Abstract
Regorafenib has been demonstrated in our previous study to trigger apoptosis through suppression of extracellular signal-regulated kinase (ERK)/nuclear factor-κB (NF-κB) activation in hepatocellular carcinoma (HCC) SK-Hep1 cells in vitro. However, the effect of regorafenib on NF-κB-modulated tumor progression in HCC in vivo is ambiguous. The aim of the present study is to investigate the effect of regorafenib on NF-κB-modulated tumor progression in HCC bearing mouse model. pGL4.50 luciferase reporter vector transfected SK-Hep1 (SK-Hep1/luc2) and Hep3B 2.1-7 tumor bearing mice were established and used for the present study. Mice were treated with vehicle or regorafenib (20 mg/kg/day by gavage) for 14 days. Effects of regorafenib on tumor growth and protein expression together with toxicity of regorafenib were evaluated with digital caliper and bioluminescence imaging (BLI), ex vivo Western blotting immunohistochemistry (IHC) staining, and measurement of body weight and pathological examination of liver tissue, respectively, in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice. The results indicated regorafenib significantly reduced tumor growth and expression of phosphorylated ERK, NF-κB p65 (Ser536), phosphorylated AKT, and tumor progression-associated proteins. In addition, we found regorafenib induced both extrinsic and intrinsic apoptotic pathways. Body weight and liver morphology were not affected by regorafenib treatment. Our findings present the mechanism of tumor progression inhibition by regorafenib is linked to suppression of ERK/NF-κB signaling in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice.
Keywords: apoptosis, Bioluminescence imaging, hepatocellular carcinoma, nuclear factor kappaB, Regorafenib
Introduction
Human hepatocellular carcinoma (HCC), or primary malignancy of the liver, is notorious for the high mortality rate [1]. Progression of HCC is characterized by several cancer hallmarks such as sustained cell proliferation, evading apoptosis, and induction of angiogenesis and metastasis [2]. Many proliferative, antiapoptotic, angiogenic, and metastatic proteins are overexpressed and associated with poor prognosis in HCC patients [3,4].
Nuclear factor-κB (NF-κB), a transcription factor, modulates immunity, inflammation, and tumorigenesis through expression of NF-κB target genes. Activation of NF-κB can be triggered by upstream kinases and lead to expression of proliferative, antiapoptotic, angiogenic, and metastatic proteins in cancer cells [5]. Constitutive activation of NF-κB signal pathway was observed in HCC and may be a therapeutic target for HCC [6]. Many anti-HCC agents not only induce cytotoxicity but also activate NF-κB signal pathway resulting in limitation of therapeutic efficacy [7]. Inhibition of NF-κB signal pathway can block tumor progression in HCC in vitro and in vivo [8]. Therefore, patients with HCC may obtain benefit from development of inhibitors of NF-κB signal pathway.
Regorafenib (Stivarga), an analog of sorafenib, has been approved to treat HCC to prolong survival in patients who progressed on sorafenib treatment [9]. Previous studies indicate regorafenib induces apoptosis and inhibits metastatic potential through suppression of NF-κB activation in HCC cells in vitro [4,7]. However, whether regorafenib reduces NF-κB-modulated tumor progression in HCC in vivo needs to be elucidated. The aim of the present study is to investigate the effects of regorafenib on NF-κB-modulated tumor progression in SK-Hep1 hepatocellular carcinoma bearing mice. Effects of regorafenib on tumor growth and expression of NF-κB modulated angiogenic, metastatic, proliferative, and antiapoptotic proteins were evaluated by using caliper, bioluminescence imaging (BLI), ex vivo Western blotting, and immunohistochemistry (IHC) staining. The toxicity of regorafenib was determined with body weight of mice and hematoxylin and eosin (H&E) staining of liver sections.
Materials and methods
Reagents and antibodies
Regorafenib was obtained from Bayer Health Care Pharmaceuticals (Whippany, NJ, U.S.A.). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), L-glutamine, and penicillin–streptomycin (PS) were bought from Gibco/Life Technologies (Carlsbad, CA, U.S.A.). Hygromycin was bought from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). JetPEI™ transfection reagent was purchased from Polyplus Transfection (Sélestat, Bas-Rhin, France). D-luciferin was bought from Promega (Madison, WI, U.S.A.). IHC Select HRP/DAB kit was purchased from Merck Millipore (Darmstadt, Hessen, Germany). Primary antibodies of NF-κB p65 (Ser536), P-AKT (Ser473), T-AKT, cellular FADD-like IL-1β-converting enzyme (FLICE)-inhibitory protein (C-FLIP), Cyclin-D1, Caspase-3 and Caspase-9 were bought from Cell Signaling Technology (Beverly, MA, U.S.A.). Primary antibodies of X-linked inhibitor of apoptosis protein (XIAP), TATA-binding protein (TBP), and Caspase-8 were purchased from Thermo Fisher Scientific (Fremont, CA, U.S.A.). Primary antibodies for matrix metallopeptidase (MMP-9) and vascular endothelial growth factor (VEGF) were bought from EMD Millipore (Billerica, MA, U.S.A.). Primary antibodies of phosphorylated extracellular signal-regulated kinase (P-ERK), T-ERK, induced myeloid leukemia cell differentiation protein (MCL-1), and Caspase-9 were purchased from Merck Millipore (Billerica, MA, U.S.A.), BioVision (Milpitas, CA, U.S.A.), and Proteintech (Chicago, IL, U.S.A.) respectively.
Cell culture
HCC SK-Hep1 cells were obtained form by professor Jing-Gung Chung at Department of Biological Science and Technology, China Medical University, (Taichung, Taiwan) and used for the present study. HCC Hep3B 2.1-7 cells were purchased from Bioresource Collection and Research Center, Food Industry Research and Development Institute, Taiwan. Cells were both maintained in DMEM containing 10% FBS, PS (100 U/ml and 100 μg/ml), and 2 mM L-glutamine and incubated at 37°C in a 95% air and 5% CO2 humidified atmosphere [10]. pGL4.50 luciferase reporter vector transfected SK-Hep1 (SK-Hep1/luc2) were cultured in the same condition with addition of 100 μg/ml hygromycin.
Plasmid transfection and stable clone selection
pGL4.50 luciferase reporter (pGL4.50[luc2/CMV]) vector was purchased from Promega (Madison, WI, U.S.A.). SK-Hep1 cell was transfected with pGL4.50 [luc2/CMV] vector using JetPEI™ transfection reagent as described previously [7]. SK-Hep1/luc2 cells stably expressing luciferase were established by selection with adding 200 μg/ml hygromycin for 2 weeks.
Animal study
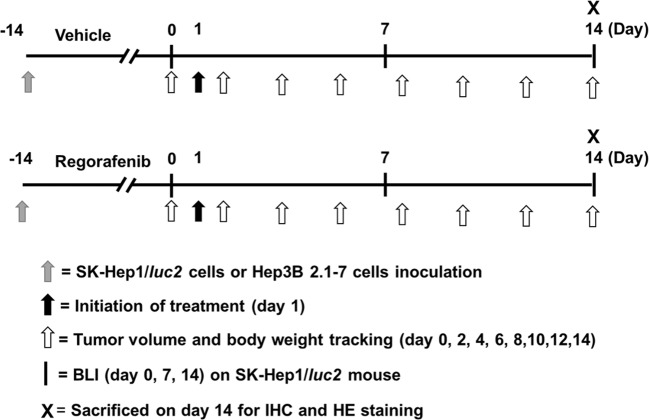
Animal study was approved by The Institutional Animal Care and Use Committee (IACUC) in Taipei Medical University, Taipei, Taiwan (IAUCU number: LAC-2016-0029). Four-week-old nude mice were obtained from the National Laboratory Animal Center, Taipei, Taiwan. SK-Hep1/luc2 (1 × 107) or Hep3B 2.1-7 cells (2 × 107) in 150 μl of mixture containing serum-free DMEM and matrigel (2:1) were inoculated subcutaneously into the right legs of nude mice [11]. When tumor volume reached about 200 mm3, mice were randomized into two groups (n = 5 for each group), vehicle group [treated with 140 μl of phosphate-buffered solution (PBS) plus 10 μl of dimethyl sulfoxide (DMSO) by gavage daily for 14 days] and regorafenib group (treated with 20 mg/kg/day by gavage for 14 days) (Figure 1). Treatment was initiated on day 1. Tumor volume was measured by digital caliper and calculated using formula 0.523 × length × width × thickness. Tumor growth was also monitored with BLI. The body weights of mice were measured after treatment together with tumor volume measurement. Mice were killed on day 14 for IHC staining of tumor tissues and pathologic examination of the liver. All animal experiments were repeated three times and complied with institutional animal care guidelines.
Figure 1. Schematic depiction of experimental protocol.
Please refers to “Material and methods” section in context for detail; HE, hematoxylin and eosin; IHC, immunohistochemistry.
In vivo bioluminescent imaging (BLI)
Mice were anesthetized using 1–3% isoflurane and injected intraperitoneally with 150 mg/kg D-luciferin 15 min before acquiring image. The photons emitted from tumor were detected with Xtreme (Bruker, Billerica, MA, U.S.A.). The image acquisition period was 10 s. Regions of interest (ROIs) of tumor were drawn and quantified by using Molecular Imaging Software (MI) version 7.2 (Bruker, Billerica, MA, U.S.A.) [8].
Immunohistochemistry (IHC) staining
Mice were killed on day 14 after treatments. Tumors were removed from mice and fixed in 4% paraformaldehyde (PFA) at 4°C. Paraffin-embedded tumor tissues were sectioned in 5 μm-thick slices by Bio-Check Laboratories Ltd (New Taipei City, Taiwan). Protein levels in tumor tissues were evaluated with IHC staining. The instructions provided with the kit were followed to perform IHC staining. The sectioned slices were immunohistochemically stained with MMP-9, VEGF, MCL-1, XIAP, C-FLIP, Cyclin-D1, anti-P-ERK, NF-κB p65 (Ser536), P-AKT (Ser473), Caspase-9, -8, and -3 antibodies respectively. The stained slides were scanned using the microscopy-based TissueFAXS platform (TissueGnostics, Vienna, Austria) and images were captured at 100× magnification [12]. ImageJ software version 1.50 (National Institutes of Health, Bethesda, MD, U.S.A.) was used to evaluate indices of positivity on IHC slides.
Ex vivo Western blotting
Proteins were extracted from mice tumors using nuclear and cytoplasmic extraction kits (Chemicon; EMD Millipore). Equal amount proteins were loaded on 10% sodium dodecyl sulfate/PAGE for electrophoresis assay under 100 voltage. After running step, proteins were transferred onto 0.2 μm polyvinylidene difluoride membrane and then blocking with 3% bovine serum albumin for 1 h. Primary antibodies of P-ERK (Thr202/Tyr204), T-ERK, NF-κB p65 (Ser536), P-AKT (Ser473), T-AKT, caspase-3, caspase-8 and caspase-9 were added on membrane and both were gently shaked on shaker in 4°C refrigerator overnight. The levels of protein were finally colored by enhanced chemiluminescence (ECL) and detected by ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc., CA, U.S.A.). Quantification of protein level was performed by Bio-Rad Image Lab [13].
Pathological examination
Mice were sacrificed on day 14 after treatments. Lobes of each mouse liver were removed and fixed in 4% PFA at 4°C. Paraffin-embedded liver tissues were sectioned in 5 μm-thick slices and then stained with hematoxylin and eosin (HE) by Bio-Check Laboratories Ltd (New Taipei City, Taiwan). The stained slides were scanned using the microscopy-based Tissue FAXS platform (TissueGnostics, Vienna, Austria) and images were captured at 100× magnification.
Statistical analysis
Student’s t-test was used to verify significance of difference between treatment and control group. Data presented were means ± standard error. Statistical significance was reached when P-value was less than 0.05. All experiments were repeated independently for three times.
Results
Regorafenib reduces tumor growth in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice
Mice bearing two different HCC cell lines SK-Hep1/luc2 and Hep3B 2.1-7 were prepared for animal studies respectively. The study procedure of animal was displayed as Figure 1. We used the digital caliper and BLI to evaluate the effect of regorafenib on tumor growth in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice in the experiment. We found regorafenib significantly inhibited both SK-Hep1/luc2 and Hep3B 2.1-7 tumor growth as compared with vehicle group (Figure 2A,B). The quantification result of tumor volume also showed marked suppression by regorafenib treatment in a SK-Hep1/luc2 and Hep3B 2.1-7 bearing model. In SK-Hep1/luc2 group, we also confirmed that photons emitted from the tumors of vehicle group were significantly higher than that of regorafenib group in Figure 2C. These results suggested that regorafenib effectively inhibited HCC tumor growth.
Figure 2. Effect of regorafenib on tumor growth in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice.
Mice were treated with vehicle (140 μl of PBS plus 10 μl of DMSO per day) or regorafenib (20 mg/kg/day) by gavage for 14 days. Tumor growth was evaluated with digital caliper and BLI. (A) SK-Hep1/luc2 tumor volume was measured by digital caliper on day 0, 2, 4, 6, 8, 10, 12, and 14. (B) Hep3B 2.1-7 tumor volume was measured by digital caliper on day 0, 2, 4, 6, 8, 10, 12, and 14. (C) SK-Hep1/luc2 tumor growth was monitored with BLI on day 0, 7, and 14; aP<0.01 and bP<0.05 as compared with vehicle group. BLI, bioluminescence imaging; DMSO, dimethyl sulfoxide; PBS, phosphate-buffered solution.
Regorafenib inhibits expression of ERK/NF-κB-modulated downstream effector proteins and triggers expression of apoptotic proteins in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice
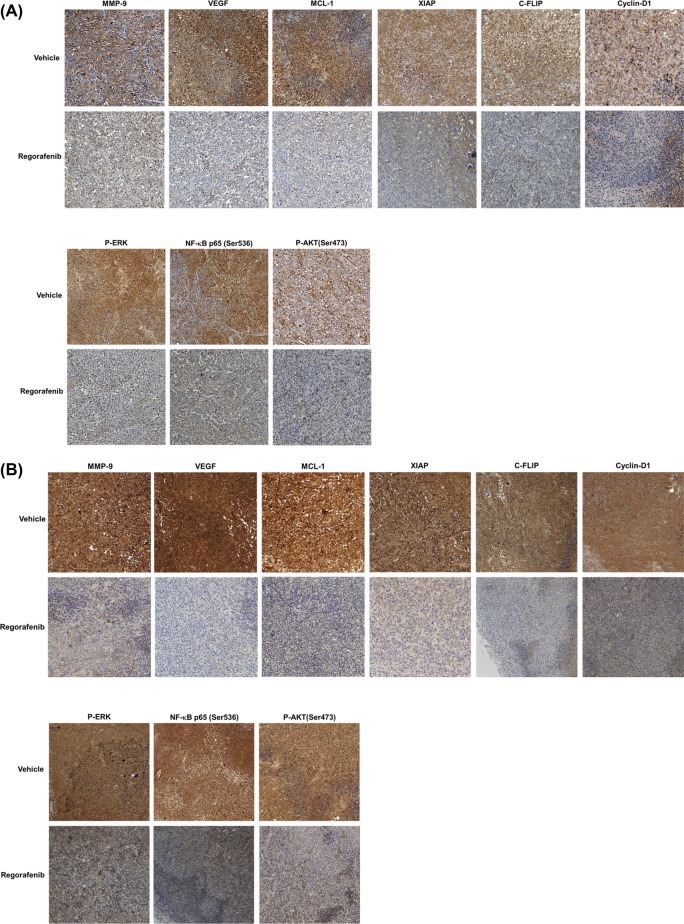
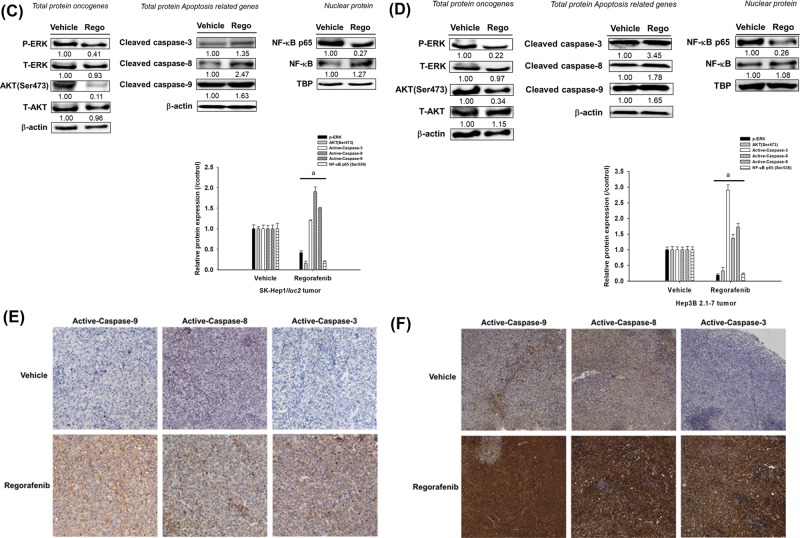
In order to measure the mechanism of regorafenib, we measured different types of proteins expression by IHC and ex vivo Western blotting. In Figure 3A,B IHC results, regorafenib significantly suppressed protein expression of MMP-9, VEGF, MCL-1, XIAP, C-FLIP, Cyclin-D1, P-ERK (Thr202/Tyr204), NF-κB p65 (Ser536), and AKT (Ser473), as compared with vehicle group. The inhibition of phospho-ERK, phospho-AKT, and NF-κB p65 proteins expression was double confirmed with ex vivo Western blotting assay (Figure 3C,D). After regorafenib treatment, the expression level of cleaved caspase-3, cleaved caspase-8, and caspase-9 was increased in two types of HCC model (Figure 3C,D). In addition, our IHC results also suggested that regorafenib may significantly induce expression of active Caspase-3, -8, and -9 as compared with vehicle group (Figure 3E,F). In sum, our results represented that regorafenib enhanced treatment efficacy of HCC via suppression of ERK/NF-κB signaling pathway.
Figure 3. Effect of regorafenib on expression of P-ERK, AKT (Ser473), NF-κB p65 (Ser473), and NF-κB-modulated downstream effector proteins in SK-Hep1/luc2 tumor and Hep3B 2.1-7 bearing mice.
Mice were killed on day 14 after treatments and protein levels in tumor tissues were evaluated with IHC staining. (A) Protein levels of MMP-9, VEGF, MCL-1, XIAP, C-FLIP, Cyclin-D1, and P-ERK, AKT (Ser473), NF-κB p65 (Ser473) on SK-Hep1/luc2 tumor by IHC. (B) IHC staining of Hep3B 2.1-7 tumor. (C) Phosphorylation oncogenes and apoptosis-related cleavage proteins expression which validated by Western blotting on SK-Hep1/luc2 tumor. (D) Western blotting of Hep3B 2.1-7 tumor. (E) Expression of antiapoptotic proteins (active Capase-9, -8, and -3) on SK-Hep1/luc2 tumor by IHC. (F) IHC staining of Hep3B 2.1-7 tumor; aP<0.01 as compared with vehicle group; C-FLIP, cellular FADD-like IL-1β-converting enzyme-inhibitory protein; IHC, immunohistochemistry; MCL, myeloid leukemia cell differentiation protein; MMP, matrix metallopeptidase; NF-κB, nuclear factor-κB; P-ERK, phosphorylated extracellular signal-regulated kinase; VEGF, vascular endothelial growth factor; XIAP, X-linked inhibitor of apoptosis protein.
General toxicity analysis of regorafenib in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice
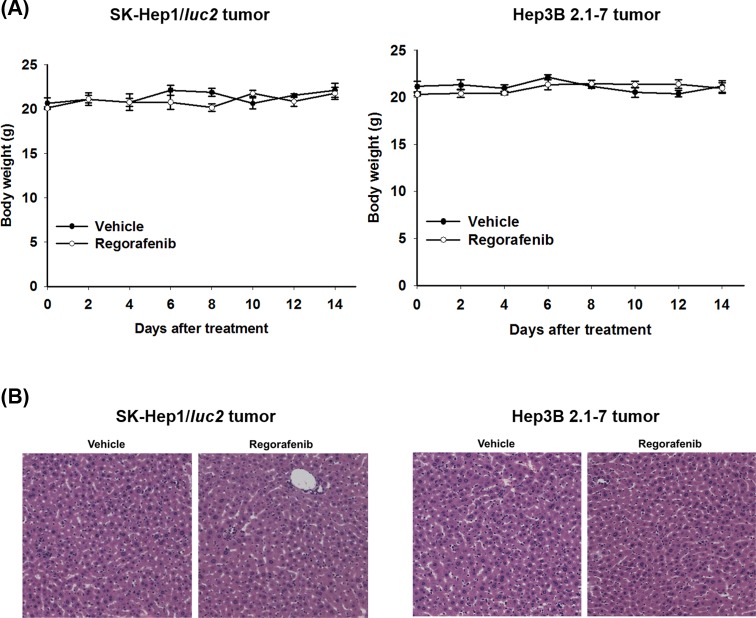
Body weight measurement and pathological examination of liver were used to monitor general toxicity of regorafenib in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice. Figure 4A presented no significant difference in body weight between regorafenib and vehicle groups. We also could not identify any obvious difference in liver morphology between two groups in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice (Figure 4B). Here, we proved that non-general toxicity was occurred after regorafenib administration.
Figure 4. Toxicity investigation of regorafenib in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice.
Toxicity of regorafenib was evaluated with body weight measurement and pathological examination of mice livers. (A) Body weight of both HCC bearing mouse were measured on day 0, 2, 4, 6, 8, 10, 12, and 14. (B) Mice were killed on day 14 after treatments and pathological examination of livers was evaluated with H&E staining; HE, hematoxylin and eosin.
Discussion
High expression of NF-κB activation, which plays key mediator of tumor progression, is associated with poor survival in HCC patients [14]. NF-κB target gene encoding proteins such as MMP-9, VEGF, MCL-1, XIAP, C-FLIP, and Cyclin-D1, promote metastasis, angiogenesis, antiapoptosis, and proliferation in HCC leading to tumor progression [4,7]. High protein levels of MMP-9, VEGF, MCL-1, XIAP, C-FLIP, and Cyclin-D1 have been suggested as biomarkers for poor prognosis and these proteins are potential therapeutic targets for the development of anticancer treatments in patients with HCC [15–18]. In the previous study, we found regorafenib, as inhibitor of NF-κB signaling, suppressed tumor growth and metastatic potential in HCC in vitro [4]. However, whether regorafenib down-regulates NF-κB-modulated tumor progression in HCC in vivo is ambiguous. Therefore, we used SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice to verify the effect of regorafenib on NF-κB-modulated tumor progression in HCC in vivo.
Regorafenib, a multikinase inhibitor, is used to treat different kinds of cancer. Su et al. [19] found regorafenib reduced protein expression of VEGF through suppression of signal transducer and activator of transcription 3 (STAT-3) activation in triple negative breast cancer cells in vitro and in vivo. Chen et al. [20] found regorafenib triggered p53-upregulated modulator of apoptosis (PUMA)-mediated apoptosis through induction of NF-κB pathway in colorectal cancer in vitro and in vivo. Tai et al. presented regorafenib induced apoptosis and inhibited expression levels of antiapoptotic and proliferative proteins (MCL-1, Survivin, and Cyclin-D1) via inhibition of STAT-3 activation. Tai et al. also presented tumor size was significant diminished by regorafenib treatment (20 mg/kg/day) as compared with vehicle group in HCC PLC tumor bearing mice [21]. In the present study, we began with finding the same dose of regorafenib as used by Tai et al. also significantly reduced tumor growth as compared with vehicle group in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice (Figure 2A,B). Next, we used ex vivo Western and IHC staining to verify the effect of regorafenib on NF-κB-modulated tumor progression in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice. The result presented regorafenib significantly reduced protein levels of P-ERK, P-AKT, NF-κB p65 (Ser536), MMP-9, VEGF, MCL-1, XIAP, C-FLIP, and Cyclin-D1 (Figure 3A–C).
Rapidly accelerated fibrosarcoma (RAF)/mitogen-activated protein kinase kinase (MEK)/ERK and phosphoinositide 3-kinase (PI3K)/AKT signaling cascades both play critical roles in the progression of HCC. NF-κB activation can be regulated by P-ERK and AKT in cancer cells [7,22]. In previous study, we presented regorafenib induced extrinsic and intrinsic apoptosis through inhibition of ERK/NF-κB activation in SK-Hep1 in vitro [7]. In contrast, Chen et al. [20] presented regorafenib inhibited ERK phosphorylation and induced expression of active-NF-κB, Caspase-3, -8, and -9 in colorectal cancer cells. Therefore, we performed the current in vivo study and Figure 3A–E indicated regorafenib not only reduced ERK, NF-κB, and AKT phosphorylation but also triggered expression of active Caspase-9, -8, and -3 in SK-Hep1/luc2 and Hep3B 2.1-7 tumor bearing mice. All these findings suggested mechanism of regorafenib anticancer effect in HCC in vivo is similar to that observed in vitro.
In conclusion, the present study demonstrated regorafenib inhibited tumor progression through suppression of ERK/NF-κB activation in HCC in vivo. We performed experiment on two different HCC bearing mice and suggested anticancer effect of regorafenib is mediated by the inhibition of ERK/NF-κB activation in HCC in vivo.
Abbreviations
- BLI
bioluminescence imaging
- C-FLIP
cellular FADD-like IL-1β-converting enzyme-inhibitory protein
- ERK
extracellular signal-regulated kinase
- HCC
hepatocellular carcinoma
- IHC
immunohistochemistry
- MCL-1
induced myeloid leukemia cell differentiation protein
- MMP9
matrix metallopeptidase
- NF-κB
nuclear factor-κB
- TBP
TATA-binding protein
- VEGF
vascular endothelial growth factor
Author Contribution
All the authors participated in the design, interpretation of the data, drafting and review of the manuscript.
Funding
We acknowledge the technical services provided by the Clinical Medicine Research Laboratory of National Yang Ming University Hospital. The study was supported by Taipei Medical University and Taipei Medical University Hospital [grant number TMU105-AE1-B49]. This project was also supported by the National Health Research Institutes [grant numbers MG-105-SP-07 and MG-106-SP-07]. The present study was also supported by a grant to J.-J.T. [grant number RD2016–020] from the National Yang-Ming University Hospital (Yilan, Taiwan).
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Zhu R.X., Seto W.K., Lai C.L. and Yuen M.F. (2016) Epidemiology of hepatocellular carcinoma in the asia-pacific region. Gut. Liver 10, 332–339 10.5009/gnl15257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu M., Jiang L. and Guan X.Y. (2014) The genetic and epigenetic alterations in human hepatocellular carcinoma: a recent update. Protein Cell 5, 673–691 10.1007/s13238-014-0065-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang I.T., Chen W.T., Tseng C.W., Chen Y.C., Kuo Y.C., Chen B.J. et al. (2017) Hyperforin inhibits cell growth by inducing intrinsic and extrinsic apoptotic pathways in hepatocellular carcinoma cells. Anticancer Res. 37, 161–167 10.21873/anticanres.11301 [DOI] [PubMed] [Google Scholar]
- 4.Liu Y.C., Wu R.H. and Wang W.S. (2017) Regorafenib diminishes the expression and secretion of angiogenesis and metastasis associated proteins and inhibits cell invasion via NF-κB inactivation in SK-Hep1 cells. Oncol. Lett. 14, 461–467 10.3892/ol.2017.6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y.C., Chiang I.T., Hsu F.T. and Hwang J.J. (2012) Using NF-κB as a molecular target for theranostics in radiation oncology research. Expert Rev. Mol. Diagn. 12, 139–146 10.1586/erm.12.2 [DOI] [PubMed] [Google Scholar]
- 6.Li W., Tan D., Zenali M.J. and Brown R.E. (2010) Constitutive activation of nuclear factor-kappa B (NF-κB) signaling pathway in fibrolamellar hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 3, 238–243 [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai J.J., Pan P.J. and Hsu F.T. (2017) Regorafenib induces extrinsic and intrinsic apoptosis through inhibition of ERK/NF-κB activation in hepatocellular carcinoma cells. Oncol. Rep. 37, 1036–1044 10.3892/or.2016.5328 [DOI] [PubMed] [Google Scholar]
- 8.Hsu F.T., Liu Y.C., Chiang I.T., Liu R.S., Wang H.E., Lin W.J. et al. (2014) Sorafenib increases efficacy of vorinostat against human hepatocellular carcinoma through transduction inhibition of vorinostat-induced ERK/NF-κB signaling. Int. J. Oncol. 45, 177–188 10.3892/ijo.2014.2423 [DOI] [PubMed] [Google Scholar]
- 9.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G. et al. (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 10.Ma C.Y., Ji W.T., Chueh F.S., Yang J.S., Chen P.Y., Yu C.C. et al. (2011) Butein inhibits the migration and invasion of SK-HEP-1 human hepatocarcinoma cells through suppressing the ERK, JNK, p38 and uPA signaling multiple pathways. J. Agric. Food. Chem. 59, 9032–9038 10.1021/jf202027n [DOI] [PubMed] [Google Scholar]
- 11.Della P.M, Badar A., Rosales C., Chokshi S., Kia A., Nathwani D. et al. (2015) Preferential targeting of disseminated liver tumors using a recombinant adeno-associated viral vector. Hum. Gene. Ther. 26, 94–103 10.1089/hum.2014.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali A.A., Hsu F.T., Hsieh C.L., Shiau C.Y., Chiang C.H., Wei Z.H. et al. (2016) Erlotinib-conjugated iron oxide nanoparticles as a smart cancer-targeted theranostic probe for MRI. Sci. Rep. 6, 36650 10.1038/srep36650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J.H., Chen W.L. and Liu Y.C. (2015) Amentoflavone induces antiangiogenic and anti-metastatic effects through suppression of NF-ĸB activation in MCF-7 cells. Anticancer Res. 35, 6685–6693 [PubMed] [Google Scholar]
- 14.Jin Y., Chen J., Feng Z., Fan W., Wang Y., Li J. et al. (2014) The expression of Survivin and NF-κB associated with prognostically worse clinicopathologic variables in hepatocellular carcinoma. Tumour Biol. 35, 9905–9910 10.1007/s13277-014-2279-0 [DOI] [PubMed] [Google Scholar]
- 15.Guo R.P., Zhong C., Shi M., Zhang C.Q., Wei W., Zhang Y.Q. et al. (2006) Clinical value of apoptosis and angiogenesis factors in estimating the prognosis of hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 132, 547–555 10.1007/s00432-006-0097-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Che Y., Ye F., Xu R., Qing H., Wang X., Yin F. et al. (2012) Co-expression of XIAP and cyclin D1 complex correlates with a poor prognosis in patients with hepatocellular carcinoma. Am. J. Pathol. 180, 1798–1807 10.1016/j.ajpath.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 17.Sieghart W., Losert D., Strommer S., Cejka D., Schmid K., Rasoul-Rockenschaub S. et al. (2006) Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J. Hepatol. 44, 151–157 10.1016/j.jhep.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 18.Du X., Bao G., He X., Zhao H., Yu F., Qiao Q. et al. (2009) Expression and biological significance of C-FLIP in human hepatocellular carcinomas. J. Exp. Clin. Cancer Res. 28, 24–31 10.1186/1756-9966-28-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su J.C., Mar A.C., Wu S.H., Tai W.T., Chu P.Y., Wu C.Y. et al. (2016) Disrupting VEGF-A paracrine and autocrine loops by targeting SHP-1 suppresses triple negative breast cancer metastasis. Sci. Rep. 6, 28888 10.1038/srep28888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen D., Wei L., Yu J. and Zhang L. (2014) Regorafenib inhibits colorectal tumor growth through PUMA-mediated apoptosis. Clin. Cancer Res. 20, 3472–3484 10.1158/1078-0432.CCR-13-2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai W.T., Chu P.Y., Shiau C.W., Chen Y.L., Li Y.S., Hung M.H. et al. (2014) STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin. Cancer Res. 20, 5768–5776 10.1158/1078-0432.CCR-14-0725 [DOI] [PubMed] [Google Scholar]
- 22.Cheng J.C., Chou C.H., Kuo M.L. and Hsieh C.Y. (2016) Radiation enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogen 25, 7009–7018 10.1038/sj.onc.1209706 [DOI] [PubMed] [Google Scholar]