Abstract
Recent studies have revealed that a combination of chemical compounds enables direct reprogramming from one somatic cell type into another without the use of transgenes by regulating cellular signaling pathways and epigenetic modifications. The generation of induced pluripotent stem (iPS) cells generally requires virus vector-mediated expression of multiple transcription factors, which might disrupt genomic integrity and proper cell functions. The direct reprogramming is a promising alternative to rapidly prepare different cell types by bypassing the pluripotent state. Because the strategy also depends on forced expression of exogenous lineage-specific transcription factors, the direct reprogramming in a chemical compound-based manner is an ideal approach to further reduce the risk for tumorigenesis. So far, a number of reported research efforts have revealed that combinations of chemical compounds and cell-type specific medium transdifferentiate somatic cells into desired cell types including neuronal cells, glial cells, neural stem cells, brown adipocytes, cardiomyocytes, somatic progenitor cells, and pluripotent stem cells. These desired cells rapidly converted from patient-derived autologous fibroblasts can be applied for their own transplantation therapy to avoid immune rejection. However, complete chemical compound-induced conversions remain challenging particularly in adult human-derived fibroblasts compared with mouse embryonic fibroblasts (MEFs). This review summarizes up-to-date progress in each specific cell type and discusses prospects for future clinical application toward cell transplantation therapy.
Keywords: chemical compound, direct reprogramming, regenerative medicine
Introduction
Terminally differentiated cells acquire a pluripotent state by forced expression of Yamanaka’s reprogramming factors [1–3]. Over the last 10 years, this discovery has provided a basic technique to manipulate cell fates in a transcription factor-based manner. The induced pluripotent stem (iPS) cells can be differentiated into numerous cell types, and are expected to be applied to drug screening, disease modeling, and transplantation therapy. However, both the reprogramming and differentiation processes raised potential safety concerns for the clinical application. The exogenous gene induction for the reprogramming might cause genomic instability and mutations [4]. In addition, contamination of residual undifferentiated cells after differentiations increases the risk for undesirable tumorigenesis after transplantation [5]. Currently, the characterization of pluripotency, differentiation potential, and genomic integrity in heterogeneous colonies of iPS cells is time-consuming and expensive, so it is not feasible that somatic cells isolated from each patient can be reprogrammed into iPS cells for their own transplantation therapy to avoid immune rejection [6]. Recently it has been reported that allogenic iPS cells with the same homozygous human leukocyte antigen (HLA) haplotype as the patient still can cause immune rejection responses in in vitro assay system [7]. The evidence also implies the importance of the use of each set of patient-derived autologous cells for their own transplantation therapy.
To solve the problems described above, direct lineage reprogramming or transdifferentiation is a promising alternative to rapidly prepare desired cell types from somatic cells by bypassing a pluripotent state. The direct reprogramming is generally achieved by forced expression of a set of lineage-specific transcription factors to establish a transcriptional network similar to the one in the specific cell type along with change in epigenetic modifications. So far accumulating evidence has demonstrated the direct reprogramming of mouse and human dermal fibroblasts into various cell types including neurones, neural stem cells, cardiomyocytes, hepatocytes, and brown adipocytes [8–12]. These reports indicate that the cell fate conversion is more flexible than we previously assumed. If desired cell types are rapidly converted within several weeks, autologous fibroblasts can be used for the patients’ own transplantation therapy. However, due to the requirement of simultaneous expression of multiple transcription factors in a single cell, the efficiency of the direct reprogramming is generally insufficient for the use of transplantation therapy without any sorting or purification steps. In addition, exogenous gene induction unexpectedly raises the risk for genomic instability and mutations, which might lead to tumor formation. The directly reprogrammed cells might be engrafted over a few years after transplantation to compensate for deficiency of tissue functions. Therefore, such a risk for tumorigenesis needs to be the lowest for the clinical applications to the maximum extent possible.
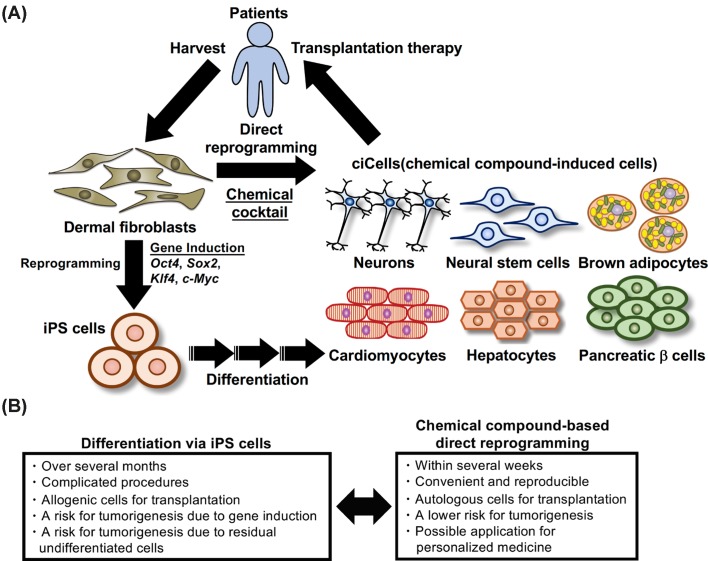
Recently, significant progress has been made in the field of direct lineage reprogramming by means of chemical compounds alone. The cell fate conversions are performed without the use of transgenes by regulating cellular signaling pathways and activity of histone/DNA modifying enzymes. In the beginning, scientists discovered a number of small molecules which significantly facilitate somatic cell reprogramming into iPS cells. Some of them enable replacement effects of the reprogramming factors such as Oct4, Sox2, and Klf4 [13–15]. Small molecules also can promote the efficiency of transcription factor-based direct reprogramming and sometimes replace the effects of transcription factors and cytokines, which is obviously helpful in preparing a large amount of cells in a defined and cost-effective manner [16,17]. They have several unique advantages in that they are preserved, highly purified, have a long half-life, are non-immunogenic, and effective at a low concentration. Accumulating evidence has demonstrated that mouse embryonic fibroblasts (MEFs) and/or human dermal fibroblasts were converted into several useful cell types including neurones, astrocytes, neural stem cells, brown adipocytes, cardiomyocytes, endoderm progenitor cells, and pluripotent stem cells (Figure 1A). However, in many cases the direct conversion is still particularly challenging in adult human fibroblasts compared with MEFs. In addition, complete chemical compound-based direct conversion into endoderm lineage cells such as hepatocytes and pancreatic β cells, the most important cell types in current regenerative medicine, has not been reported yet. Chemical compounds potentially affect wide-range gene expression and epigenetic modifications compared with the ones regulated by specific transcription factors. Nevertheless, the chemical compound-based strategy does not involve forced expression of exogenous genes as well as a pluripotent state, which could provide better cell sources for clinical applications with the lower risk for tumorigenesis (Figure 1B). This review summarizes the recent progress on chemical compound-based direct reprogramming in each specific cell type, problems to be solved, and future prospects for clinical applications.
Figure 1. Schematic figure for chemical compound-based direct reprogramming of human dermal fibroblasts into desired cell types applicable for transplantation therapy.
(A) Human dermal fibroblasts isolated from each patient are chemically converted into several desired cell types including neurones, neural stem/progenitor cells, brown adipocytes, cardiomyocytes, hepatocytes, and pancreatic β cells by regulating cellular signaling pathways and histone/DNA modification enzymes. iPS cells are reprogrammed by forced expression of transcription factors, then differentiated into these cells. (B) Compared with the differentiation via iPS cells, chemical compound-based direct reprogramming has a number of clear advantages for transplantation therapy, disease modeling, and drug development. In particular, the derivation and characterization of iPS cells for clinical applications still takes a long time and is expensive, so the use of autologous iPS cells derived from each patient might not be feasible. Chemical compound-based direct reprogramming is expected to rapidly convert autologous fibroblasts into desired cell types for the patients’ own transplantation. In addition, the chemically converted cells might have a lower risk for tumorigenesis compared with the cells prepared by forced expression of transcription factors as well as the cells inefficiently differentiated from iPS cells.
Neuronal cells
It is not feasible to prepare a sufficient amount of primary human neuronal cells to study neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS). In 2010, Vierbuchen et al. [8] first reported that MEFs were converted into neurones by forced expression of neurone-specific transcription factors, Brn2, Ascl1, and Myt1 (BAM). Later, a number of groups reported direct conversion of mouse and human fibroblasts into different neurone subtypes such as dopaminergic neurones and motor neurones by combination of BAM and each set of subtype-specific transcription factors [18–20]. These studies provided numerous insights into development of neural direct reprogramming in a chemical compound-based manner.
We first reported that the combination of six chemical compounds rapidly converted human dermal fibroblasts into chemical compound-induced neuronal cells (CiNCs) (Table 1) [21,22]. It should be noted that neonatal and adult dermal fibroblasts with different ages and genders were all converted efficiently. Not only a neuronal marker, Tuj1, but also the mature markers, MAP2 and Synapsin, were expressed in CiNCs. CiNCs were positive for both vGlut1 and GABA, suggesting that both glutamatergic (excitatory) and GABAergic (inhibitory) neurones were likely mixed in the population. The heterogeneous population of excitatory and inhibitory neurones shows the same tendency as reported in the other direct reprogramming into neuronal cells [8]. CiNCs exhibit a spike-like neuronal activity (unpublished data), suggesting that CiNCs could be functional neurones with basic electrophysiological properties. A spinal cord injury requires a rapid transplantation of neuronal cells at the subacute phase for efficient engraftment and recovery [23]. CiNCs can be rapidly prepared from autologous fibroblasts, and the risk for tumorigenesis is considered to be lower. Therefore, the transplantation of CiNCs might be one of the most promising strategies not only for treatment of spinal cord injury but also for other clinical applications. In addition, other neuronal subtypes such as cholinergic, serotonergic, and dopaminergic neurones might be generated by modifying the combination of chemical compounds, growth factors, and culture protocols.
Table 1. Summary of chemical compound-based direct reprogramming into neurones.
| Target cell type | Source cells | Generation | Chemical cocktail and cytokines | Induction/maturation medium | References |
|---|---|---|---|---|---|
| Neurone (glutamatergic neurones and GABAergic neurones) | Human dermal fibroblasts | Neonatal and adult | CHIR99021, PD0325901, LDN193189, SB431542, Pifithrin-α, and Forskolin | The N2B27 medium with the chemical cocktail for several weeks | [21] |
| Neurone (glutamatergic neurones and GABAergic neurones) | MEFs | Fetal | Forskolin, ISX9, CHIR99021, and I-BET151 | The neuronal induction medium with the chemical cocktail and basic fibroblast growth factor (bFGF) for 20 days. The maturation medium in co-culture of primary astrocytes or the neuronal induction medium containing Forskolin, bFGF, BDNF, GFNF for another 15 days | [24] |
| Neurone (glutamatergic neurones and GABAergic neurones) | Human foreskin fibroblasts, human dermal fibroblasts | Adult | VCRFSGY: VPA, CHIR99021, RepSox, Forskolin, SP600125, GO6983, and Y-27632 | The induction medium with the chemical cocktail for 8 days. The maturation medium with CHIR99021, Forskolin, and Dorsomorphin, and extra neurotrophic factors, BDNF, GDNF, and NT3 for another 6 days | [25] |
| Neurones (glutamatergic neurones) | Human primary astrocytes | Fetal | LDN193189, SB431542, TTNPB, Thiazovivin, CHIR99021, VPA, DAPT, SAG, and Purmophamine | The N2 medium with the nine chemical compounds was sequentially treated in a stepwise manner for 8 days. The neuronal differentiation medium containing three neurotrophic factors, BDNF, NT3, and IGF-1 from day 9 to 30 for the maturation | [30] |
| Neurones (glutamatergic neurones) | Human primary astrocytes | Adult | VPA, CHIR99021, RepSox, Forskolin, I-BET151, and ISX-9 | The induction medium with the chemical cocktail for 20 days. The neurone maturation medium with Forskolin and I-BET151 for further culture | [32] |
| Astrocytes | MEFs, human foreskin fibroblasts | Fetal | VAP, CHIR99021, SB431542, Tranylcypromine, and OAC1 | The induced astrocyte medium with fibroblast growth factor 2 (FGF2) and the chemical cocktail for 25 days (mouse) or 40 days (human) | [33] |
Thereafter, other groups also showed that MEFs and human dermal fibroblasts were, respectively, converted into functional neurones by a different set of chemical compounds. MEFs were converted into chemically induced neurones by four compounds including ISX9, Forskolin, CHIR99021, and I-BET151 [24]. A BET family Bromodomain inhibitor, I-BET151, repressed fibroblast specific gene expression to facilitate the direct conversion, while ISX9 is required for activation of neurone-specific gene expression. The chemically induced neurones are a mixture of glutamatergic and GABAergic neurones. Further study is required to know how the chemical combination is beneficial in the conversion of human dermal fibroblasts. Human adult fibroblasts derived from healthy individuals as well as familial Alzheimer’s disease patients were converted into neurones by seven chemical compounds, VCRFSGY, including VPA, CHIR99021, RepSox, Forskolin, SP600125, GO6983, and Y-27632 [25]. This group previously reported a conversion of MEFs into neural progenitor cells (NPCs) by combination of the VCR and hypoxia [26]. According to their hypothesis, the combination of chemicals for neuronal differentiation (FSGY) and partial reprogramming into NPCs (VCR) successfully converted human fibroblasts into neurone-like cells. After the induction of neural reprogramming, subsequent treatment with CHIR99021, Forskolin, and Dorsomorphin and extra neurotropic factors, BDNF, GDNF, and NT3, further promoted neuronal maturation and improved cell survival. The chemically induced neurones were also a mixture of glutamatergic and GABAergic neurones. The authors suggested that other neuronal subtypes might be generated with slightly modified chemical cocktails. The chemical direct reprogramming could be applied to generate patient-specific neuronal cells for neurological disease modeling, related mechanism studying, and drug screening. The amyloid β levels of the chemically induced neuronal cells were similar to those of transcription factor-induced neuronal cells derived from each familial Alzheimer’s disease patient, which suggests that chemical direct reprogramming provides an alternative approach for personalized disease modeling.
Meanwhile, several groups reported that neurones were directly converted from astrocytes by overexpression of exogenous transcription factors both in vitro and in vivo [27–29]. Astrocytes can be a cell source to regenerate neurones directly in the brain without transplantation. Zhang et al. [30] reported that sequential exposure of a set of chemical compounds directly converted primary astrocytes isolated from human fetal brain into neurones. The nine compounds used in the four steps are LDN193189, SB431542, TTNPB, Thiazovivin, CHIR99021, VPA, DAPT, SAG, and Purmophamine. Most of the astrocyte-derived neurones were glutamatergic neurones, and negative for the markers of cholinergic neurones, dopaminergic neurones, and spinal motor neurones. The neurones transplanted into mouse brain were engrafted over 1 month. Their protocol is available for human astrocytes but not mouse astrocytes, and for brain astrocytes but not spinal cord astrocytes, suggesting that the effects of chemical compounds and molecular mechanism underlying the direct reprogramming are slightly different in species and cellular subtypes. The transgene-free chemical-based technique might be further developed to regenerate neurones from astrocytes in a particular brain region in vivo. For clinical application of the in vivo reprogramming, a simpler protocol for treatment with the compounds and methods to deliver them into a particular brain region are required.
Human fetal astrocytes chemically converted into neurones, as described above, have different functional properties from adult astrocytes [31]. For the clinical application of in vivo direct reprogramming, adult astrocytes also need to be converted into neurones. Recently, Yu et al. [32] demonstrated that human adult astrocytes were more simply converted into neuronal cells by a different set of chemical compounds including VPA, CHIR99021, RepSox, Forskolin, I-BET151, and ISX-9. Tian et al. [33] reported that both mouse and human fibroblasts are directly converted into astrocytes in a chemical compound-based manner. The chemically induced astrocytes resemble primary astrocytes in terms of gene expression and functional properties. These reports expand a strategy to manipulate cell fates in fibroblasts, astrocytes, and neurones by using chemical compounds only.
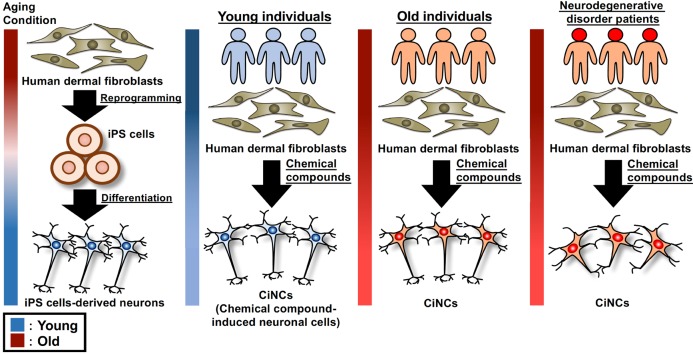
Along with increasing human longevity, the number of patients with neurological disorders is increasing. Ageing is one of the major risk factors for neurodegenerative disorders which are generally caused by abnormal accumulation of misfolded proteins in specific neurone subtypes [34,35]. It has been reported that the reprogramming into iPS cells by forced expression of Yamanaka’s factors erases most of the age-associated cellular and epigenetic marks [36]. To study a relation between ageing and neurological disorders, neuronal cells differentiated from iPS cell lines might not be appropriate as a disease model. The advantage of direct reprogramming is bypassing a pluripotent state, so the induced neuronal cells might conserve the same ageing conditions as source cells. Mertens et al. [37] demonstrated that neurones induced from human fibroblasts by transcription factors retained donor-age-dependent transcriptomic signatures and showed age-associated defects of nucleocytoplasmic compartmentalization. This observation implies that such ageing conditions might be conserved also in chemically induced neurones (Figure 2). Because forced expression of exogenous transcription factors for the direct reprogramming is supposed to unexpectedly damage proper epigenetic marks and genome integrity, such stress of chemical compound-based conversion should be milder, leading to better conservation of the ageing conditions. In the near future, the transgene-free, chemical-based strategy might be applied for personalized disease modeling to uncover the age-related pathological mechanism underlying neurodegenerative disorders.
Figure 2. Schematic figure for a possible application of chemical compound-induced neurones to uncover age-associated pathological mechanism.
The CiNCs are generated from human dermal fibroblasts isolated from young individuals, old individuals, and neurodegenerative disorder patients, respectively. The CiNCs might conserve donor-dependent ageing conditions, while it has been reported that iPS cells derived from the fibroblasts do not retain the ageing conditions. Comparison of transcriptome and repressive histone modifications in the induced neurones derived from young and old individuals might lead to identifying novel age-associated gene targets and the pathological mechanism underlying neurodegenerative disorders.
Neural stem cells
Cheng et al. [26] first reported direct conversion of MEFs, tail-tip fibroblasts, and human urinary cells into NPCs by a chemical cocktail consisting of VPA, CHIR99021, and RepSox under physiological hypoxic condition (5% O2) (Table 2). After the induction, the lineage specification in the chemically induced NPCs was gradually promoted by passaging in the neural expansion medium containing epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). The NPCs expressed major neural stem cell marker genes, Sox2 and Nestin, and could be differentiated into neurones, astrocytes, and oligodendrocytes. These results indicate that the induced NPCs resemble typical neural stem cells. A physiological hypoxia condition is likely beneficial in both the induction of NPCs and the proliferation. Such a hypoxic condition has been known to promote the reprogramming efficiency from fibroblasts into iPS cells by changing metabolic switch from oxidative to glycolytic metabolism [38]. In addition, the hypoxia condition might facilitate the conversion by activating the WNT signaling pathway, which is also activated by CHIR99021, an inhibitor of glycogen synthase kinase 3β (GSK3β) [39].
Table 2. Summary of chemical compound-based direct reprogramming into neuronal stem/progenitor cells.
| Target cell type | Source cells | Generation | Chemical cocktail and cytokines | Induction/maturation medium | References |
|---|---|---|---|---|---|
| NPCs | MEFs, mouse tail-tip fibroblasts, human urinary cells | Fetal neonatal adult | VPA, CHIR99021, and RepSox | The KSR medium (15% KSR) with the chemical cocktail for 10 days under 5% O2 condition | [26] |
| Neural stem cells | MEFs. mouse tail-tip fibroblasts | Fetal adult | VPA, BIX01294, RG108, PD0325901, CHIR99021, vitamin C, and A83-01 | The stem cell culture medium with the chemical cocktail for 1 day, then the medium without the compounds for 2 days. This cycle was repeated for a total of six times. The cells were cultured in the neural stem cell medium by suspending culture for another 2 weeks | [40] |
| Neural stem cells | MEFs | Fetal | A83-01, Thiazovivin, Purmophamine, and VPA | The standard NSC medium with the chemical cocktail for 10–12 days | [41] |
| Neural stem cells | MEFs | Fetal | CHIR99021, LDN193189, A83-01, RG108, Parnate, SMER28, retinoic acid, Hh-Ag1.5, and bFGF | The neural reprogramming basal medium with the chemical cocktail for 14 days under 5% O2 condition. After picking up colonies, the NSC medium under normal O2 condition for further culture | [42] |
| Neural stem cells | Human adipose-derived mesenchymal stem cells | Adult (23–26 years old) | SB431542, Noggin, and LDN193289 | The NSC medium containing the chemical cocktail and 3% KSR for 8 days. The B27N2 medium without EGF and fibroblast growth factor 2 (FGF2) for another 5 days. The B27N2 medium with EGF and FGF2 for another 7 days | [43] |
| Neural stem cells | Primary murine astrocytes | Adult | bFGF | The N2B27 medium including bFGF for 8 days | [44] |
Han et al. [40] reported direct reprogramming of MEFs into neural stem cells by using chemical compounds only. The culture protocol is uniquely repeating change of the medium with or without the chemical cocktail containing VPA, BIX01294, RG108, PD0325901, CHIR99021, vitamin C, and A83-01 for a total of six times. Then the 2-week suspension culture formed neurospheres expressed typical neural stem cell markers. The treatment with either chemical compounds such as A83-01, Thiazovivin, Purmophamine, VPA, or combinations of their substituents also induced the direct reprogramming from MEFs into neural stem cell-like cells [41]. The chemically induced neural stem cells described in these reports were tripotent and differentiated into neurones, astrocytes, and oligodendrocytes. Several common and different compounds were utilized in these various studies, so further studies are required to elucidate how signaling pathways regulated by each of them enable the direct conversion of MEFs into neural stem cells.
A different set of eight chemical compounds including CHIR99021, LDN193189, A83-01, RG108, Parnate, SMER28, retinoic acid, Hh-Ag1.5, and a growth factor, bFGF, was used for the direct reprogramming of MEFs into neural stem cells [42]. By genetic lineage tracing, the authors clearly demonstrated that the fibroblasts were converted into neural stem cells. They also identified signaling pathways and downstream transcription factors required for the direct conversion. They are Elk1 and Gli2 which are located at the downstream of the mitogen-activated protein kinase (MAPK) signaling pathway activated by bFGF and Sonic hedgehog (SHH) signaling pathway activated by Hh-Ag1.5, respectively. They were both recruited to the promoter region of Sox2, a master transcription factor for neural stem cells. The inhibition of these signaling pathways with other chemical compounds greatly reduced the reprogramming efficiency, suggesting that the activation of MAPK and SHH signaling pathways plays a pivotal role in the chemical direct reprogramming into neural stem cells. The present study provides great insights into the molecular mechanism underlying chemical induction of neural stem cells from mouse fibroblasts.
Most of these studies converted MEFs into neural stem cells, but for the clinical application, human adult fibroblasts have to be converted in a chemical compound-based manner. MEFs might have a relatively higher differentiation potential and plasticity than adult fibroblasts. Moreover, gene expression regulated by each chemical compound through signaling pathways and downstream transcription factors is supposed to be slightly different between mouse and human fibroblasts. Nevertheless, the identification of chemical compounds required for the conversion into neural stem/progenitor cells helps us further develop a combination of chemical compounds for human adult fibroblasts. The next step is to identify the combination showing a broad utility to the direct conversion in human fibroblasts. The chemically induced neural stem cell is a promising cell source for future transplantation therapy to treat neural injuries and degenerative diseases.
Mesenchymal stem cells (MSCs) are another cell source for direct reprogramming or transdifferentiation induced by chemical compounds. Previously, several reports have demonstrated that neural stem cell-like cells were converted from MSCs by culture medium including growth factors and chemical compounds without the use of any transgene, but the reproducibility and efficiency appeared not to be always sufficient. Recently, human adipose-derived MSCs were converted into neural stem cells by using SB431542, Noggin, and LDN193289, which are inhibitors of transforming growth factor β (TGFβ) and bone morphogenetic protein (BMP) signaling pathways [43]. This affords an efficient generation of functionally induced neural stem cells, and can be a cell source for transplantation therapy. Neural stem cells were also induced from mouse ES cell-derived astrocytes as well as primary astrocytes by a treatment with fibroblast growth factor 2 (FGF2) for 8 days [44]. The treatment with FGF2 promoted both proliferation and expression of Nestin, a neural stem cell marker, in the astrocytes, suggesting that downstream signaling pathways regulated by FGF2 play a critical role in the induction of neural stem cells.
Brown adipocytes
Obesity is a major risk concern for metabolic diseases including diabetes and cardiovascular diseases. Previous studies have revealed that brown adipocytes in our body also play an important role in physiological regulation of energy metabolism and heat production, which leads to prevention of obesity. Interestingly, it has been demonstrated that white adipocytes can be transdifferentiated into brown adipocytes, so-called beige adipocytes, to consume the storing lipid [45]. It still remains controversial whether white adipocytes or specific progenitor cells are changed into beige adipocytes in vivo [46]. So the direct reprogramming into brown adipocytes is an interesting approach to uncover the molecular mechanism underlying the transdifferentiation and proliferation in our body.
The traditional brown adipocytes are differentiated from myogenic precursor cells expressing Myf5 [47]. Recent studies have reported that functional brown adipocytes were prepared from iPS cells, MSCs, and myoblasts in a transcription factor-based or growth factor-based manner [48–50]. In 2009, Kajimura et al. [12] reported that forced expression of PRDM16 and C/EBP-β converted MEFs to induced brown adipocyte-like cells. Recently, human dermal fibroblasts were converted into brown adipocytes by forced expression of two transcription factors, C/EBP-β and C-MYC [51]. For the clinical application, a novel strategy without the use of transgenes had been anticipated to prepare brown adipocytes from human dermal fibroblasts.
Chemical compound-based direct reprogramming into brown adipocytes is a useful strategy to reveal how brown fat mass is chemically increased in our body. Recently, Nie et al. [52] reported that Bexarotene (Bex), a retinoid X receptor (RXR) agonist, directly converted myoblasts, one of the precursor cell types, into brown-like adipocytes (Table 3). Screening of 20000 chemicals in C2C12 cells, mouse skeletal myoblasts, identified that Bex treatment at 10 μM for 2 days induced brown adipogenic reprogramming. Oral administration of Bex into mice for 4 weeks along with feeding of a high-fat diet resulted in a slight increase in brown fat mass, which is associated with prevention of weight gain, increase in oxygen consumption rate, glucose/insulin tolerance, and cold tolerance. In addition, increase in brown adipocyte cell number and expression of the specific genes were observed in subcutaneous white adipose tissue (WAT), suggesting that Bex has a browning effect through RXR activation.
Table 3. Summary of chemical compound-based direct reprogramming into brown adipocytes and cardiomyocytes.
| Target cell type | Source cells | Generation | Chemical cocktail cytokines | Induction/maturation medium | References |
|---|---|---|---|---|---|
| Brown adipocyte | C2C12 cells (mouse skeletal myoblasts) | - | Bex | The basal adipogenesis medium with Bex for 2 days. The culture under basal adipogenesis conditions for another 4 days | [52] |
| Brown adipocyte | Human dermal fibroblasts | Adult (0, 38, and 49 years old) | SB431542, LDN193189, Forskolin, Dorsomorphin, and Rosiglitazone | The commercial adipocyte medium with the chemical cocktail for 3 weeks. The medium without the compounds, except for Rosiglitazone, for another 1 week | [53] |
| Cardiomyocyte | MEFs | Fetal | CHIR99021, RepSox Forskolin, VPA, Parnate, and TTNPB | The Cardiac Reprogramming Medium (CRM) with the chemical cocktail for 16 days. The Cardiomyocyte-maintaining medium (CMM) with 2i (CHIR99021 and PD0325901), leukemia inhibitory factor (LIF), and insulin for another 8 days | [63] |
| Cardiomyocyte | Human foreskin fibroblasts, human fetal lung fibroblasts | Neonatal fetal | CHIR99021, A83-01, BIX01294, AS8351, SC1, Y-27632, OAC2, SU16F, and JNJ10198409 | The chemical reprogramming medium (CRM) with the chemical cocktail for 6 days. The cardiac induction medium (CIM) for another 5 days. The conditioned medium incubated with H9 hESCs-derived cardiomyocytes for another 19 days | [65] |
We recently reported that human dermal fibroblasts were converted into chemical compound-induced brown adipocytes (ciBAs) [53]. Commercially available adipocyte medium in combination with the chemical cocktail consisting of SB431542, LDN193189, Dorsomorphin, Forskolin, and Rosiglitazone for 3–4 weeks efficiently converted human dermal fibroblasts into brown adipocytes (Figure 3). It should be noted that the chemical conversion is not dependent on age, gender, and isolation sites of the fibroblasts. ciBAs were sensitive to β-adrenergic receptor signaling activated by treatment with either isoproterenol or Forskolin, and showed elevated oxygen consumption rates. In the present study, the activation of WNT signaling pathway by CHIR99021 strongly inhibited the brown adipogenesis. According to a previous report [54], TGFβ signaling pathway negatively regulates adipogenesis, so the inhibition by SB431542 facilitated the direct conversion. The inhibition of BMP signaling pathways by LDN193189 and Dorsomorphin could be involved in the modulation of downstream Smad protein activities in collaboration with the TGFβ signaling pathway. Both peroxisome proliferator-activated receptor γ (PPARγ) activation by Rosiglitazone and cAMP induction by Forskolin play an important role in brown adipogenesis and the functional induction. The accumulation of these lines of evidence would lead to better strategies for the direct conversion and differentiation of brown adipocytes by chemical compounds. The ciBAs might be applied for not only transplantation therapy but also drug development to prevent obesity and metabolic diseases by activation of energy metabolism.
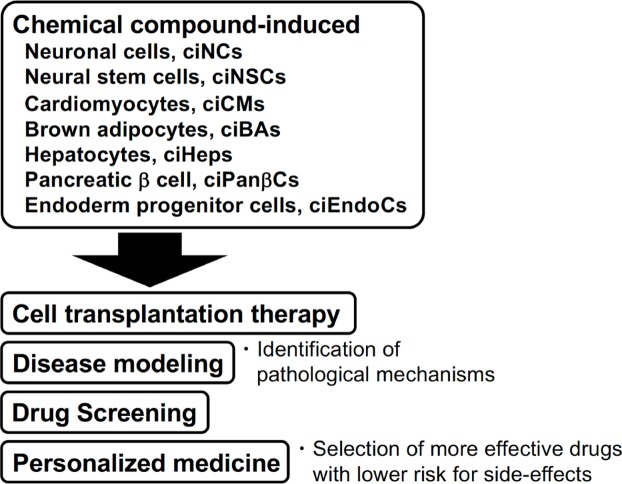
Figure 3. Possible applications of chemical compound-induced cells.
Chemical compound-induced cells (ciCells) rapidly prepared from autologous dermal fibroblasts can be a primary source for cell transplantation therapy. At the preclinical phase, the engraftment and tumorigenicity of ciCells need to be confirmed by using animal models for disease and injury. ciCells derived from each patient would be available as disease models to identify pathological mechanisms. Personalized medicine could be performed to select more effective drugs with lower risk for side effects using various kinds of ciCells. Thus, ciCells have a number of clear advantages for the clinical applications.
In vivo induction of brown adipogenic reprogramming has been performed by delivering browning agents using a microneedle-based patch [55]. Nanoparticles encapsulating Rosiglitazone were integrated with the microneedle array and they were subcutaneously injected into mice in a safe and effective manner. The subcutaneous adipose tissues were successfully converted into brown (beige) adipocytes, which is associated with increased energy expenditure, fatty acid oxidation, and improved insulin sensitivity. This technique is expected to allow clinical delivery of a chemical cocktail to locally induce direct reprogramming and to minimize side effects in other organs compared with oral and intravenous administrations.
Cardiomyocytes
Ieda et al. [10] first reported that cardiac fibroblasts that constituted over 50% of the cells in the heart were directly converted into cardiomyocyte-like cells by forced expression of a set of cardiac transcription factors, Gata4, Mef2c, and Tbx5. After the report, modified combinations of transcription factors and miRNAs converted mouse and human dermal fibroblasts into cardiomyocyte-like cells [56–58]. However, their conversion efficiencies appear not to be sufficient for clinical purposes. In an effort to improve the conversion efficiency, a number of small molecules and growth factors have been identified. A combination of FGF2, FGF10, and vascular EGF (VEGF) promoted the cardiac reprogramming of MEFs under serum-free defined medium [59–60]. In the other reports, CHIR99021, SB431542, Forskolin, Parnate, and Y-27632, which function as WNT signaling activator, TGFβ signaling inhibitor, cAMP inducer, lysine-specific demethylase 1 (LSD1) inhibitor, and Rho-associated kinase inhibitor (ROCK), respectively, promoted the direct reprogramming in combination with the cardiac transcription factors [61,62]. Such information obtained from the study on transcription factor-based direct reprogramming is greatly useful for the development of the chemical compound-based conversion into cardiomyocytes.
The transplantation of induced cardiomyocytes is a promising approach to retrieve cardiac functions if the loss of native cardiomyocytes is the fundamental pathology, e.g. cardiac infarction. For the clinical application, forced expression of exogenous genes by virus vectors should be avoided to reduce safety concerns. Chemical compounds potentially replace the effects of transcription factors in cardiac direct reprogramming. In 2015, Fu et al. [63] reported that MEFs were converted into cardiomyocyte-like cells by a combination of six chemical compounds (CRFVPT) including CHIR99021, RepSox, Forskolin, VPA, Parnate, and TTNPB. They unexpectedly found spontaneously contracting cells after the treatment with the chemical cocktail that had been already reported to reprogram MEFs into pluripotent stem cells [64]. The beating cells and clusters appeared 6 days after the treatment. Then for the maturation, first medium including the compounds was replaced with the cardiomyocyte maintaining medium supplemented with 2i (CHIR99021 and PD0325901) and leukemia inhibitory factor (LIF), whose combination was often used to keep a pluripotent state in mouse pluripotent stem cells. Amongst the compounds, CHIR99021, RepSox, Forskolin, and VPA were most critical for the cardiac reprogramming, indicating that the activation of the WNT signaling pathway and cellular cAMP-PKA pathway in combination with the inhibition of TGFβ signaling pathway is required. VPA functions as a histone deacetylase (HDAC) inhibitor, suggesting that the activation of gene expression through epigenetic changes further supports the cardiac direct reprogramming.
A combination of seven chemical compounds, CHIR99021, A8301, BIX01294, AS8351, SC1, Y-27632, and OAC2, successfully converted human foreskin fibroblasts into cardiomyocyte-like cells [65]. The authors also found that two inhibitors of platelet-derived growth factor receptors (PDGFRs), SU16F and JNJ10198409, enhanced the conversion efficiency by repressing fibroblast specific gene expression. After generation of spontaneously beating cells, the cells were further required to undergo a maturation step with the conditioned medium that was incubated with purified cardiomyocytes differentiated from human pluripotent stem cells. Without the conditioned medium, the beating cells did not strongly induce the expression of cardiomyocyte-specific genes such as Tnnt2 and Nkx2.5. Since the conditioned medium includes unknown factors secreted from the pluripotent stem cell-derived cardiomyocytes, the next step is to replace it with more defined medium containing chemical compounds and/or growth factors, particularly for a large-scale preparation of chemically induced cardiomyocytes for the transplantation therapy. In addition, several kinds of human adult fibroblasts with different ages, genders, and races need to be efficiently converted into the cardiomyocytes for the clinical use. There might be room for increase in the conversion efficiency by optimizing combinations of chemical compounds and experimental procedures.
So far these two groups have succeeded in the complete chemical conversion of MEFs and human fibroblasts into cardiomyocytes [64,65]. CHIR99021, GSK3β inhibitor, and RepSox or A83-01, TGFβ signaling inhibitors, were compounds common to the studies, suggesting that WNT activation and TGFβ inhibition are key pathways for the cardiac reprogramming. Forskolin was essential for the conversion of MEFs but for human fibroblasts Forskolin is not necessary, which might reflect a difference between species or culture conditions. The chemical cardiac reprogramming of human fibroblasts provides more insights into the molecular mechanism underlying the direct conversion. β-catenin and Smad1, which are major effectors in the downstream of WNT and BMP signaling pathways, respectively, are recruited to the promoter regions of heart developmental genes such as Mesp1, Gata4, Nkx2.5 [65]. Although how TGFβ inhibitors affect the BMP signaling pathway and the activity of Smad protein complex with Smad1 is unclear, these results suggest that the downstream effectors of specific signaling pathways play a critical role in the cardiac direct reprogramming. In addition, the treatment with the chemical cocktail enhanced active chromatin marks, H3K4me3 and H3K27ac, and a decreased repressive chromatin mark, H3K27me3. The early molecular events were likely related to enhanced accessibility of β-catenin and Smad1 to the heart developmental genes. According to the observation, epigenetic modulators, Bix01294 and AS8351, also play a critical role in the cardiac reprogramming because the loss of one of these compounds strongly reduced the reprogramming efficiency. In the case of MEFs, both cardiac precursor and lineage markers are immediately activated after the treatment with the chemicals, while in the case of human fibroblasts most of these cardiac markers are activated after the treatment with the conditioned medium of pluripotent stem cell-derived cardiomyocytes. In both cases, the fibroblasts were first converted into beating clusters by the chemical treatments, then a different step was required for the maturation. Further studies are required to improve the experimental protocol for efficient and large-scale preparation of the cardiomyocytes and to expand availability of the chemical cocktail in human adult dermal fibroblasts isolated from patients with various backgrounds.
Hepatocytes and pancreatic β cells
In chronic liver diseases such as hepatic cirrhosis and cancer, severely damaged hepatocytes cannot regenerate. Due to limited source of organ donors, cell transplantation therapy is a promising approach to treat such serious liver diseases by repopulating functional hepatocytes. So far, many trials have been made to generate induced hepatocytes from other cell sources such as ES/iPS cells, MSCs, and fibroblasts by forced expression of transcription factors as well as treatment with growth factors and small molecules [66–68]. Sekiya and Suzuki [11] first reported that hepatocytes can be converted from MEFs by forced expression of two transcription factors, Hnf4α and either Foxa1, Foxa2, or Foxa3. Modified combinations of transcription factors have been reported in human dermal fibroblasts [69,70]. The induced human hepatocytes were engrafted in immunodeficient mouse livers and restored the hepatic functions. Although hepatocytes without the use of transgene are desirable for the clinical application, the direct conversion from fibroblasts by chemical compounds only has not yet been reported.
Accumulating evidence has suggested that several chemical compounds play a pivotal role in hepatic differentiation and promotion of the direct reprogramming. Dexamethasone, an agonist for glucocorticoid receptor (GR), and nicotinamide are widely used for hepatic differentiation, maturation, and proliferation. One of the bile acids, tauroursodeoxycholic acid, enhanced hepatic differentiation of rat MSCs. Tauroursodeoxycholic acid functions through Farnesoid X receptor (FXR) and transmembrane G-protein-coupled bile acid receptor 5 (Table 4) [67]. Recently forced expression of Hnf1α in combination with CHIR99021 and A83-01 directly converted MEFs into hepatocyte-like cells [71]. These compounds promoted mesenchymal to epithelial transition (MET) which is required for the conversion from fibroblasts into induced hepatocytes. More recently, forced expression of either Foxa1, Foxa2, or Foxa3 in combination with seven chemical compounds, VPA, CHIR99021, RepSox, Forskolin, Parnate, DZNep, and TTNPB converted MEFs into hepatocytes [72]. These two reports suggest that combinations of chemical compounds could replace the effects of either Hnf1α or Foxa1–3, which might lead to further development of the hepatic reprogramming by chemical compounds only. The chemical approach for the hepatic reprogramming is promising for facilitating the transplantation for the treatment of chronic liver diseases.
Table 4. Direct reprogramming into hepatocyte-like cells by chemical compounds and exogenous gene induction.
| Target cell type | Source cells | Generation | Gene induction and chemical cocktail | Induction/maturation medium | References |
|---|---|---|---|---|---|
| Hepatocyte | Rat bone-marrow derived MSCs | Adult | Tauroursodeoxycholic acid | The IMDM-based medium with tauroursodeoxycholic acid for several weeks | [67] |
| Hepatocyte | MEFs | Fetal | Hnf1α and CHIR99021, A83-01 | After infection of retrovirus expressing Hnf1α, the hepatocyte culture medium with the compounds was incubated for 5 weeks | [71] |
| Hepatocyte | MEFs | Fetal | Foxa1, Foxa2, or Foxa3, and VPA, CHIR99021, RepSox, Forskolin, Parnate, DZNep, TTNPB | After infection with retrovirus expressing either Foxa1, Foxa2, or Foxa3, the hepatic reprogramming medium (HRM) with the chemical cocktail was incubated for 15 days. The hepatocyte maintaining medium (HMM) with the chemical cocktail for further culture | [72] |
The number of patients suffering from diabetes mellitus has been rapidly expanding all over the world, but the fundamental treatment is difficult without transplantation of islet or functional β cells. So the development of induced pancreatic β cells from other somatic cells is one of the most attractive strategies to treat diabetes. A number of studies have reported that functional β cells were transdifferentiated from pancreatic exocrine cells, pancreatic ductal cells, intestinal crypt cells, stomach cells, and hepatocytes by forced expression of β cell lineage-specific transcription factors such as Pdx1, Ngn3, and MafA [73–76]. These studies utilized developmentally relevant cells such as pancreatic cells and other endoderm lineage cells, suggesting that the starting cell types are critical for the direct reprogramming into β cells. Corresponding to the complicated developmental pathway of pancreatic β cells in vivo, proper differentiation from pluripotent stem cells requires many kinds of culture media, growth factors, and small molecules at each step of the differentiation [77,78].
In consideration of these background factors, chemical compound-based direct conversion of human dermal fibroblasts into functional β cells is challenging. In practice, such chemical conversion into β cells has not yet been reported. Sheng Ding and colleagues demonstrated that transient expression of the reprogramming factors such as Oct4, Sox2, Klf4, and c-Myc or P53 shRNA converted mouse and human fibroblasts into an intermediate [79,80]. Then, many steps using various combinations of chemical compounds and growth factors converted the intermediate into β-like cells via definitive endodermal progenitor cells and pancreatic endodermal progenitor cells. Now it is unclear whether it is feasible to directly convert human dermal fibroblasts into functional β cells by using a specific set of chemical compounds. However, such a chemical strategy is worthwhile for trying to rapidly prepare functional β cells from autologous fibroblasts for the transplantation to patients suffering from type I and type II diabetes.
The high-throughput screening of small molecules from several groups have identified candidate compounds for chemical compound-based pancreatic reprogramming. BRD7389, an inhibitor of ribosomal S6 kinase (RSK), changed the cell shape of mouse pancreatic α cells to a more β cell-like one and increased the expression of β cell-specific genes, Ins2 and Pdx1 [81]. BRD7552 was identified amongst 60000 compounds using PANC-1 cell, a human pancreatic ductal carcinoma cell line [82]. BRD7552 increased the expression of Pdx1 gene in both primary human islets and the ductal cells. 5′-Azadeoxycytidine (AZA), a DNA methyltransferase inhibitor, induced Ngn3 expression in PANC-1 cells and promoted an endocrine differentiation in the cells [83]. Harmine induced proliferation of murine and human β cells through inhibition of dual-specificity tyrosine-regulated kinase-1a (DYRK1A) [84]. More recently, one of the screened small molecules, AT7867, induced proliferation of Pdx1-positive pancreatic progenitor cells differentiated from human iPS cells [85].
Endoderm progenitor cells and somatic stem cells
Challenges to converting either differentiated endoderm cells or fibroblasts into the progenitor cells have been noted by several groups. Wang et al. [86] reported that human gastric epithelial cells (hGECs) isolated from the stomach were chemically converted into endoderm progenitor cells with tissue-specific mesenchymal feeder cells (Table 5). The human induced endoderm progenitor cells (hiEndoPCs) were proliferative and expressed endoderm markers, Sox17 and Foxa2. hiEndoPCs were subsequently differentiated into hepatocytes, pancreatic endocrine cells, and intestinal epithelial cells. The conversion requires human gastric subepithelial myofibroblasts (GSEMFs) as feeder cells, and a surgery to prepare these hGECs and GSEMFs from the stomach. Such partial reprogramming of fully differentiated endoderm cells is promising for preparing other important endoderm cells such as hepatocytes and β cells, which could be applied for transplantation therapy to treat chronic liver diseases and diabetes.
Table 5. Summary of chemical compound-based direct reprogramming into endoderm progenitor cells and somatic stem cells.
| Target cell type | Source cells | Generation | Chemical cocktail cytokines | Induction/maturation medium | References |
|---|---|---|---|---|---|
| Endoderm progenitor cells | hGECs | Adult (35–78 years old) | Bix01294, RG108, Bay k 8644, and SB431542 | The DMEM-F12 based medium with the chemical cocktail using human GSEMFs (hGSEMFs) as feeder cells for 7–15 days. The DMEM-F12 based medium with A83-01, Wnt3a, and bFGF for further culture and passage | [86] |
| Endoderm progenitor cells | MEFs | Fetal | RepSox, Forskolin, Y-27632, CHIR99021, TTNPB, bFGF, BMP4, and Activin A | The N2B27 medium with the chemical cocktail including TTNPB for 4 days (Stage 1). The N2B27 medium with the chemical cocktail including high concentration of CHIR99021 and Activin A instead of TTNPB for another 10 days (Stage 2). The modified medium mixed with the commercial HCM medium for further culture (Stage 3). | [87] |
| Chemically induced liver progenitors (CLiPs) | Rodent primary hepatocytes | Adult | Y-27632, A83-01, and CHIR99021 | Rodent primary hepatocytes under the culture treated with the three chemicals become proliferative without obvious phenotypic alterations. | [88] |
| MSC | Human dermal fibroblasts | Fetal adult | SP600125, SB202190, GO6983, Y-27632, PD0325901, and CHIR99021 | The chemical cocktail medium with the six chemicals and TGF-β1, bFGF, and LIF for 6 days. After FACS sorting, the MSC expansion medium for further culture and expansion | [91] |
Recently MEFs were chemically converted into endoderm progenitor cells by two sets of combinations of chemical compounds and growth factors [87]. MEFs were first treated with RepSox, Forskolin, Y-27632, a low concentration of CHIR99021, TTNPB, and two kinds of cytokines, bFGF and BMP4. In the second step, a high concentration of CHIR99021 and Activin A were added to the first chemical cocktail without TTNPB. The chemically induced endoderm progenitor cells (ciEPCs) formed colonies, and they were stably expanded over 13 passages. ciEPCs expressed endoderm marker genes such as Cdh1, Sox17, FoxoA2, and HNF4α. ciEPCs could be differentiated into hepatocyte-like cells in the specialized medium including chemical compounds and growth factors, DAPT, Dexamethasone, A83-01, hepatic growth factor (HGF), and oncostatin M (OSM). The hepatocyte-like cells differentiated from ciEPCs exhibited an expression pattern similar to the one of iPS cells-derived hepatocytes. ciEPCs were also differentiated into pancreatic progenitor cells expressing Pdx1; however, insulin expression was not confirmed. It is also a promising strategy to prepare clinically applicable hepatocytes and pancreatic β cells if human adult fibroblasts are successfully converted into the endoderm progenitor cells as well. Similar combinations of the chemicals and the protocols might be useful for performing the conversion of human fibroblasts into ciEPCs.
Katsuda et al. [88] successfully generated expandable hepatocyte progenitor cells by culturing rodent primary hepatocytes under the treatment with three chemicals, Y-27632, A83-01, and CHIR99021. The chemically induced liver progenitors (CLiPs) were stably proliferative under the chemical cocktail without any phenotypic alterations. CLiPs were bipotent and could be differentiated into both mature hepatocytes and biliary epithelial cells. In addition, CLiPs repopulated chronically injured liver tissues at a high rate (75–90%). The next step for the clinical application is to develop CLiPs from human primary hepatocytes; however, human CLiPs have not been generated with the same compounds, which is possibly due to difference in the molecular mechanisms between human and rodent [89]. A different set of chemical compounds might be required. The transplantation of CLiPs is a promising therapeutic strategy for liver regenerative medicine.
MSCs are multipotent somatic stem cells and can be differentiated into osteocytes, chondrocytes, and adipocytes. MSCs are generally isolated from the tissues such as bone marrow, adipose tissue, and umbilical cord blood. MSCs also have immunomodulatory effects to suppress immune response and participate in tissue repair by secreting multiple cytokines [90]. Human dermal fibroblasts were chemically converted into MSC-like cells at approximately 38% in just 6 days [91]. The chemical cocktail contains six compounds, SP600125, SB202190, GO6983, Y-27632, PD0325901, and CHIR99021, in combination with three growth factors, TGFβ1, bFGF, and LIF. The induced MSCs had a higher proliferation rate than fibroblasts, which was maintained for at least eight passages. The induced MSCs were differentiated into osteoblasts, adipocytes, and chondrocytes, similar to the multipotent capacity in MSCs isolated from human tissues. This technique is obviously useful to generate expandable MSCs from dermal fibroblasts isolated from each patient. The induced MSCs present little safety concern about tumorigenesis, so transplantation therapy using the induced MSCs themselves and differentiated osteocytes and chondrocytes might be available for future regenerative medicine.
Pluripotent stem cells
In 2013, Hou et al. [64] first reported that pluripotent stem cells were chemically reprogrammed from MEFs without any induction of transcription factors (Table 6). The treatment with a chemical cocktail containing seven compounds, VPA, CHIR99021, E-616452 (also named as RepSox), Tranylcypromine, Forskolin, DZNep, and TTNPB, generated chemically induced pluripotent stem cells (CiPSCs). They first identified that the effects of expression of Oct4, one of the reprogramming factors, could be obtained with substitution with Forskolin. Their group has already reported that the combination of VC6T (VPA, CHIR99021, E-616452, Tranylcypromine) in addition to forced expression of Oct4 could replace the effects of other reprogramming factors and reprogrammed MEFs toward iPS cells [15]. Therefore Forskolin, a potential substituent for Oct4, was combined with the VC6T. To achieve complete reprogramming into CiPSCs, DZNep was further added 16 days after the treatment with VC6TF. The generated CiPSCs were integrated with early embryos, and successfully generated viable chimera mice, suggesting that CiPSCs have a differentiation potential similar to iPS cells and ES cells.
Table 6. Summary of chemical compound-based reprogramming into induced pluripotent stem cells.
| Target cell type | Source cells | Generation | Chemical cocktail cytokines | Induction/maturation medium | References |
|---|---|---|---|---|---|
| Induced pluripotent stem cells | MEFs | Fetal | VC6TF: VPA, CHIR99021, E-616452, Tranylcypromine, and Forskolin VC6TFZ: VC6TF and DZNep | The chemical reprogramming medium with the chemical cocktail (-DZNep) for 16–20 days, followed by the medium with the chemical cocktail (+DZNep) for another 12–16 days. The medium supplemented with 2i, CHIR99021 and PD0325901, and LIF for further culture | [64] |
| Induced pluripotent stem cells | MEFs | Fetal | VC6TFAE: VPA, CHIR99021, E-616452, Tranylcypromine, Forskolin, AM580, and EPZ004777 VC6TFZASD: VC6TFZA and SGC0946, 5-Aza-dC | The chemical reprogramming medium with the chemical cocktail, VC6TFAE, for 16 days, followed by the medium with the chemical cocktail, VC6TFZASD, for another 12 days. The N2B27 medium with 2i (CHIR99021 and PD0325901) and LIF for another 12 days | [92] |
| Induced pluripotent stem cells | Mouse neural stem cells | Fetal and postnatal | VC6TFE5: VPA, CHIR99021, E-616452, Tranylcypromine, Forskolin, EPZ004777, and Ch55 VC6TFE5Z: VC6TFE5 and DZNep | The chemical reprogramming medium with the chemical cocktail, VC6TFE5 (E-616452: 2 or 5 μM), for 20 days, followed by the medium with the chemical cocktail, VC6TFE5Z (E-616452: 5 μM), for another 20 days. The N2B27 medium with 2i CHIR99021 and PD0325901 for further culture | [93] |
| Induced pluripotent stem cells | Mouse small intestinal epithelial cells | Fetal | VC6TFA: VPA, CHIR99021, E-616452, Tranylcypromine, Forskolin, and AM580 VC6TFZ: VC6TF and DZNep | The chemical reprogramming medium with the chemical cocktail, VC6TFA (E-616452: 20 μM), for 16 days, followed by the medium with the chemical cocktail, VC6TFZ (E-616452: 10 μM), for another 24 days. The N2B27 medium with 2i, CHIR99021 and PD0325901, for further culture | [93] |
Approximately 2 years later, the same group showed that extraembryonic endoderm (XEN)-like cells were an intermediate in the process of CiPSC generation [92]. The XEN-like cells strongly expressed two master genes for pluripotency, Sall4 and Lin28a, which makes the pluripotent state more accessible. The transition via XEN-like cells in the reprogramming process is unique for chemical-based, but not observed in transcription factor-based, reprogramming. In addition, the effects of several new compounds such as AM580, EPZ004777, SGC0946, and 5-Aza-dC on the chemical reprogramming were investigated. The retinoic acid receptor agonist and epigenetic modulators in combination with VC6TF increased the efficiency up to 1000-fold greater than that described in their previous reports. Not only MEFs but also neonatal dermal fibroblasts and adult lung fibroblasts were chemically reprogrammed, suggesting a broad availability of the chemical cocktail for the reprogramming. Further studies are required to reveal in more detail the molecular mechanism by which each chemical compound facilitates the chemical reprogramming through regulation of targetted signaling pathways and epigenetic modifications. The identification of an intermediate state is an important step to further improve the experimental protocol and the combination of chemical compounds for rapid and better reprogramming to a pluripotent state that resembles that of ES cells.
Moreover, their group also successfully converted mouse neural stem cells and small intestinal epithelial cells into CiPSCs [93]. Similar but slightly different combinations of the chemical compounds were required in these cell types, suggesting that a similar molecular mechanism underlying the chemical reprogramming might be conserved amongst diverse cell types. Although the combination of the core chemicals (VC6TF: VPA, CHIR99021, RepSox, Tranylcypromine, and Forskolin) was not changed, fine-tuning of the concentrations and treatment period was required in the chemical reprogramming of different cell types. In particular, RepSox, a potential substituent for Sox2 overexpression, is likely less required in the neural stem cells than the intestinal epithelial cells, probably because Sox2 is already expressed at a high level in the neural stem cells. The CiPSCs derived from the different cell types contributed to mouse chimera formation. Another report suggested that the chemically induced XEN-like cells were expandable without any genome instability and were directly converted into functional neurones and hepatocytes [94]. The XEN-like state provides an alternative strategy to prepare desired cell types by bypassing a pluripotent state.
CiPSCs might have higher developmental potentials than iPSCs induced by the reprogramming transcription factors [64,93,95]. CiPSCs might more efficiently contribute to chimera formation than the conventional iPSCs. Genome-wide analysis of DNA methylation revealed that CiPSCs were more hypomethylated than the conventional iPSCs, particularly in several regions of imprinted gene clusters [96]. The authors suggested that the hypomethylation status of CiPSC was closer to that of mouse ES cells, which might explain the higher developmental potentials of CiPSCs.
Because human somatic cells have not been reprogrammed into CiPSCs by chemical compounds only, the generation will have a great impact on this field. Several differences of signaling pathways between mouse and human pluripotent stem cells have been reported [97,98]. One of the most characteristic differences is that mouse ES cells maintain a naïve pluripotent state under the 2i (CHIR99021 and PD0325901) and LIF condition, while modification of multiple signaling pathways by chemical compounds was required to maintain such naïve pluripotency in human ES cells [99]. Therefore, the molecular mechanism underlying the chemical reprogramming into human CiPSCs might be more complicated than and different from that in the mouse. Possibly, the reprogramming requires a different set of chemical compounds. The chemical compound-based reprogramming in human somatic cells will be worth pursuing if CiPSCs have clear advantages in terms of safety, developmental potentials, and the derivation process.
Chemical compounds for direct reprogramming
In most cases, the combination of chemical compounds required for each direct conversion were empirically determined, and the detailed molecular mechanisms of the direct conversions remain largely unknown. However, identification of the chemical compounds provides great insights into how signaling pathways regulate the direct conversion. In addition, we need to understand how multiple signaling pathways could synergistically change one cell fate to another. A better understanding of the reprogramming process is obviously helpful to improve the efficiency and to perform complete epigenetic reprogramming into various cell types which are more similar to the in vivo ones.
A number of common chemicals are frequently used in the direct conversions from both mouse and human fibroblasts. CHIR99021 is one of the most frequently used compounds in various conversions into neurones, neural stem cells, cardiomyocytes, endoderm progenitor cells, and iPS cells, suggesting that activation of WNT signaling pathway by inhibiting GSK3 might potentially increase plasticity for cell fate changes in fibroblasts. On the other hand, our report indicated that CHIR99021 severely inhibited the brown adipogenic reprogramming, which suggests that a cell type-specific regulation of signaling pathways is required for chemical direct reprogramming [53]. TGFβ signaling pathway inhibitors such as SB431542, RepSox, and A83-01 were frequently used for the direct conversions into various cell types. Previous reports have suggested that the TGFβ inhibition is associated with facilitation of MET as well as suppression of profibrotic signals [100,101]. ROCK inhibitors such as Y-27632 and Thiazovivin were also used in the conversions into neurones, neural stem cells, cardiomyocytes, and endoderm and liver progenitor cells. A recent report demonstrated that ROCK inhibitor ameliorated cellular senescence and improved mitochondrial function by promoting metabolic reprogramming from glycolysis to oxidative phosphorylation in human fibroblasts [102]. In addition, the ROCK inhibition activated chromatin remodeling genes, which might be associated with a change in chromatin structures and epigenetic modifications. As shown in the cardiac direct reprogramming by transcription factors and small molecules [101], the inhibition of ROCK and TGFβ signaling pathways led to repressed expression of cytoskeletal and extracellular matrix proteins, which can facilitate direct reprogramming into other cell types, particularly from fibroblasts.
A few articles have reported a possible molecular mechanism underlying chemical direct reprogramming. A study on neural stem cell reprogramming suggested that downstream effectors, Elk1 and Gli2, in MAPK and SHH signaling pathways, respectively, were both directly bound to the promoter of Sox2 gene and activated the expression [42]. In human cardiac reprogramming, β-catenin and Smad1 in the downstream of the WNT and BMP signaling pathways are directly recruited to cardiac developmental genes [65]. In addition, the chemical cocktail required for the cardiac reprogramming opened a heterochromatin state into a euchromatin state, leading to enhanced chromatin accessibility of the downstream transcription factors. In both cases, the regulation of specific signaling pathways by chemical compounds could endogenously activate key developmental genes, leading to establishment of the cell type-specific transcriptional network. The combination of simultaneous activation of chromatin and specific signaling pathways is likely required to accomplish direct reprogramming. At the moment, this could be one of the most rational explanations for the molecular mechanism underlying complete chemical direct reprogramming.
One of the characteristics in chemically induced cells is low tumorigenicity, although whether it is really lower than that for the cells converted by transcription factors remains unknown. More careful studies will be required to evaluate the tumorigenicity in chemically induced cells by transplantation into animal models. The treatment with chemical compounds likely changes broad gene expression via downstream transcription factors of the related signaling pathways, which might also lead to an irreversible disruption of proper epigenetic states. It might increase the risk for tumorigenesis although this should be dependent on the properties of chemicals, concentration, incubation time, and so on.
Compared with gene expression regulated by specific transcription factors, chemical compounds do not always activate expression of a specific set of transcription factors required for the cell fate conversion and have an influence on a wide range of gene expression and epigenetic modifications. Therefore, to more specifically and selectively regulate signaling pathways and histone-modifying enzymes, the strategy of chemical biology is required to identify novel compounds from amongst a tremendous array of chemicals. Such novel compounds might improve on the direct reprogramming efficiency, and lead to the conversions into more kinds of cell types that have not yet been reported.
Conclusion
This review provides an overview of recent advances in chemical compound-based direct reprogramming. This field has rapidly developed based upon numerous studies on transcription factor-based direct reprogramming. The combinations of chemical compounds and the cell type-specific medium including growth factors will likely enable more cell fate conversions of somatic cells in the future. The use of chemical compounds instead of forced expression of transcription factors resolves several barriers which have been raised in the use of iPS cells and cells directly reprogrammed by transcription factors. Such small molecule-based direct conversions in a chemically defined medium will robustly facilitate the cell-based study on transplantation therapy, disease modeling, drug screening, and personalized medicine (Figure 3).
Further studies are required to more fully manipulate cell fates by chemical compounds only. In addition, most reported protocols for chemical compound-based direct reprogramming are performed and repeated by the same groups. To increase the availability in other laboratories as well as hospitals, the same or similar experiments need to be actively repeated by independent groups. Particularly, the chemical conversion of human fibroblasts into endoderm lineage cells such as hepatocytes and pancreatic β cells is clinically anticipated. The chemical compound-based strategy could become one of the main sources for cell transplantation therapy in the near future.
Abbreviations
- BAM
Brn2, Ascl1, and Myt1
- BDNF
brain-derived neurotrophic factor
- Bex
bexarotene
- bFGF
basic fibroblast growth factor
- BMP
bone morphogenetic protein
- ciBA
chemical compound-induced brown adipocyte
- ciEPC
chemically induced endoderm progenitor cell
- CiNC
chemical compound-induced neuronal cell
- CiPSC
chemically induced pluripotent stem cell
- CLiP
chemically induced liver progenitor
- EGF
epidermal growth factor
- ESCs
embryonic stem cells
- FGF2
fibroblast growth factor 2
- GABA
gamma-amino butyric acid
- GDNF
glial cell line-derived neurotrophic factor
- GSEMF
gastric subepithelial myofibroblast
- GSK3β
glycogen synthase kinase 3β
- H3K4me3
histone 3 lysine 4 trimethylation
- H3K27ac
histone 3 lysine 27 aectylation
- H3K27me3
histone 3 lysine 27 trimethylation
- hGEC
human gastric epithelial cell
- hiEndoPC
human induced endoderm progenitor cell
- LIF
leukemia inhibitory factor
- MAPK
mitogen-activated protein kinase
- MEF
mouse embryonic fibroblast
- MET
mesenchymal to epithelial transition
- MSC
mesenchymal stem cell
- NPC
neural progenitor cell
- NT3
Neurotrophin 3
- PKA
protein kinase A
- ROCK
Rho-associated kinase inhibitor
- RXR
retinoid X receptor
- SHH
sonic hedgehog
- TGFβ
transforming growth factor β
- XEN
extraembryonic endoderm
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Takahashi K. and Yamanaka S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 3.Maekawa M., Yamaguchi K., Nakamura T., Shibukawa R., Kodanaka I., Ichisaka T. et al. (2011) Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature 474, 225–229 10.1038/nature10106 [DOI] [PubMed] [Google Scholar]
- 4.Yoshihara M., Hayashizaki Y. and Murakawa Y. (2017) Genomic instability of iPSCs: challenges towards their clinical applications. Stem Cell Rev. 13, 7–16 10.1007/s12015-016-9680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masuda S., Miyagawa S., Fukushima S., Sougawa N., Okimoto K., Tada C. et al. (2015) Eliminating residual iPS cells for safety in clinical application. Protein Cell 6, 469–471 10.1007/s13238-015-0170-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Antonio M., Woodruff G., Nathanson J.L., D’Antonio-Chronowska A., Arias A., Matsui H. et al. (2017) High-throughput and cost-effective characterization of induced pluripotent stem cells. Stem Cell Rep. 8, 1101–1111 10.1016/j.stemcr.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozaki M., Iwanami A., Nagoshi N., Kohyama J., Itakura G., Iwai H. et al. (2017) Evaluation of the immunogenicity of human iPS cell-derived neural stem/progenitor cells in vitro. Stem Cell Res. 19, 128–138 10.1016/j.scr.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 8.Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Südhof T.C. and Wernig M. (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 10.1038/nature08797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ring K.L., Tong L.M., Balestra M.E., Javier R., Andrews-Zwilling Y., Li G. et al. (2012) Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 11, 100–109 10.1016/j.stem.2012.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ieda M., Fu J.D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B.G. et al. (2010) Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 10.1016/j.cell.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekiya S. and Suzuki A. (2011) Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 475, 390–393 10.1038/nature10263 [DOI] [PubMed] [Google Scholar]
- 12.Kajimura S., Seale P., Kubota K., Lunsford E., Frangioni J.V., Gygi S.P. et al. (2009) Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-β transcriptional complex. Nature 460, 1154–1158 10.1038/nature08262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huangfu D., Maehr R., Guo W., Eijkelenboom A., Snitow M., Chen A.E. et al. (2008) Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 26, 795–797 10.1038/nbt1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu S., Li W., Zhou H., Wei W., Ambasudhan R., Lin T. et al. (2010) Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 7, 651–655 10.1016/j.stem.2010.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y., Zhang Q., Yin X., Yang W., Du Y., Hou P. et al. (2011) Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 21, 196–204 10.1038/cr.2010.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu S., Ambasudhan R., Sun W., Kim H.J., Talantova M., Wang X. et al. (2014) Small molecules enable OCT4-mediated direct reprogramming into expandable human neural stem cells. Cell Res. 24, 126–129 10.1038/cr.2013.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minami I., Yamada K., Otsuji T.G., Yamamoto T., Shen Y., Otsuka S. et al. (2012) A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep. 2, 1448–1460 10.1016/j.celrep.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 18.Caiazzo M., Dell’Anno M.T., Dvoretskova E., Lazarevic D., Taverna S., Leo D. et al. (2011) Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227 10.1038/nature10284 [DOI] [PubMed] [Google Scholar]
- 19.Pang Z.P., Yang N., Vierbuchen T., Ostermeier A., Fuentes D.R., Yang T.Q. et al. (2011) Induction of human neuronal cells by defined transcription factors. Nature 476, 220–223 10.1038/nature10202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Son E.Y., Ichida J.K., Wainger B.J., Toma J.S., Rafuse V.F., Woolf C.J. et al. (2011) Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9, 205–218 10.1016/j.stem.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai P., Harada Y. and Takamatsu T. (2015) Highly efficient direct conversion of human fibroblasts to neuronal cells by chemical compounds. J. Clin. Biochem. Nutr. 56, 166–170 10.3164/jcbn.15-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toyokuni S. (2015) Chemical conversion of human fibroblasts into neuronal cells : dawn of future clinical trials. J. Clin. Biochem. Nutr. 56, 5086 10.3164/jcbn.56-3-editorial [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagoshi N. and Okano H. (2018) iPSC-derived neural precursor cells: potential for cell transplantation therapy in spinal cord injury. Cell Mol. Life Sci. 75, 989–1000 10.1007/s00018-017-2676-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., Zuo X., Jing J., Ma Y., Wang J., Liu D. et al. (2015) Small-molecule-driven direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell 17, 195–203 10.1016/j.stem.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 25.Hu W., Qiu B., Guan W., Wang Q., Wang M., Li W. et al. (2015) Direct conversion of normal and Alzheimer’s disease human fibroblasts into neuronal cells by small molecules. Cell Stem Cell 17, 204–212 10.1016/j.stem.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 26.Cheng L., Hu W., Qiu B., Zhao J., Yu Y., Guan W. et al. (2014) Generation of neural progenitor cells by chemical cocktails and hypoxia. Cell Res. 24, 665–679 10.1038/cr.2014.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinrich C., Blum R., Gascón S., Masserdotti G., Tripathi P., Sánchez R. et al. (2010) Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 8, e1000373 10.1371/journal.pbio.1000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torper O., Pfisterer U., Wolf D.A., Pereira M., Lau S., Jakobsson J. et al. (2013) Generation of induced neurons via direct conversion in vivo. Proc. Natl. Acad. Sci. U.S.A. 110, 7038–7043 10.1073/pnas.1303829110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Z., Zhang L., Wu Z., Chen Y., Wang F. and Chen G. (2014) In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 14, 188–202 10.1016/j.stem.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L., Yin J.C., Yeh H., Ma N.X., Lee G., Chen X.A. et al. (2015) Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell 17, 735–747 10.1016/j.stem.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Sloan S.A., Clarke L.E., Grant G.A., Gephart M.G.H., Barres B.A. et al. (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53 10.1016/j.neuron.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Y., Ping Y., Bian X., Shen L. and Pei G. (2017) Direct generation of human neuronal cells from adult astrocytes by small molecules. Stem Cell Rep. 8, 538–547 10.1016/j.stemcr.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian E., Sun G., Sun G., Chao J., Ye P., Warden C. et al. (2016) Small-molecule-based lineage reprogramming creates functional astrocytes. Cell Rep. 16, 781–792 10.1016/j.celrep.2016.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyss-Coray T. (2016) Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180–186 10.1038/nature20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Q., Huang T., Zhang L., Zhou Y., Luo H., Xu H. et al. (2016) Dysregulation of ubiquitin-proteasome system in neurodegenerative diseases. Front. Aging Neurosci. 8, 303 10.3389/fnagi.2016.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ocampo A., Reddy P., Martinez-Redondo P., Platero-Luengo A., Hatanaka F., Hishida T. et al. (2016) In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167, 1719–1733 10.1016/j.cell.2016.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mertens J., Paquola A.C.M., Ku M., Hatch E., Böhnke L., Ladjevardi S. et al. (2015) Directly reprogrammed human neurons retain aging-associated transcriptomic signatures and reveal age-related nucleocytoplasmic defects. Cell Stem Cell 17, 705–718 10.1016/j.stem.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathieu J., Zhou W., Xing Y., Sperber H., Ferreccio A., Agoston Z. et al. (2014) Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell 14, 592–605 10.1016/j.stem.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braunschweig L., Meyer A.K., Wagenführ L. and Storch A. (2015) Oxygen regulates proliferation of neural stem cells through Wnt/β-catenin signalling. Mol. Cell. Neurosci. 67, 84–92 10.1016/j.mcn.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 40.Han Y., Lim Y., Duffieldl M.D., Li H., Liu J., Pavathuparambil N. et al. (2016) Direct reprogramming of mouse fibroblasts to neural stem cells by small molecules. Stem Cells Int. 2016, 4304916 10.1155/2016/4304916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng J., Choi K.A., Kang P.J., Hyeon S., Kwon S., Moon J.H. et al. (2016) A combination of small molecules directly reprograms mouse fibroblasts into neural stem cells. Biochem. Biophys. Res. Commun. 476, 42–48 10.1016/j.bbrc.2016.05.080 [DOI] [PubMed] [Google Scholar]
- 42.Zhang M., Lin Y.H., Sun Y.J., Zhu S., Zheng J., Liu K. et al. (2016) Pharmacological reprogramming of fibroblasts into neural stem cells by signaling-directed transcriptional activation. Cell Stem Cell 18, 653–667 10.1016/j.stem.2016.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J., Lee N., Lee J., Choe E.K., Kim M.K., Byun M.S. et al. (2017) Small molecule-based lineage switch of human adipose-derived stem cells into neural stem cells and functional GABAergic neurons. Sci. Rep. 7, 10166 10.1038/s41598-017-10394-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleiderman S., Gutbier S., Tufekci K.U., Ortega F., Sá J.V, Teixeira A.P. et al. (2016) Conversion of non-proliferating astrocytes into neurogenic neural stem cells: control by FGF2 and IFN-gamma. Stem Cells 34, 2861–2874 10.1002/stem.2483 [DOI] [PubMed] [Google Scholar]
- 45.Wu J., Boström P., Sparks L.M., Ye L., Choi J.H., Giang A. et al. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenwald M. and Wolfrum C. (2017) The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 3, 4–9 10.4161/adip.26232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S. et al. (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 10.1038/nature07182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishio M., Yoneshiro T., Nakahara M., Suzuki S., Saeki K., Hasegawa M. et al. (2012) Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 16, 394–406 10.1016/j.cmet.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 49.Modica S., Straub L.G., Balaz M., Sun W., Wolfrum C., Varga L. et al. (2016) Bmp4 promotes a brown to white-like adipocyte shift. Cell Rep. 16, 2243–2258 10.1016/j.celrep.2016.07.048 [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y., Yang R., McLenithan J., Yu D., Wang H., Wang Y. et al. (2015) Direct conversion of human myoblasts into brown-like adipocytes by engineered super-active PPARγ. Obesity 23, 1014–1021 10.1002/oby.21062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kishida T., Ejima A., Yamamoto K., Tanaka S., Yamamoto T. and Mazda O. (2015) Reprogrammed functional brown adipocytes ameliorate insulin resistance and dyslipidemia in diet-induced obesity and type 2 diabetes. Stem Cell Rep. 5, 569–581 10.1016/j.stemcr.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nie B., Nie T., Hui X., Gu P., Mao L., Li K. et al. (2017) Brown adipogenic reprogramming induced by a small molecule. Cell Rep. 18, 624–635 10.1016/j.celrep.2016.12.062 [DOI] [PubMed] [Google Scholar]
- 53.Takeda Y., Harada Y., Yoshikawa T. and Dai P. (2017) Direct conversion of human fibroblasts to brown adipocytes by small chemical compounds. Sci. Rep. 7, 4304 10.1038/s41598-017-04665-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsurutani Y., Fujimoto M., Takemoto M., Irisuna H., Koshizaka M., Onishi S. et al. (2011) The roles of transforming growth factor-β and Smad3 signaling in adipocyte differentiation and obesity. Biochem. Biophys. Res. Commun. 407, 68–73 10.1016/j.bbrc.2011.02.106 [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y., Liu Q., Yu J., Yu S., Wang J., Qiang L. et al. (2017) Locally induced adipose tissue browning by microneedle patch for obesity treatment. ACS Nano 11, 9223–9230 10.1021/acsnano.7b04348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu J.D., Stone N.R., Liu L., Spencer C.I., Qian L., Hayashi Y. et al. (2013) Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 1, 235–247 10.1016/j.stemcr.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muraoka N., Yamakawa H., Miyamoto K., Sadahiro T., Umei T., Isomi M. et al. (2014) MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J. 33, 1565–1581 10.15252/embj.201387605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nam Y.J., Song K., Luo X., Daniel E., Lambeth K., West K. et al. (2013) Reprogramming of human fibroblasts toward a cardiac fate. Proc. Natl. Acad. Sci. U.S.A. 110, 5588–5593 10.1073/pnas.1301019110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamakawa H., Muraoka N., Miyamoto K., Sadahiro T., Isomi M., Haginiwa S. et al. (2015) Fibroblast growth factors and vascular endothelial growth factor promote cardiac reprogramming under defined conditions. Stem Cell Rep. 5, 1128–1142 10.1016/j.stemcr.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Umei T.C., Yamakawa H., Muraoka N., Sadahiro T., Isomi M., Haginiwa S. et al. (2017) Single-construct polycistronic doxycycline-inducible vectors improve direct cardiac reprogramming and can be used to identify the critical timing of transgene expression. Int. J. Mol. Sci. 18, 1–14 10.3390/ijms18081805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H., Cao N., Spencer C.I., Nie B., Ma T., Xu T. et al. (2017) Small molecules enable cardiac reprogramming of mouse fibroblasts with a single factor, Oct4. Cell Rep. 6, 951–960 10.1016/j.celrep.2014.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y., Londono P., Cao Y., Sharpe E.J., Proenza C., O’Rourke R. et al. (2015) High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nat. Commun. 6, 8243 10.1038/ncomms9243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu Y., Huang C., Xu X., Gu H., Ye Y., Jiang C. et al. (2015) Direct reprogramming of mouse fibroblasts into cardiomyocytes with chemical cocktails. Cell Res. 25, 1013–1024 10.1038/cr.2015.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hou P., Li Y., Zhang X., Liu C., Guan J., Li H. et al. (2013) Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 341, 651–654 10.1126/science.1239278 [DOI] [PubMed] [Google Scholar]
- 65.Cao N., Huang Y., Zheng J., Spencer C.I., Zhang Y., Fu J.D. et al. (2016) Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science 352, 1216–1220 10.1126/science.aaf1502 [DOI] [PubMed] [Google Scholar]
- 66.Li Q., Hutchins A.P., Chen Y., Li S., Shan Y., Liao B. et al. (2017) A sequential EMT-MET mechanism drives the differentiation of human embryonic stem cells towards hepatocytes. Nat. Commun. 8, 15166 10.1038/ncomms15166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sawitza I., Kordes C., Götze S., Herebian D. and Häussinger D. (2015) Bile acids induce hepatic differentiation of mesenchymal stem cells. Sci. Rep. 5, 13320 10.1038/srep13320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lysy P.A., Smets F., Sibille C., Najimi M. and Sokal E.M. (2007) Human skin fibroblasts: from mesodermal to hepatocyte-like differentiation. Hepatology 46, 1574–1585 10.1002/hep.21839 [DOI] [PubMed] [Google Scholar]
- 69.Huang P., Zhang L., Gao Y., He Z., Yao D., Wu Z. et al. (2014) Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 14, 370–384 10.1016/j.stem.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 70.Du Y., Wang J., Jia J., Song N., Xiang C., Xu J. et al. (2014) Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell 14, 394–403 10.1016/j.stem.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 71.Lim K.T., Lee S.C., Gao Y., Kim K.P., Song G., An S.Y. et al. (2016) Small molecules facilitate single factor-mediated hepatic reprogramming. Cell Rep. 15, 814–829 10.1016/j.celrep.2016.03.071 [DOI] [PubMed] [Google Scholar]
- 72.Guo R., Tang W., Yuan Q., Hui L., Wang X. and Xie X. (2017) Chemical cocktails enable hepatic reprogramming of mouse fibroblasts with a single transcription factor. Stem Cell Rep. 9, 499–512 10.1016/j.stemcr.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Q., Brown J., Kanarek A., Rajagopal J. and Melton D.A. (2008) In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455, 627–632 10.1038/nature07314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J., Sugiyama T., Liu Y., Wang J., Gu X., Lei J. et al. (2013) Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife 2, e00940 10.7554/eLife.00940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y. J., Finkbeiner S.R., Weinblatt D., Emmett M. J., Tameire F., Yousefi M. et al. (2014) De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Rep. 6, 1046–1058 10.1016/j.celrep.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ariyachet C., Tovaglieri A., Xiang G., Lu J., Shah M.S., Richmond C.A. et al. (2016) Reprogrammed stomach tissue as a renewable source of functional β cells for blood glucose regulation. Cell Stem Cell 18, 410–421 10.1016/j.stem.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pagliuca F.W., Millman J.R., Gürtler M., Segel M., Van Dervort A., Ryu J.H. et al. (2014) Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 10.1016/j.cell.2014.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Millman J.R., Xie C., Van Dervort A., Gürtler M., Pagliuca F.W. and Melton D.A. (2016) Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat. Commun. 7, 11463 10.1038/ncomms11463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li K., Zhu S., Russ H.A., Xu S., Xu T., Zhang Y. et al. (2014) Small molecules facilitate the reprogramming of mouse fibroblasts into pancreatic lineages. Cell Stem Cell 14, 228–236 10.1016/j.stem.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu S., Russ H.A., Wang X., Zhang M., Ma T., Xu T. et al. (2016) Human pancreatic beta-like cells converted from fibroblasts. Nat. Commun. 7, 10080 10.1038/ncomms10080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fomina-Yadlin D., Kubicek S., Walpita D., Dancik V., Hecksher-Sorensen J., Bittker J.A. et al. (2010) Small-molecule inducers of insulin expression in pancreatic alpha-cells. Proc. Natl. Acad. Sci. U.S.A. 107, 15099–15104 10.1073/pnas.1010018107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan Y., Hartland K., Boskovic Z., Wang Y., Walpita D., Lysy P.A. et al. (2013) A small-molecule inducer of pdx1 expression identified by high-throughput screening. Chem. Biol. 20, 1513–1522 10.1016/j.chembiol.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lefebvre B., Belaich S., Longue J., Vandewalle B., Oberholzer J., Gmyr V. et al. (2010) 5′-AZA induces Ngn3 expression and endocrine differentiation in the PANC-1 human ductal cell line. Biochem. Biophys. Res. Commun. 391, 305–309 10.1016/j.bbrc.2009.11.054 [DOI] [PubMed] [Google Scholar]
- 84.Wang P., Alvarez-Perez J.C., Felsenfeld D.P., Liu H., Sivendran S., Bender A. et al. (2015) A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat. Med. 21, 383–388 10.1038/nm.3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kimura A., Toyoda T., Nishi Y., Nasu M., Ohta A. and Osafune K. (2017) Small molecule AT7867 proliferates PDX1-expressing pancreatic progenitor cells derived from human pluripotent stem cells. Stem Cell Res. 24, 61–68 10.1016/j.scr.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 86.Wang Y., Qin J., Wang S., Zhang W., Duan J., Zhang J. et al. (2016) Conversion of human gastric epithelial cells to multipotent endodermal progenitors using defined small molecules. Cell Stem Cell 19, 449–461 10.1016/j.stem.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 87.Cao S., Yu S., Chen Y., Wang X., Zhou C., Liu Y. et al. (2017) Chemical reprogramming of mouse embryonic and adult fibroblast into endoderm lineage. J. Biol. Chem. 292, 19122–19132 10.1074/jbc.M117.812537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katsuda T., Kawamata M., Hagiwara K., Takahashi R., Yamamoto Y., Camargo F.D. et al. (2017) Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell 20, 41–55 10.1016/j.stem.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 89.Katsuda T. and Ochiya T. (2017) Biological and clinical insights offered by chemically induced liver progenitors (CLiPs). Stem Cell Invest. 4, 68 10.21037/sci.2017.08.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y., Chen X., Cao W. and Shi Y. (2014) Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 15, 1009–1016 10.1038/ni.3002 [DOI] [PubMed] [Google Scholar]
- 91.Lai P.L., Lin H., Chen S.F., Yang S.C., Hung K.H., Chang C.F. et al. (2017) Efficient generation of chemically induced mesenchymal stem cells from human dermal fibroblasts. Sci. Rep. 7, 44534 10.1038/srep44534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao Y., Zhao T., Guan J., Zhang X., Fu Y., Ye J. et al. (2015) A XEN-like state bridges somatic cells to pluripotency during chemical reprogramming. Cell 163, 1678–1691 10.1016/j.cell.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 93.Ye J., Ge J., Zhang X., Cheng L., Zhang Z., He S. et al. (2016) Pluripotent stem cells induced from mouse neural stem cells and small intestinal epithelial cells by small molecule compounds. Cell Res. 26, 34–45 10.1038/cr.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X., Liu D., Ma Y., Du X., Jing J., Wang L. et al. (2017) Direct reprogramming of fibroblasts via a chemically induced XEN-like state. Cell Stem Cell 21, 264–273 10.1016/j.stem.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 95.Stadtfeld M., Apostolou E., Akutsu H., Fukuda A., Follett P., Natesan S. et al. (2010) Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature 465, 175–181 10.1038/nature09017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ping W., Hu J., Hu G., Song Y., Xia Q., Yao M. et al. (2018) Genome-wide DNA methylation analysis reveals that mouse chemical iPSCs have closer epigenetic features to mESCs than OSKM-integrated iPSCs. Cell Death Dis. 9, 187 10.1038/s41419-017-0234-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van der Jeught M., Heindryckx B., O’Leary T., Duggal G., Ghimire S., Lierman S. et al. (2014) Treatment of human embryos with the TGFβ inhibitor SB431542 increases epiblast proliferation and permits successful human embryonic stem cell derivation. Hum. Reprod. 29, 41–48 10.1093/humrep/det400 [DOI] [PubMed] [Google Scholar]
- 98.Cai J., Xie D., Fan Z., Chipperfield H., Marden J., Wong W.H. et al. (2010) Modeling co-expression across species for complex traits: Insights to the difference of human and mouse embryonic stem cells. PLoS Comput. Biol. 6, e1000707 10.1371/journal.pcbi.1000707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gafni O., Weinberger L., Mansour A.A., Manor Y.S., Chomsky E., Ben-Yosef D. et al. (2013) Derivation of novel human ground state naive pluripotent stem cells. Nature 504, 282–286 10.1038/nature12745 [DOI] [PubMed] [Google Scholar]
- 100.Verrecchia F., Chu M. L. and Mauviel A. (2001) Identification of novel TGF-β/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J. Biol. Chem. 276, 17058–17062 10.1074/jbc.M100754200 [DOI] [PubMed] [Google Scholar]
- 101.Zhao Y., Londono P., Cao Y., Sharpe E.J., Proenza C., O’Rourke R. et al. (2015) High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nat. Commun. 6, 8243 10.1038/ncomms9243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park J.T., Kang H.T., Park C.H., Lee Y.S., Cho K.A. and Park S.C. (2018) A crucial role of ROCK for alleviation of senescence-associated phenotype. Exp. Gerontol. 106, 8–15 10.1016/j.exger.2018.02.012 [DOI] [PubMed] [Google Scholar]