Abstract
Background and aims
Risk of infections is increased in patients with Acute Liver Failure (ALF) and Decompensated Chronic Liver Disease (DCLD). We evaluated the frequency, site, type and risk-factors for bacterial infections in children with ALF and DCLD and its effect on outcome.
Methods
ALF or DCLD children were enrolled prospectively. Clinical and laboratory details were recorded. Cultures (blood, urine and ascites) and chest X-ray were done at admission followed by weekly surveillance cultures.
Results
173 patients, 68 ALF and 105 DCLD were enrolled. Infections were more common in DCLD than ALF (60/105 [57.1%] vs. 27/68 [39.7%]; P = 0.02). Ascitic fluid infection, pneumonia, urinary tract infection and bacteremia were seen in 19%, 17.9%, 13.2% and 12.1% patients respectively. Healthcare-Associated (HCA) infections were most frequent (39/87, 44.8%), followed by Nosocomial (NC, 32%) and Community-Acquired (CA, 23%). Nearly 3/4th of bacterial isolates were resistant to cephalosporins and quinolones, 23% being Multiresistant Bacteria (MRB). DCLD patients with infection had higher Child–Pugh Score (10 [6–14] vs. 7 [6–14]; OR 3.2 [1.77–5.10]: P = 0.007), need for ICU care (26/60 vs. 3/45; OR 10.70 [2.98–38.42]: P = 0.01), in-hospital mortality (24/60 vs. 8/45;OR 3.08 [1.22–7.75]: P = 0.04) and mortality at 3 month follow-up (32/60 vs. 9/45; OR 4.57 [1.87–11.12]: P = 0.00). Infection did not affect the outcome in ALF.
Conclusion
Infections develop in 40% ALF and 57% DCLD children. HCA and NC infections account for 77% of infections. Most culture isolates are resistant to cephalosporins and fluoroquinolones and 23% have MRB. Risk of infections is higher in DCLD patients with advanced liver disease.
Abbreviations: ALF, Acute Liver Failure; CA, Community Acquired; DCLD, Decompensated Chronic Liver Diseases; GIB, Gastrointestinal Bleeding; GNB, Gram Negative Bacilli; GPC, Gram Positive Cocci; HCA, Healthcare Associated; HE, Hepatic Encephalopathy; ICU, Intensive Care Unit; INR, International Normalized Ratio; MRB, Multiresistant Bacteria; NC, Nosocomial; SBP, Spontaneous Bacterial Peritonitis; UTI, Urinary Tract Infection
Keywords: infections, liver failure, chronic liver disease
Bacterial infections are common in patients with Acute Liver Failure (ALF) and Decompensated Chronic Liver Disease (DCLD)1, 2 and are often fatal either by itself or by precipitation of renal failure, shock or Hepatic Encephalopathy (HE).3 Spontaneous Bacterial Peritonitis (SBP), Urinary Tract Infection (UTI), pneumonia and bacteremia are the most frequently encountered infections.4
There are reports of increasing antibiotic resistance overall and specifically in patients with liver disease.5 Antibiotics need to be used judiciously based on the likely organism and sensitivity pattern, to provide effective coverage and cost effective treatment while avoiding the development of resistance. Adult data cannot be extrapolated to children and unfortunately, data regarding infections in pediatric liver disease is scarce or unavailable.6, 7
The objectives of our study were to evaluate (1) the frequency, site and risk factors for bacterial infections in children with ALF and DCLD, (2) nature of bacterial isolates, their sensitivity pattern and effect on in-hospital outcome.
Patients and Methods
Prospective evaluation of bacterial infections occurring in children admitted with ALF or DCLD in our unit between March 2013 and November 2014 was done. ALF was defined as biochemical evidence of liver injury, no history of known chronic liver disease and coagulopathy not corrected by vitamin K administration i.e. INR > 1.5 in patients with Hepatic Encephalopathy [HE], or INR > 2.0 in patients without HE.8 The diagnosis of CLD was based on clinical, laboratory, ultrasonographic and endoscopic [≥grade II esophageal varices] findings with or without liver histology. Decompensation was defined as the presence of ascites, encephalopathy and/or gastrointestinal bleeding. After admission a detailed history was taken and clinical details including previous hospital admission and treatment given were recorded. The Child–Pugh score was calculated at admission.9
Cultures were drawn from blood, urine and ascitic fluid [if present]. Cultures were taken at admission prior to commencement of anti-microbial therapy. A chest X-ray was obtained if there was a clinical suspicion of pneumonia. Thereafter, surveillance cultures were sent on a weekly basis or earlier if there was a clinical suspicion of sepsis. In ventilated patients tracheal aspirates were taken on a weekly basis.
Blood cultures were routinely obtained from peripheral veins. Central line cultures were taken for suspected line sepsis or if unable to obtain peripheral cultures. Aseptic precautions were taken and an adequate blood volume was ensured.10 Urine samples were mid-stream clean catch or catheterized as per standard recommendations.11 Ascitic fluid was inoculated into the culture bottle at the patient's bedside as per guidelines.12 Blood and ascitic fluid cultures were inoculated into BACTEC culture media [Becton Dickinson Diagnostics, New Jersey, USA]. Urine cultures were inoculated on Crome media [HiMedia, Mumbai, India]. Isolated organisms were identified by standard methods and tested for antimicrobial susceptibility by both the disk-diffusion method and the BD Phoenix automated ID system [Becton Dickinson Diagnostics, New Jersey, USA] according to the recommendations of the Clinical and Laboratory Standards institute.13 Organisms were labeled as Multiresistant [MRB] if they were resistant to 3 different antibiotic families, including β-lactams.14
Infections were diagnosed on the basis of standard criteria. Presence of ascitic fluid infection and its type i.e. Spontaneous Bacterial Peritonitis [SBP], culture negative neutrocytic ascites and mono-microbial non-neutrocytic bacteriascites were defined as per standard definitions.12 Blood stream infection was diagnosed in presence of positive blood culture and Urinary Tract Infections [UTI] in the presence of both pyuria and a positive culture with a colony count of ≥105 organisms/ml. Pneumonia was diagnosed in patients with clinical features and suggestive chest X-ray.5
Infections diagnosed at admission or within 48 h of admission were classified as Healthcare Associated [HCA] if the patient had a history of hospitalization for at least 2 days in the previous 90 days or Community Acquired [CA] if no history of hospitalization in the previous 90 days was present.15 Infection was labeled as Nosocomial [NC] when the diagnosis of infection was made beyond 48 h of admission. A patient was labeled to have had a “recent” antibiotic exposure if he had received antibiotics for a period of >48 h in the preceding 90 days prior to admission.15 Urinary catheterization, ascitic fluid paracentesis, pleural tap, central line insertion, endoscopic sclerotherapy or band ligation or invasive ventilation were classified as invasive procedures for this study.
The Institute's Ethics Committee approved the study. Written informed consent was obtained from the parent or guardian of all participants.
Statistical Analysis
Data is represented as median [range] and percentages. SPSS [version 20.0; SPSS, Inc., Chicago, IL] was used for statistical analysis. Inter-group comparisons were performed using Mann–Whitney U test, Fisher's exact test or a one-way ANOVA. Odd's ratio was calculated where appropriate. Differences were considered significant at the level of 0.05.
A multivariate logistic regression model was used to predict the risk of infections in DCLD patients with the significant factors (P < 0.05) identified by univariate analysis. Main effects logistic regression model was used and scoring systems were not included in it because a score comprises of many parameters.
Results
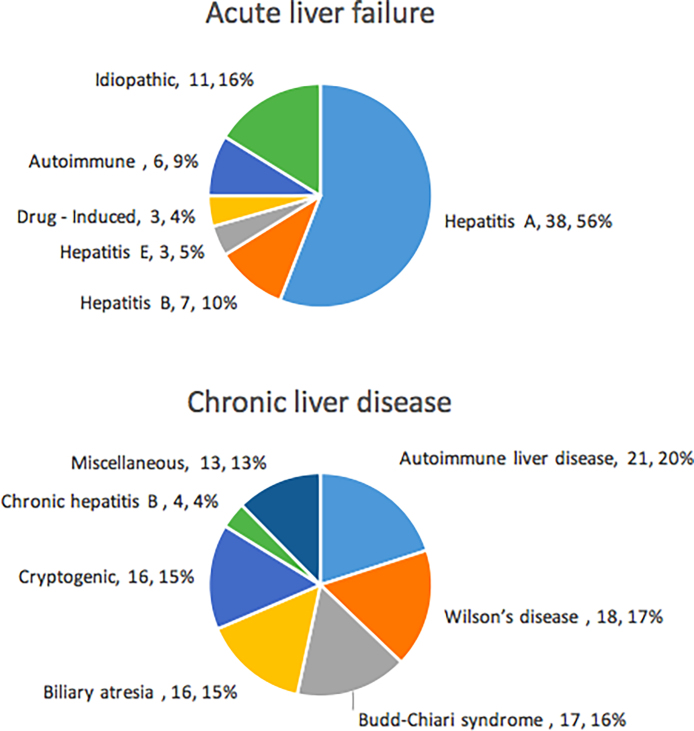
A total of 173 patients, 68 ALF [48 boys, age 72 [0.5–192] months] and 105 DCLD [70 boys, age 84 [3–204] months] were enrolled in the study. The etiology of liver disease in both the groups is illustrated in Figure 1.
Figure 1.
Etiology of liver disease in the patients enrolled in the study.
Prevalence and Site of Infection
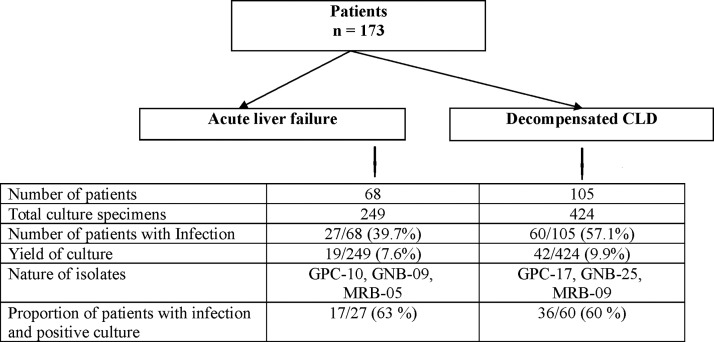
In the ALF group [n = 68], a total of 249 [4 (2–14) per patient] culture specimens [blood = 96, urine = 104, ascitic fluid = 44, tracheal aspirate = 5] were obtained (Figure 2). Twenty-seven [39.7%] patients had bacterial infection; 24 [35.2%] patients had single site and 3 [4.4%] had multiple site infection. UTI [11/68, 16.1%] was the most common site followed by pneumonia [10, 14.7%], ascitic fluid infection [8, 11.7%] and blood stream infection [6, 8.8%]. Bacterial infections were documented in 16/27 [59.2%] patients within 48 h of admission at our hospital [12 (75%) HCA and 4 (25%) CA]. Patients with HCA infections had a median hospital stay of 4 [2–24] days prior to coming to our center. NC infections were observed in 11 [40.7%] patients and were identified after 9 [7–14] days of hospitalization. UTI was the commonest HCA and CA infection seen in 8/12 [67%] and 4/4 [100%] patients respectively while pneumonia was the commonest NC infection [8/11 children].
Figure 2.
An overview of the patients with infections and the nature of their isolated pathogens.
In the DCLD group [n = 105], 424 [5 (3–22) per patient] culture specimens [blood = 180, urine = 184, ascites = 59, tracheal aspirate = 1] were obtained. Sixty [57.1%] DCLD patients had bacterial infection: ascitic fluid infection [25, 23.8%], pneumonia [21, 20%], blood stream infection [15, 14.2%] and UTI [12, 11.4%]. Single site infection was present in 46 [43.8%] patients and 14 [13.3%] had infections at multiple sites. Majority of the infections were HCA [27/60, 45%] with a median hospital stay of 6 [4–11] days prior to coming to our center. In patients with NC infections [n = 17/60, 28.3%], the infection was identified after a median of 11.5 [6–22] days post-admission to our hospital. Ascitic fluid infection was the commonest site of infection in CA, HCA and NC infections accounting for 31.2% [5/16], 51.8% [14/27] and 35.2% [6/17] cases respectively.
On comparison of the infection profile in ALF and DCLD patients, we found that infections were more common in DCLD [60 (57.1%) vs. 27 (39.7%), P = 0.02]. There was no difference in the site and type of infection between the groups.
Culture Isolates and Antibiotic Resistance
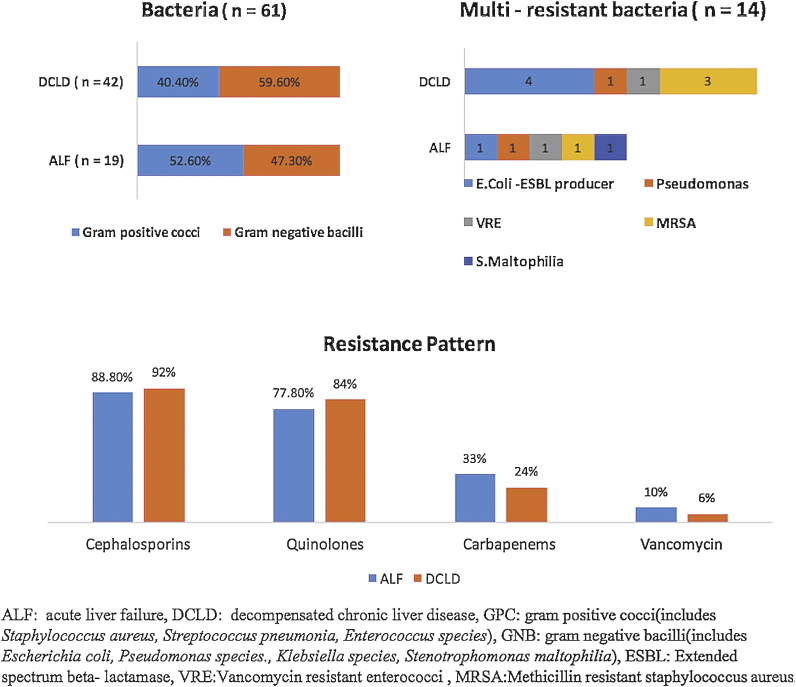
There were 19 positive culture isolates in ALF group and 42 in DCLD patients. Gram-Positive Cocci [GPC] were identified in 10/19 [52.6%] and 17/42 [40.4%] isolates while Gram-Negative Bacilli [GNB] accounted for 9 [47.3%] and 25 [59.5%] isolates in ALF and DCLD groups respectively (Figure 3). Overall the commonest isolated organism was Enterococcus faecalis [n = 7] in the ALF group and Escherichia coli [n = 16] in the DCLD group. GPC and GNB were equally distributed between HCA, CA and NC (Table 1).
Figure 3.
Type of isolated bacteria and antibiotic resistance in patients with acute liver failure and decompensated chronic liver disease.
Table 1.
Distribution of Isolated Pathogens by Type of Infection.
| CA (n = 14) | HCA (n = 29) | NC (n = 18) | P value | |
|---|---|---|---|---|
| Gram-positive cocci | 4 (3 + 1) | 15 (4 + 11) | 8 (3 + 5) | 0.32 |
| Gram negative bacilli | 10 (2 + 8) | 14 (6 + 8) | 10 (1 + 9) | 0.29 |
| Multi-resistant Bacteria |
1 (0 + 1) | 9 (3 + 6) | 4 (2 + 2) | 0.16 |
CA: Community Acquired, HCA: Health Care Associated, NC: Nosocomial.
ALF and DCLD separated by “+” in all columns. Groups were compared by ANOVA.
The resistance pattern to third generation cephalosporin, quinolones, carbapenems and vancomycin in ALF and DCLD children is shown in Figure 3. ALF and DCLD were similar in terms of isolated organisms and resistance pattern. Very high rates of resistance to third-generation cephalosporins and quinolones were seen in HCA [14/14 (100%) and 13/14 (92%) respectively] and NC infections [10/10 (100%) and 8/10 (80%)]. In DCLD patients with SBP, most GNB isolates 10/12 [83.3%] were resistant to third-generation cephalosporins and 7/10 [70%] were resistant to quinolones. Multi-Resistant Bacteria [MRB] were seen in 5/19 [26.3%] and 9/42 [21.4%] of overall isolates in ALF and DCLD respectively. There was no difference between the groups [5/19 vs. 9/42, P = 0.74].
Risk Factors for Infection
The prevalence of risk factors for infection in patients with DCLD is shown in Table 2. DCLD patients with infection had more advanced liver disease, as evident by a lower albumin [2.4 [0.9–5.7] vs. 2.9 [1.6–4.1] g/dL; P = 0.001], higher INR [2.2 [0.98–10] vs.1.76 [1–9.2]; P = 0.01] and higher Child–Pugh score [10 [6–14] vs. 7 [6–14]; P = 0.007] than those without infection. There was no difference in the nutritional status (height Z-score) [−0.1 (±0.8) vs. −0.3 (±0.9); P = 0.37] of children with and without an infection. On multivariate analysis, serum albumin levels [OR–0.73 (95% CI 0.57–0.93); P = 0.008] remained independently associated with developing infections, after tests for interaction.
Table 2.
Risk Factors of Infections in Patients With Decompensated Chronic Liver Disease.
| Infection present (n = 60) | No infection (n = 45) | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|
| Age (months) | 78 (3–180) | 87 (7–204) | 0.92 (0.67–1.22) | 0.24 |
| Known CLDa | 24 (40%) | 13 (29%) | 1.64 (0.71–3.74) | 0.87 |
| h/o prior decompensationb | 19 (31.7%) | 11 (24.4%) | 1.43 (0.59–3.42) | 0.41 |
| On SBP prophylaxis | 7 (11.7%) | 2 (4.4%) | 2.83 (0.56–14.38) | 0.48 |
| On Immuno-suppressive medicationsc | 8 (13.3%) | 4 (8.9%) | 1.57 (0.44–5.60) | 0.28 |
| Gastrointestinal bleeding | 10 (16.7%) | 6 (13.3%) | 1.30 (0.43–3.88) | 0.59 |
| Ascites | 57 (95%) | 42 (93.3%) | 1.35 (0.26–7.06) | 1.0 |
| Hepatic encephalopathy | 29 (48.3%) | 15 (33.3%) | 1.87 (0.84–4.16) | 0.16 |
| Invasive procedure | 27 (45%) | 15 (34%) | 1.63 (0.73–3.64) | 0.57 |
| Serum bilirubin (0.2–1 mg/dL) | 10.1 (0.5–33.5) | 5.6 (0.4–45.5) | 1.98 (0.32–6.77) | 0.39 |
| Serum albumin (3.5–5.5 g/dL) | 2.4 (0.9–5.7) | 2.9 (1.6–4.1) | 0.64 (0.21–0.88) | 0.001 |
| INR (0.9–1.2) | 2.2 (0.98–10) | 1.7 (1–9.2) | 1.44 (1.10–2.08) | 0.01 |
| Child–Pugh score | 10 (6–14) | 7 (6–14) | 3.2 (1.77–5.10) | 0.007 |
Continuous variables are shown as median (range); CI: Confidence Interval.
Known CLD: Patients who were diagnosed cases of chronic liver disease on follow-up and presented with decompensation.
h/o prior decompensation in the form of hepatic encephalopathy, gastrointestinal bleeding or ascites, from which the patient had recovered.
Autoimmune liver disease on therapy (n = 10), Langerhans cell histiocytosis (n = 1), Budd-Chiari Syndrome patient received prednisolone (2 mg/kg for 4 weeks) for unrelated cause; SBP-spontaneous bacterial peritonitis.
We also looked at risk factors for specific sites of infections. Need of ventilation [8/21 vs. 10/84; P = 0.02], presence of gastrointestinal bleeding [5/21 vs. 11/84; P = 0.33] and HE [12/21 vs. 27/84; P = 0.14] were compared for pneumonia. For UTI, female gender [7/12 vs. 28/93; P = 0.09] and urinary catheterization [5/12 vs. 20/93; P = 0.15] were compared and for ascitic fluid infection a history of paracentesis in the immediate past [13/25 vs. 20/80; P = 0.01] was evaluated.
In children with DCLD and infection subgroup [n = 60], 9 cases had MRB infection. Patients with MRB were more likely to have undergone an invasive procedure [9/9 vs. 33/51; P = 0.04] than those without MRB. Prior exposure to antibiotics [7/9 vs. 40/51; P = 1] and ongoing quinolone prophylaxis [2/9 vs. 6/51; P = 0.6] was similar between patients with and without MRB.
Infection and Effect on Outcome
DCLD patients with infection had a higher in-hospital mortality [24/60 [40%] vs. 8/45 [17.7%]; OR 3.08 95% CI (1.22–7.75), P = 0.01] and need for ICU admission [26/60 [43.3%] vs. 3/45 [6.6%]; OR 10.70 95%CI (2.98–38.42), P = 0.01] as compared to those without infection. However, there was no difference in the hospital stay in the two groups [7.5 [1–65] days vs. 8 [3–45] days; P = 0.9]. Amongst DCLD children, the mortality was higher in HCA [15/27] and NC [7/17] infections as compared to CA [2/16]. However, only HCA reached statistical significance [HCA vs. CA; P = 0.008, NC vs. CA; P = 0.11, HCA vs. NC; P = 0.53]. Amongst patients with infections, patients with MRB had a higher mortality rate, but it did not reach statistical significance [6/9 (66.7%) vs. 18/51 (35.3%); OR 3.66 95% CI (0.81–16.43) P = 0.08]. Site of infection did not have a bearing on mortality [ascitic fluid infection—7/25 (28%) vs. blood stream infection 5/15 (33%) vs. pneumonia 10/21 (47.6%) vs. UTI 2/12 (17%)]. At 3 months’ follow-up after discharge, DCLD patients in the infection group had a higher mortality [8/36 vs. 1/37; OR 4.57 95% CI (1.87–11.12), P = 0.01].
In ALF, presence of infection did not increase the mortality [12/27 vs. 21/41; P = 0.22]. Mortality was not affected by type of infection [HCA—5/12, NC—7/11, CA—0/4, HCA vs. CA; P = 0.24, NC vs. CA; P = 0.07, HCA vs. NC; P = 0.41]. Presence of MRB [1/5 vs. 11/22; P = 0.34] did not contribute to the mortality. Patients with infections had a hospital stay of 12.5 [2–45] days as compared to 17.5 [2–19] days without infection [P = 0.07].
Discussion
In this prospective study of 173 children with ALF and DCLD and a total of 673 culture specimens, a total of 108 infections were identified in 87/173 [50%] patients. 53/87 patients with infection had positive cultures with 61 culture-positive isolates (8 cases had isolates from >1 site) (Figure 2).
Overall 40% [27/68] of our ALF patients had infection. Of the 2 pediatric ALF studies, Godbole et al.6 in a retrospective review from UK reported infections in 25% children while Mekhala et al.,7 from Pondicherry, India reported an infection rate of 69%. Infections did not contribute to the mortality in ALF in our study, which is similar to the observation of the previous two pediatric studies.6, 7
Infection was present in 57% of our DCLD cases. There is no pediatric data available for comparison but the prevalence in adults ranges from 25% to 34%,4, 5 with a similar prevalence in the developed and developing world.16 In adults with cirrhosis the common infections are SBP [25%], UTI [20%], pneumonia [15%], bacteremia following a therapeutic procedure, cellulitis, and spontaneous bacteremia [17]. This is similar to our observation. AFI was the commonest site [23.8%], 64% [16/25] of all AFI were present at admission and E. coli was the commonest isolate. The prevalence of AFI in adult cirrhotic patients admitted to the hospital ranges from 10% to 30% and 50% of these are present at admission,17 which is similar to our findings. Amongst DCLD patients, 60% infections were due to GNB and 40% due to GPC. In adults with CLD, GNB ranged from 70% to 80%, with an increase in GPC isolates in the recent years.18 This phenomenon may be related to the current high rates of instrumentation and antibiotic usage in cirrhotic patients.
MRB were present in 9/60 [15%] patients with infection and accounted for 21% [9/42; BSI-3, AFI-3, UTI-3] of all positive cultures in patients with DCLD. In adults with cirrhosis, MRB are seen in 28–47% of positive culture isolates in tertiary care hospitals and have been associated with prior antibiotic exposure, long term quinolone prophylaxis and a prior history of infections.5, 19 However, these factors were not associated with MRB in our study. Reasons for a higher prevalence of MRB in adults as compared to children could be a longer disease duration possibly translating into more antibiotic exposure and hospitalizations and presence of co-morbidities like diabetes mellitus or chronic obstructive pulmonary disease etc. in adults. The mortality was highest in children with MRB in our study. Similarly, Salerno et al.20 reported higher mortality with MRB infections as compared to antibiotic susceptible infections (38% [24/63] vs. 22.8% [32/140]; P = 0.02) in adults with cirrhosis. MRB were more common in children who had undergone an invasive procedure and 93% [13/14] of the MRB infections were either HCA or NC. This suggests that children got infected with MRB itself, possibly transmitted during the procedure and highlights the importance of aseptic techniques.
Evaluation of risk factors of infection is necessary for risk stratification. DCLD patients with higher CPS were at an increased risk of infections. In patients with advanced cirrhosis, presence of gut dysbiosis, increased bacterial translocation and cirrhosis-associated immune dysfunction increases the susceptibility to infections.3 Obstien et al. reported a linear correlation between occurrence of SBP and MELD score in adults with cirrhosis, with every point increase in MELD increasing the risk of SBP by 11%.21
DCLD patients with infection had significantly higher requirement of ICU care, in-hospital mortality and mortality at 3 month follow-up after discharge as compared to patients without infection. This is similar to the observation in adults with cirrhosis, where in a compilation of 18 studies [11 prospective], 40.4% patients with infection and 19.5% without infection [P = 0.00001] died during the follow-up.22 This shows that prognosis is affected adversely even after resolution of infection in cirrhosis. It has been proposed that infections should be added as a clinical stage (stage 5) in the natural course of cirrhosis.22
The resistance spectrum of pathogens varies in different regions and local resistance patterns have to be known for appropriate antimicrobial use. We found a high prevalence of antibiotic resistance in our study. Third generation cephalosporin has been the antibiotic of choice for SBP treatment since the last decade.12, 23 Alarmingly, we found that most isolated gram-negative bacilli in AFI were resistant to it. In a study on adults, Fernandez et al. found a low efficacy of empirical therapy in SBP and 85% resistance to quinolones.5 We also found a high degree of resistance to fluoroquinolones that questions its use for SBP prophylaxis in our region.
Only 23% of the infections in our cohort were community-acquired, while in the study by Fernandez et al. the figure was 32%,5 bringing to light the fact that majority of infections are acquired from health-care settings. Simple preventive measures like hand washing, barrier nursing, aseptic precautions during invasive procedures, chest physiotherapy in ventilated patients and care of invasive catheters are helpful in reducing infections. There is a need to strike a balance between growing resistance amongst organisms with inappropriate antibiotic use and poor outcome in patients by using an inappropriate antibiotic. The first step would be to regularly send cultures prior to initiation of antibiotic therapy, even in primary health centers. Empirical broad spectrum antibiotics should be started based on the local sensitivity pattern and then de-escalated if required as escalation of therapy after unsuccessful empiric therapy to which the organism is insensitive carries an increased risk of mortality.24
Our study is the first prospective study of infections in a large number of children with DCLD and ALF of varying disease severity. The limitation is that our findings may be applicable to referral hospitals of the developing world only. However, it does highlight the increasing rates of infection, especially with resistant organisms even in children with liver disease. This highlights the urgent need to have continuous microbiology input to help select antibiotics as per the local isolates and resistance and not just follow the guidelines in sick children with liver disease.
To conclude, 40% patients with ALF and 57% with DCLD cases have bacterial infection. HCA infections are most common followed by NC infections and together they account for 77% of all infections. DCLD children with high Child Pugh score are more susceptible to infections and patients with infection have a poorer outcome both in terms of immediate and 3 months post discharge mortality. Most culture isolates are resistant to third generation cephalosporins and fluoroquinolones. 23% of all culture isolates are MRB, present more often in HCA and NC infection than CA. There is an urgent need to modify the empiric first line therapy in children with liver disease based on the local sensitivity patterns.
Author Contributions
RB: data collection, analysis and interpretation of data and drafting of the article. AS: data collection, concept and design, co-drafted the manuscript, approval of final version of the manuscript. RM: data collection, reviewing of manuscript, approval of final version of the manuscript SKY: critical revision of the manuscript for important intellectual content. UP: critical revision of the manuscript for important intellectual content.
Conflicts of Interest
The authors have none to declare.
References
- 1.Fernandez J., Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56:S1–S12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 2.Rolando N., Harvey F., Brahm J. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11:49–53. doi: 10.1002/hep.1840110110. [DOI] [PubMed] [Google Scholar]
- 3.Wong F., Bernardi M., Balk R. International Ascites Club. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718–725. doi: 10.1136/gut.2004.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borzio M., Salerno F., Piantoni L., Cazzaniga M., Angeli P., Bissoli F. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41–48. doi: 10.1016/s1590-8658(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez J., Acevedo J., Castro M. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 6.Godbole G., Shanmugam N., Dhawan A., Verma A. Infectious complications in pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2011;53:320–325. doi: 10.1097/MPG.0b013e318222b0cd. [DOI] [PubMed] [Google Scholar]
- 7.Mekala S., Jagadisan B., Parija S.C., Lakshminarayanan S. Surveillance for infectious complications in pediatric acute liver failure – a prospective study. Indian J Pediatr. 2015;82:260–266. doi: 10.1007/s12098-014-1497-1. [DOI] [PubMed] [Google Scholar]
- 8.Squires R.H., Shneider B.L., Bucuvalas J. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugh R.N., Murray-Lyon I.M., Dawson J.L. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 10.Buttery J.P. Blood cultures in newborns and children: optimizing an everyday test. Arch Dis Child Fetal Neonatal. 2002;87:25–28. doi: 10.1136/fn.87.1.F25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts K.B. Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595–610. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 12.Runyon B.A., AASLD Practice Guidelines Committee Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute . Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2014. Performance standards for antimicrobial susceptibility testing; Twentieth Informational Supplement, CLSI document M100-S24. [Google Scholar]
- 14.Magiorakos A.P., Srinivasan A., Carey R.B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 15.Friedman N.D., Kaye K.S., Stout J.E. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 16.Baijal R., Amarapurkar D., Praveen Kumar H.R. A multicenter prospective study of infections related morbidity and mortality in cirrhosis of liver. Indian J Gastroenterol. 2014;33:336–342. doi: 10.1007/s12664-014-0461-3. [DOI] [PubMed] [Google Scholar]
- 17.Caly W.R., Strauss E. A prospective study of bacterial infection in patients with cirrhosis. J Hepatol. 1993;18:353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez J., Navasa M., Gomez J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 19.Tandon P., Delisle A., Topal J.E., Garcia-Tsao G. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol. 2012;10:1291–1298. doi: 10.1016/j.cgh.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salerno F., Borzio M., Pedicino C. AISF Investigators. The impact of infection by multidrug-resistant agents in patients with cirrhosis. A multicenter prospective study. Liver Int. 2017;37:71–79. doi: 10.1111/liv.13195. [DOI] [PubMed] [Google Scholar]
- 21.Obstein K.L., Campbell M.S., Reddy K.R., Yang Y.X. Association between model for end-stage liver disease and spontaneous bacterial peritonitis. Am J Gastroenterol. 2007;102:2732–2736. doi: 10.1111/j.1572-0241.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 22.Arvaniti V., D’Amico G., Fede G., Manousou P., Tsochatzis E., Pleguezuelo M. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 23.European Association for the Study of the Liver EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Umgelter A., Reindl W., Miedaner M., Schmid R.M., Huber W. Failure of current antibiotic first-line regimens and mortality in hospitalized patients with spontaneous bacterial peritonitis. Infection. 2009;37:2–8. doi: 10.1007/s15010-008-8060-9. [DOI] [PubMed] [Google Scholar]