renal disease (9–33%), infection (35%), liver failure (13–30%), malignancies (NHL 13% and hepatocellular carcinoma 10%), cardiovascular disease (17%), and diffuse vasculitis (13%).35, 59
Diagnosis
Diagnosis of MC is based on clinical, pathological and laboratory work-up including cryoglobulin testing, quantitative serum protein and globulins, complement levels, virologic markers, and urine analysis. The cryoglobulin determination is crucial and can be associated with false negative and false positive results. The most important variable confounding standardization of cryoglobulin testing is improper sample handling.72 For the correct evaluation of serum cryoglobulins, laboratories should ensure that samples are collected and maintained in tubes pre-warmed to 37 °C (from phlebotomy until the serum is separated by centrifugation). Cryocrit determination and cryoglobulin characterization should be conducted at 4 °C (after 7 days). Anticoagulants should not be used in order to avoid false positive results from cryofibrinogen.48, 73 Biopsies from purpuric skin lesions showing Leukocytoclastic Vasculitis (LCV), can often be helpful. Kidney biopsy should be reserved for patients with renal disease. The presence of a clonal expansion of B-lymphocytes in peripheral blood, bone marrow, and liver confirms the lymphoproliferative nature of MC.74, 75 Ferri et al. has proposed a standardized criteria for the diagnosis and classification of MC48, 76 (Table 1). Cryoglobulin levels, RF activity, and decreased C4 levels all weakly correlate with MC disease activity.73, 77 Despite these challenges, these tests are useful for predicting treatment response.
Table 1.
Proposed Criteria for the Diagnosis and Classification of Patients with Mixed Cryoglobulinemia.48
| Criteria | Major | Minor |
|---|---|---|
| Serological | Mixed cryoglobulins Low C4 |
Rheumatoid factor+ HCV+ HBV+ |
| Pathological | Leukocytoclastic vasculitis | Clonal B-cell infiltrates (liver and/or bone marrow) |
| Clinical | Purpura | Chronic hepatitis Membranoproliferative GN Peripheral neuropathy Skin ulcers |
HCV+ or HBV+, markers of Hepatitis C Virus or Hepatitis B Virus infection (anti-HCV ± HCV-RNA; HBV-DNA or HBsAg); C, Complement; GN, Glomerulonephritis.
“Definite” mixed cryoglobulinemia syndrome: (a) Serum mixed cryoglobulins (±low C4) + purpura + leukocytoclastic vasculitis. (b) Serum mixed cryoglobulins (±low C4) + 2 minor clinical symptoms + 2 minor serologic/pathologic findings.
“Incomplete” or “possible” mixed cryoglobulinemia syndrome: (a) Mixed cryoglobulins or low C4 + 1 minor clinical symptom + 1 minor serologic ± pathologic findings. (b) Purpura and/or leukocytoclastic vasculitis + 1 minor clinical symptom + 1 minor serologic ± pathologic findings. (c) Two minor clinical symptoms + 2 minor serological ± pathologic findings.
“Essential” or “secondary” mixed cryoglobulinemia syndrome: Absence or presence of well known disorders (infectious, immunological, or neoplastic) at the time of the diagnosis.
Treatment of HCV-Related Mixed Cryoglobulinemia
Before HCV infection was identified as an important etiology in MC, a variable combination of anti-inflammatory and immunosuppressive agents had been used as the main treatment strategy of MC. Soon after the importance of HCV and B-cell clonal expansion were recognized in the pathogenesis of MC, several studies assessed the effects of antiviral therapy for HCV and biological therapies targeting B-cell proliferation.
Pegylated-Interferon (Peg-IFN) and Ribavirin (RBV)
Pegylated-Interferon (Peg-IFN) and Ribavirin (RBV) therapy has been associated with improvement in clinical MC syndrome and immunologic parameters, such as cryoglobulins, IgM, RF, and complement levels. Following a Sustained Virologic Response (SVR), interferon-based therapy yielded a beneficial effect in patients with HCV-related MC (62–78%)78, 79, 80, 81 (Table 2). However, patients with MC do not respond as well to Peg-IFN and RBV combination therapy compared to patients without MC. In a meta-analysis of combination therapy for HCV-associated MC (10 clinical studies included 100 unique patients; 4–39% had baseline renal involvement), Peg-IFN and RBV yielded a virologic response rate of 52% (95% CI: 40–63%)82 compared to 50% and 80% for genotype 1/4 and 2/3 patients without MC, respectively.83, 84 Patients with MC tend to be older and have several other comorbidities (i.e. kidney disease, nephropathy, thrombocytopenia, etc.) which could influence SVR.82 Until recently, a long-term outcome following HCV eradication in patients with MC has been reported. In a large prospective cohort, 253 HCV patients with symptomatic/asymptomatic MC, and 158 HCV patients without MC were followed-up for 92.5 (35–124) months following Peg-IFN plus RBV therapy. Overall SVR rate was significantly lower in patients with MC compared to those without MC (48.6% vs. 61.4%; P = 0.014). In the majority (57%) of SVR patients all MCS symptoms persistently disappeared, whereas all non-SVR patients were also clinical nonresponders, in spite of a transient improvement in some cases, thus suggesting that SVR is associated with amelioration of the clinical manifestations of MC.85 Further, eradication of HCV by IFN-based therapy can lead to the resolution of MC-related low-grade lymphomas—particularly splenic villous lymphomas and immunocytomas.86, 87, 88 It seems plausible that the anti-proliferative activity of IFN could help contain B-cell clonal expansion in addition to eradicating HCV and preventing B-cell antigenic stimulation.89
Table 2.
Effects of Pegylated-Interferon Plus Ribavirin in HCV-Related Mixed Cryoglobulinemia.
| Author (year) | N | Population | Treatment | Response |
|---|---|---|---|---|
| Alric et al. (2004)78 | 25 | MC, active GN nephritic range proteinuria Refractory to CS/PF/diuretic - Divided into 2 groups; [A] n = 18, [B] n = 7 |
[A] Peg-IFN 1.5 μg/kg/week + RBV 600–1000 mg/day for ≥24 week [B] conventional Rx FU ≥ 6 months after Rx (IS/PF are allowed) |
SVR 66.7% (12/18) Decreased proteinuria and cryoglob. in responders Increased serum albumin in responders No change in serum Cr Improved purpura 12/12(SVR), 3/6(NR), and 1/7[B] |
| Cacoub et al. (2005)79 | 9 | Active MC, IFN naïve (7) and IFN NR (3) |
Peg-IFN 1.5 μg/kg/week + RBV 800–1200 mg/day for ≥24 week (short-term, low-dose CS in 2 pt.) | SVR 78%, partial response 11%, relapse 11% Complete clinical response 89% Complete/partial immunologic response 56%/44% |
| Mazzaro et al. (2005)80 | 18 | Active MC | Peg-IFN 1.0 μg/kg/week+ RBV 1000–1200 mg/day for 48 week | SVR 44%, EOT 83% Purpura and cryocrit improved in responders |
| Saadoun et al. (2006)81 | 72 | Active MC, IFN naïve (52) and IFN NR (20) - Divided into 2 groups; [A] n = 40, seen in 2001 or later; [B] n = 32, seen prior to 2001 |
[A] Peg-IFN 1.5 μg/kg/week + RBV 800–1200 mg/day ≥ 24 week [B] IFN 3 mU × 3/week ≥ 24 week Mean FU 39.7 ± 24.4 months after Rx (CS/IS/PF are allowed) |
SVR; [A] = 62.5%, [B] = 53.1% Complete CR; [A] = 67.5%, [B] = 56.3% Complete IR; [A] = 57.5%, [B] = 31.3% PF and IS were less likely to be used in [A] Factors associate with CR = EVR (OR 3.53; 95% CI: 1.18–10.59) and GFR ≤70 ml/min (OR 0.18; 95% CI: 0.05–0.67) |
| Saadoun et al. (2015)98 | 30 | Active MC, IFN naïve (7) and IFN NR (23) |
Telaprevir 12 week (17) or boceprevir 44 week (13) + Peg-IFN/RBV 48 week | SVR 67% Complete CR 67%; PR 23% Cryoglobulin clearance 56%; significant improvement in BVAS, serum RF, C4 levels |
MC, Mixed Cryoglobulinemias; RTX, Rituximab; Peg, Pegylated; IFN, Interferon; FU, Follow-Up; CS, Corticosteroid; IS, Immunosuppressive agents; PF, Plasmapheresis; CLD, Chronic Liver Disease; CR, Clinical Remission; IR, Immunological Response; SVR, Sustained Virological Response; EVR, Early Virological Response; NR, Non-Responder; GFR, Glomerular Filtration Rate; Cr, Creatinine; BVAS, Birmingham Vasculitis Activity Score; RF, Rheumatoid Factor.
DAA Therapy
DAA therapy has vastly improved SVR rates in patients with and without cryoglobulinemia vasculitis. Among patients with cryoglobulinemia vasculitis, DAA regimens yielded SVR rates of 74–100% (Table 3). In almost all studies, a complete or partial reduction in MC clinical symptoms occurred during or after DAA administration and was correlated with SVR. In these studies, DAA therapy eliminated or ameliorated clinical symptoms of MC in 61–100% of patients who achieved SVR12. A complete clinical response was defined as improvement of all affected organs and/or a Birmingham Vasculitis Activity Score (version 3) of 0. The highest clinical response rate was demonstrated in a prospective study by Saadoun et al. in which all patients (n = 41) achieved SVR and a complete (90%) or partial (10%) clinical response after 12 or 24 weeks of Sofosbuvir (SOF) and Daclatasvir (DAC). 90 Similar observations were reported in a prospective study by Gragnani et al. in which 93% of patients (n = 41/44) achieved a complete or partial response in MC vasculitis in the background of 100% achieving SVR. At SVR24, the cohort clinical responses improved to 100% with 77% of patients achieving a complete clinical response.89 Interestingly, one study showed a higher clinical response rate compared to the SVR rate; 74% of patients achieved SVR after 24 weeks of SOF + RBV (n = 24), while 87% of patients achieved a complete clinical response.91 Meanwhile, Sollima et al. showed that MC-related vasculitis can persist or relapse in high proportion of patients who achieve SVR. This study included 7 patients who were treated with DAA regimens and achieved SVR. Five patients had nephropathy and 6 patients failed previous antiviral therapy and/or rituximab. At the end of DAA treatment, cyrocrit levels were undetected in 4 patients, but upon follow-up, only 1 patient exhibited cryoglobulin disappearance and a partial clinical response.92 These results suggest that longer follow-up is needed to assess the course of MC, and especially in patients with late stage, multi-phase cryoglobulinemia vasculitis.93 Overall, vasculitis symptoms improved after the administration of DAA therapy and subsequent viral clearance. The type of DAA regimen used throughout all studies might have different impacts on clinical responses to MC. For instance, higher SVR rates and clinical responses have been reported by Saadoun et al. among patients treated with SOF and DAC in comparison to patients treated with SOF and RBV.90, 91
Table 3.
Virological and Clinical Responses to DAA Therapy in Patients with Cryoglobulinemic Vasculitis.
| Author (year) | N | DAA regimens | RTX (n) | SVR (%) | Clinical response (%) at 12 week post-treatment |
Complete cryoglobulin reduction (%) | |
|---|---|---|---|---|---|---|---|
| Complete | Partial | ||||||
| Saadoun et al. (2016)91 | 24 | SOF/RBV × 24 week | 4 | 74 | 87 | - | 46 |
| Sise et al. (2016)97 | 12 | SOF/SIM (n = 8) SOF/RBV (n = 4) |
4 | 83 | 33 | 33 | 44 (n = 4/9) |
| Bonacci et al. (2016)99 | 35 | 3D (n = 10) SOF/LDV (n = 10) SOF/SIM (n = 2) DAC/SIM (n = 3) SOF/DAC (n = 2) Peg-IFN/DAA (n = 5) Others (n = 3) Use of RBV (n = 24) |
0 | 94 | 71 | 14 | 45 |
| Gragnani et al. (2017)a 93 | 17 | 3D (n = 5) 3D/RBV (n = 6) SOF/RBV (n = 5) SOF/DAC (n = 1) |
- | 100 (week 8) | 30 (week 8) | 50 (week 8) | 35 (week 8) |
| Gragnani et al. (2016)89 | 44 | SOF/RBV (n = 18) SOF/SIM/ ± RBV (n = 12) SOF/DAC/±RBV (n = 4) SOF/LDV/±RBV (n = 10) |
2 | 100 | 66 | 27 | 32 |
| Saadoun et al. (2017)90 | 41 | SOF/DAC (n = 32) × 12 week SOF/DAC (n = 9) × 24 week |
2 | 100 | 90 | 10 | 50 |
| Hegazy et al.b 137 | 35 | SOF/RBV (n = 13) × 24 week SOF/IFN/RBV (n = 8) × 12 week SOF/DAC (n = 5) × 12 week SOF/SIM (n = 9) × 12 week |
- | 100 | 84–100 for each symptom (EOT) | - | - |
| Emery et al. (2017)96 | 18 | DAAs ± IFN | 3 | 89 | 39 | 22 | 29 |
| Sollima et al. (2016)c 92 | 7 | 3D (n = 2) SOF/RBV (n = 2) SOF/DAC (n = 2) SOF/SIM (n = 1) |
- | 100 | 0 | 14 | 14 |
| Tsuge et al. (2016)105 | 1 | DAC/ASU × 24 week | 0 | 100 | 100 | 0 | 0 |
| Obata et al. (2017)104 | 1 | DAC/ASU × 24 week | 0 | 100 | 100 | 0 | 0 |
Assessments at treatment week 8.
Clinical assessments at end of treatment.
Clinical assessments at last assessment post-treatment.
DAA, Direct Acting Antivirals; RTX, Rituximab; SOF, Sofosbuvir; LDV, Ledipasvir; DAC, Daclatasvir; ASU, Asunaprevir; RBV, Ribavirin; SIM, Simeprevir; 3D, paritaprevir/ritonavir/ombitasvir/dasabuvir; IFN, Fnterferon; SVR, Sustained Virological Response; EOT, End-of-Treatment.
An evidence-based review and recommendations has been performed by the International Study Group of Extrahepatic Manifestations related to HCV (ISG-EHCV) in 2016. Among 170 HCV patients with extrahepatic manifestations, mainly MC, 50 patients being treated with IFN-free regimens and 120 with IFN-containing regimens, there was a clearly higher rate of SVR in patients treated with IFN-free regimens (92% vs. 68%), but with slightly lower rates of complete clinical response (68% vs. 76%) and cryoglobulin clearance (47% vs. 56%) compared with patients treated with IFN-containing regimens.94 Caution on the interpretation of these data must be exercised due to the large degree of heterogeneity in patient characteristics, the different DAA regimens used and the uncontrolled design of the studies. Nevertheless, DAA-based, IFN-free regimens should be considered as a first-line therapy for HCV patients with MC (with similar regimens as recommended for non-MC patients) due to their superior safety and efficacy profiles, particularly in terms of viral clearance, as compared with IFN-based regimens.94 The benefits of the addition of RBV in DAA regimens remain debatable. Based on a systematic review of 120 patients with MC treated with multiple DAA regimens with or without RBV, a few more patients treated with RBV-containing regimens had a complete clinical response (74% vs. 64%), with similar rate of cryoglobulin clearance (47% vs. 48%) but with a lower rate of SVR (88% vs. 97%) compared with those treated with RBV-free regimens.94
Clinical and Immunologic Responses
In a prospective clinical trial of patients with HCV-related MC, DAA-based therapy has shown to restore disturbances in peripheral B- and T-cell homeostasis.95 However, clinical symptoms of cryoglobulinemia vasculitis may resolve at different rates. In a retrospective study by Emery et al., skin manifestations resolved at a higher rate (39%) compared to renal and neurological manifestations (11%) (n = 18).96 Gragnani et al. assessed clinical responses at 12 and 24-weeks post-treatment and showed that palpable purpura, kidney disease, and skin ulcers resolved over a faster duration of time compared to fatigue, sicca syndrome and peripheral neuropathy (n = 44).89 Neurological symptoms and sicca syndrome might not resolve at all in patients with chronic HCV-associated MC and irreversible nerve/gland damage.
Most studies observed reduced cryocrit levels after HCV treatment; nevertheless, cryoglobulin levels can persist in 20–100% of patients in spite of viral clearance (immunological responses are highly variable among studies).78, 80, 81, 90, 91, 93, 96, 97, 98 After multivariate analysis, Bonacci et al. found that a baseline cryocrit level below 2.7% was independently associated with a complete immunological response—defined as the absence of circulating cryoglobulins and normalized levels of complement and/or RF.99 The mechanisms underlying the persistence of cryoglobulin production and its manifestation after HCV clearance are not clear. In cryoglobulinemia vasculitis, B-cell proliferation may eventually reach an HCV-independent autonomous phase, evidenced by the persistence of t(14;18) positive B-cell clones and small quantities of HCV-RNA in the lymphatic system even after successful antiviral therapy.100, 101, 102 Among patients who have been cured of HCV, but maintain clinical manifestations of MC, a different underlying condition can be considered—such as B-cell NHL etiology.103
Renal Involvement
HCV eradication has been associated with improvement in renal function among patients with HCV-related MC. Thus far, several studies have shown positive effects of DAA therapy in patients with MPGN or other HCV related renal manifestations.89, 91, 97, 99, 104, 105 Sise et al. showed significant improvement in creatinine levels and proteinuria post-DAA treatment in 7 patients with renal involvement (5 confirmed MPGN by biopsy and cryoglobulin deposits noted on electron microscopy).97 Similar results were seen in a study by Bonacci et al. where 7 patients with renal involvement (5 confirmed MPGN by biopsy) experienced improvement in hematuria (P = 0.03) and estimated glomerular filtration rate (eGFR) (P = 0.03).99 Additionally, Gragnani et al. reported that 4 patients with renal involvement (1 confirmed MPGN by biopsy) experienced improvement in eGFR and improvement in proteinuria.89
Although DAA regimens are effective for patients with MC, SOF remains contraindicated in patients with chronic kidney disease stage 4–5 because its inactive metabolite GS-331007 accumulates as it is mostly filtered and eliminated by the kidneys.106 Therefore, non-SOF-based DAA regimens should be considered for patients with MC in the context of severe renal impairment. So far, the safety and efficacy of DAC and Asunaprevir (ASU) has been explored in genotype 1b patients undergoing hemodialysis.107, 108 In two case studies from Japan, a 24-week regimen of DAC/ASU achieved positive clinical and virologic response in patients with HCV-associated cryoglobulinemic MPGN/renal impairment. In one study, a 70-year-old man with MC and MPGN achieved SVR after completing a 24-week regimen of DAC/ASU. During treatment, his hematuria and proteinuria dramatically decreased while his creatinine, total protein, and complements slowly normalized; azilsartan was added for persistent proteinuria. During follow-up, his HCV and MPGN completely resolved despite persistent cryoglobulinemia.104 In another study, a 51-year-old woman with cirrhosis, chronic renal failure and chronic heart failure achieved SVR after 24 weeks of DAC/ASU. At treatment initiation, she exhibited mild ascites, severe leg edema, mild anemia, proteinuria and hematuria. During treatment, her ALT and creatinine levels initially spiked and then normalized. At SVR12, her leg edema and albuminuria almost completely resolved, and her serum cryoglobulin decreased.105 Recently, highly effective DAA regimens including, grazoprevir/elbasvir and glecaprevir/pibrentasvir, have been approved for use in patients with severe renal impairment with expected SVR rates similar to that of patients with normal renal function. However, the experience on their use in HCV patients with MC and severe renal impairment are very limited. It should be kept in mind that although these case series present promising results for patients with HCV-associated MC, more studies with larger sample sizes and longer follow-up are needed to evaluate DAA treatment for patients with MPGN/renal impairment.
Adverse Events
Adverse events were observed frequently with IFN-containing regimens ranging from 49–100%. Anemia was reported in 17–31% of patients, and blood transfusions were required in patients with severe anemia).78, 80, 81, 98 RBV dose reductions for anemia occurred frequently; suspension of therapy was less common. There had been a potential risk of developing or worsening of autoimmune diseases due to IFN use, particularly in patients with extrahepatic manifestations who may already have had or had some increased susceptibility to autoimmune disorders; a scenario unlikely with IFN-free regimens. IFN-free DAA therapy with or without RBV is generally well-tolerated in HCV-related MC patients.90, 91, 93, 96, 97 The common side effects of DAA therapy in MC vasculitis included anemia, fatigue, insomnia, and nausea. A small number of serious adverse events were reported in a few studies. Notably, treatment with DAA regimens must be individualized, especially in the setting of severe renal and liver disease. RBV ideally should not be given if baseline hemoglobin levels are <10 g/dL, particularly since MC is associated with anemia (severe autoimmune cytopenias and severe glomerulonephritis).94 If RBV is used, the dose should be adjusted preemptively according to the patient's eGFR and then should be closely monitored during the treatment.
Rituximab
Rituximab is a chimeric monoclonal antibody directed at CD20 antigen on the surface of B-lymphocytes, and it is highly effective in eliminating B-cells through complement-dependent and antibody-mediated cellular toxicity.109 By depleting B-cells, rituximab has the potential to reduce the clonal B-cell expansion, and the development of plasma cells, thereby limiting cryoglobulin production. Rituximab at the standard dose of 375 mg/m2 weekly for 4 weeks has proved to be safe and effective in the treatment of HCV-related MC (Table 3, Table 4). Rituximab is indicated for patients with progressive renal disease, mononeuritis multiplex, and skin ulcers.110 In patients with disease refractory to immunosuppressive agents and/or antiviral therapy, rituximab monotherapy leads to significant clinical (skin, renal, and neuropathy) and immunological (cryoglobulins, IgM, and RF activity, complements, and anti-HCV titers) improvement in 80–100% of patients.111, 112, 113, 114, 115 Reversal of B-cell expansion in both peripheral blood and bone marrow were reported in most patients.111, 112, 113, 114, 115 Interestingly, complete remission of MC-related NHL was reported in 2/3 of patients in one study.112 Rituximab has been associated with significantly elevated HCV viremia, presumably due to partial reduction of humoral control.116 These changes are generally transient and do not appear to have an adverse effect on liver function.114 Clinical benefits of rituximab therapy are usually observed within 5 months after treatment and can last up to 1–2 years. Although B-cell repletion and vasculitis may develop in up to one third of patients, these symptoms can still be responsive to rituximab retreatment.111, 112, 117 Among patients who had a relapse in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, rituximab and glucocorticoid retreatment was noted to be successful.118
Table 4.
Effects of Rituximab Therapy in HCV-Related Mixed Cryoglobulinemia.
| Author (year) | N | Population | Treatment | Response |
|---|---|---|---|---|
| Sansonno et al. (2003)111 | 20 | Active MC, refractory to IFN | RTX 375 mg/m2 weekly × 4 FU 12 months (no IS were added) |
CR 80% (reduced cryocrit + clinical improvement) Overall response rate occurred in 5 months Decreased RF, anti-HCV titer and B-cell HCV-RNA increased (×2) in responders, and remained much the same in non-responders |
| Zaja et al. (2003)112 | 12 | Active MC, refractory to CS/IS/IFN/PF 3 pt. had NHL |
RTX 375 mg/m2 weekly × 4 FU response at 6 months with add. 3–28 months FU (low-mod. dose CS are allowed) |
Improved purpura and neuropathy 90–100% Decreased cryocrit, RF and IgM 100% Streroid-sparing effect 100% B-cell depletion in PB (100%), in BM (2/7) Clinical relapse 33% In NHL: 2/3 CR, 1/3 partial response |
| Roccatello et al. (2008)113 | 12 | Active MC, resistance (6), or intolerance (3) to conventional Rx, or significant BM infiltrates (3) | RTX 375 mg/m2 weekly × 4 + add. 2 doses monthly FU at least 18 months (no IS were added) |
Improvement of clinical signs and symptoms Decreased cryocrit, ESR, IgM, and proteinuria HCV-RNA and IgG remained stable BM abnormalities reversed to normal 100% (3/3) |
| Patrarca et al. (2010)114 | 19 | Active MC with CLD (F3-4) intolerant or contraindicated to IFN (15 pt. had cirrhosis, 6 pt. had ascites) | RTX 375 mg/m2 weekly × 4 FU 6–48 months (low-mod. dose CS are allowed) |
Clinical improved (CR 12, partial response 7) 9 negative cryocrit, 5 decreased cryocrit HCV-RNA increased during Rx—3 months after Rx Improved liver synthetic functions and ascites |
| Saadoun et al. (2008)119 | 16 | Active MC, resistance (11) or relapser (5) to previous Peg-IFN or IFN + RBV | RTX 375 mg/m2 weekly × 4, then Peg-IFN 1.5 μg/kg/week + RBV 600–1200 mg/day FU ≥ 6 months after Rx (mean 19.4 months) |
Clinical and immunological improved 93.7% CR 62.5% (all had SVR), clinical relapse 18.8% SVR 68.7% Predictors of CR = shorter vasculitis duration before Rx and lower HCV-RNA at 3 months |
| Terrier et al. (2009)117 | 32 | Active MC - Divided into 2 groups; [A] n = 20, IFN naïve (9), NR or relapser (11); [B] n = 12, failed previous Peg-IFN Rx or IFN intolerant |
[A] RTX 375 mg/m2 weekly × 4 or 100 mg on day 1 and day 15, then Peg-IFN 1.5 μg/kg/week + RBV 600–1200 mg/day for 12 months (range 3–20) [B] RTX alone FU 23 ± 12 months |
SVR; [A] 55%, [B] 0%* Clinical response; [A] = 95%, [B] = 67%* Immunological response; [A] = 100%, [B] = 82%* Clinical relapse; [A] = 15%, [B] 33%* Immunological relapse; [A] = 25%, [B] = 50%* All relapses associated with no SVR 6 pt. had re-Rx by RTX—clinical response 100% *P = ns |
| Dammacco et al. (2010)120 | 37 | Active MC, naïve to IFN/IS - Randomized into 2 groups; [A] n = 22, [B] n = 15 |
[A] RTX 375 mg/m2 weekly × 4 + add. 2 doses 5-monthly, with Peg-IFN alfa-2b 1.5 μg/kg/week or alfa-2a 180 μg/week + RBV for 48 week [B] Peg-IFN + RBV FU 36 months after Rx |
CCR at 12 months; [A] = 54.5%, [B] = 33.3%** CCR at 36 months; [A] = 83.3%(10/12), [B] = 40%(2/5)*** **P < 0.05, ***P < 0.01 Cryoglobulins persisted at 36 months; [A] = 22.7%, [B]33.3% |
| Visentini et al. (2011)138 | 27 | Active MC, resistance (6), or intolerance (3) to | RTX 250 mg/m2 × 2 week | CR 79% Relapse 42% (mean time of relapse 6.5 months) |
MC, Mixed Cryoglobulinemias; RTX, Rituximab; Peg, Pegylated; IFN, Interferon; FU, Follow-Up; CS, Corticosteroid; IS, Immunosuppressive agents; PF, Plasmapheresis; BM, Bone Marrow; PB, Peripheral Blood; CLD, Chronic Liver Disease; NHL, Non-Hodgkin's Lymphoma; SVR, Sustained Virological Response; CR, Clinical Remission; CCR, Complete Clinical Response (disappearance of symptoms, cryoglobulins, serum HCV-RNA, and B-cell clonalities from the blood).
The effectiveness of combination therapy with rituximab and Peg-IFN/RBV has been demonstrated in many studies (Table 4). Despite a variation in study populations and treatment regimens, treatment seemed to be safe and offered additional benefits beyond each agent, in terms of clinical/immunological and virological responses, in both treatment naïve and treatment refractory patients.117, 119, 120 Treatment was well-tolerated with no infectious complications. When SVR is achieved, a long-term clinical-immunological response is usually observed. A randomized controlled trial showed that Peg-IFN/RBV combined with rituximab was more effective than Peg-IFN/RBV alone in MC patients and its effect lasted for more than 3 years.120 In a few recent studies, a small proportion of patients with MC received rituximab in conjunction with DAA treatment (Table 4). These patients had progressive forms of MC, and no significant differences were found in virologic responses to DAA treatment alone compared to patients who received additional rituximab.89, 91, 96, 97
Rituximab is currently considered the best biological target option for patients with MC and its use should be exercised with a reasonable individualized assessment of the benefits and risks. It is still debatable whether it should be administered concomitantly with DAAs or sequentially.94 The advantage of administering DAAs and rituximab to patients at the same time includes lowering the autoimmune response triggered by the virus while also lowering the viral load. Moreover, rituximab has a non-immediate pharmacodynamic profile; thus, earlier administration of rituximab could lead to a better outcome. However, hematological toxicity remains a potential risk with concomitant use of both therapies. The sequential administration of rituximab and DAAs could be undertaken to mitigate major adverse events. Rituximab administration before DAA therapy could further increase the odds of achieving SVR by depleting B cells—a potential reservoir for the virus.94
Rituximab is often associated with mild infusion reactions such as fever, chill, nausea, vomiting, bronchospasm, urticaria, and orthostatic hypotension. However, in a few MC patients, particularly with high baseline cryoglobulin levels, rituximab can form a complex with RF-positive IgMkappa leading to accelerated cryoprecipitation which may eventually cause severe systemic drug reactions, flare of MC vasculitis and serum sickness syndrome.121 Thus, it is suggested that rituximab be administered with caution in MC vasculitis, with use of the 375 mg protocol and plasma exchanges prior to rituximab infusion in patients with high baseline levels of mixed cryoglobulin.121
Immunosuppressive and Cytotoxic Agents
Systemic Corticosteroids (CS), either high-dose oral prednisone or intravenous methylprednisolone, have successfully been used to treat the acute phase of vasculitis symptoms. Cytotoxic drugs, particularly cyclophosphamide, in combination with CS, have been shown to be effective in inducing clinical remission in severe MC patients.77, 122, 123 However, these agents are not curative and are associated with significant side effects, liver toxicity, and subsequent increase in HCV viremia.124, 125, 126, 127 Although severe exacerbations of HCV in non-transplanted immunosuppressive settings is uncommon, fatal cases of Fibrosing Cholestatic Hepatitis (FCH) with conventional cytotoxic agents have been reported.128, 129 A meta-analysis of controlled clinical trials suggested that standard IFN was more effective than immunosuppressive agents in lowering proteinuria in HCV-related cryoglobulinemic glomerulonephritis.130 Therefore, CS and cyclophosphamide should be reserved for patients with severe vasculitis with relatively preserved liver function and should be administered for a short-term until vasculitis activity is controlled. Other agents, such as mycophenolate mofetil,77, 131 anti-tumor necrotic factor (TNF) antibodies,132 and cyclosporine,133 have been used for MC patients; however, supporting data are limited.
Plasmapheresis
Removal of circulating cryoglobulins by therapeutic plasmapheresis is accepted as an adjunctive therapy for severe exacerbation of vasculitis, especially with renal insufficiency. In combination with immunosuppressive agents, it generally induces temporary clinical remission. Several apheretic procedures have been used in MC, including non-selective plasma exchange,134 more selective procedure such as double-filtrating plasma exchange,135 or immunoadsorption apheresis.136
Summary
HCV-related MC is a unique condition with complex pathogenesis that involves HCV antigen-driven B-lymphocyte clonal proliferation and mutagenesis. Clinical spectrum of MC ranges from asymptomatic state to clinically-apparent vasculitis involving multiple organs. In the era of DAA therapy, patients with HCV-related MC achieve high rates of viral clearance that is commonly accompanied by an improvement in clinical symptoms as well as immunological profiles. Rituximab, either alone or in combination with DAA, has also been shown to be effective. Nevertheless, there have been limited and somewhat conflicting data, particularly over the long-term, regarding the rate and degree of clinical response of MC following DAA therapy. It appears that we have come quite a long way in the last decade with this condition. As with non MC related HCV, undoubtedly long term outcome data will be forthcoming over the next few years. As we move forward successful therapy of HCV is not likely to be a challenge in contrast to access to therapy.
Conflicts of Interest
The authors have none to declare.
Acknowledgement
The authors would like to thank Julia Palecki and Kelly Borges for their help with review of the literature.
See Editorial on Pages 2-3.
References
- 1.Zignego A.L., Ferri C., Pileri S.A., Caini P., Bianchi F.B. Extrahepatic manifestations of hepatitis C virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. 2007;39:2–17. doi: 10.1016/j.dld.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Zignego A.L., Macchia D., Monti M. Infection of peripheral mononuclear blood cells by hepatitis C virus. J Hepatol. 1992;15:382–386. doi: 10.1016/0168-8278(92)90073-x. [DOI] [PubMed] [Google Scholar]
- 3.Roque Afonso A.M., Jiang J., Penin F. Nonrandom distribution of hepatitis C virus quasispecies in plasma and peripheral blood mononuclear cell subsets. J Virol. 1999;73:9213–9221. doi: 10.1128/jvi.73.11.9213-9221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flint M., McKeating J.A. The role of the hepatitis C virus glycoproteins in infection. Rev Med Virol. 2000;10:101–117. doi: 10.1002/(sici)1099-1654(200003/04)10:2<101::aid-rmv268>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Quinn E.R., Chan C.H., Hadlock K.G., Foung S.K., Flint M., Levy S. The B-cell receptor of a hepatitis C virus (HCV)-associated non-Hodgkin lymphoma binds the viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood. 2001;98:3745–3749. doi: 10.1182/blood.v98.13.3745. [DOI] [PubMed] [Google Scholar]
- 6.Ito M., Murakami K., Suzuki T. Enhanced expression of lymphomagenesis-related genes in peripheral blood B cells of chronic hepatitis C patients. Clin Immunol. 2010;135:459–465. doi: 10.1016/j.clim.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Ivanovski M., Silvestri F., Pozzato G. Somatic hypermutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood. 1998;91:2433–2442. [PubMed] [Google Scholar]
- 8.Machida K., Cheng K.T., Pavio N., Sung V.M., Lai M.M. Hepatitis C virus E2-CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol. 2005;79:8079–8089. doi: 10.1128/JVI.79.13.8079-8089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machida K., Cheng K.T., Lai C.K., Jeng K.S., Sung V.M., Lai M.M. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. J Virol. 2006;80:7199–7207. doi: 10.1128/JVI.00321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zignego A.L., Craxi A. Extrahepatic manifestations of hepatitis C virus infection. Clin Liver Dis. 2008;12:611–636. doi: 10.1016/j.cld.2008.03.012. ix. [DOI] [PubMed] [Google Scholar]
- 11.Machida K., Cheng K.T., Sung V.M. Hepatitis C virus induces a mutator phenotype: enhanced mutations of immunoglobulin and protooncogenes. Proc Natl Acad Sci U S A. 2004;101:4262–4267. doi: 10.1073/pnas.0303971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machida K., Cheng K.T., Sung V.M., Lee K.J., Levine A.M., Lai M.M. Hepatitis C virus infection activates the immunologic (type II) isoform of nitric oxide synthase and thereby enhances DNA damage and mutations of cellular genes. J Virol. 2004;78:8835–8843. doi: 10.1128/JVI.78.16.8835-8843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoury T., Chen S., Adar T., Jacob E.O., Mizrahi M. Hepatitis C infection and lymphoproliferative disease: accidental comorbidities? World J Gastroenterol. 2014;20:16197–16202. doi: 10.3748/wjg.v20.i43.16197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed J.C. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood. 2008;111:3322–3330. doi: 10.1182/blood-2007-09-078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 16.Kitay-Cohen Y., Amiel A., Hilzenrat N. Bcl-2 rearrangement in patients with chronic hepatitis C associated with essential mixed cryoglobulinemia type II. Blood. 2000;96:2910–2912. [PubMed] [Google Scholar]
- 17.Zignego A.L., Giannelli F., Marrocchi M.E. T(14;18) translocation in chronic hepatitis C virus infection. Hepatology (Baltimore, Md) 2000;31:474–479. doi: 10.1002/hep.510310230. [DOI] [PubMed] [Google Scholar]
- 18.Zignego A.L., Ferri C., Giannelli F. Prevalence of bcl-2 rearrangement in patients with hepatitis C virus-related mixed cryoglobulinemia with or without B-cell lymphomas. Ann Intern Med. 2002;137:571–580. doi: 10.7326/0003-4819-137-7-200210010-00008. [DOI] [PubMed] [Google Scholar]
- 19.Libra M., De Re V., Gloghini A. Frequency of bcl-2/IgH translocation in patients with non-Hodgkin's lymphoma and chronic hepatitis C virus infection. Minerva Gastroenterol Dietol. 2005;51:165–170. [PubMed] [Google Scholar]
- 20.Libra M., Gloghini A., Malaponte G. Association of t(14;18) translocation with HCV infection in gastrointestinal MALT lymphomas. J Hepatol. 2008;49:170–174. doi: 10.1016/j.jhep.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Ferri C., Antonelli A., Mascia M.T. HCV-related autoimmune and neoplastic disorders: the HCV syndrome. Dig Liver Dis. 2007;39(suppl 1):S13–S21. doi: 10.1016/s1590-8658(07)80005-3. [DOI] [PubMed] [Google Scholar]
- 22.Zignego A.L., Giannini C., Ferri C. Hepatitis C virus-related lymphoproliferative disorders: an overview. World J Gastroenterol. 2007;13:2467–2478. doi: 10.3748/wjg.v13.i17.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fornasieri A., Bernasconi P., Ribero M.L. Hepatitis C virus (HCV) in lymphocyte subsets and in B lymphocytes expressing rheumatoid factor cross-reacting idiotype in type II mixed cryoglobulinaemia. Clin Exp Immunol. 2000;122:400–403. doi: 10.1046/j.1365-2249.2000.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loffreda S., Muratori P., Muratori L., Mele L., Bianchi F.B., Lenzi M. Enhanced monocyte Th1 cytokine production in HCV-infected cryoglobulinemic patients. J Hepatol. 2003;38:230–236. doi: 10.1016/s0168-8278(02)00353-7. [DOI] [PubMed] [Google Scholar]
- 25.Saadoun D., Boyer O., Trebeden-Negre H. Predominance of type 1 (Th1) cytokine production in the liver of patients with HCV-associated mixed cryoglobulinemia vasculitis. J Hepatol. 2004;41:1031–1037. doi: 10.1016/j.jhep.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Boyer O., Saadoun D., Abriol J. CD4+CD25+ regulatory T-cell deficiency in patients with hepatitis C-mixed cryoglobulinemia vasculitis. Blood. 2004;103:3428–3430. doi: 10.1182/blood-2003-07-2598. [DOI] [PubMed] [Google Scholar]
- 27.Saadoun D., Rosenzwajg M., Joly F. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 28.Horcajada J.P., Garcia-Bengoechea M., Cilla G., Etxaniz P., Cuadrado E., Arenas J.I. Mixed cryoglobulinaemia in patients with chronic hepatitis C infection: prevalence, significance and relationship with different viral genotypes. Ann Med. 1999;31:352–358. doi: 10.3109/07853899908995902. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann H., Schott P., Polzien F. Cryoglobulinemia in chronic hepatitis C virus infection: prevalence, clinical manifestations, response to interferon treatment and analysis of cryoprecipitates. Z Gastroenterol. 1995;33:643–650. [PubMed] [Google Scholar]
- 30.Maillard P., Lavergne J.P., Siberil S. Fcgamma receptor-like activity of hepatitis C virus core protein. J Biol Chem. 2004;279:2430–2437. doi: 10.1074/jbc.M311470200. [DOI] [PubMed] [Google Scholar]
- 31.Sansonno D., Dammacco F. Hepatitis C virus, cryoglobulinaemia, and vasculitis: immune complex relations. Lancet Infect Dis. 2005;5:227–236. doi: 10.1016/S1473-3099(05)70053-0. [DOI] [PubMed] [Google Scholar]
- 32.Brouet J.C., Clauvel J.P., Danon F., Klein M., Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57:775–788. doi: 10.1016/0002-9343(74)90852-3. [DOI] [PubMed] [Google Scholar]
- 33.Monti G., Galli M., Invernizzi F. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC. Italian Group for the Study of Cryoglobulinaemias. QJM. 1995;88:115–126. [PubMed] [Google Scholar]
- 34.Agnello V., Chung R.T., Kaplan L.M. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 35.Ferri C., Sebastiani M., Giuggioli D. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33:355–374. doi: 10.1016/j.semarthrit.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Pawlotsky J.M., Roudot-Thoraval F., Simmonds P. Extrahepatic immunologic manifestations in chronic hepatitis C and hepatitis C virus serotypes. Ann Intern Med. 1995;122:169–173. doi: 10.7326/0003-4819-122-3-199502010-00002. [DOI] [PubMed] [Google Scholar]
- 37.Frangeul L., Musset L., Cresta P., Cacoub P., Huraux J.M., Lunel F. Hepatitis C virus genotypes and subtypes in patients with hepatitis C, with and without cryoglobulinemia. J Hepatol. 1996;25:427–432. doi: 10.1016/s0168-8278(96)80200-5. [DOI] [PubMed] [Google Scholar]
- 38.Siagris D., Christofidou M., Tsamandas A., Lekkou A., Thomopoulos K., Labropoulou-Karatza C. Cryoglobulinemia and progression of fibrosis in chronic HCV infection: cause or effect? J Infect. 2004;49:236–241. doi: 10.1016/j.jinf.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Lunel F., Musset L., Cacoub P. Cryoglobulinemia in chronic liver diseases: role of hepatitis C virus and liver damage. Gastroenterology. 1994;106:1291–1300. doi: 10.1016/0016-5085(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 40.Cacoub P., Poynard T., Ghillani P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204–2212. doi: 10.1002/1529-0131(199910)42:10<2204::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 41.Cicardi M., Cesana B., Del Ninno E. Prevalence and risk factors for the presence of serum cryoglobulins in patients with chronic hepatitis C. J Viral Hepat. 2000;7:138–143. doi: 10.1046/j.1365-2893.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- 42.Wong V.S., Egner W., Elsey T., Brown D., Alexander G.J. Incidence, character and clinical relevance of mixed cryoglobulinaemia in patients with chronic hepatitis C virus infection. Clin Exp Immunol. 1996;104:25–31. doi: 10.1046/j.1365-2249.1996.d01-639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sene D., Ghillani-Dalbin P., Thibault V. Longterm course of mixed cryoglobulinemia in patients infected with hepatitis C virus. J Rheumatol. 2004;31:2199–2206. [PubMed] [Google Scholar]
- 44.Younossi Z., Park H., Henry L., Adeyemi A., Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150:1599–1608. doi: 10.1053/j.gastro.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 45.Mahale P., Engels E.A., Li R. The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut. 2017 doi: 10.1136/gutjnl-2017-313983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kayali Z., Buckwold V.E., Zimmerman B., Schmidt W.N. Hepatitis C, cryoglobulinemia, and cirrhosis: a meta-analysis. Hepatology (Baltimore, Md) 2002;36:978–985. doi: 10.1053/jhep.2002.35620. [DOI] [PubMed] [Google Scholar]
- 47.Giordano T.P., Henderson L., Landgren O. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010–2017. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 48.Ferri C., Zignego A.L., Pileri S.A. Cryoglobulins. J Clin Pathol. 2002;55:4–13. doi: 10.1136/jcp.55.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charles E.D., Dustin L.B. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 2009;76:818–824. doi: 10.1038/ki.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Congia M., Clemente M.G., Dessi C. HLA class II genes in chronic hepatitis C virus-infection and associated immunological disorders. Hepatology (Baltimore, Md) 1996;24:1338–1341. doi: 10.1002/hep.510240603. [DOI] [PubMed] [Google Scholar]
- 51.Amoroso A., Berrino M., Canale L. Are HLA class II and immunoglobulin constant region genes involved in the pathogenesis of mixed cryoglobulinemia type II after hepatitis C virus infection? J Hepatol. 1998;29:36–44. doi: 10.1016/s0168-8278(98)80176-1. [DOI] [PubMed] [Google Scholar]
- 52.Lenzi M., Frisoni M., Mantovani V. Haplotype HLA-B8-DR3 confers susceptibility to hepatitis C virus-related mixed cryoglobulinemia. Blood. 1998;91:2062–2066. [PubMed] [Google Scholar]
- 53.Nagasaka A., Takahashi T., Sasaki T. Cryoglobulinemia in Japanese patients with chronic hepatitis C virus infection: host genetic and virological study. J Med Virol. 2001;65:52–57. [PubMed] [Google Scholar]
- 54.Hwang S.J., Chu C.W., Huang D.F., Lan K.H., Chang F.Y., Lee S.D. Genetic predispositions for the presence of cryoglobulinemia and serum autoantibodies in Chinese patients with chronic hepatitis C. Tissue Antigens. 2002;59:31–37. doi: 10.1034/j.1399-0039.2002.590106.x. [DOI] [PubMed] [Google Scholar]
- 55.Sebastiani G.D., Bellisai F., Caudai C. Association of extrahepatic manifestations with HLA class II alleles and with virus genotype in HCV infected patients. J Biol Regul Homeost Agents. 2005;19:17–22. [PubMed] [Google Scholar]
- 56.De Re V., Caggiari L., De Vita S. Genetic insights into the disease mechanisms of type II mixed cryoglobulinemia induced by hepatitis C virus. Dig Liver Dis. 2007;39(suppl 1):S65–S71. doi: 10.1016/s1590-8658(07)80014-4. [DOI] [PubMed] [Google Scholar]
- 57.Zignego A.L., Wojcik G.L., Cacoub P. Genome-wide association study of hepatitis C virus- and cryoglobulin-related vasculitis. Genes Immunity. 2014;15:500–505. doi: 10.1038/gene.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gragnani L., Piluso A., Giannini C. Genetic determinants in hepatitis C virus-associated mixed cryoglobulinemia: role of polymorphic variants of BAFF promoter and Fcgamma receptors. Arthritis Rheum. 2011;63:1446–1451. doi: 10.1002/art.30274. [DOI] [PubMed] [Google Scholar]
- 59.Landau D.A., Scerra S., Sene D., Resche-Rigon M., Saadoun D., Cacoub P. Causes and predictive factors of mortality in a cohort of patients with hepatitis C virus-related cryoglobulinemic vasculitis treated with antiviral therapy. J Rheumatol. 2010;37:615–621. doi: 10.3899/jrheum.090790. [DOI] [PubMed] [Google Scholar]
- 60.Ramos-Casals M., Loustaud-Ratti V., De Vita S. Sjogren syndrome associated with hepatitis C virus: a multicenter analysis of 137 cases. Medicine (Baltimore) 2005;84:81–89. doi: 10.1097/01.md.0000157397.30055.c9. [DOI] [PubMed] [Google Scholar]
- 61.Ioannidis J.P., Vassiliou V.A., Moutsopoulos H.M. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjogren's syndrome. Arthritis Rheum. 2002;46:741–747. doi: 10.1002/art.10221. [DOI] [PubMed] [Google Scholar]
- 62.Ramos-Casals M., De Vita S., Tzioufas A.G. Hepatitis C virus, Sjogren's syndrome and B-cell lymphoma: linking infection, autoimmunity and cancer. Autoimmun Rev. 2005;4:8–15. doi: 10.1016/j.autrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Ramos-Casals M., Brito-Zeron P., Yague J. Hypocomplementaemia as an immunological marker of morbidity and mortality in patients with primary Sjogren's syndrome. Rheumatology (Oxford) 2005;44:89–94. doi: 10.1093/rheumatology/keh407. [DOI] [PubMed] [Google Scholar]
- 64.Tarantino A., Campise M., Banfi G. Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int. 1995;47:618–623. doi: 10.1038/ki.1995.78. [DOI] [PubMed] [Google Scholar]
- 65.Daghestani L., Pomeroy C. Renal manifestations of hepatitis C infection. Am J Med. 1999;106:347–354. doi: 10.1016/s0002-9343(99)00014-5. [DOI] [PubMed] [Google Scholar]
- 66.Roccatello D., Fornasieri A., Giachino O. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis. 2007;49:69–82. doi: 10.1053/j.ajkd.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 67.Johnson R.J., Willson R., Yamabe H. Renal manifestations of hepatitis C virus infection. Kidney Int. 1994;46:1255–1263. doi: 10.1038/ki.1994.393. [DOI] [PubMed] [Google Scholar]
- 68.Beddhu S., Bastacky S., Johnson J.P. The clinical and morphologic spectrum of renal cryoglobulinemia. Medicine (Baltimore) 2002;81:398–409. doi: 10.1097/00005792-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Saadoun D., Asselah T., Resche-Rigon M. Cryoglobulinemia is associated with steatosis and fibrosis in chronic hepatitis C. Hepatology (Baltimore, Md) 2006;43:1337–1345. doi: 10.1002/hep.21190. [DOI] [PubMed] [Google Scholar]
- 70.Gisbert J.P., Garcia-Buey L., Pajares J.M., Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin's lymphoma: systematic review and meta-analysis. Gastroenterology. 2003;125:1723–1732. doi: 10.1053/j.gastro.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 71.Monti G., Pioltelli P., Saccardo F. Incidence and characteristics of non-Hodgkin lymphomas in a multicenter case file of patients with hepatitis C virus-related symptomatic mixed cryoglobulinemias. Arch Intern Med. 2005;165:101–105. doi: 10.1001/archinte.165.1.101. [DOI] [PubMed] [Google Scholar]
- 72.Sargur R., White P., Egner W. Cryoglobulin evaluation: best practice? Ann Clin Biochem. 2010;47:8–16. doi: 10.1258/acb.2009.009180. [DOI] [PubMed] [Google Scholar]
- 73.Shihabi Z.K. Cryoglobulins: an important but neglected clinical test. Ann Clin Lab Sci. 2006;36:395–408. [PubMed] [Google Scholar]
- 74.Vallat L., Benhamou Y., Gutierrez M. Clonal B cell populations in the blood and liver of patients with chronic hepatitis C virus infection. Arthritis Rheum. 2004;50:3668–3678. doi: 10.1002/art.20594. [DOI] [PubMed] [Google Scholar]
- 75.Quartuccio L., Fabris M., Salvin S. Bone marrow B-cell clonal expansion in type II mixed cryoglobulinaemia: association with nephritis. Rheumatology (Oxford) 2007;46:1657–1661. doi: 10.1093/rheumatology/kem209. [DOI] [PubMed] [Google Scholar]
- 76.Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25. doi: 10.1186/1750-1172-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iannuzzella F., Vaglio A., Garini G. Management of hepatitis C virus-related mixed cryoglobulinemia. Am J Med. 2010;123:400–408. doi: 10.1016/j.amjmed.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 78.Alric L., Plaisier E., Thebault S. Influence of antiviral therapy in hepatitis C virus-associated cryoglobulinemic MPGN. Am J Kidney Dis. 2004;43:617–623. doi: 10.1053/j.ajkd.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 79.Cacoub P., Saadoun D., Limal N., Sene D., Lidove O., Piette J.C. PEGylated interferon alfa-2b and ribavirin treatment in patients with hepatitis C virus-related systemic vasculitis. Arthritis Rheum. 2005;52:911–915. doi: 10.1002/art.20958. [DOI] [PubMed] [Google Scholar]
- 80.Mazzaro C., Zorat F., Caizzi M. Treatment with peg-interferon alfa-2b and ribavirin of hepatitis C virus-associated mixed cryoglobulinemia: a pilot study. J Hepatol. 2005;42:632–638. doi: 10.1016/j.jhep.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 81.Saadoun D., Resche-Rigon M., Thibault V., Piette J.C., Cacoub P. Antiviral therapy for hepatitis C virus-associated mixed cryoglobulinemia vasculitis: a long-term followup study. Arthritis Rheum. 2006;54:3696–3706. doi: 10.1002/art.22168. [DOI] [PubMed] [Google Scholar]
- 82.Fabrizi F., Dixit V., Messa P. Antiviral therapy of symptomatic HCV-associated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019–1027. doi: 10.1002/jmv.23562. [DOI] [PubMed] [Google Scholar]
- 83.Manns M.P., McHutchison J.G., Gordon S.C. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet (London, England) 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 84.Fried M.W., Shiffman M.L., Reddy K.R. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 85.Gragnani L., Fognani E., Piluso A. Long-term effect of HCV eradication in patients with mixed cryoglobulinemia: a prospective, controlled, open-label, cohort study. Hepatology (Baltimore, Md) 2015;61:1145–1153. doi: 10.1002/hep.27623. [DOI] [PubMed] [Google Scholar]
- 86.Mazzaro C., Franzin F., Tulissi P. Regression of monoclonal B-cell expansion in patients affected by mixed cryoglobulinemia responsive to alpha-interferon therapy. Cancer. 1996;77:2604–2613. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2604::AID-CNCR26>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 87.Hermine O., Lefrere F., Bronowicki J.P. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- 88.Vallisa D., Bernuzzi P., Arcaini L. Role of anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade, B-cell, non-Hodgkin's lymphoma: a multicenter Italian experience. J Clin Oncol. 2005;23:468–473. doi: 10.1200/JCO.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 89.Gragnani L., Visentini M., Fognani E. Prospective study of guideline-tailored therapy with direct-acting antivirals for hepatitis C virus-associated mixed cryoglobulinemia. Hepatology (Baltimore, Md) 2016;64:1473–1482. doi: 10.1002/hep.28753. [DOI] [PubMed] [Google Scholar]
- 90.Saadoun D., Pol S., Ferfar Y. Efficacy and safety of sofosbuvir plus daclatasvir for treatment of HCV-associated cryoglobulinemia vasculitis. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 91.Saadoun D., Thibault V., Si Ahmed S.N. Sofosbuvir plus ribavirin for hepatitis C virus-associated cryoglobulinaemia vasculitis: VASCUVALDIC study. Ann Rheum Dis. 2016;75:1777–1782. doi: 10.1136/annrheumdis-2015-208339. [DOI] [PubMed] [Google Scholar]
- 92.Sollima S., Milazzo L., Peri A.M., Torre A., Antinori S., Galli M. Persistent mixed cryoglobulinaemia vasculitis despite hepatitis C virus eradication after interferon-free antiviral therapy. Rheumatology (Oxford) 2016;55:2084–2085. doi: 10.1093/rheumatology/kew268. [DOI] [PubMed] [Google Scholar]
- 93.Gragnani L., Piluso A., Urraro T. Virological and clinical response to interferon-free regimens in patients with HCV-related mixed cryoglobulinemia: preliminary results of a prospective pilot study. Curr Drug Targets. 2017;18:772–785. doi: 10.2174/1389450117666160208145432. [DOI] [PubMed] [Google Scholar]
- 94.Ramos-Casals M., Zignego A.L., Ferri C. Evidence-based recommendations on the management of extrahepatic manifestations of chronic hepatitis C virus infection. J Hepatol. 2017;66:1282–1299. doi: 10.1016/j.jhep.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 95.Comarmond C., Garrido M., Pol S. Direct-acting antiviral therapy restores immune tolerance to patients with hepatitis C virus-induced cryoglobulinemia vasculitis. Gastroenterology. 2017;152 doi: 10.1053/j.gastro.2017.02.037. 2052–2062.e2052. [DOI] [PubMed] [Google Scholar]
- 96.Emery J.S., Kuczynski M., La D. Efficacy and safety of direct acting antivirals for the treatment of mixed cryoglobulinemia. Am J Gastroenterol. 2017 doi: 10.1038/ajg.2017.49. [DOI] [PubMed] [Google Scholar]
- 97.Sise M.E., Bloom A.K., Wisocky J. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology (Baltimore, Md) 2016;63:408–417. doi: 10.1002/hep.28297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saadoun D., Resche Rigon M., Pol S. PegIFNalpha/ribavirin/protease inhibitor combination in severe hepatitis C virus-associated mixed cryoglobulinemia vasculitis. J Hepatol. 2015;62:24–30. doi: 10.1016/j.jhep.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 99.Bonacci M., Lens S., Londono M.C. Virologic, clinical, and immune response outcomes of patients with hepatitis C virus-associated cryoglobulinemia treated with direct-acting antivirals. Clin Gastroenterol Hepatol. 2017;15:575–583. doi: 10.1016/j.cgh.2016.09.158. e571. [DOI] [PubMed] [Google Scholar]
- 100.Giannelli F., Moscarella S., Giannini C. Effect of antiviral treatment in patients with chronic HCV infection and t(14;18) translocation. Blood. 2003;102:1196–1201. doi: 10.1182/blood-2002-05-1537. [DOI] [PubMed] [Google Scholar]
- 101.Radkowski M., Gallegos-Orozco J.F., Jablonska J. Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatology (Baltimore, Md) 2005;41:106–114. doi: 10.1002/hep.20518. [DOI] [PubMed] [Google Scholar]
- 102.Giannini C., Petrarca A., Monti M. Association between persistent lymphatic infection by hepatitis C virus after antiviral treatment and mixed cryoglobulinemia. Blood. 2008;111:2943–2945. doi: 10.1182/blood-2007-09-112490. [DOI] [PubMed] [Google Scholar]
- 103.Landau D.A., Saadoun D., Halfon P. Relapse of hepatitis C virus-associated mixed cryoglobulinemia vasculitis in patients with sustained viral response. Arthritis Rheum. 2008;58:604–611. doi: 10.1002/art.23305. [DOI] [PubMed] [Google Scholar]
- 104.Obata F., Murakami T., Miyagi J. A case of rapid amelioration of hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis treated by interferon-free directly acting antivirals for HCV in the absence of immunosuppressant. CEN Case Rep. 2017;6:55–60. doi: 10.1007/s13730-016-0244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsuge M., Hiramatsu A., Shinohara F. Improvement of renal dysfunction in a patient with hepatitis C virus-related liver cirrhosis by daclatasvir and asunaprevir combination therapy: a case report. Hepatol Res. 2016;46:944–948. doi: 10.1111/hepr.12629. [DOI] [PubMed] [Google Scholar]
- 106.Kirby B.J., Symonds W.T., Kearney B.P., Mathias A.A. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Clin Pharmacokinet. 2015;54:677–690. doi: 10.1007/s40262-015-0261-7. [DOI] [PubMed] [Google Scholar]
- 107.Toyoda H., Kumada T., Tada T. Safety and efficacy of dual direct-acting antiviral therapy (daclatasvir and asunaprevir) for chronic hepatitis C virus genotype 1 infection in patients on hemodialysis. J Gastroenterol. 2016;51:741–747. doi: 10.1007/s00535-016-1174-4. [DOI] [PubMed] [Google Scholar]
- 108.Kawakami Y., Imamura M., Ikeda H. Pharmacokinetics, efficacy and safety of daclatasvir plus asunaprevir in dialysis patients with chronic hepatitis C: pilot study. J Viral Hepat. 2016;23:850–856. doi: 10.1111/jvh.12553. [DOI] [PubMed] [Google Scholar]
- 109.Weiner G.J. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cacoub P., Comarmond C. New insights into HCV-related rheumatologic disorders: a review. J Adv Res. 2017;8:89–97. doi: 10.1016/j.jare.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sansonno D., De Re V., Lauletta G., Tucci F.A., Boiocchi M., Dammacco F. Monoclonal antibody treatment of mixed cryoglobulinemia resistant to interferon alpha with an anti-CD20. Blood. 2003;101:3818–3826. doi: 10.1182/blood-2002-10-3162. [DOI] [PubMed] [Google Scholar]
- 112.Zaja F., De Vita S., Mazzaro C. Efficacy and safety of rituximab in type II mixed cryoglobulinemia. Blood. 2003;101:3827–3834. doi: 10.1182/blood-2002-09-2856. [DOI] [PubMed] [Google Scholar]
- 113.Roccatello D., Baldovino S., Rossi D. Rituximab as a therapeutic tool in severe mixed cryoglobulinemia. Clin Rev Allergy Immunol. 2008;34:111–117. doi: 10.1007/s12016-007-8019-0. [DOI] [PubMed] [Google Scholar]
- 114.Petrarca A., Rigacci L., Caini P. Safety and efficacy of rituximab in patients with hepatitis C virus-related mixed cryoglobulinemia and severe liver disease. Blood. 2010;116:335–342. doi: 10.1182/blood-2009-11-253948. [DOI] [PubMed] [Google Scholar]
- 115.De Vita S., Quartuccio L., Isola M. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:843–853. doi: 10.1002/art.34331. [DOI] [PubMed] [Google Scholar]
- 116.Lake-Bakaar G., Dustin L., McKeating J., Newton K., Freeman V., Frost S.D. Hepatitis C virus and alanine aminotransferase kinetics following B-lymphocyte depletion with rituximab: evidence for a significant role of humoral immunity in the control of viremia in chronic HCV liver disease. Blood. 2007;109:845–846. doi: 10.1182/blood-2006-08-041525. [DOI] [PubMed] [Google Scholar]
- 117.Terrier B., Saadoun D., Sene D. Efficacy and tolerability of rituximab with or without PEGylated interferon alfa-2b plus ribavirin in severe hepatitis C virus-related vasculitis: a long-term followup study of thirty-two patients. Arthritis Rheum. 2009;60:2531–2540. doi: 10.1002/art.24703. [DOI] [PubMed] [Google Scholar]
- 118.Miloslavsky E.M., Specks U., Merkel P.A. Rituximab for the treatment of relapses in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2014;66:3151–3159. doi: 10.1002/art.38788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saadoun D., Resche-Rigon M., Sene D., Perard L., Karras A., Cacoub P. Rituximab combined with Peg-interferon-ribavirin in refractory hepatitis C virus-associated cryoglobulinaemia vasculitis. Ann Rheum Dis. 2008;67:1431–1436. doi: 10.1136/ard.2007.081653. [DOI] [PubMed] [Google Scholar]
- 120.Dammacco F., Tucci F.A., Lauletta G. Pegylated interferon-alpha, ribavirin, and rituximab combined therapy of hepatitis C virus-related mixed cryoglobulinemia: a long-term study. Blood. 2010;116:343–353. doi: 10.1182/blood-2009-10-245878. [DOI] [PubMed] [Google Scholar]
- 121.Sene D., Ghillani-Dalbin P., Amoura Z., Musset L., Cacoub P. Rituximab may form a complex with IgMkappa mixed cryoglobulin and induce severe systemic reactions in patients with hepatitis C virus-induced vasculitis. Arthritis Rheum. 2009;60:3848–3855. doi: 10.1002/art.25000. [DOI] [PubMed] [Google Scholar]
- 122.Lamprecht P., Gause A., Gross W.L. Cryoglobulinemic vasculitis. Arthritis Rheum. 1999;42:2507–2516. doi: 10.1002/1529-0131(199912)42:12<2507::AID-ANR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 123.Fabrizi F., Lunghi G., Messa P., Martin P. Therapy of hepatitis C virus-associated glomerulonephritis: current approaches. J Nephrol. 2008;21:813–825. [PubMed] [Google Scholar]
- 124.Magy N., Cribier B., Schmitt C. Effects of corticosteroids on HCV infection. Int J Immunopharmacol. 1999;21:253–261. doi: 10.1016/s0192-0561(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 125.Henry S.D., Metselaar H.J., Van Dijck J., Tilanus H.W., Van Der Laan L.J. Impact of steroids on hepatitis C virus replication in vivo and in vitro. Ann N Y Acad Sci. 2007;1110:439–447. doi: 10.1196/annals.1423.046. [DOI] [PubMed] [Google Scholar]
- 126.Dizdar O., Tapan U., Aksoy S., Harputluoglu H., Kilickap S., Barista I. Liver dysfunction after chemotherapy in lymphoma patients infected with hepatitis C. Eur J Haematol. 2008;80:381–385. doi: 10.1111/j.1600-0609.2008.01039.x. [DOI] [PubMed] [Google Scholar]
- 127.Della Rossa A., Baldini C., Tavoni A., Bombardieri S. How HCV has changed the approach to mixed cryoglobulinemia. Clin Exp Rheumatol. 2009;27:S115–S123. [PubMed] [Google Scholar]
- 128.Saleh F., Ko H.H., Davis J.E. Fatal hepatitis C associated fibrosing cholestatic hepatitis as a complication of cyclophosphamide and corticosteroid treatment of active glomerulonephritis. Ann Hepatol. 2007;6:186–189. [PubMed] [Google Scholar]
- 129.Ceballos-Viro J., Lopez-Picazo J.M., Perez-Gracia J.L., Sola J.J., Aisa G., Gil-Bazo I. Fibrosing cholestatic hepatitis following cytotoxic chemotherapy for small-cell lung cancer. World J Gastroenterol. 2009;15:2290–2292. doi: 10.3748/wjg.15.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fabrizi F., Bruchfeld A., Mangano S., Dixit V., Messa P., Martin P. Interferon therapy for HCV-associated glomerulonephritis: meta-analysis of controlled trials. Int J Artif Organs. 2007;30:212–219. doi: 10.1177/039139880703000306. [DOI] [PubMed] [Google Scholar]
- 131.Gladstone D.E., Golightly M.G., Zamkoff K.W. Severe, refractory type II essential mixed cryoglobulinemia treated with 2-chlorodeoxyadenosine and mycophenolate mofetil. Rheumatol Int. 2005;25:635–636. doi: 10.1007/s00296-004-0577-3. [DOI] [PubMed] [Google Scholar]
- 132.Chandesris M.O., Gayet S., Schleinitz N., Doudier B., Harle J.R., Kaplanski G. Infliximab in the treatment of refractory vasculitis secondary to hepatitis C-associated mixed cryoglobulinaemia. Rheumatology (Oxford) 2004;43:532–533. doi: 10.1093/rheumatology/keh080. [DOI] [PubMed] [Google Scholar]
- 133.Ballare M., Bobbio F., Poggi S. A pilot study on the effectiveness of cyclosporine in type II mixed cryoglobulinemia. Clin Exp Rheumatol. 1995;13(suppl 13):S201–S203. [PubMed] [Google Scholar]
- 134.Rockx M.A., Clark W.F. Plasma exchange for treating cryoglobulinemia: a descriptive analysis. Transfus Apher Sci. 2010;42:247–251. doi: 10.1016/j.transci.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 135.Ramunni A., Lauletta G., Brescia P. Double-filtration plasmapheresis in the treatment of leg ulcers in cryoglobulinemia. J Clin Apher. 2008;23:118–122. doi: 10.1002/jca.20166. [DOI] [PubMed] [Google Scholar]
- 136.Stefanutti C., Vivenzio A., Di Giacomo S. Immunoadsorption apheresis and immunosuppressive drug therapy in the treatment of complicated HCV-related cryoglobulinemia. J Clin Apher. 2009;24:241–246. doi: 10.1002/jca.20222. [DOI] [PubMed] [Google Scholar]
- 137.Hegazy M.T., Hussein M.A., Quartuccio L., Fawzy M., Zoheir N., Ellawindi M.I. Treatment of cryoglobulinemic vasculitis with sofosbuvir in four combination protocols. Arthritis Rheumatol. 2016;68:2977A. [Google Scholar]
- 138.Visentini M., Ludovisi S., Petrarca A. A phase II, single-arm multicenter study of low-dose rituximab for refractory mixed cryoglobulinemia secondary to hepatitis C virus infection. Autoimmun Rev. 2011;10:714–719. doi: 10.1016/j.autrev.2011.04.033. [DOI] [PubMed] [Google Scholar]